1. Introduction
The human hERG K
+ channel (Kv11.1) plays an important role in the repolarization of the cardiac action potential [
1]. Many non-antiarrhythmic drugs were found to inhibit this channel determining the so-called ‘QT prolongation’, i.e., an anomalously long time spent by the heart muscle to contract and relax. Consequently, the study of in vitro interactions of drugs with hERG has become an important part of ‘safety pharmacology’. QT prolongation, in itself, is not toxic; nonetheless, this phenomenon has often been observed in humans just prior to the onset of fatal cardiac events, such as ventricular arrhythmias leading to sudden cardiac arrest and death [
2]. This concomitance has been a major cause of several drug withdrawals and of the implantation of cardioverter-defibrillators in the chest to prevent this ‘sudden death’.
Even though hERG contains a K
+-selective pore and four voltage sensing domains like the
Shaker K
+ channel and other voltage-gated K
+ channels, its gating properties and functional activity are quite different [
3,
4]. Both
Shaker and hERG consist of four unconnected ‘subunits’ of high internal homology, each composed of six membrane-spanning
α-helices (S1–S6), which are circularly arranged around a transmembrane central pore filled with water. The four S1–S4 helices of each subunit form a voltage sensing domain, with the S4 helix bearing seven positive charges in
Shaker [
5] and five in hERG [
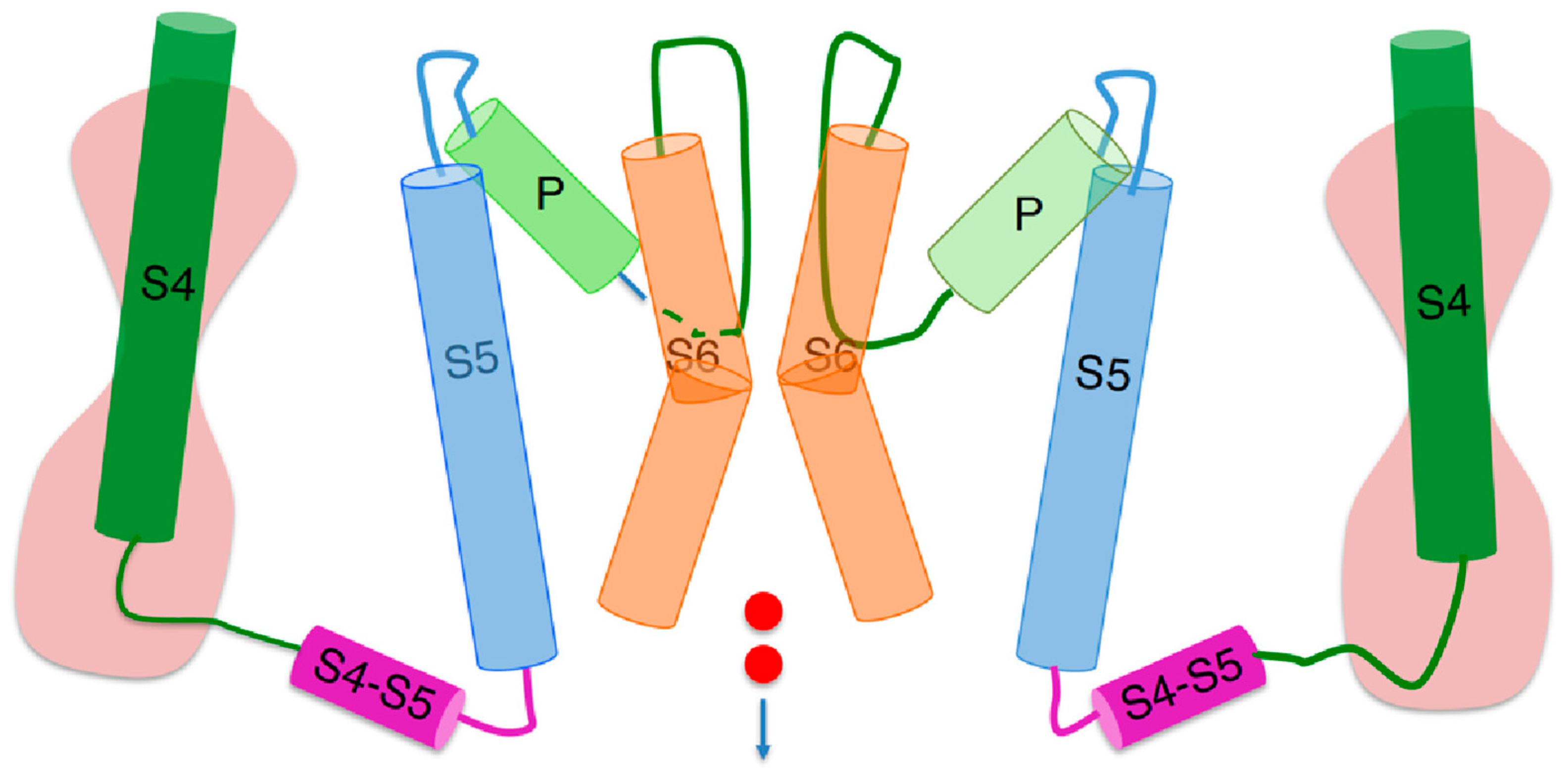
3], and with the totality of the three other helices bearing a net negative charge. The three S1–S3 helices of each voltage sensing domain are arranged to form a hydrophobic gasket called the ‘gating pore’, along which the corresponding S4 segment can move. The gating pore is interposed between two hydrophilic vestibules. Each S4 segment is covalently linked to the corresponding S5 segment by an S4–S5 linker located on the intracellular side of the membrane, as shown in
Figure 1. In turn, on the extracellular side of the membrane, each S5 segment is covalently linked to the corresponding S6 segment by a loop (the ‘P loop’), which is partially folded back into the central pore, lining its upper portion and forming a funnel-like vestibule whose narrow section constitutes the ‘selectivity filter’. The latter is so called because it contains a highly conserved amino acid sequence (the ‘signature sequence’), whose carbonyl oxygen atoms can replace the hydration shell of the permeant cation, allowing its flow along the selectivity filter.
