Assessment of Various Food Proteins as Structural Materials for Delivery of Hydrophobic Polyphenols Using a Novel Co-Precipitation Method
Abstract
1. Introduction
- -
- They most often give low encapsulation efficiency and/or loading capacity;
- -
- Some of them are not suitable for use in food formulations (e.g., use of toxic solvents);
- -
- Some use expensive or inconvenient processing methods that are difficult or inefficient to adapt and scale up in the food industry.
2. Results and Discussion
2.1. Powder Characteristics, Entrapment Efficiency, and Loading Capacity
2.2. Morphology of Polyphenol–Protein Co-Precipitates
2.3. Crystallinity of the Polyphenol–Protein Co-Precipitates
2.4. Dispersibility and Solubility of the Polyphenol–Protein Co-Precipitates
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Manufacture of the Polyphenol–Protein Co-Precipitates
3.3. Entrapment Efficiency (EE) and Loading Capacity (LC) Determination
3.4. Morphology of the Co-Precipitates
3.5. Powder X-ray Diffraction (XRD)
3.6. Dispersibility of the Co-Precipitates in Water
3.7. Solubility of the Co-Precipitates in Water
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kawabata, K.; Mukai, R.; Ishisaka, A. Quercetin and related polyphenols: New insights and implications for their bioactivity and bioavailability. Food Funct. 2015, 6, 1399–1417. [Google Scholar] [CrossRef]
- Choi, S.-S.; Park, H.-R.; Lee, K.-A. A Comparative Study of Rutin and Rutin Glycoside: Antioxidant Activity, Anti-Inflammatory Effect, Effect on Platelet Aggregation and Blood Coagulation. Antioxidants 2021, 10, 1696. [Google Scholar] [CrossRef] [PubMed]
