The Applications of Electrochemical Immunosensors in the Detection of Disease Biomarkers: A Review
Abstract
1. Introduction
2. Types of Electrochemical Immunosensors
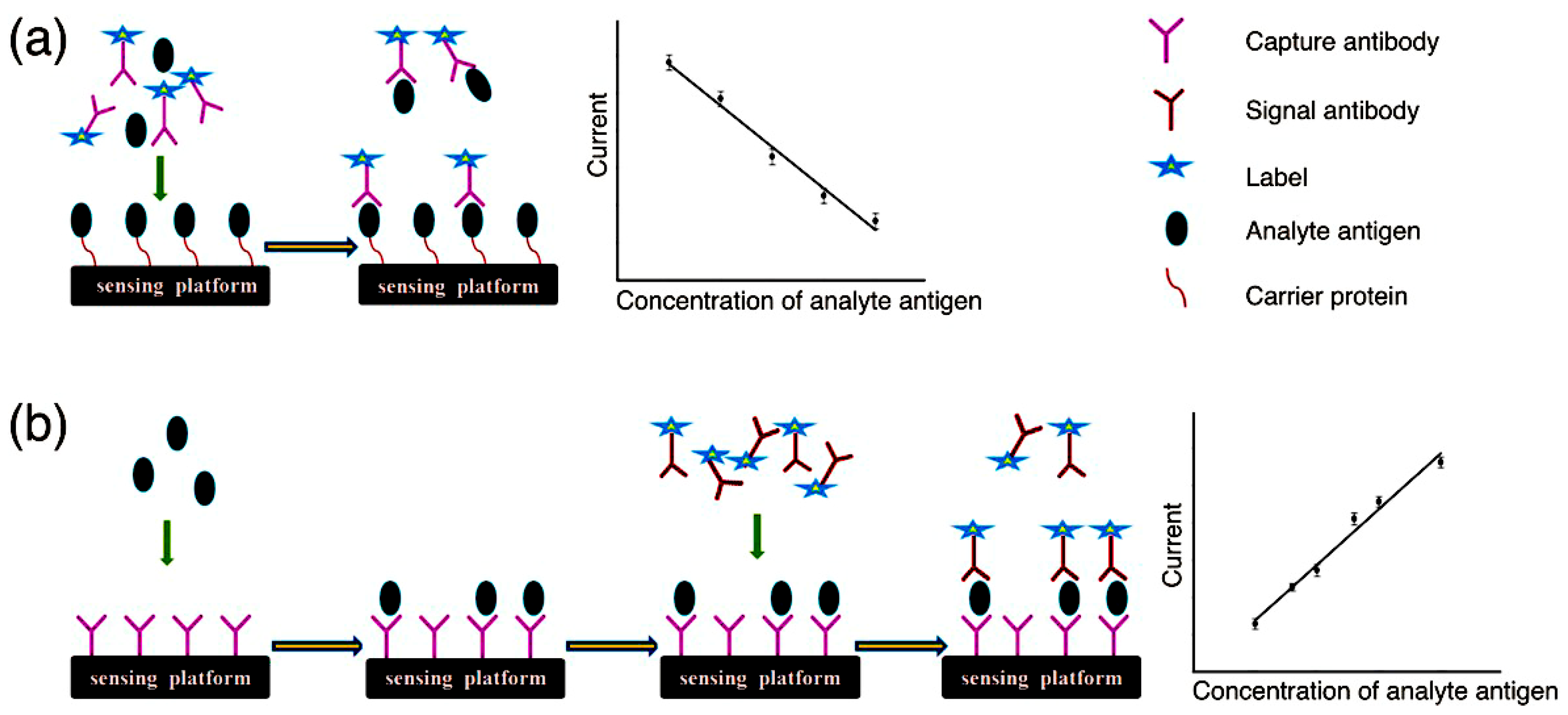
2.1. Competitive Electrochemical Immunosensors
2.2. Noncompetitive Electrochemical Immunosensors
3. Competitive Immunosensors
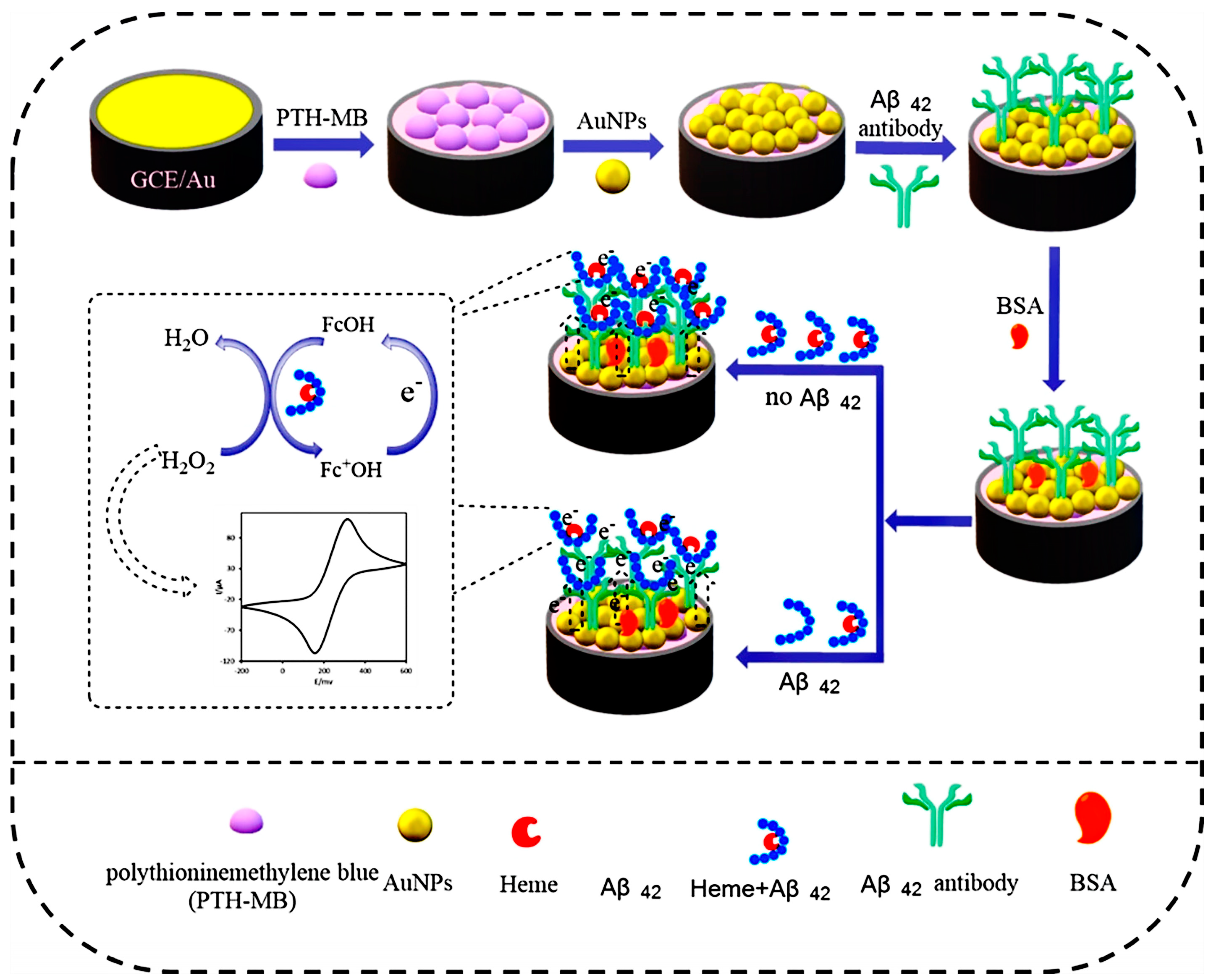
3.1. Redox-Couple-Labeled Electrochemical Immunosensors
3.2. Enzyme-Labeled Electrochemical Immunosensors
3.2.1. Horseradish Peroxidase
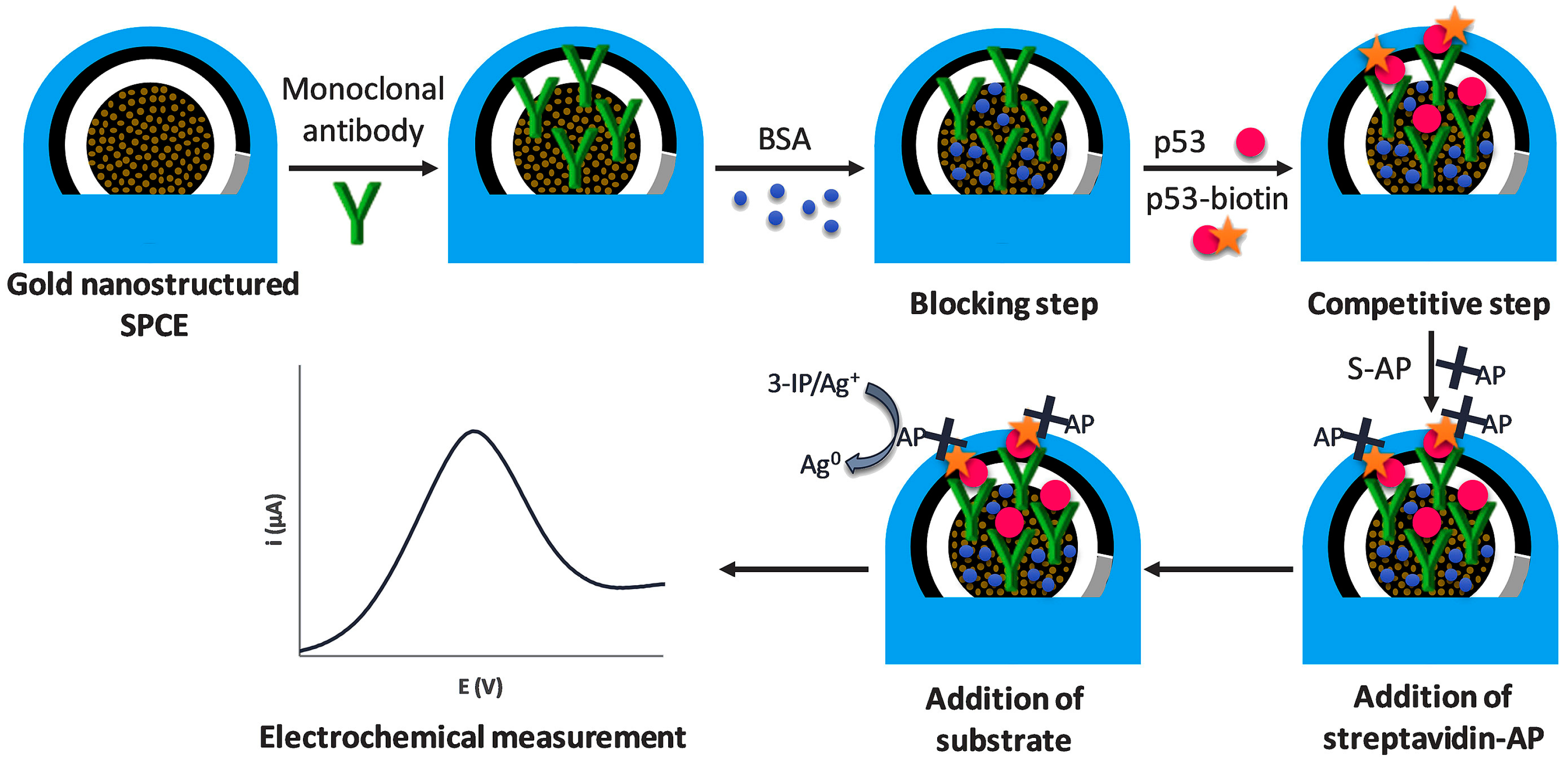
3.2.2. Alkaline Phosphatase
3.3. Nanomaterial-Based Electrochemical Immunosensors
3.3.1. Metal Nanomaterials
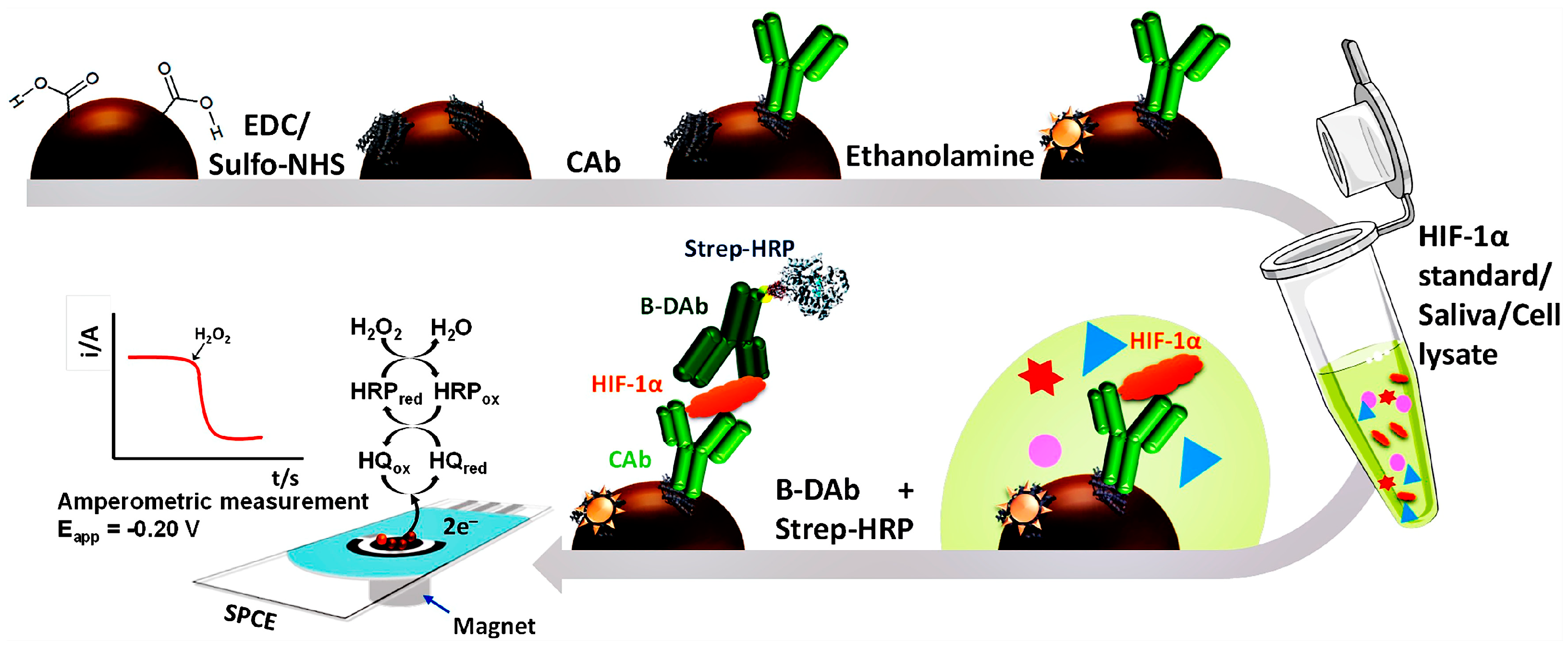
3.3.2. Magnetic Nanomaterials
4. Noncompetitive Immunosensors
4.1. Redox-Couple-Labeled Electrochemical Immunosensors
4.2. Enzyme-Labeled Electrochemical Immunosensor
4.2.1. Horseradish Peroxidase
4.2.2. Alkaline Phosphatase
4.2.3. Urease
4.3. Nanomaterial-Based Electrochemical Immunosensors
4.3.1. Metal Nanomaterials
4.3.2. Magnetic Nanomaterials
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brazaca, L.C.; Moreto, J.R.; Martin, A.; Tehrani, F.; Wang, J.; Zucolotto, V. Colorimetric paper-based immunosensor for simultaneous determination of fetuin B and clusterin toward early Alzheimer’s diagnosis. ACS Nano 2019, 13, 13325–13332. [Google Scholar] [CrossRef] [PubMed]
- Asensio, M.G.; Aranguez, A.G.; Povedano, E.; Montiel, V.R.V.; Poves, C.; Acenero, M.J.F.; Calle, A.M.; Fernandez, G.S.; Diez, S.F.; Camps, J. Multiplexed monitoring of a novel autoantibody diagnostic signature of colorectal cancer using HaloTag technology-based electrochemical immunosensing platform. Theranostics 2020, 10, 3022–3034. [Google Scholar] [CrossRef] [PubMed]
- Bahavarnia, F.; Saadati, A.; Hassanpour, S.; Hasanzadeh, M.; Shadjou, N.; Hassanzadeh, A. Paper based immunosensing of ovarian cancer tumor protein CA 125 using novel nano-ink: A new platform for efficient diagnosis of cancer and biomedical analysis using microfluidic paper-based analytical devices (muPAD). Int. J. Biol. Macromol. 2019, 138, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Ma, Z. Multiple functional strategies for amplifying sensitivity of amperometric immunoassay for tumor markers: A review. Biosens. Bioelectron. 2017, 98, 100–112. [Google Scholar] [CrossRef]
- Jones, A.; Dhanapala, L.; Kankanamage, R.N.T.; Kumar, C.V.; Rusling, J.F. Multiplexed immunosensors and immunoarrays. Anal. Chem. 2020, 92, 345–362. [Google Scholar] [CrossRef]
- Qi, M.; Zhang, Y.; Cao, C.; Zhang, M.; Liu, S.; Liu, G. Decoration of reduced graphene oxide nanosheets with aryldiazonium salts and gold nanoparticles toward a label-free amperometric immunosensor for detecting cytokine tumor necrosis factor-alpha in live cells. Anal. Chem. 2016, 88, 9614–9621. [Google Scholar] [CrossRef]
- Farzin, L.; Shamsipur, M. Recent advances in design of electrochemical affinity biosensors for low level detection of cancer protein biomarkers using nanomaterial-assisted signal enhancement strategies. J. Pharm. Biomed. Anal. 2018, 147, 185–210. [Google Scholar] [CrossRef]
- Cao, Y.; Dai, Y.; Chen, H.; Tang, Y.; Chen, X.; Wang, Y.; Zhao, J.; Zhu, X. Integration of fluorescence imaging and electrochemical biosensing for both qualitative location and quantitative detection of cancer cells. Biosens. Bioelectron. 2019, 130, 132–138. [Google Scholar] [CrossRef]
- Lim, S.A.; Ahmed, M.U. Electrochemical immunosensors and their recent nanomaterial-based signal amplification strategies: A review. RSC Adv. 2016, 6, 24995–25014. [Google Scholar] [CrossRef]
