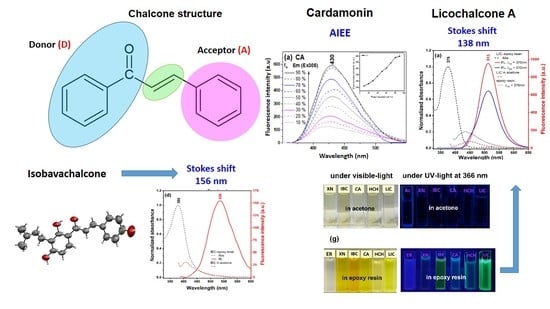
Naturally Occurring Chalcones with Aggregation-Induced Emission Enhancement Characteristics
Abstract
1. Introduction
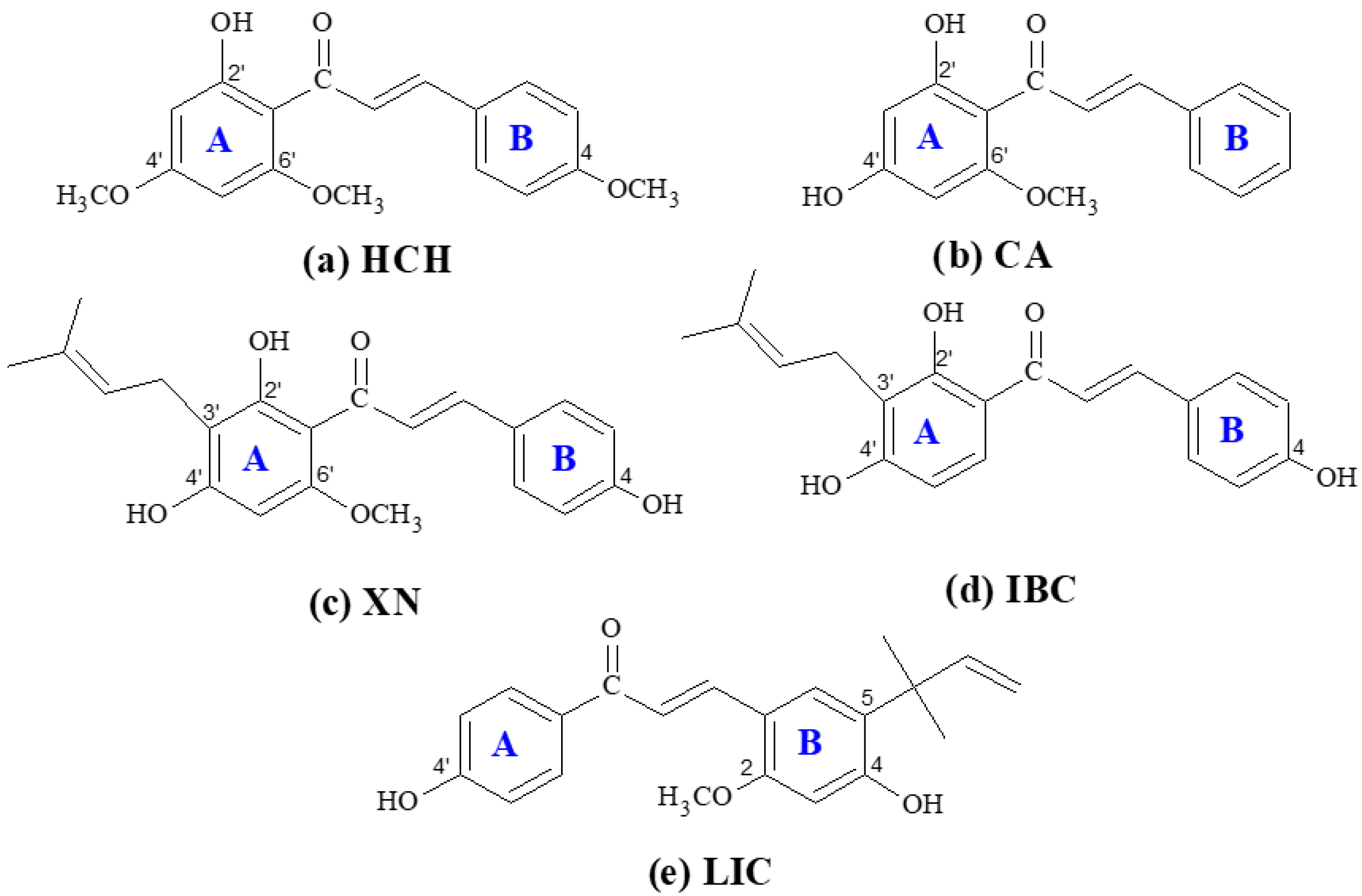
2. Results and Discussion
2.1. Spectral Properties in Solution
Fluorescence Quantum Yield
2.2. Spectral Properties in the Solid State
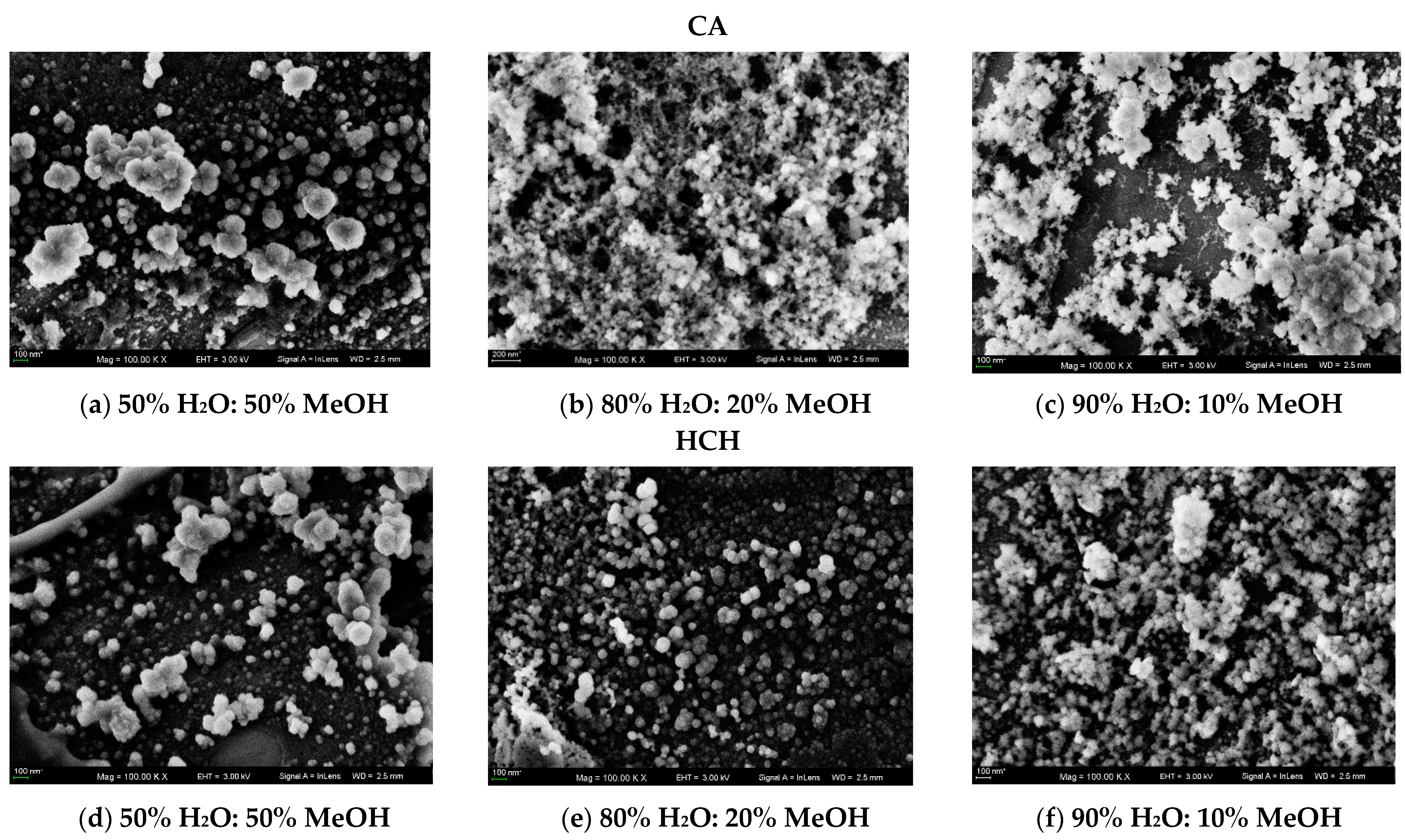
2.3. SEM
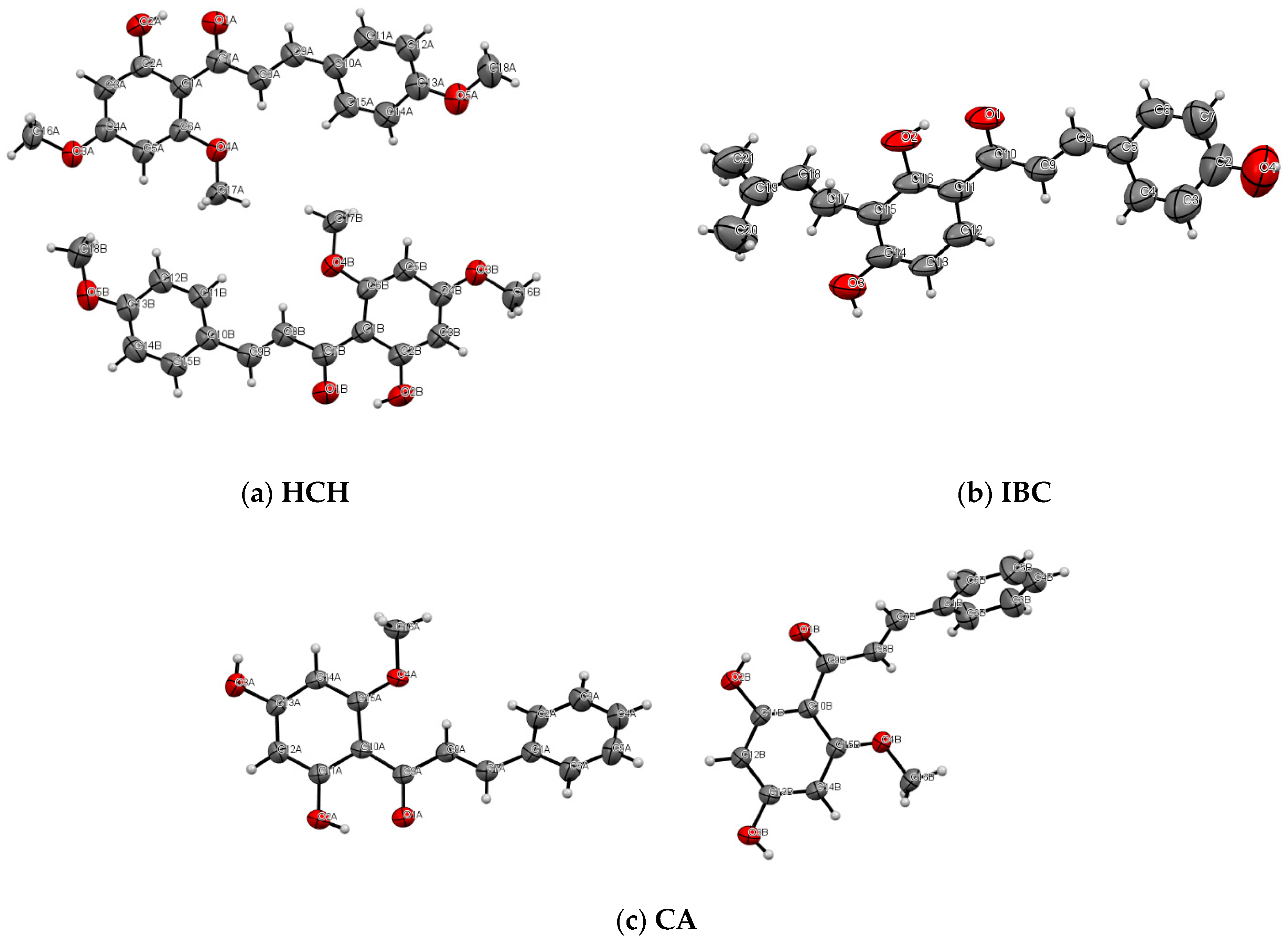
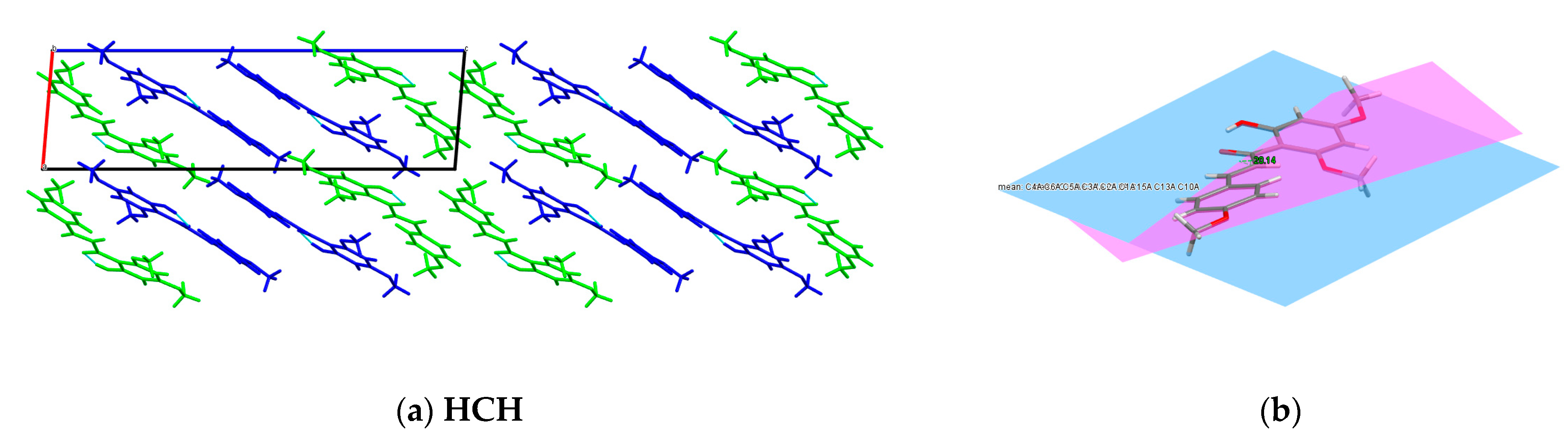
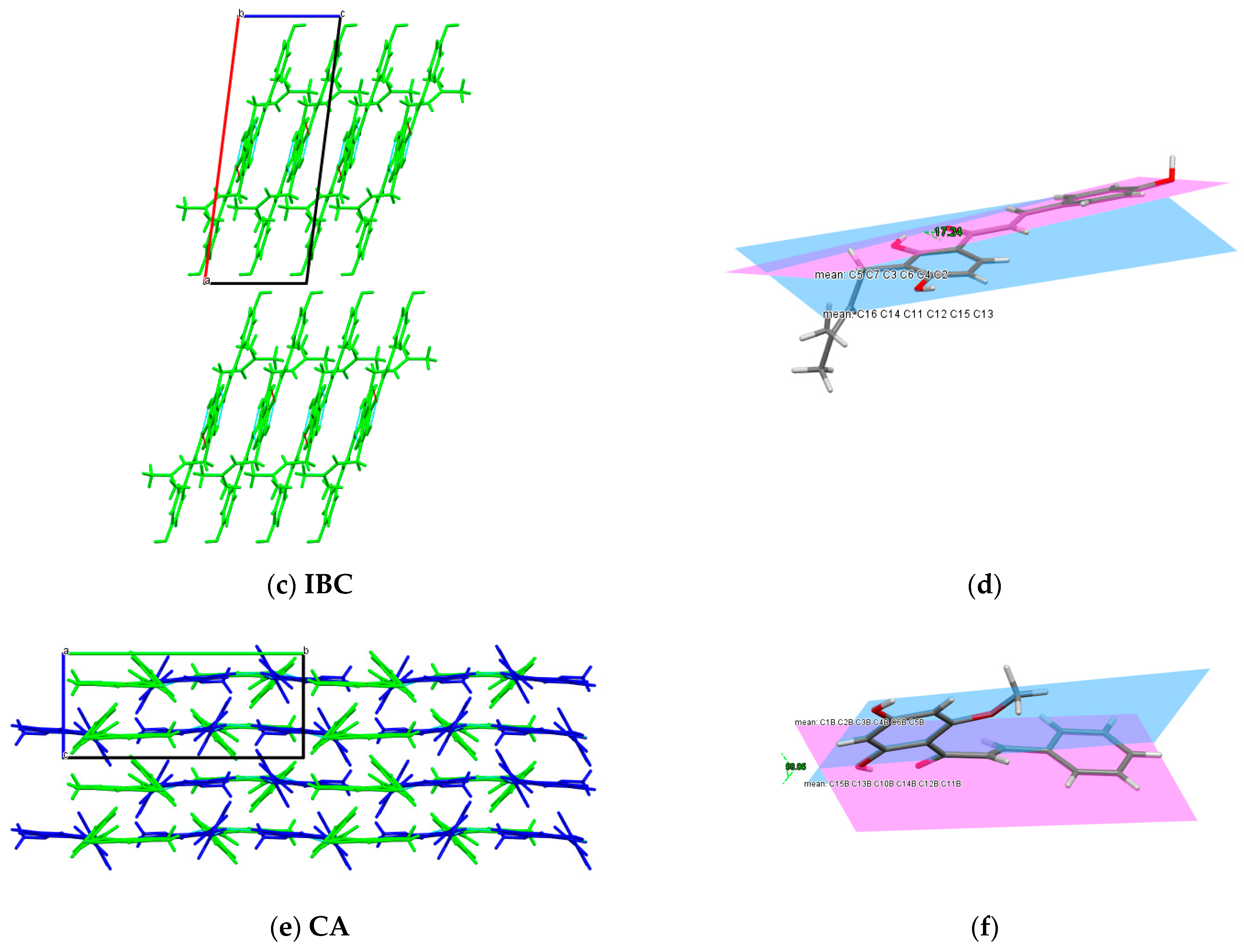
2.4. Single X-ray Diffraction
2.5. DPPH Activity
2.6. AChE and BuChE Inhibition Evaluation
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Electronic Absorption and Fluorescence Spectra
3.2.2. Fluorescence Quantum Yield
3.2.3. SEM Measurements
3.2.4. Single X-ray Diffraction
3.2.5. DPPH Activity
3.2.6. AChE and BChE Inhibitor Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chen, C.J.; Liu, B. Enhancing the performance of pure organic room-temperature phosphorescent luminophores. Nat. Commun. 2019, 10, 2111. [Google Scholar] [CrossRef]
- Zhao, T.P.; Wang, C.M.; Hu, S.Y.; Ji, S.H.; Hu, C.; Xu, K.; Teng, B. Structural design and characterization of a chalcone derivative crystal DAMO with strong SHG efficiency for NLO applications. Opt. Mater. 2021, 112, 110765. [Google Scholar] [CrossRef]