The lumen of the transmembrane ‘central pore’ is lined by the S6 segments of the four subunits, which are flanked by the corresponding S5 segments non-covalently connected to them by many amino acid contacts throughout the entire membrane. The central pore is filled with water and shows a widening at about half its full length, called the ‘central cavity’. The S6 segments are widely separated near the extracellular membrane surface, while converging at the intracellular membrane surface, where they form a sort of bundle crossing, called ‘activation gate’, which may be open or closed. Differently from
Shaker, under the circumstances specified below, the hERG potassium channel assumes a peculiar open conformation characterized by a central pore with an atypically small cross section of radius < 1 Å. This appreciable narrowing of the central pore occurs midway along its length, as revealed by a cryo-EM structure of the open hERG [
3]; it is surrounded by four deep hydrophobic pockets, which may explain the channel unusual sensitivity to many drugs.
A sufficiently positive transmembrane potential step from a far negative ‘resting’ (‘holding’) transmembrane potential
ϕ determines a movement of the four S4 segments from an ‘in’ to an ‘out’ position with respect to the extracellular fluid. The total number of positive charges of the four S4 segments that pass across the corresponding gating pores following their outward movement constitutes the overall ‘gating charge’. Similarly to the
Shaker K
+ channel [
6], the outward movement of each S4 helix of hERG is transmitted through its intracellular S4–S5 linker to the corresponding S5 helix, which in turn shifts the respective S6 helix slightly away from the axis of the central pore. The activation gate is completely closed (C) when all four S4 segments are in the ‘in’ position and completely open (O) when they are all in the ‘out’ position. In moving outwards, each S4 segment rotates to form transient ion pairs with the negative charges located in the corresponding S1–S3 segments, according to the so-called ‘sliding helix model’ [
7].
A depolarizing pulse induces the hERG K
+ channel to move the S4 segments outwards with respect to the extracellular fluid. This movement is about two orders of magnitude slower than in the
Shaker K
+ channel [
8] and is referred to as ‘
slow activation’. By attaching a fluorescent probe to the extracellular S3–S4 linker of hERG, Smith and Yellen [
9] verified that the kinetics of the fluorescence changes is low and correlates well with the kinetics of the slow activation of the K
+ outward current. This provides clear evidence that the kinetics of the slow activation must be attributed to the slow outward S4 movements. This behavior is at least partially ascribable to the S1–S3 segments of hERG being more negatively charged than those of
Shaker [
10], thus exerting a stronger electrostatic attraction upon the corresponding S4 segments. Tryptophan scanning mutagenesis demonstrated that each S4 segment is loosely packed within its own voltage sensing domain [
11]. The slow activation is followed by a ‘
fast inactivation’, probably ascribed to a subtle rearrangement in the hERG selectivity filter with respect to
Shaker [
3]. This implies that the latter two processes proceed in parallel almost simultaneously, leading to a rapid decay of the K
+ outward current, without the intervention of the hERG ‘endogenous blocker’, which is in charge of the plugging of the central pore. The hERG potassium channel fast inactivation is considered to be similar to the
Shaker ‘C-type inactivation’ [
12], which consists of a collapse of the central pore; this collapse is positioned in the proximity of the selectivity filter, whose molecular arrangement is distorted, determining its inactivation [
13,
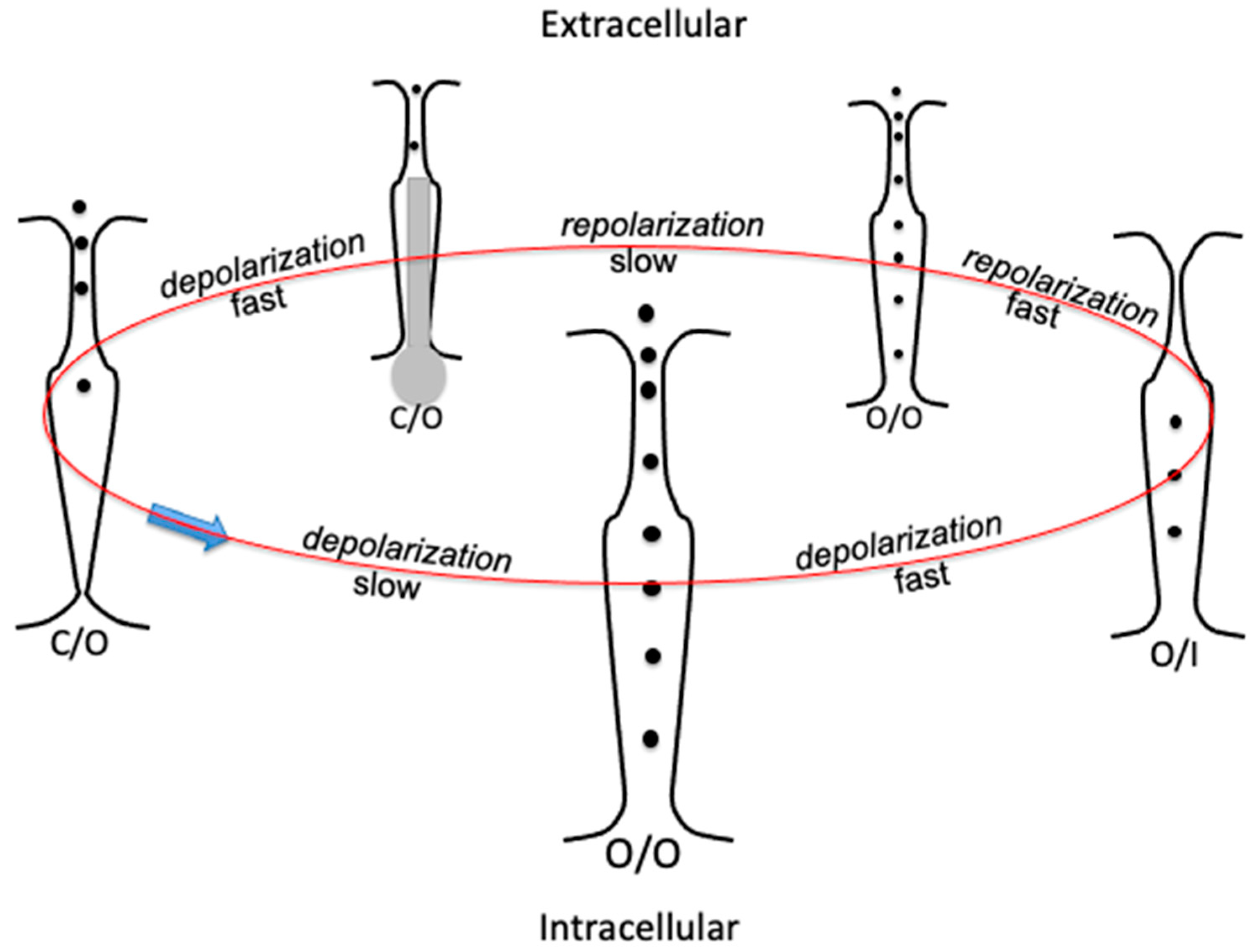
14]. Under these conditions, the selectivity filter is said to be ‘inactive’ and denoted by I. The conformation of any voltage-gated tetrameric ion channel can be conveniently described by specifying between parentheses the O or C state of the activation gate, followed by the O or I state of the selectivity filter, and separating the two states with a slash. This notation, adopted in [
15,
16,
17], is clear and straightforward.
A repolarization induced by a sufficiently negative potential step is rapid in the hERG channel (
fast repolarization), causing the inactive selectivity filter (sometimes called the ‘inactivation gate’ [