- El-Desoky, A.H.; Abdel-Rahman, R.F.; Ahmed, O.K.; El-Beltagi, H.S.; Hattori, M. Anti-inflammatory and antioxidant activities of naringin isolated from Carissa carandas L.: In vitro and in vivo evidence. Phytomedicine 2018, 42, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Kim, N.; Shin, Y.; Kim, S.-Y.; Kim, Y.-J. Activity of catechins and their applications. Biomed. Dermatol. 2020, 4, 1–10. [Google Scholar] [CrossRef]
- Nakazawa, Y.; Aoki, M.; Ishiwa, S.; Morishita, N.; Endo, S.; Nagai, N.; Yamamoto, N.; Funakoshi-Tago, M.; Tamura, H. Oral intake of α-glucosyl-hesperidin ameliorates selenite-induced cataract formation. Mol. Med. Rep. 2020, 21, 1258–1266. [Google Scholar] [CrossRef]
- Cao, H.; Saroglu, O.; Karadag, A.; Diaconeasa, Z.; Zoccatelli, G.; Conte-Junior, C.A.; Gonzalez-Aguilar, G.A.; Ou, J.; Bai, W.; Zamarioli, C.M. Available technologies on improving the stability of polyphenols in food processing. Food Front. 2021, 2, 109–139. [Google Scholar] [CrossRef]
- Deng, J.; Yang, H.; Capanoglu, E.; Cao, H.; Xiao, J. Technological aspects and stability of polyphenols. In Polyphenols: Properties, Recovery, and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 295–323. [Google Scholar]
- Rashidinejad, A.; Birch, E.J.; Everett, D.W. Antioxidant activity and recovery of green tea catechins in full-fat cheese following gastrointestinal simulated digestion. J. Food Compos. Anal. 2016, 48, 13–24. [Google Scholar] [CrossRef]
- Rashidinejad, A. Cheese as a Delivery Vehicle for Green Tea Catechins; University of Otago: Dunedin, New Zealand, 2015. [Google Scholar]
- Adrar, N.S.; Madani, K.; Adrar, S. Impact of the inhibition of proteins activities and the chemical aspect of polyphenols-proteins interactions. PharmaNutrition 2019, 7, 100142. [Google Scholar] [CrossRef]