- Zhang, J.X.; Chen, W.N.; Rojas, A.J.; Jucov, E.V.; Timofeeva, T.V.; Parker, T.C.; Barlow, S.; Marder, S.R. Controllable Direct Arylation: Fast Route to Symmetrical and Unsymmetrical 4,7-Diaryl-5,6-difluoro-2,1,3-benzothiadiazole Derivatives for Organic Optoelectronic Materials. J. Am. Chem. Soc. 2013, 135, 16376–16379. [Google Scholar] [CrossRef]
- Liu, D.; De, J.B.; Gao, H.K.; Ma, S.Q.; Ou, Q.; Li, S.; Qin, Z.S.; Dong, H.L.; Liao, Q.; Xu, B.; et al. Organic Laser Molecule with High Mobility, High Photoluminescence Quantum Yield, and Deep-Blue Lasing Characteristics. J. Am. Chem. Soc. 2020, 142, 6332–6339. [Google Scholar] [CrossRef] [PubMed]
- Weil, T.; Vosch, T.; Hofkens, J.; Peneva, K.; Mullen, K. The Rylene Colorant Family-Tailored Nanoemitters for Photonics Research and Applications. Angew. Chem. Int. Edit. 2010, 49, 9068–9093. [Google Scholar] [CrossRef] [PubMed]
- Tagare, J.; Dubey, D.K.; Yadav, R.A.K.; Jou, J.H.; Vaidyanathan, S. Triphenylamine-imidazole-based luminophores for deep-blue organic light-emitting diodes: Experimental and theoretical investigations. Mater. Adv. 2020, 1, 666–679. [Google Scholar] [CrossRef]
- Li, N.; Liu, L.Y.; Luo, H.Q.; Wang, H.Q.; Yang, D.P.; He, F. Flavanone-Based Fluorophores with Aggregation-Induced Emission Enhancement Characteristics for Mitochondria-Imaging and Zebrafish-Imaging. Molecules 2020, 25, 3298. [Google Scholar] [CrossRef]
