Interface Bonding Properties of CrAlSiN-Coated Cemented Carbides Doped with CeO2 and Y2O3 Rare Earth Oxides
Abstract
:1. Introduction
2. Parameter Selection for Simulation Analysis and Parameter Calculation of Interface Bonding Properties
2.1. Parameter Selection for Simulation Analysis
2.2. Parameter Calculation of Interface Bonding Properties
3. Analysis of Interface Bonding Properties of the CrAlSiN/WC-Co Model Non-Doped with CeO2 or Y2O3
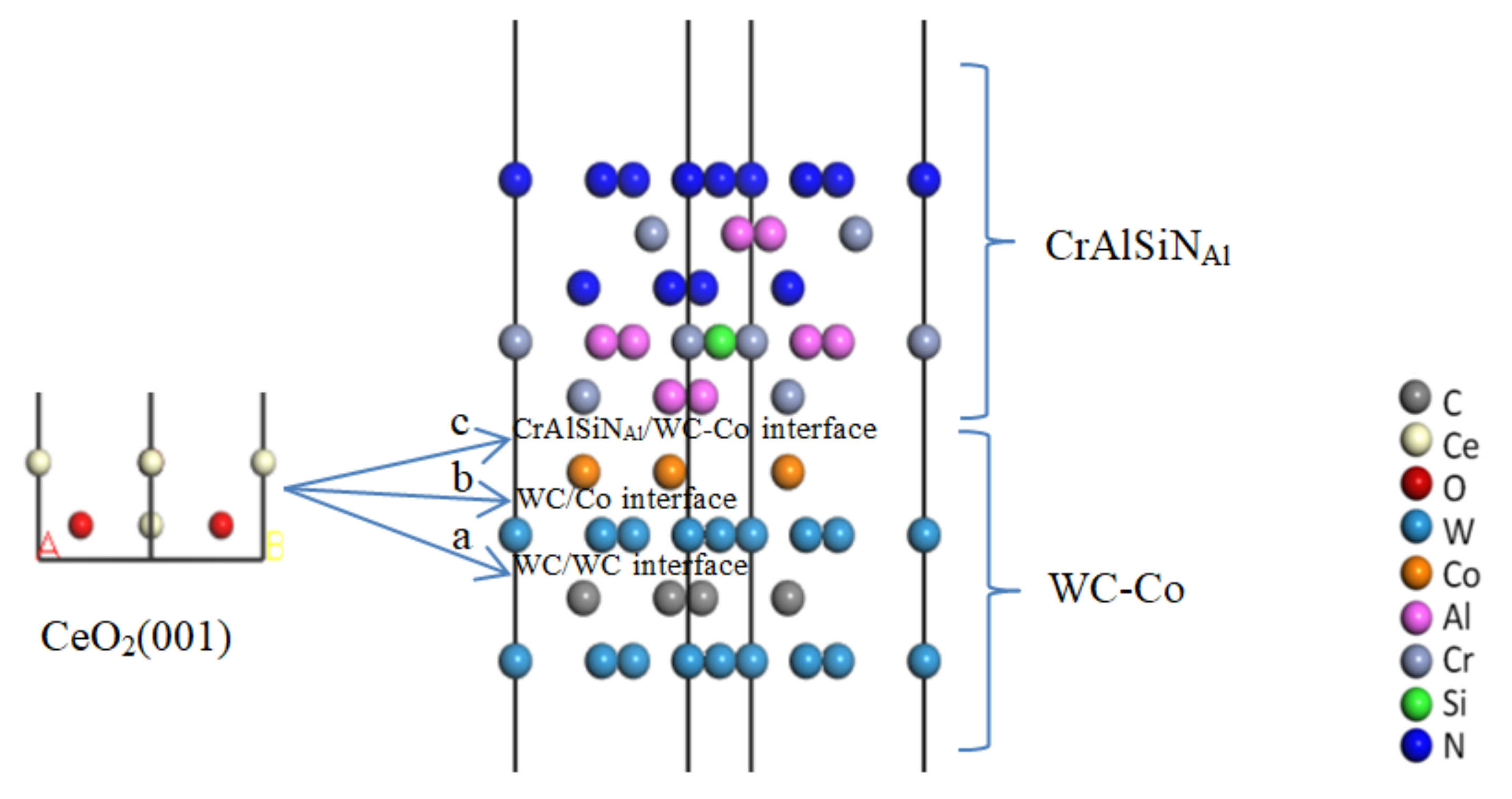
3.1. Construction of the CrAlSiN/WC-Co Model
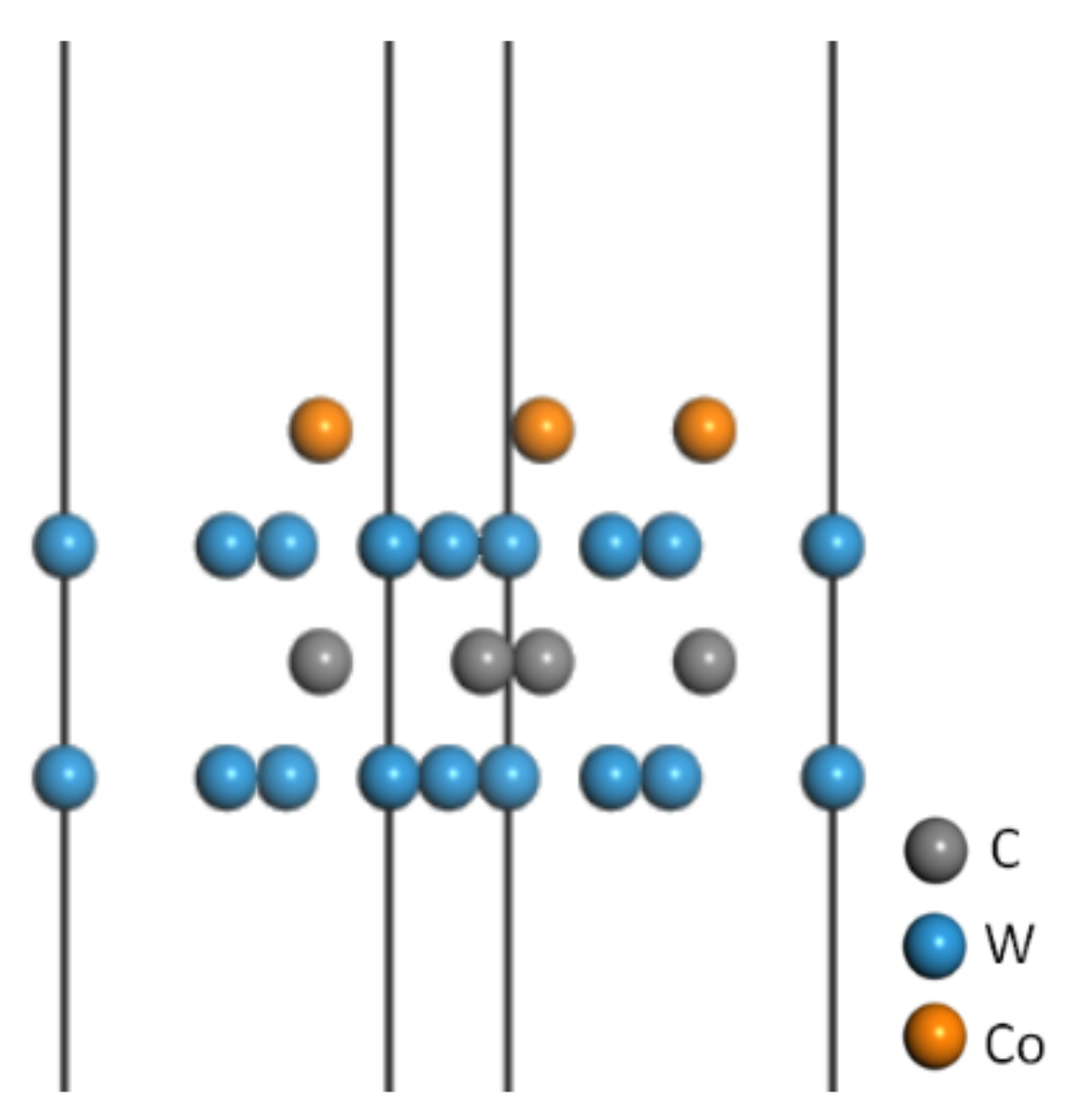
3.1.1. Construction of the WC-Co Cemented Carbide Matrix Model
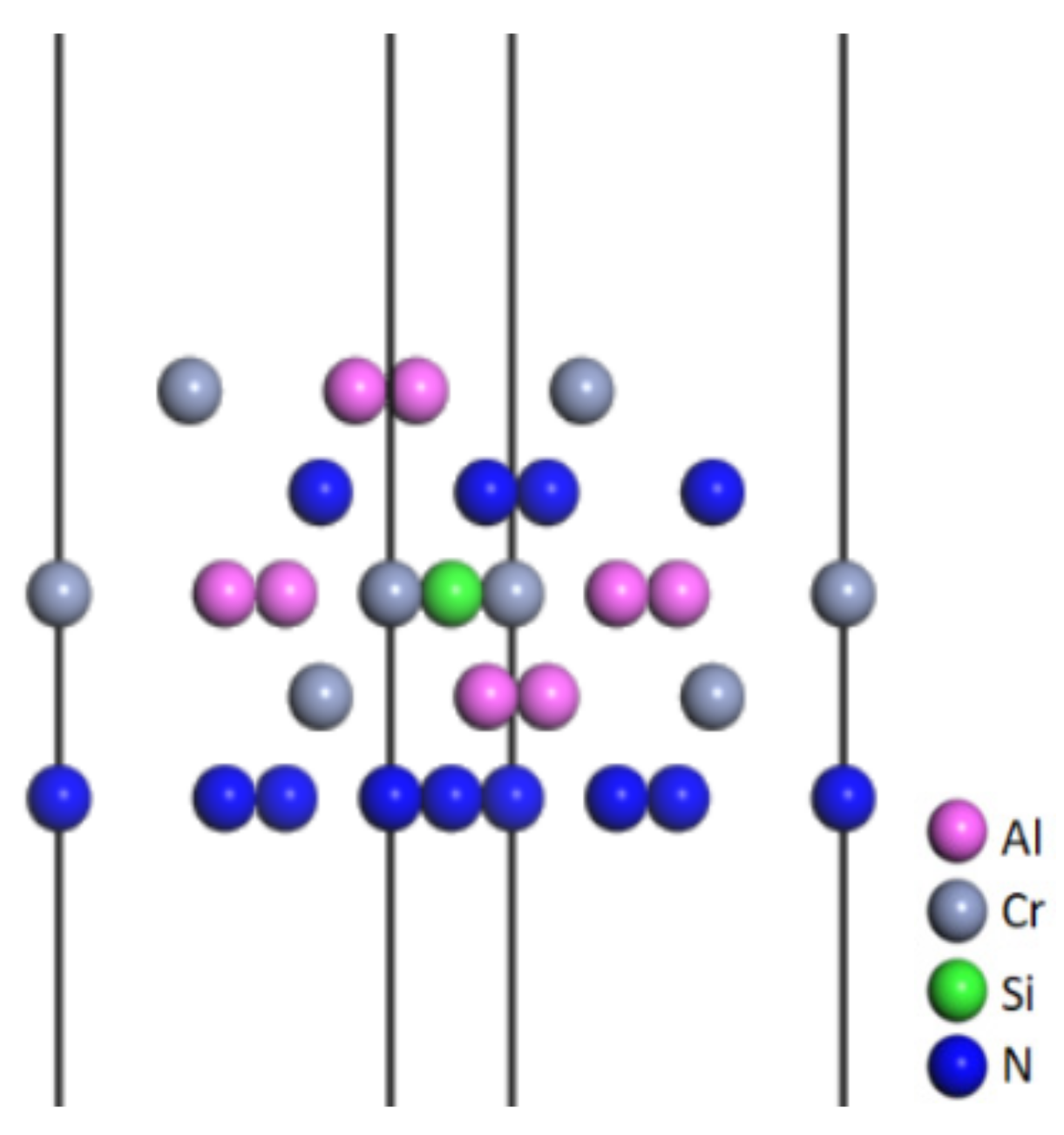
3.1.2. Construction of the CrAlSiN Coating Model
3.1.3. Construction of the CrAlSiN/WC-Co Models with Different Terminal Atoms
3.2. Interface Bonding Property Analysis