18]) to open almost instantaneously by removal of the collapsed configuration of the central pore and of the nearby selectivity filter. However, the resulting (O/O) conformation must deal with an almost concomitant
slow deactivation. The latter slowly moves the endogenous blocker, which comes into play for the first time since the initial slow activation and slowly fits into the central pore from the open activation gate. This blocker consists of the hERG N terminus and is similar to the ‘ball-and-chain’ motif of the
Shaker N terminus. Of the ~390 residues of the hERG N terminus, the first 135 form the ‘eag domain’, which bears a net positive charge and whose removal results in active hERG K
+ channels with altered gating properties [
19]. This strongly suggests that, here too, like in all other known tetrameric cation channels [
20,
21,
22], the determinant of central pore plugging by the blocker resides in the net positive charge of the latter. Deletion and point mutation studies [
19,
23] in the N-terminus domain also concur in identifying this domain with the endogenous blocker.
3. Modeling of hERG
The available literature on the kinetics and mechanism of the hERG potassium channel is based on an arbitrary interpretation of the groundbreaking results obtained by Hodgkin and Huxley [
27] in their investigation of squid axon sodium and potassium currents. The heavy parametrization of their results was believed to indicate that any proposed sequence of closed states must terminate with a single open state. This ‘dogma’ of Hodgkin and Huxley parametrization has influenced the formulation of countless mechanistic models of voltage-gated tetrameric ion channels. These models are mainly stochastic and require a high number of free parameters and of unspecified conformational states [
17]. Some models hypothesize that an inactivated state may stem not only from an open state at strongly depolarized potentials, but also from pre-open closed states at hyperpolarized or modestly depolarized potentials (‘closed-state inactivation’) [
28]. The fact that the same set of parameters and initial conditions can yield an ensemble of different outputs imparts an inherent flexibility to stochastic models, allowing them to interpret a great number of experimental features, albeit at the expense of a high number of free parameters. Moreover, the introduction of too many states in stochastic models makes it practically impossible to ascribe a clear conformation to all of them.
Unlike the other tetrameric ion channels, hERG exhibits outward and inward K
+ currents, both elicited by progressively increasing depolarizing potentials
ϕ. Consequently, the corresponding stochastic models must introduce two open states: one for the K
+ outward currents and the other for the inward ones [
29]. However, even in this case, a quantitative analysis of the activation and inactivation kinetics of the hERG channel requires a high number of free parameters (e.g., ten for the activation and eight for the inactivation in [
29]). A closed-state inactivation was also hypothesized by Kiehn et al. [
30] for an hERG K
+ channel.
We have shown that the main features of the squid axon [
31] and
Shaker potassium channels [
20], the squid axon sodium channel [
21], and the Ca
v3.1 calcium channel [
22] can be simulated with two or, at most, three free parameters by assuming a stepwise outward movement of the four S4 segments of these tetrameric ion channels, which determines a concomitant stepwise increase in the flow of the permeant cation. This very low number of free parameters also minimizes the number of distinct conformational states (four in the schematic of
Figure 2), which can be clearly defined by simply specifying the open or closed state of the activation gate and the open or inactive state of the selectivity filter. In support of this stepwise mechanism, the conductance of the I
Ks ion channel pore, which provides a reserve of cardiac action potential repolarization at times of stress, was recently proposed to result from the outward movement of individual voltage sensing domains [
32]. We will follow an analogous procedure with the hERG channel, using two distinct computations, one for the outward K
+ currents and the other for the inward K
+ currents, with the latter starting from the onset of a fast repolarization unaffected by the slow deactivation. This hypothetical situation is closely approached by inward K
+ currents at transmembrane potentials where the steady-state inactivation curve is already very low.