- Liu, L.; Lei, Y.; Zhang, J.; Li, N.; Zhang, F.; Wang, H.; He, F. Rational Design for Multicolor Flavone-Based Fluorophores with Aggregation-Induced Emission Enhancement Characteristics and Applications in Mitochondria-Imaging. Molecules 2018, 23, 2290. [Google Scholar] [CrossRef]
- Luo, H.Q.; Li, N.; Liu, L.Y.; Wang, H.Q.; He, F. Synthesis of New AIEE-Active Chalcones for Imaging of Mitochondria in Living Cells and Zebrafish In Vivo. Int. J. Mol. Sci. 2021, 22, 8949. [Google Scholar] [CrossRef]
- Wangngae, S.; Chansaenpak, K.; Nootem, J.; Ngivprom, U.; Aryamueang, S.; Lai, R.Y.; Kamkaew, A. Photophysical Study and Biological Applications of Synthetic Chalcone-Based Fluorescent Dyes. Molecules 2021, 26, 2979. [Google Scholar] [CrossRef]
- Kagatikar, S.; Sunil, D. Aggregation induced emission of chalcones. Chem. Pap. 2021, 75, 6147–6156. [Google Scholar] [CrossRef]
- Liu, B.; Cui, W.B.; Zhou, J.L.; Wang, H.Q. A Novel Triphenylamine-Based Flavonoid Fluorescent Probe with High Selectivity for Uranyl in Acid and High Water Systems. Sensors 2022, 22, 6987. [Google Scholar] [CrossRef] [PubMed]
- Budziak-Wieczorek, I.; Slusarczyk, L.; Mysliwa-Kurdziel, B.; Kurdziel, M.; Srebro-Hooper, M.; Korona-Glowniak, I.; Gagos, M.; Gladyszewski, G.; Stepulak, A.; Kluczyk, D.; et al. Spectroscopic characterization and assessment of microbiological potential of 1,3,4-thiadiazole derivative showing ESIPT dual fluorescence enhanced by aggregation effects. Sci. Rep. 2022, 12, 22140. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Luo, Z.J.; Si, S.F.; Zhou, X.F.; Pan, C.J.; Wang, L. A photostable triphenylamine-based flavonoid dye: Solvatochromism, aggregation-induced emission enhancement, fabrication of organic nanodots, and cell imaging applications. Dye. Pigment. 2017, 142, 32–38. [Google Scholar] [CrossRef]
- Mao, L.C.; Liu, Y.Z.; Yang, S.J.; Li, Y.X.; Zhang, X.Y.; Wei, Y. Recent advances and progress of fluorescent bio-/chemosensors based on aggregation-induced emission molecules. Dye. Pigment. 2019, 162, 611–623. [Google Scholar] [CrossRef]
- Tang, Y.J.; Zhang, D.; Zhang, Y.X.; Liu, Y.L.; Cai, L.R.; Plaster, E.; Zheng, J. Fundamentals and exploration of aggregation-induced emission molecules for amyloid protein aggregation. J. Mater. Chem. B 2022, 10, 2280–2295. [Google Scholar] [CrossRef]
- Budziak, I.; Karcz, D.; Makowski, M.; Mysliwa-Kurdziel, B.; Kasprzak, K.; Matwijczuk, A.; Chrusciel, E.; Oniszczuk, A.; Adwent, L.; Matwijczuk, A. Spectroscopic and theoretical investigation into substituent- and aggregation-related dual fluorescence effects in the selected 2-amino-1,3,4-thiadiazoles. J. Mol. Liq. 2019, 291, 111261. [Google Scholar] [CrossRef]
- Wang, L.Y.; Yang, L.L.; Cao, D.R. Application of Aggregation-Induced Emission (AIE) Systems in Sensing and Bioimaging. Curr. Org. Chem. 2014, 18, 1028–1049. [Google Scholar] [CrossRef]