- Rodriguez, R.M.T.; Martín, C.M.S.; Gamella, M.; Pedrero, M.; Bosch, N.M.; Navarro, P.; Frutos, P.G.d.; Pingarron, J.M.; Campuzano, S. Electrochemical immunosensing of ST2: A checkpoint target in cancer diseases. Biosensors 2021, 11, 202. [Google Scholar] [CrossRef]
- Khanmohammadi, A.; Aghaie, A.; Vahedi, E.; Qazvini, A.; Ghanei, M.; Afkhami, A.; Hajian, A.; Bagheri, H. Electrochemical biosensors for the detection of lung cancer biomarkers: A review. Talanta 2020, 206, 120251. [Google Scholar] [CrossRef]
- Bolotsky, A.; Butler, D.; Dong, C.; Gerace, K.; Glavin, N.R.; Muratore, C.; Robinson, J.A.; Ebrahimi, A. Two-dimensional materials in biosensing and healthcare: From in vitro diagnostics to optogenetics and beyond. ACS Nano 2019, 13, 9781–9810. [Google Scholar] [CrossRef]
- Ding, M.D.; Shu, Q.; Zhang, N.; Yan, C.; Niu, H.; Li, X.; Guan, P.; Hu, X. Electrochemical immunosensor for the sensitive detection of Alzheimer’s biomarker amyloid-β (1–42) using the heme-amyloid-β (1–42) complex as the signal source. Electroanalysis 2022, 34, 263–274. [Google Scholar] [CrossRef]
- Gutierrez, O.A.; Rama, E.C.; Varas, N.A.; Rodriguez, C.M.; Novelli, A.; Sanchez, M.T.F.; Garcia, A.C. Competitive electrochemical immunosensor for the detection of unfolded p53 protein in blood as biomarker for Alzheimer’s disease. Clin. Chim. Acta 2020, 1093, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Malla, P.; Liao, H.P.; Liu, C.H.; Wu, W.C. Electrochemical immunoassay for serum parathyroid hormone using screenprinted carbon electrode and magnetic beads. J. Electroanal. Chem. 2021, 895, 115463. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, J.; Zhou, B.; Ian, H.; Huaiyu, S. Advanced sensitivity amplification strategies for voltammetric immunosensors of tumor marker: State of the art. Biosens. Bioelectron. 2021, 178, 113021. [Google Scholar] [CrossRef]
- Chen, G.; Roy, I.; Yang, C.; Prasad, P.N. Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy. Chem. Rev. 2016, 116, 2826–2885. [Google Scholar] [CrossRef]
- Li, F.; Feng, Y.; Dong, P.; Tanga, B. Gold nanoparticles modified electrode via a mercapto-diazoaminobenzene monolayer and its development in DNA electrochemical biosensor. Biosens. Bioelectron. 2010, 25, 2084–2088. [Google Scholar] [CrossRef]
- Nie, R.; Xu, X.; Cui, X.; Chen, Y.; Yang, L. A highly sensitive capillary-based immunosensor by combining with peroxidase nanocomplex-mediated signal amplification for detection of procalcitonin in human serum. ACS Omega 2019, 4, 6210–6217. [Google Scholar] [CrossRef]
- Khan, M.Z.H.; Hasan, M.R.; Hossain, S.I.; Ahommed, M.S.; Daizy, M. Ultrasensitive detection of pathogenic viruses with electrochemical biosensor: State of the art. Biosens. Bioelectron. 2020, 166, 112431. [Google Scholar] [CrossRef]
- Zhu, G.; Yin, X.; Jin, D.; Zhang, B.; Gu, Y.; An, Y. Paper-based immunosensors: Current trends in the types and applied detection techniques. TrAC Trends Anal. Chem. 2019, 111, 100–117. [Google Scholar] [CrossRef]
- Peng, C.; Hua, M.Y.; Li, N.S.; Hsu, Y.P.; Chen, Y.T.; Chuang, C.K.; Pang, S.T.; Yang, H.W. A colorimetric immunosensor based on self-linkable dual-nanozyme for ultrasensitive bladder cancer diagnosis and prognosis monitoring. Biosens. Bioelectron. 2019, 126, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Cesewski, E.; Johnson, B.N. Electrochemical biosensors or pathogen detection. Biosens. Bioelectron. 2020, 159, 112214. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Thalmayer, A.S.; Zeising, S.; Fischer, G.; Lubke, M. Commercial and scientific solutions for blood glucose monitoring-a review. Sensors 2022, 22, 425. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.M.; Guzman, M.M.; Lopez, M.A.; Escarpa, A. An array-based electrochemical magneto-immunosensor for early neonatal sepsis diagnostic: Fast and accurate determination of C-reactive protein in whole blood and plasma samples. Microchem. J. 2020, 157, 104913. [Google Scholar] [CrossRef]