- Macedo, A.S.; Quelhas, S.; Silva, A.M.; Souto, E.B. Nanoemulsions for delivery of flavonoids: Formulation and in vitro release of rutin as model drug. Pharm. Dev. Technol. 2014, 19, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Shadnoush, M.; Sohrabvandi, S.; Khorshidian, N.; Mortazavian, A.M. Encapsulation Systems for Delivery of Flavonoids: A Review. Biointerface Res. Appl. Chem. 2021, 11, 13934–13951. [Google Scholar]
- Rashidinejad, A.; Birch, E.J.; Sun-Waterhouse, D.; Everett, D.W. Delivery of green tea catechin and epigallocatechin gallate in liposomes incorporated into low-fat hard cheese. Food Chem. 2014, 156, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Mauludin, R.; Müller, R.H.; Keck, C.M. Kinetic solubility and dissolution velocity of rutin nanocrystals. Eur. J. Pharm. Sci. 2009, 36, 502–510. [Google Scholar] [CrossRef]
- Mehranfar, F.; Bordbar, A.-K.; Parastar, H. A combined spectroscopic, molecular docking and molecular dynamic simulation study on the interaction of quercetin with β-casein nanoparticles. J. Photochem. Photobiol. B Biol. 2013, 127, 100–107. [Google Scholar] [CrossRef]
- Pan, K.; Zhong, Q.; Baek, S.J. Enhanced dispersibility and bioactivity of curcumin by encapsulation in casein nanocapsules. J. Agric. Food Chem. 2013, 61, 6036–6043. [Google Scholar] [CrossRef]
- Shpigelman, A.; Shoham, Y.; Israeli-Lev, G.; Livney, Y.D. β-Lactoglobulin–naringenin complexes: Nano-vehicles for the delivery of a hydrophobic nutraceutical. Food Hydrocoll. 2014, 40, 214–224. [Google Scholar] [CrossRef]
- Tian, Y.; Xu, G.; Cao, W.; Li, J.; Taha, A.; Hu, H.; Pan, S. Interaction between pH-shifted β-conglycinin and flavonoids hesperetin/hesperidin: Characterization of nanocomplexes and binding mechanism. LWT 2021, 140, 110698. [Google Scholar] [CrossRef]