- Zhu, C.L.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: A Trailblazing Journey to the Field of Biomedicine. Acs. Appl. Bio. Mater. 2018, 1, 1768–1786. [Google Scholar] [CrossRef]
- Tong, H.; Hong, Y.N.; Dong, Y.Q.; Haussler, M.; Lam, J.W.Y.; Li, Z.; Guo, Z.F.; Guo, Z.H.; Tang, B.Z. Fluorescent “light-up” bioprobes based on tetraphenylethylene derivatives with aggregation-induced emission characteristics. Chem. Commun. 2006, 35, 3705–3707. [Google Scholar] [CrossRef]
- Wang, M.; Gu, X.G.; Zhang, G.X.; Zhang, D.Q.; Zhu, D.B. Convenient and Continuous Fluorometric Assay Method for Acetylcholinesterase and Inhibitor Screening Based on the Aggregation-Induced Emission. Anal. Chem. 2009, 81, 4444–4449. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Tang, B.Z. AIE Luminogens for Bioimaging and Theranostics: From Organelles to Animals. Chem 2017, 3, 56–91. [Google Scholar] [CrossRef]
- Lin, N.; Wu, X.H.; Cai, Z.X.; Shi, J.B.; Tong, B.; Dong, Y.P. The fluorescence properties of 4′-Methoxychalcone derivates modified by substituents and investigation of lysosomal imaging. Dye. Pigment. 2022, 199, 110091. [Google Scholar] [CrossRef]
- Dai, F.F.; Zhao, M.; Yang, F.Z.; Wang, T.T.; Wang, C. An ESIPT coupled AIE fluorescent probe for biothiols detection and imaging based on a chalcone fluorophore. Dye. Pigment. 2020, 183, 108627. [Google Scholar] [CrossRef]
