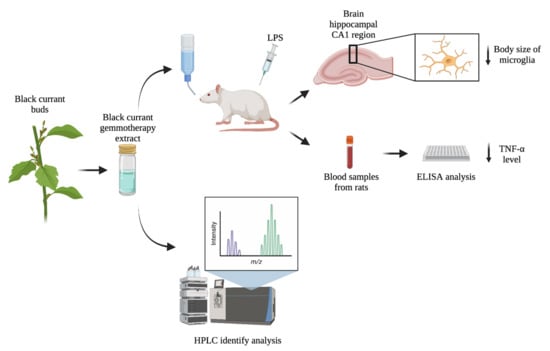
The Flavonoid Rich Black Currant (Ribes nigrum) Ethanolic Gemmotherapy Extract Elicits Neuroprotective Effect by Preventing Microglial Body Swelling in Hippocampus and Reduces Serum TNF-α Level: Pilot Study
Abstract
1. Introduction
2. Results
2.1. The BC-GTE Contains Important Flavonoids and Features Relevant In Vitro Antioxidant Properties
2.2. The BC-GTE Does Not Feature Cytotoxicity in the Case of Drosophila melanogaster
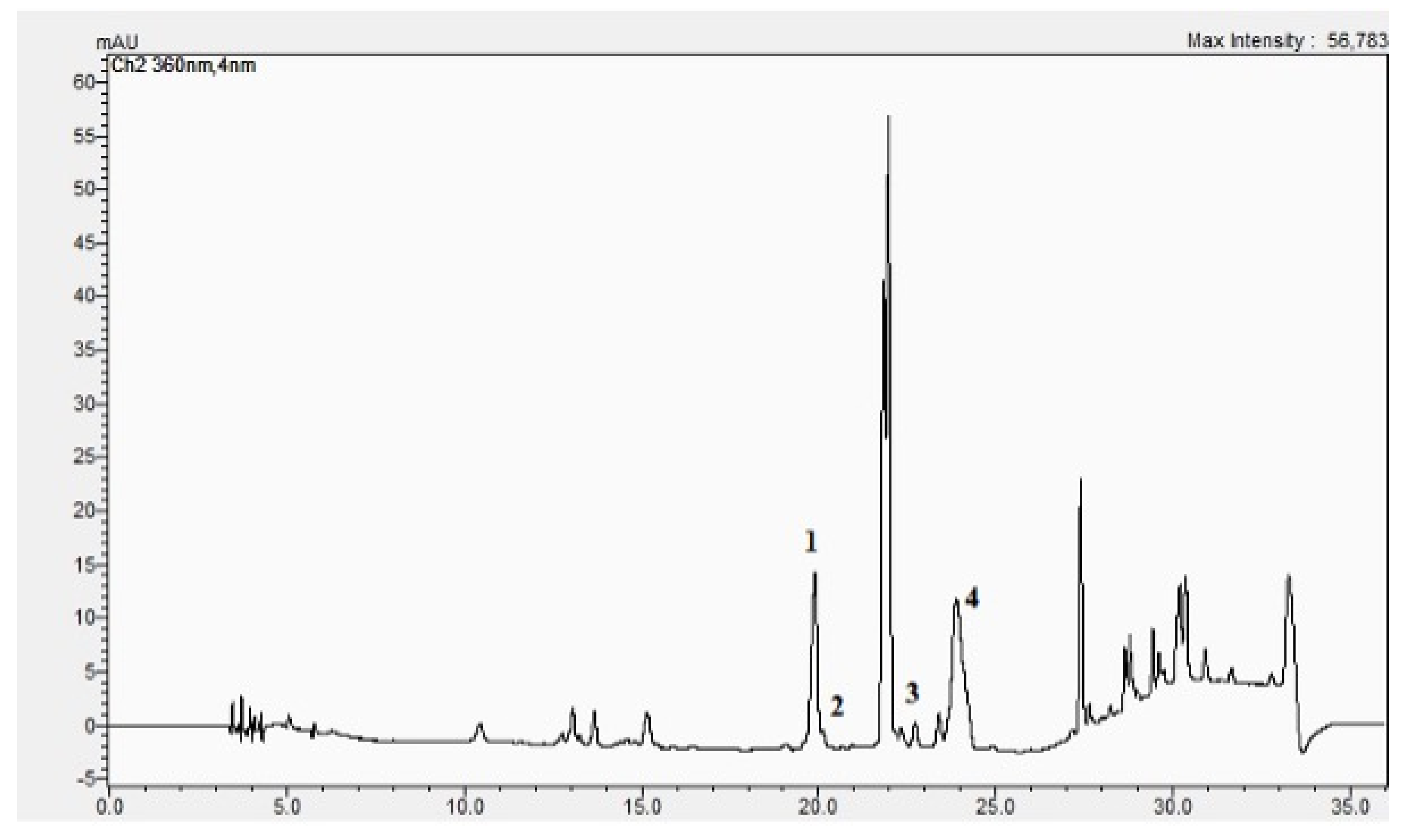
2.3. The BC-GTE Contains Many Phytonutrients with Reported Anti-Inflammatory and Neuroprotective Properties
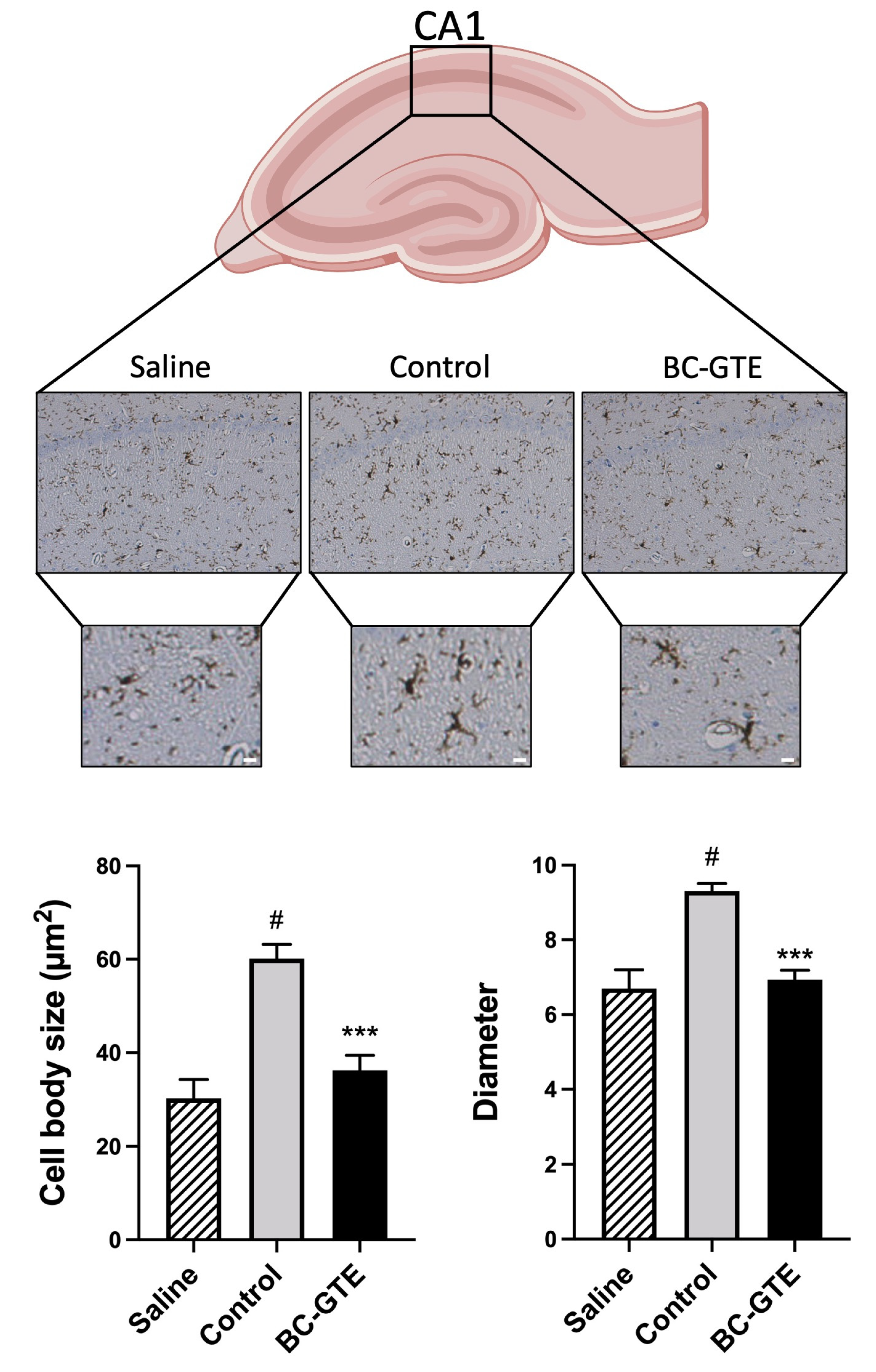
2.4. The BC-GTE Pretreatment Overcomes the Swelling of Rat Microglial Cell Body, following LPS Activation
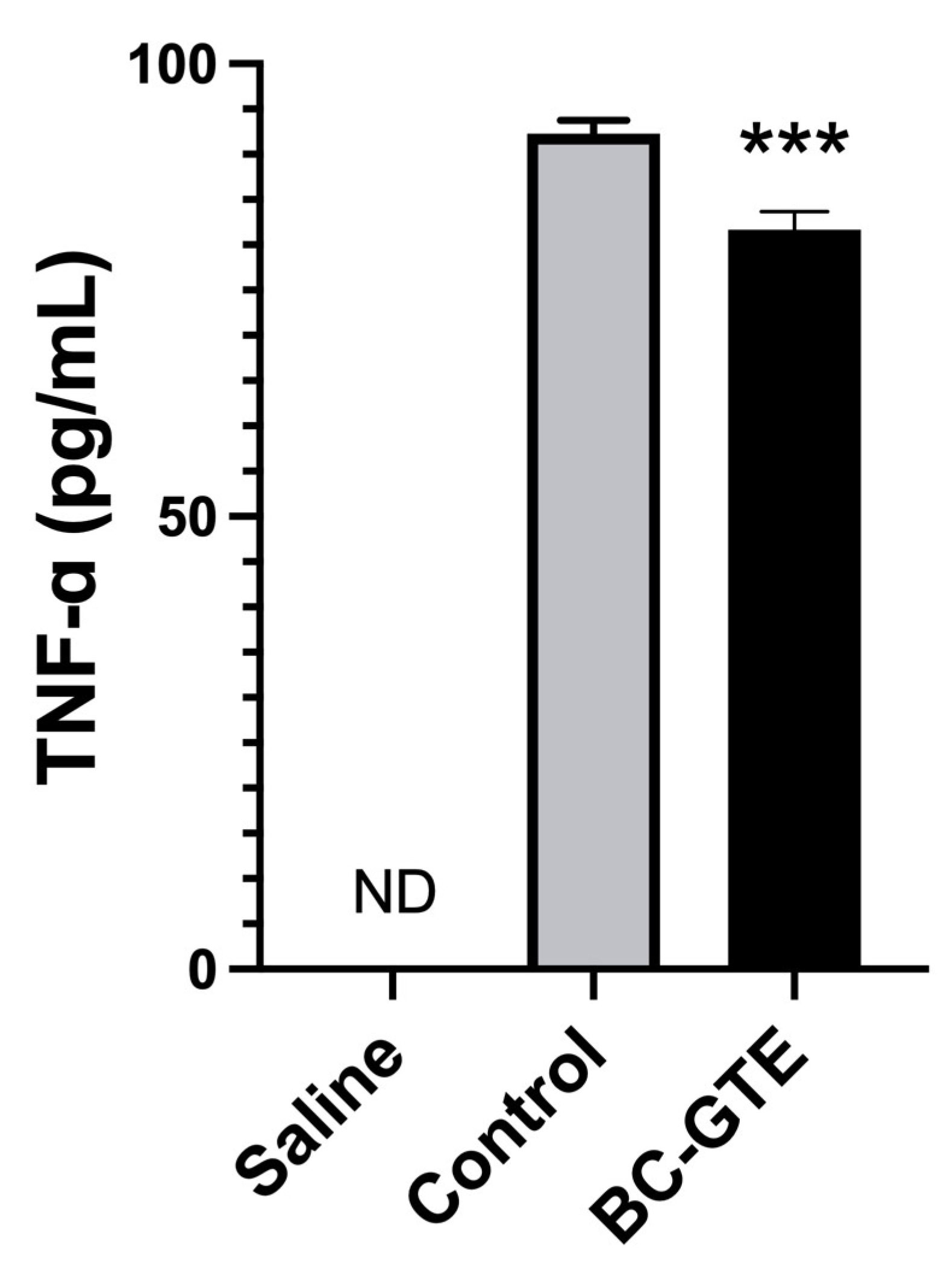
2.5. The BC-GTE Pretreatment Leads to Diminished Serum TNF-α Level in the Serum
3. Materials and Methods
3.1. Preparation of BC-GTE
3.2. UHPLC–ESI-MS Analysis of BC-GTE
3.3. Phytochemical Analysis of BC-GTE
3.4. Cytotoxicity Studies on Drosophila Melanogaster
3.5. Rattus Norvegicus Based Experimental Design
3.6. The LPS-Induced Inflammatory Reaction in Rats
3.7. Quantification of the Serum Specific TNF-α Levels
3.8. Immunohistochemistry Analysis
3.9. Analysis of Microglial Activation
3.10. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5465813/ (accessed on 18 November 2022). [CrossRef]
- Calis, Z.; Mogulkoc, R.; Baltaci, A.K. The Roles of Flavonols/Flavonoids in Neurodegeneration and Neuroinflammation. Mini Rev. Med. Chem. 2020, 20, 1475–1488. Available online: https://pubmed.ncbi.nlm.nih.gov/31288717/ (accessed on 18 November 2022). [CrossRef]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 5276130. Available online: /pmc/articles/PMC5075620/ (accessed on 18 November 2022). [CrossRef]
- Golan, H.; Levav, T.; Mendelsohn, A.; Huleihel, M. Involvement of Tumor Necrosis Factor Alpha in Hippocampal Development and Function. Cereb. Cortex 2004, 14, 97–105. Available online: https://academic.oup.com/cercor/article/14/1/97/433504 (accessed on 18 November 2022). [CrossRef]
- Spencer, J.P.E. Flavonoids and brain health: Multiple effects underpinned by common mechanisms. Genes Nutr. 2009, 4, 243. Available online: /pmc/articles/PMC2775888/ (accessed on 18 November 2022). [CrossRef] [PubMed]
- Vauzour, D.; Vafeiadou, K.; Rodriguez-Mateos, A.; Rendeiro, C.; Spencer, J.P.E. The neuroprotective potential of flavonoids: A multiplicity of effects. Genes Nutr. 2008, 3, 115–126. Available online: https://genesandnutrition.biomedcentral.com/articles/10.1007/s12263-008-0091-4 (accessed on 18 November 2022). [CrossRef]
- Rendeiro, C.; Spencer, J.P.E.; Vauzour, D.; Butler, L.T.; Ellis, J.A.; Williams, C.M. The impact of flavonoids on spatial memory in rodents: From behaviour to underlying hippocampal mechanisms. Genes Nutr. 2009, 4, 251. Available online: /pmc/articles/PMC2775889/ (accessed on 18 November 2022). [CrossRef]
- Brás, J.P.; Bravo, J.; Freitas, J.; Barbosa, M.A.; Santos, S.G.; Summavielle, T.; Almeida, M.I. TNF-alpha-induced microglia activation requires miR-342: Impact on NF-kB signaling and neurotoxicity. Cell Death Dis. 2020, 11, 415. Available online: https://www.nature.com/articles/s41419-020-2626-6 (accessed on 17 November 2022). [CrossRef] [PubMed]
- Cortez, R.E.; Mejia, E.G. de Blackcurrants (Ribes nigrum): A Review on Chemistry, Processing, and Health Benefits. J. Food Sci. 2019, 84, 2387–2401. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/1750-3841.14781 (accessed on 11 October 2022). [CrossRef]