- Sanati, A.; Jalali, M.; Raeissi, K.; Karimzadeh, F.; Kharaziha, M.; Mahshid, S.S.; Mahshid, S. A review on recent advancements in electrochemical biosensing using carbonaceous nanomaterials. Microchim. Acta 2019, 186, 773. [Google Scholar] [CrossRef]
- Aguilar, M.V.; Jofre, C.F.; Baldo, F.M.A.; Alonso, A.; Angel, S.; Raba, J.; Pereira, S.V.; Messina, G.A. Serological diagnosis of toxoplasmosis disease using a fluorescent immunosensor with chitosan-ZnO-nanoparticles. Anal. Biochem. 2019, 564−565, 116–122. [Google Scholar] [CrossRef]
- Moura, S.L.; Rusinol, A.P.; Sappia, L.; Martí, M.; Pividori, M.P. The activity of alkaline phosphatase in breast cancer exsomes simplifies the biosensing design. Biosens. Bioelectron. 2022, 198, 113826. [Google Scholar] [CrossRef]
- Biljana, M.; Larsen, S.; Olsen, D.A.; Madsen, J.S.; Brandslund, I.; Alatraktchi, F.A.A. Rapid SARS-CoV-2 detection using electrochemical immunosensor. Sensors 2021, 21, 390. [Google Scholar] [CrossRef]
- Kim, J.; Park, M. Recent progress in electrochemical immunosensors. Biosensors 2021, 11, 360. [Google Scholar] [CrossRef]
- Huo, X.; Liu, X.; Liu, J.; Sukumaran, P.; Alwarappan, S.; Wong, D.K.Y. Strategic applications of nanomaterials as sensing platforms and signal amplification markers at electrochemical immunosensors. Electroanalysis 2016, 28, 1730–1749. [Google Scholar] [CrossRef]
- Luppa, B.; Sokoll, J.; Chan, W. Immunosensors-principles and applications to clinical chemistry. Clin. Chim. Acta 2001, 314, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Zhou, L.; Feng, L.; Ding, Y.; Shi, H.; Wang, L.; Gee, S.J.; Hammock, B.D.; Wang, M. Competitive and noncompetitive phage immunoassays for the determination of benzothiostrobin. Anal. Chim. Acta 2015, 890, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Zhu, D.Z.; Gan, H.; Yao, Z.; Fu, Q.; Zhang, X.J. Prospects and challenges of using electrochemical immunosensors as an alternative detection method for SARS-CoV-2 wastewater-based epidemiology. Sci. Total Environ. 2021, 777, 146239. [Google Scholar] [CrossRef]
- Seddaoui, N.; Amine, A. Smartphone-based competitive immunoassay for quantitative on-site detection of meat adulteration. Talanta 2021, 230, 122346. [Google Scholar] [CrossRef]
- Wang, X.; Cohen, L.; Wang, J.; Walt, D.R. Competitive immunoassays for the detection of small molecules using single molecule Arrays. J. Am. Chem. Soc. 2018, 140, 18132–18139. [Google Scholar] [CrossRef]
- Liua, A.; Anfossib, L.; Shena, L.; Lia, C.; Wang, X. Non-competitive immunoassay for low-molecular-weight contaminant detection in food, feed and agricultural products: A mini-review. Trends Food Sci. Technol. 2018, 71, 181–187. [Google Scholar] [CrossRef]
- Khan, M.S.; Ameer, H.; Chi, Y. Label-free and sensitive electrochemiluminescent immunosensor based on novel luminophores of Zn2SnO4 nanorods. Sens. Actuators B Chem. 2021, 337, 129761. [Google Scholar] [CrossRef]
- Warsinke, A.; Benkert, A.; Scheller, F.W. Electrochemical immunoassays. Fresenius J. Anal. Chem. 2000, 366, 622–634. [Google Scholar] [CrossRef]
