Amino Acid Derivatives of Chlorin-e6—A Review
Abstract
1. Introduction
2. Photodynamic Therapy Using NPe6
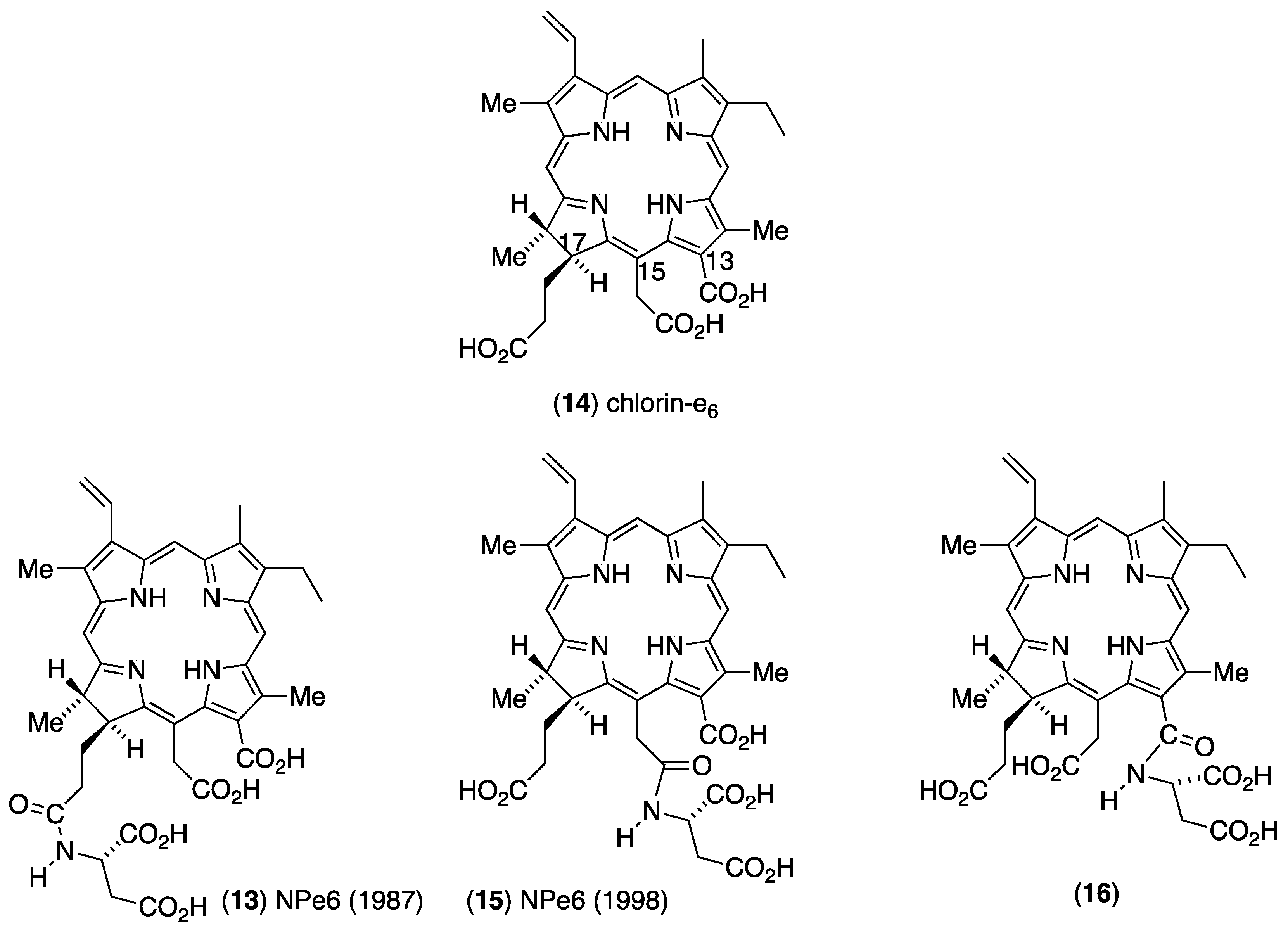
3. Structure Elucidation of NPe6
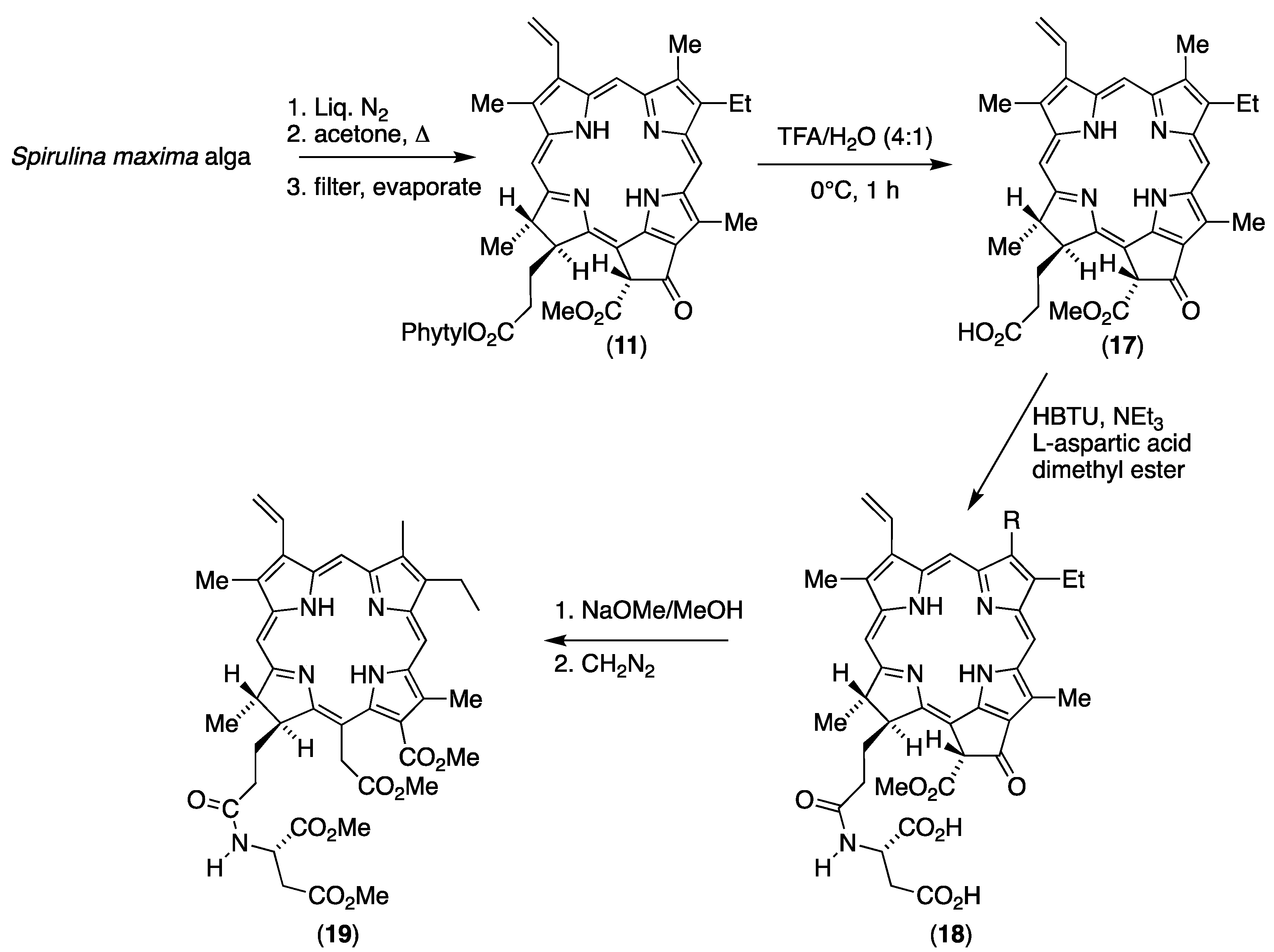
3.1. Unambiguous Partial Synthesis of 173-Aspartylchlorin-e6 (13)
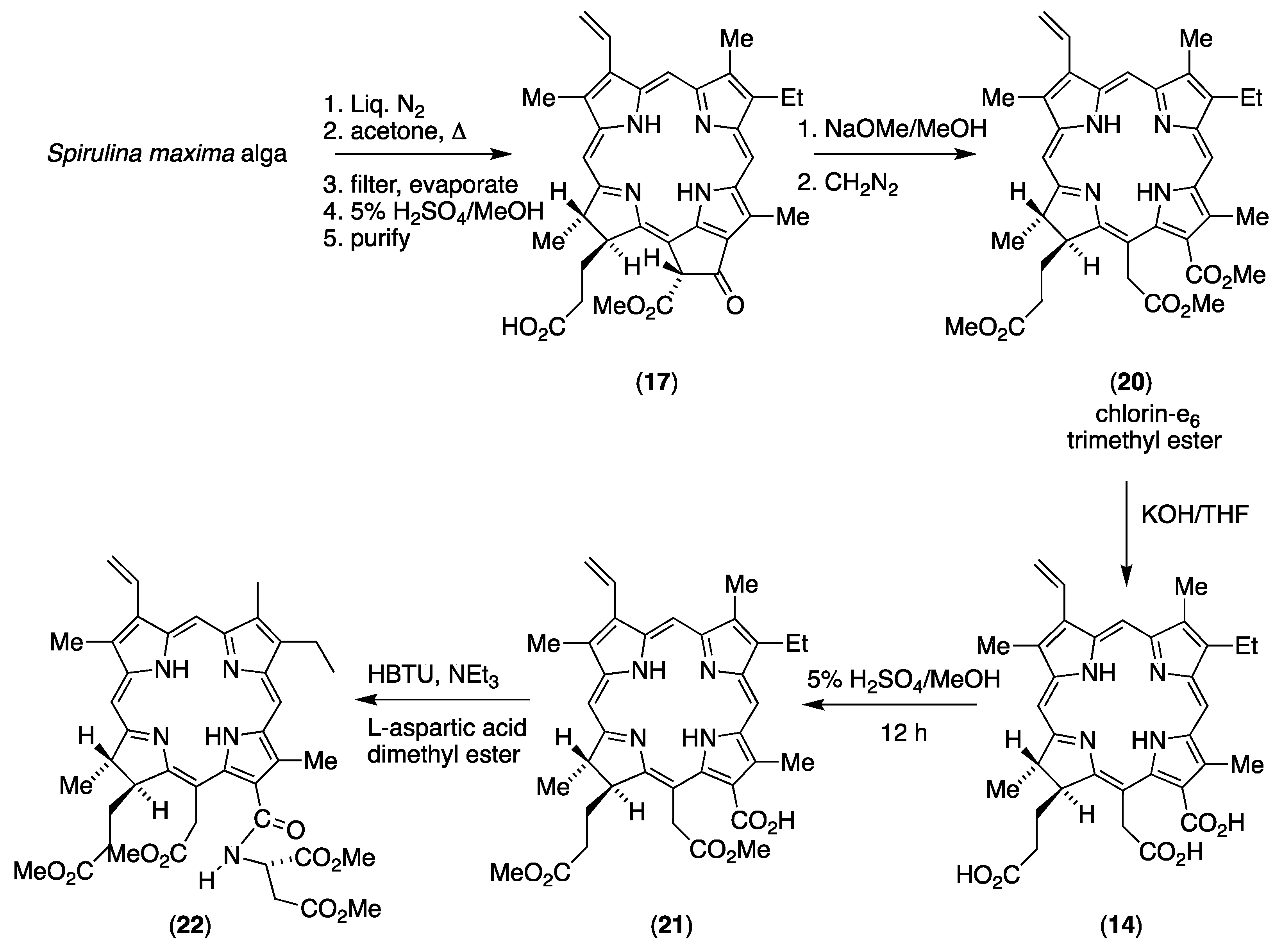
3.2. Unambiguous Partial Synthesis of 131-Aspartylchlorin-e6 (16)
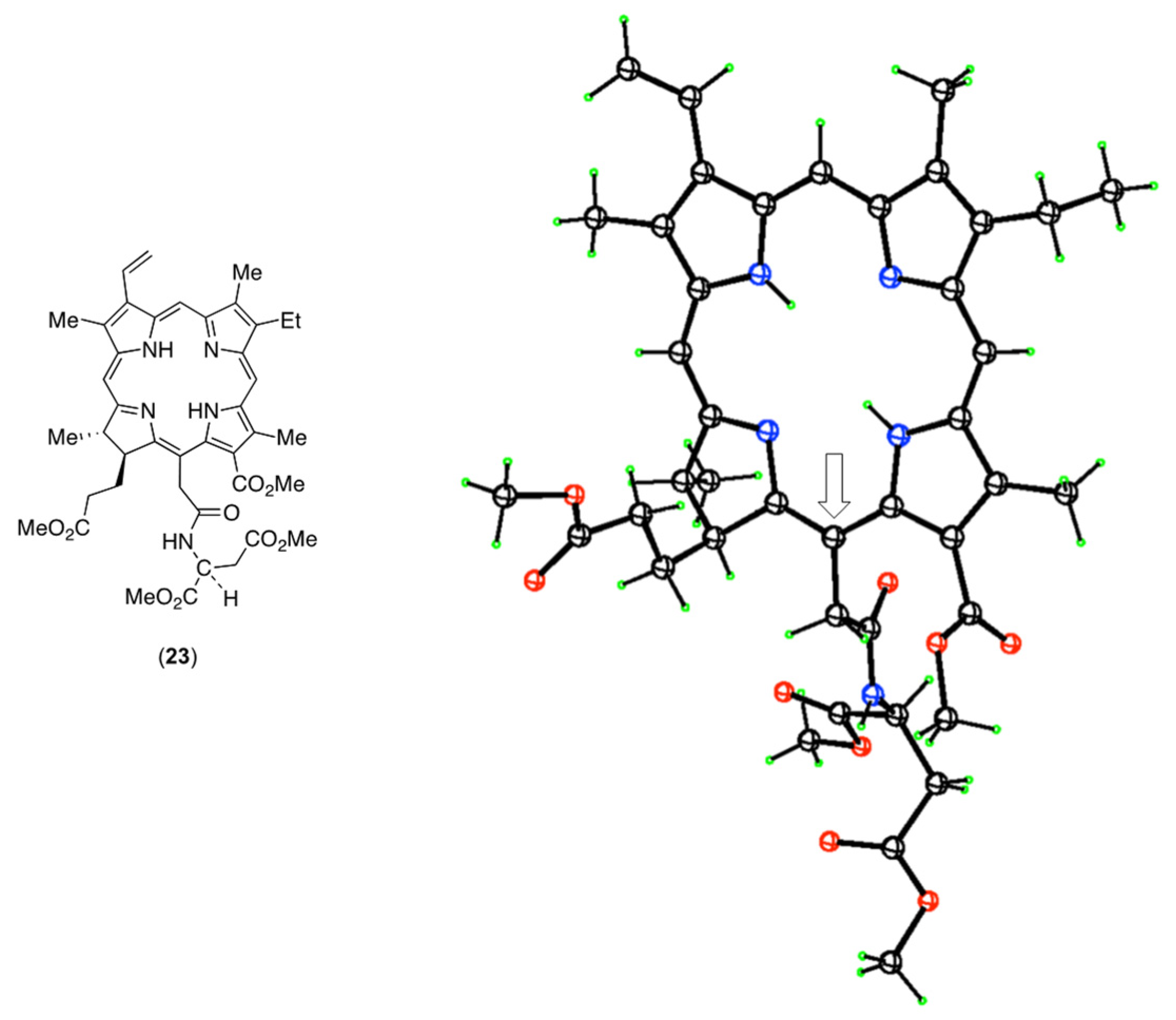
3.3. X-ray Crystal Structure of Authentic NPe6 Tetramethyl Ester (23)
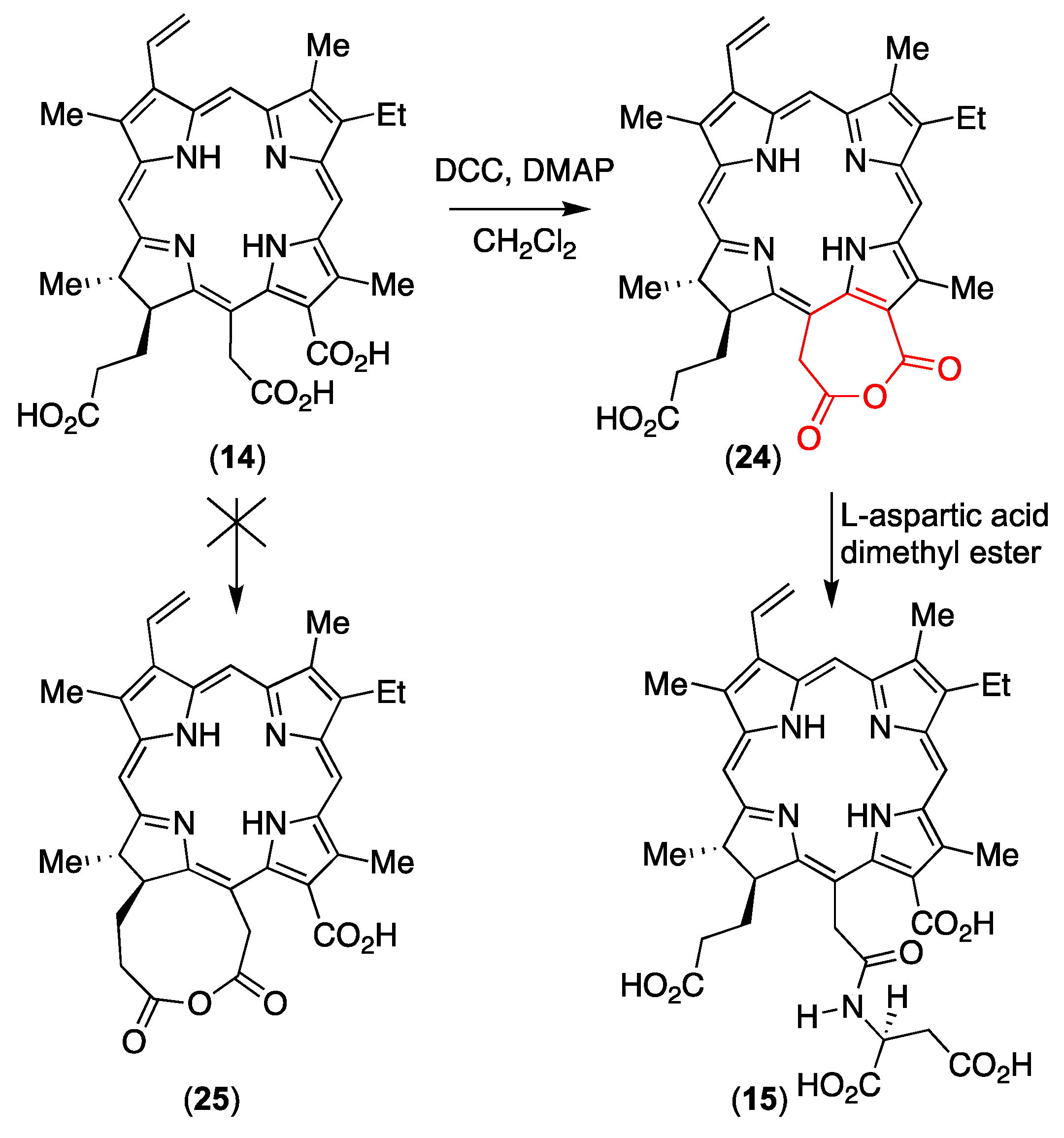
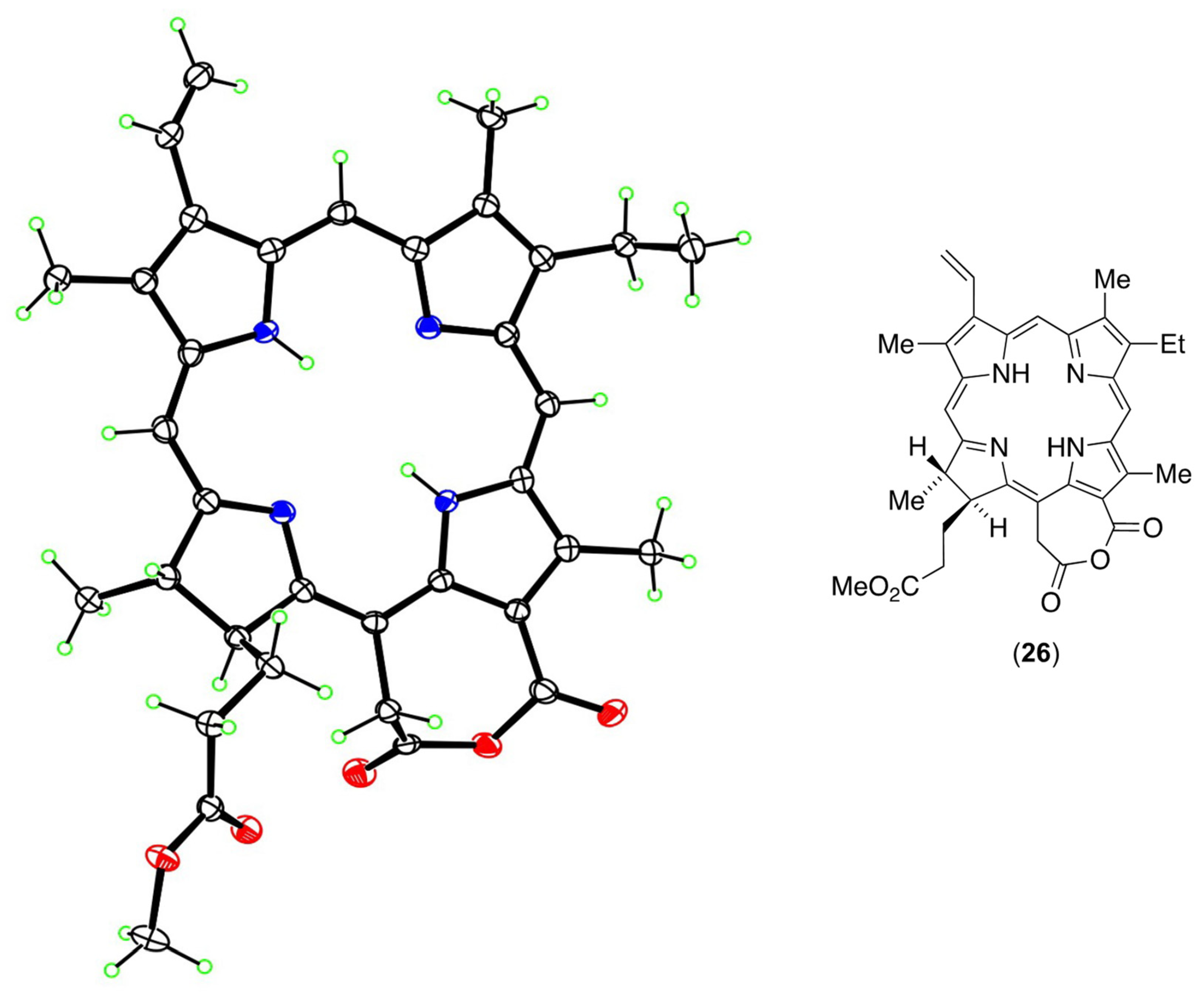
3.4. An Anhydride Intermediate (24) in the Formation of NPe6
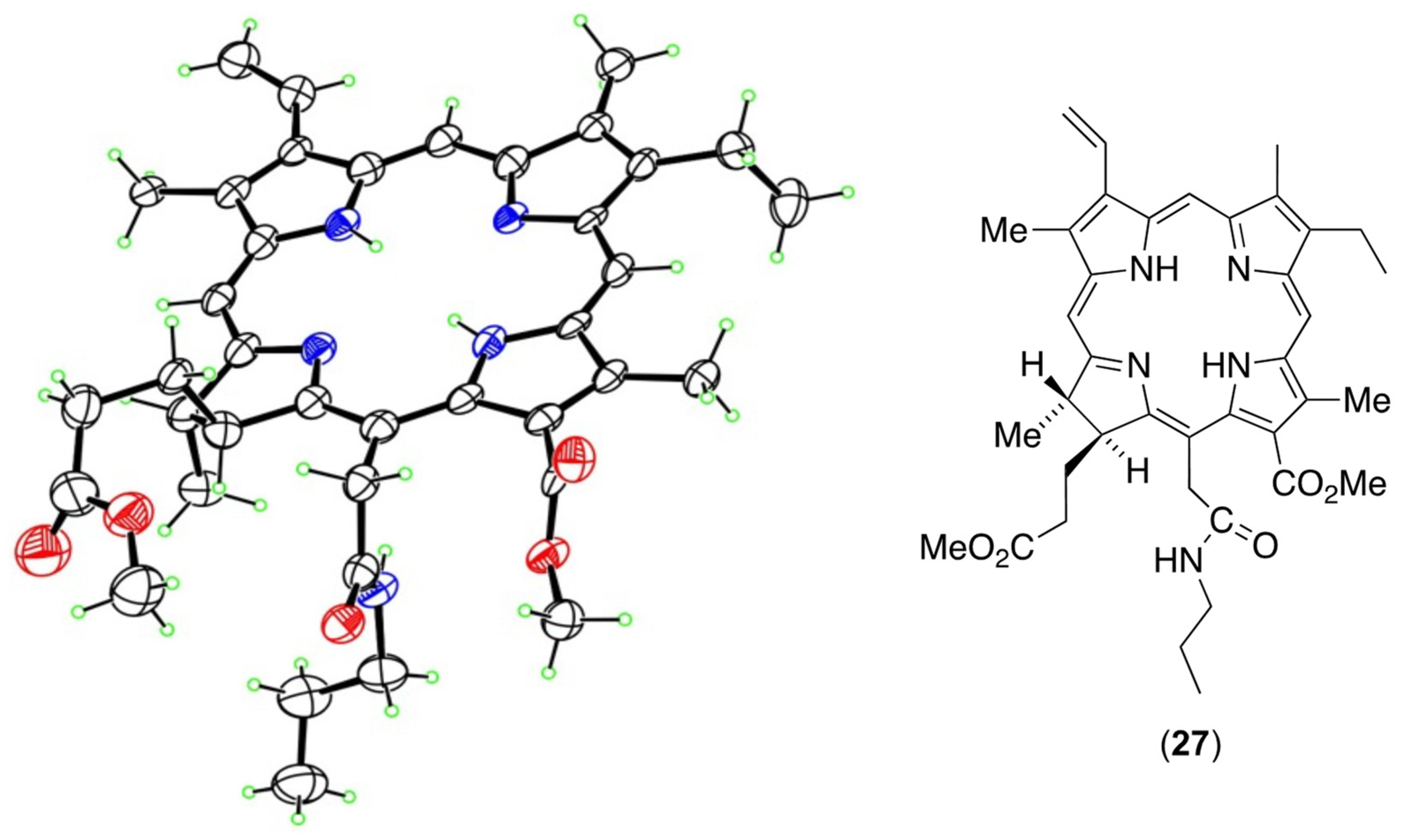
3.5. The Coup de Grace—Synthesis of NPe6 Tetramethyl Ester (23) from the Anhydride (26)
4. Phototoxicity of NPe6 Amino Acid Conjugates
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Fischer, H.; Orth, H. Die Chemie des Pyrrols; Akademische Verlagsgesellschaft: Leipzig, Germany, 1934; Volume I. [Google Scholar]
- Fischer, H.; Orth, H. Die Chemie des Pyrrols; Akademische Verlagsgesellschaft: Leipzig, Germany, 1937; Volume II, Part i. [Google Scholar]
- Fischer, H.; Stern, A. Die Chemie des Pyrrols; Akademische Verlagsgesellschaft: Leipzig, Germany, 1940; Volume II, Part ii. [Google Scholar]
