Altered Glycosylation in Progression and Management of Bladder Cancer
Abstract
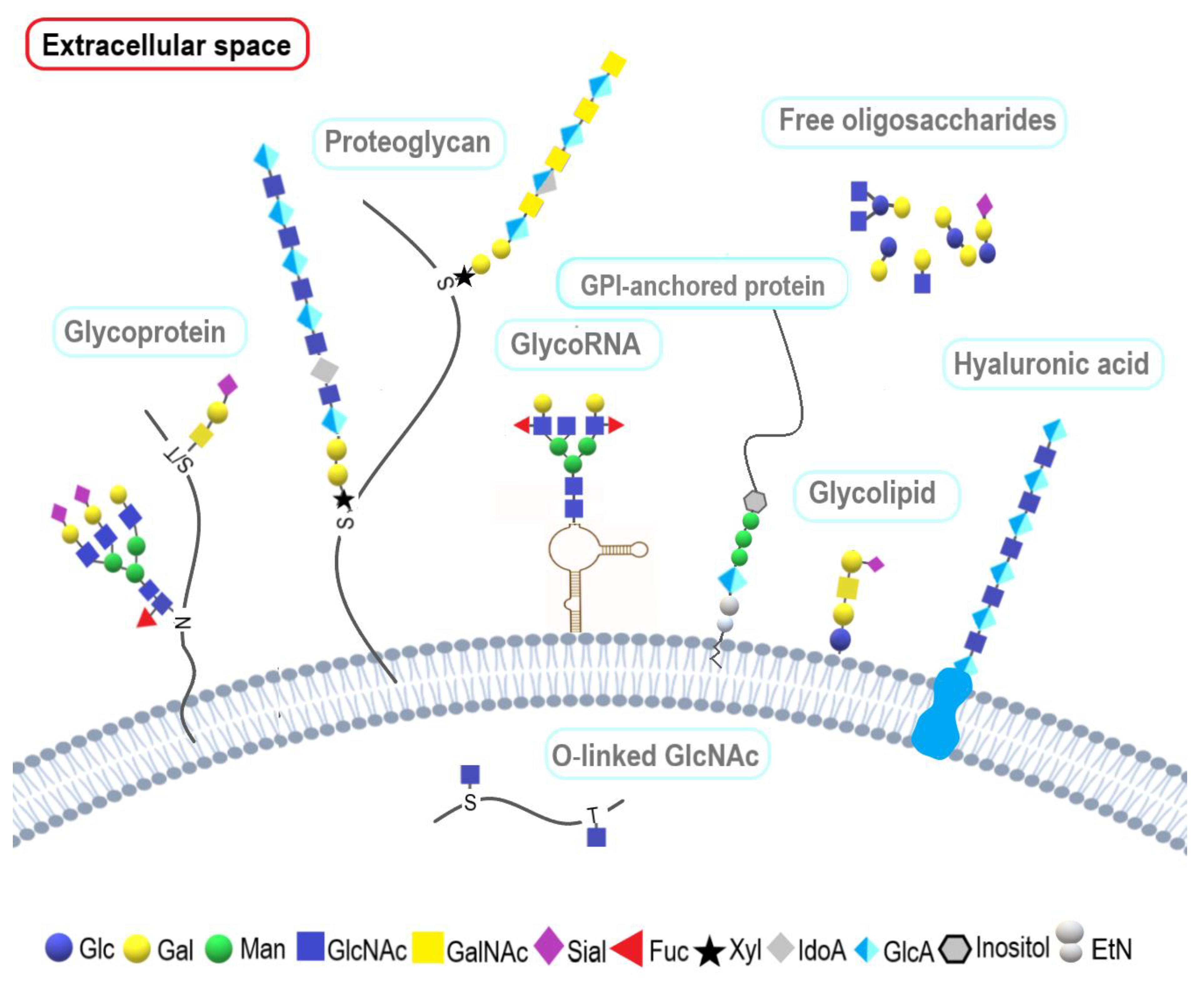
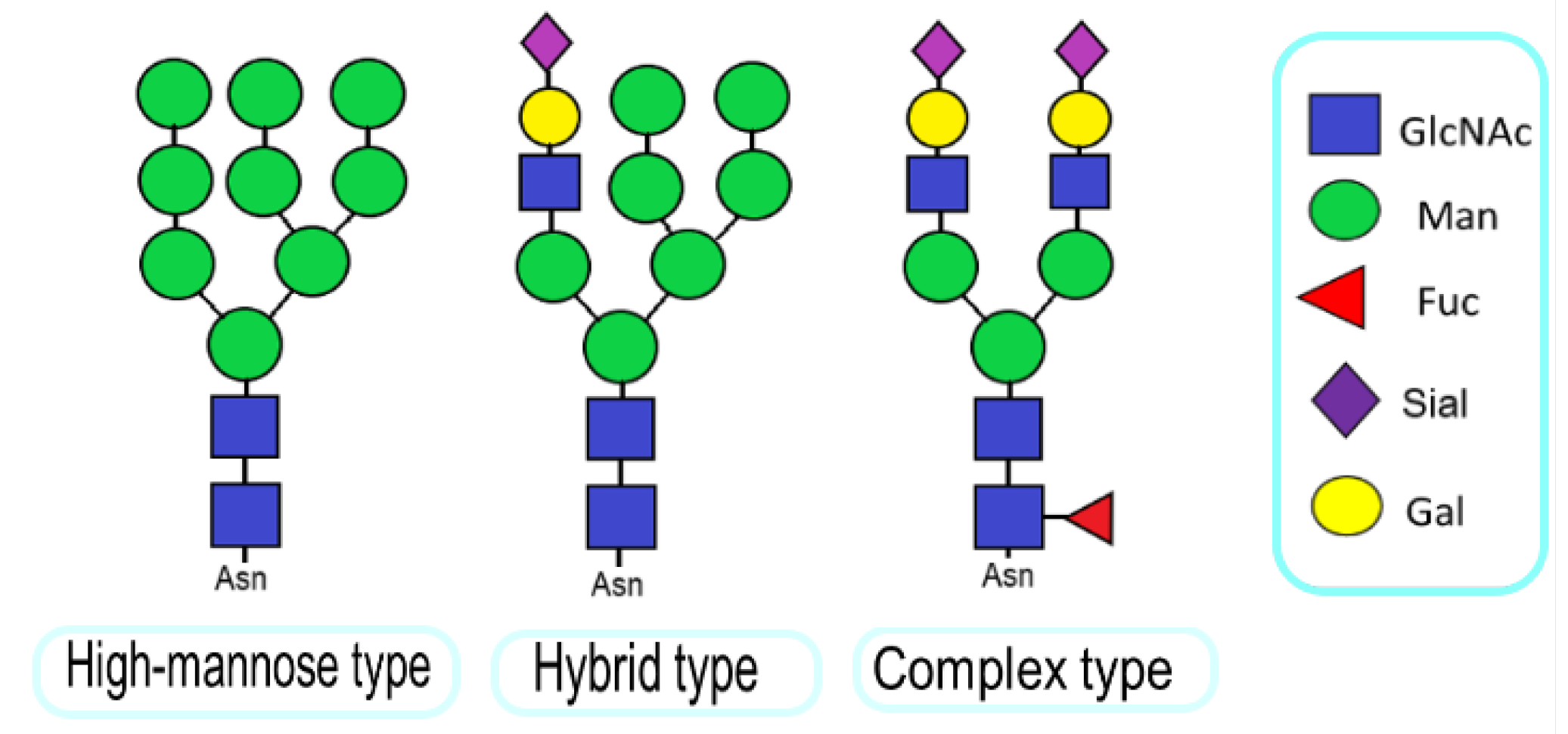
1. Introduction
2. Alteration in Glycosylation Observed in Cancer
| Change in Glycosylation | Role in Cancer | References |
|---|---|---|
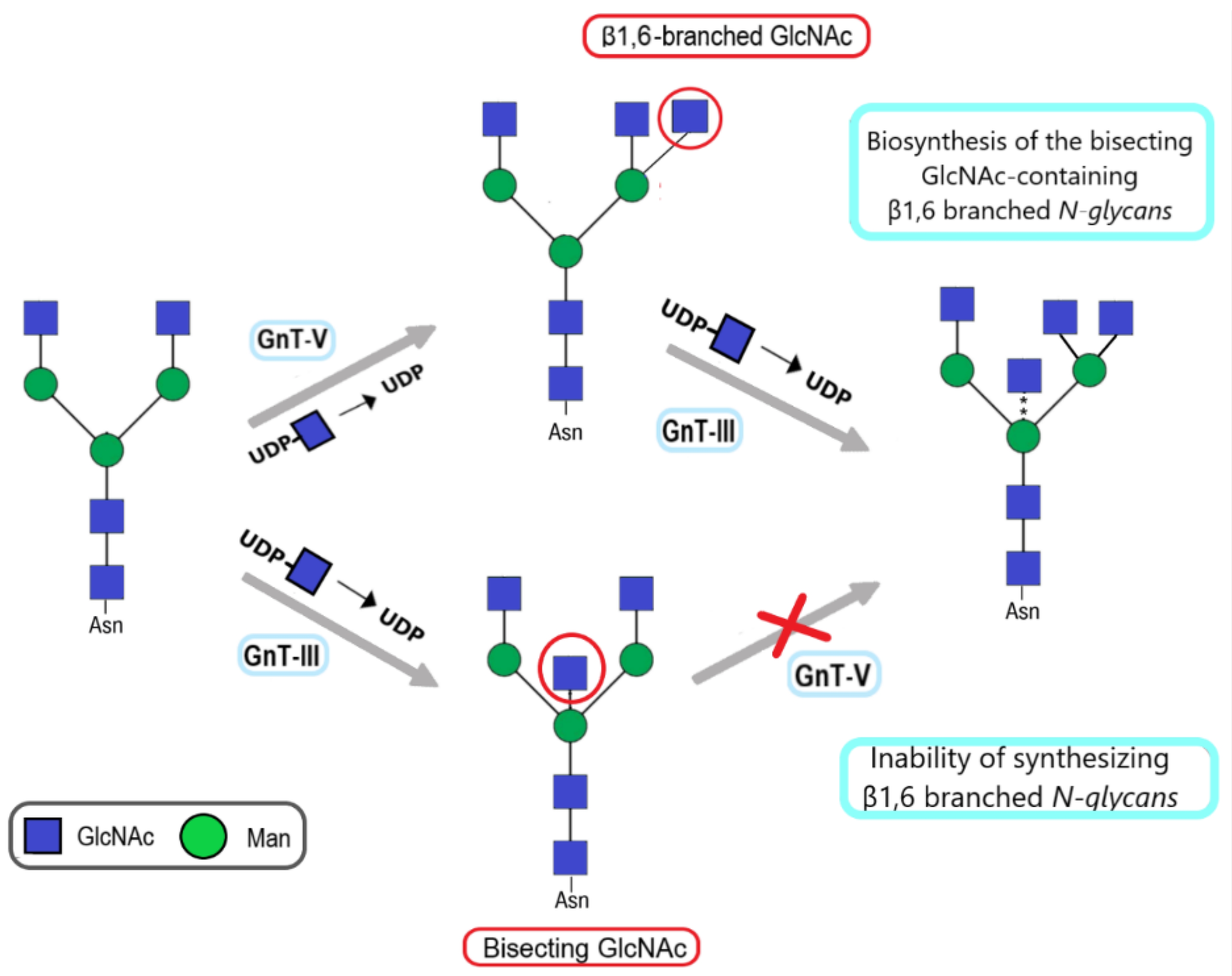
| Increase in β1,6-branched N-glycans due to overexpression of N-acetylglucosaminyltransferase V (gnt-V) | Increased rate of metastases in mice | [19,21,22,23] |
| Increase in β1,4-branched tetra-antennary N-glycan due to overexpression of N-acetylglucosaminyltransferase IV (gnt-IV) | Enhanced tumor progression by lattice formation via galectin binding to poly-N-acetyllactosamines (lacnac), and the formation of sialyl-Lewis X (slex) | [19,23,24] |
| Increase in N-glycans core fucosylation due to overexpression of α-1,6-fucosyltransferase (FUT8) | Promotion of lung cancer and melanoma progression, a critical role in antibody-dependent cellular cytotoxicity (ADCC) and immune evasion, the regulation of TGF-β, EGF, α3β1 integrin, and E-cadherin function | [19,23,25] |
| Increase in bisecting glcnac in N-glycans due to overexpression of N-acetylglucosaminyltransferase III (gnt-III) | Suppression of tumor progression in melanoma and mouse mammary tumors | [23,26] |
| Presence of Tn and T antigens and their sialylated glycoforms, sialyl-Tn (stn) and sialyl-T (ST), respectively | Interference with immune cell recognition and blocking or masking of antigenic peptides presentation by major histocompatibility complex (MHC) molecules; enhanced tumorigenic and invasive properties and promotion of immunosuppression | [23] |
| Increase in N-glycan α2,6 sialylation due to β-galactoside α2,6-sialyltransferase 1 (ST6GAL1) up-regulation | Increased integrin-mediated cell motility and protection from apoptosis induced by galectins, death receptor ligands, and chemotherapeutic drugs | [27] |
| A2,8-linked polysialic acids (polysia) | Reduced tumor cell anchorage to extracellular matrix components, making it easier for cancer cells to enter the bloodstream and interact with platelets | [14] |
| N-acetylglucosaminylacylation (O-glcnacylation) | Cancer risk factor, excessive nutritional intake | [28] |
| O-acetylated gangliosides | Protection from apoptosis | [23] |
| Metabolic incorporation of diet-derived N-glycolylneuraminic acid (Neu5Gc) into human glycans | Promotion of tumor growth by enhancing chronic inflammation and angiogenesis | [23] |
| Sialylation of Lewisx and Lewisx/a | Crucial in extravasation | [14] |
| Slex and slea epitopes on glycosphingolipids | Metastatic potential in mice and tumor progression, metastatic spread, and poor prognosis of patients | [19] |
| Sialyl-Lewis-related structures | Influencing tumor progression by interacting with Siglecs that have immunosuppressive functions | [23] |
| Complete loss of glycosylphosphatidylinositol (GPI)-anchored proteins | Observed in some cases of malignant and premalignant states involving the hematopoietic system | [23] |
3. The Role of β1,6-Branched N-Glycans in Bladder Cancer
4. The Role of Bisecting N-Acetylglucosamine in Bladder Cancer
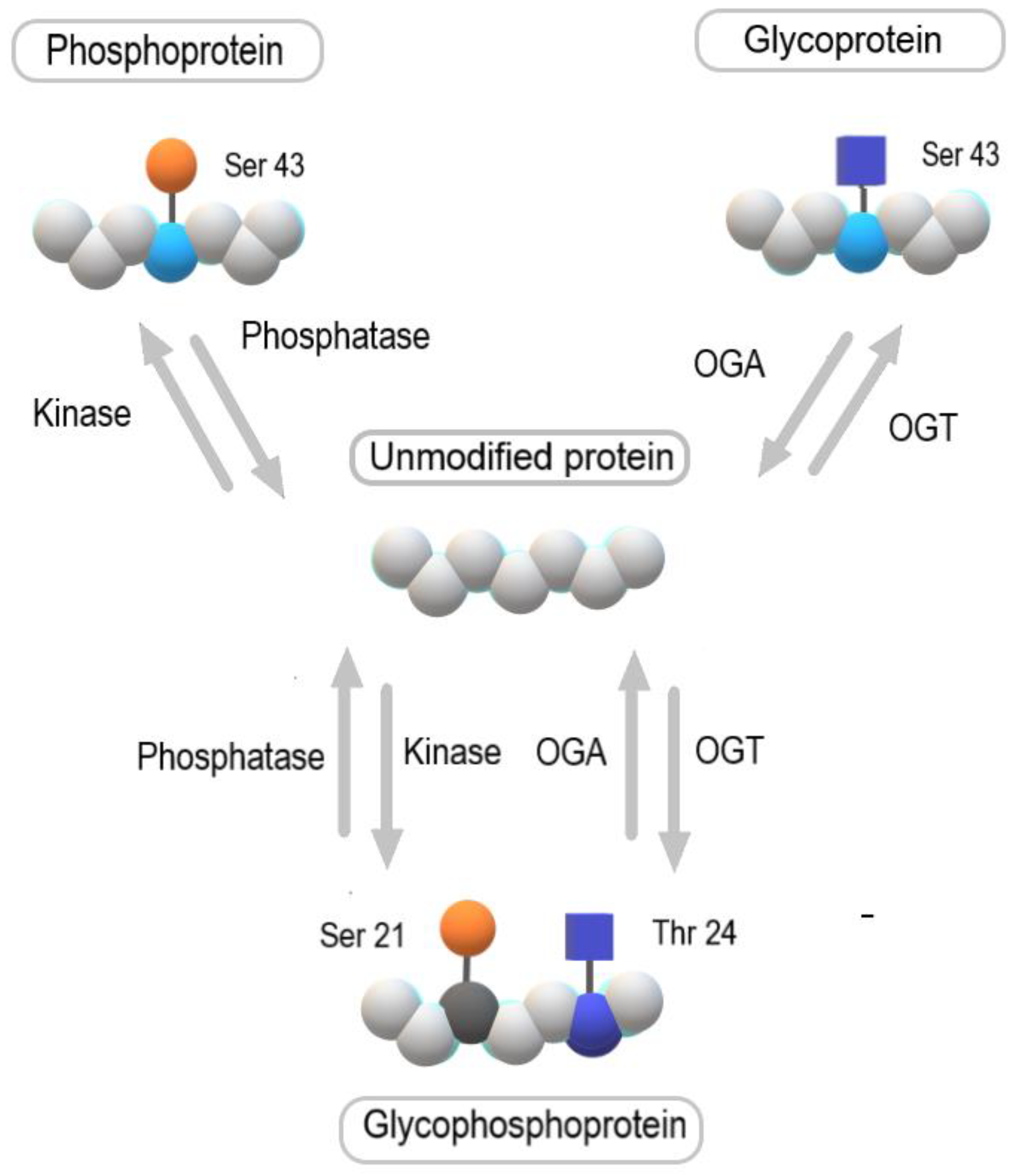
5. The Role of O-GlcNAcylation in Bladder Cancer
6. The Role of Total Sialylation in Bladder Cancer
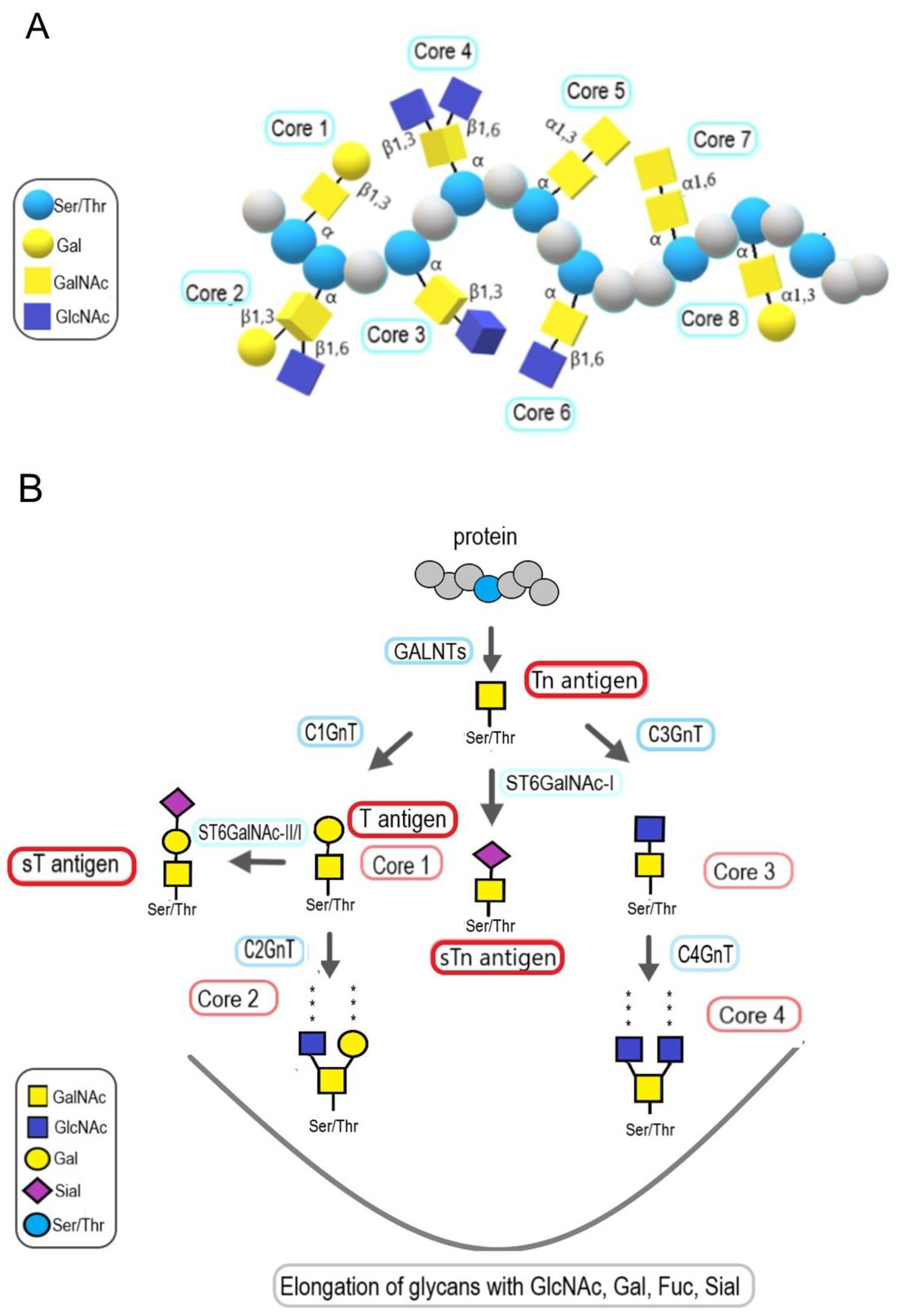
7. The Role of Mucin-Type O-Glycans in Bladder Cancer
7.1. The Role of Mucin Expression in Bladder Cancer
7.2. The Role of Tn and T Antigens in Bladder Cancer
7.3. The Role of Core 2 Structures in Bladder Cancer
8. Fucosylation in Bladder Cancer
9. Lewis Antigens in Bladder Cancer
10. Glycosylation of Extracellular Vesicles in Bladder Cancer
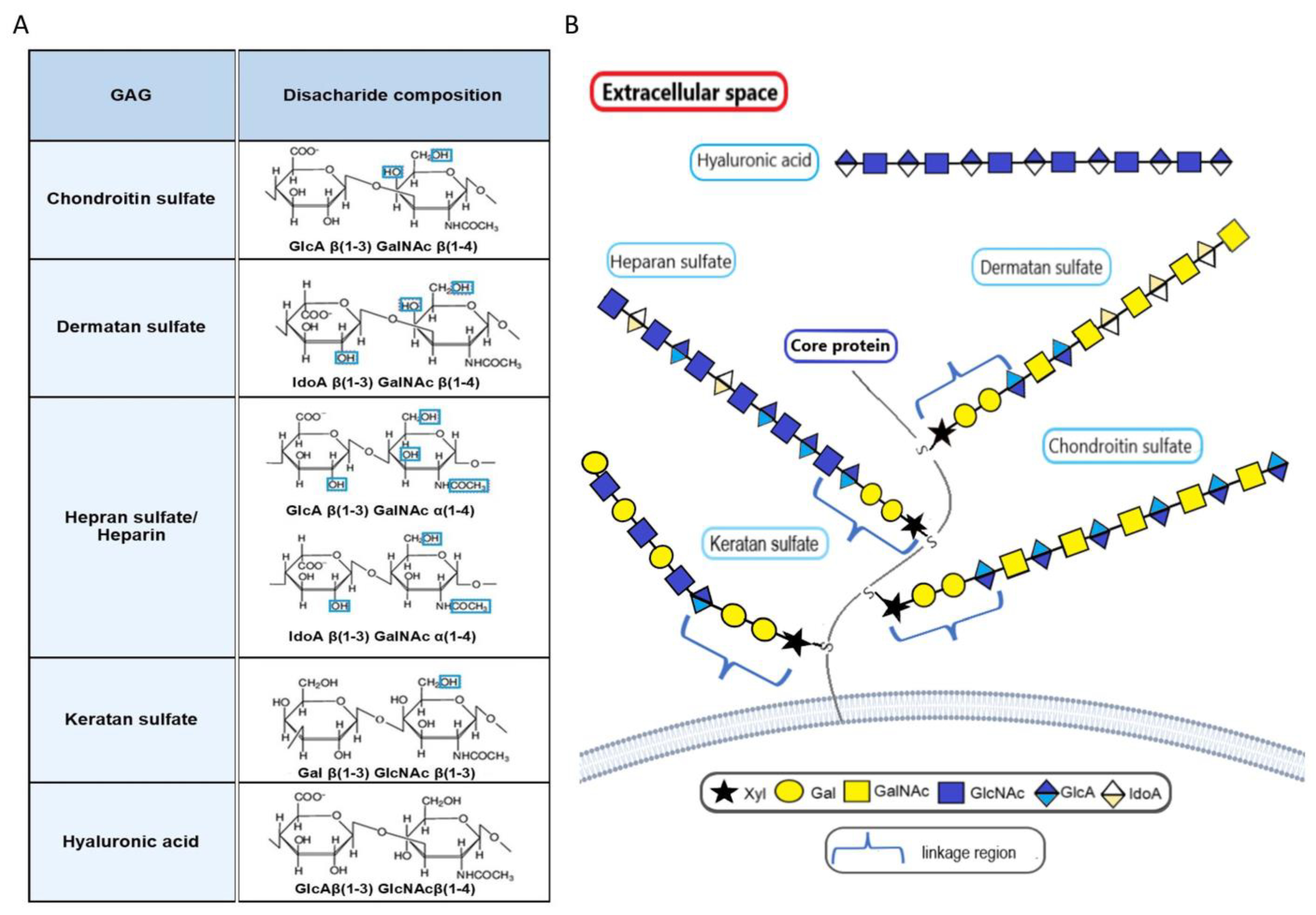
11. Proteoglycans in Bladder Cancer
12. Manipulation of Glycome as a Therapeutic Tool in Bladder Cancer
13. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| β4GalT1 | β1:4-galactosyltransferase-1 |
| 2DG | 2-deoxy-d-glucose |
| ADCC | antibody-dependent cellular cytotoxicity |
| AGP | α-1-Acid glycoprotein |
| BC | Bladder cancer |
| BCG | Bacillus Calmette-Guérin |
| C1GnT | Core 1 β1,3-galactosyltransferase |
| CAR T | Chimeric antigen receptor T |
| CD44 | Cluster of Differentiation 44 |
| CDK5 | Cyclin-dependent-like kinase 5 |
| CRT | Calreticulin |
| CSCs | Cancer stem cells |
| CTCs | Circulating tumor cells |
| CTLs | Cytotoxic T-lymphocytes |
| DCs | Dendritic cells |
| ECM | Extracellular matrix |
| EGF | Epidermal growth factor |
| EMT | Epithelial-mesenchymal transition |
| EPI | Epirubicin |
| EVs | Extracellular vesicles |
| FUTs | Fucosyltransferases |
| GlcNAc | N-acetylglucosamine |
| GnT III | N-acetylglucosaminyltransferase III |
| GnT IV | N-acetylglucosaminyltransferase IV |
| GnT V | N-acetylglucosaminyltransferase V |
| GPI | Glycosylphosphatidylinositol |
| GPI-IL-2 | Glycosyl-phosphatidylinositol-anchored interleukin 2 |
| HLA | Human leukocyte antigen |
| Igs | Immunoglobulins |
| ITGA3 | α3 integrin subunit |
| Lea | Lewisa |
| Leb | Lewisb |
| Lex | Lewisx |
| Ley | Lewisy |
| MIBC | Muscle-infiltrating bladder cancer |
| Neu5Ac | N-acetyl-neuraminic acid |
| Neu5Gc | N-glycolyl-neuraminic acid |
| NK | Natural killer |
| NMIBC | Non-muscle-infiltrating bladder cancer |
| OGT | O-GlcNAc transferase |
| PHAL | Phaseolus vulgaris leucoagglutinin |
| Sia | Sialic acids |
| SLex | sialyl-Lewis X |
| ST antigen | Sialilated T antigen |
| ST6GALNAC-I | ST6 N-acetylgalactosaminide α-2,6-sialyltransferase 1 |
| TACA | Tumor-associated carbohydrate antigens |
| TGFβ | Transforming growth factor β |
| TURBT | Transurethral resection of bladder tumor |
| UC | Urothelial carcinomas |
| UEA | Ulex europaeus agglutinin |
References
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Padala, S.A.; Barsouk, A. Epidemiology of bladder cancer. Med. Sci. 2020, 8, 15. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 7, 209–249. [Google Scholar] [CrossRef]
- Chalasani, V.; Chin, J.L.; Izawa, J.I. Histologic variants of urothelial bladder cancer and nonurothelial histology in bladder cancer. Can. Urol. Assoc. J. 2009, 3 (Suppl. 4), S193–S198. [Google Scholar] [CrossRef] [PubMed]
- Yousef, P.G.; Gabril, M.Y. An update on the molecular pathology of urinary bladder tumors. Pathol. Res. Pract. 2017, 214, 1–6. [Google Scholar] [CrossRef]
- Nadal, R.; Bellmunt, J. Management of metastatic bladder cancer. Cancer Treat. Rev. 2019, 76, 10–21. [Google Scholar] [CrossRef]
- Sternberg, C.N.; Bellmunt, J.; Sonpavde, G.; Siefker-Radtke, A.O.; Stadler, W.M.; Bajorin, D.F.; Dreicer, R.; George, D.J.; Milowsky, M.I.; Theodorescu, D.; et al. ICUD-EAU International Consultation on Bladder Cancer 2012: Chemotherapy for urothelial carcinoma-neoadjuvant and adjuvant settings. Eur. Urol. 2013, 63, 58–66. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, W.J. Can we use methylation markers as diagnostic and prognostic indicators for bladder cancer? Investig. Clin. Urol. 2016, 57 (Suppl. 1), S77–S88. [Google Scholar] [CrossRef] [PubMed]
- Flynn, R.A.; Pedram, K.; Malaker, S.A.; Batista, P.J.; Smith, B.A.H.; Johnson, A.G.; George, B.M.; Majzoub, K.; Villalta, P.W.; Carette, J.E.; et al. Small RNAs are modified with N-glycans and displayed on the surface of living cells. Cells 2021, 184, 3109–3124.e22. [Google Scholar] [CrossRef] [PubMed]
- Varki, A. Biological roles of glycans. Glycobiology 2017, 27, 3–49. [Google Scholar] [CrossRef]
- Stambuk, T.; Klasic, M.; Zoldos, V.; Lauc, G. N-glycans as functional effectors of genetic and epigenetic disease risk. Mol. Asp. Med. 2021, 79, 100891. [Google Scholar] [CrossRef]
