Preparation and Anti-Lung Cancer Activity Analysis of Guaiacyl-Type Dehydrogenation Polymer
Abstract
1. Introduction
2. Results and Discussion
2.1. 13C-NMR Spectral Analysis of DHP
2.2. Analysis of Molecular Weights of the DHP Fractions
2.3. Analysis of the Total Phenolic Content (TPC) of DHP-Classified Fractions
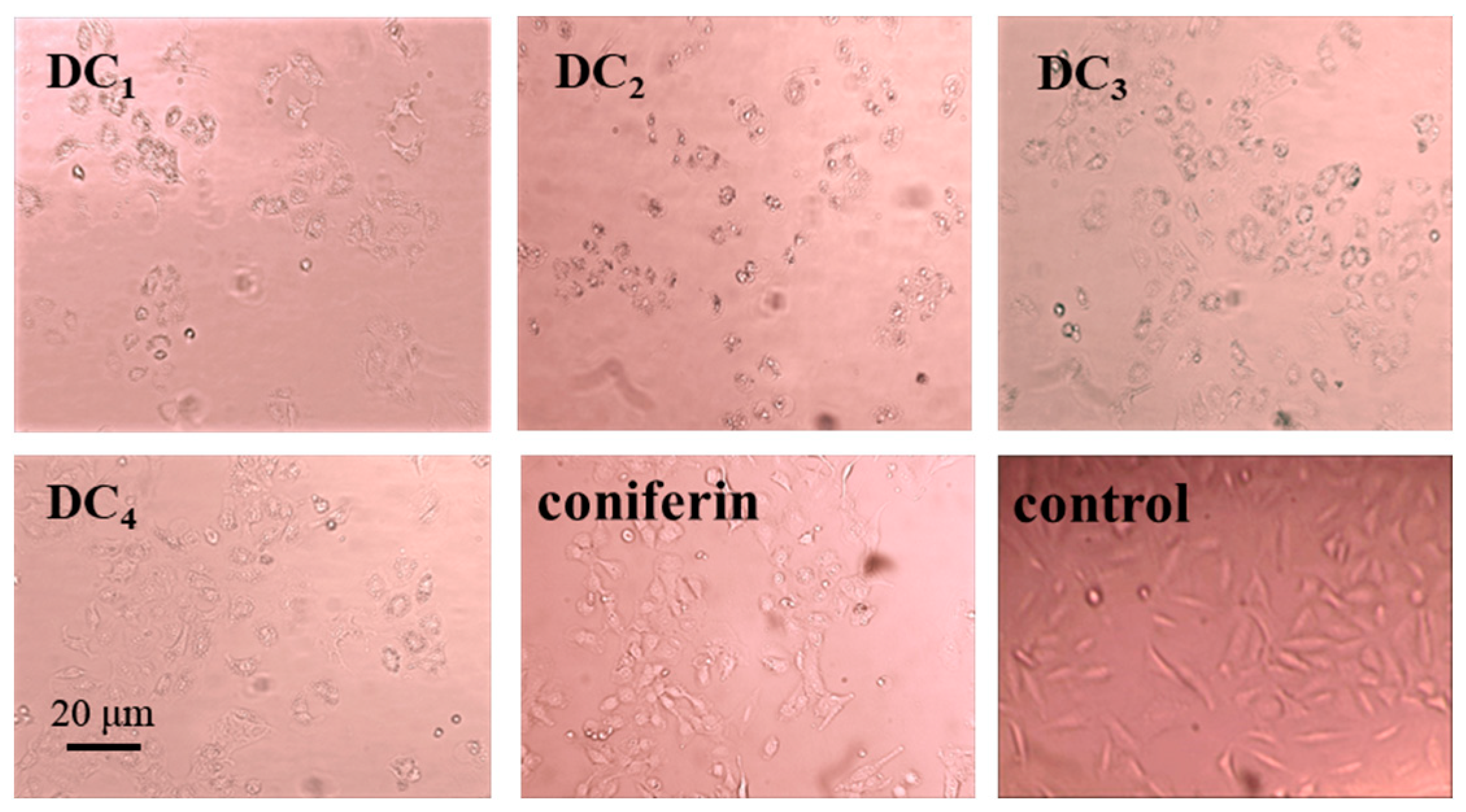
2.4. Analysis of the Anti-Tumor Activities of the G-DHP Classified Fractions