- Yang, R.; Zhou, Z.; Sun, G.; Gao, Y.; Xu, J.; Strappe, P.; Blanchard, C.; Cheng, Y.; Ding, X. Synthesis of homogeneous protein-stabilized rutin nanodispersions by reversible assembly of soybean (Glycine max) seed ferritin. RSC Adv. 2015, 5, 31533–31540. [Google Scholar] [CrossRef]
- Wijaya, W.; Harfieyanto, R.C.; Dewettinck, K.; Patel, A.R.; Van der Meeren, P. Whey protein isolate–low methoxyl pectin nanocomplexes improve physicochemical and stability properties of quercetin in a model fat-free beverage. Food Funct. 2019, 10, 986–996. [Google Scholar] [CrossRef]
- Rashidinejad, A.; Loveday, S.M.; Jameson, G.B.; Hindmarsh, J.P.; Singh, H. Rutin-casein co-precipitates as potential delivery vehicles for flavonoid rutin. Food Hydrocoll. 2019, 96, 451–462. [Google Scholar] [CrossRef]
- Rashidinejad, A.; Acevedo-Fani, A.; Singh, H.; Loveday, S.; Thompson, A.; Niu, Z. Flavonoid Delivery. System. Patent WO2020095238A1 (PCT/IB2019/059560), 14 May 2020. [Google Scholar]
- Konecsni, K.; Low, N.; Nickerson, M. Chitosan–tripolyphosphate submicron particles as the carrier of entrapped rutin. Food Chem. 2012, 134, 1775–1779. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, L.; Yin, W.; Liu, Z.; Shi, L.; Tang, M. A Novel Drug Delivery System: The Encapsulation of Naringenin in Metal-Organic Frameworks into Liposomes. AAPS PharmSciTech 2021, 22, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Liao, W.; Charcosset, C. Recent advances in encapsulation of curcumin in nanoemulsions: A review of encapsulation technologies, bioaccessibility and applications. Food Res. Int. 2020, 132, 109035. [Google Scholar] [CrossRef] [PubMed]