- Song, Z.G.; Kwok, R.T.K.; Zhao, E.G.; He, Z.K.; Hong, Y.N.; Lam, J.W.Y.; Liu, B.; Tang, B.Z. A Ratiometric Fluorescent Probe Based on ESIPT and AIE Processes for Alkaline Phosphatase Activity Assay and Visualization in Living Cells. Acs. Appl. Mater. Inter. 2014, 6, 17245–17254. [Google Scholar] [CrossRef]
- Budziak, I.; Arczewska, M.; Kaminski, D.M. Formation of Prenylated Chalcone Xanthohumol Cocrystals: Single Crystal X-ray Diffraction, Vibrational Spectroscopic Study Coupled with Multivariate Analysis. Molecules 2019, 24, 4245. [Google Scholar] [CrossRef]
- Budziak, I.; Arczewska, M.; Kaminski, D.M. Structure and Physical Properties of Cardamonin: A Spectroscopic and Computational Approach. Molecules 2020, 25, 4070. [Google Scholar] [CrossRef]
- Orlikova, B.; Tasdemir, D.; Golais, F.; Dicato, M.; Diederich, M. Dietary chalcones with chemopreventive and chemotherapeutic potential. Genes Nutr. 2011, 6, 125–147. [Google Scholar] [CrossRef]
- Zhuang, C.L.; Zhang, W.; Sheng, C.Q.; Zhang, W.N.; Xing, C.G.; Miao, Z.Y. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef]
- Constantinescu, T.; Lungu, C.N. Anticancer Activity of Natural and Synthetic Chalcones. Int. J. Mol. Sci. 2021, 22, 11306. [Google Scholar] [CrossRef]
- Constantinescu, T.; Mihis, A.G. Two Important Anticancer Mechanisms of Natural and Synthetic Chalcones. Int. J. Mol. Sci. 2022, 23, 11595. [Google Scholar] [CrossRef]
