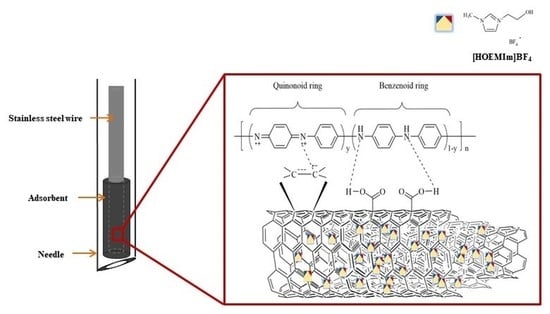
Synthesis and Characterization of a Multi-Walled Carbon Nanotube–Ionic Liquid/Polyaniline Adsorbent for a Solvent-Free In-Needle Microextraction Method
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of the HS–INME–MWCNT–IL/PANI Coating Layer
2.1.1. Effect of Synthesis Conditions on Extraction
2.1.2. Effect of the Extraction Conditions of the HS–INME Method on Extraction
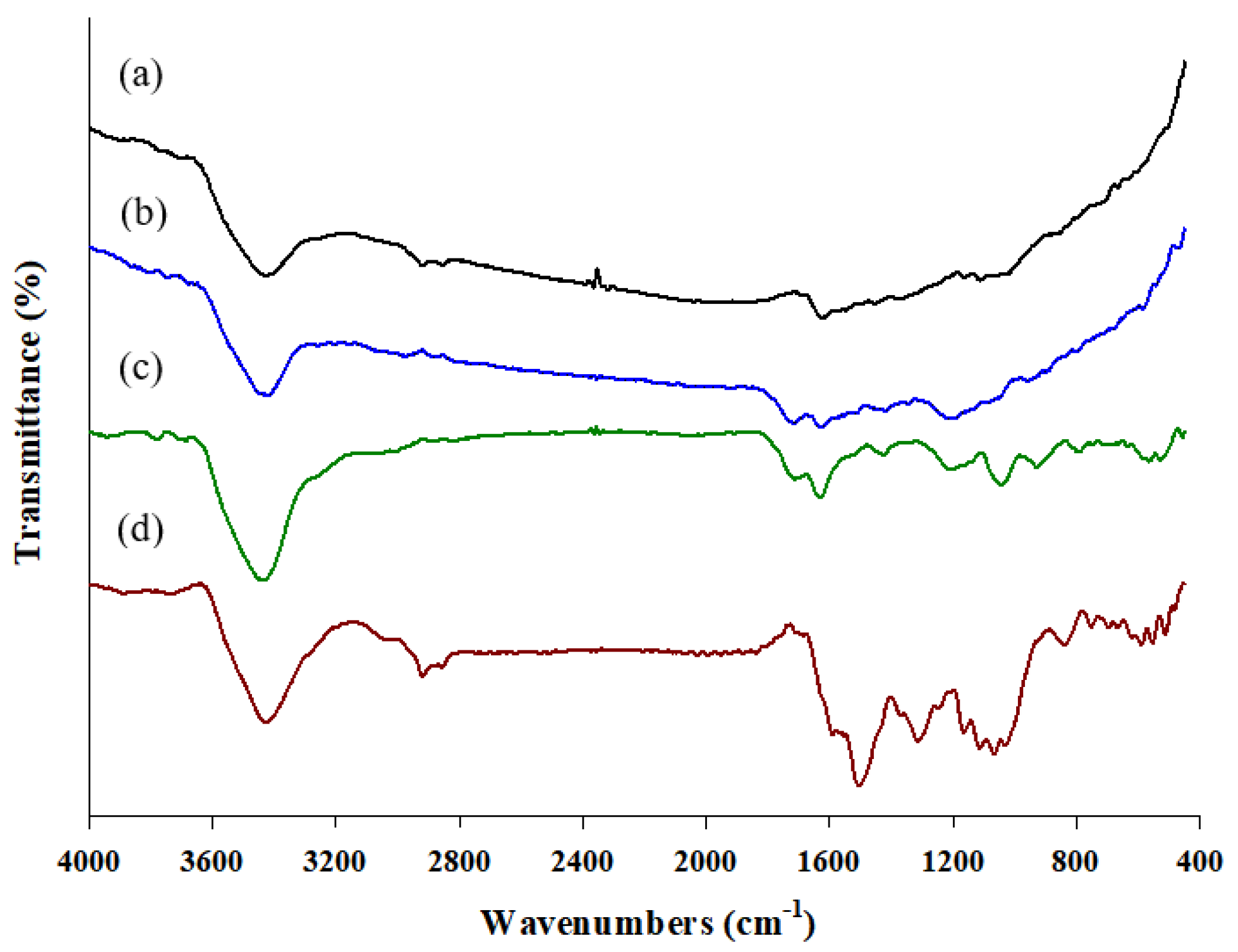
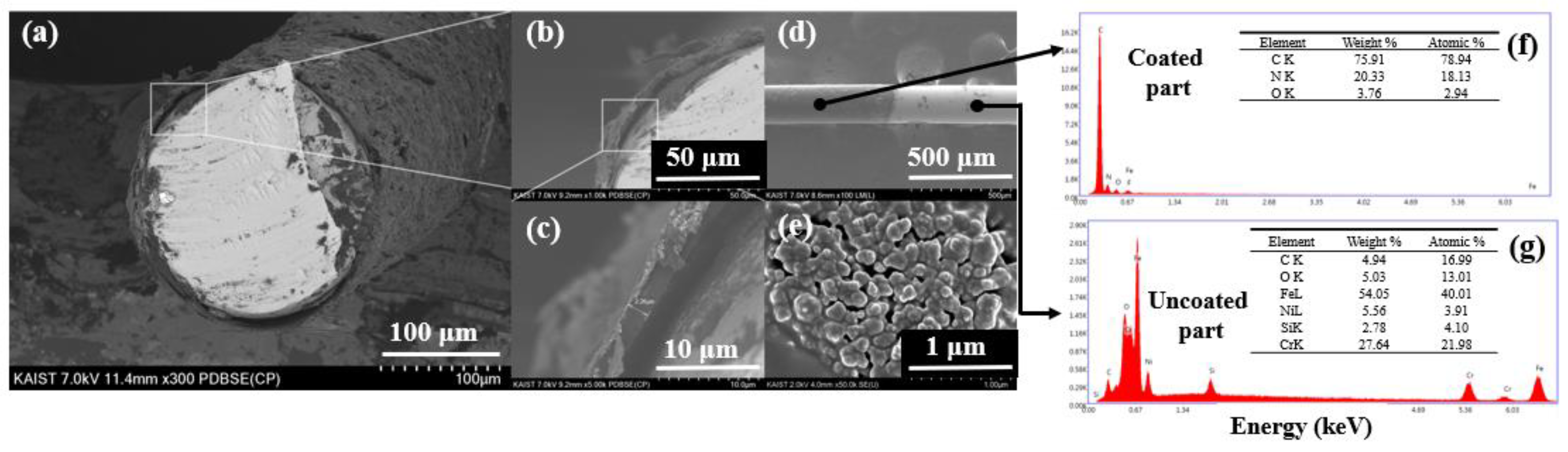
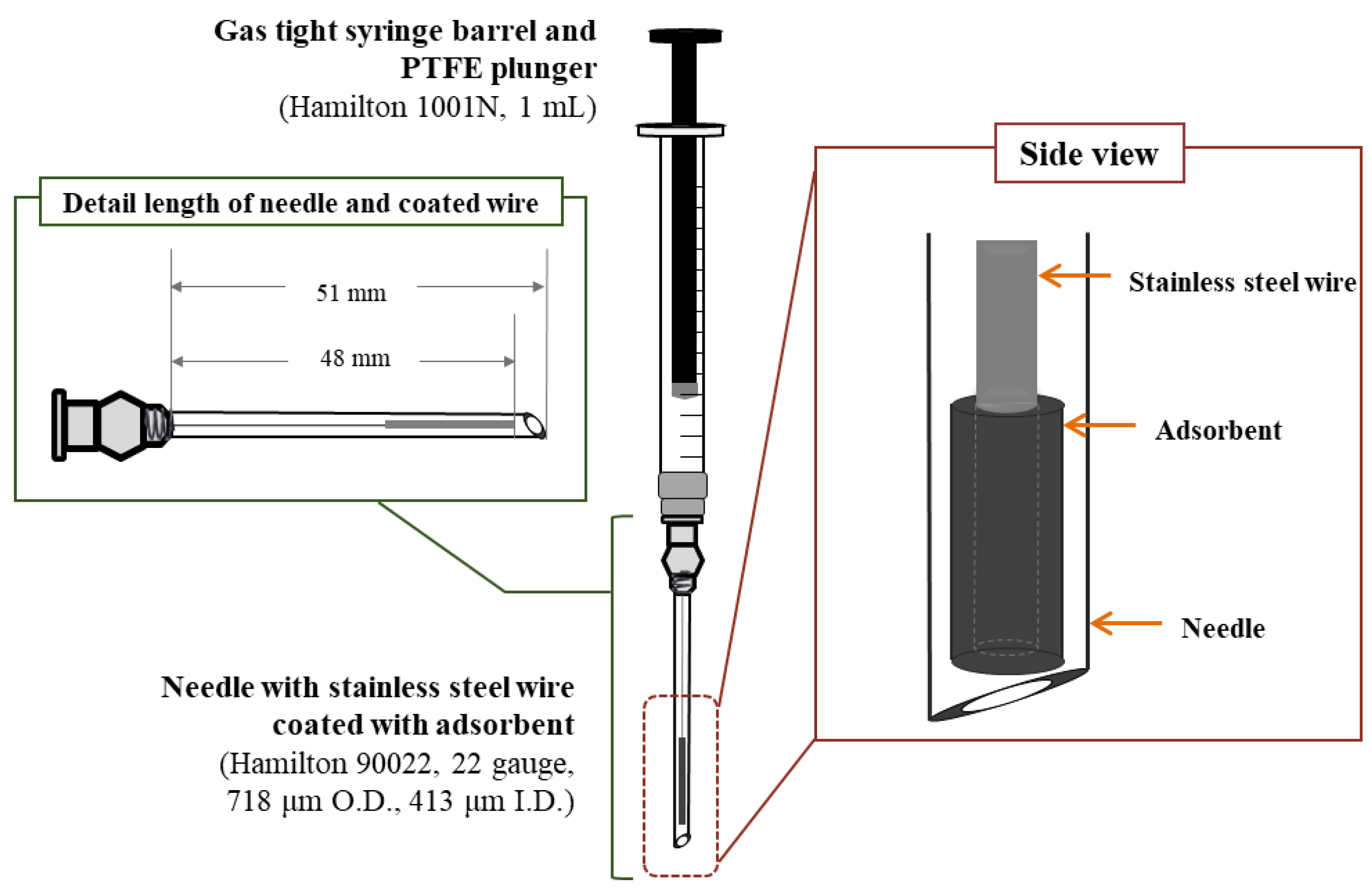
2.2. Synthesis and Characteristics of the MWCNT–IL/PANI Layer on a Wire
2.3. Validation of the Analytical Method
2.4. Comparison of Extraction Efficiency
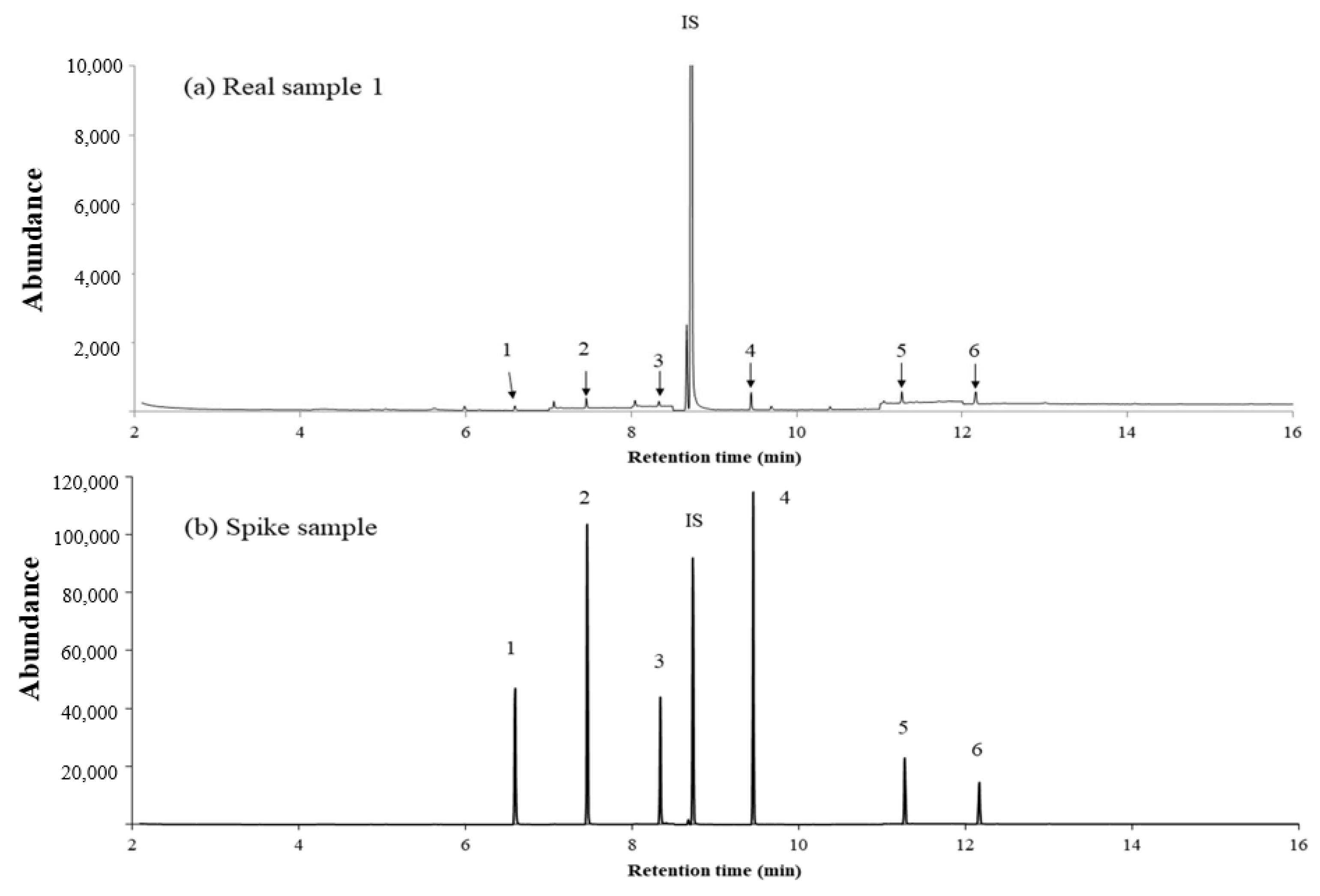
2.5. Application of HS–INME–MWCNT–IL/PANI to an Aqueous Sample
3. Materials and Methods
3.1. Reagents and Materials
3.2. Preparation of the MWCNT-IL/PANI Layer Coated on a Wire
3.3. Characterization of the MWCNT–IL/PANI Coating Layer
3.4. Headspace In–Needle Microextraction Procedure
3.5. Optimization of HS–INME–MWCNT–IL/PANI
3.6. Validation of HS–INME–MWCNT–IL/PANI
3.7. Gas Chromatography/Mass Spectrometry (GC/MS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Aly, A.A.; Górecki, T. Green Approaches to Sample Preparation Based on Extraction Techniques. Molecules 2020, 25, 1719. [Google Scholar] [CrossRef] [PubMed]
- Câmara, J.S.; Perestrelo, R.; Berenguer, C.V.; Andrade, C.F.P.; Gomes, T.M.; Olayanju, B.; Kabir, A.; MR Rocha, C.; Teixeira, J.A.; Pereira, J.A.M. Green Extraction Techniques as Advanced Sample Preparation Approaches in Biological, Food, and Environmental Matrices: A Review. Molecules 2022, 27, 2953. [Google Scholar] [CrossRef] [PubMed]
- Picó, Y. Comprehensive Sampling and Sample Preparation; Pawliszyn, J., Ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 3, Chapter 3; p. 569. [Google Scholar]