- Huebbe, P.; Giller, K.; de Pascual-Teresa, S.; Arkenau, A.; Adolphi, B.; Portius, S.; Arkenau, C.N.; Rimbach, G. Effects of blackcurrant-based juice on atherosclerosis-related biomarkers in cultured macrophages and in human subjects after consumption of a high-energy meal. Br. J. Nutr. 2012, 108, 234–244. Available online: http://www.ncbi.nlm.nih.gov/pubmed/22011640 (accessed on 22 May 2019). [CrossRef] [PubMed]
- Benn, T.; Kim, B.; Park, Y.K.; Wegner, C.J.; Harness, E.; Nam, T.G.; Kim, D.O.; Lee, J.S.; Lee, J.Y. Polyphenol-rich blackcurrant extract prevents inflammation in diet-induced obese mice. J. Nutr. Biochem. 2014, 25, 1019–1025. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, J.-Y. Blackcurrant (Ribes nigrum) Extract Exerts an Anti-Inflammatory Action by Modulating Macrophage Phenotypes. Nutrients 2019, 11, 975. Available online: http://www.ncbi.nlm.nih.gov/pubmed/31035378 (accessed on 4 June 2019). [CrossRef]
- Hoogland, I.C.M.; Houbolt, C.; van Westerloo, D.J.; van Gool, W.A.; van de Beek, D. Systemic inflammation and microglial activation: Systematic review of animal experiments. J. Neuroinflamm. 2015, 12, 114. Available online: https://pubmed.ncbi.nlm.nih.gov/26048578/ (accessed on 22 November 2022). [CrossRef]
- Chen, Y.; Peng, F.; Xing, Z.; Chen, J.; Peng, C.; Li, D. Beneficial effects of natural flavonoids on neuroinflammation. Front. Immunol 2022, 13, 6267. Available online: https://pubmed.ncbi.nlm.nih.gov/36353622/ (accessed on 13 March 2023). [CrossRef]
- Wadhwa, G.; Krishna, K.V.; Taliyan, R.; Tandon, N.; Yadav, S.S.; Banerjee, D.; Narwaria, A.; Katiyar, C.; Dubey, S.K. A novel UPLC-MS/MS method for simultaneous quantification of trigonelline, 4-hydroxyisoleucine, and diosgenin from Trigonella foenum-graecum extract: Application to pharmacokinetic study in healthy and type 2 diabetic rats. Biomed. Chromatogr. 2022, 36, e5275. Available online: https://pubmed.ncbi.nlm.nih.gov/34738247/ (accessed on 13 March 2023). [CrossRef] [PubMed]
- Ostapiuk, A.; Urbanska, E.M. Kynurenic acid in neurodegenerative disorders-unique neuroprotection or double-edged sword? CNS Neurosci. Ther. 2022, 28, 19–35. Available online: https://pubmed.ncbi.nlm.nih.gov/34862742/ (accessed on 13 March 2023). [CrossRef]
- Gong, B.; Zheng, Y.; Li, J.; Lei, H.; Liu, K.; Tang, J.; Peng, Y. Luteolin activates M2 macrophages and suppresses M1 macrophages by upregulation of hsa_circ_0001326 in THP-1 derived macrophages. Bioengineered 2022, 13, 5079–5090. Available online: https://pubmed.ncbi.nlm.nih.gov/35152837/ (accessed on 13 March 2023). [CrossRef] [PubMed]
- Zhang, J.X.; Xing, J.G.; Wang, L.L.; Jiang, H.L.; Guo, S.L.; Liu, R. Luteolin Inhibits Fibrillary β-Amyloid1-40-Induced Inflammation in a Human Blood-Brain Barrier Model by Suppressing the p38 MAPK-Mediated NF-κB Signaling Pathways. Molecules 2017, 22, 334. Available online: https://pubmed.ncbi.nlm.nih.gov/28245546/ (accessed on 13 March 2023). [CrossRef] [PubMed]
- Zhang, K.K.; Wang, H.; Qu, D.; Chen, L.J.; Wang, L.B.; Li, J.H.; Liu, J.L.; Xu, L.L.; Yoshida, J.S.; Xu, J.T.; et al. Luteolin Alleviates Methamphetamine-Induced Hepatotoxicity by Suppressing the p53 Pathway-Mediated Apoptosis, Autophagy, and Inflammation in Rats. Front. Pharmacol. 2021, 12, 641917. Available online: https://pubmed.ncbi.nlm.nih.gov/33679421/ (accessed on 13 March 2023). [CrossRef]