- Dammak, I.; do Amaral Sobral, P.J. Formulation optimization of lecithin-enhanced pickering emulsions stabilized by chitosan nanoparticles for hesperidin encapsulation. J. Food Eng. 2018, 229, 2–11. [Google Scholar] [CrossRef]
- Semalty, A.; Semalty, M.; Singh, D.; Rawat, M. Preparation and characterization of phospholipid complexes of naringenin for effective drug delivery. J. Incl. Phenom. Macrocycl. Chem. 2010, 67, 253–260. [Google Scholar] [CrossRef]
- Wei, Q.; Keck, C.M.; Müller, R.H. Oral hesperidin—Amorphization and improved dissolution properties by controlled loading onto porous silica. Int. J. Pharm. 2017, 518, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Dutta, P. Green synthesis, characterization and biological evaluation of chitin glucan based zinc oxide nanoparticles and its curcumin conjugation. Int. J. Biol. Macromol. 2020, 156, 514–521. [Google Scholar] [CrossRef]
- Shruthi, P.A.; Pushpadass, H.A.; Magdaline Eljeeva Emerald, F.; Surendra Nath, B.; Laxmana Naik, N. Formulation and characterization of catechin-loaded proniosomes for food fortification. J. Sci. Food Agric. 2021, 101, 2439–2448. [Google Scholar] [CrossRef]
- Yildirim-Elikoglu, S.; Erdem, Y.K. Interactions between milk proteins and polyphenols: Binding mechanisms, related changes, and the future trends in the dairy industry. Food Rev. Int. 2018, 34, 665–697. [Google Scholar] [CrossRef]
- Zhao, Q.; Yu, X.; Zhou, C.; Yagoub, A.E.A.; Ma, H. Effects of collagen and casein with phenolic compounds interactions on protein in vitro digestion and antioxidation. LWT 2020, 124, 109192. [Google Scholar] [CrossRef]
- Pujara, N.; Jambhrunkar, S.; Wong, K.Y.; McGuckin, M.; Popat, A. Enhanced colloidal stability, solubility and rapid dissolution of resveratrol by nanocomplexation with soy protein isolate. J. Colloid Interface Sci. 2017, 488, 303–308. [Google Scholar] [CrossRef]