- Fu, H.J.; Chen, Z.J.; Wang, H.; Luo, L.; Wang, Y.; Huang, R.M.; Xu, Z.L.; Hammock, B. Development of a sensitive non-competitive immunoassay via immunocomplex binding peptide or the determination of ethyl carbamate in wine samples. J. Hazard. Mater. 2021, 406, 124288. [Google Scholar] [CrossRef]
- Soto, D.; Orozco, J. Hybrid Nanobioengineered nanomaterial-based electrochemical biosensors. Molecules 2022, 27, 3841. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Kim, Y.R.; Kang, C.M.; Chung, T.D. Electrochemical signal amplification for immunosensor based on 3D interdigitated array electrodes. Anal. Chem. 2014, 86, 5991–5998. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, D.R.; Grabowsk, I. Antibody-ferrocene conjugates as a platform for electro-chemical detection of low-density lipoprotein. Molecules 2022, 27, 5492. [Google Scholar] [CrossRef]
- Parate, K.; Rangnekar, S.V.; Jing, D.; Mendivelso, P.D.L.; Ding, S.; Secor, E.B.; Smith, E.A.; Hostetter, J.M.; Hersam, M.C.; Claussen, J.C. Aerosol-jet-printed graphene immunosensor for label-free cytokine monitoring in serum. ACS Appl. Mater. Interfaces 2020, 12, 8592–8603. [Google Scholar] [CrossRef]
- Ma, C.; Zhao, C.; Zhang, L.; Li, W.; Song, Y.; Chen, S.; Hong, C.; Qi, Y. Construction of a “signal on” electrochemical immunosensor based on light induction. ACS Sustain. Chem. Eng. 2021, 9, 13788–13797. [Google Scholar] [CrossRef]
- Kordash, H.K.; Hasanzadeh, M. Biomedical analysis of exosomes using biosensing methods: Recent progress. Anal. Methods 2020, 12, 2795–2811. [Google Scholar] [CrossRef]
- Nishiyama, K.; Fukuyama, M.; Maeki, M.; Ishida, A.; Tani, H.; Hibara, A.; Tokeshi, M. One-step non-competitive fluorescence polarization immunoassay based on a Fab ragment or C-reactive protein quantifcation. Sens. Actuators B Chem. 2021, 326, 128982. [Google Scholar] [CrossRef]
- Kalyani, T.; Sangili, A.; Nanda, A.; Prakash, S.; Kaushik, A.; Kumar Jana, S. Bio-nanocomposite based highly sensitive and label-free electrochemical immunosensor for endometriosis diagnostics application. Bioelectrochemistry 2021, 139, 107740. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhang, C.; Xi, F. Disposable amperometric label-free immunosensor on chitosan–graphene-modified patterned ITO electrodes for prostate specific antigen. Molecules 2022, 27, 5895. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Li, M.; Li, H.; Li, C.; Li, P.; Qian, L.; Yang, B. Sandwich-type electrochemical immunosensor for carcinoembryonic antigen detection based on the cooperation of a gold-vertical graphene electrode and gold@silica–methylene blue. J. Mater. Chem. B 2020, 8, 298–307. [Google Scholar] [CrossRef]
- Yadav, A.K.; Verma, D.; Kumar, A.; Kumar, P.; Solanki, P.R. The perspectives of biomarker-based electrochemical immunosensors, artificial intelligence and the internet of medical things toward COVID-19 diagnosis and management. Mater. Today Chem. 2021, 20, 100443. [Google Scholar] [CrossRef]
- Yan, T.; Wu, T.; Wei, S.; Wang, H.; Sun, M.; Yan, L.; Wei, Q.; Ju, H. Photoelectrochemical competitive immunosensor for 17β-estradiol detection based on ZnIn2S4@NH2-MIL-125(Ti) amplified by PDA NS/Mn:ZnCdS. Biosens. Bioelectron. 2020, 148, 111739. [Google Scholar] [CrossRef]
