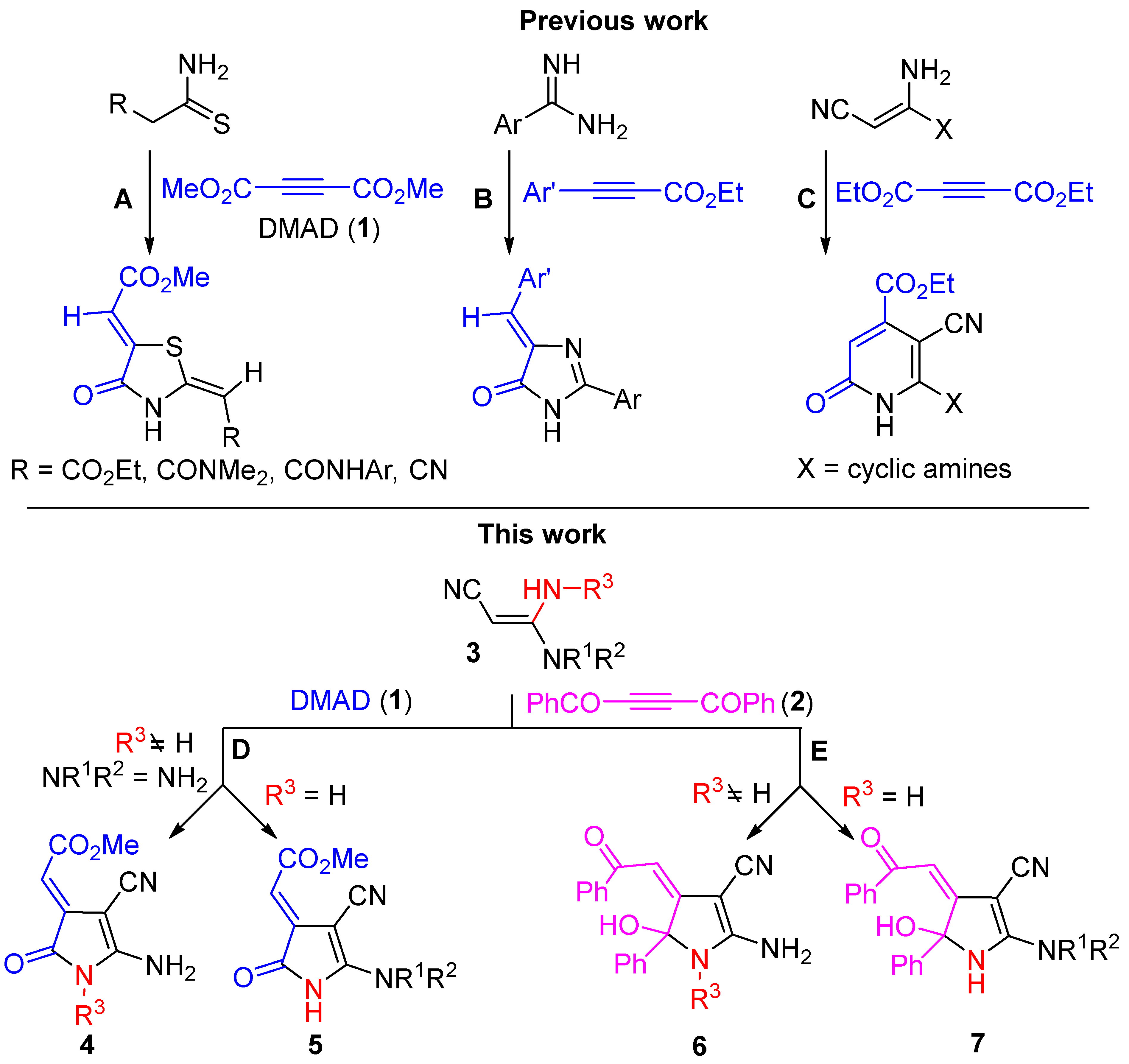
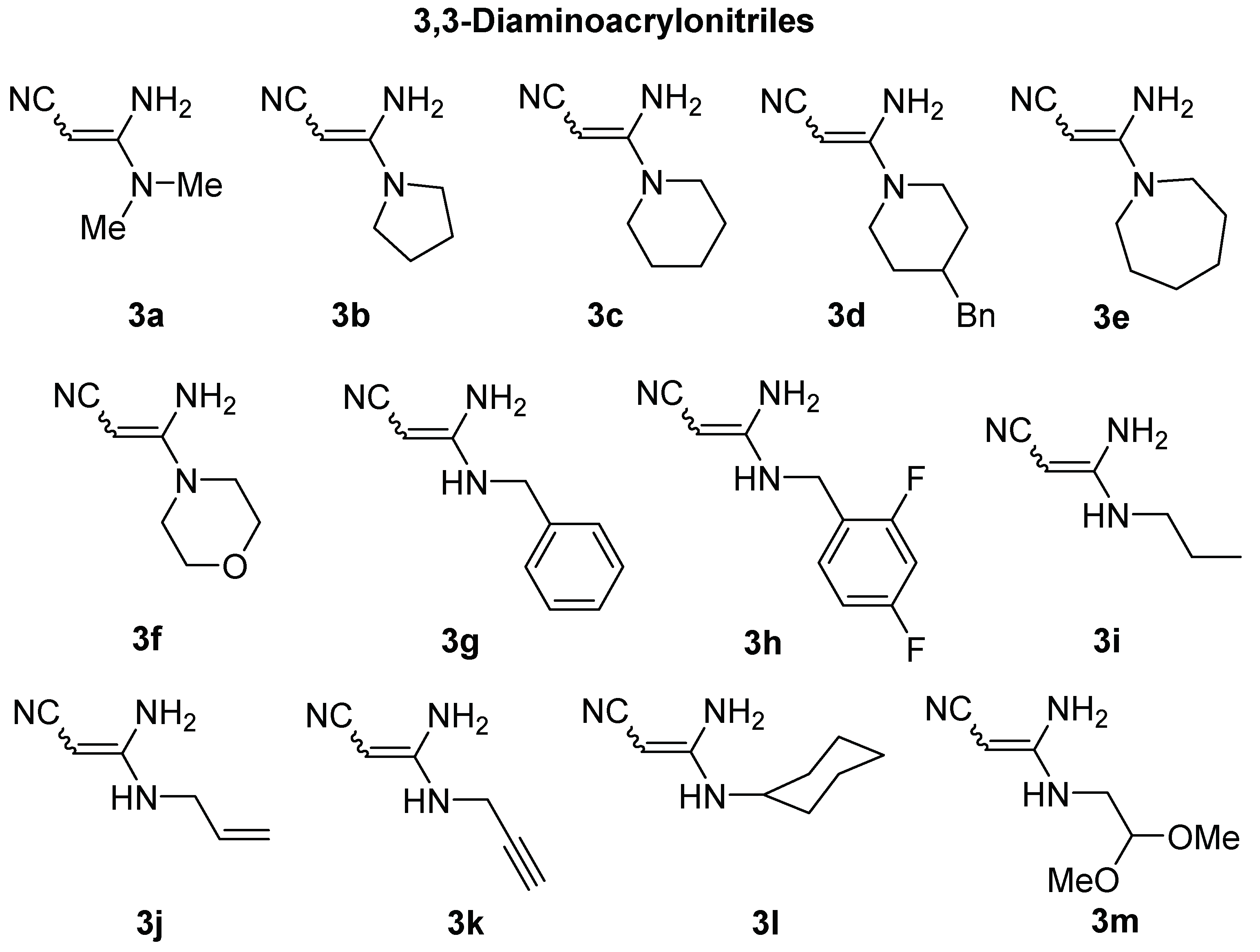
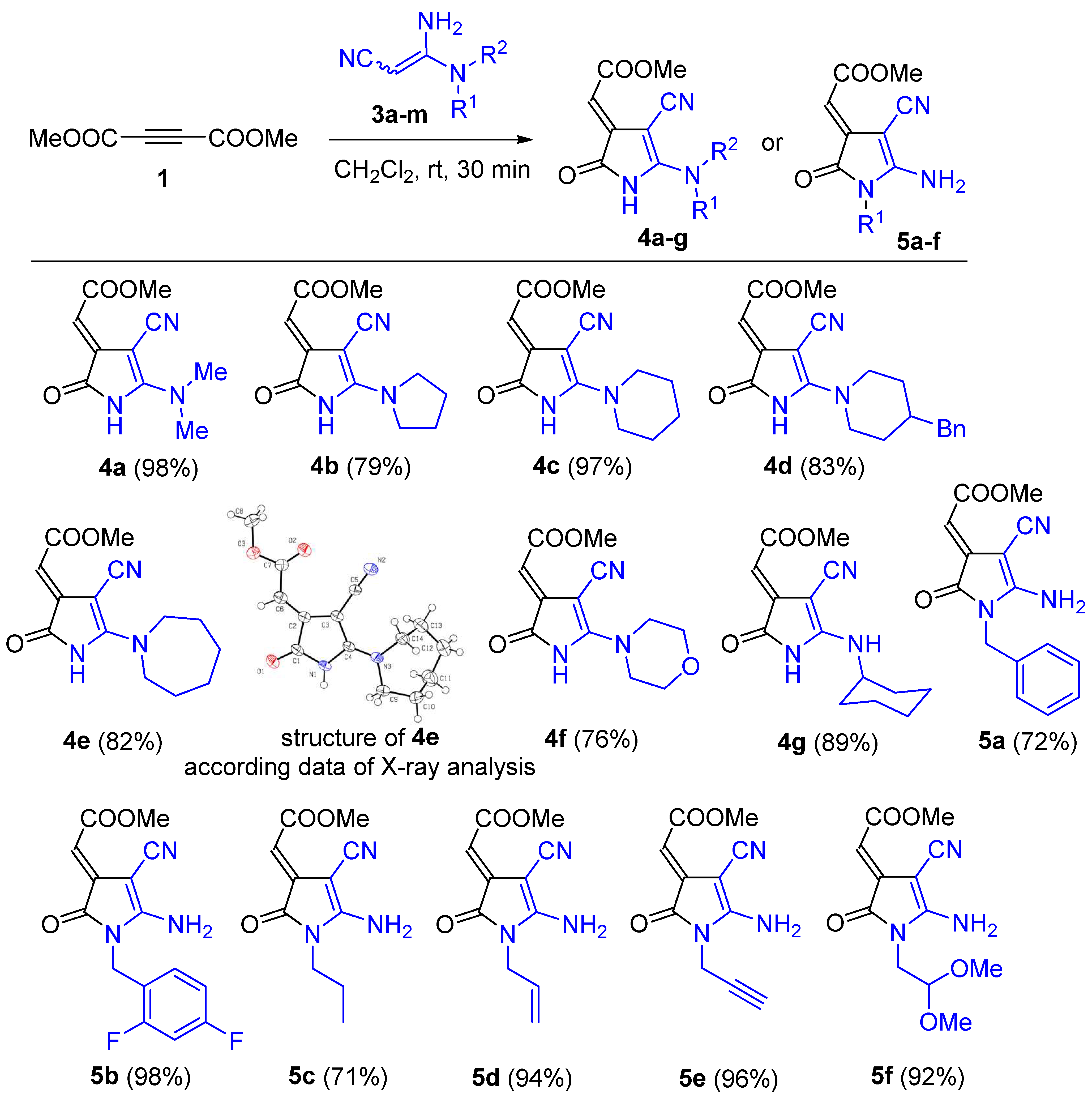
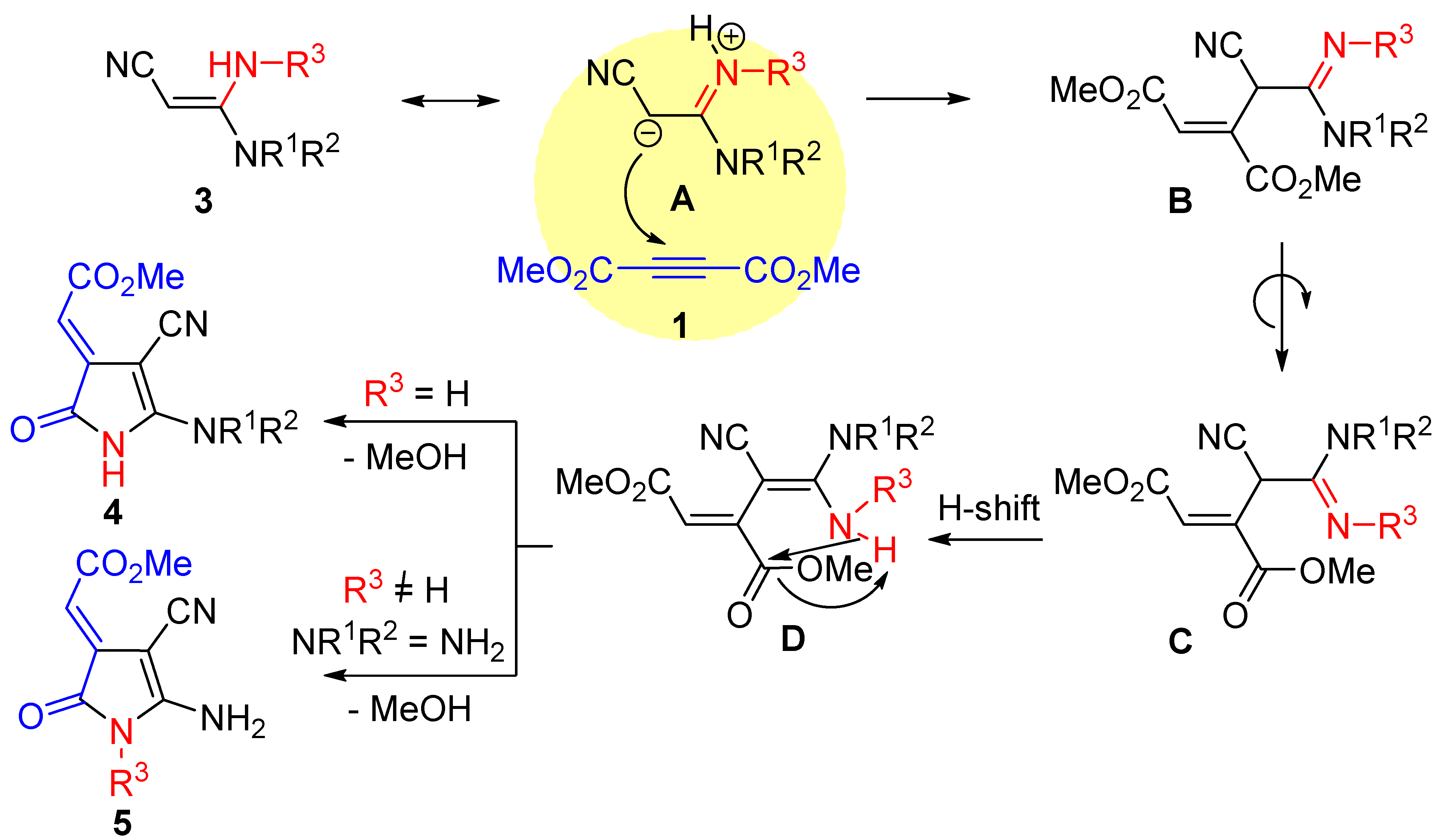
3.1.2. Synthesis of Pyrroles 4a–g, 5a–f. General Procedure
DMAD (1) (0.5 mmol, 71 mg) was added to the solution of corresponding 3,3-diaminoacrylonitryle 3 (0.5 mmol) in DCM (2 mL) at room temperature. The reaction mixture was stirred for 30 min at room temperature, then PE (10 mL) was added, and the resulting solution was stirred for 5 min more. The formed precipitate was filtered off, washed with DCM/PE (1:5) and dried.