- Jackson, A.H.; Kenner, G.W.; Sach, G.S. Stepwise synthesis of porphyrins through a-oxobilanes. J. Chem. Soc. C 1967, 2045–2059. [Google Scholar] [CrossRef]
- Jackson, A.H.; Kenner, G.W.; McGillivray, G.; Smith, K.M. Porphyrin syntheses through b-oxobilanes and oxophlorins (oxyporphyrins). J. Chem. Soc. C 1968, 294–302. [Google Scholar] [CrossRef]
- Jackson, A.H.; Kenner, G.W.; Smith, K.M. Porphyrin syntheses through b-bilenes. J. Chem. Soc. C 1971, 502–509. [Google Scholar] [CrossRef]
- Smith, K.M. Development of porphyrin syntheses. New J. Chem. 2016, 40, 5647. [Google Scholar] [CrossRef]
- Kenner, G.W.; McCombie, S.W.; Smith, K.M. Separation and oxidative degradation of chlorophyll derivatives. J. Chem. Soc. Perkin Trans. 1 1973, 2517–2523. [Google Scholar] [CrossRef] [PubMed]
- Wasielewski, M.R.; Svec, W.A. Syntheses of covalently linked dimeric derivatives of chlorophyll a, pyrochlorophyll a, chlorophyll b, and bacteriochlorophyll a. J. Org. Chem. 1980, 45, 1969–1974. [Google Scholar] [CrossRef]
- Smith, K.M.; Goff, D.A.; Simpson, D.J. Meso-substitution of chlorophyll derivatives: Direct route for transformation of bacteriopheophorbides-d into bacteriopheophorbides-c. J. Am. Chem. Soc. 1985, 107, 4946–4954. [Google Scholar] [CrossRef]