- Jon, C.-S.; Meng, L.-Y.; Li, D. Recent review on carbon nanomaterials functionalized with ionic liquids in sample pretreatment application. TrAC Trends Anal. Chem. 2019, 120, 115641. [Google Scholar] [CrossRef]
- Prokůpková, G.; Holadová, K.; Poustka, J.; Hajšlová, J. Development of a solid-phase microextraction method for the determination of phthalic acid esters in water. Anal. Chim. Acta 2002, 457, 211–223. [Google Scholar] [CrossRef]
- Serôdio, P.; Nogueira, J. Considerations on ultra-trace analysis of phthalates in drinking water. Water Res. 2006, 40, 2572–2582. [Google Scholar] [CrossRef]
- Jobling, S.; Reynolds, T.; White, R.; Parker, M.G.; Sumpter, J.P. A variety of environmentally persistent chemicals, including some phthalate plasticizers, are weakly estrogenic. Environ. Health Perspect. 1995, 103, 582–587. [Google Scholar] [CrossRef]
- Castillo, M.; Oubina, A.; Barceló, D. Evaluation of ELISA kits followed by liquid chromatography-atmospheric pressure chemical ionization-mass spectrometry for the determination of organic pollutants in industrial effluents. Environ. Sci. Technol. 1998, 32, 2180–2184. [Google Scholar] [CrossRef]
- Potter, D.W.; Pawliszyn, J. Rapid determination of polyaromatic hydrocarbons and polychlorinated biphenyls in water using solid-phase microextraction and GC/MS. Environ. Sci. Technol. 1994, 28, 298–305. [Google Scholar] [CrossRef]
- Colon, I.; Dimandja, J.D. High-throughput analysis of phthalate esters in human serum by direct immersion SPME followed by isotope dilution–fast GC/MS. Anal. Bioanal. Chem. 2004, 380, 275–283. [Google Scholar] [CrossRef]
- Lee, S.Y.; Yoon, J.H.; Bae, S.; Lee, D.S. In-needle microextraction coupled with gas chromatography/mass spectrometry for the analysis of phthalates generating from food containers. Food Anal. Methods 2018, 11, 2767–2777. [Google Scholar] [CrossRef]