Methyl (E)-2-(4-cyano-5-(dimethylamino)-2-oxo-1,2-dihydro-3H-pyrrol-3-ylidene)acetate (4a). Compound 4a was obtained at a 98% yield (109 mg), according to the general procedure (amidine 3a: 56 mg, 0.5 mmol; acetylene 1: 71 mg, 0.5 mmol; DCM (2 mL)) as a yellow solid, mp 241–243 °C. 1H NMR (400 MHz, DMSO-d6): δ 3.29 (s, 6H), 3.64 (s, 3H), 5.71 (s, 1H), 11.27 (br. s, 1H). 13C NMR (100 MHz, DMSO-d6): δ 40.7, 50.4, 60.7, 102.4, 117.4, 139.1, 161.5, 165.8, 168.3. IR (ATR, ZnSe, cm−1): ν, 3131, 3057, 2776, 2202, 1744, 1696, 1637, 1596, 1449, 1395, 1352, 1297, 1154, 1137, 1011. HRMS (ESI-TOF) m/z: [M + H]+ Calcd. for C10H12N3O3 222.0873; Found: 222.0880.
Methyl (E)-2-(4-cyano-2-oxo-5-(pyrrolidin-1-yl)-1,2-dihydro-3H-pyrrol-3-ylidene)acetate (4b). Compound 4b was obtained at a 79% yield (97 mg), according to the general procedure (amidine 3b: 69 mg, 0.5 mmol; acetylene 1: 71 mg, 0.5 mmol; DCM (2 mL)) as a yellow solid, mp 254–255 °C. 1H NMR (400 MHz, DMSO-d6): δ 1.91–1.98 (m, 4H), 3.57 (br. s, 2H), 3.63 (s, 3H), 3.88 (br. s, 2H), 5.66 (s, 1H), 11.32 (br. s, 1H). 13C NMR (100 MHz, DMSO-d6): δ 24.0, 25.2, 49.2, 50.4, 50.9, 60.9, 101.7, 117.5, 139.0, 158.8, 166.0, 168.5. IR (ATR, ZnSe, cm−1): ν, 3131, 3057, 2776, 2202, 1744, 1696, 1637, 1596, 1449, 1395, 1352, 1297, 1154, 1137, 1011. HRMS (ESI-TOF) m/z: [M + H]+ Calcd. for C12H14N3O3 248.1030; Found: 248.1028.
Methyl (E)-2-(4-cyano-2-oxo-5-(piperidin-1-yl)-1,2-dihydro-3H-pyrrol-3-ylidene)acetate (4c). Compound 4c was obtained at a 97% yield (128 mg), according to the general procedure (amidine 3c: 76 mg, 0.5 mmol; acetylene 1: 71 mg, 0.5 mmol; DCM (2 mL)) as a yellow solid, mp 216–218 °C. 1H NMR (400 MHz, DMSO-d6): δ 1.66 (br. s, 6H), 3.64 (s, 3H), 3.76 (br. s, 4H), 5.71 (s, 1H), 11.34 (br. s, 1H). 13C NMR (100 MHz, DMSO-d6): δ 23.1, 25.7, 49.4, 50.5, 60.7, 102.4, 117.2, 139.3, 160.2, 165.8, 168.4. IR (ATR, ZnSe, cm−1): ν, 3271, 3062, 2929, 2854, 2196, 1744, 1694, 1634, 1589, 1558, 1447, 1368, 1256, 1151, 1020. HRMS (ESI-TOF) m/z: [M + H]+ Calcd. for C13H16N3O3 262.1186; Found: 262.1188.
Methyl (E)-2-(5-(4-benzylpiperidin-1-yl)-4-cyano-2-oxo-1,2-dihydro-3H-pyrrol-3-ylidene)acetate (4d). Compound 4d was obtained at an 83% yield (146 mg), according to the general procedure (amidine 3d: 121 mg, 0.5 mmol; acetylene 1: 71 mg, 0.5 mmol; DCM (2 mL)) as a yellow solid, mp 232–234 °C. 1H NMR (400 MHz, DMSO-d6): δ 1.27–1.37 (m, 2H), 1.70–1.73 (m, 2H), 1.86–1.97 (m, 1H), 2.53 (d, J = 8 Hz, 2H), 3.22–3.25 (m, 2H), 3.64 (s, 3H), 4.26 (br. s, 2H), 5.73 (s, 1H), 7.17–7.21 (m, 3H), 7.27–7.31 (m, 2H), 11.29 (br. s, 1H). 13C NMR (100 MHz, DMSO-d6): δ 31.5, 36.2, 41.4, 48.4, 50.4, 60.8, 102.7, 117.0, 125.9, 128.1, 128.9, 139.1, 139.6, 160.2, 165.7, 168.3. IR (ATR, ZnSe, cm−1): ν 3149, 3083, 2918, 2200, 1718, 1688, 1627, 1597, 1493, 1452, 1375, 1281, 1185, 1153, 1111, 1091, 1072, 1040. HRMS (ESI-TOF) m/z: [M + Na]+ Calcd. for C20H21N3NaO3 374.1475; Found: 374.1472.