- Oseroff, A.R.; Ohuoha, D.; Hasan, T.; Bommer, J.C.; Yarmush, M.L. Antibody-targeted photolysis: Selective photodestruction of human T-cell leukemia cells using monoclonal antibody-chlorine6 conjugates. Proc. Natl. Acad. Sci. USA 1986, 83, 8744–8748. [Google Scholar] [CrossRef]
- Bommer, J.C.; Ogden, B.F. Tetrapyrrole Therapeutic Agents. U.S. Patent 4,693,885, 23 June 1987. [Google Scholar]
- Gomi, S.; Nishizuka, T.; Ushiroda, O.; Ushida, N.; Takahashi, H.; Sumi, S. The structure of mono-L-aspartyl chlorin e6 and its related compounds. Heterocycles 1998, 48, 2231–2243. [Google Scholar] [CrossRef]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Ethirajan, M.; Chen, Y.; Joshi, P.; Pandey, R.K. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem. Soc. Rev. 2011, 40, 340–362. [Google Scholar] [CrossRef] [PubMed]
- Kessel, D.; Reiners, J.J. Photodynamic therapy: Autophagy and mitophagy, apoptosis and paraptosis. Autophagy 2020, 16, 2098–2101. [Google Scholar] [CrossRef] [PubMed]
- Spikes, J.; Bommer, J. Photosensitizing properties of mono-L-aspartyl chlorin e6 (NPe6): A candidate sensitizer for the photodynamic therapy of tumors. J. Photochem. Photobiol. B 1993, 17, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.S.; Roberts, W.G.; Berns, M.W. In vivo studies on the utilization of mono-L-aspartyl chlorin (NPe6) for photodynamic therapy. Cancer Res. 1987, 47, 4681–4685. [Google Scholar]