- Jeon, H.L.; Son, H.H.; Bae, S.; Lee, D.S. Use of polyacrylic acid and polydimethylsiloxane mixture for in-needle microextraction of volatile aroma compounds in essential oils. Bull. Korean Chem. Soc. 2015, 36, 2730–2739. [Google Scholar] [CrossRef]
- Lee, E.J.; Lee, D.S. Fabrication of in-needle microextraction device using nichrome wire coated with poly(ethylene glycol) and poly(dimethylsiloxane) for determination of volatile compounds in lavender oils. Bull. Korean Chem. Soc. 2014, 35, 211–217. [Google Scholar] [CrossRef]
- Bang, Y.J.; Hwang, Y.R.; Lee, S.Y.; Park, S.M.; Bae, S. Sol–gel-adsorbent-coated extraction needles to detect volatile compounds in spoiled fish. J. Sep. Sci. 2017, 40, 3839–3847. [Google Scholar] [CrossRef]
- Hwang, Y.; Lee, Y.; Ahn, S.; Bae, S. Electrochemically polyaniline-coated microextraction needle for phthalates in water. Anal. Sci. Technol. 2020, 33, 76–85. [Google Scholar]
- Kim, S.; Bae, S.; Lee, D.-S. Characterization of scents from Juniperus chinensis by headspace in-needle microextraction using graphene oxide-polyaniline nanocomposite coated wire followed by gas chromatography-mass spectrometry. Talanta 2022, 245, 123463. [Google Scholar] [CrossRef]
- Kim, S.; Bae, S. In vitro and in vivo human body odor analysis method using GO:PANI/ZNRs/ZIF-8 adsorbent followed by GC/MS. Molecules 2022, 27, 4795. [Google Scholar] [CrossRef]
- Motitswe, M.G.; Badmus, K.O.; Khotseng, L. Development of Adsorptive Materials for Selective Removal of Toxic Metals in Wastewater: A Review. Catalysts 2022, 12, 1057. [Google Scholar] [CrossRef]
- Qu, L.; Dai, L. Substrate-enhanced electroless deposition of metal nanoparticles on carbon nanotubes. J. Am. Chem. Soc. 2005, 127, 10806–10807. [Google Scholar] [CrossRef]
- Randriamahazaka, H.; Ghilane, J. Electrografting and controlled surface functionalization of carbon based surfaces for electroanalysis. Electroanalysis 2016, 28, 13–26. [Google Scholar] [CrossRef]
