Functionalization of Framboidal Phenylboronic Acid-Containing Nanoparticles via Aqueous Suzuki–Miyaura Coupling Reactions
Abstract
1. Introduction
2. Results
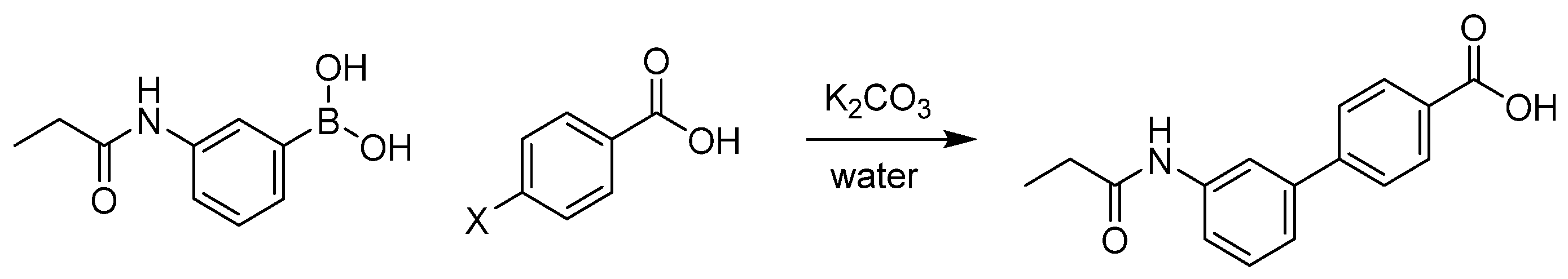
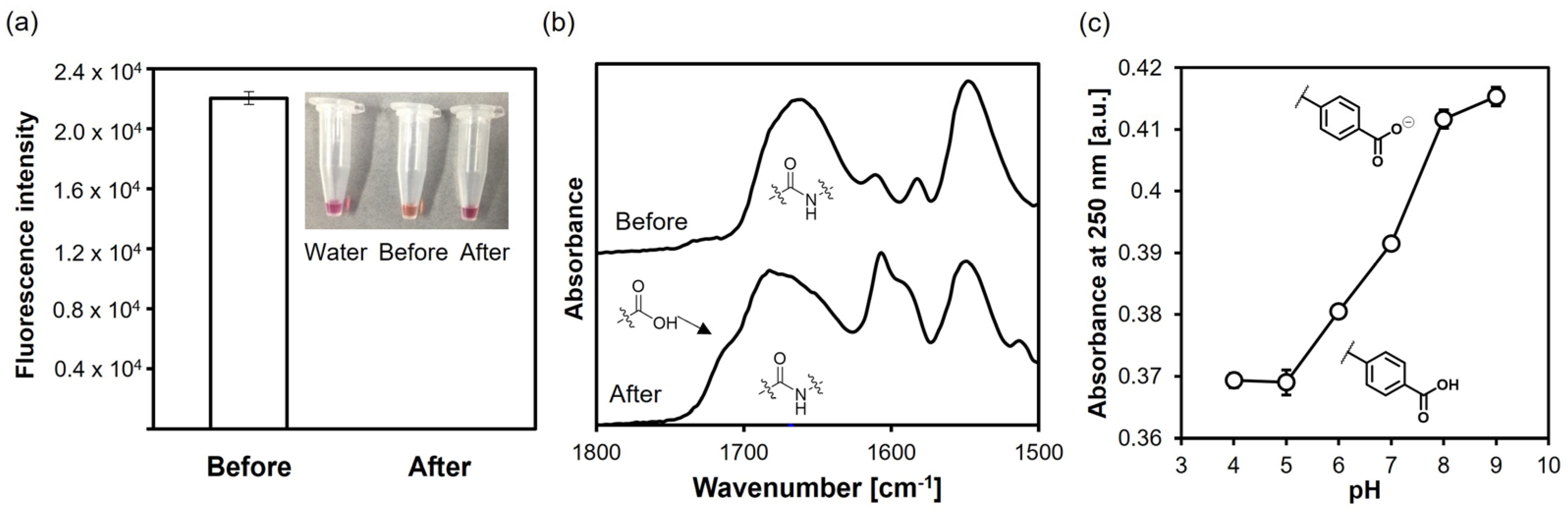
2.1. Aqueous Suzuki–Miyaura Coupling Model Reactions
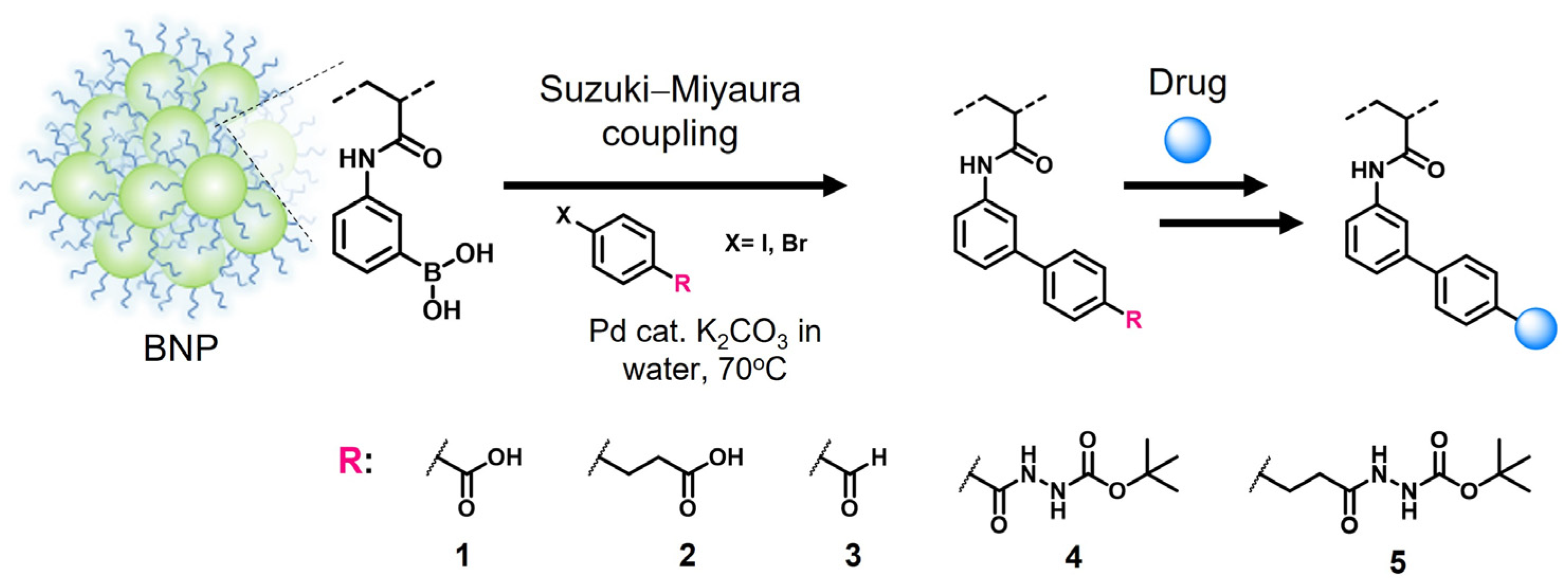
2.2. BNP Modification via Aqueous Suzuki–Miyaura Coupling Reaction
2.3. Framboidal Structures of Suzuki–Miyaura Coupling-Functionalized BNP
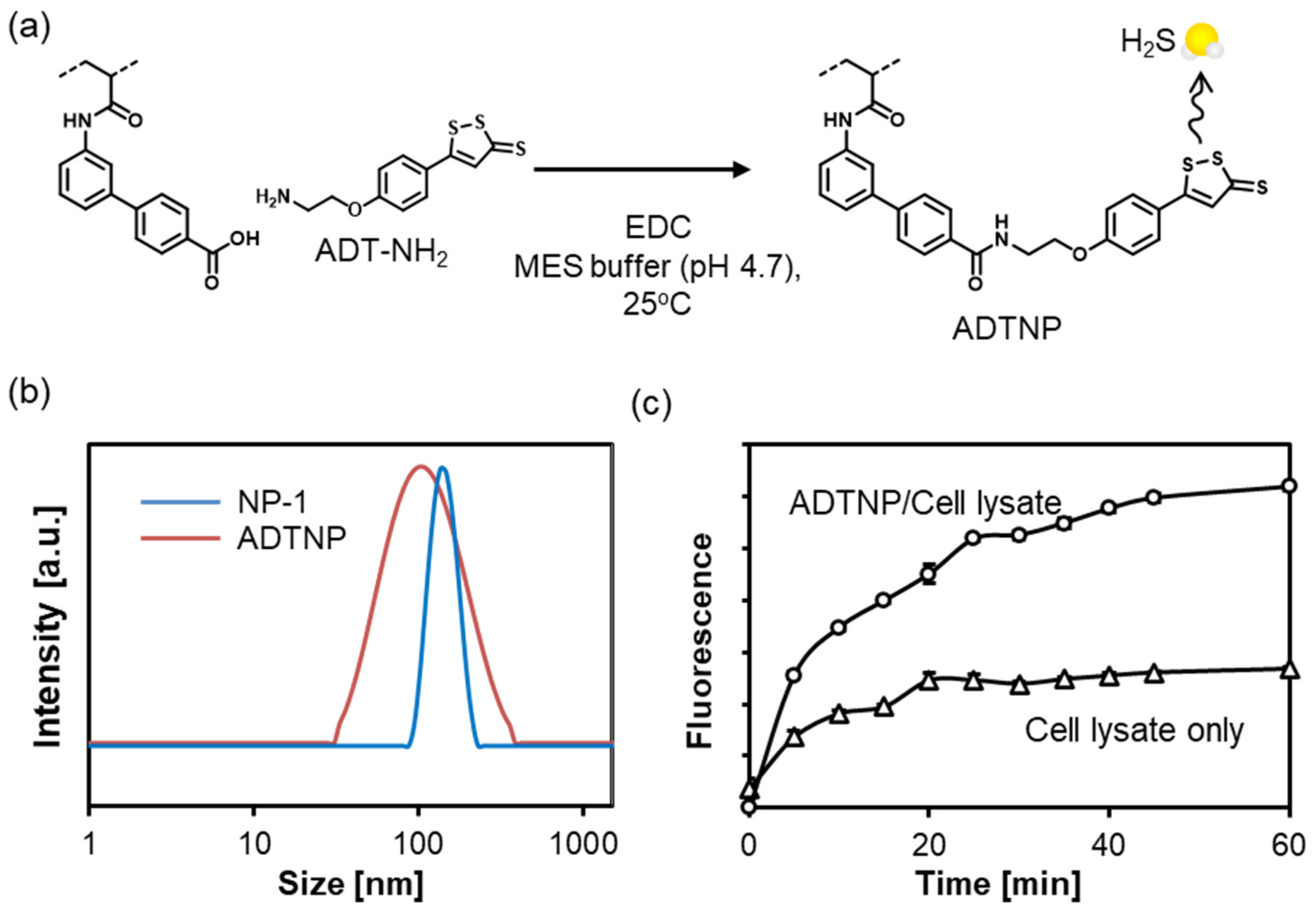
2.4. Functionalization of Carboxylate NP (NP-1) with ADT
3. Materials and Methods
3.1. Instrumentation
3.2. Suzuki–Miyaura Coupling Model Reactions with 4-Iodo- and 4-Bromo-Benzoic Acid
3.3. Synthesis of BNPs
3.4. Alizarin Red Fluorescence Assay
3.5. BNP Functionalization with 4-Iodo-Benzoic Acid (NP-1)
3.6. BNP Functionalization with 3-(4-Bromophenyl)propionic Acid (NP-2)
3.7. BNP Functionalization with 4-Iodobenzaldehyde (NP-3)
3.8. BNP Functionalization with Suzuki–Miyaura Coupling Partner 4 (NP-4)
3.9. BNP Functionalization with Suzuki–Miyaura Coupling Partner 5 (NP-5)
3.10. Modification of NP-1 with ADT-NH2 (ADTNP)
3.11. Quantification of ADT
3.12. Cell Lysate Preparation
3.13. Measurement of H2S Release in Cell Lysate via WSP-1 Fluorescent H2S Detection Dye
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hasegawa, U.; Nishida, T.; van der Vlies, A.J. Dual Stimuli-Responsive Phenylboronic Acid-Containing Framboidal Nanoparticles by One-Step Aqueous Dispersion Polymerization. Macromolecules 2015, 48, 4388–4393. [Google Scholar] [CrossRef]