- Lauc, G.; Krištić, J.; Zoldoš, V. Glycans—The third revolution in evolution. Front. Genet. 2014, 5, 145. [Google Scholar] [CrossRef] [PubMed]
- Kristic, J.; Zaytseva, O.O.; Ram, R.; Nguyen, Q.; Novokmet, M.; Vuckovic, F.; Vilaj, M.; Trbojevic-Akmacic, I.; Pezer, M.; Davern, K.M.; et al. Profiling and genetic control of the murine immunoglobulin glycome. Nat. Chem. Biol. 2018, 14, 516–524. [Google Scholar] [CrossRef]
- Reis, C.A.; Osorio, H.; Silva, L.; Gomes, C.; David, L. Alterations in glycosylation as biomarkers for cancer detection. J. Clin. Pathol. 2010, 63, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Hoja-Łukowicz, D.; Przybyło, M.; Duda, M.; Pocheć, E.; Bubka, M. On the trail of the glycan codes stored in cancer-related cell adhesion proteins. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 3237–3257. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef]
- RodrÍguez, E.; Schetters, S.; van Kooyk, Y. The tumour glyco-code as a novel immune checkpoint for immunotherapy. Nat. Rev. Immunol. 2018, 18, 204–211. [Google Scholar] [CrossRef]
- Mereiter, S.; Balmaña, M.; Campos, D.; Gomes, J.; Reis, C.A. Glycosylation in the era of cancer-targeted therapy: Where are we heading? Cancer Cell 2019, 36, 6–16. [Google Scholar] [CrossRef]
- Drickamer, K.; Taylor, M.E. Introduction to Glycobiology, 2nd ed.; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Stanley, P.; Moremen, K.W.; Lewis, N.E.; Taniguchi, N.; Aebi, M. N-glycans. In Essentials of Glycobiology, 4th ed.; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Mohnen, D., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press: Long Island, NY, USA, 2022; Chapter 9. [Google Scholar] [CrossRef]
- Zoqlam, R.; Lazauskaite, S.; Glickman, S.; Zaitseva, L.; Ilie, P.C.; Qi, S. Emerging molecular mechanisms and genetic targets for developing novel therapeutic strategies for treating bladder diseases. Eur. J. Pharm. Sci. 2022, 173, 106167. [Google Scholar] [CrossRef]
- Miyoshi, E.; Terao, M.; Kamada, Y. Physiological roles of N-acetylglucosaminyltransferase V (GnT-V) in mice. BMB Rep. 2012, 45, 554–559. [Google Scholar] [CrossRef]
- Nagae, M.; Kizuka, Y.; Mihara, E.; Kitago, Y.; Hanashima, S.; Ito, Y.; Takagi, J.; Taniguchi, N.; Yamaguchi, Y. Structure and mechanism of cancer-associated N-acetylglucosaminyltransferase-V. Nat. Commun. 2018, 9, 3380. [Google Scholar] [CrossRef]
- Bellis, S.L.; Reis, C.A.; Varki, A.; Kannagi, R.; Stanley, P. Glycosylation Changes in Cancer. In Essentials of Glycobiology, 4th ed.; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Mohnen, D., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press: Long Island, NY, USA, 2022; Chapter 47. [Google Scholar] [CrossRef]
- Kizuka, Y.; Taniguchi, N. Enzymes for N-glycan branching and their genetic and nongenetic regulation in cancer. Biomolecules 2016, 6, 25. [Google Scholar] [CrossRef]
- Bastian, K.; Scott, E.; Elliott, D.J.; Munkley, J. FUT8 alpha-(1,6)-fucosyltransferase in cancer. Int. J. Mol. Sci. 2021, 22, 455. [Google Scholar] [CrossRef] [PubMed]
- Link-Lenczowski, P.; Bubka, M.; Balog, C.I.A.; Koeleman, C.A.M.; Butters, T.D.; Wuhrer, M.; Lityńska, A. The glycomic effect of N-acetylglucosaminyltransferase III overexpression in metastatic melanoma cells. GnT-III modifies highly branched N-glycans. Glycoconj. J. 2018, 35, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Garnham, R.; Scott, E.; Livermore, K.E.; Munkley, J. ST6GAL1: A key player in cancer. Oncol. Lett. 2019, 20, 983–989. [Google Scholar] [CrossRef]
- Fardini, Y.; Dehennaut, V.; Lefebvre, T.; Issad, T. O-GlcNAcylation: A new cancer hallmark? Front. Endocrinol. 2013, 4, 99. [Google Scholar] [CrossRef] [PubMed]
- Hirata, T.; Nagae, M.; Osuka, R.F.; Mishra, S.K.; Yamada, M.; Kizuka, Y. Recognition of glycan and protein substrates by N-acetylglucosaminyltransferase-V. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129726. [Google Scholar] [CrossRef]
- Osuka, R.F.; Hirata, T.; Nagae, M.; Nakano, M.; Shibata, H.; Okamoto, R.; Kizuka, Y. N-acetylglucosaminyltransferase-V requires a specific noncatalytic luminal domain for its activity toward glycoprotein substrates. J. Biol. Chem. 2022, 298, 101666. [Google Scholar] [CrossRef]
- Demetriou, M.; Granovsky, M.; Quaggin, S.; Dennis, J.W. Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature 2001, 409, 733–739. [Google Scholar] [CrossRef]
- Taniguchi, N.; Kizuka, Y. Glycans and cancer: Role of N-Glycans in cancer biomarker, progression and metastasis, and therapeutics. Adv. Cancer Res. 2015, 126, 11–51. [Google Scholar] [CrossRef]
- Zhao, Y.; Sato, Y.; Isaji, T.; Fukuda, T.; Matsumoto, A.; Miyoshi, E.; Gu, J.; Taniguchi, N. Branched N-glycans regulate the biological functions of integrins and cadherins. FEBS J. 2008, 275, 1939–1948. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Takahashi, M.; Gu, J.G.; Miyoshi, E.; Matsumoto, A.; Kitazume, S.; Taniguchi, N. Functional roles of N-glycans in cell signaling and cell adhesion in cancer. Cancer Sci. 2008, 99, 1304–1310. [Google Scholar] [CrossRef]
- Przybyło, M.; Lityńska, A.; Pocheć, E. Different adhesion and migration properties of human HCV29 non-malignant urothelial and T24 bladder cancer cells: Role of glycosylation. Biochimie 2005, 87, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Olden, K.; Breton, P.; Grzegorzewski, K.; Yasuda, Y.; Gause, B.L.; Oredipe, O.A.; Newton, S.A.; White, S.L. The potential importance of swainsonine in therapy of cancer and immunology. Pharmacol. Ther. 1991, 50, 285–290. [Google Scholar] [CrossRef]
- Lityńska, A.; Przybyło, M.; Pocheć, E.; Laidler, P. Adhesion properties of human bladder cell lines with extracellular matrix components: The role of integrins and glycosylation. Acta Biochim. Pol. 2002, 49, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Pocheć, E.; Lityńska, A.; Bubka, M.; Amoresano, A.; Casbarra, A. Characterization of the oligosaccharide component of α3β1 integrin from human bladder carcinoma cell line T24 and its role in adhesion and migration. Eur. J. Cell Biol. 2006, 85, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Lityńska, A.; Przybyło, M.; Ksiazek, D.; Laidler, P. Differences of α3β1 integrin glycans from different human bladder cell lines. Acta Biochim. Pol. 2000, 47, 427–434. [Google Scholar] [CrossRef]
- Laidler, P.; Gil, D.; Pituch-Noworolska, A.; Ciołczyk, D.; Ksiazek, D.; Przybyło, M.; Lityńska, A. Expression of β1-integrins and N-cadherin in bladder cancer and melanoma cell lines. Acta Biochim. Pol. 2000, 47, 1159–1170. [Google Scholar] [CrossRef]
- Przybyło, M.; Hoja-Lukowicz, D.; Litynska, A.; Laidler, P. Different glycosylation of cadherins from human bladder non-malignant and cancer cell lines. Cancer Cell Int. 2002, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Ishimura, H.; Takahashi, T.; Nakagawa, H.; Nishimura, S.I.; Arai, Y.; Horikawa, Y.; Habuchi, T.; Miyoshi, E.; Kyan, A.; Hagisawa, S.; et al. N-acetylglucosaminyltransferase V and β1-6 branching N-linked oligosaccharides are associated with good prognosis of patients with bladder cancer. Clin. Cancer Res. 2006, 12, 2506–2511. [Google Scholar] [CrossRef]