- Dobrowolski, R.; Mróz, A.; Dąbrowska, M.; Olszański, P. Solid sampling high-resolution continuum source graphite furnace atomic absorption spectrometry for gold determination in geological samples after preconcentration onto carbon nanotubes. Spectrochim. Acta Part B At. Spectrosc. 2017, 132, 13–18. [Google Scholar] [CrossRef]
- Guan, Z.; Huang, Y.; Wang, W. Carboxyl modified multi-walled carbon nanotubes as solid-phase extraction adsorbents combined with high-performance liquid chromatography for analysis of linear alkylbenzene sulfonates. Anal. Chim. Acta 2008, 627, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Zhao, F.; Zeng, B. Novel multiwalled carbon nanotubes-polyaniline composite film coated platinum wire for headspace solid-phase microextraction and gas chromatographic determination of phenolic compounds. J. Chromatogr. A 2009, 1216, 3751–3757. [Google Scholar] [CrossRef] [PubMed]
- Stege, P.W.; Lapierre, A.V.; Martinez, L.D.; Messina, G.A.; Sombra, L.L. A combination of single-drop microextraction and open tubular capillary electrochromatography with carbon nanotubes as stationary phase for the determination of low concentration of illicit drugs in horse urine. Talanta 2011, 30, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Jun, L.Y.; Mubarak, N.M.; Yee, M.J.; Yon, L.S.; Bing, C.H.; Khalid, M.; Abdullah, E.C. An Overview of functionalised carbon nanomaterial for organic pollutant removal. J. Ind. Eng. Chem. 2018, 67, 175–186. [Google Scholar] [CrossRef]
- Tunckol, M.; Durand, J.; Serp, P. Carbon nanomaterial-ionic liquid hybrids. Carbon 2012, 50, 4303–4334. [Google Scholar] [CrossRef]
- Bellayer, S.; Gilman, J.W.; Eidelman, N.; Bourbigot, S.; Flambard, X.; Fox, D.M.; De Long, H.C.; Trulove, P.C. Preparation of homogeneously dispersed multiwalled carbon nanotube/polystyrene nanocomposites via melt extrusion using trialkyl imidazolium compatibilizer. Adv. Funct. Mater. 2005, 5, 910–916. [Google Scholar] [CrossRef]