- Alexeev, V.L.; Sharma, A.C.; Goponenko, A.V.; Das, S.; Lednev, I.K.; Wilcox, C.S.; Finegold, D.N.; Asher, S.A. High Ionic Strength Glucose-Sensing Photonic Crystal. Anal. Chem. 2003, 75, 2316–2323. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, U.; Inubushi, R.; Uyama, H.; Uematsu, T.; Kuwabata, S.; van der Vlies, A.J. Mannose-displaying fluorescent framboidal nanoparticles containing phenylboronic acid groups as a potential drug carrier for macrophage targeting. Colloids Surf. B Biointerfaces 2015, 136, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Van der Vlies, A.J.; Inubushi, R.; Uyama, H.; Hasegawa, U. Polymeric Framboidal Nanoparticles Loaded with a Carbon Monoxide Donor via Phenylboronic Acid-Catechol Complexation. Bioconj. Chem. 2016, 27, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Van der Vlies, A.J.; Morisaki, M.; Neng, H.I.; Hansen, E.M.; Hasegawa, U. Framboidal Nanoparticles Containing a Curcumin–Phenylboronic Acid Complex with Antiangiogenic and Anticancer Activities. Bioconj. Chem. 2019, 30, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A. Recent advances in the cross-coupling reactions of organoboron derivatives with organic electrophiles, 1995–1998. J. Organomet. Chem. 1999, 576, 147–168. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Decottignies, A.; Len, C.; Fihri, A. Suzuki–Miyaura Cross-Coupling Reactions in Aqueous Media: Green and Sustainable Syntheses of Biaryls. ChemSusChem 2010, 3, 502–522. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.; Zhao, J.Y.; Shi, X.; Duan, X.H. An efficient water-soluble surfactant-type palladium catalyst for Suzuki cross-coupling reactions in pure water at room temperature. New J. Chem. 2016, 40, 6568–6572. [Google Scholar] [CrossRef]
- Wang, J.-C.; Liu, C.-X.; Kan, X.; Wu, X.-W.; Kan, J.-L.; Dong, Y.-B. Pd@COF-QA: A phase transfer composite catalyst for aqueous Suzuki–Miyaura coupling reaction. Gr. Chem. 2020, 22, 1150–1155. [Google Scholar] [CrossRef]
- Qi, M.; Tan, P.Z.; Xue, F.; Malhi, H.S.; Zhang, Z.-X.; Young, D.J.; Hor, T.S.A. A supramolecular recyclable catalyst for aqueous Suzuki–Miyaura coupling. RSC Adv. 2015, 5, 3590–3596. [Google Scholar] [CrossRef]
- Shaughnessy, K.H. Hydrophilic Ligands and Their Application in Aqueous-Phase Metal-Catalyzed Reactions. Chem. Rev. 2009, 109, 643–710. [Google Scholar] [CrossRef] [PubMed]
- Karna, P.; Okeke, M.; Meira, D.M.; Finfrock, Z.; Yang, D.-S. Water-Soluble Palladium Nanoclusters as Catalysts in Ligand-Free Suzuki–Miyaura Cross-Coupling Reactions. ACS Appl. Nano Mater. 2022, 5, 3188–3193. [Google Scholar] [CrossRef]
- Jasim, S.A.; Ansari, M.J.; Majdi, H.S.; Opulencia, M.J.C.; Uktamov, K.F. Nanomagnetic Salamo-based-Pd(0) Complex: An efficient heterogeneous catalyst for Suzuki–Miyaura and Heck cross-coupling reactions in aqueous medium. J. Mol. Struct. 2022, 1261, 132930. [Google Scholar] [CrossRef]