- Aizawa, K.; Okunaka, T.; Ohtani, T.; Kawabe, H.; Yasunaka, Y.; O’Hata, S.; Saito, T. Localization of mono-L-aspartyl chlorin e6 (NPe6) in mouse tissue. Photochem. Photobiol. 1987, 46, 789–793. [Google Scholar] [CrossRef]
- Ferrario, A.; Kessel, D.; Gomer, C.J. Metabolic properties and photosensitizing responsiveness of mono-L-aspartyl-chlorin-e6 in a mouse tumor model. Cancer Res. 1992, 52, 2890–2893. [Google Scholar]
- Kessel, D. Pharmacokinetics of N-aspartyl chlorin e6 in cancer patients. J. Photochem. Photobiol. B Biol. 1997, 39, 81–83. [Google Scholar] [CrossRef]
- Sheyhedin, L.; Aizawa, K.; Araake, M.; Kumasaka, H.; Okunaka, T.; Kato, H. The effects of serum on cellular uptake and phototoxicity of mono-L-aspartyl chlorin e6 (NPe6) in vitro. Photochem. Photobiol. 1998, 68, 110–114. [Google Scholar] [CrossRef]
- Kanda, T.; Sugihara, T.; Takata, T.; Mae, Y.; Kinoshita, H.; Sakaguchi, T.; Hasegawa, T.; Kurumi, H.; Ikebuchi, Y.; Murakami, T.; et al. Low-density lipoprotein receptor expression is involved in the beneficial effect of photodynamic therapy using talaporfin sodium on gastric cancer cells. Oncol. Lett. 2019, 17, 3261–3266. [Google Scholar] [CrossRef]
- Saito, T.; Tsukahara, T.; Kubo, T.; Kanaseki, T.; Hirohashi, Y.; Torigoe, T.; Li, L. Elucidation of intracellular uptake and degradation mechanism of photosensitizer talaporfin. Mol. Cryst. Liq. Cryst. 2020, 707, 81–87. [Google Scholar] [CrossRef]