- Patel, A.; Hu, Y.; Tiwari, J.K.; Velikov, K.P. Synthesis and characterisation of zein–curcumin colloidal particles. Soft Matter 2010, 6, 6192–6199. [Google Scholar] [CrossRef]
- Keppler, J.K.; Schwarz, K.; van der Goot, A.J. Covalent modification of food proteins by plant-based ingredients (polyphenols and organosulphur compounds): A commonplace reaction with novel utilization potential. Trends Food Sci. Technol. 2020, 101, 38–49. [Google Scholar] [CrossRef]
- Strauss, G.; Gibson, S.M. Plant phenolics as cross-linkers of gelatin gels and gelatin-based coacervates for use as food ingredients. Food Hydrocoll. 2004, 18, 81–89. [Google Scholar] [CrossRef]
- Rawel, H.M.; Rohn, S.; Kroll, J. Influence of a sugar moiety (rhamnosylglucoside) at 3-O position on the reactivity of quercetin with whey proteins. Int. J. Biol. Macromol. 2003, 32, 109–120. [Google Scholar] [CrossRef]
- Liu, T.; Yao, G.; Liu, X.; Yin, H. Preparation nanocrystals of poorly soluble plant compounds using an ultra-small-scale approach. Aaps Pharmscitech 2017, 18, 2610–2617. [Google Scholar] [CrossRef]
- Ji, P.; Yu, T.; Liu, Y.; Jiang, J.; Xu, J.; Zhao, Y.; Hao, Y.; Qiu, Y.; Zhao, W.; Wu, C. Naringenin-loaded solid lipid nanoparticles: Preparation, controlled delivery, cellular uptake, and pulmonary pharmacokinetics. Drug Des. Dev. Ther. 2016, 10, 911. [Google Scholar]
- Ali, S.H.; Sulaiman, G.M.; Al-Halbosiy, M.M.; Jabir, M.S.; Hameed, A.H. Fabrication of hesperidin nanoparticles loaded by poly lactic co-Glycolic acid for improved therapeutic efficiency and cytotoxicity. Artif. Cells Nanomed. Biotechnol. 2019, 47, 378–394. [Google Scholar] [CrossRef]