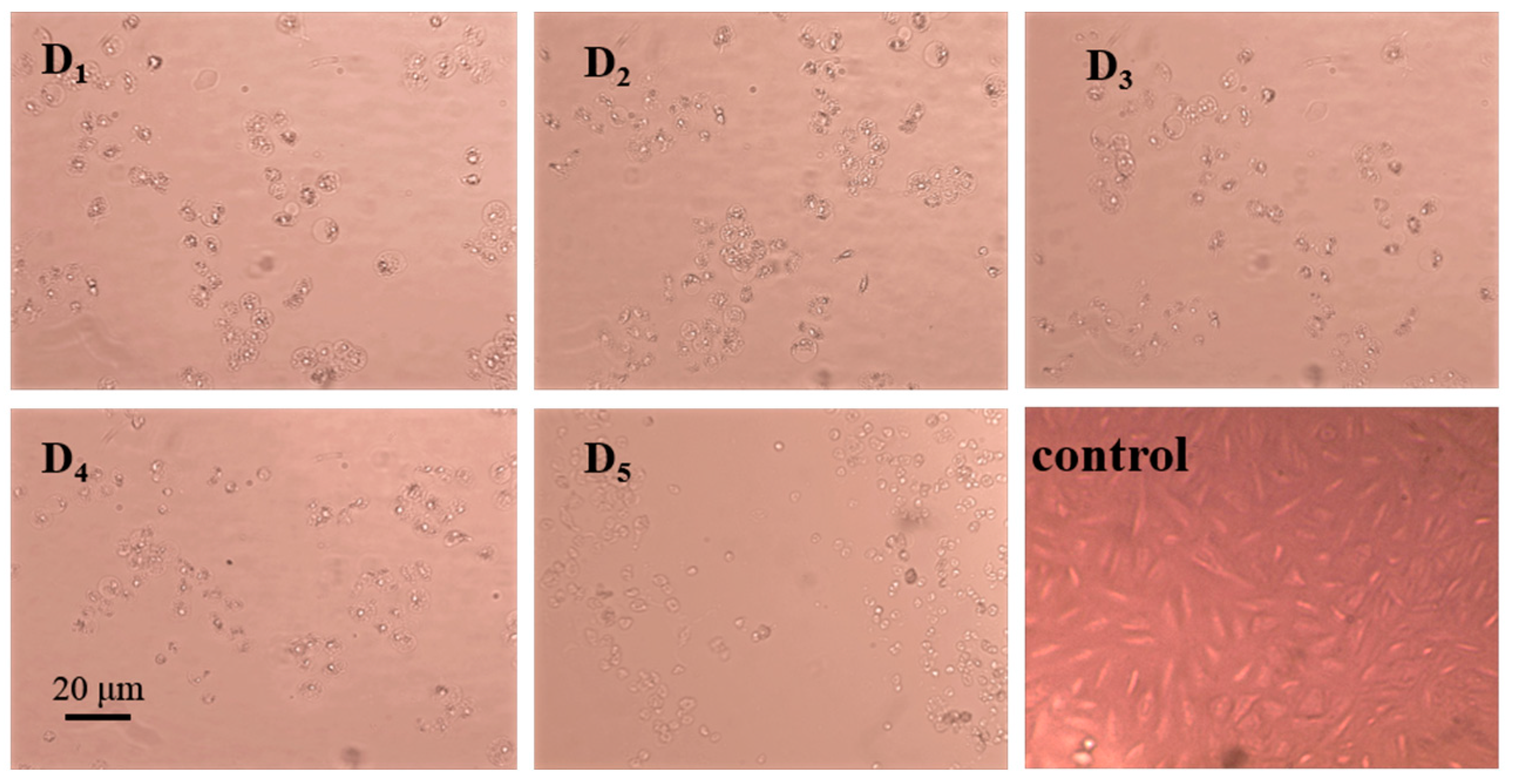
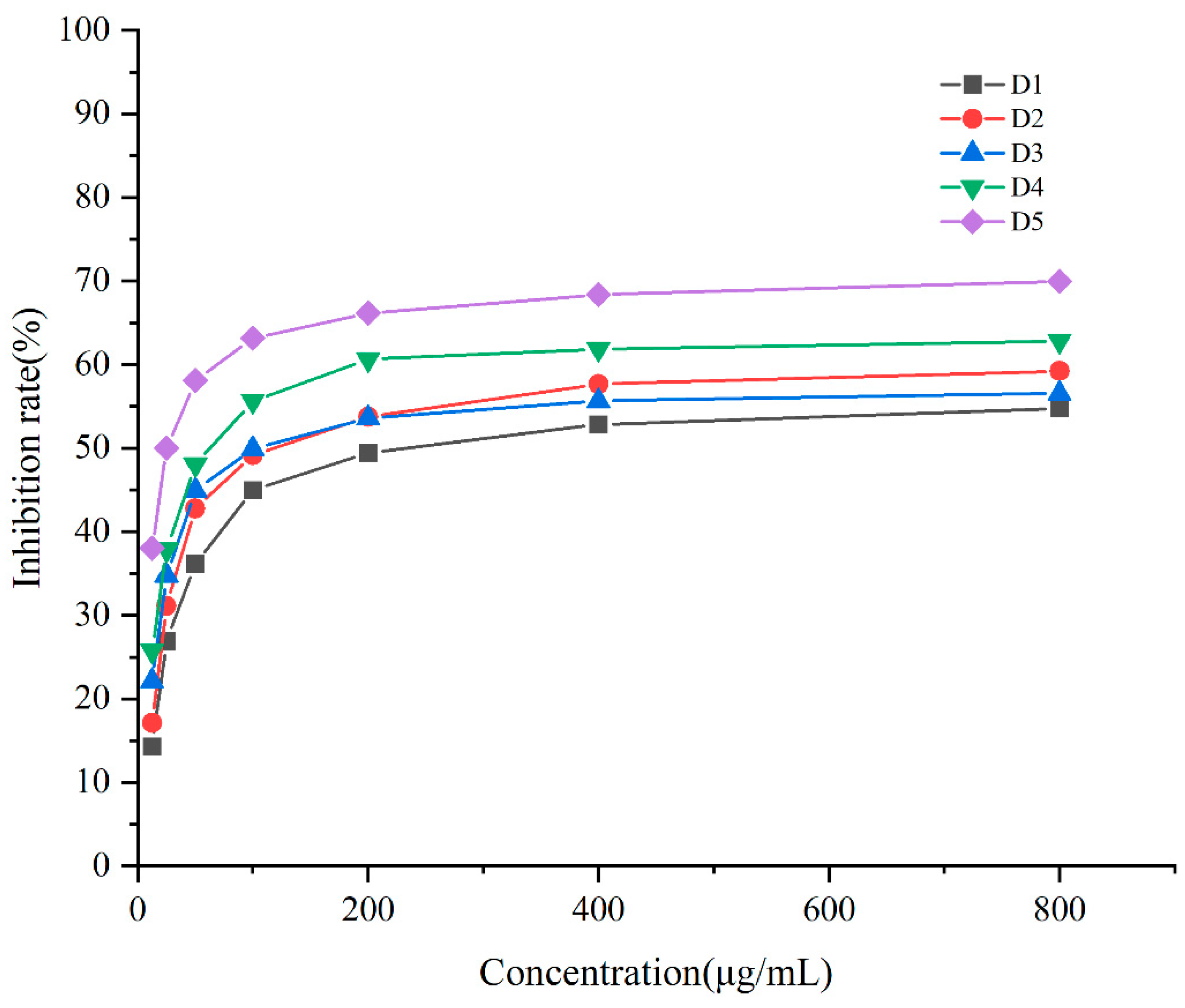
2.5. Analysis of the Anti-Tumor Activity of the Purified Substances of G-DHP Ether-Soluble Fraction
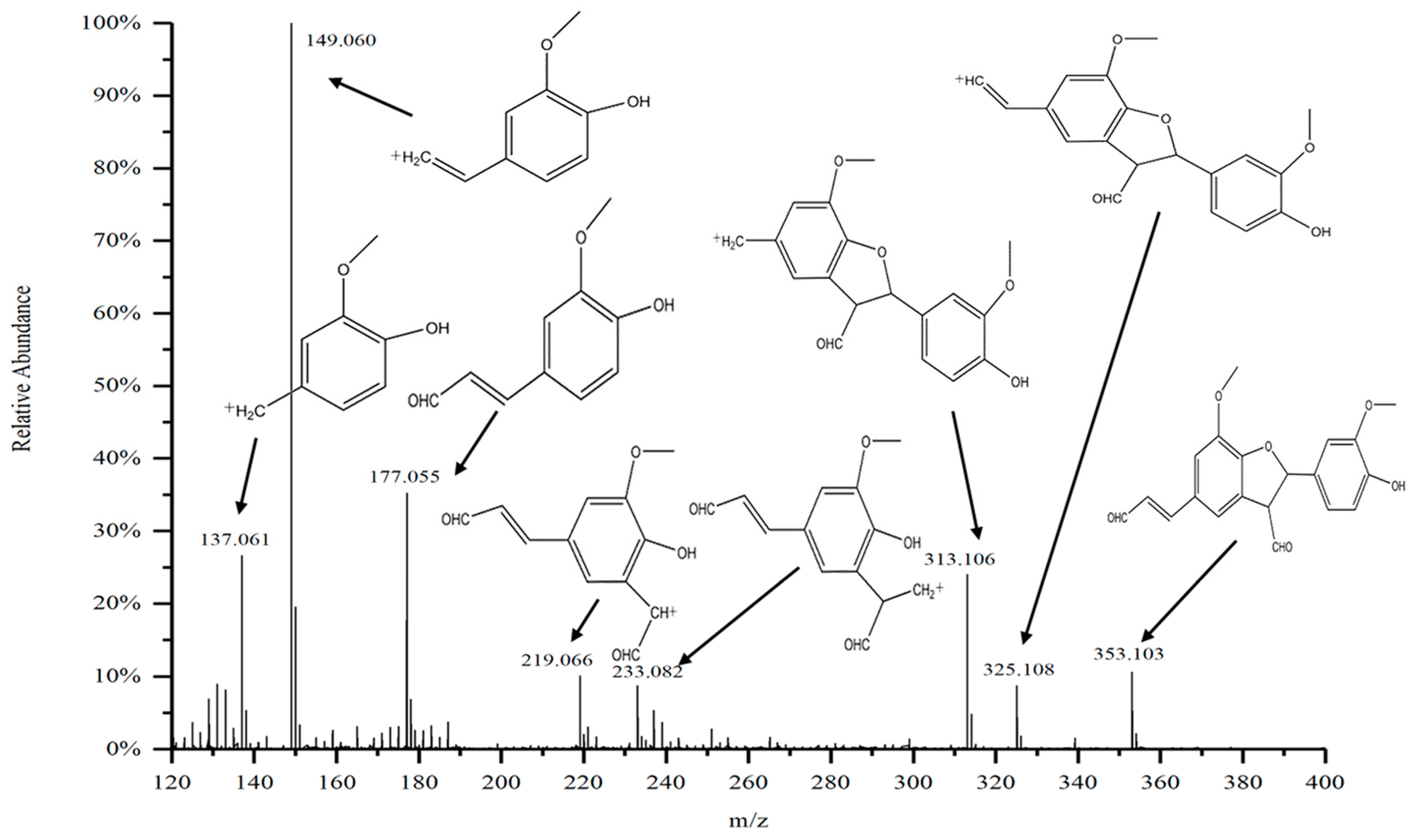
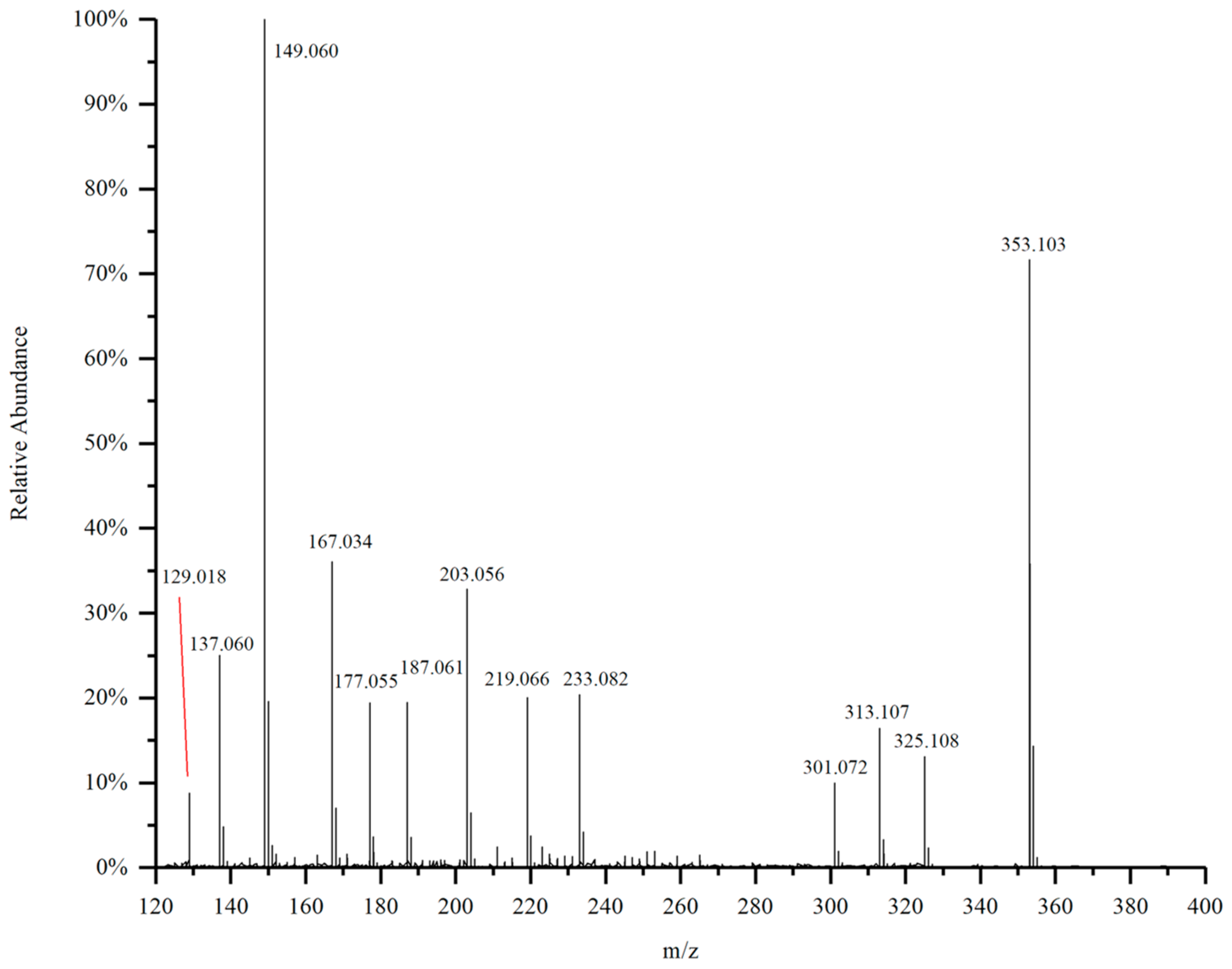
2.6. HESI-MS Mass Spectrometry Analysis of the Purified Substances
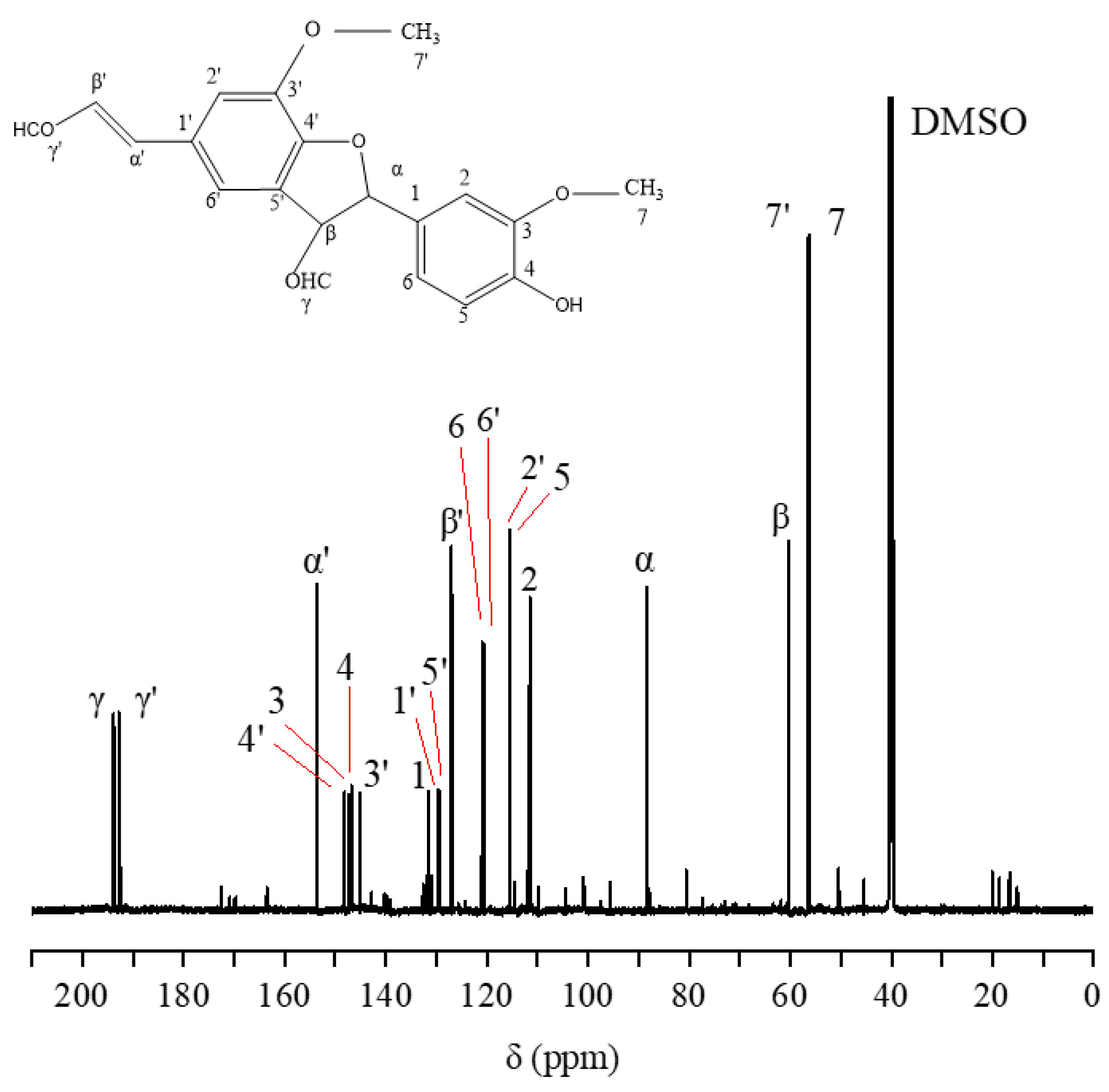
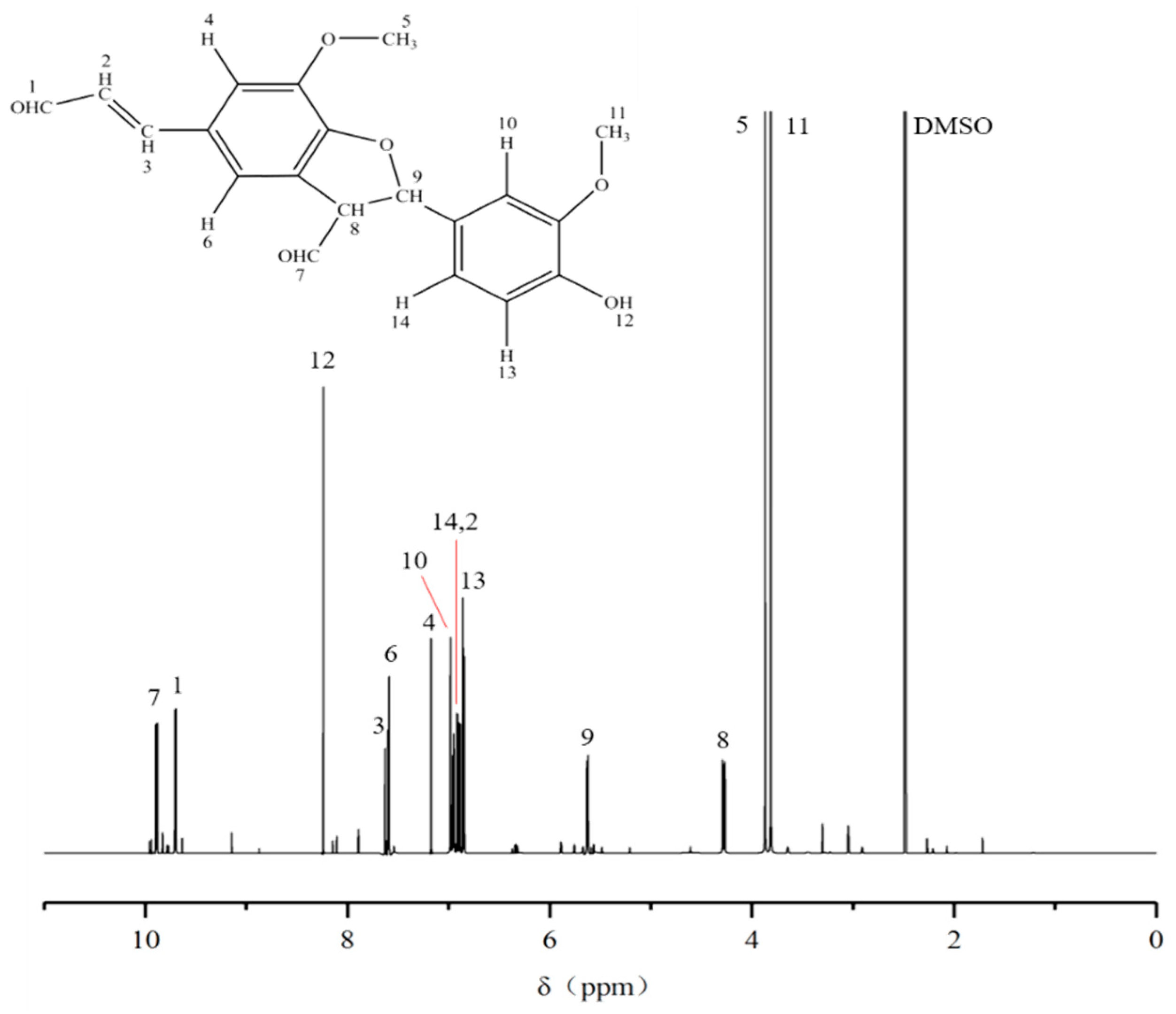