4. Analysis of the Interfaces Bonding Properties of the Al Terminal Model Doped with CeO2 or Y2O3
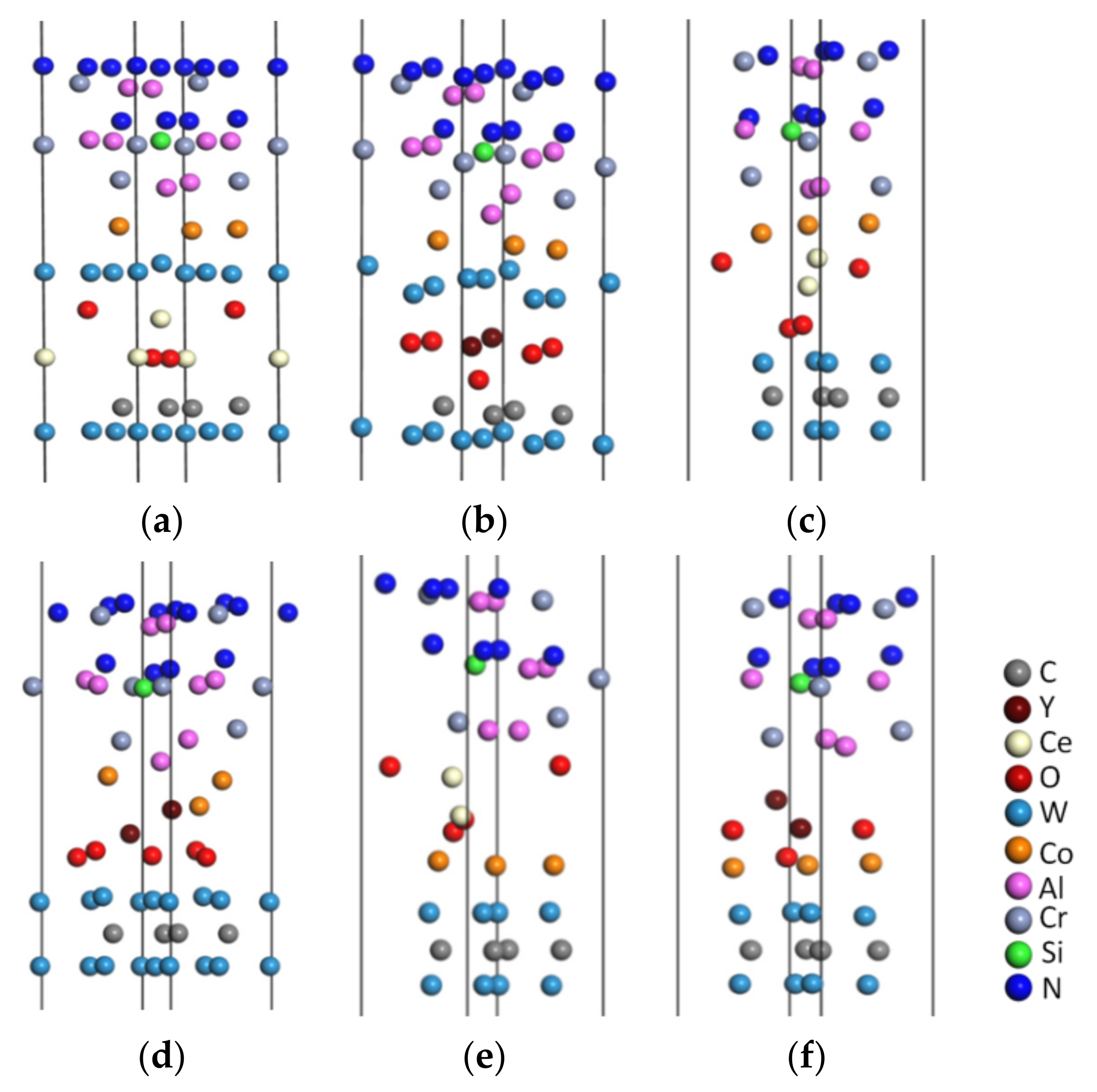
4.1. Construction of Doped Models
4.1.1. Construction of the CeO2 and Y2O3 Models
4.1.2. Construction of Al Terminal Models Doped with CeO2 or Y2O3
4.2. Geometric Optimization of the Doped Models
4.3. Analysis of the Interface Bonding Properties of the Al Terminal Model Doped with CeO2 or Y2O3