- Takahashi, T.; Hagisawa, S.; Yoshikawa, K.; Tezuka, F.; Kaku, M.; Ohyama, C. Predictive value of N-acetylglucosaminyltransferase-V for superficial bladder cancer recurrence. J. Urol. 2006, 175, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.M.; Zhang, X.Y.; Chen, H.L.; Wang, G.M.; Zhang, Y.K. Structural alterations of sugar chains in urine fibronectin from bladder cancer patients and its enzymatic mechanism. J. Cancer Res. Clin. Oncol. 2001, 127, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Karavana, S.Y.; Şenyiğit, Z.A.; Çalışkan, C.; Sevin, G.; Özdemir, D.I.; Erzurumlu, Y.; Şen, S.; Baloğlu, E. Gemcitabine hydrochloride microspheres used for intravesical treatment of superficial bladder cancer: A comprehensive in vitro/ex vivo/in vivo evaluation. Drug Des. Dev. Ther. 2018, 12, 1959–1975. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Cong, X.; Wang, S.; Fang, S.; Dong, X.; Yuan, Y.; Fan, J. GnT-V promotes chemosensitivity to gemcitabine in bladder cancer cells through β1,6 GlcNAc branch modification of human equilibrative nucleoside transporter 1. Biochem. Biophys. Res. Commun. 2018, 503, 3142–3148. [Google Scholar] [CrossRef] [PubMed]
- Stanley, P. Biological consequences of overexpressing or eliminating N-acetylglucosaminyltransferase-TIII in the mouse. Biochim. Biophys. Acta Gen. Subj. 2002, 1573, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Hasegawa, Y.; Maeda, K.; Kitano, M.; Taniguchi, N. Role of glycosyltransferases in carcinogenesis; growth factor signaling and EMT/MET programs. Glycoconj. J. 2022, 39, 167–176. [Google Scholar] [CrossRef]
- Tanaka, T.; Yoneyama, T.; Noro, D.; Imanishi, K.; Kojima, Y.; Hatakeyama, S.; Tobisawa, Y.; Mori, K.; Yamamoto, H.; Imai, A.; et al. Aberrant N-glycosylation profile of serum immunoglobulins is a diagnostic biomarker of urothelial carcinomas. Int. J. Mol. Sci. 2017, 18, 2632. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Qian, K. Proteins O-GlcNAcylation: Emerging mechanisms and functions. Nat. Rev. Mol. Cell Biol. 2017, 18, 452–465. [Google Scholar] [CrossRef]
- Hart, G.W. Nutrient regulation of signaling and transcription. J. Biol. Chem. 2019, 294, 2211–2231. [Google Scholar] [CrossRef] [PubMed]
- Mueller, T.; Ouyang, X.; Johnson, M.S.; Qian, W.J.; Chatham, J.C.V.; Darley-Usmar, V.; Zhang, J. New insights into the biology of protein o-glcnacylation: Approaches and observations. Front. Aging 2021, 1, 620382. [Google Scholar] [CrossRef]
- Elbatrawy, A.A.; Kim, E.J.; Nam, G. O-GlcNAcase: Emerging Mechanism, Substrate Recognition and Small-Molecule Inhibitors. Chem. Med. Chem. 2020, 14, 1244–1257. [Google Scholar] [CrossRef]
- Hart, G.W.; Slawson, C.; Ramirez-Correa, G.; Lagerlof, O. Cross talk between O-GlcNAcylation and phosphorylation: Roles in signaling, transcription, and chronic disease. Annu. Rev. Biochem. 2011, 80, 825–858. [Google Scholar] [CrossRef]
- Bond, M.R.; Hanover, J.A. A little sugar goes a long way: The cell biology of O-GlcNAc. J. Cell Biol. 2015, 208, 869–880. [Google Scholar] [CrossRef]
- Nie, H.; Yi, W. O-GlcNAcylation, a sweet link to the pathology of diseases. J. Zhejiang Univ. Sci. B 2019, 20, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Pyo, K.H.; Kim, H.R. Role and Function of O-GlcNAcylation in Cancer. Cancers 2021, 13, 5365. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Zhang, X.; Liang, T.; Bai, X. O-GlcNAcylation: An important post-translational modification and a potential therapeutic target for cancer therapy. Mol. Med. 2022, 28, 115. [Google Scholar] [CrossRef] [PubMed]
- Rozanski, W.; Krzeslak, A.; Forma, E.; Brys, M.; Blewniewski, M.; Wozniak, P.; Lipinski, M. Prediction of bladder cancer based on urinary content of MGEA5 and OGT mRNA level. Clin. Lab. 2012, 58, 579–583. [Google Scholar] [PubMed]
- Wang, L.; Chen, S.; Zhang, Z.; Zhang, J.; Mao, S.; Zheng, J.; Xuan, Y.; Liu, M.; Cai, K.; Zhang, W.; et al. Suppressed OGT expression inhibits cell proliferation while inducing cell apoptosis in bladder cancer. BMC Cancer 2018, 18, 1141. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Lu, M.H.; Dai, G.C.; Yao, Q.; Xiang, H.; Wang, L.X.; Xue, B.X.; Liu, X. O-GlcNAcylation promotes malignant phenotypes of bladder cancer cells. Neoplasma 2020, 67, 880–888. [Google Scholar] [CrossRef]
- Wu, J.; Tan, Z.; Li, H.; Lin, M.; Jiang, Y.; Liang, L.; Ma, O.; Gou, J.; Ning, L.; Li, X.; et al. Melatonin reduces proliferation and promotes apoptosis of bladder cancer cells by suppressing O-GlcNAcylation of cyclin-dependent-like kinase 5. J. Pineal Res. 2021, 71, e12765. [Google Scholar] [CrossRef]
- Jin, L.; Yuan, F.; Dai, G.; Yao, Q.; Xiang, H.; Wang, L.; Xue, B.; Shan, Y.; Liu, X. Blockage of O-linked GlcNAcylation induces AMPK-dependent autophagy in bladder cancer cells. Cell. Mol. Biol. Lett. 2020, 5, 17. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, J.; Zhang, W.; Kadier, A.; Wang, R.; Zhang, H.; Yao, X. O-GlcNAcylation enhances NUSAP1 stability and promotes bladder cancer aggressiveness. Onco. Targets Ther. 2021, 14, 445–454. [Google Scholar] [CrossRef]
- Lee, H.W.; Kang, M.J.; Kwon, Y.J.; Nansa, S.A.; Jung, E.H.; Kim, S.H.; Lee, S.J.; Jeong, K.C.; Kim, Y.; Cheong, H.; et al. Targeted inhibition of O-linked β-N-acetylglucosamine transferase as a promising therapeutic strategy to restore chemosensitivity and attenuate aggressive tumor traits in chemoresistant urothelial carcinoma of the bladder. Biomedicines 2021, 10, 1162. [Google Scholar] [CrossRef]
- Harduin-Lepers, A.; Krzewinski-Recchi, M.A.; Colomb, F.; Foulquier, F.; Groux-Degroote, S.; Delannoy, P. Sialyltransferases functions in cancers. Front. Biosci. 2012, 4, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, X. Sialic acid metabolism and sialyltransferases: Natural functions and applications. Appl. Microbiol. Biotechnol. 2012, 94, 887–905. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, T.; Takahashi, K.; Hata, K.; Shiozaki, K.; Yamaguchi, K. Sialidase significance for cancer progression. Glycoconj. J. 2012, 29, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Vajaria, B.N.; Patel, K.R.; Begum, R.; Patel, P.S. Sialylation: An avenue to target cancer cells. Pathol. Oncol. Res. 2016, 22, 443–447. [Google Scholar] [CrossRef]
- Kolasińska, E.; Przybyło, M.; Janik, M.; Lityńska, A. Towards understanding the role of sialylation in melanoma progression. Acta Biochim. Pol. 2016, 63, 533–541. [Google Scholar] [CrossRef]
- Bordron, A.; Bagacean, C.; Mohr, A.; Tempescul, A.; Bendaoud, B.; Deshayes, S.; Dalbies, F.; Buors, C.; Saad, H.; Berthou, C.; et al. Resistance to complement activation, cell membrane hypersialylation and relapses in chronic lymphocytic leukemia patients treated with rituximab and chemotherapy. Oncotarget 2018, 9, 31590–31605. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Ding, J. Sialylation is involved in cell fate decision during development, reprogramming and cancer progression. Protein Cell 2019, 10, 550–565. [Google Scholar] [CrossRef] [PubMed]
- Dobie, C.; Skropeta, D. Insights into the role of sialylation in cancer progression and metastasis. Br. J. Cancer 2021, 124, 76–90. [Google Scholar] [CrossRef]
- Xu, C.; Wang, S.; Wu, Y.; Sun, X.; Yang, D.S. Recent advance in understanding the roles of sialyltransferases in tumor angiogenesis and metastasis. Glycoconj. J. 2021, 38, 119–127. [Google Scholar] [CrossRef]
- Dalangood, S.; Zhu, Z.; Ma, Z.; Li, J.; Zeng, Q.; Yan, Y.; Shen, B.; Yan, J.; Huang, R. Identification of glycogene-type and validation of ST3GAL6 as a biomarker predicts clinical outcome and cancer cell invasion in urinary bladder cancer. Theranostics 2020, 10, 10078–10091. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.S.; Volkmer, J.P.; Weissman, I. Cancer stem cells in bladder cancer: A revisited and evolving concept. Curr. Opin. Urol. 2010, 20, 393–397. [Google Scholar] [CrossRef]