2.7. 13C-NMR and 1H-NMR Analysis of the Purified Compound
2.8. Discussion of the Structure–Effect Relationships of the Purified Substances
3. Experiment
3.1. Materials
3.2. Methods
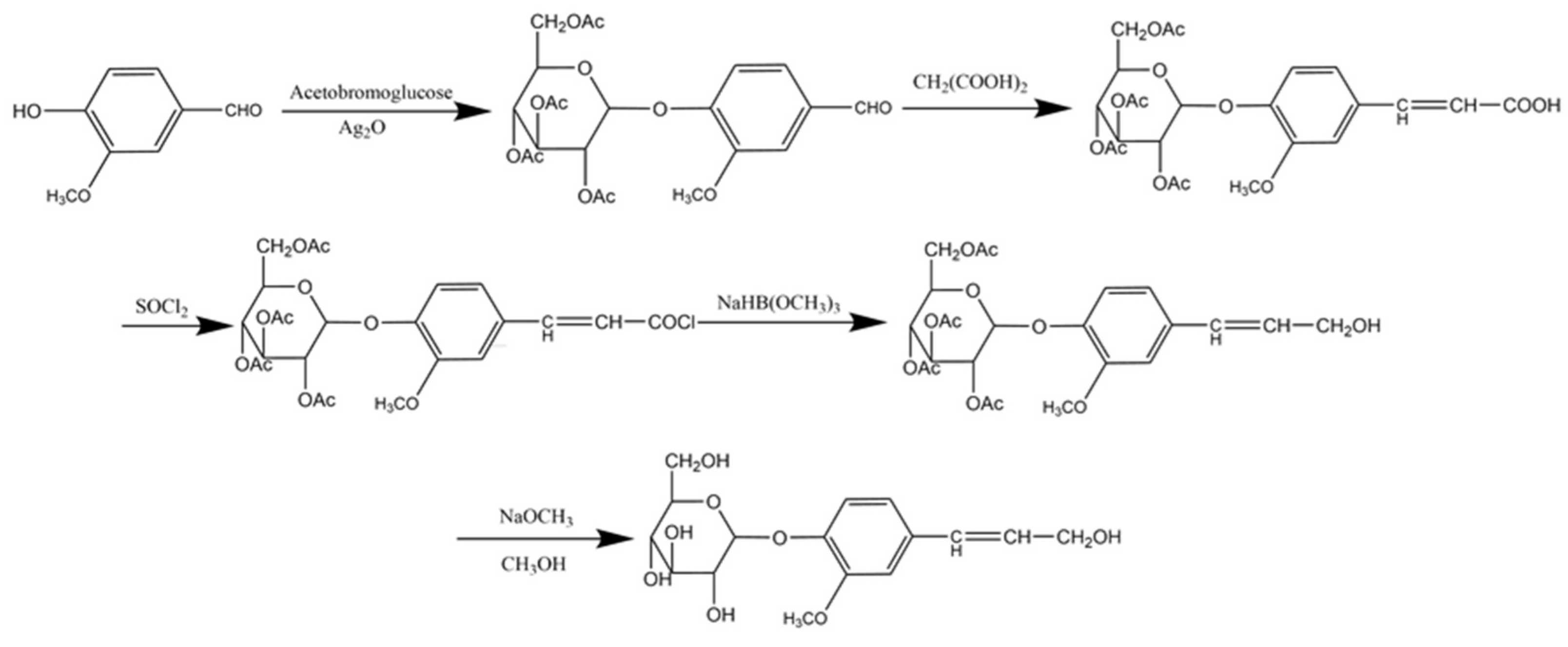
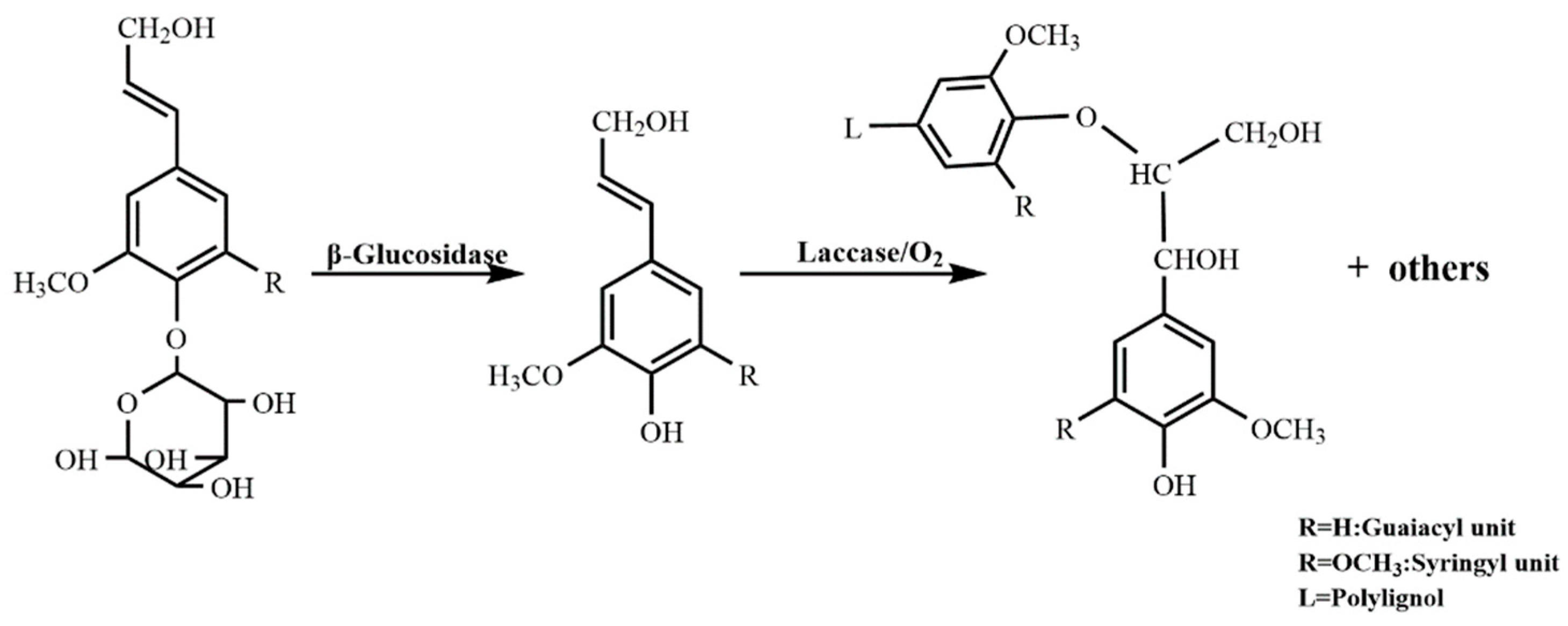
3.2.1. Synthesis of DHP
3.2.2. 13C-NMR Measurement of G-DHP
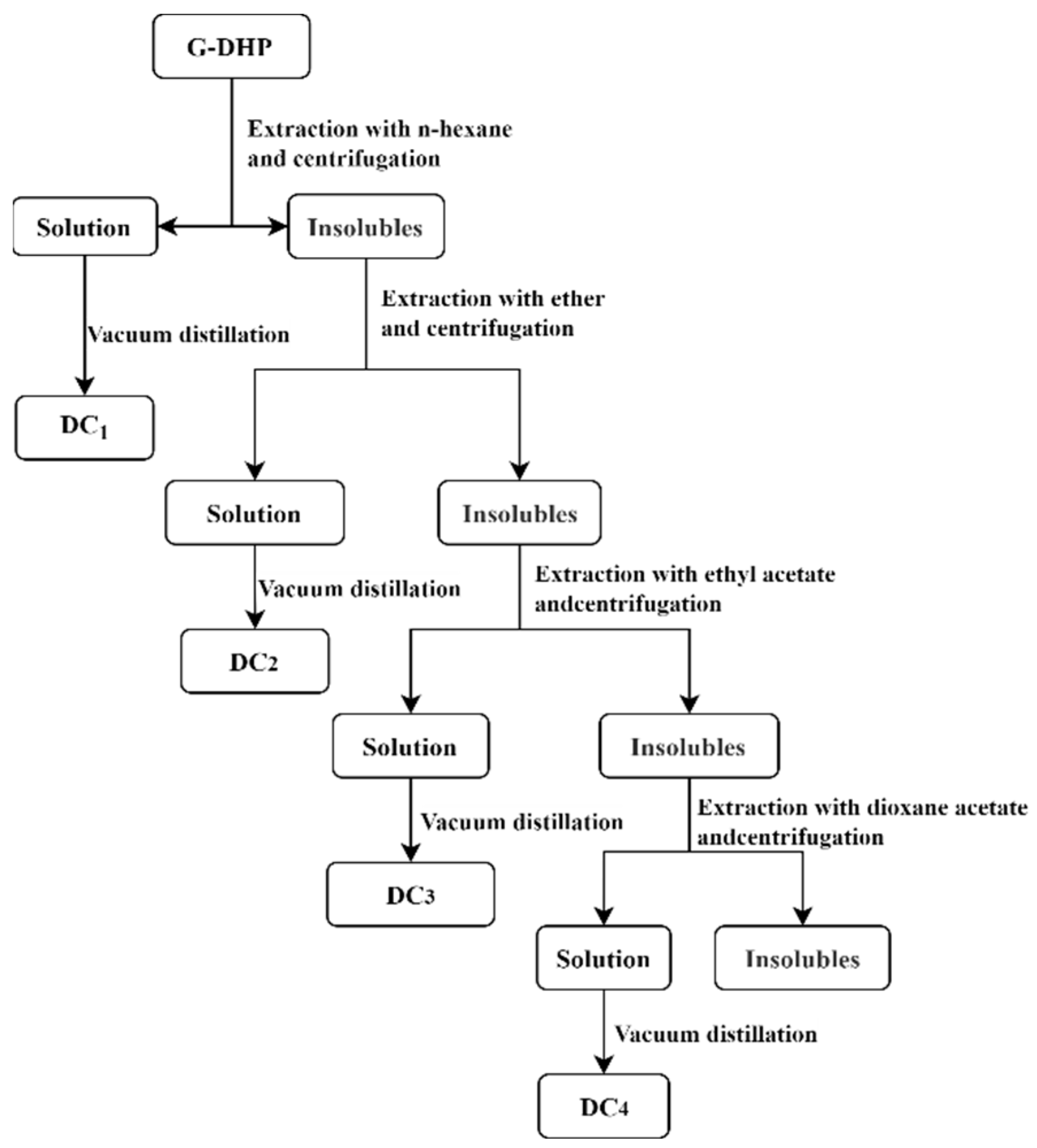
3.2.3. Classification of G-DHP
3.2.4. Determination of Molecular Weight of the G-DHP Fractions
