Submicron Nonporous Silica Particles for Enhanced Separation Performance in pCEC
Abstract
1. Introduction
2. Results and Discussion
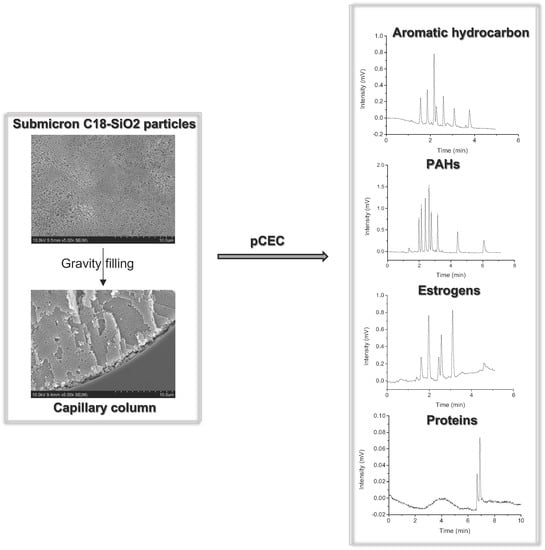
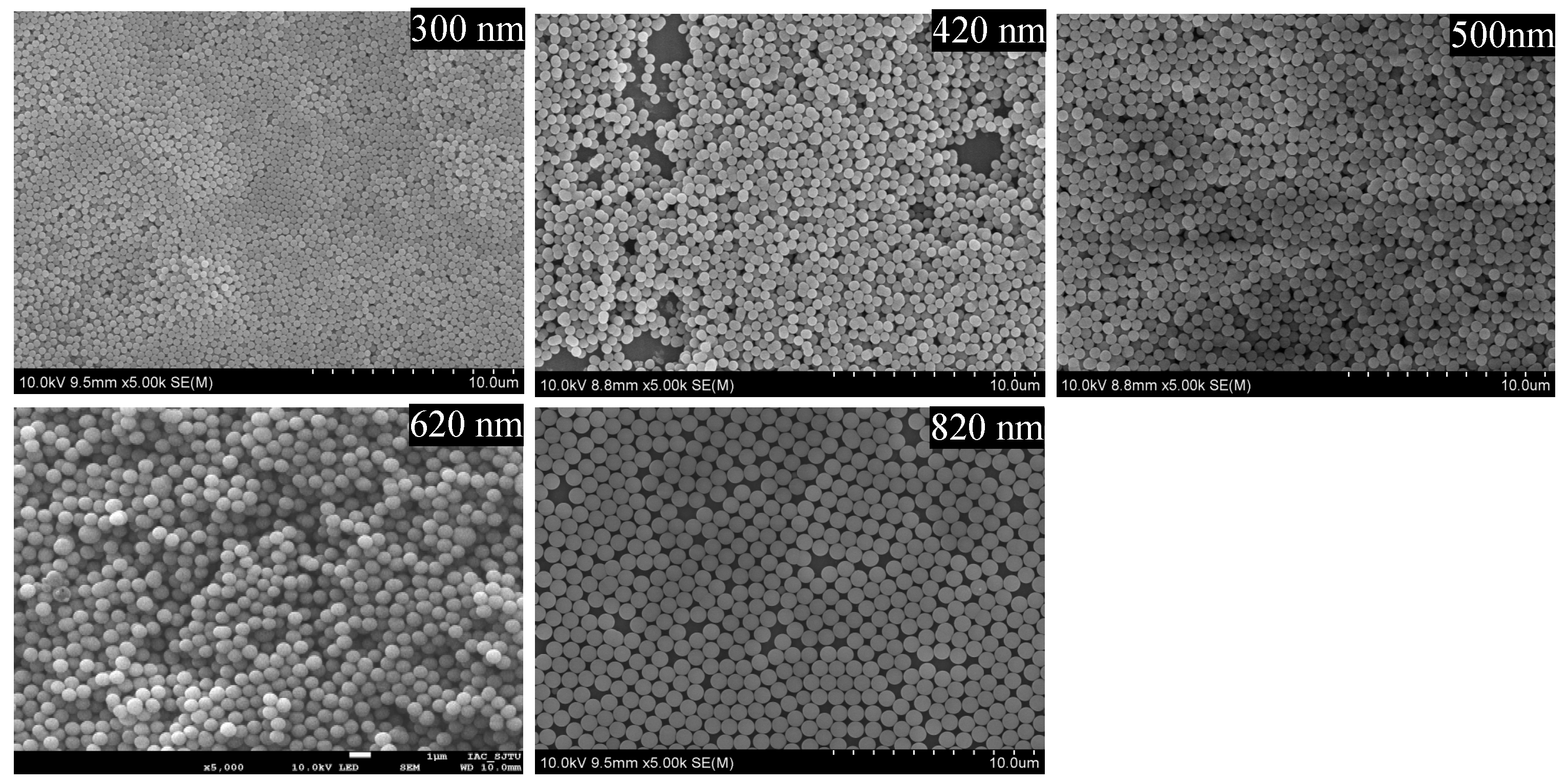
2.1. Characterization
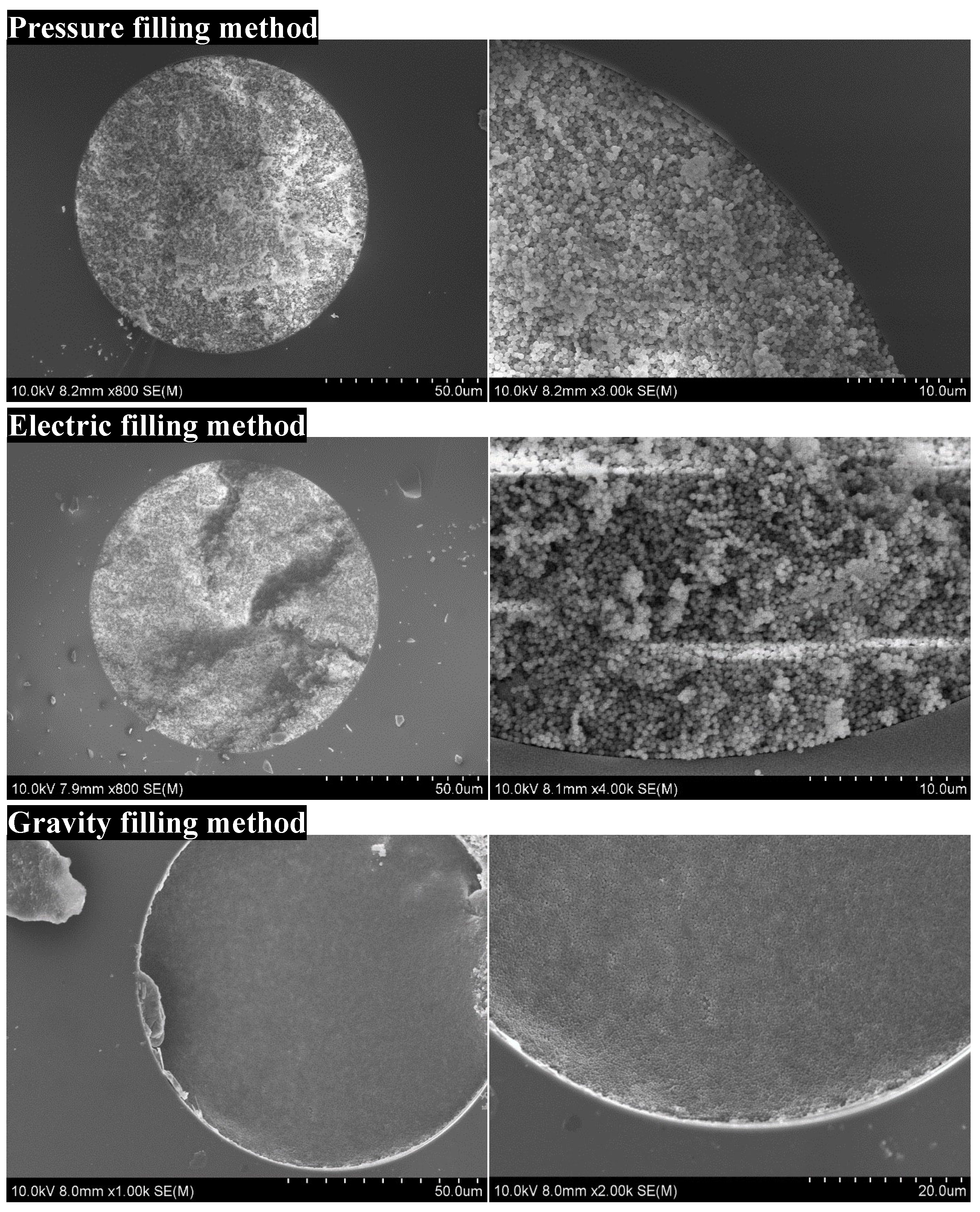
2.2. Optimization of the Preparational Method for a Packed Column
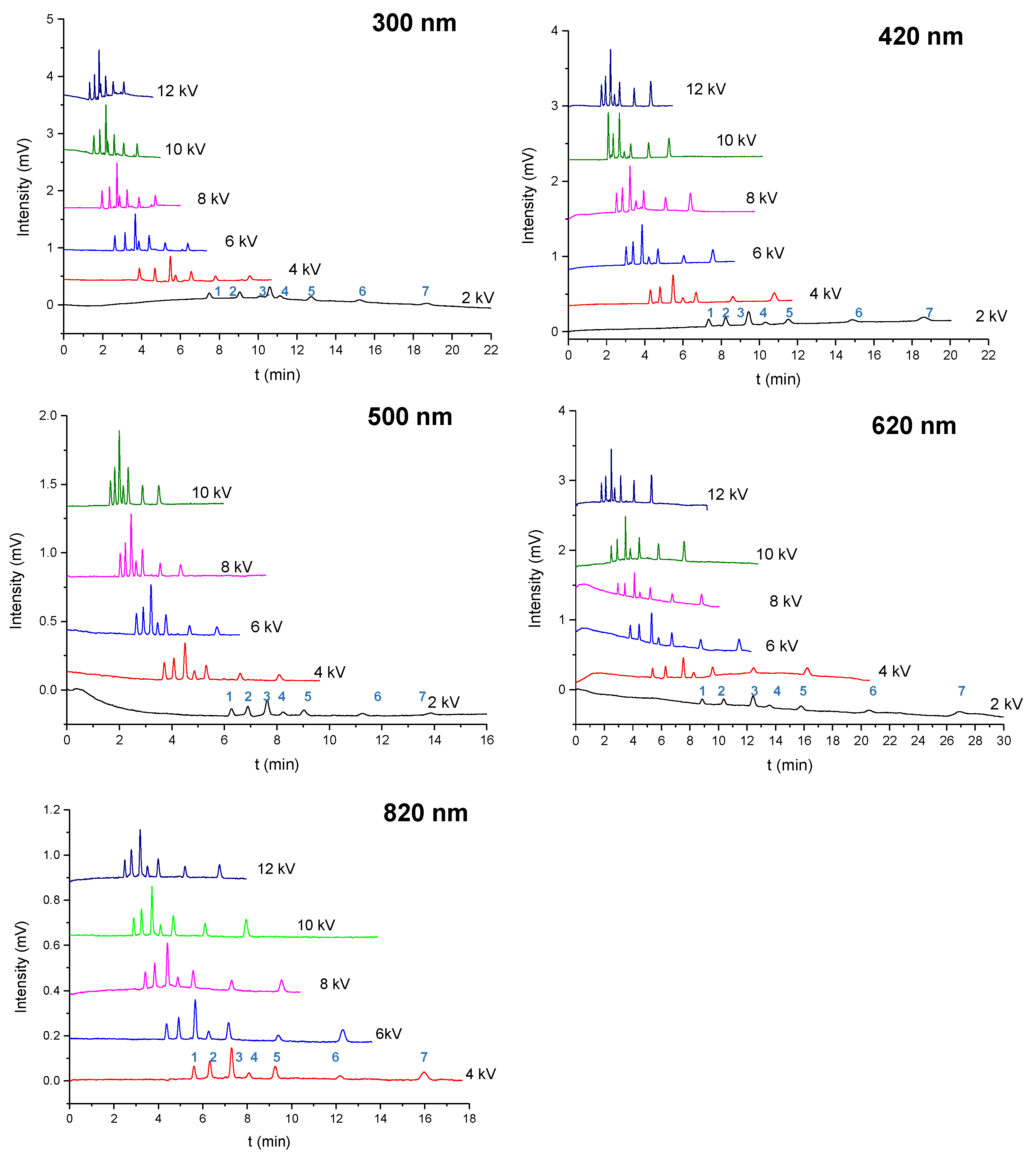
2.3. Separation of the Aromatic Compounds
2.4. Separation of PAHs
2.5. Separation of Estrogens
2.6. Analysis of Proteins
3. Materials and Methods
3.1. Instruments
3.2. Reagents
3.3. Materials
3.4. Synthesis of the Monodispersed C18-SiO2 Spheres
3.5. Preparation of the Capillaries Packed with Submicron Silica Particles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical-Physical Applications to Nanomedicine. Molecules 2019, 25, 112. [Google Scholar] [CrossRef] [PubMed]
- Gama, M.R.; Collins, C.H.; Bottoli, C.B. Nano-liquid chromatography in pharmaceutical and biomedical research. J. Chromatogr. Sci. 2013, 51, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Kuger, L.; Arlt, C.R.; Franzreb, M. Magnetic/flow controlled continuous size fractionation of magnetic nanoparticles using simulated moving bed chromatography. Talanta 2022, 240, 123160. [Google Scholar] [CrossRef] [PubMed]