- Chen, D.; Li, D.; Cui, Z.; Zhang, C.; Zhang, Z.; Yan, L. Evaluation of the value of preoperative sialic acid levels in diagnosis and localization of urothelial tumors. J. Cancer 2021, 12, 5066–5075. [Google Scholar] [CrossRef] [PubMed]
- Bennett, E.P.; Mandel, U.; Clausen, H.; Gerken, T.A.; Fritz, T.A.; Tabak, L.A. Control of mucin-type O-glycosylation: A classification of the polypeptide GalNAc-transferase gene family. Glycobiology 2012, 22, 736–756. [Google Scholar] [CrossRef]
- Dhanisha, S.S.; Guruvayoorappan, C.; Drishya, S.; Abeesh, P. Mucins: Structural diversity, biosynthesis, its role in pathogenesis and as possible therapeutic targets. Crit. Rev. Oncol. Hematol. 2018, 122, 98–122. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Du, Y.; Yang, Z.; He, L.; Wang, Y.; Hao, L.; Ding, M.; Yan, R.; Wang, J.; Fan, Z. GALNT1- mediated glycosylation and activation of Sonic Hedgehog Signaling maintains the self-renewal and tumor-initiating capacity of bladder cancer stem cells. Cancer Res. 2016, 76, 1273–1283. [Google Scholar] [CrossRef]
- Bafna, S.; Kaur, S.; Batra, S.K. Membrane-bound mucins: The mechanistic basis for alterations in the growth and survival of cancer cells. Oncogene 2010, 29, 2893–2904. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.T.; Ten Hagen, K.G. Mucin-type O-glycosylation during development. J. Biol. Chem. 2013, 288, 6921–6929. [Google Scholar] [CrossRef]
- Corfield, A.P. Mucins: A biologically relevant glycan barrier in mucosal protection. Biochim. Biophys. Acta 2015, 1850, 236–252. [Google Scholar] [CrossRef]
- Melhem, H.; Regan-Komito, D.; Niess, J.H. Mucins dynamics in physiological and pathological conditions. Int. J. Mol. Sci. 2021, 22, 13642. [Google Scholar] [CrossRef]
- Sheng, Y.H.; Hasnain, S.Z. Mucus and mucins: The underappreciated host defence system. Front. Cell. Infect. Microbiol. 2022, 12, 856962. [Google Scholar] [CrossRef]
- Jonckheere, N.; Skrypek, N.; Van Seuningen, I. Mucins and tumor resistance to chemotherapeutic drugs. Biochim. Biophys. Acta 2014, 1846, 142–151. [Google Scholar] [CrossRef]
- Chugh, S.; Gnanapragassam, V.S.; Jain, M.; Rachagani, S.; Ponnusamy, M.P.; Batra, S.K. Pathobiological implications of mucin glycans in cancer: Sweet poison and novel targets. Biochim. Biophys. Acta 2015, 1856, 211–225. [Google Scholar] [CrossRef]
- Hanson, R.L.; Hollingsworth, M.A. Functional consequences of differential O-glycosylation of MUC1, MUC4, and MUC16 (downstream effects on signaling). Biomolecules 2016, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- van Putten, J.P.M.; Strijbis, K. Transmembrane mucins: Signaling receptors at the intersection of inflammation and cancer. J. Innate Immun. 2017, 9, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Aithal, A.; Rauth, S.; Kshirsagar, P.; Shah, A.; Lakshmanan, I.; Junker, W.M.; Jain, M.; Ponnusamy, M.P.; Batra, S.K. MUC16 as a novel target for cancer therapy. Expert Opin. Ther. Targets 2018, 22, 675–686. [Google Scholar] [CrossRef]
- Bhatia, R.; Gautam, S.K.; Cannon, A.; Thompson, C.; Hall, B.R.; Aithal, A.; Banerjee, K.; Jain, M.; Solheim, J.C.; Kumar, S.; et al. Cancer-associated mucins: Role in immune modulation and metastasis. Cancer Metastasis Rev. 2019, 38, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Leon, F.; Rauth, S.; Batra, S.K.; Ponnusamy, M.P. A systematic review on the implications of O-linked glycan branching and truncating enzymes on cancer progression and metastasis. Cells 2020, 9, 446. [Google Scholar] [CrossRef]
- Ganguly, K.; Rauth, S.; Marimuthu, S.; Kumar, S.; Batra, S.K. Unraveling mucin domains in cancer and metastasis: When protectors become predators. Cancer Metastasis Rev. 2020, 39, 647–659. [Google Scholar] [CrossRef]
- Brockhausen, I.; Melamed, J. Mucins as anti-cancer targets: Perspectives of the glycobiologist. Glycoconj. J. 2021, 38, 459–474. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, Z.; Zhang, S.; Zhu, P.; Ko, J.K.; Yung, K.K. MUC1: Structure, function, and clinical application in epithelial cancers. Int. J. Mol. Sci. 2021, 22, 6567. [Google Scholar] [CrossRef] [PubMed]
- Marimuthu, S.; Rauth, S.; Ganguly, K.; Zhang, C.; Lakshmanan, I.; Batra, S.K.; Ponnusamy, M.P. Mucins reprogram stemness, metabolism and promote chemoresistance during cancer progression. Cancer Metastasis Rev. 2021, 40, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Ratan, C.; Cicily, K.D.D.; Nair, B.; Nath, L.R. MUC glycoproteins: Potential biomarkers and molecular targets for cancer therapy. Curr. Cancer Drug Targets 2021, 21, 132–152. [Google Scholar] [CrossRef]
- Wi, D.H.; Cha, J.H.; Jung, Y.S. Mucin in cancer: A stealth cloak for cancer cells. BMB Rep. 2021, 54, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Qing, L.; Li, O.; Yang, Y.; Xu, W.; Dong, Z. A prognosis marker MUC1 correlates with metabolism and drug resistance in bladder cancer: A bioinformatics research. BMC Urol. 2022, 22, 114. [Google Scholar] [CrossRef]
- Zhang, W.; Li, P.; Ding, L.; Yan, C. Mannose-targeting Concanavalin A-Epirubicin Conjugate for Targeted Intravesical Chemotherapy of Bladder Cancer. Chem. Asian J. 2022, 17, e202200342. [Google Scholar] [CrossRef]
- N’Dow, J.; Pearson, J.P.; Bennett, M.K.; Neal, D.E.; Robson, C.N. Mucin gene expression in human urothelium and in intestinal segments transposed into the urinary tract. J. Urol. 2000, 164, 1398–1404. [Google Scholar] [CrossRef]
- Lau, S.K.; Weiss, L.M.; Chu, P.G. Differential expression of MUC1, MUC2, and MUC5AC in carcinomas of various sites: An immunohistochemical study. Am. J. Clin. Pathol. 2004, 122, 61–69. [Google Scholar] [CrossRef]
- Garbar, C.; Mascaux, C. Expression of MUC1 (Ma695) in noninvasive papillary urothelial neoplasm according to the 2004 World Health Organization classification of the noninvasive urothelial neoplasm. An immunologic tool for the pathologist? Anal. Quant. Cytol. Histol. 2011, 33, 277–282. [Google Scholar]
- Kaur, S.; Momi, N.; Chakraborty, S.; Wagner, D.G.; Horn, A.J.; Lele, S.M.; Theodorescu, D.; Batra, S.K. Altered expression of transmembrane mucins, MUC1 and MUC4, in bladder cancer: Pathological implications in diagnosis. PLoS ONE 2014, 9, e92742. [Google Scholar] [CrossRef] [PubMed]
- Stojnev, S.; Ristic-Petrovic, A.; Jankovic Velickovic, L.; Krstic, M.; Bogdanovic, D.; Khanh, D.T.; Ristic, A.; Conic, I.; Stefanovic, V. Prognostic significance of mucin expression in urothelial bladder cancer. Int. J. Clin. Exp. Pathol. 2014, 7, 4945–4958. [Google Scholar] [PubMed]
- Kaymaz, E.; Ozer, E.; Unverdi, H.; Hucumenglu, S. Evaluation of MUC1 and P53 expressions in noninvasive papillary urothelial neoplasms of bladder, their relationship with tumor grade and role in the differential diagnosis. Indian J. Pathol. Microbiol. 2017, 60, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, G.; Hayashi, T.; Sentani, K.; Takemoto, K.; Sekino, Y.; Uraoka, N.; Hanamoto, M.; Nose, H.; Teishima, J.; Arihiro, K.; et al. Clinicopathological significance of the overexpression of MUC1 in upper tract urothelial carcinoma and possible application as a diagnostic marker. Pathol. Int. 2022, 72, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Retz, M.; Lehmann, J.; Amann, E.; Wullich, B.; Röder, C.; Stöckle, M. Mucin 7 and cytokeratin 20 as new diagnostic urinary markers for bladder tumor. J. Urol. 2003, 169, 86–89. [Google Scholar] [CrossRef]
- Okegawa, T.; Kinjo, M.; Horie, S.; Nutahara, K.; Higashihara, E. Detection of mucin 7 gene expression in exfoliated cells in urine from patients with bladder tumor. Urology 2003, 62, 182–186. [Google Scholar] [CrossRef]