- Tan, X.H.; Zhang, K.K.; Xu, J.T.; Qu, D.; Chen, L.J.; Li, J.H.; Wang, Q.; Wang, H.J.; Xie, X.L. Luteolin alleviates methamphetamine-induced neurotoxicity by suppressing PI3K/Akt pathway-modulated apoptosis and autophagy in rats. Food Chem. Toxicol. 2020, 137, 111179. Available online: https://pubmed.ncbi.nlm.nih.gov/32035215/ (accessed on 13 March 2023). [CrossRef]
- Tsai, C.F.; Chen, G.W.; Chen, Y.C.; Shen, C.K.; Lu, D.Y.; Yang, L.Y.; Chen, J.H.; Yeh, W.L. Regulatory Effects of Quercetin on M1/M2 Macrophage Polarization and Oxidative/Antioxidative Balance. Nutrients 2021, 14, 67. Available online: https://pubmed.ncbi.nlm.nih.gov/35010945/ (accessed on 13 March 2023). [CrossRef] [PubMed]
- Fan, H.; Tang, H.B.; Shan, L.Q.; Liu, S.C.; Huang, D.G.; Chen, X.; Chen, Z.; Yang, M.; Yin, X.H.; Yang, H.; et al. Quercetin prevents necroptosis of oligodendrocytes by inhibiting macrophages/microglia polarization to M1 phenotype after spinal cord injury in rats. J. Neuroinflamm. 2019, 16, 206. Available online: https://pubmed.ncbi.nlm.nih.gov/31699098/ (accessed on 13 March 2023). [CrossRef] [PubMed]
- Xiong, G.; Ji, W.; Wang, F.; Zhang, F.; Xue, P.; Cheng, M.; Sun, Y.; Wang, X.; Zhang, T. Quercetin Inhibits Inflammatory Response Induced by LPS from Porphyromonas gingivalis in Human Gingival Fibroblasts via Suppressing NF-κB Signaling Pathway. Biomed Res. Int. 2019, 2019, 6282635. Available online: /pmc/articles/PMC6720363/ (accessed on 13 March 2023). [CrossRef] [PubMed]
- Cai, S.Q.; Zhang, Q.; Zhao, X.H.; Shi, J. The In Vitro Anti-Inflammatory Activities of Galangin and Quercetin towards the LPS-Injured Rat Intestinal Epithelial (IEC-6) Cells as Affected by Heat Treatment. Molecules 2021, 26, 7495. Available online: https://pubmed.ncbi.nlm.nih.gov/34946578/ (accessed on 13 March 2023). [CrossRef] [PubMed]
- Chen, P.; Huo, X.; Liu, W.; Li, K.; Sun, Z.; Tian, J. Apigenin exhibits anti-inflammatory effects in LPS-stimulated BV2 microglia through activating GSK3β/Nrf2 signaling pathway. Immunopharmacol. Immunotoxicol. 2020, 42, 9–16. Available online: https://pubmed.ncbi.nlm.nih.gov/31760890/ (accessed on 13 March 2023). [CrossRef] [PubMed]
- Kurniati, D.; Hirai, S.; Egashira, Y. Effect of apigenin on tryptophan metabolic key enzymes expression in lipopolysaccharide-induced microglial cells and its mechanism. Heliyon 2022, 9, e12743. Available online: https://pubmed.ncbi.nlm.nih.gov/36685364/ (accessed on 13 March 2023). [CrossRef]
- Chang, S.; Li, X.; Zheng, Y.; Shi, H.; Zhang, D.; Jing, B.; Chen, Z.; Qian, G.; Zhao, G. Kaempferol exerts a neuroprotective effect to reduce neuropathic pain through TLR4/NF-ĸB signaling pathway. Phytother. Res. 2022, 36, 1678–1691. Available online: https://pubmed.ncbi.nlm.nih.gov/35234314/ (accessed on 13 March 2023). [CrossRef]
- Park, S.E.; Sapkota, K.; Kim, S.; Kim, H.; Kim, S.J. Kaempferol acts through mitogen-activated protein kinases and protein kinase B/AKT to elicit protection in a model of neuroinflammation in BV2 microglial cells. Br. J. Pharmacol. 2011, 164, 1008–1025. Available online: https://pubmed.ncbi.nlm.nih.gov/21449918/ (accessed on 13 March 2023). [CrossRef]
- Zhuang, Z.; Ye, G.; Huang, B. Kaempferol Alleviates the Interleukin-1β-Induced Inflammation in Rat Osteoarthritis Chondrocytes via Suppression of NF-κB. Med. Sci. Monit. 2017, 23, 3925. Available online: /pmc/articles/PMC5566200/ (accessed on 13 March 2023). [CrossRef]