- Ryu, J.-H.; Jang, C.-J.; Yoo, Y.-S.; Lim, S.-G.; Lee, M. Supramolecular reactor in an aqueous environment: Aromatic cross Suzuki coupling reaction at room temperature. J. Org. Chem. 2005, 70, 8956–8962. [Google Scholar] [CrossRef] [PubMed]
- Mattiello, S.; Rooney, M.; Sanzone, A.; Brazzo, P.; Sassi, M.; Beverina, L. Suzuki–Miyaura Micellar Cross-Coupling in Water, at Room Temperature, and under Aerobic Atmosphere. Org. Lett. 2017, 19, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Wallow, T.I.; Novak, B.M. In aqua synthesis of water-soluble poly(p-phenylene) derivatives. J. Am. Chem. Soc. 1991, 113, 7411–7412. [Google Scholar] [CrossRef]
- Hasegawa, U.; van der Vlies, A.J. Polymeric micelles for hydrogen sulfide delivery. MedChemComm 2015, 6, 273–276. [Google Scholar] [CrossRef]
- Hasegawa, U.; van der Vlies, A.J. Design and Synthesis of Polymeric Hydrogen Sulfide Donors. Bioconj. Chem. 2014, 25, 1290–1300. [Google Scholar] [CrossRef] [PubMed]

| Entry | Pd/B [mol %] 1 | T [°C] 2 | X | Conversion [%] 3 | Yield [%] 4 |
|---|---|---|---|---|---|
| 1 | 1 | 70 | I | 100 | 94 |
| 2 | 0.1 | 70 | I | 100 | 79 |
| 3 | 0.01 | 70 | I | 100 | 93 |
| 4 | 1 | 25 | I | 100 | 98 |
| 5 | 0.1 | 25 | I | 100 | 91 |
| 6 | 0.01 | 25 | I | 47 | n.d. |
| 7 | 1 | 25 | Br | 78 | n.d. |
| 8 | 1 | 70 | Br | 100 | 98 |
| 9 | 0.1 | 70 | Br | 100 | 99 |
| 10 | 0.01 | 70 | Br | 100 | 100 |