Methyl (E)-2-(5-(azepan-1-yl)-4-cyano-2-oxo-1,2-dihydro-3H-pyrrol-3-ylidene)acetate (4e). Compound 4e was obtained at an 82% yield (113 mg), according to the general procedure (amidine 3e: 83 mg, 0.5 mmol; acetylene 1: 71 mg, 0.5 mmol; DCM (2 mL)) as a yellow solid, mp 235–236 °C. 1H NMR (400 MHz, DMSO-d6): δ 1.55 (br. s, 4H), 1.64–1.80 (m, 4H), 3.64 (s, 3H), 3.68–3.95 (m, 4H), 5.72 (s, 1H), 11.25 (br. s, 1H). 13C NMR (100 MHz, DMSO-d6): δ 25.7, 27.7, 50.4, 51.2, 60.1, 102.6, 117.1, 138.9, 160.4, 165.8, 168.3. IR (ATR, ZnSe, cm−1): ν 3159, 3089, 2923, 2857, 2184, 1725, 1688, 1631, 1585, 1451, 1403, 1355, 1261, 1158, 1097, 1045. HRMS (ESI-TOF) m/z: [M + H]+ Calcd. for C14H18N3O3 276.1343; Found: 276.1347.
Methyl (E)-2-(4-cyano-5-morpholino-2-oxo-1,2-dihydro-3H-pyrrol-3-ylidene)acetate (4f). Compound 4f was obtained at a 76% yield (100 mg), according to the general procedure (amidine 3f: 77 mg, 0.5 mmol; acetylene 1: 71 mg, 0.5 mmol; DCM (2 mL)) as a yellow solid, mp 235–237 °C. 1H NMR (400 MHz, DMSO-d6): δ 3.65 (s, 3H), 3.73–3.75 (m, 4H), 3.78–3.80 (m, 4H), 5.78 (s, 1H); 11.35 (br. s, 1H). 13C NMR (100 MHz, DMSO-d6): δ 48.3, 50.5, 61.0, 65.5, 103.6, 117.0, 138.8, 160.8, 165.7, 168.1. IR (ATR, ZnSe, cm−1): ν 3184, 2981, 2875, 2187, 1755, 1713, 1700, 1575, 1461, 1432, 1357, 1297, 1266, 1202, 1145, 1114, 1066, 1002. HRMS (ESI-TOF) m/z: [M + Na]+ Calcd. for C12H13N3NaO4 286.0798; Found: 286.0800.
Methyl (E)-2-(4-cyano-5-(cyclohexylamino)-2-oxo-1,2-dihydro-3H-pyrrol-3-ylidene)acetate (4g). Compound 4g was obtained at an 89% yield (122 mg), according to the general procedure (amidine 3l: 83 mg, 0.5 mmol; acetylene 1: 71 mg, 0.5 mmol; DCM (2 mL)) as a yellow solid, mp 236–237 °C. 1H NMR (400 MHz, DMSO-d6): δ 1.04–1.11 (m, 1H), 1.21–1.31 (m, 2H), 1.41–1.49 (m, 2H), 1.57–1.60 (m, 1H), 1.70–1.82 (m, 4H), 3.53 (br. s, 1H), 3.63 (s, 3H), 5.60 (s, 1H), 8.78 (br. s, 1H), 11.45 (br. s, 1H). 13C NMR (100 MHz, DMSO-d6): δ 24.4, 24.5, 32.1, 50.2, 53.5, 60.8, 100.1, 116.0, 138.0, 161.9, 166.3, 169.1. IR (ATR, ZnSe, cm−1): ν 3210, 3139, 3026, 2951, 2937, 2859, 2199, 1731, 1705, 1650, 1598, 1517, 1452, 1439, 1398, 1357, 1334, 1306, 1272, 1258, 1192, 1171, 1150, 1123, 1077, 1053, 1044. HRMS (ESI-TOF) m/z: [M + Na]+ Calcd. for C14H17N3NaO3 298.1162; Found: 298.1159.
Methyl (E)-2-(5-amino-1-benzyl-4-cyano-2-oxo-1,2-dihydro-3H-pyrrol-3-ylidene)acetate (5a). Compound 5a was obtained at a 72% yield (102 mg), according to the general procedure (amidine 3g: 87 mg, 0.5 mmol; acetylene 1: 71 mg, 0.5 mmol; DCM (2 mL)) as a yellow solid, mp 192–193 °C. 1H NMR (400 MHz, DMSO-d6): δ 3.66 (s, 3H), 4.88 (s, 2H), 5.76 (s, 1H), 7.18–7.28 (m, 2H), 7.30–7.37 (m, 3H), 8.97 (br. s, 2H). 13C NMR (100 MHz, DMSO-d6): δ 42.1, 50.5, 60.5, 102.7, 115.7, 126.7, 127.5, 128.6, 135.7, 136.6, 163.7, 166.1, 167.6. IR (ATR, ZnSe, cm−1): ν 3140, 3117, 2991, 2198, 1736, 1707, 1651, 1607, 1555, 1495, 1441, 1408, 1362, 1317, 1169, 1118, 1079, 1047, 1025. HRMS (ESI-TOF) m/z: [M + Na]+ Calcd. for C15H13N3NaO3 306.0849; Found: 306.0847.
Methyl (E)-2-(5-amino-4-cyano-1-(2,4-difluorobenzyl)-2-oxo-1,2-dihydro-3H-pyrrol-3-ylidene)acetate (5b). Compound 5b was obtained at a 98% yield (78 mg), according to the general procedure (amidine 3h: 105 mg, 0.5 mmol; acetylene 1: 71 mg, 0.5 mmol; DCM (2 mL)) as a yellow solid, mp 208–210 °C. 1H NMR (600 MHz, DMSO-d6): δ 3.66/3.61* (s, 3H), 4.89/4.82* (s, 2H), 5.74/5.69* (s, 1H), 7.03–7.07 (m, 1H), 7.13–7.19 (m, 1H), 7.24–7.29 (m, 1H), 8.97 (br. s, 2H). 13C NMR (100 MHz, DMSO-d6): δ 37.1/37.0*, 50.5, 60.7, 102.7, 104.1 (t, J = 25.7 Hz), 111.5 (dd, J = 21.2, 3.5 Hz), 115.7, 118.9 (dd, J = 14.7, 3.6 Hz), 129.3 (dd, J = 10.0, 5.6 Hz), 136.5, 159.8 (dd, J = 248.4, 12.4 Hz), 161.7 (dd, J = 246.2, 12.2 Hz), 163.6, 166.1, 167.4. IR (ATR, ZnSe, cm−1): ν 3153, 2947, 2824, 2197, 1742, 1691, 1632, 1591, 1436, 1421, 1376, 1279, 1163, 1080, 1084, 1072, 1065, 1044. HRMS (ESI-TOF) m/z: [M + H]+ Calcd. for C15H12F2N3O3 320.0841; Found: 320.0843.