- Enogieru, A.B.; Haylett, W.; Hiss, D.C.; Bardien, S.; Ekpo, O.E. Rutin as a Potent Antioxidant: Implications for Neurodegenerative Disorders. Oxid. Med. Cell. Longev. 2018, 2018, 6241017. Available online: https://pubmed.ncbi.nlm.nih.gov/30050657/ (accessed on 13 March 2023). [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2017, 25, 149–164. Available online: https://pubmed.ncbi.nlm.nih.gov/28344465/ (accessed on 13 March 2023). [CrossRef] [PubMed]
- Hernández-Aquino, E.; Muriel, P. Beneficial effects of naringenin in liver diseases: Molecular mechanisms. World J. Gastroenterol. 2018, 24, 1679–1707. Available online: https://pubmed.ncbi.nlm.nih.gov/29713125/ (accessed on 13 March 2023). [CrossRef] [PubMed]
- Nouri, Z.; Fakhri, S.; El-Senduny, F.F.; Sanadgol, N.; Abd-Elghani, G.E.; Farzaei, M.H.; Chen, J.T. On the Neuroprotective Effects of Naringenin: Pharmacological Targets, Signaling Pathways, Molecular Mechanisms, and Clinical Perspective. Biomolecules 2019, 9, 690. Available online: https://pubmed.ncbi.nlm.nih.gov/31684142/ (accessed on 13 March 2023). [CrossRef] [PubMed]
- Hartogh, D.J.D.; Tsiani, E. Antidiabetic Properties of Naringenin: A Citrus Fruit Polyphenol. Biomolecules 2019, 9, 99. Available online: /pmc/articles/PMC6468535/ (accessed on 13 March 2023). [CrossRef]
- Zielińska, D.; Zieliński, H.; Laparra-Llopis, J.M.; Szawara-Nowak, D.; Honke, J.; Giménez-Bastida, J.A. Caffeic Acid Modulates Processes Associated with Intestinal Inflammation. Nutrients 2021, 13, 554. Available online: https://pubmed.ncbi.nlm.nih.gov/33567596/ (accessed on 13 March 2023). [CrossRef]
- Muhammad Abdul Kadar, N.N.; Ahmad, F.; Teoh, S.L.; Yahaya, M.F. Caffeic Acid on Metabolic Syndrome: A Review. Molecules 2021, 26, 5490. Available online: https://pubmed.ncbi.nlm.nih.gov/34576959/ (accessed on 13 March 2023). [CrossRef]
- Stompor-Gorący, M.; Machaczka, M. Recent Advances in Biological Activity, New Formulations and Prodrugs of Ferulic Acid. Int. J. Mol. Sci. 2021, 22, 12889. Available online: https://pubmed.ncbi.nlm.nih.gov/34884693/ (accessed on 13 March 2023). [CrossRef]
- Jarocka-karpowicz, I.; Markowska, A. Therapeutic Potential of Jasmonic Acid and Its Derivatives. Int. J. Mol. Sci. 2021, 22, 8437. Available online: https://pubmed.ncbi.nlm.nih.gov/34445138/ (accessed on 13 March 2023). [CrossRef]
- Lee, T.K.; Kang, I.J.; Kim, B.; Sim, H.J.; Kim, D.W.; Ahn, J.H.; Lee, J.C.; Ryoo, S.; Shin, M.C.; Cho, J.H.; et al. Experimental Pretreatment with Chlorogenic Acid Prevents Transient Ischemia-Induced Cognitive Decline and Neuronal Damage in the Hippocampus through Anti-Oxidative and Anti-Inflammatory Effects. Molecules 2020, 25, 3578. Available online: https://pubmed.ncbi.nlm.nih.gov/32781658/ (accessed on 13 March 2023). [CrossRef]
- Fukutomi, R.; Ohishi, T.; Koyama, Y.; Pervin, M.; Nakamura, Y.; Isemura, M. Beneficial Effects of Epigallocatechin-3- O-Gallate, Chlorogenic Acid, Resveratrol, and Curcumin on Neurodegenerative Diseases. Molecules 2021, 26, 415. Available online: https://pubmed.ncbi.nlm.nih.gov/33466849/ (accessed on 13 March 2023). [CrossRef]
- Piermartiri, T.; Pan, H.; Figueiredo, T.H.; Marini, A.M. α-Linolenic Acid, A Nutraceutical with Pleiotropic Properties That Targets Endogenous Neuroprotective Pathways to Protect against Organophosphate Nerve Agent-Induced Neuropathology. Molecules 2015, 20, 20355. Available online: /pmc/articles/PMC6332275/ (accessed on 13 March 2023). [CrossRef] [PubMed]