- Sasaki, M.; Tanaka, M.; Kojima, Y.; Nishie, H.; Shimura, T.; Kubota, E.; Kataoka, H. Anti-tumor immunity enhancement by photodynamic therapy with talaporfin sodium and anti-programmed death 1 antibody. Mol. Ther. Oncolytics 2023, 28, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Miyajima, K.; Kojika, M.; Kono, T.; Kato, H. Photodynamic Therapy (PDT) with Chemotherapy for Advanced Lung Cancer with Airway Stenosis. Int. J. Mol. Sci. 2015, 16, 25466–25475. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, T.; Matsumoto, Y.; Imabayashi, T.; Uchimura, K.; Sasada, S. Photodynamic therapy can be safely performed with Talaporfin sodium as a day treatment for central-type early-stage lung cancer. Photodiagnosis Photodyn. Ther. 2022, 38, 102836. [Google Scholar] [CrossRef] [PubMed]
- Yano, T.; Kasai, H.; Horimatsu, T.; Yoshimura, K.; Teramukai, S.; Morita, S.; Tada, H.; Yamamoto, Y.; Kataoka, H.; Kakushima, N.; et al. A multicenter phase II study of salvage photodynamic therapy using talaporfin sodium (ME2906) and a diode laser (PNL6405EPG) for local failure after chemoradiotherapy or radiotherapy for esophageal cancer. Oncotarget 2017, 8, 22135–22144. [Google Scholar] [CrossRef]
- Inoue, T.; Ishihara, R. Photodynamic therapy for esophageal cancer. Clin. Endosc. 2021, 54, 494–498. [Google Scholar] [CrossRef]