Both outward and inward K
+ currents of the hERG channel can be simulated with procedures analogous to those adopted for the
Shaker K
+ channel [
20]. The opening of tetrameric K
+ ion channels, no matter if
Shaker or hERG, involves the stepwise outward movement of its S4 segments, which pass from an ‘in’ state to an ‘out’ state. In view of the known structure of tetrameric ion channels [
6], it is reasonable to expect that the stepwise outward movement of each S4 segment will be transmitted to the corresponding S6 segment of the bundle crossing constituting the activation gate, eliciting a K
+ flow through this partially open gate.
We will assume that this ion flow increases in proportion to the number of ‘out’ S4 segments. It is worth noting that it is only thanks to this assumption that the deterministic model succeeds in interpreting the shape and potential dependence of families of current-time (
I-
t) curves using only two or, at most, three free parameters. This assumption conflicts with a dogma for most electrophysiologists, according to which an ion channel opens only after all four subunits (or domains) of the ion channel are activated [
17]. Conversely, in our view, an ion channel opens gradually via the progressive outward movement of the four S4 segments participating in its tetrameric structure, giving rise to an increasing ‘out’ cluster. This clustering will be referred to as an
aggregation, to signify a progressive building up of the open channel.
The models used to interpret all salient features of squid axon Na
+ [
21], squid axon K
+ [
31], and
Shaker K
+ channels [
20], as well as the Ca
v3.1 calcium channel [
22], are two-state models, in that they consider the S4 segments of the four voltage sensing domains in either an ‘in’ or ‘out’ position with respect to the extracellular side of the membrane. Nonetheless, this two-state model is not rigid, because the four S4 segments of a channel are allowed to pass from the ‘in’ to the ‘out’ position step by step. From now on, to simplify the notation, the subunits of a K
+ channel with their ‘out’ (‘in’) S4 segment will be briefly called ‘out’ (‘in’) subunits. The ‘in’ subunits are formally regarded as non-aggregated and the ‘out’ subunits as aggregated, even though they are part of the same tetrameric structure.
The outward movement of ‘in’ subunits is simulated based on two quite reasonable assumptions. The first assumption consists in ascribing a circular shape to the cross-sectional area A of the ‘out’ subunits that are aggregated after moving outwards. This assumption is combined with a second reasonable assumption, according to which the rate of growth of the radius R of an ‘out’ cluster is proportional to the frequency of the favorable impacts of the cluster with the ‘in’ subunits that are moving outwards. By favorable impacts we mean those impacts in which the lateral orientation of the ‘in’ subunits that are trying to join an ‘out’ cluster by moving outwards complies with that of the subunits composing the progressively growing ‘out’ cluster. These two assumptions immediately yield a proportional relationship between the rate of growth, dR/dt, of the cluster radius R and the surface coverage [M] by the ‘in’ subunits.
To this end, let us denote by
x1 and
x2 the mole fractions of the ‘in’ and ‘out’ subunits in the membrane, respectively; differently stated,
x2 is the ratio of the number of ‘out’ subunits in the membrane unit surface area to the totality of all subunits, irrespective of their ‘in’ or ‘out’ position relative to the extracellular fluid; (
x1 +
x2) is clearly equal to unity. Denoting by
θ the fraction of the membrane unit surface area covered by all subunits, irrespective of their ‘out’ or ‘in’ state, [M] is given by
θpx1, where
p is the steady-state open probability at the given depolarization potential. Upon setting
S =
x2/(
x1 +
x2) =
x2 for convenience, we then have:
On the other hand, the surface coverage by the ‘out’ subunits, denoted by [C], is clearly given by:
and is proportional to the K
+ outward current.
The quantity
S should not be confused with
p. In fact, even though
S attains the unit value at any given depolarization potential
ϕ as the time
t from the onset of the depolarizing pulse becomes sufficiently long, such a unit value refers to the maximum mole fraction of ‘out’ subunits permitted by the potential-dependent probability
p at the given
ϕ value, and not to the unitary mole fraction of ‘out’ subunits in the whole membrane. The latter situation is only achieved when
p equals unity at sufficiently positive transmembrane potentials
ϕ. It is important to reiterate that the ‘out’ or ‘in’ position of a subunit refers exclusively to its S4 segment, and not to the whole subunit, as clearly shown in
Figure 4. Upon ascribing a circular shape of radius
R to the cross-sectional area
A of the growing ‘out’ cluster and assuming that the growth rate of
A is proportional to the frequency of impacts of the M subunits with the circumference of
A, it immediately follows that:
Here, kR is the proportionality constant for the radial growth of the ‘out’ clusters over time.
The radial growth expression in Equation (4) can be inserted into the well-known formalism of the kinetics of nucleation and growth, which requires only one free parameter, i.e., the product of the nucleation rate constant (
kN) and the square of
kR [
20,
21,
22,
31]. In practice, in all investigated systems relying on the above expression for the rate of radial growth [
20,
21,
22,
31], the best fit by the model to the experimental behavior is attained by setting the number of ‘out’ subunits composing the ‘nucleus’ equal to unity. This simply means that, in practice, no real nucleation process occurs. In this case, the nucleation rate can be interpreted as the rate at which an ‘in’ subunit M moves outwards, and can be regarded as proportional to the surface coverage [M] by the ‘in’ subunits:
where
kN is a proportionality constant.