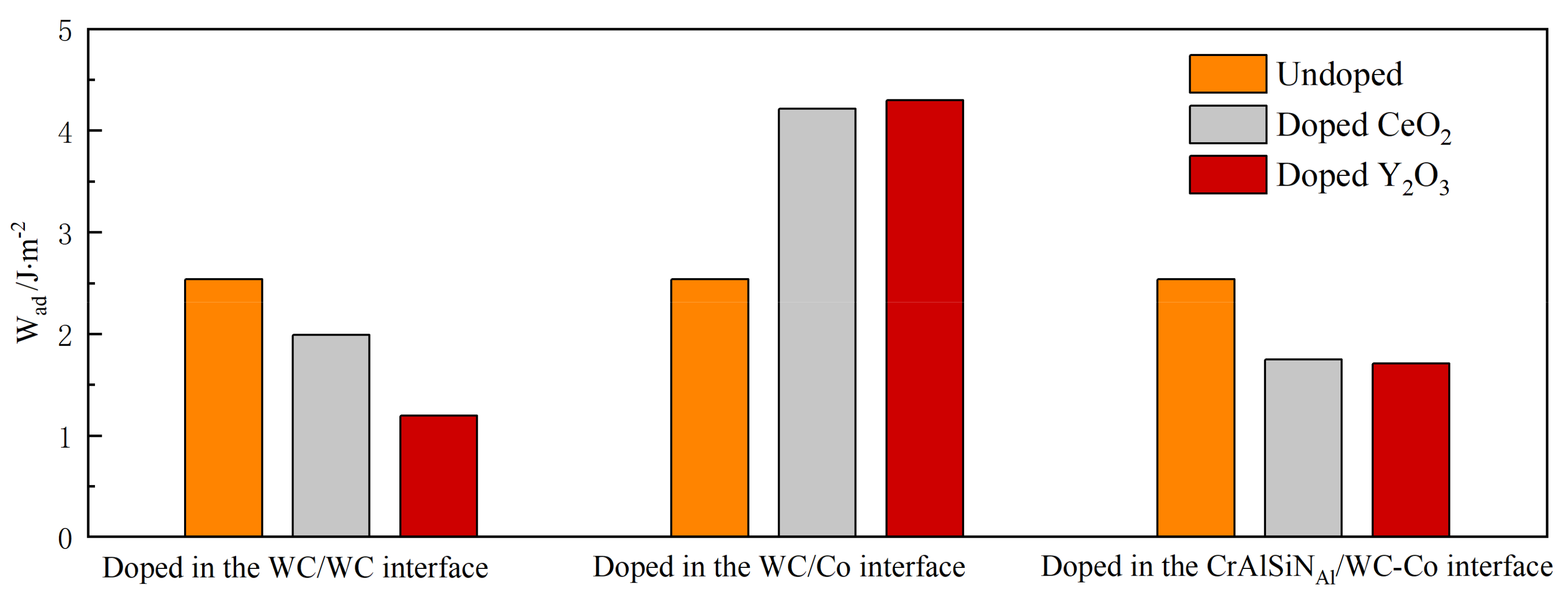
4.3.1. Adhesion Work Analysis
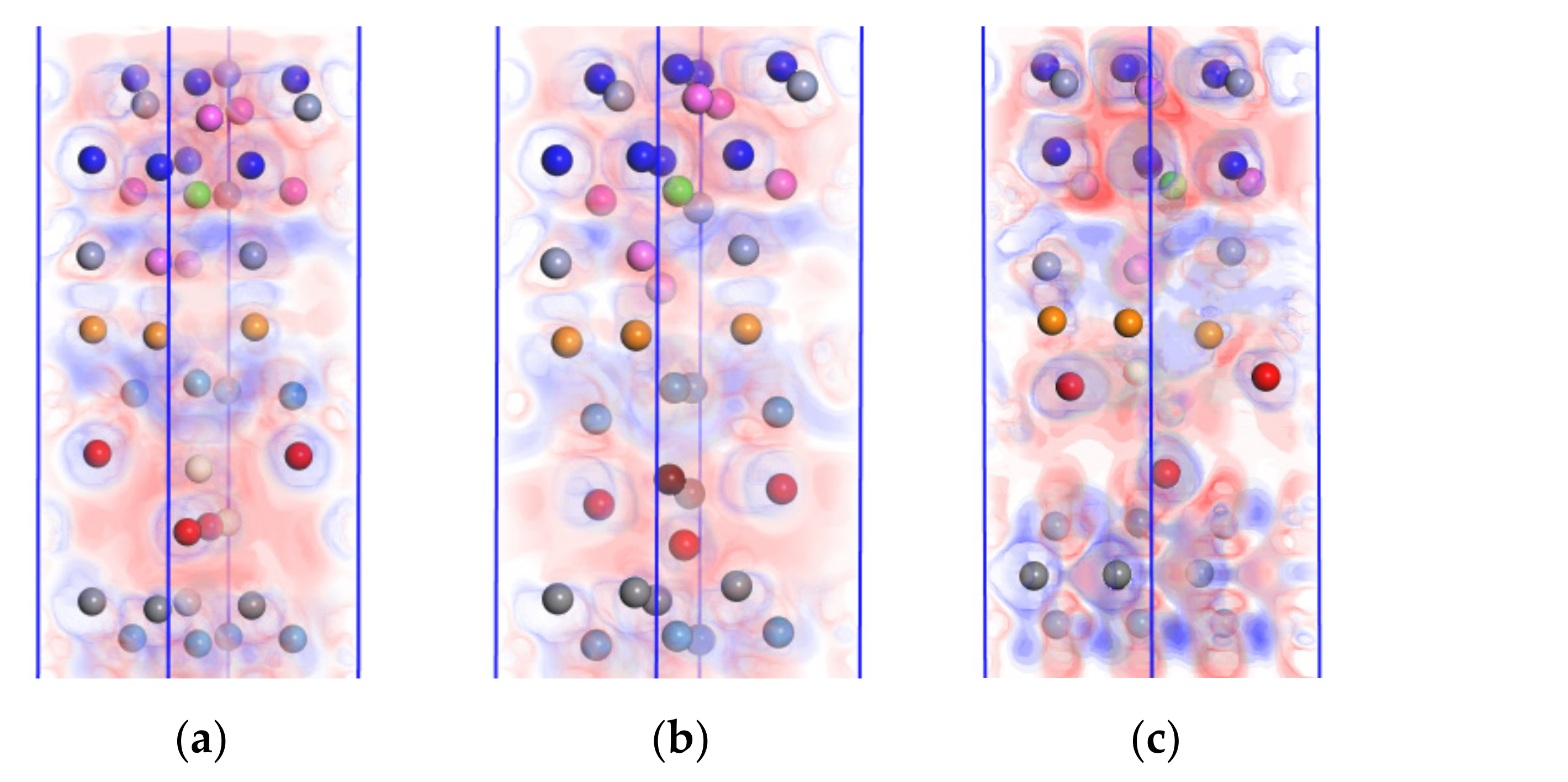
4.3.2. Charge Density Difference Analysis
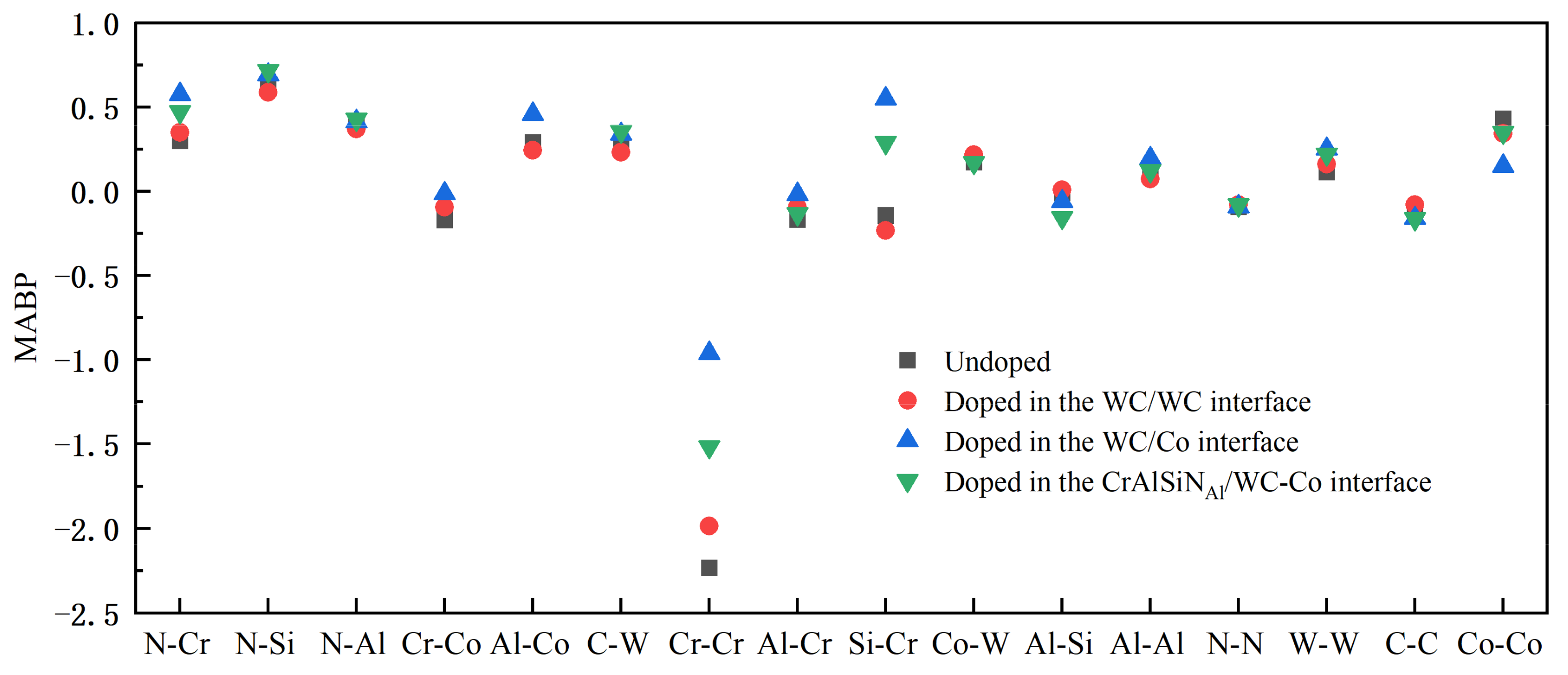
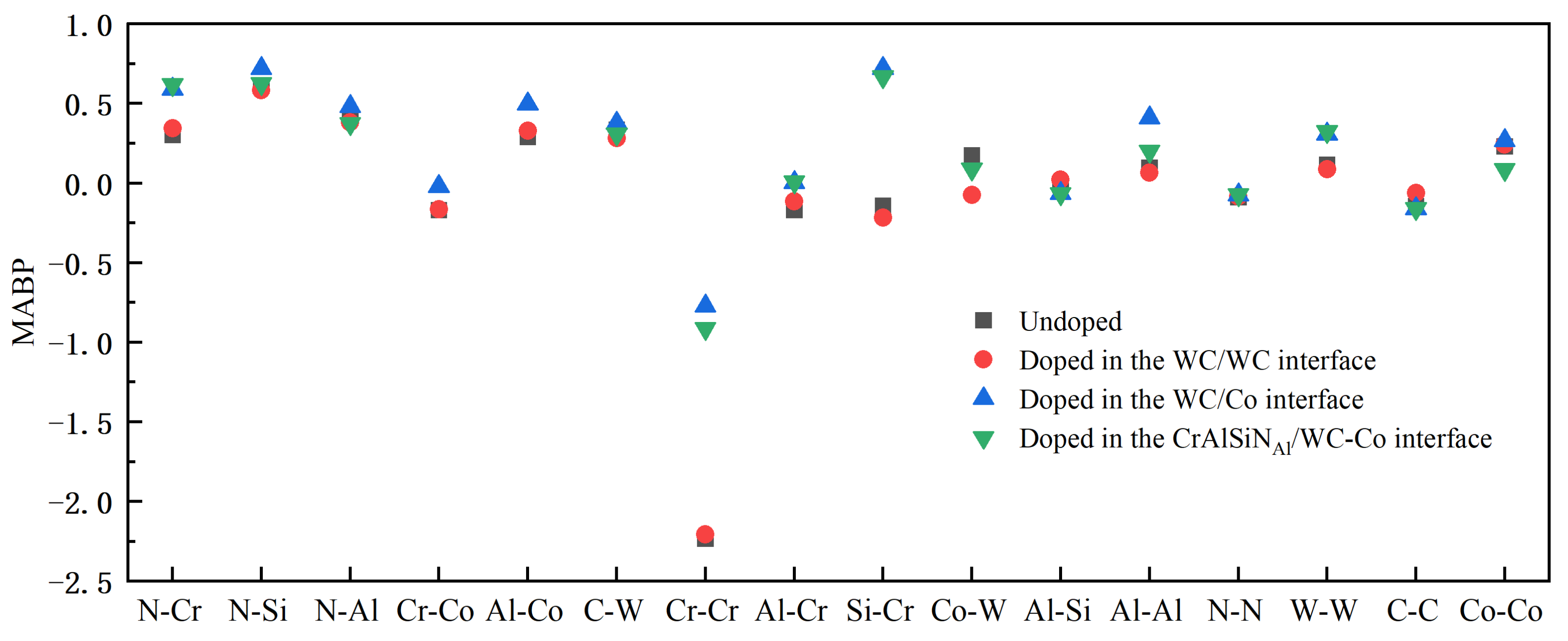
4.3.3. Mulliken Average Bond Population Analysis
5. Conclusions
- (1)
- The adhesion work values were calculated for three interface models with various terminal atoms, namely CrAlSiNSi/WC-Co, CrAlSiNN/WC-Co, and CrAlSiNAl/WC-Co. The analysis showed that the adhesion work was the highest at the CrAlSiNSi/WC-Co interface (4.312 J·m−2) and the lowest at the CrAlSiNAl/WC-Co interface (2.536 J·m−2).
- (2)
- Based on the CrAlSiNAl/WC-Co interface model with the lowest interface bonding strength, we doped CeO2 or Y2O3 into the WC/WC, WC/Co, and CrAlSiNAl/WC-Co interfaces to obtain the doped models.