- Kinjo, M.; Okegawa, T.; Horie, S.; Nutahara, K.; Higashihara, E. Detection of circulating MUC7-positive cells by reverse transcription-polymerase chain reaction in bladder cancer patients. Int. J. Urol. 2004, 11, 38–43. [Google Scholar] [CrossRef]
- Pu, X.Y.; Wang, Z.P.; Chen, Y.R.; Wang, X.H.; Wu, Y.L.; Wang, H.P. The value of combined use of survivin, cytokeratin 20 and mucin 7 mRNA for bladder cancer detection in voided urine. J. Cancer Res. Clin. Oncol. 2008, 134, 659–665. [Google Scholar] [CrossRef]
- Cotton, S.; Azevedo, R.; Gaiteiro, C.; Ferreira, D.; Lima, L.; Peixoto, A.; Fernandes, E.; Neves, M.; Neves, D.; Amaro, T.; et al. Targeted O-glycoproteomics explored increased sialylation and identified MUC16 as a poor prognosis biomarker in advanced-stage bladder tumours. Mol. Oncol. 2017, 11, 895–912. [Google Scholar] [CrossRef]
- Yamashita, T.; Higashi, M.; Sugiyama, H.; Morozumi, M.; Momose, S.; Tamaru, J.I. Cancer antigen 125 expression enhances the gemcitabine/cisplatin-resistant tumor microenvironment in bladder cancer. Am. J. Pathol. 2022, 193, 350–361. [Google Scholar] [CrossRef]
- Zhang, X.G.; Zhang, T.; Li, C.Y.; Zhang, M.H.; Chen, F.M. CD164 promotes tumor progression and predicts the poor prognosis of bladder cancer. Cancer Med. 2018, 7, 3763–3772. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Matsumoto, H.; Matsuyama, H.; Fujii, N.; Inoue, R.; Yamamoto, Y.; Nagao, K. Clinical significance of CD44 variant 9 expression as a prognostic indicator in bladder cancer. Oncol. Rep. 2016, 36, 2852–2860. [Google Scholar] [CrossRef]
- Wu, C.T.; Lin, W.Y.; Chang, Y.H.; Chen, W.C.; Chen, M.F. Impact of CD44 expression on radiation response for bladder cancer. J. Cancer 2017, 8, 1137–1144. [Google Scholar] [CrossRef]
- Wu, C.T.; Lin, W.Y.; Chen, W.C.; Chen, M.F. Predictive value of CD44 in muscle-invasive bladder cancer and its relationship with IL-6 signaling. Ann. Surg. Oncol. 2018, 25, 3518–3526. [Google Scholar] [CrossRef]
- Gaiteiro, C.; Soares, J.; Relvas-Santos, M.; Peixoto, A.; Ferreira, D.; Paulo, P.; Brandão, A.; Fernandes, E.; Azevedo, R.; Palmeira, C.; et al. Glycoproteogenomics characterizes the CD44 splicing code associated with bladder cancer invasion. Theranostics 2022, 12, 3150–3177. [Google Scholar] [CrossRef]
- Liao, C.; Wang, Q.; An, J.; Chen, J.; Li, X.; Long, Q.; Xiao, L.; Guan, X.; Liu, J. CD44 glycosylation as a therapeutic target in oncology. Front. Oncol. 2022, 12, 883831. [Google Scholar] [CrossRef]
- Shigeta, K.; Hasegawa, M.; Kikuchi, E.; Yasumizu, Y.; Kosaka, T.; Mizuno, R.; Mikami, S.; Miyajima, A.; Kufe, D.; Oya, M. Role of the MUC1-C oncoprotein in the acquisition of cisplatin resistance by urothelial carcinoma. Cancer Sci. 2020, 111, 3639–3652. [Google Scholar] [CrossRef]
- Nielsen, T.O.; Borre, M.; Nexo, E.; Sorensen, B.S. Co-expression of HER3 and MUC1 is associated with a favourable prognosis in patients with bladder cancer. BJU Int. 2015, 115, 163–165. [Google Scholar] [CrossRef] [PubMed]
- Memon, A.A.; Sorensen, B.S.; Melgard, P.; Fokdal, L.; Thykjaer, T.; Nexo, E. Expression of HER3, HER4 and their ligand heregulin-4 is associated with better survival in bladder cancer patients. Br. J. Cancer 2004, 91, 2034–2041. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.A.; Videira, P.A.; Lima, L.; Pereira, S.; Silva, M.; Carrascal, M.; Severino, P.F.; Fernandes, E.; Almeida, A.; Costa, C.; et al. Overexpression of tumour-associated carbohydrate antigen sialyl-Tn in advanced bladder tumours. Mol. Oncol. 2013, 7, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.F.; Silva, M.; Carrascal, M.; Malagolini, N.; Chiricolo, M.; Venturi, G.; Astolfi, A.; Catera, M.; Videira, P.A.; Dall’Olio, F. Expression of sialylTn sugar antigen in bladder cancer cells affects response to Bacillus Calmette Guérin (BCG) and to oxidative damage. Oncotarget 2017, 8, 54506–54517. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.; Severino, P.F.; Silva, M.; Miranda, A.; Tavares, A.; Pereira, S.; Fernandes, E.; Cruz, R.; Amaro, T.; Reis, C.A.; et al. Response of high-risk of recurrence/progression bladder tumours expressing sialyl-Tn and sialyl-6-T to BCG immunotherapy. Br. J. Cancer 2013, 109, 2106–2114. [Google Scholar] [CrossRef]
- Costa, C.; Pereira, S.; Lima, L.; Peixoto, A.; Fernandes, E.; Neves, D.; Neves, M.; Gaiteiro, C.; Tavares, A.; Gil da Costa, R.M.; et al. Abnormal protein glycosylation and activated PI3K/Akt/mTOR pathway: Role in bladder cancer prognosis and targeted therapeutics. PLoS ONE 2015, 10, e0141253. [Google Scholar] [CrossRef]
- Peixoto, A.; Fernandes, E.; Gaiteiro, C.; Lima, L.; Azevedo, R.; Soares, J.; Cotton, S.; Parreira, B.; Neves, M.; Amaro, T.; et al. Hypoxia enhances the malignant nature of bladder cancer cells and concomitantly antagonizes protein O-glycosylation extension. Oncotarget 2016, 7, 63138–63157. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.; Neves, M.; Oliveira, M.I.; Dieguez, L.; Freitas, R.; Azevedo, R.; Gaiteiro, C.; Soares, J.; Ferreira, D.; Peixoto, A.; et al. Sialyl-Tn identifies muscle-invasive bladder cancer basal and luminal subtypes facing decreased survival, being expressed by circulating tumor cells and metastases. Urol. Oncol. 2017, 35, 675.e1–675.e8. [Google Scholar] [CrossRef] [PubMed]
- Neves, M.; Azevedo, R.; Lima, L.; Oliveira, M.I.; Peixoto, A.; Ferreira, D.; Soares, J.; Fernandes, E.; Gaiteiro, C.; Palmeira, C.; et al. Exploring sialyl-Tn expression in microfluidic-isolated circulating tumour cells: A novel biomarker and an analytical tool for precision oncology applications. N. Biothchnol. 2019, 49, 77–87. [Google Scholar] [CrossRef]
- Carrascal, M.A.; Severino, P.F.; Guadalupe Cabral, M.; Silva, M.; Ferreira, J.A.; Calais, F.; Quinto, H.; Pen, C.; Ligeiro, D.; Santos, L.L.; et al. Sialyl Tn-expressing bladder cancer cells induce a tolerogenic phenotype in innate and adaptive immune cells. Mol. Oncol. 2014, 8, 753–765. [Google Scholar] [CrossRef]
- Ogata, S.; Maimonis, P.J.; Itzkowitz, S.H. Mucins bearing the cancer-associated sialosyl-Tn antigen mediate inhibition of natural killer cell cytotoxicity. Cancer Res. 1992, 52, 4741–4746. [Google Scholar]
- Bernardo, C.; Costa, C.; Amaro, T.; Goncalves, M.; Lopes, P.; Freitas, R.; Gartner, F.; Amado, F.; Ferreira, J.A.; Santos, L. Patient-derived sialyl-Tn-positive invasive bladder cancer xenografts in nude mice: An exploratory model study. Anticancer Res. 2014, 34, 735–744. [Google Scholar]
- Theodoropoulos, V.E.; Lazaris, A.; Sofras, F.; Gerzelis, I.; Tsoukala, V.; Ghikonti, I.; Manikas, K.; Kastriotis, I. Hypoxiainducible factor 1α expression correlates with angiogenesis and unfavorable prognosis in bladder cancer. Eur. Urol. 2004, 46, 200–208. [Google Scholar] [CrossRef]
- Chai, C.Y.; Chen, W.T.; Hung, W.C.; Kang, W.Y.; Huang, Y.C.; Su, Y.C.; Yang, C.H. Hypoxia-inducible factor-1α expression correlates with focal macrophage infiltration, angiogenesis and unfavourable prognosis in urothelial carcinoma. J. Clin. Pathol. 2008, 61, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, A.; Freitas, R.; Ferreira, D.; Relvas-Santos, M.; Paulo, P.; Cardoso, M.; Soares, J.; Gaiteiro, C.; Palmeira, C.; Teixeira, F.; et al. Metabolomics, transcriptomics and functional glycomics reveals bladder cancer cells plasticity and enhanced aggressiveness facing hypoxia and glucose deprivation. BioRxiv, 2021; preprint. [Google Scholar] [CrossRef]
- Peixoto, A.; Ferreira, D.; Azevedo, R.; Freitas, R.; Fernandes, E.; Relvas-Santos, M.; Gaiteiro, C.; Soares, J.; Cotton, S.; Teixeira, B.; et al. Glycoproteomics identifies HOMER3 as a potentially targetable biomarker triggered by hypoxia and glucose deprivation in bladder cancer. J. Exp. Clin. Cancer Res. 2021, 40, 191. [Google Scholar] [CrossRef] [PubMed]