- Zhong, H.; Zhao, C.; Chen, J.; Chen, M.; Luo, T.; Tang, W.; Liu, J. Electrochemical immunosensor with surfaceconfined probe for sensitive and reagentless detection of breast cancer biomarker. RSC Adv. 2020, 10, 22291–22296. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Huang, R.; Wang, J.; Chang, B. Sensitive electrochemical immunoassay of a biomarker based on biotin-avidin conjugated DNAzyme concatamer with signal tagging. RSC Adv. 2013, 3, 13451–13456. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, A.J.; Yuan, P.X.; Luo, X.; Xue, Y.; Feng, J.J. Three dimensional sea-urchin-like PdAuCu nanocrystals/ferrocene-graftedpolylysine as an efficient probe to amplify the electrochemical signals for ultrasensitive immunoassay of carcinoembryonic antigen. Biosens. Bioelectron. 2019, 132, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Z.; Li, A.W.X.; Zou, X.; Huang, Q.; Zhoud, L. Competitive electrochemical sensing for cancer cell evaluation based on thionine-interlinked signal probes. Analyst 2023, 148, 912–918. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, X.; Tu, F.; Yao, C. Ultrasensitive enzyme-free electrochemical immunoassay for free thyroxine based on three dimensionally ordered macroporous chitosan-Au nanoparticles hybrid film. Biosens. Bioelectron. 2014, 59, 377–383. [Google Scholar] [CrossRef]
- Kondzior, M.; Grabowska, I. Antibody-electroactive probe conjugates based electrochemical immunosensors. Sensors 2020, 20, 2014. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wan, Y.; Deng, S.; Yang, S.; Su, Y.; Fan, C.; Aldalbahi, A.; Zuo, X. Aptamer-initiated on-particle template-independent enzymatic polymerization (aptamer-OTEP) for electrochemical analysis of tumor biomarkers. Biosens. Bioelectron. 2016, 86, 536–541. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, G.; Mao, X.; Yang, S.; Ruyck, K.D.; Wu, Y. High sensitivity immunoassays for small molecule compounds detection-novel noncompetitive immunoassay designs. Trends Anal. Chem. 2018, 103, 198–208. [Google Scholar] [CrossRef]
- Suna, B.; Wang, Y.; Lia, D.; Li, W.; Gou, X.; Gou, Y.; Hu, F. Development of a sensitive electrochemical immunosensor using polyaniline functionalized graphene quantum dots for detecting a depression marker. Mater. Sci. Eng. 2020, 111, 110797. [Google Scholar] [CrossRef]
- Freire, L.D.S.; Ruzo, C.M.; Salgado, B.B.; Gandarilla, A.M.D.; Barcelay, B.Y.; Tavares, A.P.M.; Sales, M.G.F.; Cordeiro, I.; Lalwani, J.D.B.; Matos, R.; et al. An electrochemical immunosensor based on carboxylated graphene/SPCE for IgG-SARS-CoV-2 nucleocapsid determination. Biosensors 2022, 12, 1161. [Google Scholar] [CrossRef] [PubMed]
- Kanno, Y.; Zhou, Y.; Takeshi, F.; Takahashi, Y. Alkaline phosphatase-based electrochemical analysis for point-of-care testing. Electroanalysis 2022, 34, 161–167. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Deng, D.; Li, M.; Zhang, C.; Luo, L. NiO-coated CuCo2O4 nanoneedle arrays on carbon cloth for nonenzymatic glucose sensing. ACS Appl. Nano Mater. 2021, 4, 9821–9830. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wang, E. Noble metal nanomaterials: Controllable synthesis and application in fuel cells and analytical sensors. Nano Today 2011, 6, 240–264. [Google Scholar] [CrossRef]
- Sakthivel, R.; Prasanna, S.B.; Tseng, C.L.; Lin, L.Y.; Duann, Y.F.; He, J.H.; Chung, R.J. A sandwich-type electrochemical immunosensor for insulin detection based on Au-adhered Cu5Zn8 hollow porous carbon nanocubes and AuNP deposited nitrogen-doped holey graphene. Small 2022, 18, 2202516. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, W.; Ma, C.; Sun, Y.; Qiao, J.; Li, H.; Hong, C. First use of inorganic copper silicate-transduced enzyme-free electrochemical immunosensor for carcinoembryonic antigen detection. Sens. Actuators B Chem. 2020, 319, 128311. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, N.; He, M.; Luo, P.; Tan, L. A nanozymebased competitive electrochemical immunosensor for the determination of E-selectin. Microchim. Acta 2022, 189, 406. [Google Scholar] [CrossRef]