- Shim, Y.; Kim, H.J. Solvation of carbon nanotubes in a room-temperature ionic liquid. ACS Nano 2009, 3, 1693–1702. [Google Scholar] [CrossRef]
- Li, L.; Wu, M.; Feng, Y.; Zhao, F.; Zeng, B. Doping of three-dimensional porous carbon nanotube-graphene-ionic liquid composite into polyaniline for the headspace solid-phase microextraction and gas chromatography determination of alcohols. Anal. Chim. Acta 2016, 948, 48–54. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, H.; Zhao, F.; Zeng, B. A novel poly(3,4-ethylenedioxythiophene)-ionic liquid composite coating for the headspace solid-phase microextraction and gas chromatography determination of several alcohols in soft drinks. Anal. Chim. Acta 2014, 850, 41–48. [Google Scholar] [CrossRef]
- Fukushima, T.; Kosaka, A.; Ishimura, Y.; Yamamoto, T.; Takigawa, T.; Ishii, N.; Aida, T. Molecular ordering of organic molten salts triggered by single-walled carbon nanotubes. Science 2003, 3000, 2072–2074. [Google Scholar] [CrossRef]
- Pawliszyn, J. Theory of solid-phase microextraction. J. Chromatogr. Sci. 2000, 38, 270–278. [Google Scholar] [CrossRef]
- Ping, Z. In situ FT-IR-attenuated total reflection spectroscopic investigations on the base-acid transitions of polyaniline. base-acid transition in the emeraldine form of polyaniline. J. Chem. Soc. Faraday Trans. 1996, 92, 3063–3067. [Google Scholar] [CrossRef]
- Furukawa, Y.; Ueda, F.; Hyodo, Y.; Harada, I.; Nakajima, T.; Kawagoe, T. Vibrational spectra and structure of polyaniline. Macromolecules 1988, 21, 1297–1305. [Google Scholar] [CrossRef]
- Trchová, M.; Stejskal, J. Polyaniline: The infrared spectroscopy of conducting polymer nanotubes (IUPAC technical report). Pure Appl. Chem. 2011, 83, 1803–1817. [Google Scholar] [CrossRef]