- Skrzypczak-Wiercioch, A.; Sałat, K. Lipopolysaccharide-Induced Model of Neuroinflammation: Mechanisms of Action, Research Application and Future Directions for Its Use. Molecules 2022, 27, 5481. Available online: https://pubmed.ncbi.nlm.nih.gov/36080253/ (accessed on 13 March 2023). [CrossRef] [PubMed]
- Wu, S.Y.; Wang, T.F.; Yu, L.; Jen, C.J.; Chuang, J.I.; Wu, F.S.; Wu, C.W.; Kuo, Y.M. Running exercise protects the substantia nigra dopaminergic neurons against inflammation-induced degeneration via the activation of BDNF signaling pathway. Brain. Behav. Immun. 2011, 25, 135–146. Available online: http://dx.doi.org/10.1016/j.bbi.2010.09.006 (accessed on 23 November 2022). [CrossRef] [PubMed]
- Hoogland, I.C.M.; Westhoff, D.; Engelen-Lee, J.Y.; Melief, J.; Valls Serón, M.; Houben-Weerts, J.H.M.P.; Huitinga, I.; van Westerloo, D.J.; van der Poll, T.; van Gool, W.A.; et al. Microglial Activation After Systemic Stimulation With Lipopolysaccharide and Escherichia coli. Front. Cell. Neurosci. 2018, 12, 110. Available online: https://pubmed.ncbi.nlm.nih.gov/29755322/ (accessed on 22 November 2022). [CrossRef] [PubMed]
- Hovens, I.B.; van Leeuwen, B.L.; Nyakas, C.; Heineman, E.; van der Zee, E.A.; Schoemaker, R.G. Postoperative cognitive dysfunction and microglial activation in associated brain regions in old rats. Neurobiol. Learn. Mem. 2015, 118, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Arjona, M. del M.; Grondona, J.M.; Granados-Durán, P.; Fernández-Llebrez, P.; López-Ávalos, M.D. Microglia Morphological Categorization in a Rat Model of Neuroinflammation by Hierarchical Cluster and Principal Components Analysis. Front. Cell. Neurosci. 2017, 11, 235. [Google Scholar] [CrossRef]
- Godbout, J.P.; Chen, J.; Abraham, J.; Richwine, A.F.; Berg, B.M.; Kelley, K.W.; Johnson, R.W. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J. 2005, 19, 1329–1331. Available online: https://pubmed.ncbi.nlm.nih.gov/15919760/ (accessed on 11 October 2021). [CrossRef]
- Heppner, F.L.; Ransohoff, R.M.; Becher, B. Immune attack: The role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 2015, 16, 358–372. Available online: https://www.nature.com/articles/nrn3880 (accessed on 11 October 2021). [CrossRef]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. Available online: https://pubmed.ncbi.nlm.nih.gov/9916193/ (accessed on 11 October 2021).
- Apak, R.; Güçlü, K.; Özyürek, M.; Çelik, S.E. Mechanism of antioxidant capacity assays and the CUPRAC (cupric ion reducing antioxidant capacity) assay. Microchim. Acta 2007, 160, 413–419. Available online: https://link.springer.com/article/10.1007/s00604-007-0777-0 (accessed on 11 October 2021). [CrossRef]
- Tetau, M. Gemmotherapy: A Clinical guide; Editions Similia: 2010; ISBN 9782842510503. Available online: https://www.abebooks.com/Gemmotherapy-Clinical-guide-2010-Edition-Max/31290232315/bd (accessed on 1 March 2023).
- Liu, L.; Zhang, Q.; Cai, Y.; Sun, D.; He, X.; Wang, L.; Yu, D.; Li, X.; Xiong, X.; Xu, H.; et al. Resveratrol counteracts lipopolysaccharide-induced depressivelike behaviors via enhanced hippocampal neurogenesis. Oncotarget 2016, 7, 56045–56059. Available online: http://www.ncbi.nlm.nih.gov/pubmed/27517628 (accessed on 14 January 2020). [CrossRef]
- Lin, H.Y.; Huang, C.C.; Chang, K.F. Lipopolysaccharide Preconditioning Reduces Neuroinflammation Against Hypoxic Ischemia and Provides Long-Term Outcome of Neuroprotection in Neonatal Rat. Pediatr. Res. 2009, 66, 254–259. Available online: https://www.nature.com/articles/pr2009197 (accessed on 17 November 2022). [CrossRef] [PubMed]