The kinetic treatment of aggregation starts from considering that d
N in Equation (5) expresses the number of ‘in’ subunits per unit surface area of the membrane that are moving outwards in an infinitesimal time interval between
y and
y + d
y, interposed between the onset (
t = 0) of the depolarizing pulse and a given ‘observation time’
t. Upon again ascribing a circular shape to the clusters, the area
A of the membrane unit surface covered by the clusters generated by d
N during the finite time interval between
y and
t is given by:
The parameter
z is an auxiliary variable having the dimensions of time, which is allowed to vary between
y and the observation time
t. Upon integrating Equation (6) over time between the limits of integration
y = 0 and
y =
t, we obtain the ratio of the membrane area covered by the ‘out’ clusters (eventually yielding the ion channels) to that covered by all subunits, no matter if aggregated or not:
The above dimensionless quantity is equal to the ratio,
S, defined in Equation (2). However, the integration of Equation (6), leading to Equation (7), ignores the possible overlapping of the progressively growing clusters. Hence, the resulting quantity
Sx, called
extended area, is greater than
S and is distinguished from it by the subscript
x. To obtain
S from
Sx, a simplified approach leading to the rigorous expression derived by Avrami [
33,
34] is as follows. We will make the reasonable assumption that the growth rate of
Sx, d
Sx/d
t, is proportional to the total unit surface area of the membrane,
ST = 1, whereas the growth rate of
S, d
S/d
t, is proportional to the fraction (
ST–S) of the unit surface area of the membrane still unoccupied, according to the same proportionality constant,
kg. Consequently, we have:
The last differential equation in Equation (8) allows
S to be calculated from
Sx.
Replacement into Equation (7) of
vR and
vN from Equations (4) and (5), with [M] =
θp(1 −
S) in view of Equation (2), and repeated differentiation of the resulting equation with respect to time
t via the generalized Leibnitz formula yields the following differential equations:
The system of the three differential equations in Equation (9) and of the additional differential equation in Equation (8) can be solved by the fourth-order Runge–Kutta method, allowing the calculation of
S as a function of time
t and of the transmembrane potential
ϕ [
20,
21,
22].
It is only at this point that hERG starts differing from
Shaker by exhibiting a fast inactivation (O/O) → (O/I), which is concomitant with the slow activation and is expressed by the experimental damping factor of Equation (1). Hence, the outward K
+ current of hERG is obtained by multiplying the outward current proportional to [C] in Equation (3) by the damping factor in Equation (1), yielding:
Here, p is the open probability (i.e., the steady-state activation curve normalized to unity).
The parameter
θ is not a free parameter. In fact, it can be ignored by formally setting it equal to unity and using a suitable conversion factor to compare a family of calculated K
+ outward current vs. time (
I-
t) curves at different transmembrane potentials with the corresponding experimental ones. The well-known formalism of the kinetics of ‘nucleation and growth’ [
20,
22,
31,
35] requires only a single free parameter, i.e., the product of the nucleation rate constant
kN and the square of the rate constant
kR of growth of the radius of the cross-sectional area of the clusters of ‘out’ subunits. In what follows,
kN and
kR will be constantly set equal to 10
2 s
−1 and 10
5 s
−1, i.e., two orders of magnitude smaller than those in the
Shaker potassium channel [
20]. The set of the four differential equations in Equations (8) and (9) can be solved by the Runge–Kutta method of the fourth order.
At this point, a distinction must be made between the K+ outward current, which is not affected by the endogenous blocker, and the K+ inward current. The mathematical treatment underlying the derivation of the K+ inward current is identical with that used for the outward K+ current, except for the change in the sign of the current and the notable effect induced by the intervention of the endogenous blocker.
We already said that the endogenous blockers of
Shaker K
+, Na
+, and Ca
v3.1 cation channels bear a net positive charge. This is also the case with the hERG potassium channel N terminus [
19]. Upon observing that all endogenous blockers bear a net positive charge, during the slow deactivation phase they are expected to be progressively attracted along the central pore by the increasingly negative charge that the step-by-step outward movement of the four S4 segments leaves behind on the intracellular side of the hydrophobic gating pores in the corresponding voltage sensing domains. The average number density,
Nbk, of blockers bound to the receptor, which is located within the pore and close to the selectivity filter, is related to the total number density of blockers,
Nbk,t, in the membrane via a one-sided Boltzmann-like function [
21]:
Here,
Wbk is the work of formation of bound blockers. It consists of a structural component,
Ubk, and of a Coulomb-attractive component proportional to the time dependent charge, −
q, progressively left on the cytoplasmic side of the gating pores by the stepwise outward movement of the four S4 segments, multiplied by the constant positive charge of the blocker:
The parameter
k″ is a positive proportionality constant for the Coulomb-attractive interaction. Since the blocker is immersed in the intracellular fluid before folding into the channel structure, the effect of the transmembrane potential is negligible and can be ignored. If we make the reasonable assumption that the structural component
Ubk is much greater than
kT, Equation (11) simplifies as follows:
In the above equation, account is taken of the fact that the charge −q progressively left on the cytoplasmic side of the gating pores by the stepwise outward movement of the four S4 segments is equal in magnitude and opposite in sign to the positive charge moved by the S4 segments passing across their relative gating pores, i.e., the true positive gating charge q. Conversely, S is the total gating charge normalized to unity defined in Equation (2), which attains the unit value for sufficiently long times at all depolarization potentials, and hence, it is proportional to q according to a proportionality constant. The product of the latter proportionality constant and k″ yields a different proportionality constant k′.