- Kannan, K.; Mukherjee, J.; Mishra, P.; Gupta, M.N. Nickel Ferrite Nanoparticles as an Adsorbent for Immobilized Metal Affinity Chromatography of Proteins. J. Chromatogr. Sci. 2021, 59, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, M.A.; Luyet, C.; Kumar, P.; Elvati, P.; VanEpps, J.S.; Violi, A.; Kotov, N.A. Chiral chromatography and surface chirality of carbon nanoparticles. Chirality 2022, 34, 1494–1502. [Google Scholar] [CrossRef]
- Liu, Q.; Yan, K.; Chen, Q.; Cheddah, S.; Shen, L.; Xiao, H.; Wang, Y.; Yan, C. Preparation of silica colloidal crystal column and its application in pressurized capillary electrochromatography. J. Chromatogr. A 2019, 1587, 172–179. [Google Scholar] [CrossRef]
- Billen, J.; Desmet, G. Understanding and design of existing and future chromatographic support formats. J. Chromatogr. A 2007, 1168, 73–99; discussion 71–72. [Google Scholar] [CrossRef]
- Wei, B.; Rogers, B.J.; Wirth, M.J. Slip Flow in Colloidal Crystals for Ultraefficient Chromatography. J. Am. Chem. Soc. 2012, 134, 10780–10782. [Google Scholar] [CrossRef]
- Rogers, B.J.; Wirth, M.J. Slip Flow through Colloidal Crystals of Varying Particle Diameter. ACS Nano 2013, 7, 725–731. [Google Scholar] [CrossRef]
- Wu, Z.; Rogers, B.J.; Wei, B.; Wirth, M.J. Insights from theory and experiments on slip flow in chromatography. J. Sep. Sci. 2013, 36, 1871–1876. [Google Scholar] [CrossRef]