- Pérez-Cano, F.J.; Castell, M. Flavonoids, Inflammation and Immune System. Nutrients 2016, 8, 659. Available online: /pmc/articles/PMC5084045/ (accessed on 13 March 2023). [CrossRef] [PubMed]
- Di Matteo, V.; Esposito, E. Biochemical and therapeutic effects of antioxidants in the treatment of Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis. Curr. Drug Targets. CNS Neurol. Disord. 2003, 2, 95–107. Available online: https://pubmed.ncbi.nlm.nih.gov/12769802/ (accessed on 18 November 2022). [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. Available online: /pmc/articles/PMC7697716/ (accessed on 18 November 2022). [CrossRef]
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011, 89, 217–233. [Google Scholar] [CrossRef]
- Hambali, A.; Kumar, J.; Hashim, N.F.M.; Maniam, S.; Mehat, M.Z.; Cheema, M.S.; Mustapha, M.; Adenan, M.I.; Stanslas, J.; Hamid, H.A. Hypoxia-Induced Neuroinflammation in Alzheimer’s Disease: Potential Neuroprotective Effects of Centella asiatica. Front. Physiol. 2021, 12, 1698. [Google Scholar] [CrossRef]
- Pranzatelli, M.R. Advances in biomarker-guided therapy for pediatric- and adult-onset neuroinflammatory disorders: Targeting chemokines/cytokines. Front. Immunol. 2018, 9, 557. [Google Scholar] [CrossRef]
- Kölliker-Frers, R.; Udovin, L.; Otero-Losada, M.; Kobiec, T.; Herrera, M.I.; Palacios, J.; Razzitte, G.; Capani, F. Neuroinflammation: An Integrating Overview of Reactive-Neuroimmune Cell Interactions in Health and Disease. Mediators Inflamm. 2021, 2021, 9999146. Available online: https://pubmed.ncbi.nlm.nih.gov/34158806/ (accessed on 13 March 2023). [CrossRef]
- Deus, C.M.; Tavares, H.; Beatriz, M.; Mota, S.; Lopes, C. Mitochondrial Damage-Associated Molecular Patterns Content in Extracellular Vesicles Promotes Early Inflammation in Neurodegenerative Disorders. Cells 2022, 11, 2364. Available online: https://pubmed.ncbi.nlm.nih.gov/35954208/ (accessed on 13 March 2023). [CrossRef] [PubMed]
- Wang, S.; Cao, M.; Xu, S.; Shi, J.; Mao, X.; Yao, X.; Liu, C. Luteolin Alters Macrophage Polarization to Inhibit Inflammation. Inflammation 2020, 43, 95–108. Available online: https://pubmed.ncbi.nlm.nih.gov/31673976/ (accessed on 13 March 2023). [CrossRef] [PubMed]
- Zhang, B.; Wei, Y.Z.; Wang, G.Q.; Li, D.D.; Shi, J.S.; Zhang, F. Targeting MAPK pathways by naringenin modulates microglia M1/M2 polarization in lipopolysaccharide-stimulated cultures. Front. Cell. Neurosci. 2019, 12, 531. [Google Scholar] [CrossRef] [PubMed]
- Welser-Alves, J.V.; Milner, R. Microglia are the major source of TNF-α and TGF-β in postnatal glial cultures; regulation by cytokines, lipopolysaccharide, and vitronectin. Neurochem. Int. 2013, 63, 47–53. Available online: /pmc/articles/PMC3819935/ (accessed on 18 November 2022). [CrossRef] [PubMed]
- Chen, C.; Wei, Y.Z.; He, X.M.; Li, D.D.; Wang, G.Q.; Li, J.J.; Zhang, F. Naringenin Produces Neuroprotection Against LPS-Induced Dopamine Neurotoxicity via the Inhibition of Microglial NLRP3 Inflammasome Activation. Front. Immunol. 2019, 10, 936. Available online: https://pubmed.ncbi.nlm.nih.gov/31118933/ (accessed on 13 March 2023). [CrossRef] [PubMed]

| Chemical Classification | Bioactive Compounds |
|---|---|
| Alkaloids | Kynurenic acid |
| Trigonelline | |
| Amino Acids | 4-Guanidinobutyric acid |
| 4-Hydroxyisoleucine | |
| Asparagine | |
| Glutamic acid | |
| Isoleucine or Leucine | |
| Phenylalanine | |
| Proline | |
| Threonine | |