The ‘driving force’ of the slow deactivation can be regarded as proportional to the difference between the maximum value attained by
Nbk at sufficiently long times, when
S equals unity, and its value at any given time
t. From Equation (13) and upon normalization to unity, we have:
Since the ion flow takes place on the fraction of the membrane unit surface area covered by the open ion channels, [C] =
θpS (see Equation (3)), the K
+ inward current,
incurr, is proportional to this fraction multiplied by the driving force of Equation (14):
As S increases gradually over time from 0 to unity following a sufficiently long depolarizing pulse, the first factor of proportionality, θpS, increases rapidly over time and is responsible for the initial rising section of the K+ inward ionic current. Conversely, the second factor of proportionality, , decreases much more slowly and accounts for the slow exponential decay of the K+ inward current, typical of hERG slow deactivation. The current decay is ascribed to the stepwise outward movement of the endogenous blocker along the central pore, which is induced by the concomitant stepwise outward movement of the S4 segments of the four hERG subunits, gradually plugging the central pore.
Here, kh is a free dimensionless parameter required to simulate the exponential decay of the K+ inward current. The probability p in Equation (15) is the experimental ‘open probability of the inward K+ current’, which contributes to the depolarization of the cardiac action potential by injecting positive charges on the intracellular side of the membrane. The simulation of the K+ inward current requires only two free parameters, i.e., the overall rate constant of aggregation of the four ‘out’ subunits, kNkR2, and the free parameter kh expressing the rate constant for the exponential decay of the K+ inward current.
Simulation of the hERG Potassium Channel in Piper et al. [8]
We will now examine in detail the paper by Piper et al. [
8], which provides the experimental values of both outward and inward K
+ currents while maintaining physiological K
+ concentrations in both the intracellular and extracellular solutions. The five transmembrane potentials for both the outward and inward K
+ currents (i.e., −50, −30, −10, +10, and +30 mV) are unequivocally ascribable to these currents by using five different colors for the five
I-
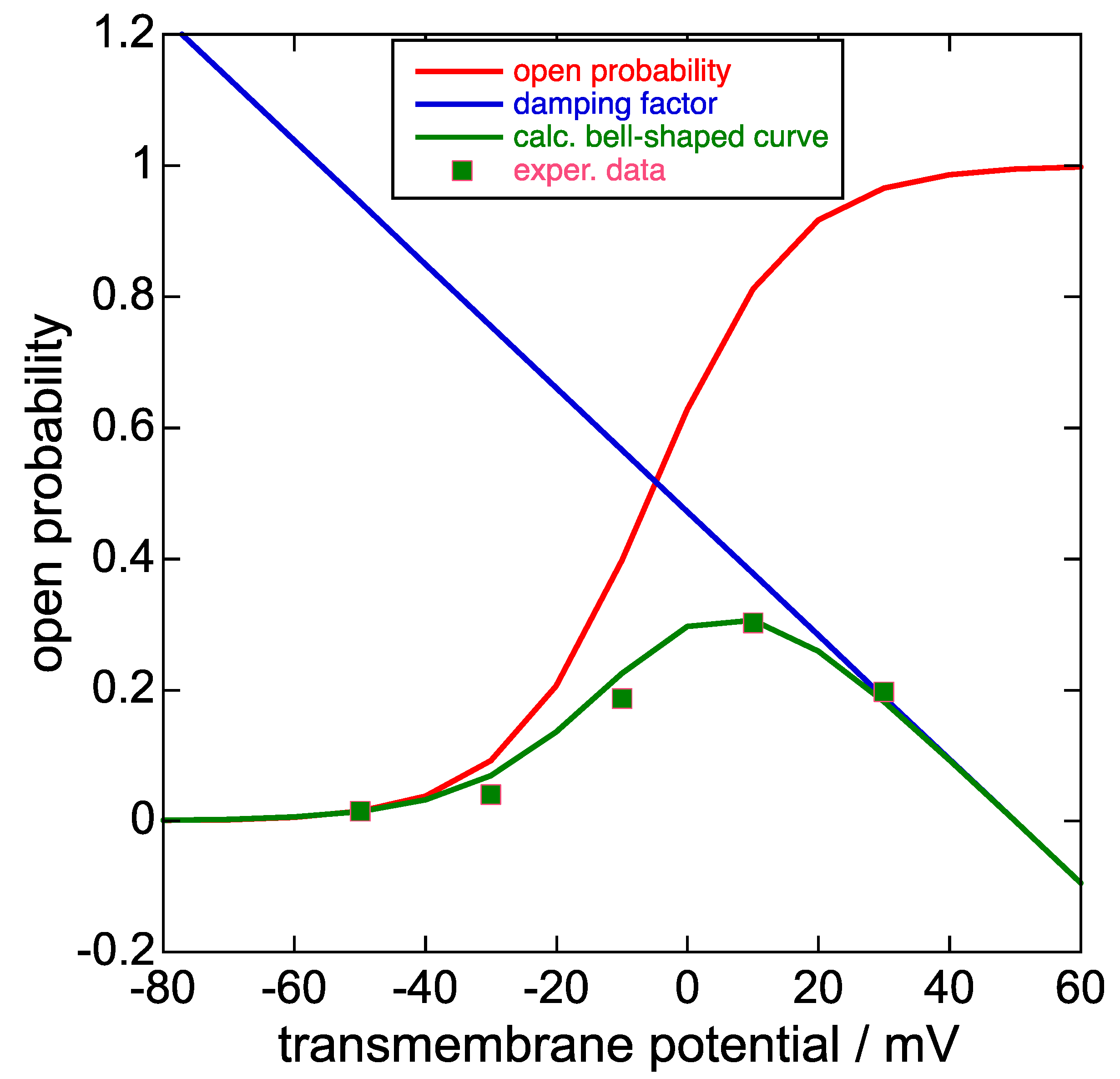
t curves. This paper provides the Boltzmann parameters of the experimental steady-state activation curve in Figure 2c of [
8] (
z = 2.4,
ϕ1/2 = −5.6 mV,
a = exp[−
zϕ1/2/25.6 mV] = 1.69), and those of the experimental steady-state inactivation curve in Figure 4a of [
8] (
z = 1.1,
ϕ1/2 = −82 mV
, a = 33.9). Piper et al. [
8] also provide the Boltzmann parameters for the ‘open probability of the inward K
+ current’ predicted by their stochastic model in Figure 5f of [
8] (
z = 2.2,
ϕ1/2 = −9 mV
, a = 2.16).