- Malkin, D.S.; Wei, B.; Fogiel, A.J.; Staats, S.L.; Wirth, M.J. Submicrometer plate heights for capillaries packed with silica colloidal crystals. Anal. Chem. 2010, 82, 2175–2177. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.; Guo, Z.; Li, J.; Liu, M.; Chen, Y. One-step packing of anti-voltage photonic crystals into microfluidic channels for ultra-fast separation of amino acids and peptides. Lab. Chip 2013, 13, 706–713. [Google Scholar] [CrossRef]
- Chen, Y.; Liao, T.; Hu, C. Fast assembly of anti-voltage photonic crystals in microfluidic channels for ultrafast separation of amino acids and peptides. Methods Mol. Biol. 2015, 1274, 119–135. [Google Scholar]
- Wei, B.; Malkin, D.S.; Wirth, M.J. Plate heights below 50 nm for protein electrochromatography using silica colloidal crystals. Anal. Chem. 2010, 82, 10216–10221. [Google Scholar] [CrossRef]
- Zhang, H.; Wirth, M.J. Electromigration of single molecules of DNA in a crystalline array of 300-nm silica colloids. Anal. Chem. 2005, 77, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wei, B.; Zhang, X.; Wirth, M.J. Efficient separations of intact proteins using slip-flow with nano-liquid chromatography-mass spectrometry. Anal. Chem. 2014, 86, 1592–1598. [Google Scholar] [CrossRef]
- Anspach, J.A.; Maloney, T.D.; Brice, R.W.; Colón, L.A. Injection valve for ultrahigh-pressure liquid chromatography. Anal. Chem. 2005, 77, 7489–7494. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, W.; Jia, M.; Liu, R.; Liu, Q.; Xiao, H.; Li, J.; Xue, Y.; Wang, Y.; Yan, C. Recent advances in microscale separation. Electrophoresis 2018, 39, 8–33. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Gu, X.; Chen, Y.; Yan, C. Preparation of hydrophilic interaction monolithic column and its application in the analysis of melamine in dairy products using pressurized capillary electrochromatography. Se Pu 2010, 28, 231–235. [Google Scholar] [CrossRef]
- Yan, C.; Schaufelberger, D.; Erni, F. Electrochromatography and micro high-performance liquid chromatography with 320 μm I.D. packed columns. J. Chromatogr. A 1994, 670, 15–23. [Google Scholar] [CrossRef]
- Perchepied, S.; Ritchie, H.; Desmet, G.; Eeltink, S. Insights in column packing processes of narrow bore and capillary-scale columns: Methodologies, driving forces, and separation performance—A tutorial review. Anal. Chim. Acta 2022, 1235, 340563. [Google Scholar] [CrossRef]
- Yan, C.; Dadoo, R.; Zhao, H.; Zare, R.N.; Rakestraw, D.J. Capillary Electrochromatography: Analysis of Polycyclic Aromatic Hydrocarbons. Anal. Chem. 1995, 67, 2026–2029. [Google Scholar] [CrossRef]
- Dadoo, R.; Yan, C.; Zare, R.N.; Anex, D.S.; Rakestraw, D.J.; Hux, G.A. Advances toward the routine use of capillary electrochromatography. LC GC Liq. Chromatogr. Gas Chromatogr. 1997, 15, 630–635. [Google Scholar]
- Galukhin, A.; Bolmatenkov, D.; Emelianova, A.; Zharov, I.; Gor, G.Y. Porous Structure of Silica Colloidal Crystals. Langmuir 2019, 35, 2230–2235. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Ye, Y.H.; Zhou, Z.; Xu, H. Gravity-assisted convective assembly of centimeter-sized uniform two-dimensional colloidal crystals. Langmuir 2013, 29, 1796–1801. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Huang, S.; Huang, S.; Lin, J.; Huang, B.; Kuo, C. Improvement of the Centrifugal Force in Gravity Driven Method for the Fabrication of Highly Ordered and Submillimeter-Thick Colloidal Crystal. Polymers 2021, 13, 692. [Google Scholar] [CrossRef]
- Qu, Q.; Lu, X.; Huang, X.; Hu, X.; Zhang, Y.; Yan, C. Preparation and evaluation of C18-bonded 1-microm silica particles for pressurized capillary electrochromatography. Electrophoresis 2006, 27, 3981–3987. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Yan, C.; Wang, Y. Submicron Nonporous Silica Particles for Enhanced Separation Performance in pCEC. Molecules 2023, 28, 3542. https://doi.org/10.3390/molecules28083542
Liu Q, Yan C, Wang Y. Submicron Nonporous Silica Particles for Enhanced Separation Performance in pCEC. Molecules. 2023; 28(8):3542. https://doi.org/10.3390/molecules28083542
Chicago/Turabian StyleLiu, Qing, Chao Yan, and Yan Wang. 2023. "Submicron Nonporous Silica Particles for Enhanced Separation Performance in pCEC" Molecules 28, no. 8: 3542. https://doi.org/10.3390/molecules28083542
APA StyleLiu, Q., Yan, C., & Wang, Y. (2023). Submicron Nonporous Silica Particles for Enhanced Separation Performance in pCEC. Molecules, 28(8), 3542. https://doi.org/10.3390/molecules28083542