Methyl (E)-2-(5-amino-4-cyano-2-oxo-1-propyl-1,2-dihydro-3H-pyrrol-3-ylidene)acetate (5c). Compound 5c was obtained at a 71% yield (83 mg), according to the general procedure (amidine 3i: 63 mg, 0.5 mmol; acetylene 1: 71 mg, 0.5 mmol; DCM (2 mL)) as a yellow solid, mp 182–183 °C. 1H NMR (400 MHz, DMSO-d6): δ 0.82/0.86* (t, J = 7.2 Hz, 3H), 1.46–1.51 (m, 2H), 3.56 (t, J = 7.2 Hz, 2H), 3.65/3.63* (s, 3H), 5.72 (s, 1H), 8.82 (br. s, 2H). 13C NMR (100 MHz, DMSO-d6): δ 10.6, 21.2/22.7*, 40.5, 50.4/50.3*, 60.1, 102.2, 115.8, 136.8, 163.9, 166.1, 167.5. IR (ATR, ZnSe, cm−1): ν 3320, 3279, 3236, 3198, 3169, 2969, 2951, 2879, 2197, 1748, 1730, 1706, 1658,1614, 1561, 1499, 1451, 1412, 1385, 1348, 1318, 1277, 1199, 1177, 1115, 1051, 1042, 1025. HRMS (ESI-TOF) m/z: [M + H]+ Calcd. for C11H14N3O3 236.1030; Found: 236.1027.
Methyl (E)-2-(1-allyl-5-amino-4-cyano-2-oxo-1,2-dihydro-3H-pyrrol-3-ylidene)acetate (5d). Compound 5d was obtained at a 94% yield (110 mg), according to the general procedure (amidine 3j: 62 mg, 0.5 mmol; acetylene 1: 71 mg, 0.5 mmol; DCM (2 mL)) as a yellow solid, mp 176–177 °C. 1H NMR (400 MHz, DMSO-d6): δ 3.66/3.64* (s, 3H), 4.25–4.27 (m, 2H), 5.00–5.05 (m, 1H), 5.12–5.15 (m, 1H), 5.73–5.83 (m, 2H), 8.81 (br. s, 2H). 13C NMR (100 MHz, DMSO-d6): δ 40.9, 50.4, 60.2, 102.5, 115.7, 116.3, 131.6/133.5*, 136.6, 163.6, 166.1, 167.2. IR (ATR, ZnSe, cm−1): ν 3395, 3316, 3279, 3238, 3199, 3169, 2950, 2197, 1749, 1730, 1706, 1697, 1657, 1613, 1562, 1494, 1448, 1411, 1385, 1319, 1201, 1183, 1136, 1116, 1050. HRMS (ESI-TOF) m/z: [M + H]+ Calcd. for C11H12N3O3 234.0873; Found: 234.0853.
Methyl (E)-2-(5-amino-4-cyano-2-oxo-1-(prop-2-yn-1-yl)-1,2-dihydro-3H-pyrrol-3-ylidene)acetate (5e). Compound 5e was obtained at a 96% yield (111 mg), according to the general procedure (amidine 3k: 61 mg, 0.5 mmol; acetylene 1: 71 mg, 0.5 mmol; DCM (2 mL)) as a yellow solid, mp 202–203 °C. 1H NMR (400 MHz, DMSO-d6): δ 3.66 (s, 3H), 4.47 (d, J = 2.2 Hz, 2H), 5.76 (s, 1H), 8.97 (br. s, 2H). 13C NMR (100 MHz, DMSO-d6): δ 28.9, 50.5, 60.7, 74.8, 77.3, 103.0, 115.4, 136.3, 162.7, 165.9, 166.7. IR (ATR, ZnSe, cm−1): ν 3382, 3301, 3281, 3238, 3205, 3175, 2950, 2201, 2193, 2129, 1753, 1704, 1665, 1618, 1563, 1498, 1450, 1427, 1408, 1385, 1323, 1305, 1197, 1182, 1167, 1137, 1089. HRMS (ESI-TOF) m/z: [M + H]+ Calcd. for C11H10N3O3 232.0717; Found: 232.0710.
Methyl (E)-2-(5-amino-4-cyano-1-(2,2-dimethoxyethyl)-2-oxo-1,2-dihydro-3H-pyrrol-3-ylidene)acetate (5f). Compound 5f was obtained at a 92% yield (129 mg), according to the general procedure (amidine 3m: 86 mg, 0.5 mmol; acetylene 1: 71 mg, 0.5 mmol; DCM (2 mL)) as a yellow solid, mp 178–179 °C. 1H NMR (400 MHz, DMSO-d6): δ 3.29 (s, 6H), 3.65 (s, 3H), 3.76 (d, J = 5.5 Hz, 2H), 4.50 (t, J = 5.5 Hz, 1H), 5.73 (s, 1H), 8.78 (br. s, 2H). 13C NMR (100 MHz, DMSO-d6): δ 40.9, 50.4, 54.3, 60.4, 100.7, 102.4, 115.6, 136.5, 163.9, 166.1, 167.5. IR (ATR, ZnSe, cm−1): ν 3371, 3293, 3141, 3006, 2962, 2944, 2841, 2801, 2202, 1756, 1706, 1686, 1623, 1567, 1502, 1464, 1435, 1416, 1402, 1385, 1361, 1318, 1219, 1198, 1175, 1134, 1100, 1037, 1006. HRMS (ESI-TOF) m/z: [M + H]+ Calcd. for C12H16N3O5 282.1084; Found: 282.1089.