- Akimoto, J. Photodynamic therapy for malignant brain tumors. Neurol. Med. Chir. (Tokyo) 2016, 56, 151–157. [Google Scholar] [CrossRef]
- Muragaki, Y.; Akimoto, J.; Maruyama, T.; Iseki, H.; Ikuta, S.; Nitta, M.; Maebayashi, K.; Saito, T.; Okada, Y.; Kaneko, S.; et al. Phase II clinical study on intraoperative photodynamic therapy with talaporfin sodium and semiconductor laser in patients with malignant brain tumors. J. Neurosurg. 2013, 119, 845–852. [Google Scholar] [CrossRef]
- Suvorov, N.; Pogorilyy, V.; Diachkova, E.; Vasil’ev, Y.; Mironov, A.; Grin, M. Derivatives of natural chlorophylls as agents for antimicrobial photodynamic therapy. Int. J. Mol. Sci. 2021, 22, 6392. [Google Scholar] [CrossRef]
- Hargus, J.A.; Fronczek, F.R.; Vicente, M.G.H.; Smith, K.M. Mono-(L)-aspartylchlorin-e6. Photochem. Photobiol. 2007, 83, 1006–1015. [Google Scholar] [CrossRef]
- Cox, M.T.; Jackson, A.H.; Kenner, G.W.; McCombie, S.W.; Smith, K.M. Vinylporphyrin b-ketoesters. J. Chem. Soc. Perkin Trans. 1 1974, 516–527. [Google Scholar] [CrossRef]
- Jinadasa, R.G.W.; Hu, X.; Vicente, M.G.H.; Smith, K.M. Syntheses and cellular investigations of 173-, 152-, and 131-amino acid derivatives of chlorin e6. J. Med. Chem. 2011, 54, 7464–7476. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Jinadasa, R.G.W.; Jiao, L.; Fronczek, F.R.; Nguyen, A.L.; Smith, K.M. Chlorin-e6 131:152-anhydride: A key intermediate in conjugation reactions of chlorin-e6. Eur. J. Org. Chem. 2015, 2015, 3661–3665. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Humble, S.W.; Jinadasa, R.G.W.; Zhou, Z.; Nguyen, A.L.; Vicente, M.G.H.; Smith, K.M. Syntheses and PDT activity of new mono- and di-conjugated derivatives of chlorin e6. J. Porphyr. Phthalocyanines 2017, 21, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, L.; Wang, S.; Li, Y.; Zhang, F.; Song, B.; Zhao, W. Syntheses of new chlorin derivatives containing maleimide functional group and their photodynamic activity evaluation. Bioorg. Med. Chem. Lett. 2015, 25, 4078–4081. [Google Scholar] [CrossRef]
- Zhang, X.-J.; Han, G.-Y.; Guo, C.-Y.; Ma, Z.-Q.; Lin, M.-Y.; Wang, Y.; Miao, Z.-Y.; Zhang, W.-N.; Sheng, C.-Q.; Yao, J.-Z. Design, synthesis, and biological evaluation of novel 31-hexyloxychlorin-e6 based 152- or 131-amino acid derivatives as potent photosensitizers for photodynamic therapy. Eur. J. Med. Chem. 2020, 207, 112715. [Google Scholar] [CrossRef]
- Kustov, A.V.; Morshnev, P.K.; Kukushkina, N.V.; Smirnova, N.L.; Berezin, D.B.; Karimov, D.R.; Shukhto, O.V.; Kustova, T.V.; Belykh, D.V.; Mal’shakova, M.V.; et al. Solvation, cancer cell photoinactivation and the interaction of chlorin photosensitizers with a potential passive carrier non-ionic surfactant Tween 80. Int. J. Mol. Sci. 2022, 23, 5294. [Google Scholar] [CrossRef]