- Luo, Z.J.; Lv, T.Y.Z.; Zhu, K.N.; Li, Y.; Wang, L.; Gooding, J.J.; Liu, G.Z.; Liu, B. Paper-Based Ratiometric Fluorescence Analytical Devices towards Point-of-Care Testing of Human Serum Albumin. Angew. Chem. Int. Edit. 2020, 59, 3131–3136. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.L.; Liu, G.; Qiao, M.Y.; Li, Y.Y.; Yi, X.Y. Biosensors for the Detection of Enzymes Based on Aggregation-Induced Emission. Biosensors 2022, 12, 953. [Google Scholar] [CrossRef]
- Burmaoglu, S.; Yilmaz, A.O.; Polat, M.F.; Kaya, R.; Gulcin, I.; Algul, O. Synthesis and biological evaluation of novel tris-chalcones as potent carbonic anhydrase, acetylcholinesterase, butyrylcholinesterase and alpha-glycosidase inhibitors. Bioorg. Chem. 2019, 85, 191–197. [Google Scholar] [CrossRef] [PubMed]
- George, G.; Koyiparambath, V.P.; Sukumaran, S.; Nair, A.S.; Pappachan, L.K.; Al-Sehemi, A.G.; Kim, H.; Mathew, B. Structural Modifications on Chalcone Framework for Developing New Class of Cholinesterase Inhibitors. Int. J. Mol. Sci. 2022, 23, 3121. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.R.; Zhou, C.; Fan, H.Q.; Tang, J.J.; Liu, L.B.; Gao, X.H.; Wang, Q.A.; Liu, W.K. Novel Potent and Selective Acetylcholinesterase Inhibitors as Potential Drugs for the Treatment of Alzheimer’s Disease: Synthesis, Pharmacological Evaluation, and Molecular Modeling of Amino-Alkyl-Substituted Fluoro-Chalcones Derivatives. Chem. Biol. Drug Des. 2015, 86, 517–522. [Google Scholar] [CrossRef]