- Boyer, M.I.; Quillard, S.; Rebourt, E.; Louarn, G.; Buisson, J.P.; Monkman, A.; Lefrant, S. Vibrational Analysis of Polyaniline: A model compound approach. J. Phys. Chem. B 1998, 102, 7382–7392. [Google Scholar] [CrossRef]
- Zheng, W.; Angelopoulos, M.; Epstein, A.J.; MacDiarmid, A.G. Experimental evidence for hydrogen bonding in polyaniline: Mechanism of aggregate formation and dependency on oxidation state. Macromolecules 1997, 30, 2953–2955. [Google Scholar] [CrossRef]
- Tammer, M.G. Sokrates: Infrared and Raman characteristic group frequencies: Tables and charts. Colloid Polym. Sci. 2004, 283, 235. [Google Scholar] [CrossRef]
- Dhand, C.; Arya, S.K.; Singh, S.P.; Singh, B.P.; Datta, M.; Malhotra, B. Preparation of polyaniline/multiwalled carbon nanotube composite by novel electrophoretic route. Carbon 2008, 46, 1727–1735. [Google Scholar] [CrossRef]
- Ngo, C.L.; Le, Q.T.; Ngo, T.T.; Nguyen, D.N.; Vu, M.T. Surface modification and functionalization of carbon nanotube with some organic compounds. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 035017. [Google Scholar]
- Kumar, B.R.; Rao, T.S. AFM Studies on Surface Morphology, Topography and Texture of Nanostructured Zinc Aluminum Oxide Thin Films. Dig. J. Nanomater. Biostruct. 2012, 7, 1881–1889. [Google Scholar]
- Thompson, M.; Ellison, S.L.R.; Wood, R. Harmonized guidelines for single-laboratory (IUPAC technical report). Pure Appl. Chem. 2002, 74, 835–855. [Google Scholar] [CrossRef]
- Currie, L.A. Nomenclature in evaluation of analytical methods including detection and quantification capabilities. Pure Appl. Chem. 1995, 67, 1699–1723. [Google Scholar] [CrossRef]
- Fasano, E.; Bono-Blay, F.; Cirillo, T.; Montuori, P.; Lacorte, S. Migration of phthalates, alkylphenols, bisphenol A and di(2-ethylhexyl)adipate from food packaging. Food Control 2012, 27, 132–138. [Google Scholar] [CrossRef]