- Krishnaswamy, K.; Orsat, V.; Thangavel, K. Synthesis and characterization of nano-encapsulated catechin by molecular inclusion with beta-cyclodextrin. J. Food Eng. 2012, 111, 255–264. [Google Scholar] [CrossRef]
- Lv, L.; Fu, C.; Zhang, F.; Wang, S. Thermally-induced whey protein isolate-daidzein co-assemblies: Protein-based nanocomplexes as an inhibitor of precipitation/crystallization for hydrophobic drug. Food Chem. 2019, 275, 273–281. [Google Scholar] [CrossRef]
- Maity, S.; Mukhopadhyay, P.; Kundu, P.P.; Chakraborti, A.S. Alginate coated chitosan core-shell nanoparticles for efficient oral delivery of naringenin in diabetic animals—An in vitro and in vivo approach. Carbohydr. Polym. 2017, 170, 124–132. [Google Scholar] [CrossRef]
- Rashidinejad, A.; Jameson, G.B.; Singh, H. The effect of pH and sodium caseinate on the aqueous solubility, stability, and crystallinity of rutin towards concentrated colloidally stable particles for the incorporation into functional foods. Molecules 2022, 27, 534. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.; McGarry, K.; Chaw, C.S.; Elkordy, A.A. Feasibility of using gluconolactone, trehalose and hydroxy-propyl gamma cyclodextrin to enhance bendroflumethiazide dissolution using lyophilisation and physical mixing techniques. Pharmaceutics 2018, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Sabet, S.; Rashidinejad, A.; Qazi, H.J.; McGillivray, D.J. An efficient small intestine-targeted curcumin delivery system based on the positive-negative-negative colloidal interactions. Food Hydrocoll. 2021, 111, 106375. [Google Scholar] [CrossRef]
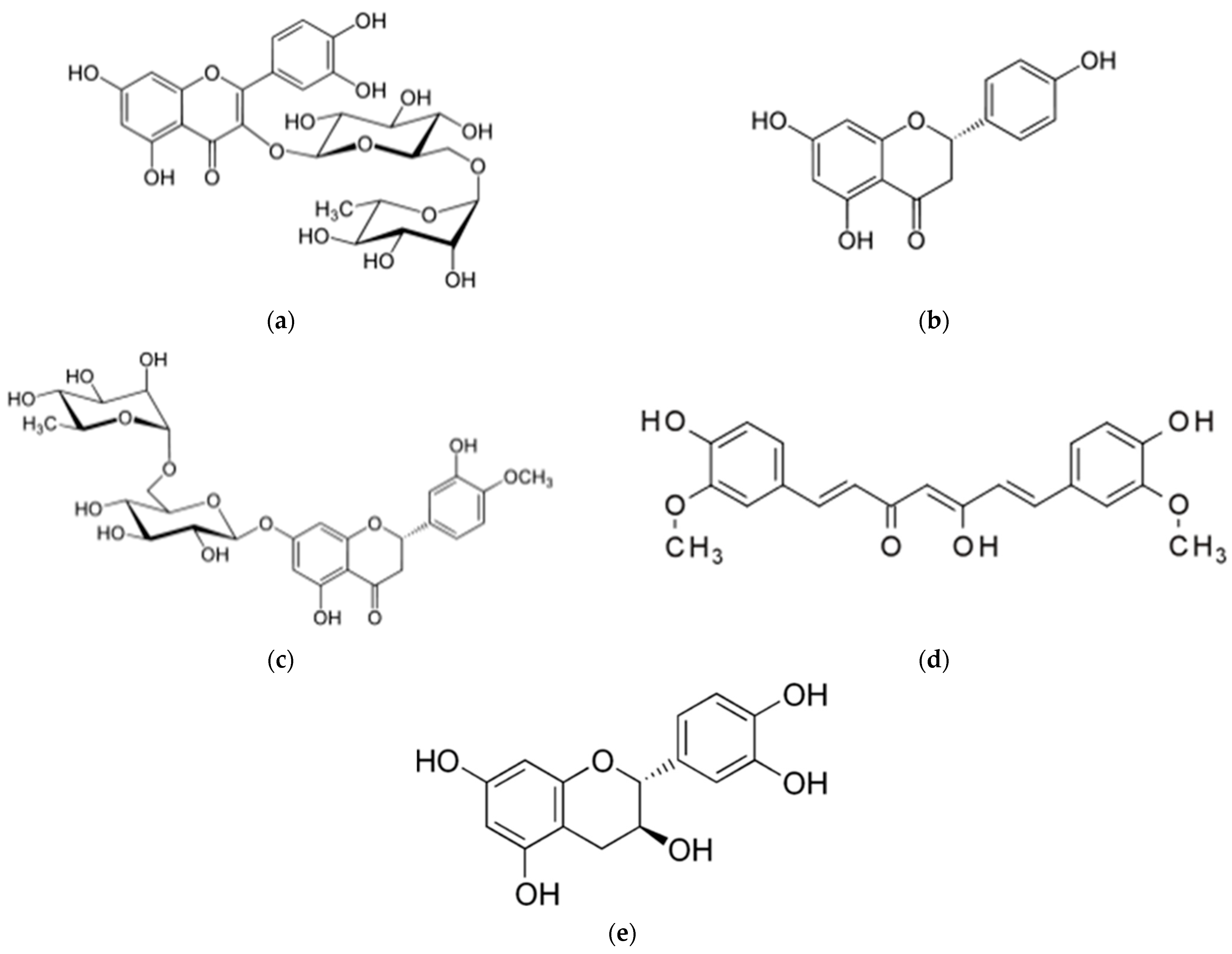
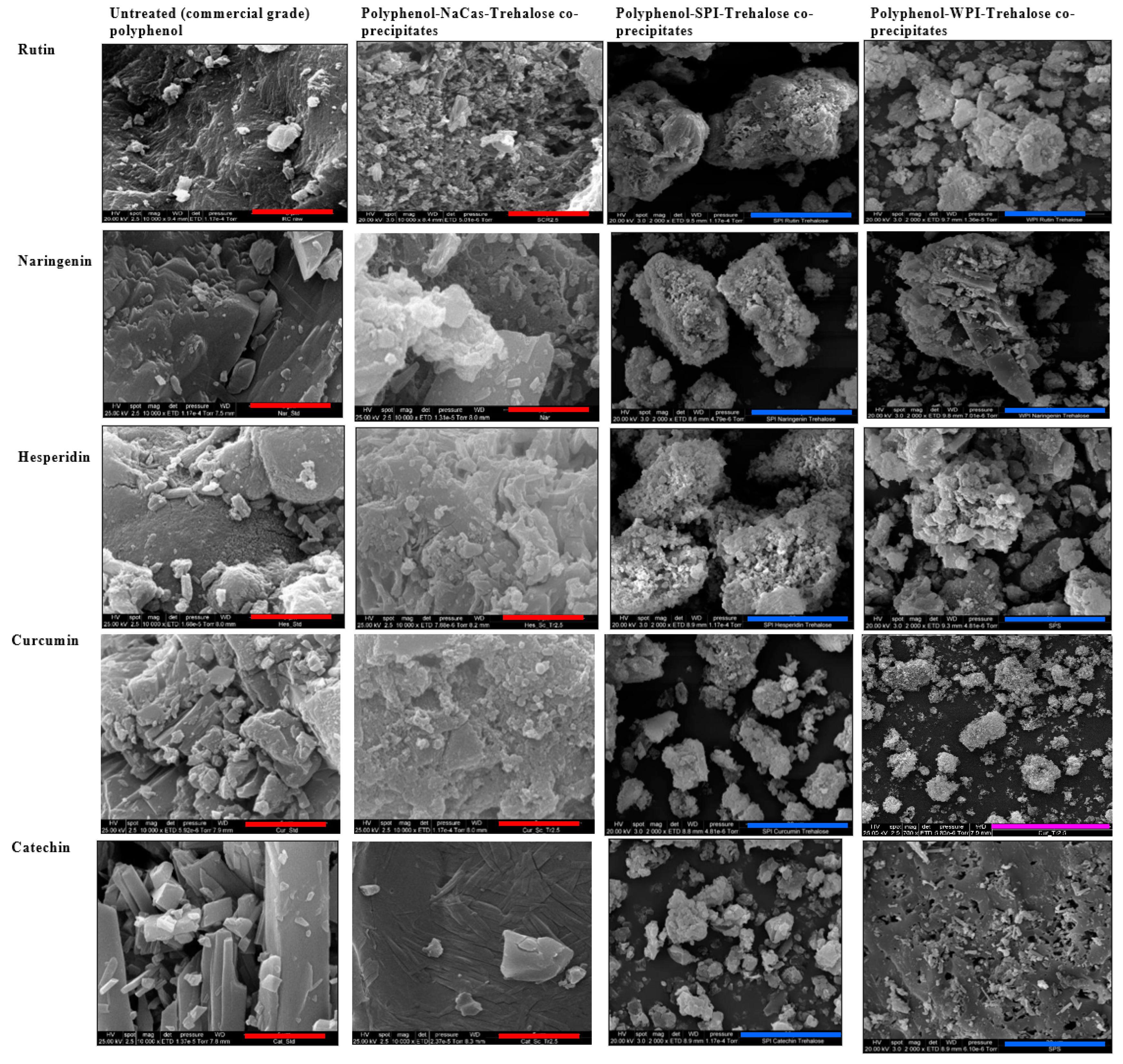
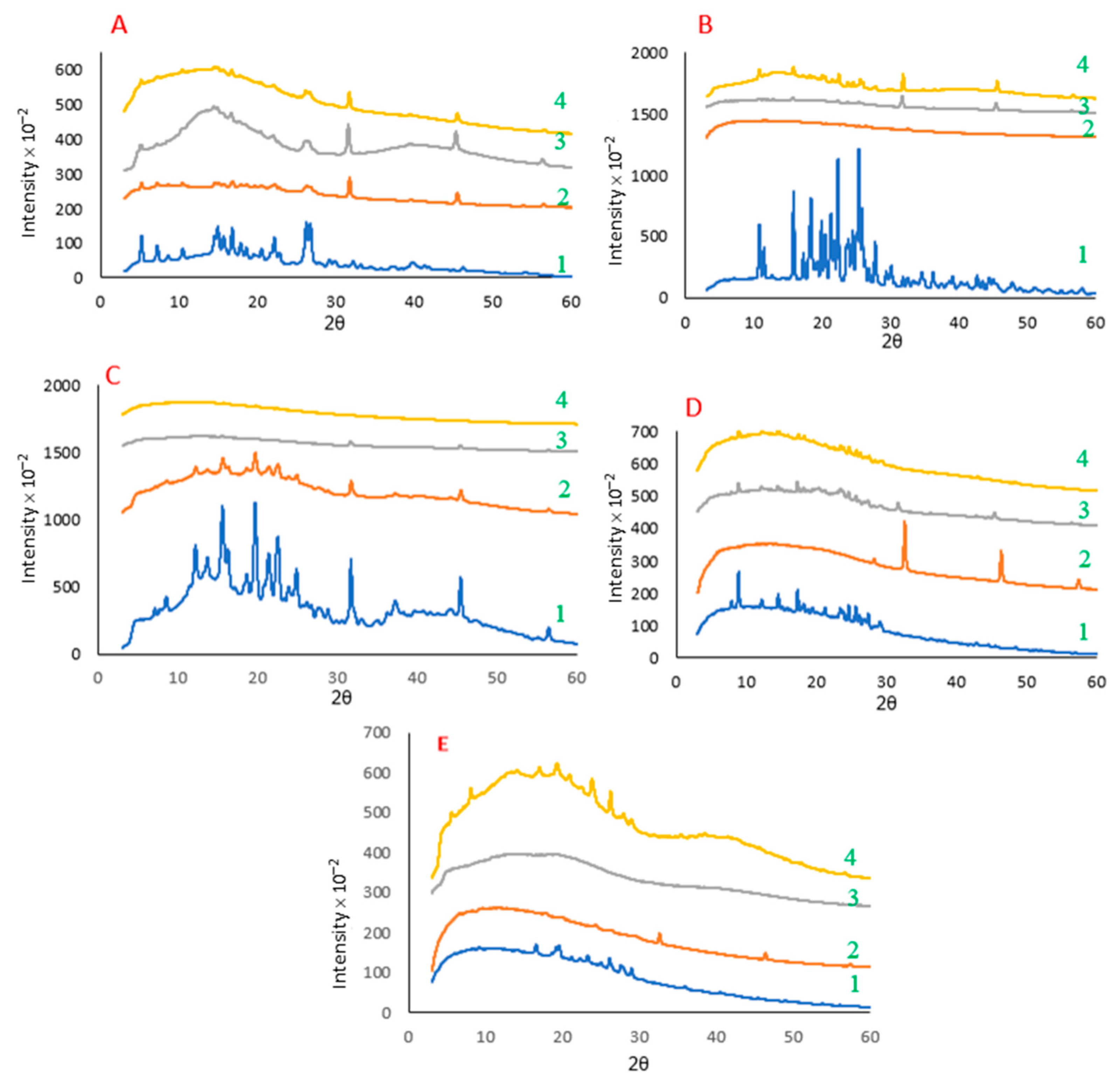
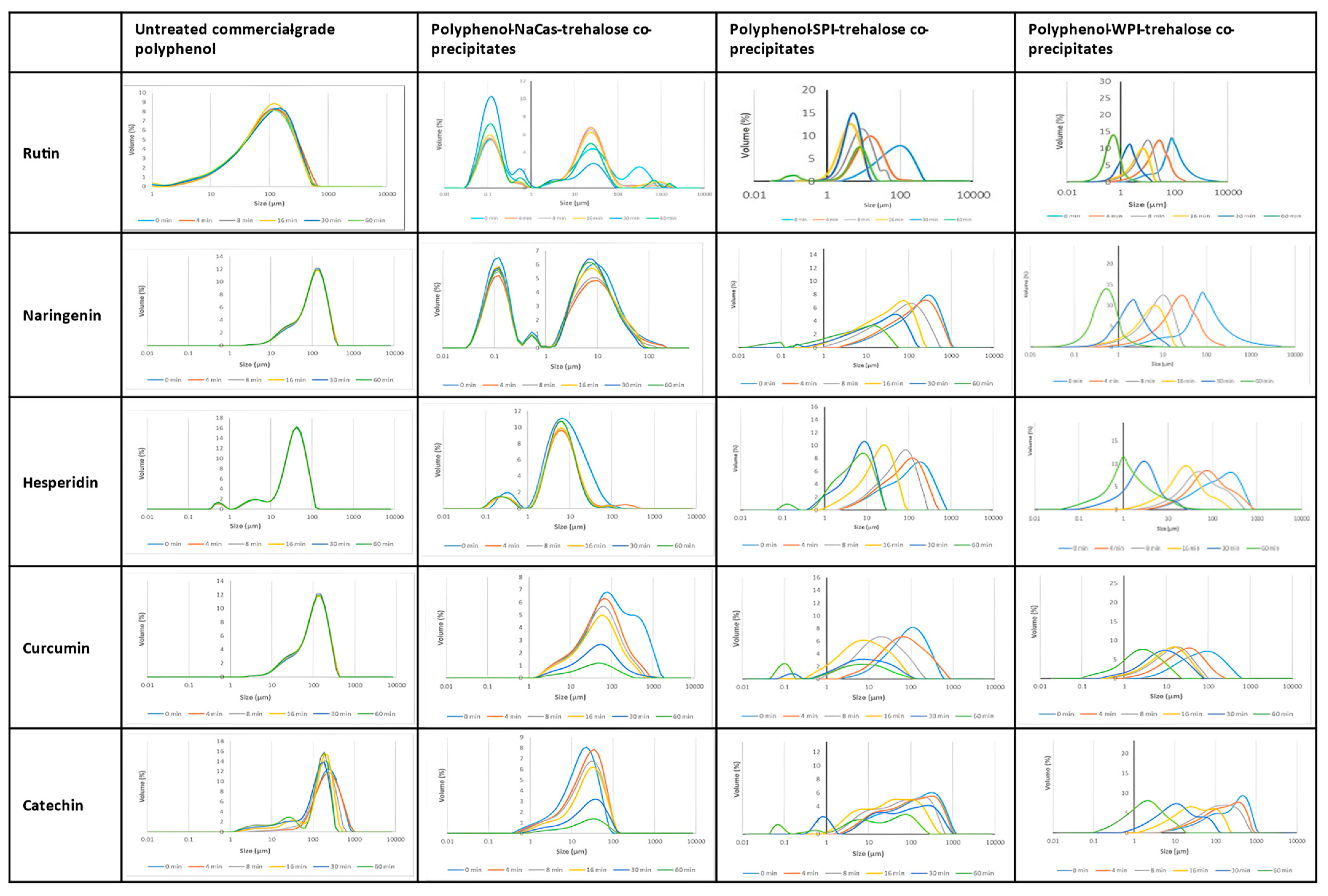