3.1.3. Synthesis of Pyrroles 6a–g, 7a,b. General Procedure
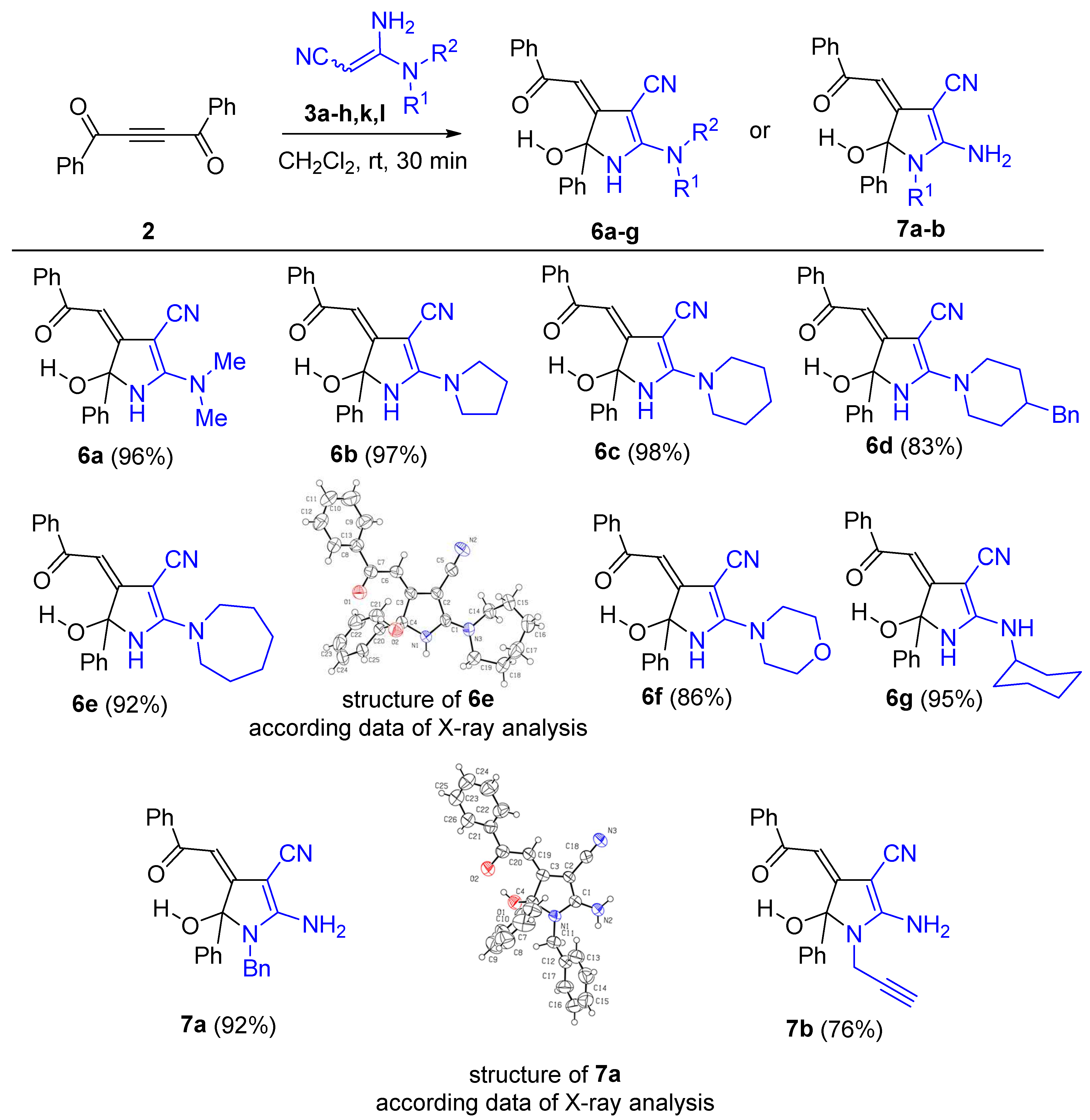
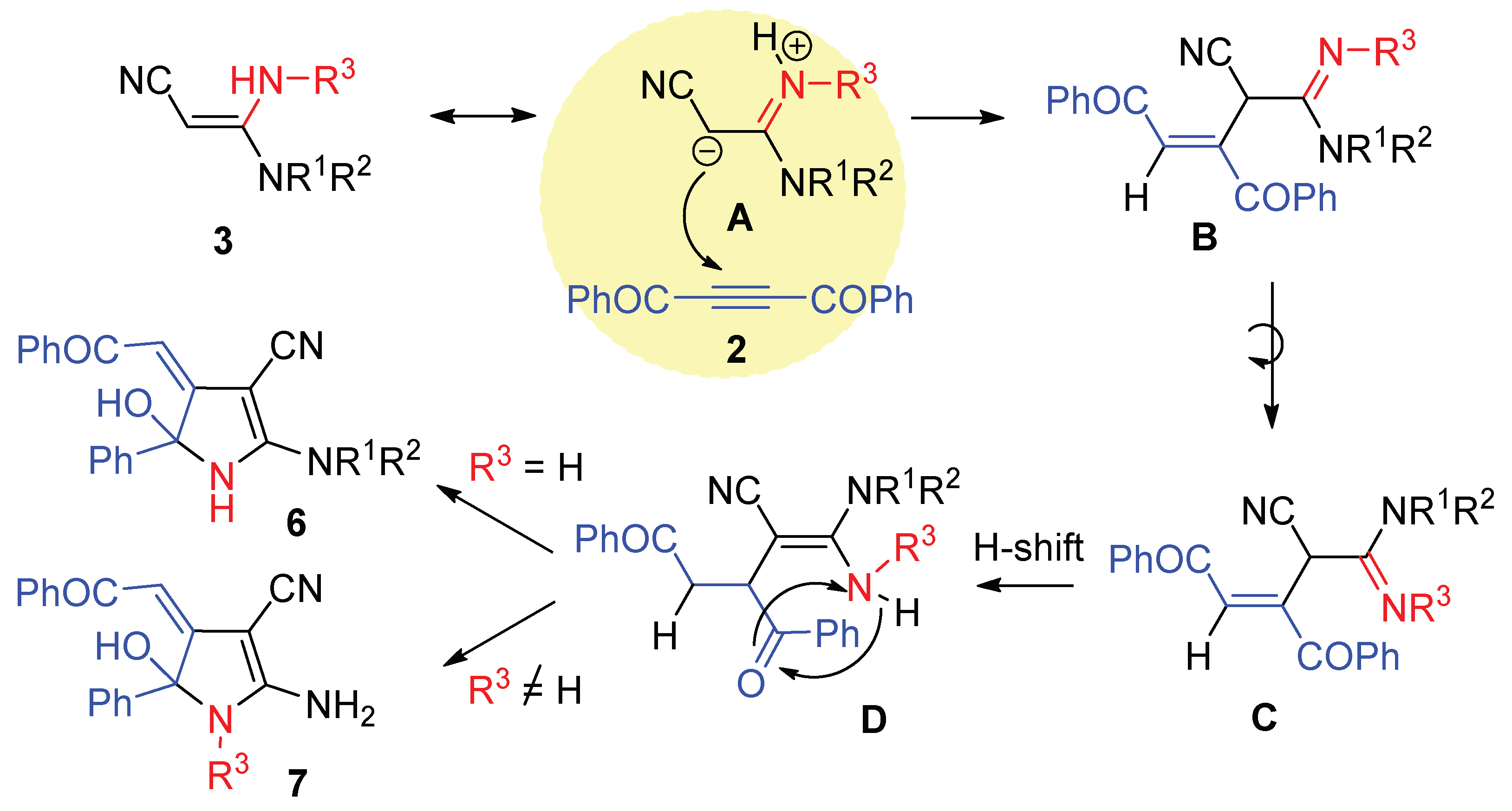
Corresponding 3,3-diaminoacrylonitryle 3 (0.5 mmol) was added to the solution of dibenzoylacetylene 2 (0.5 mmol, 117 mg) in DCM (2 mL) at room temperature. The reaction mixture was stirred for 30 min at room temperature, then ethanol (4 mL) was added, and the resulting solution was stirred for 5 min more. The formed precipitate was filtered off, washed with cold ethanol (1:5) and dried.
(Z)-2-(Dimethylamino)-5-hydroxy-4-(2-oxo-2-phenylethylidene)-5-phenyl-4,5-dihydro-1H-pyrrole-3-carbonitrile (6a). Compound 6a was obtained at a 96% yield (158 mg), according to the general procedure (amidine 3a: 56 mg, 0.5 mmol; acetylene 2: 117 mg, 0.5 mmol; DCM (2 mL)) as a yellow solid, mp 162–164 °C. 1H NMR (400 MHz, CDCl3): δ 3.26 (br. s, 6H), 5.78 (br. s, 1H), 6.65 (s, 1H), 7.26–7.36 (m, 5H), 7.41–7.45 (m, 1H), 7.59 (d, J 8.0 Hz, 2H), 7.82 (d, J 8.0 Hz, 2H), 9.24 (br. s, 1H). 13C NMR (100 MHz, CDCl3): δ 40.2, 68.4, 92.0, 101.4, 117.4, 124.9, 128.3, 128.4, 128.6, 128.8, 132.0, 139.4, 141.4, 161.9, 169.6, 188.9. IR (ATR, ZnSe, cm−1): ν 3414, 3228, 3059, 3025, 2937, 2190, 1638, 1596, 1573, 1521, 1458, 1432, 1400, 1329, 1307, 1218, 1199, 1177, 1158, 1132, 1069, 1047, 1024, 1001. HRMS (ESI-TOF) m/z: [M + H]+ Calcd. for C21H20N3O2 346.1550; Found: 346.1545.