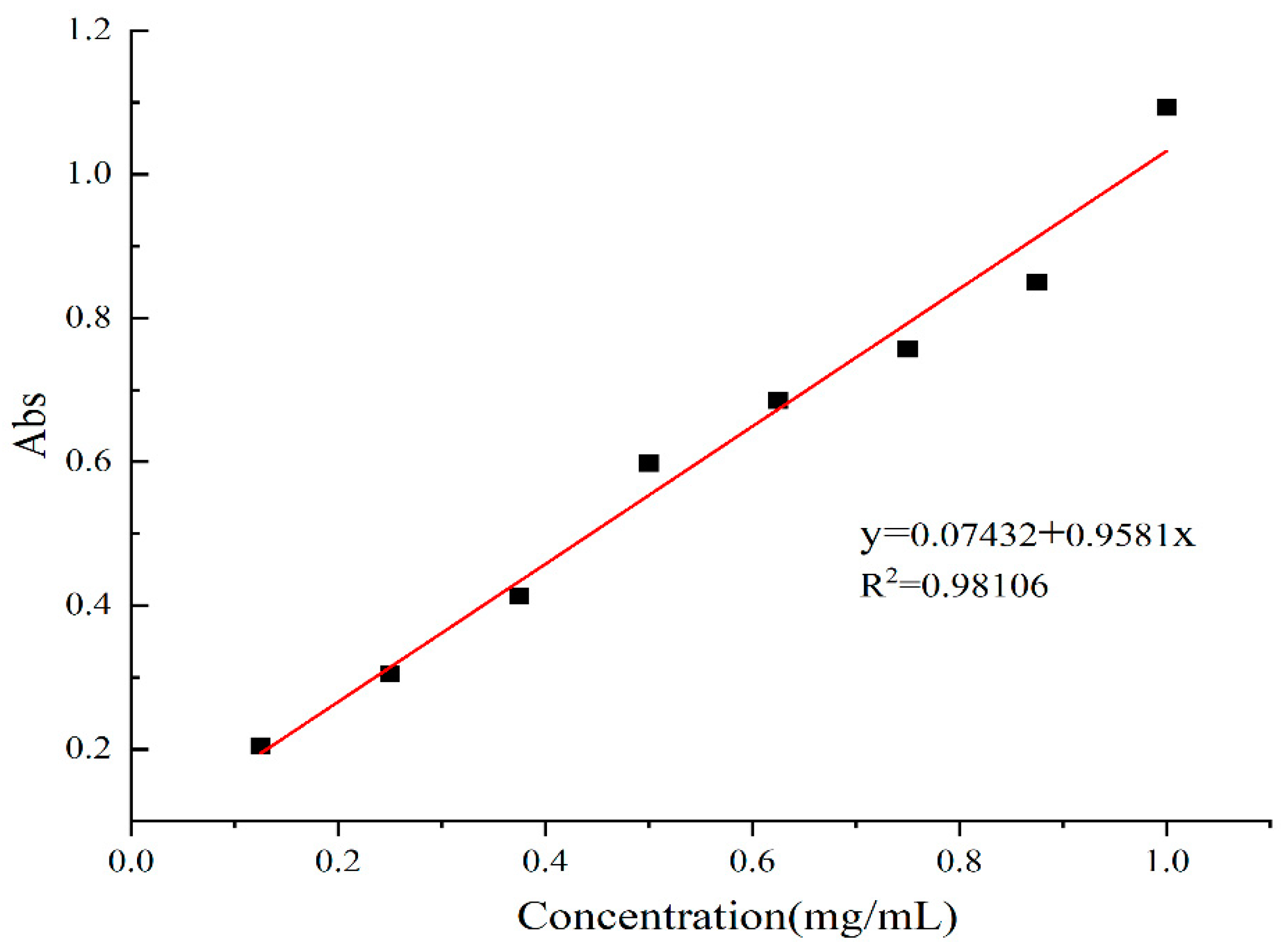
3.2.5. Determination of Total Phenol Content (TPC)
3.2.6. Determination of the Anti-Tumor Activities of DHP Fractions and Purified Compounds
3.2.7. Purification of the Ether-Soluble Fraction of DHP with Preparative Column Chromatography
3.2.8. Mass Spectrometry Analysis of the Structure of the DHP Purified Compound
3.2.9. 13C-NMR and 1H-NMR Measurement of the Purified Substance D5
13C-NMR Measurement of the Purified Substance D5
1H-NMR Measurement of the Purified Substance D5
4. Conclusions
- (1)
- 13C-NMR determination revealed that the structure of G-DHP was relatively similar to that of the gymnosperm ginkgo MWL, which was dominated by β-O-4, β-5, β-1, β-β, and 5-5 substructures.
- (2)
- G-DHP was classified with different polar solvents to obtain a hexane-soluble fraction (DC1), ether-soluble fraction (DC2), ethyl acetate-soluble fraction (DC3), and dioxane-soluble fraction (DC4). The GPC results and total phenolic content measurements showed that the total phenolic content of fractions decreased with an increase in molecular weight. The bioactivity assay showed that the ether-soluble fraction (DC2) had the strongest inhibitory effect on lung cancer A549 with an IC50 of 181.46 ± 28.01 μg/mL.