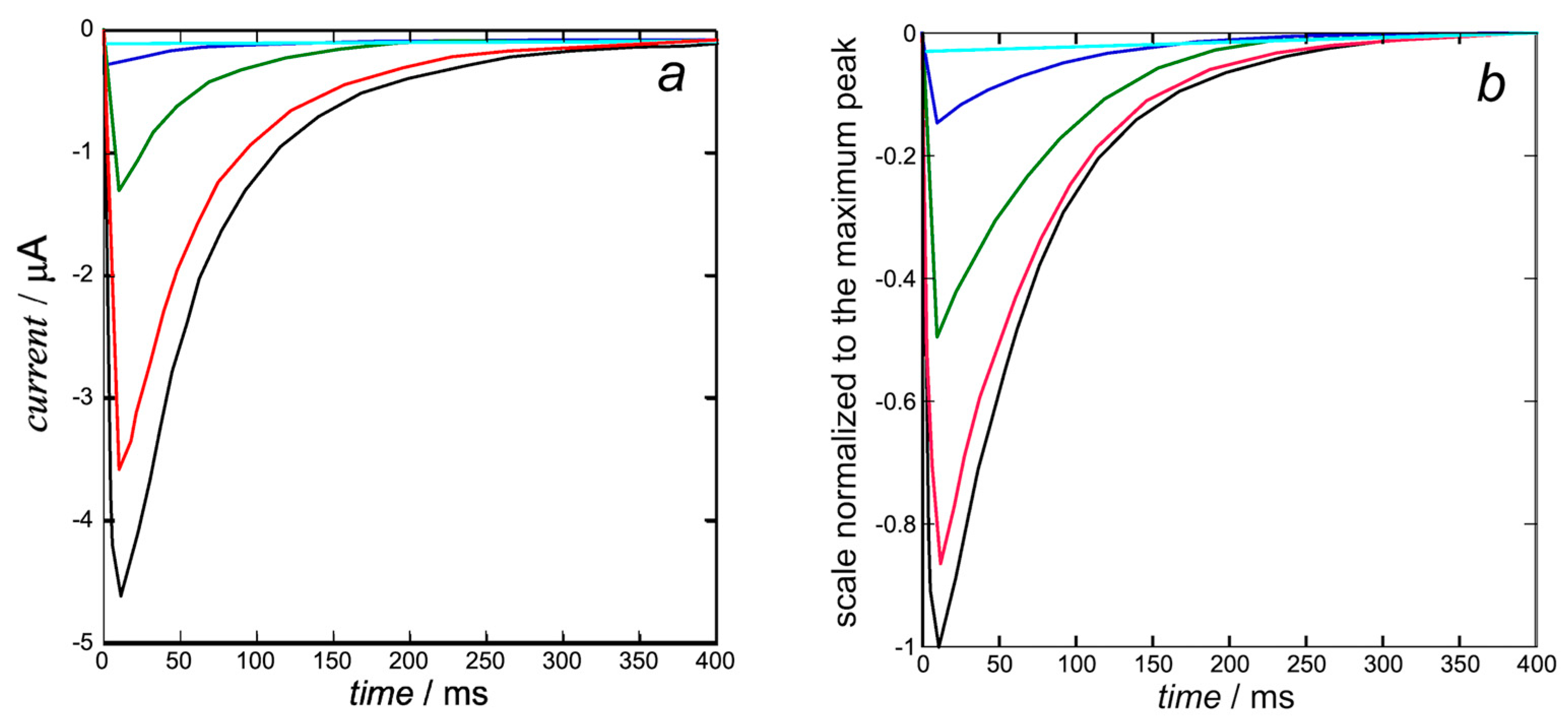
In view of the substantial independence of the inward currents from the outward ones, sanctioned by the very fast repolarization (see also
Figure 2), they can be treated by compiling two separate Fortran text files. The K
+ outward
I-
t curves are calculated from Equation (10). Upon using the experimental steady-state activation curve as the open probability, the resulting calculated K
+ outward
I-
t curves in
Figure 5b only require the single free parameter expressing the overall rate constant
kNkR2 = 1 × 10
12 s
−3 of cluster aggregation. They are moderately close to those calculated by Piper et al. in Figure 5b (right) of [
8] and shown in
Figure 5a below, whose computation with the stochastic model requires more than 40 free parameters, as summarized in Table 1 of [
8].
The K
+ inward
I-
t curves in
Figure 6b are calculated from Equation (15) with the same rate constant
kNkR2 = 1 × 10
12 s
−3 of cluster aggregation used in
Figure 5 and with
kh = 1. They are quite close to those calculated by Piper et al. in Figure 5b (right) of [
8]. The open probability
p in this equation is the ‘open probability of the inward K
+ current’ estimated in [
8].
At the holding potential of −110 mV, the hERG ion channel is closed and starts to open at about −50 mV, where the steady-state inactivation curve in Figure 4a of [
8] is already relatively low. This is even more true for the K
+ inward
I-
t curves in
Figure 6b at more positive transmembrane potentials, where the steady-state inactivation curve becomes vanishingly small. The K
+ ions are pushed inwards rapidly, but the open channel tends to close more slowly due to the endogenous blocker, which sneaks into the activation gate, inducing slow deactivation.
A peculiar feature is reported in Figure 1b of [
8], where the inward K
+ current obtained by stepping from a holding potential of −110 mV decreases as the transmembrane potential
ϕ becomes progressively less negative and becomes outward as soon as
ϕ becomes positive. Evidently, this behavior, which is consistent with the combined effect of electric and osmotic forces, strongly suggests a disruption of the structural feature described by Wang and MacKinnon in [
3] and responsible for the K
+ inward
I-
t curves in
Figure 6.