- Takamiya, R.; Ohtsubo, K.; Takamatsu, S.; Taniguchi, N.; Angata, T. The interaction between Siglec-15 and tumor-associated sialyl-Tn antigen enhances TGF-β secretion from monocytes/macrophages through the DAP12-Syk pathway. Glycobiology 2013, 23, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, L.R.; Feldmann, A.; Bergmann, R.; Koristka, S.; Berndt, N.; Máthé, D.; Hegedüs, N.; Szigeti, K.; Videira, P.A.; Bachmann, M.; et al. Extended half-life target module for sustainable UniCAR T-cell treatment of STn-expressing cancers. J. Exp. Clin. Cancer Res. 2020, 39, 77. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.; Fernandes, E.; Ferreira, J.A.; Lima, L.; Tavares, A.; Peixoto, A.; Parreira, B.; Correia da Costa, J.M.; Brindley, P.J.; Lopes, C.; et al. P53 and cancer-associated sialylated glycans are surrogate markers of cancerization of the bladder associated with Schistosoma haematobium infection. PLoS Negl. Trop. Dis. 2014, 8, e3329. [Google Scholar] [CrossRef]
- Liu, F.; Fu, J.; Bergstrom, K.; Shan, X.; McDaniel, J.M.; McGee, S.; Bai, X.; Chen, W.; Xia, L. Core 1-derived mucin-type O-glycosylation protects against spontaneous gastritis and gastric cancer. J. Exp. Med. 2020, 217, e20182325. [Google Scholar] [CrossRef]
- Chugh, S.; Barkeer, S.; Rachagani, S.; Nimmakayala, R.K.; Perumal, N.; Pothuraju, R.; Atri, P.; Mahapatra, S.; Thapa, I.; Talmon, G.A.; et al. Disruption of C1galt1 gene promotes development and metastasis of pancreatic adenocarcinomas in mice. Gastroenterology 2018, 155, 1608–1624. [Google Scholar] [CrossRef]
- Leon, F.; Seshacharyulu, P.; Nimmakayala, R.K.; Chugh, S.; Karmakar, S.; Nallasamy, P.; Vengoji, R.; Rachagani, S.; Cox, J.L.; Mallya, K.; et al. Reduction in O-glycome induces diferentially glycosylated CD44 to promote stemness and metastasis in pancreatic cancer. Oncogene 2022, 41, 57–71. [Google Scholar] [CrossRef]
- Tan, Z.; Jiang, Y.; Liang, L.; Wu, J.; Cao, L.; Zhou, X.; Song, Z.; Ye, Z.; Zhao, Z.; Feng, H.; et al. Dysregulation and prometastatic function of glycosyltransferase C1GALT1 modulated by cHP1BP3/ miR-1-3p axis in bladder cancer. J. Exp. Clin. Cancer Res. 2022, 41, 228. [Google Scholar] [CrossRef]
- Xiang, T.; Qiao, M.; Xie, J.; Li, Z.; Xie, H. Emerging roles of the unique molecular chaperone Cosmc in the regulation of health and disease. Biomolecules 2022, 12, 1732. [Google Scholar] [CrossRef]
- Wang, Y.; Ju, T.; Ding, X.; Xia, B.; Wang, W.; Xia, L.; He, M.; Cummings, R.D. Cosmc is an essential chaperone for correct protein O-glycosylation. Proc. Natl. Acad. Sci. USA 2010, 107, 9228–9233. [Google Scholar] [CrossRef] [PubMed]
- Klatte, T.; Xylinas, E.; Rieken, M.; Kluth, L.A.; Rouprêt, M.; Pycha, A.; Fajkovic, H.; Seitz, C.; Karakiewicz, P.I.; Lotan, Y.; et al. Impact of ABO blood type on outcomes in patients with primary nonmuscle invasive bladder cancer. J. Urol. 2014, 191, 1238–1243. [Google Scholar] [CrossRef] [PubMed]
- Videira, P.A.; Correia, M.; Malagolini, N.; Crespo, H.C.; Ligeiro, D.; Calais, F.M.; Trindade, H.; Dall’Olio, F. ST3Gal.I sialyltransferase relevance in bladder cancer tissues and cell lines. BMC Cancer 2009, 9, 357. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.F.; Silva, M.; Carrascal, M.; Malagolini, N.; Chiricolo, M.; Venturi, G.; Barbaro Forleo, R.; Astolfi, A.; Catera, M.; Videira, P.A.; et al. Oxidative damage and response to Bacillus Calmette-Guérin in bladder cancer cells expressing sialyltransferase ST3GAL1. BMC Cancer 2018, 18, 198. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Amano, M.; Kitamura, H.; Tsukamoto, T.; Masumori, N.; Hirose, K.; Ohashi, T.; Nishimura, S.I. N- and O-glycome analysis of serum and urine from bladder cancer patients using a high-throughput glycoblotting method. J. Glycom. Lipidom. 2013, 3, 1000108. [Google Scholar] [CrossRef]
- Suzuki, Y.; Sutoh, M.; Hatakeyama, S.; Mori, K.; Yamamoto, H.; Koie, T.; Saitoh, H.; Yamaya, K.; Funyu, T.; Habuchi, T.; et al. MUC1 carrying core 2 O-glycans functions as a molecular shield against NK cell attack, promoting bladder tumor metastasis. Int. J. Oncol. 2012, 40, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, S.; Sutoh, M.; Hatakeyama, S.; Hiraoka, N.; Habuchi, T.; Horikawa, Y.; Hashimoto, Y.; Yoneyama, T.; Mori, K.; Koie, T.; et al. A novel strategy for evasion of NK cell immunity by tumours expressing core2 O-glycans. EMBO J. 2011, 30, 3173–3185. [Google Scholar] [CrossRef]
- Sutoh Yoneyama, M.; Tobisawa, Y.; Hatakeyama, S.; Sato, M.; Tone, K.; Tatara, Y.; Kakizaki, I.; Funyu, T.; Fukuda, M.; Hoshi, S.; et al. A mechanism for evasion of CTL immunity by altered O-glycosylation of HLA class I. J. Biochem. 2017, 161, 479–492. [Google Scholar] [CrossRef]
- Li, H.; Yang, F.; Chang, K.; Yu, X.; Guan, F.; Li, X. The synergistic function of long and short forms of β4GalT1 in p53-mediated drug resistance in bladder cancer cells. Biochim. Biophys. Acta. Mol. Cell Res. 2023, 1870, 119409. [Google Scholar] [CrossRef]
- Jia, L.; Zhang, J.; Ma, T.; Guo, Y.; Yu, Y.; Cui, J. The Function of Fucosylation in Progression of Lung Cancer. Front. Oncol. 2018, 8, 565. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Haltiwanger, R.S. O-fucosylation of notch occurs in the endoplasmic reticulum. J. Biol. Chem. 2005, 280, 11289–11294. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, Y.; Saitoh, A.; Suzuki, Y.; Igarashi, K.; Oguro, M.; Yokota, T.; Mori, S.; Suda, T.; Isemura, M.; Asakura, H. Fucosylation index of alpha-fetoprotein, a possible aid in the early recognition of hepatocellular carcinoma in patients with cirrhosis. Hepatology 1993, 17, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Shan, M.; Yang, D.; Dou, H.; Zhang, L. Fucosylation in cancer biology and its clinical applications. Prog. Mol. Biol. Transl. Sci. 2019, 162, 93–119. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Tan, Z.; Lu, W.; Guo, J.; Yu, H.; Yu, J.; Sun, C.; Qi, X.; Li, Z.; Guan, F. Quantitative glycome analysis of N-glycan patterns in bladder cancer vs normal bladder cells using an integrated strategy. J. Proteome Res. 2015, 14, 639–653. [Google Scholar] [CrossRef]
- Ferdosi, S.; Ho, T.H.; Castle, E.P.; Stanton, M.L.; Borges, C.R. Behavior of blood plasma glycan features in bladder cancer. PLoS ONE 2018, 13, e0201208. [Google Scholar] [CrossRef]
- Guo, J.; Xiang, L.; Zengqi, T.; Wei, L.; Ganglong, Y. Alteration of N-glycans and Expression of Their Related Glycogenes in the Epithelial-Mesenchymal Transition of HCV29 Bladder Epithelial Cells. Molecules 2014, 19, 20073–20090. [Google Scholar] [CrossRef]
- Lu, Y.C.; Chen, C.N.; Chu, C.Y.; Lu, J.; Wang, B.J.; Chen, C.H.; Huang, M.C.; Lin, T.H.; Pan, C.C.; Chen, S.S.; et al. Calreticulin activates β1 integrin via fucosylation by fucosyltransferase 1 in J82 human bladder cancer cells. Biochem. J. 2014, 460, 69–78. [Google Scholar] [CrossRef]
- Liu, M.; Zheng, Q.; Chen, S.; Liu, J.; Li, S. Fut7 promotes the epithelial–mesenchymal transition and immune infiltration in bladder urothelial carcinoma. J. Inflamm. Res. 2021, 14, 1069–1084. [Google Scholar] [CrossRef]
- Numahata, K.; Satoh, M.; Handa, K.; Saito, S.; Ohyama, C.; Ito, A.; Takahashi, T.; Hoshi, S.; Orikasa, S.; Hakomori, S.I. Sialosyl-Le(x) expression defines invasive and metastatic properties of bladder carcinoma. Cancer 2002, 94, 673–685. [Google Scholar] [CrossRef]