(Z)-5-Hydroxy-4-(2-oxo-2-phenylethylidene)-5-phenyl-2-(pyrrolidin-1-yl)-4,5-dihydro-1H-pyrrole-3-carbonitrile (6b). Compound 6b was obtained at a 97% yield (170 mg), according to the general procedure (amidine 3b: 70 mg, 0.5 mmol; acetylene 2: 117 mg, 0.5 mmol; DCM (2 mL)) as a yellow solid, mp 205–206 °C. 1H NMR (400 MHz, CDCl3): δ 1.88–1.94 (m, 4H), 3.18 (br. s, 1H), 3.35 (br. s, 1H), 3.90 (br. s, 2H), 6.44 (s, 1H), 6.51 (s, 1H), 7.19–7.27 (m, 5H), 7.34 (t, 1H, J = 7.2 Hz), 7.50 (d, 2H, J = 6.7 Hz), 7.71 (d, 2H, J = 7.4 Hz), 8.45 (br. s, 1H). 13C NMR (100 MHz, CDCl3): δ 24.8, 25.9, 49.2, 68.9, 92.4, 100.3, 117.4, 125.1, 128.2, 128.3, 128.4, 128.5, 131.8, 139.6, 141.5, 159.1, 170.2, 188.3. IR (ATR, ZnSe, cm−1): ν 3419, 3268, 3216, 3171, 3057, 2991, 2960, 2881, 2200, 1639, 1594, 1568, 1524, 1490, 1451, 1432, 1386, 1355, 1325, 1301, 1244, 1230, 1214, 1194, 1180, 1156, 1137, 1111, 1084, 1072, 1065, 1048, 1025. HRMS (ESI-TOF) m/z: [M + H]+ Calcd. for C23H22N3O2 372.1707; Found: 372.1704.
(Z)-5-Hydroxy-4-(2-oxo-2-phenylethylidene)-5-phenyl-2-(piperidin-1-yl)-4,5-dihydro-1H-pyrrole-3-carbonitrile (6c). Compound 6c was obtained at a 98% yield (180 mg), according to the general procedure (amidine 3c: 76 mg, 0.5 mmol; acetylene 2: 117 mg, 0.5 mmol; DCM (2 mL)) as a yellow solid, mp 148–150 °C. 1H NMR (400 MHz, CDCl3): δ 1.63 (s, 6H), 3.46–3.67 (m, 4H), 6.36 (s, 1H), 6.55 (br. s, 1H), 7.19–7.28 (m, 5H), 7.35 (t, J = 7.4 Hz, 1H), 7.51 (d, J 6.8 Hz, 2H), 7.73 (d, J = 7.2 Hz, 2H,), 8.48 (br. s, 1H). 13C NMR (100 MHz, CDCl3): δ 23.7, 25.9, 49.2, 68.6, 91.9, 100.6, 117.3, 125.0, 128.3, 128.3, 128.5, 128.7, 131.9, 139.5, 141.4, 160.5, 170.3, 188.5. IR (ATR, ZnSe, cm−1): ν 3418, 3195, 3061, 3031, 2941, 2858, 2189, 1622, 1595, 1569, 1519, 1491, 1450, 1435, 1400, 1385, 1355, 1326, 1300, 1232, 1219, 1198, 1178, 1131, 1085, 1071, 1051, 1022, 1004. HRMS (ESI-TOF) m/z: [M + H]+ Calcd. for C24H24N3O2 386.1863; Found: 386.1861.
(Z)-2-(4-Benzylpiperidin-1-yl)-5-hydroxy-4-(2-oxo-2-phenylethylidene)-5-phenyl-4,5-dihydro-1H-pyrrole-3-carbonitrile (6d). Compound 6d was obtained at an 83% yield (198 mg), according to the general procedure (amidine 3d: 120 mg, 0.5 mmol; acetylene 2: 117 mg, 0.5 mmol; DCM (2 mL)) as a yellow solid, mp 143–145 °C. 1H NMR (400 MHz, CDCl3): δ 1.20–1.31 (m, 2H), 1.67–1.81 (m, 3H), 2.50 (d, J = 8.0 Hz, 2H), 2.92–3.01 (m, 2H), 4.02–4.25 (m, 2H), 6.32 (br. s, 1H), 6.56 (s, 1H), 7.04 (d, J = 7.4 Hz, 2H), 7.12 (t, J = 7.6 Hz, 1H), 7.18–7.28 (m, 7H), 7.36 (t, J = 7.6 Hz, 1H), 7.51 (d, J = 6.9 Hz, 2H), 7.72 (d, J = 7.4 Hz, 2H), 8.69 (br. s, 1H). 13C NMR (100 MHz, CDCl3): δ 31.9, 32.0, 37.6, 42.7, 48.4, 48.5, 68.7, 91.9, 100.8, 117.2, 125.0, 126.5, 128.28, 128.34, 128.5, 128.6, 128.7, 129.2, 132.0, 139.2, 139.4, 141.4, 160.5, 170.1, 188.6. IR (ATR, ZnSe, cm−1): ν 3417, 3169, 3060, 3025, 2919, 2851, 2191, 1623, 1596, 1571, 1517, 1491, 1451, 1429, 1401, 1384, 1326, 1299, 1223, 1198, 1178, 1156, 1085, 1069, 1051, 1025, 1001. HRMS (ESI-TOF) m/z: [M + H]+ Calcd. for C31H30N3O2 476.2332; Found: 476.2332.
(Z)-2-(Azepan-1-yl)-5-hydroxy-4-(2-oxo-2-phenylethylidene)-5-phenyl-4,5-dihydro-1H-pyrrole-3-carbonitrile (6e). Compound 6e was obtained at a 92% yield (174 mg), according to the general procedure (amidine 3e: 82 mg, 0.5 mmol; acetylene 2: 117 mg, 0.5 mmol; DCM (2 mL)) as a yellow solid, mp 212–214 °C. 1H NMR (400 MHz, CDCl3): δ 1.50–1.83 (m, 8H), 3.36–3.91 (m, 4H), 6.52 (s, 1H), 6.67 (br. s, 1H), 7.17–7.29 (m, 5H), 7.34 (t, J = 7.6 Hz, 1H), 7.49 (d, J = 8.3 Hz, 2H), 7.72 (d, J = 8.3 Hz, 2H), 8.58 (br. s, 1H). 13C NMR (100 MHz, CDCl3): δ 26.9, 29.4, 50.6, 68.5, 91.9, 100.2, 117.3, 125.0, 128.2, 128.3, 128.4, 128.6, 131.8, 139.5, 141.3, 161.0, 170.6, 188.3. IR (ATR, ZnSe, cm−1): ν 3430, 3227, 3060, 2928, 2915, 2859, 2192, 1630, 1595, 1573, 1521, 1493, 1470, 1453, 1437, 1420, 1402, 1385, 1368, 1345, 1309, 1221, 1201, 1177, 1157, 1119, 1101, 1083, 1068, 1051, 1027, 1013. HRMS (ESI-TOF) m/z: [M + H]+ Calcd. for C25H26N3O2 400.2020; Found: 400.2015.