- Asadollahzadeh, H.; Noroozian, E.; Maghsoudi, S. Solid-phase microextraction of phthalate esters from aqueous media by electrochemically deposited carbon nanotube/polypyrrole composite on a stainless steel fiber. Anal. Chim. Acta 2010, 669, 32–38. [Google Scholar] [CrossRef]
- Sun, Y.; Fang, Z.; Wang, C.; Zhou, A.; Duan, H. Incorporating nanoporous polyaniline into layer-by-layer ionic liquid-carbon nanotube-graphene paper: Towards freestanding flexible electrodes with improved supercapacitive performance. Nanotechnology 2015, 26, 374002. [Google Scholar] [CrossRef]
- El Rhazi, M.; Majid, S.; Elbasri, M.; Salih, F.E.; Oularbi, L.; Lafdi, K. Recent progress in nanocomposites based on conducting polymer: Application as electrochemical sensors. Int. Nano Lett. 2018, 8, 79–99. [Google Scholar] [CrossRef]
- Chen, G.Z.; Shaffer, M.S.P.; Coleby, D.; Dixon, G.; Zhou, W.; Fray, D.J.; Windle, A.H. Carbon nanotube and polypyrrole composites: Coating and doping. Adv. Mater. 2000, 12, 522–526. [Google Scholar] [CrossRef]
- Son, H.H.; Bae, S.; Lee, D.S. New needle packed with polydimethylsiloxane having a micro-bore tunnel for headspace in-needle microextraction of aroma components of citrus oils. Anal. Chim. Acta 2012, 751, 86–93. [Google Scholar] [CrossRef]
- Qian, S.; Ji, H.; Wu, X.X.; Li, N.; Yang, Y.; Bu, J.; Zhang, X.; Qiao, L.; Yu, H.; Xu, N.; et al. Detection and quantification analysis of chemical migrants in plastic food contact products. PLoS ONE 2018, 13, e0208467. [Google Scholar] [CrossRef]
| Compound | Regression Equation | Coefficient of Determination (r2) | LOD (μg) | LOQ (μg) | Dynamic Range (μg) |
|---|---|---|---|---|---|
| Dimethyl phthalate | y = 2.5326x + 0.0233 | 0.9907 | 19.57 | 65.23 | 65.23~2.50 × 102 |
| Diethyl phthalate | y = 6.5383x + 0.0390 | 0.9967 | 15.84 | 52.79 | 52.79~1.00 × 103 |