The present deterministic model allows gating charges to be easily estimated starting from the parameter
S defined in Equation (2), which measures the mole fraction of ‘out’ S4 segments and tends toward unity with an increase in the length of the depolarizing pulse. This is just a measure of the gating charge normalized to unity, and its time derivative yields the corresponding gating current. As an example,
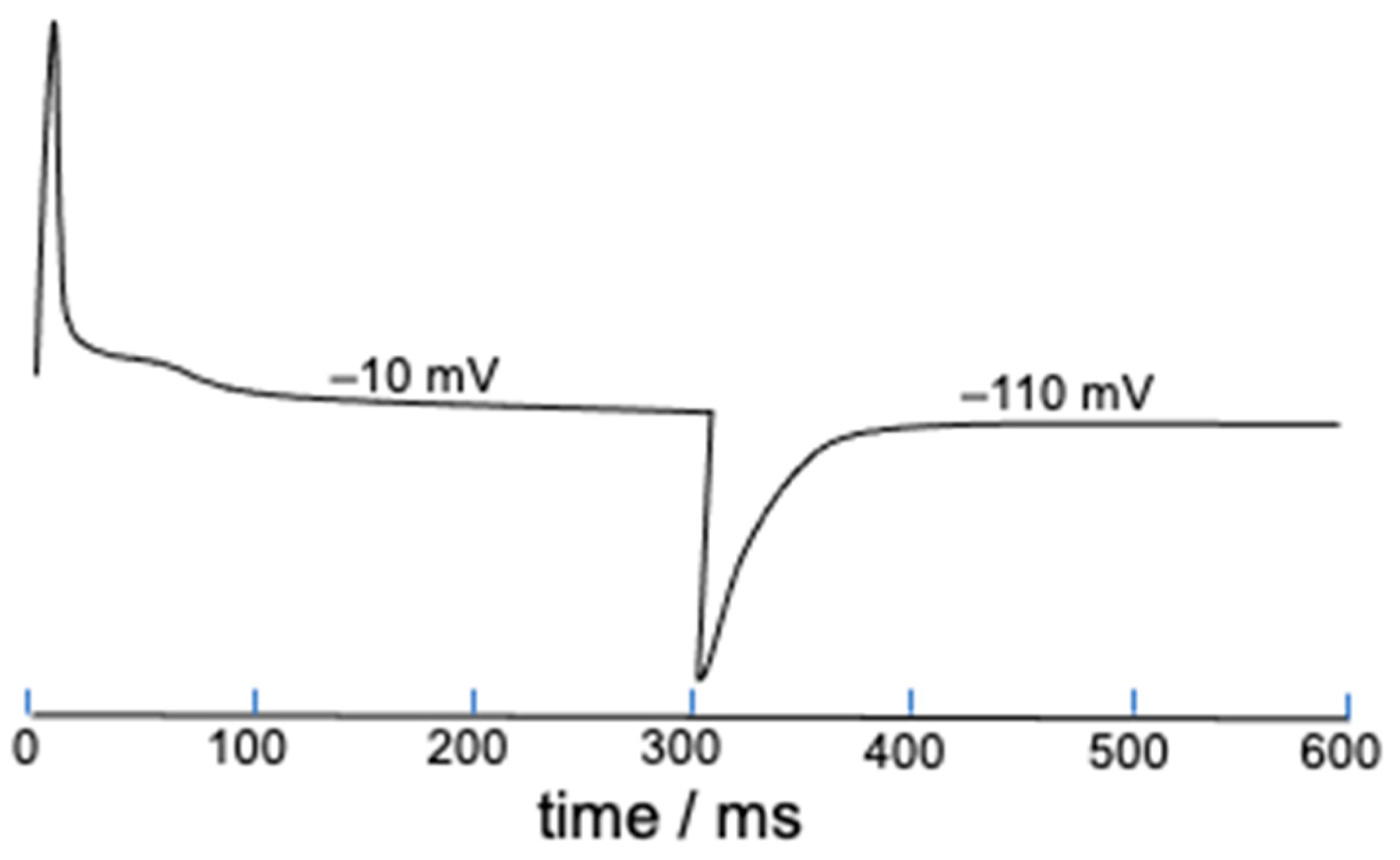
Figure 7 shows the gating current elicited by a 300 ms long square wave pulse from −110 to −10 mV and vice versa. The shoulder following the initial positive peak might possibly be induced by the slow activation.
The hERG potassium channel contributes to depolarizing the neuron membrane with its K
+ inward current and to repolarizing it with its K
+ outward current. The initial flat plateau of the cardiac action potential is determined by a small K
+ outward current that rapidly undergoes fast inactivation. A contribution to the initial depolarization comes from sodium and calcium ions, which move from the extracellular fluid to the cytosol [
36]. The subsequent rapid repolarization of the terminal portion of the cardiac action potential proceeds quickly due to the inactivation of calcium and sodium ion channels, which determines a decrease in the intracellular movement of positive charges; this effect is combined with a rapid repolarizing outward K
+ current of hERG and with a slow delayed rectifier
IKs potassium current [
37]. These currents cause an increase in the movement of positive charges out of the cell, which determines a current peak of the action potential and has a role in determining action potential duration. The increasingly more outward overall membrane current causes the cardiac action potential to move to its resting value, with a decrease in the electrochemical driving force for K
+ efflux and the slow deactivation of hERG [
38]. Loss-of-function mutations of the hERG gene prolong this final repolarization, inducing an anomalously long time spent by the heart muscle to contract and relax, i.e., the so-called ‘QT prolongation’.