- Islam, M.K.; Syed, P.; Dhondt, B.; Gidwani, K.; Pettersson, K.; Lamminmäki, U.; Leivo, J. Detection of bladder cancer with aberrantly fucosylated ITGA3. Anal. Biochem. 2021, 628, 114283. [Google Scholar] [CrossRef] [PubMed]
- Islam, K. Extracellular Vesicle Glycosylations as Novel Biomarkers of Urological Cancers: Nanoparticle-Aided Glycovariant Assay to Detect Vesicles for the Early Detection of Cancer. Ph.D. Thesis, Turun Yliopisto, Turku, Finland, 2022. [Google Scholar]
- Ongay, S.; Martín-Álvarez, P.J.; Neusüss, C.; de Frutos, M. Statistical evaluation of CZE-UV and CZE-ESI-MS data of intact α-1-acid glycoprotein isoforms for their use as potential biomarkers in bladder cancer. Electrophoresis 2010, 31, 3314–3325. [Google Scholar] [CrossRef]
- Blanas, A.; Sahasrabudhe, N.M.; Rodríguez, E.; van Kooyk, Y.; van Vliet, S.J. Fucosylated antigens in cancer: An alliance toward tumor progression, metastasis, and resistance to chemotherapy. Front. Oncol. 2018, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Sheinfeld, J.; Reuter, V.E.; Sarkis, A.S.; Cordon-Cardo, C. Blood group antigens in normal and neoplastic urothelium. J. Cell. Biochem. 1992, 50, 50–55. [Google Scholar] [CrossRef]
- Sheinfeld, J.; Reuter, V.E.; Melamed, M.R.; Fair, W.R.; Morse, M.; Sogani, P.C.; Herr, H.W.; Whitmore, W.F.; Cordon-Cardo, C. Enhanced bladder cancer detection with the Lewis X antigen as a marker of neoplastic transformation. J. Urol. 1990, 143, 285–288. [Google Scholar] [CrossRef]
- Pal, S.K.; Pham, A.; Vuong, W.; Liu, X.; Lin, Y.; Ruel, N.; Yuh, B.E.; Chan, K.; Wilson, T.; Lerner, S.P.; et al. Prognostic significance of neutrophilic infiltration in benign lymph nodes in patients with muscle-invasive bladder cancer. Eur. Urol. Focus 2017, 3, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Ezeabikwa, B.; Nandini, M.; Aristotelis, A.; Stuart, M.H.; Yasuyuki, M.; Miguel, M.C.; Sylvain, L.; Msano, M.; Ali, I.; Jamie, H.-M.; et al. Major differences in glycosylation and fucosyltransferase expression in low-grade versus high-grade bladder cancer cell lines. Glycobiology 2021, 31, 1444–1463. [Google Scholar] [CrossRef] [PubMed]
- Skorstengaard, K.; Vestergaard, E.M.; Langkilde, N.C.; Christensen, L.L.; Wolf, H.; Orntoft, T.F. Lewis antigen mediated adhesion of freshly removed human bladder tumors to E-selectin. J. Urol. 1999, 161, 1316–1323. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Magalhaes, A.; Gomes, J.; Peixoto, A.; Gaiteiro, C.; Fernandes, E.; Santos, L.L.; Reis, C.A. Protein glycosylation in gastric and colorectal cancers: Toward cancer detection and targeted therapeutics. Cancer Lett. 2017, 387, 32–45. [Google Scholar] [CrossRef]
- Subedi, P.; Schneider, M.; Philipp, J.; Azimzadeh, O.; Metzger, F.; Moertl, S. Comparison of methods to isolate proteins from extracellular vesicles for mass spectrometry-based proteomic analyses. Anal. Biochem. 2019, 584, 113390. [Google Scholar] [CrossRef]
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P.-K. Extracellular vesicles: Composition, biological relevance, and methods of study. Bioscience 2015, 65, 783–797. [Google Scholar] [CrossRef]
- Bebelman, M.P.; Smit, M.J.; Pegtel, D.M.; Baglio, S.R. Biogenesis and function of extracellular vesicles in cancer. Pharmacol. Ther. 2018, 188, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.H.; Cerione, R.A.; Antonyak, M.A. Extracellular vesicles and their roles in cancer progression. Methods Mol. Biol. 2021, 2174, 143–170. [Google Scholar] [CrossRef] [PubMed]
- Surman, M.; Wilczak, M.; Przybyło, M. Lectin-based study reveals the presence of disease-relevant glycoepitopes in bladder cancer cells and ectosomes. Int. J. Mol. Sci. 2022, 23, 14368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Y.; Luo, C.; Xia, Y.; Chen, H.; Wu, X. Glycosyl-phosphatidylinositol-anchored interleukin-2 expressed on tumor-derived exosomes induces antitumor immune response in vitro. Tumori 2010, 96, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Klingler, C.H. Glycosoaminoglycans: How much do we know about their role in the bladder. Urol. J. 2016, 83 (Suppl. 1), S11–S14. [Google Scholar] [CrossRef]
- Singleton, P.A. Hyaluronan regulation of endothelial barrier function in cancer. Adv. Cancer Res. 2014, 123, 191–209. [Google Scholar] [CrossRef]
- Morla, S. Glycosaminoglycans and glycosaminoglycan mimetics in cancer and inflammation. Int. J. Mol. Sci. 2019, 20, 1963. [Google Scholar] [CrossRef]
- Tcheocharis, A.D.; Karamanos, N.K. Proteoglycans remodeling in cancer: Underlying molecular mechanism. Matrix Biol. 2019, 75–76, 220–259. [Google Scholar] [CrossRef]
- Wei, J.; Hu, M.; Huang, K.; Lin, S.; Du, H. Roles of proteoglycans and glycosaminoglycans in cancer development and progression. Int. J. Mol. Sci. 2020, 21, 5983. [Google Scholar] [CrossRef]
- Belting, M. Glycosaminoglycans in cancer treatment. Thromb. Res. 2014, 133 (Suppl. 2), S95–S101. [Google Scholar] [CrossRef] [PubMed]
- Wieboldt, R.; Läubli, H. Glycosaminoglycans in cancer therapy. Am. J. Physiol. Cell Physiol. 2022, 322, C1187–C1200. [Google Scholar] [CrossRef]
- Rübben, H.; Friedrichs, R.; Stuhlsatz, H.; Lutzeyer, W. Glycosaminoglycans in urothelial carcinomas. Urol. Res. 1983, 11, 163–166. [Google Scholar] [CrossRef] [PubMed]
- De Klerk, D.P. The glycosaminoglycans of human bladder cancers of varying grade and stage. J. Urol. 1985, 134, 978–981. [Google Scholar] [CrossRef] [PubMed]
- Atahan, O.; Kayigil, O.; Hizel, N.; Yavuz, O.; Metin, A. Urinary glycosaminoglycan excretion in bladder carcinoma. Scand. J. Urol. Nephrol. 1996, 30, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Konukoğlu, D.; Akçay, T.; Erözenci, A. The importance urinary glycosaminoglycan as a marker for superficial bladder tumors. Cancer Biochem. Res. 1995, 15, 91–95. [Google Scholar]
- Bojanić, N.; Nale, D.; Mićić, S.; Lalić, N.; Vuksanović, A.; Tulić, C. Glycosaminoglycans in the urinary bladder mucosa, tumor tissue and mucosal tissue around tumor. Vojnosanit. Pregl. 2012, 69, 147–150. [Google Scholar] [CrossRef]
- Lokeshwar, V.B.; Obek, C.; Pham, H.T.; Wei, D.; Young, M.J.; Duncan, R.C.; Soloway, M.S.; Bloch, N.L. Urinary hyaluronic acid and hyaluronidase: Markers for bladder cancer detection and evaluation of grade. J. Urol. 2000, 163, 348–356. [Google Scholar] [CrossRef]
- Lokeshwar, V.B.; Schroeder, G.L.; Selzer, M.G.; Hautmann, S.H.; Posey, T.; Duncan, R.C.; Watson, R.; Rose, L.; Markowitz, S.; Soloway, M.S. Bladder tumor markers for monitoring recurrence and screening comparison of hyaluronic acid-hyaluronidase and BTA-Stat tests. Cancer 2002, 95, 61–72. [Google Scholar] [CrossRef]
- Schroeder, G.L.; Lorenzo-Gomez, M.F.; Hautmann, S.H.; Friedrich, M.G.; Ekici, S.; Huland, H.; Lokeshwar, V. A side by side comparison of cytology and biomarkers for bladder cancer detection. J. Urol. 2004, 172, 1123–1126. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Fu, C.; Zhang, Q.; He, C.; Zhang, F.; Wei, Q. The role of CD44 in pathological angiogenesis. FASEB J. 2020, 34, 13125–13139. [Google Scholar] [CrossRef]
- Yaghobi, Z.; Movassaghpour, A.; Talebi, M.; Abdoli Shadbad, M.; Hajiasgharzadeh, K.; Pourvahdani, S.; Baradaran, B. The role of CD44 in cancer chemoresistance: A concise review. Eur. J. Pharmacol. 2021, 903, 174147. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Yang, C.; Gao, F. The state of CD44 activation in cancer progression and therapeutic targeting. FEBS J. 2022, 289, 7970–7986. [Google Scholar] [CrossRef] [PubMed]
- Golshani, R.; Hautmann, S.H.; Estrella, V.; Cohen, B.L.; Kyle, C.C.; Manoharan, M.; Jorda, M.; Soloway, M.S.; Lokeshwar, V.B. HAS1 expression in bladder cancer and its relation to urinary HA test. Int. J. Cancer 2007, 120, 1712–1720. [Google Scholar] [CrossRef]