- (3)
- Doping CeO2 or Y2O3 into the WC/WC and CrAlSiNAl/WC-Co interfaces deteriorated the interface bonding properties of the Al terminal model; in contrast, doping into the WC/Co interface improved the bonding properties of the Al terminal model. Doping either CeO2 or Y2O3 into the WC/Co interface increased the adhesion work. Further charge density difference and MABP analyses revealed that the interfaces with higher adhesion work and improved interface bonding properties exhibited a decreased interatomic distance, a higher charge density, a larger number of charge transfers between atoms, stronger interatomic interactions, a higher MABP, and higher interatomic bonding strength.
- (4)
- Of the two rare earth oxides, Y2O3 doping into the WC/Co interface improved the interface bonding properties more significantly than CeO2 doping. In CrAlSiNAl/WC/CeO2/Co, the adhesion work at the CrAlSiNAl/WC-Co, CeO2/Co, and WC/CeO2 interfaces was 4.216, 3.235, and 4.615 J·m−2, respectively. In CrAlSiNAl/WC/Y2O3/Co, the adhesion work values at the CrAlSiNAl/WC-Co, Y2O3/Co, and WC/Y2O3 interfaces were 4.297, 3.982, and 4.724 J·m−2, respectively. The adhesion work with Y2O3 doping was consistently higher than that with CeO2 doping. The constructed charge density difference maps revealed that Y2O3 doping into each interface consistently resulted in a higher charge density, a higher number of charge transfers, and stronger interatomic interactions. The MABP of the Y2O3-doped models was consistently higher than that of the CeO2-doped models. These results strongly suggested that Y2O3 doping more significantly increased the interatomic interactions and reduced the interatomic repulsion in the Al terminal model (CrAlSiNAl/WC-Co) compared to CeO2 doping. Therefore, when rare earth oxides are doped at the WC/Co interface, the doping of Y2O3 has a better effect in terms of improving the interface bonding performance of the Al terminal model (CrAlSiNAl/WC-Co).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Chang, Y.Y.; Lai, H.M. Wear behavior and cutting performance of CrAlSiN and TiAlSiN hard coatings on cemented carbide cutting tools for Ti alloys. Surf. Coat. Technol. 2014, 259, 152–158. [Google Scholar] [CrossRef]
- Tang, Q.F.; Wang, X.; Liang, Y.M.T.; Fan, Q.X.; Wang, T.G. Study on failure mechanism of CrAlSiN-coated tool in cutting titanium alloy. J. Tianjin Univ. Technol. Educ. 2021, 31, 9–12. [Google Scholar]
- Zhang, S.H.; Wang, L.; Wang, Q.M.; Li, M.X. A superhard CrAlSiN superlattice coatingdeposited by multi-arc ion plating: I. Microstructure and mechanical properties. Surf. Coat. Technol. 2013, 214, 160–167. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, S.S.; Xiao, X.L.; Deng, X. Study on cutting performance of dry turning Ti-6Al-4V titanium alloy with AlCrSiN coated tool. J. Guangdong Univ. Technol. 2021, 38, 99–106. [Google Scholar]
- Hsu, C.H.; Huang, W.C.; Lee, Y.P.; Ho, W.Y. Effect of nitrogen atmosphere heat treatment on structure and wear behavior of CrAlSiN nanocomposite film. Surf. Coat. Technol. 2017, 320, 230–234. [Google Scholar] [CrossRef]
- Lu, M.; Wang, H.; Song, X.; Sun, F. Effect of doping level on residual stress, coating-substrate adhesion and wear resistance of boron-doped diamond coated tools. J. Manuf. Process. 2023, 88, 145–156. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, M.; Zhou, F.; Zhou, Z.; Jin, X. The toughness evaluation of CrBN coatings doped with Ni or Cu by experiment and FEM. Appl. Surf. Sci. 2022, 599, 153804. [Google Scholar] [CrossRef]
- Yu, Y.; Mei, F.; Lin, X.; Gao, J.; Yuan, T. Impact of the B/C-doping ratio on the microstructure, mechanical properties, and cutting performance of AlTiN-based coatings. Ceram. Int. 2023, 49, 2774–2785. [Google Scholar] [CrossRef]