| Compound | Phototoxicity (IC50, μM) | Dark Toxicity (IC50, μM) |
|---|---|---|
| Chlorin e6 (14) | 20.8 | >400 |
| 173-LysChlorin e6TME | 26.2 | >400 |
| 152-AspChlorin e6DME | 4.0 | 373.1 |
| 152-LysChlorin e6TME | 28.8 | >400 |
| 152-AspPdChlorin e6DME | 16.7 | 324.8 |
| 152-LysPdChlorin e6TME | 3.3 | >400 |
| 131-AspChlorin e6DME | 0.6 | 284.6 |
| 131-βAlaAspChlorin e6DME | 0.8 | 383.9 |
| 131-EDLysChlorin e6DME 1 | 1.3 | 268.4 |
| 131-LysChlorin e6TME | 0.6 | 34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vicente, M.d.G.H.; Smith, K.M. Amino Acid Derivatives of Chlorin-e6—A Review. Molecules 2023, 28, 3479. https://doi.org/10.3390/molecules28083479
Vicente MdGH, Smith KM. Amino Acid Derivatives of Chlorin-e6—A Review. Molecules. 2023; 28(8):3479. https://doi.org/10.3390/molecules28083479
Chicago/Turabian StyleVicente, Maria da Graça H., and Kevin M. Smith. 2023. "Amino Acid Derivatives of Chlorin-e6—A Review" Molecules 28, no. 8: 3479. https://doi.org/10.3390/molecules28083479
APA StyleVicente, M. d. G. H., & Smith, K. M. (2023). Amino Acid Derivatives of Chlorin-e6—A Review. Molecules, 28(8), 3479. https://doi.org/10.3390/molecules28083479