| Tryptophan | |
| Others | Hydroxybenzaldehyde |
| Indole-4-carbaldehyde | |
| Esters | Ethyl gallate |
| Sinapoyl-methoxyphenol | |
| Flavonoids | Acacetin |
| Ampelopsin (Ampeloptin, Dihydromyricetin) | |
| Apigenin | |
| Astragalin (Kaempferol-3-O-glucoside) | |
| Catechin | |
| Chrysoeriol | |
| Dihydrokaempferol (Aromadendrin, Katuranin) | |
| Dihydroxy-dimethoxyflavone isomer 1 | |
| Flavonoids (Continue)Dihydroxy-dimethoxyflavone isomer 2 | |
| Flavonoids (Continue) | Dihydroxy-dimethoxyisoflavan |
| Dihydroxy-methoxyflavanone-O-hexoside isomer 1 | |
| Dihydroxy-methoxyflavanone-O-hexoside isomer 2 | |
| Dihydroxy-trimethoxyflavone isomer 1 | |
| Dihydroxy-trimethoxyflavone isomer 2 | |
| Epicatechin | |
| Epigallocatechin | |
| Eriodictyol | |
| Genkwanin (Apigenin-7-O-methyl ether) | |
| Hydroxy-dimethoxyflavone | |
| Hydroxy-tetramethoxyflavone (Retusin) | |
| Hydroxy-trimethoxyflavone (Salvigenin) | |
| Hyperoside (Quercetin-3-O-galactoside, Hyperin) | |
| Isoquercitrin (Hirsutrin, Quercetin-3-O-glucoside) | |
| Isorhamnetin | |
| Isorhamnetin-3-O-glucoside | |
| Isorhamnetin-3-O-rutinoside (Narcissin) | |
| Isorhamnetin-O-hexoside isomer 1 | |
| Isorhamnetin-O-hexoside isomer 2 | |
| Isorhamnetin-O-hexoside-O-pentoside | |
| Kaempferol | |
| Kaempferol-3-O-rutinoside (Nicotiflorin) | |
| Kaempferol-O-hexoside | |
| Kaempferol-O-hexoside-di-O-deoxyhexoside | |
| Kaempferol-O-hexoside-O-pentoside-O-deoxyhexoside | |
| Kaempferol-O-pentoside | |
| Luteolin | |
| MyricetiFlavonoids (Continue)n | |
| Flavonoids (Continue) | Myricetin-3′-O-glucoside (Cannabiscitrin) |
| Myricetin-3-O-rutinoside | |
| Myricetin-O-(malonyl)glucoside | |
| Myricetin-O-arabinoside | |
| Myricetin-O-xyloside | |
| Naringenin | |
| Naringenin-6,8-di-C-glucoside | |
| Pentahydroxyflavanone | |
| Pentahydroxyflavanone isomer 1 | |
| Pentahydroxyflavanone isomer 2 | |
| Pentahydroxy-methoxyflavon-O-hexoside isomer 1 | |
| Pentahydroxy-methoxyflavon-O-hexoside isomer 2 | |
| Pentahydroxy-methoxyflavon-O-rutinoside | |
| Prodelphinidin B | |
| Prodelphinidin C isomer 1 | |
| Prodelphinidin C isomer 2 | |
| Prodelphinidin C isomer 3 | |
| Prunin (Naringenin 7-O-glucoside) | |
| Quercetin | |
| Quercetin-3-O-rutinoside-7-O-glucoside | |
| Quercetin-di-O-hexoside | |
| Quercetin-O-(acetyl)hexoside | |
| Quercetin-O-(coumaroyl)hexoside | |
| Quercetin-O-hexoside-di-O-deoxyhexoside | |
| Quercetin-O-hexoside-O-pentoside-O-deoxyhexoside | |
| Quercetin-O-pentoside isomer 1 | |
| Quercetin-O-pentoside isomer 2 | |
| Rutin (Quercetin-3-O-rutinoside) | |
| Sakuranetin (4′,5-Dihydroxy-7-methoxyflavanone) | |
| Taxifolin (Dihydroquercetin) | |
| Tetrahydroxychalcone (Butein) | |
| Tetrahydroxy-dimethoxyflavone isomer 1 | |
| Tetrahydroxy-dimethoxyflavone isomer 2 | |
| Tetrahydroxy-dimethoxyflavone-O-hexoside isomer 1 | |
| Tetrahydroxy-dimethoxyflavone-O-hexoside isomer 2 | |
| Tetrahydroxy-dimethoxyflavone-O-hexoside-O-deoxyhexoside | |
| Tetrahydroxyflavanone-O-hexoside | |
| Tetrahydroxyflavone-O-(pentosyl)hexoside | |
| Trihydroxy-dimethoxyflavone | |
| Trihydroxy-methoxyflavone | |
| Trihydroxy-trimethoxyflavone-O-hexoside | |
| Trihydroxy-trimethoxyflavone-O-hexoside isomer 1 | |
| Trihydroxy-trimethoxyflavone-O-hexoside isomer 2 | |
| Carboxylic Acids | Caffeic acid |
| cis-Aconitic acid | |
| Dihydroxy-methoxybenzoic acid isomer 1 | |
| Dihydroxy-methoxybenzoic acid isomer 2 | |
| Ferulic acid | |
| Hydroxyhexadecanoic acid (hydroxypalmitic acid) | |