- Kramer, M.W.; Escudero, D.O.; Lokeshwar, S.D.; Golshani, R.; Ekwenna, O.O.; Acosta, K.; Mersegurger, A.S.; Soloway, M.S.; Lokeshwar, V.B. Association of hyaluronic acid family members (HAS1, HAS2, and HYAL-1) with bladder cancer diagnosis and prognosis. Cancer 2011, 117, 1197–1209. [Google Scholar] [CrossRef]
- Golshani, R.; Lopez, L.; Estrella, V.; Kramer, M.; Iida, N.; Lokeshwar, V.B. Hyaluronic acid synthase-1 expression regulates bladder cancer growth, invasion, and angiogenesis through CD44. Cancer Res. 2008, 68, 483–491. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Brown, T.J.; Heldin, P. Silencing of hyaluronan synthase 2 suppresses the malignant phenotype of invasive breast cancer cells. Int. J. Cancer 2007, 120, 2557–2567. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, A.G.; Kovar, J.L.; Loughman, E.; Elowsky, C.; Oakley, G.G.; Simpson, M.A. Spontaneous metastasis of prostate cancer is promoted by excess hyaluronan synthesis and processing. Am. J. Pathol. 2009, 174, 1027–1036. [Google Scholar] [CrossRef]
- Guin, S.; Pollard, C.; Ru, Y.; Ritterson Lew, C.; Duex, J.E.; Dancik, G.; Owens, C.; Spencer, A.; Knight, S.; Holemon, H.; et al. Role in tumor growth of a glycogen debranching enzyme lost in glycogen storage disease. J. Natl. Cancer Inst. 2014, 106, dju062. [Google Scholar] [CrossRef]
- Guin, S.; Ru, Y.; Agarwal, N.; Lew, C.R.; Owens, C.; Comi, G.P.; Theodorescu, D. Loss of glycogen debranching enzyme AGL drives bladder tumor growth via induction of hyaluronic acid synthesis. Clin. Cancer Res. 2016, 22, 1274–1283. [Google Scholar] [CrossRef]
- Oldenburg, D.; Ru, Y.; Weinhaus, B.; Cash, S.; Theodorescu, D.; Guin, S. CD44 and RHAMM are essential for rapid growth of bladder cancer driven by loss of Glycogen Debranching Enzyme (AGL). BMC Cancer 2016, 16, 713. [Google Scholar] [CrossRef]
- Kramer, M.W.; Golshani, R.; Merseburger, A.S.; Kramer, M.W.; Golshani, R.; Merseburger, A.S.; Knapp, J.; Garcia, A.; Hennenlotter, J.; Duncan, R.C.; et al. HYAL-1 Hyaluronidase: A potential prognostic indicator for progression to muscle invasion and recurrence in bladder cancer. Eur. Urol. 2009, 57, 86–93. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, Q.; Wang, C.H.; Shi, F.; Cao, H.; Yu, Y.; Zhang, M.; Liu, X. Hyaluronic acid/ hyaluronidase as biomarkers for bladder cancer: A diagnostic meta-analysis. Neoplasma 2017, 64, 901–908. [Google Scholar] [CrossRef]
- Ferro, M.; Giuberti, G.; Zappavigna, S.; Perdonà, S.; Facchini, G.; Sperlongano, P.; Porto, S.; Di Lorenzo, G.; Buonerba, C.; Abbruzzese, A.; et al. Chondroitin sulphate enhances the antitumor activity of gemcitabine and mitomycin-C in bladder cancer cells with different mechanisms. Oncol. Rep. 2012, 27, 409–415. [Google Scholar] [CrossRef]
- Morrione, A.; Neill, T.; Iozzo, R.V. Dichotomy of decorin activity on the insulin-like growth factor-I system. FEBS J. 2013, 280, 2138–2149. [Google Scholar] [CrossRef] [PubMed]
- Appunni, S.; Anand, V.; Khandelwal, M.; Gupta, N.; Rubens, M.; Sharma, A. Small leucine rich proteoglycans (decorin, biglycan and lumican) in cancer. Clin. Chim. Acta 2019, 491, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sainio, A.; Nyman, M.; Lund, R.; Vuorikoski, S.; Boström, P.; Laato, M.; Boström, P.J.; Järveläinen, H. Lack of decorin expression by human bladder cancer cells offers new tools in the therapy of urothelial malignancies. PLoS ONE 2013, 8, e76190. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, Z.; Yang, N.; Zhang, J.; Liang, Z. Decorin inhibits proliferation and metastasis in human bladder cancer cells by upregulating P21. Medicine 2022, 101, e29760. [Google Scholar] [CrossRef]
- El Behi, M.; Krumeich, S.; Lodillinsky, C.; Kamoun, A.; Tibaldi, L.; Sugano, G.; De Reynies, A.; Chapeaublanc, E.; Laplanche, A.; Lebret, T.; et al. An essential role for decorin in bladder cancer invasiveness. EMBO Mol. Med. 2013, 5, 1835–1851. [Google Scholar] [CrossRef] [PubMed]
- Niedworok, C.; Röck, K.; Kretschmer, I.; Freudenberger, T.; Nagy, N.; Szarvas, T.; vom Dorp, F.; Reis, H.; Rübben, H.; Fischer, J.W. Inhibitory role of the small leucine-rich proteoglycan biglycan in bladder cancer. PLoS ONE 2013, 8, e80084. [Google Scholar] [CrossRef]
- Salanti, A.; Clausen, T.M.; Agerbæk, M.Ø.; Al Nakouzi, N.; Dahlbäck, M.; Oo, H.Z.; Lee, S.; Gustavsson, T.; Rich, J.R.; Hedberg, B.J.; et al. Targeting human cancer by a glycosaminoglycan binding malaria protein. Cancer Cell 2015, 28, 500–514. [Google Scholar] [CrossRef] [PubMed]
- Seiler, R.; Oo, H.Z.; Tortora, D.; Clausen, T.M.; Wang, C.K.; Kumar, G.; Pereira, M.A.; Ørum-Madsen, M.S.; Agerbæk, M.Ø.; Gustavsson, T.; et al. An oncofetal glycosaminoglycan modification provides therapeutic access to cisplatin-resistant bladder cancer. Eur. Urol. 2017, 72, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Clausen, T.M.; Pereira, M.A.; Al Nakouzi, N.; Oo, H.Z.; Agerbæk, M.Ø.; Lee, S.; Ørum-Madsen, M.S.; Kristensen, A.R.; El-Naggar, A.; Grandgenett, P.M.; et al. Oncofetal chondroitin sulfate glycosaminoglycans are key players in integrin signaling and tumor cell motility. Mol. Cancer Res. 2016, 14, 1288–1299. [Google Scholar] [CrossRef]
- Clausen, T.M.; Kumar, G.; Ibsen, E.K.; Ørum-Madsen, M.S.; Hurtado-Coll, A.; Gustavsson, T.; Agerbæk, M.Ø.; Gatto, F.; Todenhöfer, T.; Basso, U.; et al. A simple method for detecting oncofetal chondroitin sulfate glycosaminoglycans in bladder cancer urine. Cell Death Discov. 2020, 6, 65. [Google Scholar] [CrossRef]
- Greco, B.; Malacarne, V.; De Girardi, F.; Scotti, G.M.; Manfredi, F.; Angelino, E.; Sirini, C.; Camisa, B.; Falcone, L.; Moresco, M.A.; et al. Disrupting N-glycan expression on tumor cells boosts chimeric antigen receptor T cell efficacy against solid malignancies. Sci. Transl. Med. 2022, 14, eabg3072. [Google Scholar] [CrossRef]
- Kong, S.; Gong, P.; Zeng, W.F.; Jiang, B.; Hou, X.; Zhang, Y.; Zhao, H.; Liu, M.; Yan, G.; Zhou, X.; et al. pGlycoQuant with a deep residual network for quantitative glycoproteomics at intact glycopeptide level. Nat. Commun. 2022, 13, 7539. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Pan, J.; Shah, P.; Ao, M.; Thomas, S.N.; Liu, Y.; Chen, L.; Schnaubelt, M.; Clark, D.J.; Rodriguez, H.; et al. Integrated proteomic and glycoproteomic characterization of human high-grade serous ovarian carcinoma. Cell Rep. 2020, 33, 108276. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Hu, Y.; Sun, S.; Chen, L.; Schnaubelt, M.; Clark, D.; Ao, M.; Zhang, Z.; Chan, D.; Qian, J.; et al. Glycoproteomics-based signatures for tumor subtyping and clinical outcome prediction of high-grade serous ovarian cancer. Nat. Commun. 2020, 11, 6139. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilczak, M.; Surman, M.; Przybyło, M. Altered Glycosylation in Progression and Management of Bladder Cancer. Molecules 2023, 28, 3436. https://doi.org/10.3390/molecules28083436
Wilczak M, Surman M, Przybyło M. Altered Glycosylation in Progression and Management of Bladder Cancer. Molecules. 2023; 28(8):3436. https://doi.org/10.3390/molecules28083436
Chicago/Turabian StyleWilczak, Magdalena, Magdalena Surman, and Małgorzata Przybyło. 2023. "Altered Glycosylation in Progression and Management of Bladder Cancer" Molecules 28, no. 8: 3436. https://doi.org/10.3390/molecules28083436
APA StyleWilczak, M., Surman, M., & Przybyło, M. (2023). Altered Glycosylation in Progression and Management of Bladder Cancer. Molecules, 28(8), 3436. https://doi.org/10.3390/molecules28083436