- Eissa, S.; Zourob, M. Development of a low-cost cotton-tipped electrochemical immunosensor for the detection of SARS-CoV-2. Anal. Chem. 2021, 93, 1826–1833. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.; Nawaz, M.H.; Rehman, I.u.; Hayat, A.; Marty, J.L. Dopamine/mucin-1 unctionalized electro-active carbon nanotubes as a probe for direct competitive electrochemical immunosensing of breast cancer biomarker. Sens. Actuators B Chem. 2021, 330, 129351. [Google Scholar] [CrossRef]
- Bolukbaşi, O.S.; Yola, B.B.; Karaman, C.; Atar, N.; Yola, M.L. Electrochemical αfetoprotein immunosensor based on Fe3O4NPs@ covalent organic framework decorated gold nanoparticles and magnetic nanoparticles including SiO2@TiO2. Microchim. Acta 2022, 189, 242. [Google Scholar] [CrossRef]
- Pakchin, P.S.; Fathi, M.; Ghanbari, H.; Saber, R.; Omidi, Y. A novel electrochemical immunosensor for ultrasensitive detection of CA125 in ovarian cancer. Biosens. Bioelectron. 2020, 153, 112029. [Google Scholar] [CrossRef]
- Miao, J.; Du, K.; Li, X.; Xu, X.; Dong, X.; Fang, J.; Cao, W.; Wei, Q. Ratiometric electrochemical immunosensor for the detection of procalcitonin based on the ratios of SiO2-Fc–COOH–Au and UiO-66-TB complexes. Biosens. Bioelectron. 2021, 171, 112713. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, Y.; Li, Q.; Dou, L.; Liu, M.; Shao, S.; Zhu, J.; Shen, J.; Wang, Z.; Wen, K. Binding affnity-guided design of a highly sensitive noncompetitive immunoassay for small molecule detection. Food Chem. 2021, 351, 129270. [Google Scholar] [CrossRef]
- Nakhjavani, A.S.; Afsharan, H.; Khalilzadeh, B.; Ghahremani, M.H.; Carrara, S.; Omidi, Y. Gold and silver bio/nano-hybrids-based electrochemical immunosensor for ultrasensitive detection of carcinoembryonic antigen. Biosens. Bioelectron. 2019, 141, 111439. [Google Scholar] [CrossRef]
- Li, W.; Chen, S.; Yang, Y.; Song, Y.; Ma, C.; Qiao, X. Ultrasensitive electrochemical immunosensor based on the signal amplification strategy of the competitive reaction of Zn2+ and ATP ions to construct a “signal on” mode GOx-HRP enzyme cascade reaction. Microchim. Acta 2020, 188, 61. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Yan, M.; Xue, Y.; Huang, J.; Yang, X. Electrochemical immunosensor for cardiac troponin I detection based on covalent organic framework and enzyme-catalyzed signal amplification. Anal. Chem. 2021, 93, 13572–13579. [Google Scholar] [CrossRef] [PubMed]
- Valverde, A.; Serafín, V.; Montero, C.A.; Gonzalez-Cortes, A.; Barderas, R.; YanzeSedeno, P.; Campuzano, S.; Pingarron, J.M. Carbon/inorganic hybrid nanoarchitectures as carriers for signaling elements in electrochemical immunosensors: First biosensor for the determination of the inflammatory and metastatic processes biomarker RANK-ligand. ChemElectroChem 2019, 7, 810–820. [Google Scholar] [CrossRef]
- Martin, C.; Gamella, M.; Pedrero, M.; Calle, M.A.; Barderas, R.; Campuzano, S.; Pingarron, J.M. Magnetic beads-based electrochemical immunosensing of HIF-1α, a biomarker of tumoral hypoxia. Sens. Actuators B Chem. 2020, 307, 127623. [Google Scholar] [CrossRef]
- Vasquez, V.; Navas, M.C.; Jaimes, J.A.; Orozco, J. SARS-CoV-2 electrochemical immunosensor based on the spike-ACE2 complex. Clin. Chim. Acta 2022, 1205, 339718. [Google Scholar] [CrossRef]