| Jasmonic acid | |
| trans-Aconitic acid | |
| Tuberonic acid | |
| α-Linolenic acid | |
| 5-O-p-Coumaroylnigrumin | |
| Carboxylic Acids (Continue)Caffeoylglucose isomer 1 | |
| Carboxylic Acids (Continue) | Caffeoylglucose isomer 2 |
| Caffeoylglucose isomer 3 | |
| Chlorogenic acid (3-O-Caffeoylquinic acid) | |
| Chryptochlorogenic acid (4-O-Caffeoylquinic acid) | |
| Coumaroyl-glucose isomer 1 | |
| Coumaroyl-glucose isomer 2 | |
| Coumaroyl-glucose isomer 3 | |
| Coumaroylquinic acid isomer 1 | |
| Coumaroylquinic acid isomer 2 | |
| Coumaroylquinic acid isomer 3 | |
| Feruloylquinic acid isomer 1 | |
| Feruloylquinic acid isomer 2 | |
| Feruloylquinic acid isomer 3 | |
| Galloylglucose isomer 1 | |
| Galloylglucose isomer 2 | |
| Neochlorogenic acid (5-O-Caffeoylquinic acid) | |
| Phloretin | |
| Phlorizin (Phloridzin) | |
| Tetrahydroxy-methoxy chalcone | |
| Tuberonic acid glucoside | |
| Terpenes | Abscisic acid (ABA) |
| Geranylgeraniol | |
| Vitamins | Adenine (B4) |
| Nicotinic acid (Niacin, B3) | |
| Pyridoxine (B6) | |
| Riboflavin (B2) |
| BC-GTE Flavonoids Content | BC-GTE Antioxidant Capacity | |||||||
|---|---|---|---|---|---|---|---|---|
| Total Flavonoids [mg/mL] | Apigenin [mg/mL] | Kaempferol [µg/mL] | Luteolin [mg/mL] | Quercetin [mg/mL] | FRAP 1 | CUPRAC 1 | Superoxid Radical Inhibition 2 | Xanthin-Oxidase Inhibition 3 |
| 0.82 ± 0.013 | 0.152 ± 0.002 | 3.9 ± 0.04 | 0.34 ± 0.003 | 0.26 ± 0.003 | 672 ± 7.1 | 4565 ± 48.8 | 4512 ± 49.6 | 41 ± 0.4 |
| 18.5% | 41.5% | 31.7% | ||||||
| Standard | Concentration 1 | Calibration Curve Equation 2 | Correlation Factor | Detection Limit 1 | Quantification Limit 1 |
|---|---|---|---|---|---|
| Apigenin | 50–360 | 42,007 × c[µg/mL] – 218,952 | 0.9990 | 15.6 | 26.1 |
| Kaempferol | 40–300 | 38,007 × c[µg/mL] + 772,543 | 0.9799 | 40.6 | 81.3 |
| Luteolin | 40–300 | 32,470 × c[µg/mL] + 441,347 | 0.9945 | 27.2 | 54.4 |
| Quercetin | 50–400 | 10,272 × c[µg/mL] + 17,725 | 0.9926 | 3.5 | 6.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Téglás, T.; Mihok, E.; Cziáky, Z.; Oláh, N.-K.; Nyakas, C.; Máthé, E. The Flavonoid Rich Black Currant (Ribes nigrum) Ethanolic Gemmotherapy Extract Elicits Neuroprotective Effect by Preventing Microglial Body Swelling in Hippocampus and Reduces Serum TNF-α Level: Pilot Study. Molecules 2023, 28, 3571. https://doi.org/10.3390/molecules28083571
Téglás T, Mihok E, Cziáky Z, Oláh N-K, Nyakas C, Máthé E. The Flavonoid Rich Black Currant (Ribes nigrum) Ethanolic Gemmotherapy Extract Elicits Neuroprotective Effect by Preventing Microglial Body Swelling in Hippocampus and Reduces Serum TNF-α Level: Pilot Study. Molecules. 2023; 28(8):3571. https://doi.org/10.3390/molecules28083571
Chicago/Turabian StyleTéglás, Tímea, Emőke Mihok, Zoltán Cziáky, Neli-Kinga Oláh, Csaba Nyakas, and Endre Máthé. 2023. "The Flavonoid Rich Black Currant (Ribes nigrum) Ethanolic Gemmotherapy Extract Elicits Neuroprotective Effect by Preventing Microglial Body Swelling in Hippocampus and Reduces Serum TNF-α Level: Pilot Study" Molecules 28, no. 8: 3571. https://doi.org/10.3390/molecules28083571
APA StyleTéglás, T., Mihok, E., Cziáky, Z., Oláh, N.-K., Nyakas, C., & Máthé, E. (2023). The Flavonoid Rich Black Currant (Ribes nigrum) Ethanolic Gemmotherapy Extract Elicits Neuroprotective Effect by Preventing Microglial Body Swelling in Hippocampus and Reduces Serum TNF-α Level: Pilot Study. Molecules, 28(8), 3571. https://doi.org/10.3390/molecules28083571