- (3)
- Further purification of the DC2 fraction revealed that the obtained compounds D4 and D5 had significant anti-tumor activities, with IC50 values of 61.54 ± 17.10 μg/mL and 28.61 ± 8.52 μg/mL against lung cancer A549, respectively. HESI-MS measurements indicated that D5 was a coniferyl aldehyde dimer linked by β-5. The compounds D4 and D5 were similar substance, but D4 contained more impurities. 13C-NMR and 1H-NMR results also confirmed the chemical structure of D5.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Thai, A.A.; Solomon, B.J.; Sequist, L.V.; Gainor, J.F.; Heist, R.S. Lung cancer. Lancet 2021, 398, 535–554. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Garg, J.; Chiu, M.N.; Krishnan, S.; Tripathi, L.K.; Pandit, S.; Far, B.F.; Jha, N.K.; Kesari, K.K.; Tripathi, V.; Pandey, S.; et al. Applications of lignin nanoparticles for cancer drug delivery: An update. Mater. Lett. 2022, 311, 131573. [Google Scholar] [CrossRef]
- Lu, F.J.; Chu, L.H.; Gau, R.J. Free radical-scavenging properties of lignin. Nutr. Cancer 1998, 30, 31–38. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, W.; Wang, Q.; Wang, D.; Dong, D.; Mu, H.; Ye, X.S.; Duan, J. Purification, antioxidant and immunological activities of polysaccharides from Actinidia Chinensis roots. Int. J. Biol. Macromol. 2015, 72, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Lábaj, B.K.J. Antigenotoxic effect of lignin preparations from wastes of pulp and paper industry-in vitro and ex vivo activivity. Acta Fac. Xylologiae Zvolen 2010, 53, 69–76. [Google Scholar]
- Geies, A.; Abdelazim, M.; Sayed, A.M.; Ibrahim, S. Thermal, Morphological and Cytotoxicity Characterization of Hardwood Lignins Isolated by In-Situ Sodium Hydroxide-Sodium Bisulfate Method. Nat. Resour. 2020, 11, 427–438. [Google Scholar] [CrossRef]
- Sakagami, H.; Asano, K.; Yoshida, T.; Kawazoe, Y. Organ distribution and toxicity of lignin. In Vivo 1999, 13, 41–44. [Google Scholar] [PubMed]
- Sakagami, H.; Kushida, T.; Oizumi, T.; Nakashima, H.; Makino, T. Distribution of lignin-carbohydrate complex in plant kingdom and its functionality as alternative medicine. Pharm. Ther. 2010, 128, 91–105. [Google Scholar] [CrossRef]
- Ugartondo, V.; Mitjans, M.; Vinardell, M.P. Applicability of lignins from different sources as antioxidants based on the protective effects on lipid peroxidation induced by oxygen radicals. Ind. Crop. Prod. 2009, 30, 184–187. [Google Scholar] [CrossRef]
- Barapatre, A.; Meena, A.S.; Mekala, S.; Das, A.; Jha, H. In vitro evaluation of antioxidant and cytotoxic activities of lignin fractions extracted from Acacia nilotica. Int. J. Biol. Macromol. 2016, 86, 443–453. [Google Scholar] [CrossRef]
- Mitsuhashi, S.; Kishimoto, T.; Uraki, Y.; Okamato, T.; Ubukata, M. Low molecular weight lignin suppresses activation of NF-κB and HIV-1 promoter. Bioorg. Med. Chem. 2008, 16, 2645–2650. [Google Scholar] [CrossRef]
- Turliuc, M.D.; Cucu, A.I.; Carauleanu, A.; Costea, C. Efficiency and safety of microporous polysaccharide hemispheres from potato starch in brain surgery. Cellul. Chem. Technol. 2018, 52, 505–513. [Google Scholar]
- Spiridon, I.; Poni, P.; Ghica, G. Biological and pharmaceutical applications of lignin and its derivatives: A mini-review. Cellul. Chem. Technol. 2018, 52, 543–550. [Google Scholar]
- Shu, F.; Jiang, B.; Yuan, Y.; Li, M.; Wu, W.; Jin, Y.; Xiao, H. Biological activities and emerging roles of lignin and lignin-based products—A review. Biomacromolecules 2021, 22, 4905–4918. [Google Scholar] [CrossRef] [PubMed]
- Andrijevic, L.; Radotic, K.; Bogdanovic, J.; Mutavdzic, D.; Bogdanovic, G. Antiproliferative effect of synthetic lignin against human breast cancer and normal fetal lung cell lines. Potency of low molecular weight fractions. J. BUON 2008, 13, 241–244. [Google Scholar] [PubMed]
- Xie, Y.; Jiang, C.; Chen, X.; Wu, H.; Bi, S. Preparation of Oligomeric Phenolic Compounds (DHPs) from Coniferin and Syringin and Characterization of their Anticancer Properties. BioResources 2020, 15, 1791–1809. [Google Scholar] [CrossRef]
- Xie, Y.; Yasuda, S.; Wu, H.; Liu, H. Analysis of the structure of lignin-carbohydrate complexes by the specific 13C tracer method. J. Wood Sci. 2000, 46, 130–136. [Google Scholar] [CrossRef]
- Holtman, K.M.; Chang, H.M.; Kadla, J.F. Solution-state nuclear magnetic resonance study of the similarities between milled wood lignin and cellulolytic enzyme lignin. J. Agric. Food Chem. 2004, 52, 720–726. [Google Scholar] [CrossRef]
- Hage, R.E.; Brosse, N.; Chrusciel, L.; Sanchez, C.; Sannigrahi, P.; Ragauskas, A. Characterization of milled wood lignin and ethanol organosolv lignin from miscanthus. Polym. Degrad. Stab. 2009, 94, 1632–1638. [Google Scholar] [CrossRef]
- Lüdemann, H.D.; Nimz, H. Carbon-13 nuclear magnetic resonance spectra of lignins. Biochem. Biophys. Res. Commun. 1973, 52, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Robert, D.R.; Terashima, N. Selective carbon 13 enrichment of side chain carbons of ginkgolignin traced by carbon 13 nuclear magnetic resonance. Plant Physiol. Biochem. 1994, 32, 243–249. [Google Scholar]
- Terashima, N.; Atalla, R.H.; Ralph, S.A.; Landucci, L.L.; Lapierre, C.; Monties, B. New Preparations of Lignin Polymer Models under Conditions that Approximate Cell Wall Lignification. I. Synthesis of Novel Lignin Polymer Models and their Structural Characterization by 13C NMR. Holzforschung 1995, 49, 521–527. [Google Scholar] [CrossRef]
- Harman-Ware, A.E.; Happs, R.M.; Davison, B.H.; Davis, M.F. The effect of coumaryl alcohol incorporation on the structure and composition of lignin dehydrogenation polymers. Biotechnol. Biofiuels 2017, 10, 281. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Cui, S.; Xie, Y. Synthesis and Antibacterial Properties of Oligomeric Dehydrogenation Polymer from Lignin Precursors. Molecules 2022, 27, 1466. [Google Scholar] [CrossRef]
- Grever, M.R.; Schepartz, S.A.; Chabner, B.A. The National Cancer Institute: Cancer drug discovery and development program. Semin. Oncol. 1992, 19, 622–638. [Google Scholar]
- Goldin, A.; Venditti, J.M.; Macdonald, J.S.; Muggia, F.M.; Henney, J.E.; Devita, V.T., Jr. Current results of the screening program at the Division of Cancer Treatment, National Cancer Institute. Eur. J. Cancer 1981, 17, 129–142. [Google Scholar] [CrossRef]
- Geran, R.I. Protocols for screening chemical agents and natural products against animal tumors and other biological systems: Drug Evaluation Branch. Nat. Cancer Inst. 1962, 3, 1–107. [Google Scholar]