- Patel, G.K.; Khan, M.A.; Zubair, H.; Srivastava, S.K.; Khushman, M.; Singh, S.; Singh, A.P. Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Sci. Rep. 2019, 9, 5335. [Google Scholar] [CrossRef]
- Samanta, S.; Rajasingh, S.; Drosos, N.; Zhou, Z.; Dawn, B.; Rajasingh, J. Exosomes: New molecular targets of diseases. Acta Pharmacol. Sin. 2018, 39, 501–513. [Google Scholar] [CrossRef]
- Lee, S.E.; Jeong, S.E.; Hong, J.S.; Im, H.; Hwang, S.Y.; Oh, J.K.; Kim, S.E. Gold-nanoparticle-coated magnetic beads for ALP-enzyme-based electrochemical immunosensing in human plasma. Materials 2022, 15, 6875. [Google Scholar] [CrossRef]
- Kim, K.J.; Song, Y.; Park, S.; Oh, S.J.; Kwon, S.J. Immunosensor for human IgE detection using electrochemical redox cycling with ferrocene-mixed self-assembled monolayers modified Au electrode. Bull. Korean Chem. Soc. 2022, 44, 141–146. [Google Scholar] [CrossRef]
- Cao, L.; Fang, C.; Zeng, R.; Zhao, X.; Jiang, Y.; Chen, Z. Paper-based microfluidic devices for electrochemical immunofiltration analysis of human chorionic gonadotropin. Biosens. Bioelectron. 2017, 92, 87–94. [Google Scholar] [CrossRef]
- Huang, W.; Guo, P.; Fu, L.; Lin, C.T.; Yu, A.; Lai, G. Enzyme-catalyzed deposition of polydopamine for amplifying the signal inhibition to a novel Prussian blue-nanocomposite and ultrasensitive electrochemical immunosensing. J. Mater. Sci. Technol. 2022, 102, 166–173. [Google Scholar] [CrossRef]
- Rahmati, Z.; Roushani, M.; Hosseini, H.; Choobin, H. Electrochemical immunosensor with Cu2O nanocube coating for detection of SARS-CoV-2 spike protein. Microchim. Acta. 2021, 188, 105. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Deng, D.; He, H.; Yan, X.; Wang, Z.; Fan, C.; Luo, L. Effective immobilization of Au nanoparticles on TiO2 loaded graphene for a novel sandwich-type immunosensor. Biosens. Bioelectron. 2018, 102, 301–306. [Google Scholar] [CrossRef]
- Chen, S.; Yang, Y.; Li, W.; Song, Y.; Shi, L.; Hong, C. A sandwich-type electrochemical immunosensor using Ag@CeO2-Au as a lable for sensitive detection of carcinoembryonic antigen. Microchem. J. 2020, 159, 105415. [Google Scholar] [CrossRef]
- Sauzay, C.; Petit, A.; Bourgeois, A.M.; Barbare, J.C.; Chauffert, B.; Galmiche, A.; Houessinon, A. Alpha-foetoprotein (AFP): A multi-purpose marker in hepatocellular carcinoma. Clin. Chim. Acta 2016, 463, 39–44. [Google Scholar] [CrossRef]
- Ciemny, G.J.; Pankiewicz, J.; Malewski, Z.; von Kaisenberg, C.; Kocylowski, R. Alpha-fetoprotein (AFP)-new aspects of a well-known marker in perinatology. Ginekol. Pol. 2022, 93, 70–75. [Google Scholar] [CrossRef]
- Chen, H.; Li, Y.; Song, Y.; Liu, F.; Deng, D.; Zhu, X.; He, H.; Yan, X.; Luo, L. A sandwich-type electrochemical immunosensor based on spherical nucleic acids-templated Ag nanoclusters for ultrasensitive detection of tumor biomarker. Biosens. Bioelectron. 2023, 223, 115029. [Google Scholar] [CrossRef] [PubMed]
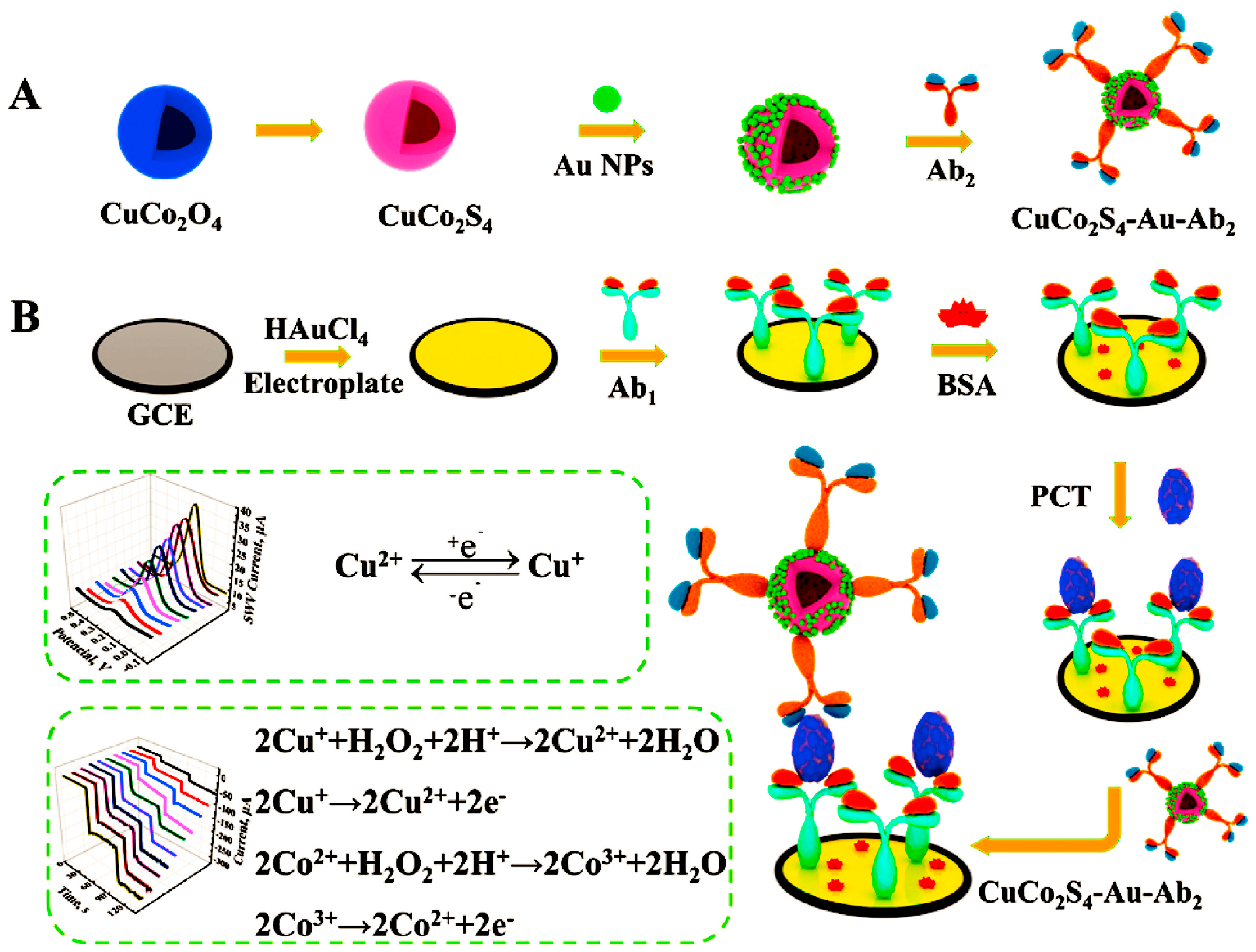
- Li, Y.; Liu, L.; Liu, X.; Ren, Y.; Xu, K.; Zhang, N.; Sun, X.; Yang, X.; Ren, X.; Wei, Q. A dual-mode PCT electrochemical immunosensor with CuCo2S4 bimetallic sulfides as enhancer. Biosens. Bioelectron. 2020, 163, 112280. [Google Scholar] [CrossRef] [PubMed]
- Medetalibeyoglu, H.; Kotan, G.; Atar, N.; Yola, M.L. A novel and ultrasensitive sandwich-type electrochemical immunosensor based on delaminated MXene@AuNPs as signal amplification for prostate specific antigen (PSA) detection and immunosensor validation. Talanta 2020, 220, 121403. [Google Scholar] [CrossRef]