5-(Z)-5-Hydroxy-2-morpholino-4-(2-oxo-2-phenylethylidene)-5-phenyl-4,5-dihydro-1H-pyrrole-3-carbonitrile (6f). Compound 6f was obtained at an 86% yield (158 mg), according to the general procedure (amidine 3f: 76 mg, 0.5 mmol; acetylene 2: 117 mg, 0.5 mmol; DCM (2 mL)) as a yellow solid, mp 207–209 °C. 1H NMR (400 MHz, CDCl3): δ 3.54–3.69 (m, 8H), 6.17 (br. s, 1H), 6.60 (s, 1H), 7.19–7.29 (m, 5H), 7.37 (t, J = 7.3 Hz, 1H), 7.52 (d, J = 8.2 Hz, 2H), 7.70 (d, J = 7.6 Hz, 2H), 8.88 (br. s, 1H). 13C NMR (100 MHz, CDCl3): δ 47.5, 66.1, 68.5, 91.9, 101.9, 117.1, 125.0, 128.3, 128.4, 128.6, 128.9, 132.2, 139.2, 141.2, 161.2, 169.4, 189.0. IR (ATR, ZnSe, cm−1): ν 3414, 3196, 3058, 2965, 2922, 2893, 2856, 2191, 1621, 1594, 1569, 1518, 1491, 1454, 1429, 1384, 1353, 1332, 1306, 1288, 1267, 1224, 1201, 1172, 1158, 1115, 1085, 1070, 1048, 1033, 1022, 1002. HRMS (ESI-TOF) m/z: [M + H]+ Calcd. for C23H22N3O3 388.1656; Found: 388.1654.
(Z)-2-(Cyclohexylamino)-5-hydroxy-4-(2-oxo-2-phenylethylidene)-5-phenyl-4,5-dihydro-1H-pyrrole-3-carbonitrile (6g). Compound 6g was obtained at a 95% yield (190 mg), according to the general procedure (amidine 3l: 82 mg, 0.5 mmol; acetylene 2: 118 mg, 0.5 mmol; DCM (2 mL)) as a yellow solid, mp 216–218 °C. 1H NMR (400 MHz, CDCl3): 1.11–1.344 (m, 5H), 1.59 (br. s, 1H), 1.75 (br. s, 2H), 1.91–1.99 (m, 2H), 3.27–3.42 (m, 1H), 5.60 (d, J = 8.2 Hz, 1H), 6.48 (br. s, 1H), 7.30–7.36 (m, 2H), 7.41–7.44 (m, 1H), 7.55 (d, J = 8.0 Hz, 2H), 7.78 (d, J = 8.0 Hz, 2H), 10.22 (br. s, 1H). 13C NMR (100 MHz, CDCl3): 24.6, 24.7, 25.0, 33.2, 33.5, 53.4, 68.2, 93.5, 115.9, 125.2, 128.2, 128.4, 128.5, 128.9, 132.0, 139.4, 140.6, 161.1, 183.4. IR (ATR, ZnSe, cm−1): ν 3298, 3229, 3182, 2185, 1638, 1575, 1484, 1359, 1325, 1298, 1247, 1117, 1044. HRMS (ESI-TOF) m/z: [M + H]+ Calcd. for C25H26N3O2 400.2020; Found: 400.2018.
(Z)-2-Amino-1-benzyl-5-hydroxy-4-(2-oxo-2-phenylethylidene)-5-phenyl-4,5-dihydro-1H-pyrrole-3-carbonitrile (7a). Compound 7a was obtained at a 92% yield (94 mg), according to the general procedure (amidine 3g: 43 mg, 250 µmol; acetylene 2: 59 mg, 250 µmol; DCM (1 mL)) as a yellow solid, mp 230–232 °C. 1H NMR (400 MHz, CDCl3): 4.16 (d, J = 16.6 Hz, 1H), 4.49 (d, J = 16.6 Hz, 1H), 5.11 (br. s, 1H), 6.55 (s, 1H), 7.17 (d, J = 6.6 Hz, 2H), 7.29–7.36 (m, 8H), 7.41–7.46 (m, 1H), 7.66 (d, J = 7.4 Hz, 2H), 7.81 (d, J = 7.4 Hz, 2H), 9.40 (s, 1H). IR (ATR, ZnSe, cm−1): ν 3445, 3333, 3275, 3181, 3056, 3027, 2197, 1683, 1674, 1595, 1573, 1543, 1473, 1439, 1383, 1357, 1337, 1325, 1314, 1304, 1296, 1258, 1215, 1201, 1172, 1155, 1126, 1109, 1076, 1059, 1026, 1003. HRMS (ESI-TOF) m/z: [M + H]+ Calcd. for C26H22N3O2 408.1707; Found: 408.1706.
5-(Z)-2-Amino-5-hydroxy-4-(2-oxo-2-phenylethylidene)-5-phenyl-1-(prop-2-yn-1-yl)-4,5-dihydro-1H-pyrrole-3-carbonitrile (7b). Compound 7b was obtained at a 76% yield (136 mg), according to the general procedure (amidine 3k: 60 mg, 0.5 mmol; acetylene 2: 117 mg, 0.5 mmol; DCM (2 mL)) as a yellow solid, mp 187–187 °C. 1H NMR (400 MHz, CDCl3): δ 2.29 (s, 1H), 3.85 (dd, J = 18.4, 2.5 Hz, 1H), 4.02 (dd, J = 18.4, 2.5 Hz, 1H,), 6.07 (br. s, 2H), 6.52 (s, 1H), 7.30–7.41 (m, 5H), 7.41–7.45 (m, 1H), 7.59 (d, J = 7.6 Hz, 2H), 7.79 (d, J = 7.6 Hz, 2H), 9.30 (s, 1H). 13C NMR (100 MHz, CDCl3): δ 30.4, 68.1, 74.7, 76.0, 95.5, 100.8, 116.0, 125.8 128.2, 128.3, 129.1, 132.0, 138.3, 139.4, 162.6, 167.6, 188.7. IR (ATR, ZnSe, cm−1): ν 3432, 3340, 3288, 3263, 3195, 2190, 1663, 1613, 1597, 1576, 1546, 1498, 1477, 1450, 1423, 1385, 1343, 1322, 1304, 1297, 1235, 1211, 1175, 1156, 1106, 1078, 1059, 1033, 1025, 1017, 1003. HRMS (ESI-TOF) m/z: [M + H]+ Calcd. for C22H18N3O2 356.1394; Found: 356.1391.