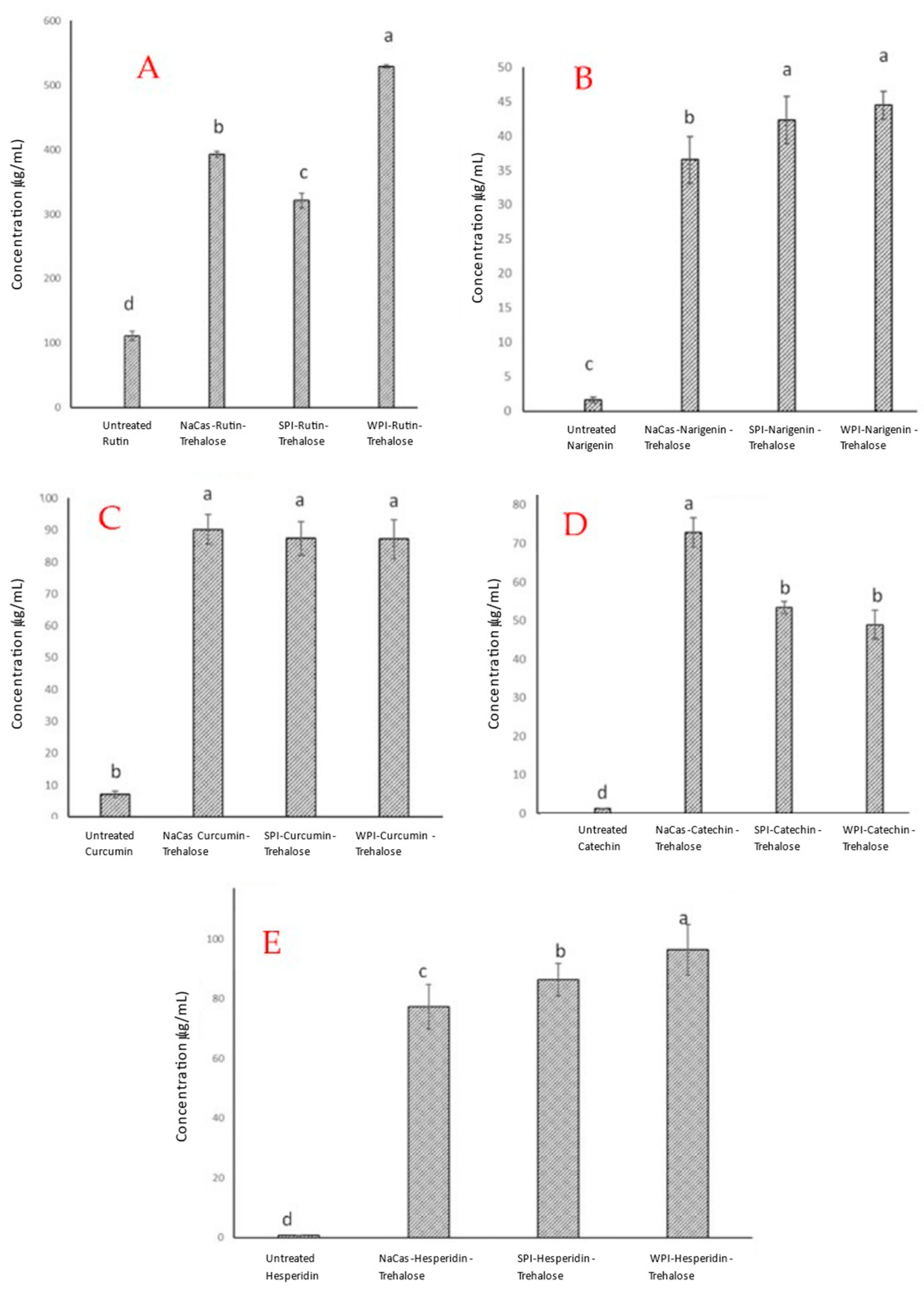
| Molecule | Hydrophobicity | Solubility a | Solubility a | Solubility a | Solubility a |
|---|---|---|---|---|---|
| (Untreated) | (NaCas) | (SPI) | (WPI) | ||
| Rutin b,c | −1.3 (266/610) d | 110 | 400 | 320 | 530 |
| Naringenin | 2.4 (87/272) | 2 | 37 | 42 | 45 |
| Curcumin | 3.2 (93/368) | 8 | 90 | 88 | 88 |
| Catechin e | 0.4 (111/308) | 1.5 | 73 | 53 | 49 |
| Hesperidin b | 1.1 (234/610) | 2 | 77 | 85 | 95 |
| Rhamnose | −2.1 (90/164) | very high | |||
| [1,1′-Biphenyl]-2,2′,4,4′-tetrol | 2.4 (81/218) | low? | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashidinejad, A.; Nieuwkoop, M.; Singh, H.; Jameson, G.B. Assessment of Various Food Proteins as Structural Materials for Delivery of Hydrophobic Polyphenols Using a Novel Co-Precipitation Method. Molecules 2023, 28, 3573. https://doi.org/10.3390/molecules28083573
Rashidinejad A, Nieuwkoop M, Singh H, Jameson GB. Assessment of Various Food Proteins as Structural Materials for Delivery of Hydrophobic Polyphenols Using a Novel Co-Precipitation Method. Molecules. 2023; 28(8):3573. https://doi.org/10.3390/molecules28083573
Chicago/Turabian StyleRashidinejad, Ali, Matthijs Nieuwkoop, Harjinder Singh, and Geoffrey B. Jameson. 2023. "Assessment of Various Food Proteins as Structural Materials for Delivery of Hydrophobic Polyphenols Using a Novel Co-Precipitation Method" Molecules 28, no. 8: 3573. https://doi.org/10.3390/molecules28083573
APA StyleRashidinejad, A., Nieuwkoop, M., Singh, H., & Jameson, G. B. (2023). Assessment of Various Food Proteins as Structural Materials for Delivery of Hydrophobic Polyphenols Using a Novel Co-Precipitation Method. Molecules, 28(8), 3573. https://doi.org/10.3390/molecules28083573