| DLS before Suzuki | DLS after Suzuki | |||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Coupling Partner | Pd/B [%] 1 | Time [h] | Conversion [%] 2 | Diameter [nm] 3 | PDI 3 | Diameter [nm] 3 | PDI 3 |
| NP-1 | 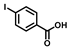 | 1 | 32 | 100 | 101.5 | 0.028 | 130.5 | 0.116 |
| NP-2 |  | 5 | 42 | 97 | 101.7 | 0.058 | 119.2 | 0.091 |
| NP-3 |  | 1 | 43 | 100 | 237.5 | 0.077 | 234.6 | 0.032 |
| NP-4 |  | 5 | 42 | 79 | 101.7 | 0.058 | 113.4 | 0.214 |
| NP-5 |  | 5 | 42 | 72 | 101.7 | 0.058 | 102.4 | 0.080 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van der Vlies, A.J.; Hasegawa, U. Functionalization of Framboidal Phenylboronic Acid-Containing Nanoparticles via Aqueous Suzuki–Miyaura Coupling Reactions. Molecules 2023, 28, 3602. https://doi.org/10.3390/molecules28083602
van der Vlies AJ, Hasegawa U. Functionalization of Framboidal Phenylboronic Acid-Containing Nanoparticles via Aqueous Suzuki–Miyaura Coupling Reactions. Molecules. 2023; 28(8):3602. https://doi.org/10.3390/molecules28083602
Chicago/Turabian Stylevan der Vlies, André J., and Urara Hasegawa. 2023. "Functionalization of Framboidal Phenylboronic Acid-Containing Nanoparticles via Aqueous Suzuki–Miyaura Coupling Reactions" Molecules 28, no. 8: 3602. https://doi.org/10.3390/molecules28083602
APA Stylevan der Vlies, A. J., & Hasegawa, U. (2023). Functionalization of Framboidal Phenylboronic Acid-Containing Nanoparticles via Aqueous Suzuki–Miyaura Coupling Reactions. Molecules, 28(8), 3602. https://doi.org/10.3390/molecules28083602