| Diallyl phthalate | y = 2.1476x + 0.0653 | 0.9959 | 16.17 | 53.91 | 53.91~5.00 × 102 |
| Dibutyl phthalate | y = 5.1304x + 0.1313 | 0.9734 | 18.9 | 63.01 | 63.01~5.00 × 102 |
| Benzyl butyl phthalate | y = 2.6991x + 0.0557 | 0.9931 | 42.03 | 140.1 | 140.1~1.00 × 103 |
| Di(2-ethylhexyl) phthalate | y = 2.6567x + 0.0994 | 0.9918 | 50.56 | 168.5 | 168.5~1.00 × 103 |
| MWCNT–IL/PANI Layer Parameter | Conditions |
|---|---|
| Percentage of MWCNT-ionic liquid (%, w/v) | 5, 10, 15 |
| Polymerization potential (V) | 1, 2, 3 |
| Electrochemical deposition time (s) | 150, 350, 500, 700 |
| Adsorbent surface length (cm) | 0.5, 1.0, 1.5 |
| HS–INME parameter | Conditions |
| Saturation time (min) | 15, 30, 45, 60 |
| Extraction temperature (°C) | 25, 50, 80 |
| Adsorption time (min) | 10, 20, 30, 60 |
| Desorption time (min) | 0.5, 1, 3, 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, S.; Bae, S. Synthesis and Characterization of a Multi-Walled Carbon Nanotube–Ionic Liquid/Polyaniline Adsorbent for a Solvent-Free In-Needle Microextraction Method. Molecules 2023, 28, 3517. https://doi.org/10.3390/molecules28083517
Ahn S, Bae S. Synthesis and Characterization of a Multi-Walled Carbon Nanotube–Ionic Liquid/Polyaniline Adsorbent for a Solvent-Free In-Needle Microextraction Method. Molecules. 2023; 28(8):3517. https://doi.org/10.3390/molecules28083517
Chicago/Turabian StyleAhn, Soyoung, and Sunyoung Bae. 2023. "Synthesis and Characterization of a Multi-Walled Carbon Nanotube–Ionic Liquid/Polyaniline Adsorbent for a Solvent-Free In-Needle Microextraction Method" Molecules 28, no. 8: 3517. https://doi.org/10.3390/molecules28083517
APA StyleAhn, S., & Bae, S. (2023). Synthesis and Characterization of a Multi-Walled Carbon Nanotube–Ionic Liquid/Polyaniline Adsorbent for a Solvent-Free In-Needle Microextraction Method. Molecules, 28(8), 3517. https://doi.org/10.3390/molecules28083517