- Liu, D.F. Study on Ductility-Enhancing and Strengthening Mechanism of Y Micro-Alloyed 6.5%Si High-Silicon Steel and Evolution of Microstructure and Texture in Hot and Warm Rolling. Master’s Thesis, Jiangxi University of Science and Technology, Ganzhou, China, 2020. [Google Scholar]
- Du, T. Role and mechanism of rare earth elements in metallic materials. Trans. Nonferrous Met. Socirty China 1996, 6, 15–20. [Google Scholar]
- Li, B.; He, R.; Yang, H.; Zou, D.; Liu, Y.J.; Liang, Y.; Yang, Q.M.; Li, Y.X. Effect of Re addition on the microstructure and mechanical properties of WC-10Co cemented carbides fabricated by chemical coating method. Int. J. Refract. Met. Hard Mater. 2020, 93, 105344. [Google Scholar] [CrossRef]
- Liu, S. Study on rare-earth doped cemented carbides in China. Int. J. Refract. Met. Hard Mater. 2009, 27, 528–534. [Google Scholar]
- Zou, Q.; Zhang, M.L.; Li, Y.G.; Luo, Y.A. Research progress and prospect of strengtheningand toughening of WC cemented carbides. J. Mech. Eng. 2021, 57, 195–204+212. [Google Scholar]
- Wang, Y.; Pan, Z.; Wang, C.; Sun, X.; Peng, Z.; Wang, B. Cutting performance of WC-Co alloys modified by nano-additives. J. Mater. Sci. Technol. 2012, 28, 205–213. [Google Scholar] [CrossRef]
- Wen, Y. Effect of Rare Earth and Copper on Microstructure and Properties of WC-10Cocemented Carbide. Master’s Thesis, Jiangxi University of Science and Technology, Ganzhou, China, 2019. [Google Scholar]
- Guo, S.D.; Bao, R.; Yang, J.G.; Chen, H.; Yi, J.H. Effect of Mo and Y2O3 additions on the microstructure and properties of fine WC-Co cemented carbides fabricated by spark plasma sintering. Int. J. Refract. Met. Hard Mater. 2017, 69, 1–10. [Google Scholar] [CrossRef]
- Wang, H.L.; Wang, L.S. Effect of Y2O3 on properties of WC-10CO cemented carbides. China Tungsten Ind. 2020, 35, 63–67. [Google Scholar]
- Hang, C.G. Effects of rare earth Y, Ce and their addition methods on microstructure and properties of cemented carbides. Mater. Sci. Eng. Powder Metall. 2014, 19, 701–706. [Google Scholar]
- Yang, Y.; Luo, L.M.; Zan, X.; Zhu, X.Y.; Zhu, L.; Wu, Y.C. Synthesis of Y2O3-doped WC-Co powders by wet chemical method and its effect on the properties of WC-Co cemented carbide alloy. Int. J. Refract. Met. Hard Mater. 2020, 92, 105324. [Google Scholar] [CrossRef]
- Qin, Y.Q.; Peng, Y.Q.; Tian, Y.; Luo, L.M.; Ma, Y.; Zan, X.; Zhu, X.Y.; Wu, Y.C. Effect of Y2O3 on microstructure and mechanical properties of WC-Co-cemented carbides prepared via solid-liquid doping method and spark plasma sintering. Mater. Today Commun. 2020, 24, 101096. [Google Scholar] [CrossRef]
- Deng, X.C.; Wang, K.F.; Zhang, G.H. Effects of oxide addition on structure and properties of WC–10Co cemented carbide obtained by in situ synthesized powder. Int. J. Appl. Ceram. Technol. 2022, 19, 1916–1928. [Google Scholar] [CrossRef]
- Yang, J.R.; Tang, M.H.; Li, S.L.; Xu, X.Z. First-principles study of WC-Co (0001)/cBN (111) interface bonding properties. J. Synth. Cryst. 2020, 49, 485–493. [Google Scholar]
- Wang, C.; Wang, C.Y. Ni/Ni3Al interface: A density functional theory study. Appl. Surf. Sci. 2009, 255, 3669–3675. [Google Scholar] [CrossRef]
- Li, Y.F.; Gao, Y.M.; Xiao, B.; Min, T.; Fan, Z.J.; Ma, S.Q.; Yi, D.W. Theoretical study on the electronic properties and stabilities of low-index surfaces of WC polymorphs. Comput. Mater. Sci. 2011, 50, 939–948. [Google Scholar] [CrossRef]
- Christensen, M.; Wahnstrӧm, G. Effects of cobalt intergranular segregation on interface energetics in WC-Co. Acta Mater. 2004, 52, 2199–2207. [Google Scholar] [CrossRef]
- Zhang, S.H.; Wang, L.; Wang, Q.M.; Li, M.X. A superhard CrAlSiN superlattice coating deposited by a multi-arc ion plating: II. Thermal stability and oxidation resistance. Surf. Coat. Technol. 2013, 214, 153–159. [Google Scholar] [CrossRef]