- Li, J.; Wang, P.; Yang, J.; Chen, F.; Xie, Y. Preparation of Porous Biological Carrier with Gingko LCC and Application in Culture of Human Hepatocytes. Chem. Ind. For. Prod. 2014, 34, 81–87. [Google Scholar]
- Zhao, H.; Feng, Q.; Xie, Y.; Li, J.; Chen, X. Preparation of Biocompatible Hydrogel from Lignin Carbohydrate Complex (LCC) as Cell Carriers. Bioresources 2017, 12, 8490–8504. [Google Scholar] [CrossRef]
- Cui, C.; Chen, X.; Liu, C.; Zhu, Y.; Ouyang, J.; Shen, Y.; Zhou, Z.; Qi, F. In Situ Reactor-Integrated Electrospray Ionization Mass Spectrometry for Heterogeneous Catalytic Reactions and Its Application in the Process Analysis of High-Pressure Liquid-Phase Lignin Depolymerization. Anal. Chem. 2021, 93, 12987–12994. [Google Scholar] [CrossRef]
- Dean, K.R.; Novak, B.; Moradipour, M.; Tong, X.; Moldovan, D.; Knutson, B.L.; Rankin, S.E.; Lynn, B.C. Complexation of Lignin Dimers with β-Cyclodextrin and Binding Stability Analysis by ESI-MS, Isothermal Titration Calorimetry, and Molecular Dynamics Simulations. J. Phys. Chem. B 2022, 126, 1655–1667. [Google Scholar] [CrossRef]
- Qi, Y.; Hempelmann, R.; Volmer, D.A. Shedding light on the structures of lignin compounds: Photo-oxidation under artificial UV light and characterization by high resolution mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 8203–8210. [Google Scholar] [CrossRef] [PubMed]
- Terashima, N.; Atalla, R.H.; Ralph, S.A.; Landucci, L.L.; Lapierre, C.; Monties, B. New preparations of lignin polymer models under conditions that approximate cell wall lignification. II. Structural characterization of the models by thioacidolysis. Holzforschung 1996, 50, 9–14. [Google Scholar] [CrossRef]
- Ye, Z.Z.; Xie, Y.M.; Wu, C.X.; Wang, P.; Le, X. Dehydrogenation polymerization of isoeugenol and formation of lignin-carbohydrate complexes with presence of polysaccharide. Chem. Ind. For. Prod. 2016, 36, 45–50. [Google Scholar]
- Chen, X.K.; Zhao, H.K.; Wu, H.F.; Ye, Z.Z.; Xie, Y.M. Preparation and antioxidant properties of dehydrogenation polymer of isoeugenol. Chem. Ind. For. Prod. 2018, 38, 87–92. [Google Scholar]
- Niu, Y.; Zhang, X.; Xiao, Z.; Song, S.; Eric, K.; Jia, C.; Yu, H.; Zhu, J. Characterization of odor-active compounds of various cherry wines by gas chromatography–mass spectrometry, gas chromatography–olfactometry and their correlation with sensory attributes. J. Chromatogr. B 2011, 879, 2287–2293. [Google Scholar] [CrossRef] [PubMed]
- Kosyakov, D.S.; Pikovskoi, I.I.; Ul’yanovskii, N.V. Dopant-assisted atmospheric pressure photoionization Orbitrap mass spectrometry–An approach to molecular characterization of lignin oligomers. Anal. Chim. Acta 2021, 1179, 338836. [Google Scholar] [CrossRef]
- Ito, T.; Hayase, R.; Kawai, S.; Ohashi, H.; Higuchi, T. Coniferyl aldehyde dimers in dehydrogenative polymerization: Model of abnormal lignin formation in cinnamyl alcohol dehydrogenase-deficient plants. J. Wood Sci. 2002, 48, 216–221. [Google Scholar] [CrossRef]
- Zemek, J.; Košíková, B.; Augustin, J.; Joniak, D. Antibiotic properties of lignin components. Folia Microbiol. 1979, 24, 483–486. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, X.; Jiang, C.; Wu, H.; Ye, Z. Preparation of oligomeric dehydrogenation polymer and characterization of its antibacterial properties. Bioresources 2019, 14, 2842–2860. [Google Scholar] [CrossRef]
- Gładkowski, W.; Skrobiszewski, A.; Mazur, M.; Siepka, M.; Pawlak, A.; Obminska-Mrukowicz, B.; Bialonska, A.; Poradowski, D.; Drynda, A.; Urbaniak, M. Synthesis and anticancer activity of novel halolactones with β-aryl substituents from simple aromatic aldehydes. Tetrahedron 2013, 69, 10414–10423. [Google Scholar] [CrossRef]
- Li, M.F.; Sun, S.N.; Xu, F.; Sun, R.C. Sequential solvent fractionation of heterogeneous bamboo organosolv lignin for value-added application. Sep. Purif. Technol. 2012, 101, 18–25. [Google Scholar] [CrossRef]
- Bonoli, M.; Verardo, V.; Marconi, E.; Caboni, M.F. Antioxidant phenols in barley (Hordeum vulgare L.) flour: Comparative spectrophotometric study among extraction methods of free and bound phenolic compounds. J. Agric. Food Chem. 2004, 52, 5195–5200. [Google Scholar] [CrossRef]
- Ahamed, M.; Ali, D.; Alhadlaq, H.A.; Akhtar, M.J. Nickel oxide nanoparticles exert cytotoxicity via oxidative stress and induce apoptotic response in human liver cells (HepG2). Chemosphere 2013, 93, 2514–2522. [Google Scholar] [CrossRef] [PubMed]
- Le, N.T.; Donadu, M.G.; Ho, D.V.; Doan, T.Q.; Le, A.T.; Raal, A.; Usai, D.; Sanna, G.; Marchetti, M.; Usai, M.; et al. Biological activities of essential oil extracted from leaves of Atalantia sessiflora Guillauminin Vietnam. J. Infect. Dev. Ctries. 2020, 14, 1054–1064. [Google Scholar] [CrossRef] [PubMed]
- Nga, N.T.H.; Ngoc, T.T.B.; Trinh, N.T.M.; Trinh, N.T.M.; Thuoc, T.L.; Thao, D.T.P. Optimization and application of MTT assay in determining density of suspension cells. Anal. Biochem. 2020, 610, 113937. [Google Scholar] [CrossRef] [PubMed]


| DHP Fractions | Mw | Mn | PDI |
|---|---|---|---|
| DC1 | 188 | 277 | 1.47 |
| DC2 | 402 | 643 | 1.60 |
| DC3 | 1203 | 1695 | 1.41 |
| DC4 | 1853 | 3486 | 1.88 |
| DHP Fractions | Mw | TPC |
|---|---|---|
| DC1 | 277 | 228.66 |
| DC2 | 643 | 186.57 |
| DC3 | 1695 | 92.21 |
| DC4 | 3486 | 70.53 |
| Classified Component | IC50 (μg/mL) |
|---|---|
| DC1 | 318.55 ± 53.49 |
| DC2 | 181.46 ± 28.01 |
| DC3 | 401.71 ± 64.27 |
| DC4 | 461.03 ± 79.53 |
| Purified Substances | IC50 (μg/mL) |
|---|---|
| D1 | 286.76 ± 47.16 |
| D2 | 179.25 ± 27.96 |
| D3 | 113.70 ± 23.55 |
| D4 | 61.54 ± 17.10 |
| D5 | 28.61 ± 8.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Yue, Y.; Wei, X.; Xie, Y. Preparation and Anti-Lung Cancer Activity Analysis of Guaiacyl-Type Dehydrogenation Polymer. Molecules 2023, 28, 3589. https://doi.org/10.3390/molecules28083589
Zhou J, Yue Y, Wei X, Xie Y. Preparation and Anti-Lung Cancer Activity Analysis of Guaiacyl-Type Dehydrogenation Polymer. Molecules. 2023; 28(8):3589. https://doi.org/10.3390/molecules28083589
Chicago/Turabian StyleZhou, Junyi, Yuanyuan Yue, Xin Wei, and Yimin Xie. 2023. "Preparation and Anti-Lung Cancer Activity Analysis of Guaiacyl-Type Dehydrogenation Polymer" Molecules 28, no. 8: 3589. https://doi.org/10.3390/molecules28083589
APA StyleZhou, J., Yue, Y., Wei, X., & Xie, Y. (2023). Preparation and Anti-Lung Cancer Activity Analysis of Guaiacyl-Type Dehydrogenation Polymer. Molecules, 28(8), 3589. https://doi.org/10.3390/molecules28083589