- Prasad, K.S.; Cao, X.; Gao, N.; Jin, Q.; Sanjay, S.T.; Henao-Pabon, G.; Li, X. A low-cost nanomaterial-based electrochemical immunosensor on paper for high-sensitivity early detection of pancreatic cancer. Sens. Actuators B Chem. 2020, 305, 127516. [Google Scholar] [CrossRef]
- Shrishty, B.; Samriddhi, M.; Kumeria, T.; Shiddiky, M.J.A.; Popat, A.; Choudhury, S.; Bose, S.; Nayak, R. Rapid fabrication of homogeneously distributed hyper-branched gold nanostructured electrode based electrochemical immunosensor for detection of protein biomarkers. Sens. Actuators B Chem. 2021, 326, 128803. [Google Scholar] [CrossRef]
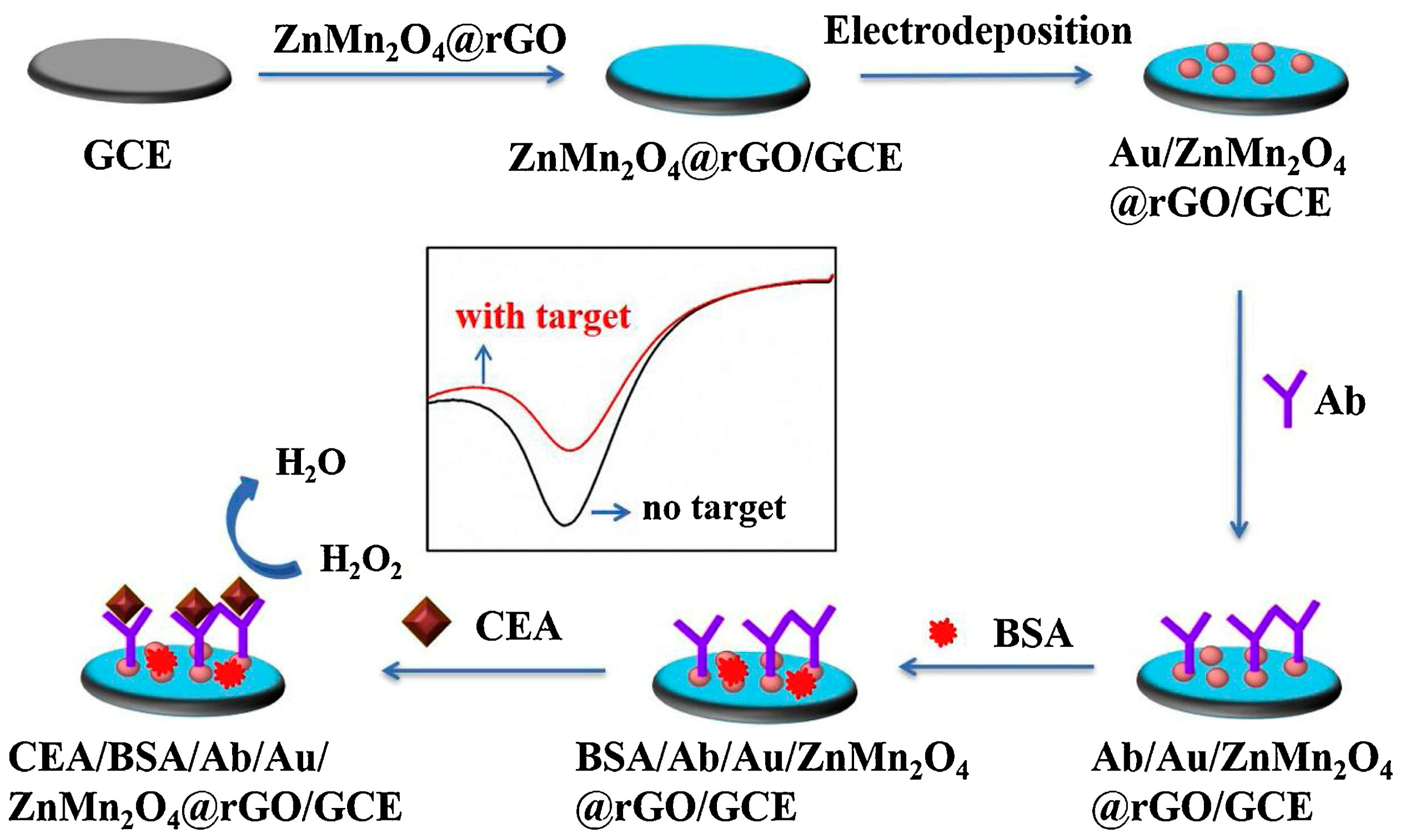
- Fan, X.; Deng, D.; Chen, Z.; Qi, J.; Li, Y.; Han, B.; Huan, K.; Luo, L. A sensitive amperometric immunosensor for the detection of carcinoembryonic antigen using ZnMn2O4@ reduced graphene oxide composites as signal amplifier. Sens. Actuators B Chem. 2021, 339, 129852. [Google Scholar] [CrossRef]
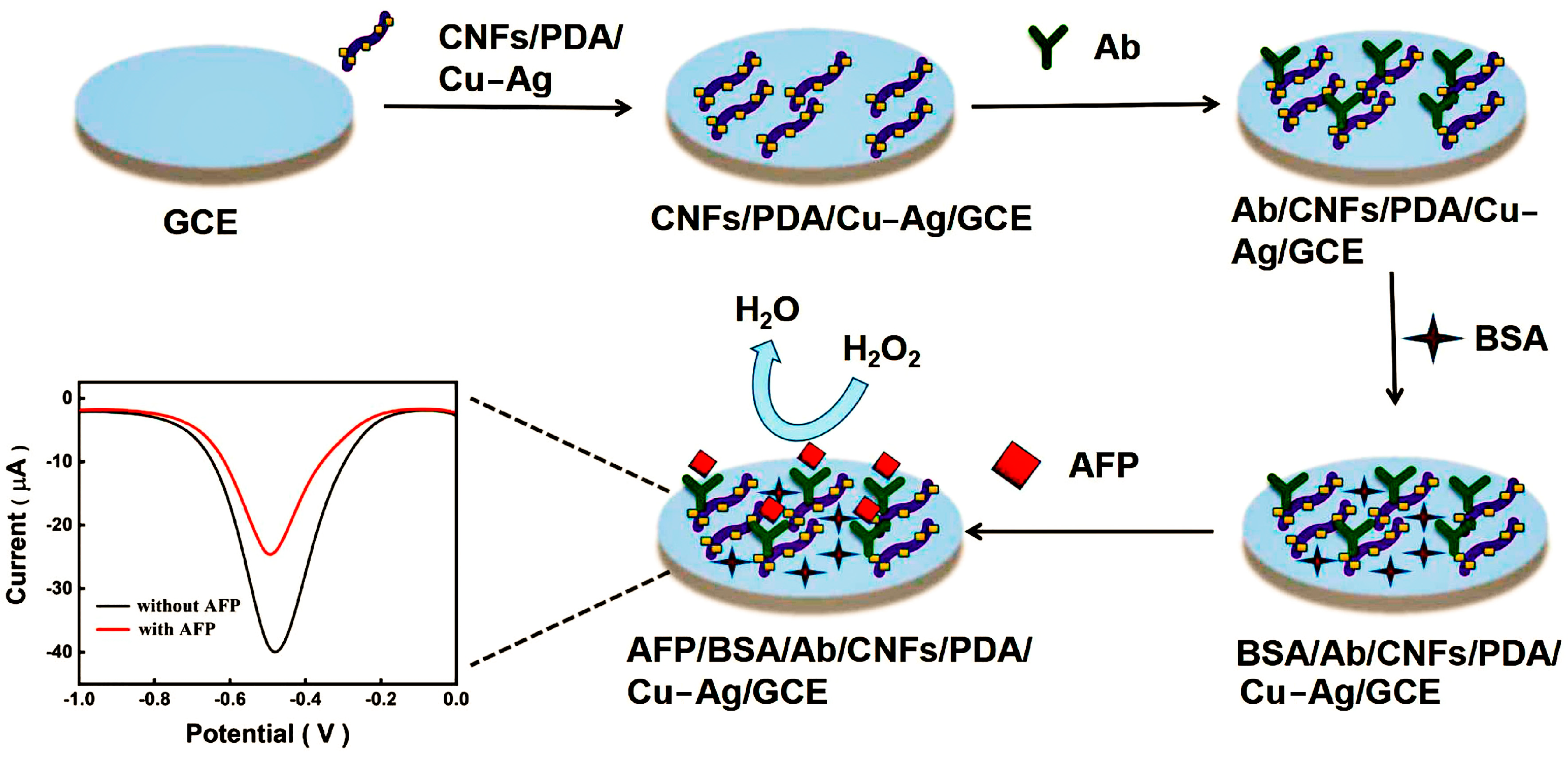
- Liu, F.; Chen, H.; Deng, D.; Fan, X.; Li, Y.; Madrakian, T.; Luo, L. An ultrasensitive immunosensor based on cellulose nanofibrils/polydopamine/Cu-Ag nanocomposite for the detection of AFP. Bioelectrochemistry 2022, 147, 108200. [Google Scholar] [CrossRef]
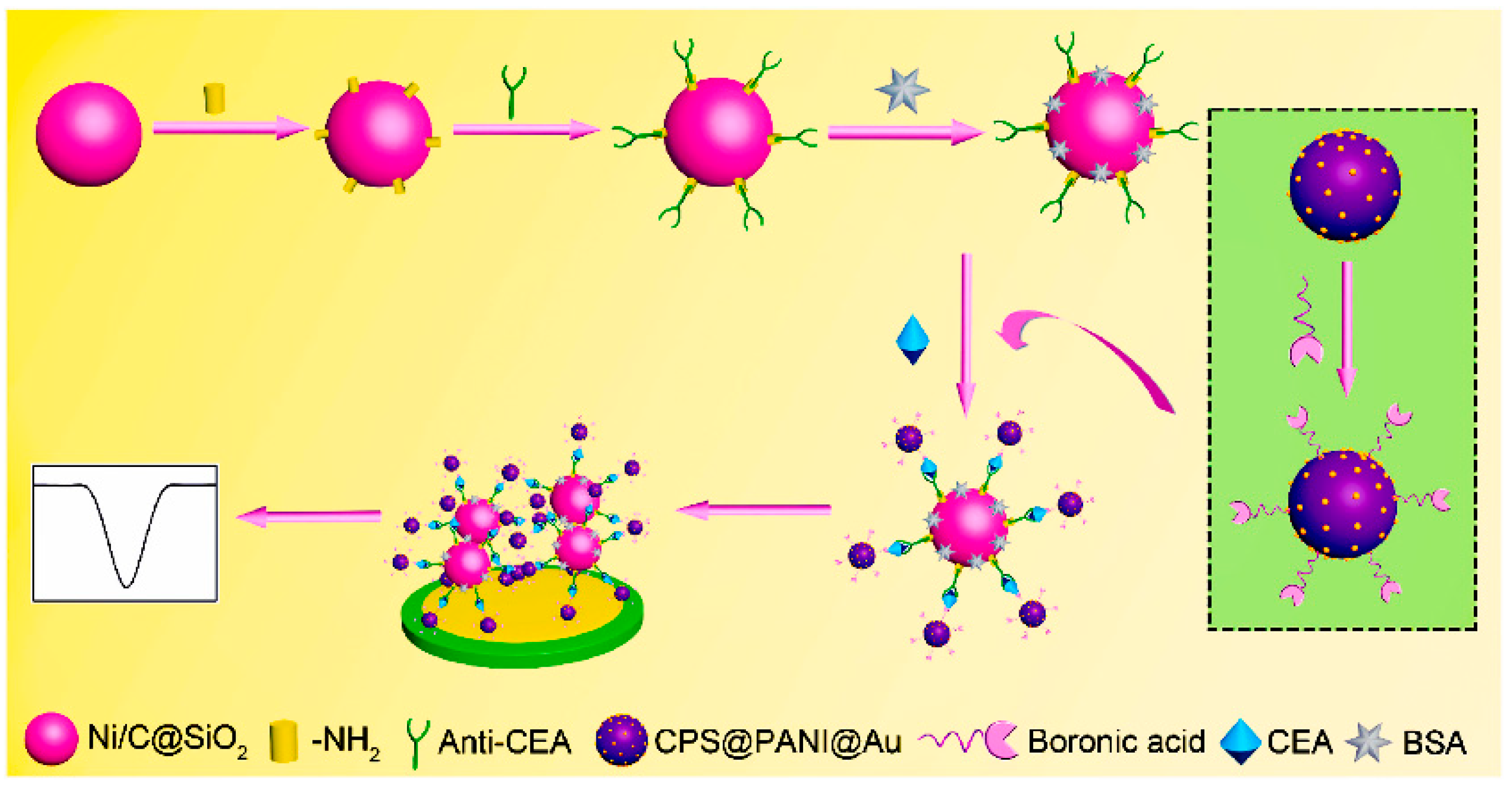
- Song, D.; Zheng, J.; Myung, N.V.; Xu, J.; Zhang, M. Sandwich-type electrochemical immunosensor for CEA detection using magnetic hollow Ni/C@SiO2 nanomatrix and boronic acid functionalized CPS@PANI@Au probe. Talanta 2021, 225, 122006. [Google Scholar] [CrossRef]
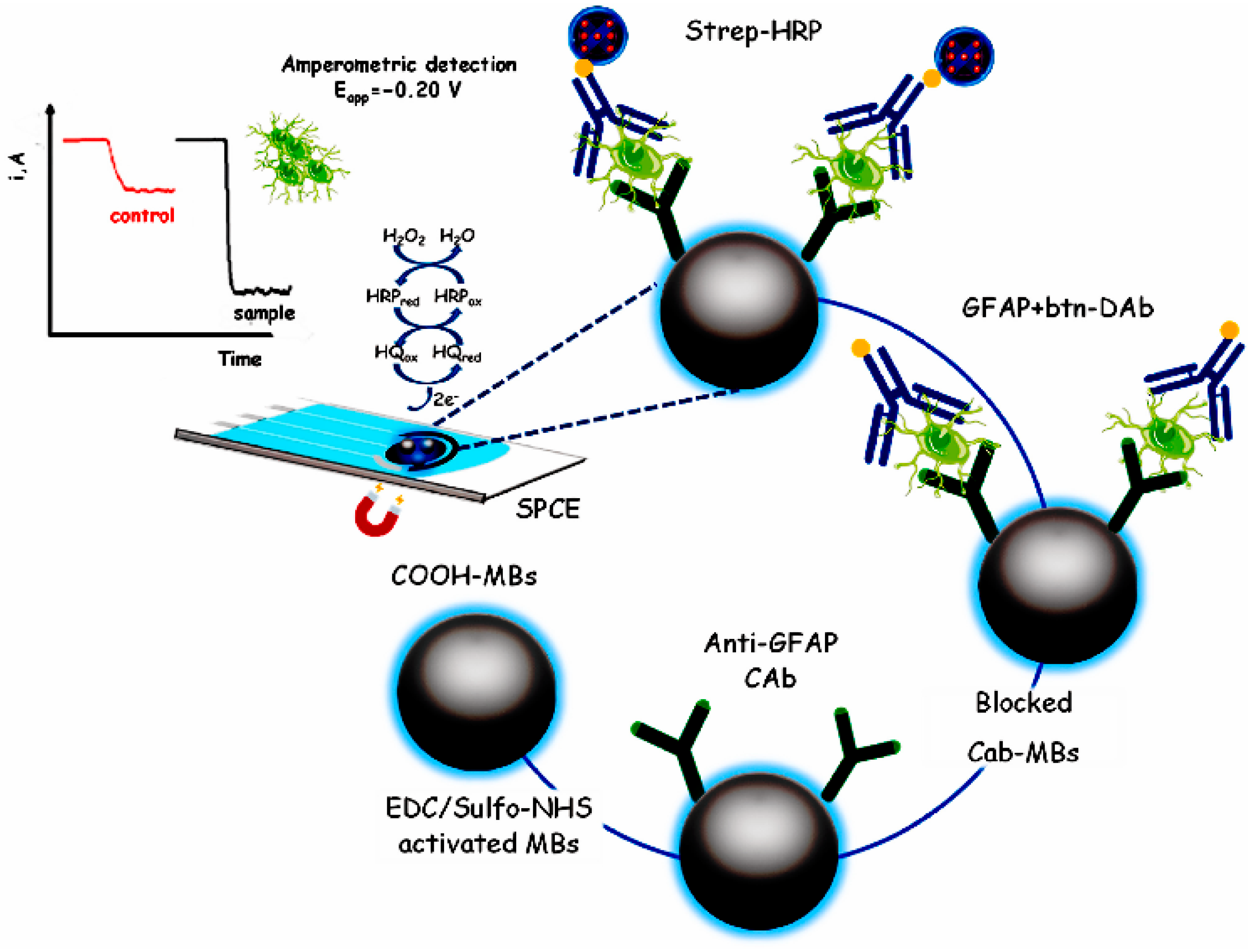
- Ozcelikay, G.; Gamella, M.; Unal, M.A.; Gucuyener, K.; Calle, A.M.; Barderas, R.; Pingarron, J.M.; Campuzano, S.; Ozkan, S.A. Assisting dementia diagnosis through the electrochemical immunosensing of glial fibrillary acidic protein. Talanta 2022, 246, 123526. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, L.; Saroglia, M.; Galat, G.; Santis, R.D.; Fillo, S.; Luca, V.; Faggioni, G.; Amore, N.D.; Regalbuto, E.; Salvatori, P.; et al. Magnetic beads combined with carbon black-based screen-printed electrodes for COVID-19: A reliable and miniaturized electrochemical immunosensor for SARS-CoV-2 detection in saliva. Biosens. Bioelectron. 2021, 171, 112686. [Google Scholar] [CrossRef] [PubMed]
- Durmus, C.; Hanoglu, S.B.; Harmanci, D.; Moulahoum, H.; Tok, K.; Ghorbanizamani, F.; Sanli, S.; Zihnioglu, F.; Evran, S.; Cicek, C.; et al. Indiscriminate SARS-CoV-2 multivariant detection using magnetic nanoparticle-based electrochemical immunosensing. Talanta 2022, 243, 123356. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Zhang, J.; Huang, R.; Wang, D.; Deng, D.; Zhang, Q.; Luo, L. The Applications of Electrochemical Immunosensors in the Detection of Disease Biomarkers: A Review. Molecules 2023, 28, 3605. https://doi.org/10.3390/molecules28083605
Chen H, Zhang J, Huang R, Wang D, Deng D, Zhang Q, Luo L. The Applications of Electrochemical Immunosensors in the Detection of Disease Biomarkers: A Review. Molecules. 2023; 28(8):3605. https://doi.org/10.3390/molecules28083605
Chicago/Turabian StyleChen, Huinan, Jialu Zhang, Rong Huang, Dejia Wang, Dongmei Deng, Qixian Zhang, and Liqiang Luo. 2023. "The Applications of Electrochemical Immunosensors in the Detection of Disease Biomarkers: A Review" Molecules 28, no. 8: 3605. https://doi.org/10.3390/molecules28083605
APA StyleChen, H., Zhang, J., Huang, R., Wang, D., Deng, D., Zhang, Q., & Luo, L. (2023). The Applications of Electrochemical Immunosensors in the Detection of Disease Biomarkers: A Review. Molecules, 28(8), 3605. https://doi.org/10.3390/molecules28083605