- Haršáni, M.; Sahul, M.; Zacková, P.; Čaplovič, L. Study of cathode current effect on the properties of CrAlSiN coatings prepared by LARC. Vacuum 2017, 139, 1–8. [Google Scholar] [CrossRef]
- Zhang, G.A. Preparation and Mechanical Properties of CrN Composite Films and Al-DLC Films. Master’s Thesis, Lanzhou University, Lanzhou, China, 2008. [Google Scholar]
- Liu, C.B.; Wang, P.; Huang, F.; Li, C. Improved mechanical and thermal properties of CrAlN coatings by Si solid solution. Vacuum 2016, 125, 180–184. [Google Scholar] [CrossRef]
- Liang YM, T.; Fan, Q.X.; Wang, X.; Wang, T.G.; Liu, Y.M.; Cao, F.T. Microstructure and properties of CrAlN nano-gradient coatings. Surf. Technol. 2021, 50, 348–355. [Google Scholar]
- Hang, Z.Q. Study on High Temperature Resistance and Interface First-principles Calculation of RareEarth Modified WC-Co Coatings. Master’s Thesis, Southwest Jiaotong University, Chengdu, China, 2018. [Google Scholar]


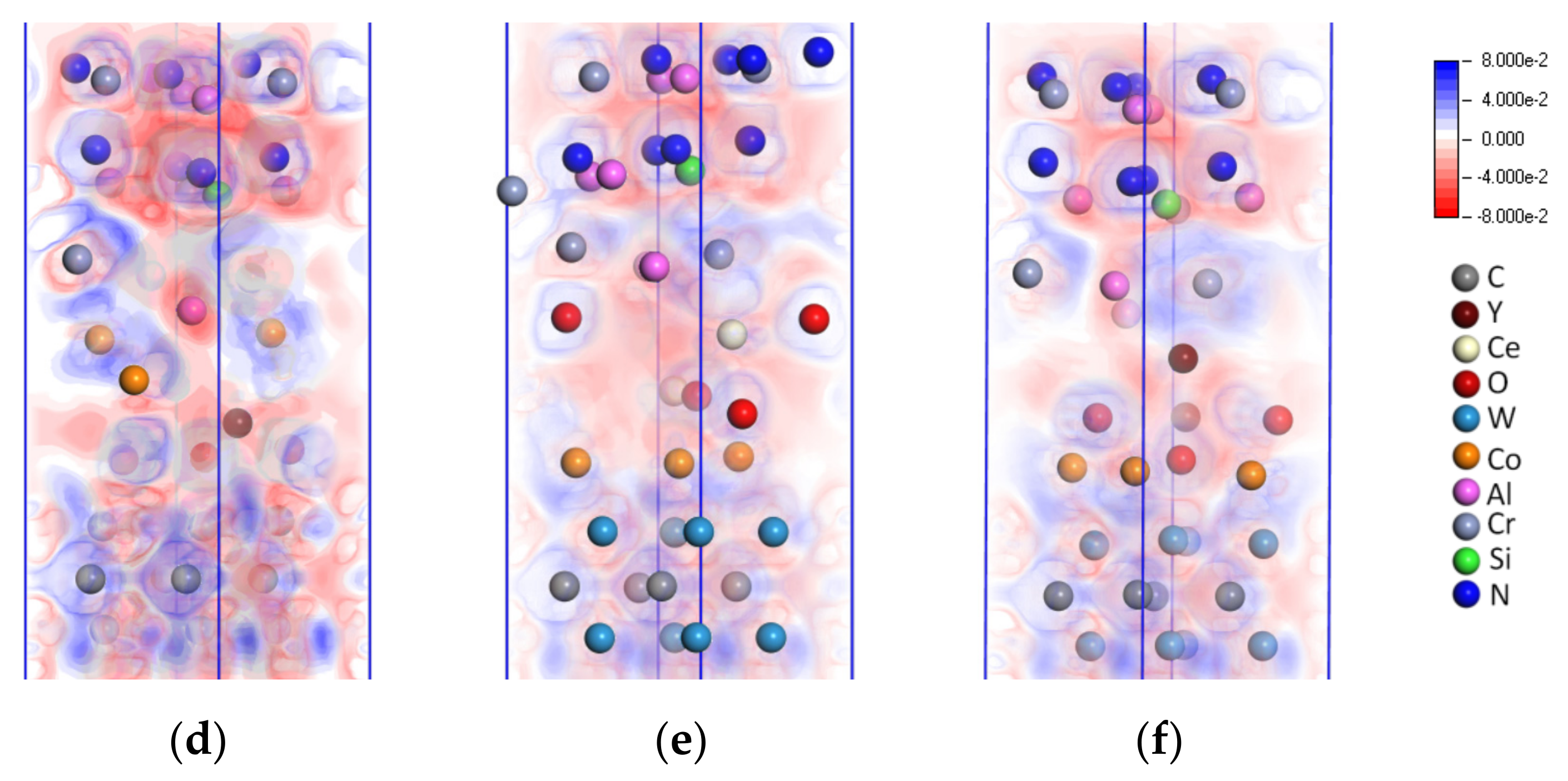

| Model | Eα/eV | Eβ/eV | Eα/β/eV | Aα/β/Å2 | Wad/J·m−2 |
|---|---|---|---|---|---|
| CrAlSiNSi/WC-Co | 14,954.098 | 19,210.462 | −34,172.070 | 27.868 | 4.312 |
| CrAlSiNN/WC-Co | 14,950.463 | 19,210.372 | −34,167.178 | 27.411 | 3.702 |
| CrAlSiNAl/WC-Co | 14,953.219 | 19,209.751 | −34,167.157 | 26.418 | 2.536 |
| Doping Type | Interface Model | Interface | Eα/(eV) | Eβ/(eV) | Eα/β/(eV) | Aα/β/(Å2) | Wad/(J·m−2) |
|---|---|---|---|---|---|---|---|
| Undoped | CrAlSiNAl/WC-Co | CrAlSiNAl/WC-Co | −14,953.219 | −19,209.751 | −34,167.157 | 26.418 | 2.536 |
| Doped CeO2 | CrAlSiNAl/WC/ CeO2/WC-Co | CrAlSiNAl/WC-Co | −14,952.436 | −23,075.467 | −38,031.572 | 29.494 | 1.990 |
| WC-Co/CeO2 | −25,809.872 | −12,214.276 | −38,031.572 | 29.494 | 4.027 | ||
| CrAlSiNAl/WC/ CeO2/Co | CrAlSiNAl/WC-Co | −82,179.012 | −15,042.382 | −97,229.165 | 29.494 | 4.216 | |
| CeO2/Co | −78,884.118 | −18,339.084 | −97,229.165 | 29.494 | 3.235 | ||
| WC/CeO2 | −74,637.363 | −22,583.294 | −97,229.165 | 29.494 | 4.615 | ||
| CrAlSiNAl/CeO2/ WC-Co | CrAlSiNAl/CeO2 | −15,041.256 | −82,183.017 | −97,229.200 | 29.494 | 2.673 | |
| CeO2/WC-Co | −77,935.625 | −19,290.353 | −97,229.200 | 29.494 | 1.748 | ||
| Doped Y2O3 | CrAlSiNAl/WC/ Y2O3/WC-Co | CrAlSiNAl/WC-Co | −14,952.029 | −20,895.258 | −35,849.532 | 30.051 | 1.195 |
| WC-Co/Y2O3 | −25,808.481 | −10,034.951 | −35,849.532 | 30.051 | 3.248 | ||
| CrAlSiNAl/WC/ Y2O3/Co | CrAlSiNAl/WC-Co | −20,905.148 | −14,955.857 | −35,869.075 | 30.051 | 4.297 | |
| Y2O3/Co | −18,082.653 | −17,778.943 | −35,869.075 | 30.051 | 3.982 | ||
| WC/Y2O3 | −16,078.979 | −19,781.223 | −35,869.075 | 30.051 | 4.724 | ||
| CrAlSiNAl/Y2O3/ WC-Co | CrAlSiNAl/Y2O3 | −14,956.840 | −20,909.197 | −35,869.241 | 30.051 | 1.706 | |
| Y2O3/WC-Co | −19,207.037 | −16,652.194 | −35,869.241 | 30.051 | 5.330 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Yue, Y.; Wang, Y.; Zhang, Y. Interface Bonding Properties of CrAlSiN-Coated Cemented Carbides Doped with CeO2 and Y2O3 Rare Earth Oxides. Molecules 2023, 28, 3584. https://doi.org/10.3390/molecules28083584
Yang J, Yue Y, Wang Y, Zhang Y. Interface Bonding Properties of CrAlSiN-Coated Cemented Carbides Doped with CeO2 and Y2O3 Rare Earth Oxides. Molecules. 2023; 28(8):3584. https://doi.org/10.3390/molecules28083584
Chicago/Turabian StyleYang, Junru, Yanping Yue, Yan Wang, and Yuekan Zhang. 2023. "Interface Bonding Properties of CrAlSiN-Coated Cemented Carbides Doped with CeO2 and Y2O3 Rare Earth Oxides" Molecules 28, no. 8: 3584. https://doi.org/10.3390/molecules28083584
APA StyleYang, J., Yue, Y., Wang, Y., & Zhang, Y. (2023). Interface Bonding Properties of CrAlSiN-Coated Cemented Carbides Doped with CeO2 and Y2O3 Rare Earth Oxides. Molecules, 28(8), 3584. https://doi.org/10.3390/molecules28083584