- Tran, T.D.; Nguyen, T.C.V.; Nguyen, N.S.; Nguyen, D.M.; Nguyen, T.T.H.; Le, M.T.; Thai, K.M. Synthesis of Novel Chalcones as Acetylcholinesterase Inhibitors. Appl. Sci. 2016, 6, 198. [Google Scholar] [CrossRef]
- Arczewska, M.; Gieroba, B.; Gladyszewska, B.; Roj, E.; Gagos, M. The effect of solvent on the spectral properties of xanthohumol. Przem. Chem. 2018, 97, 624–628. [Google Scholar]
- Arczewska, M.; Kaminski, D.M.; Gorecka, E.; Pociecha, D.; Roj, E.; Slawinska-Brych, A.; Gagos, M. The molecular organization of prenylated flavonoid xanthohumol in DPPC multibilayers: X-ray diffraction and FTIR spectroscopic studies. Bba-Biomembranes 2013, 1828, 213–222. [Google Scholar] [CrossRef]
- Kaminski, D.M.; Gaweda, K.; Arczewska, M.; Senczyna, B.; Gagos, M. A kinetic study of xanthohumol cyclization to isoxanthohumol—A role of water. J. Mol. Struct. 2017, 1139, 10–16. [Google Scholar] [CrossRef]
- Hidalgo, A.Y.; Velasco, M.; Sanchez-Lara, E.; Gomez-Rivera, A.; Vilchis-Reyes, M.A.; Alvarado, C.; Herrera-Ruiz, M.; Lopez-Rodriguez, R.; Romero-Ceronio, N.; Lobato-Garcia, C.E. Synthesis, Crystal Structures, and Molecular Properties of Three Nitro-Substituted Chalcones. Crystals 2021, 11, 1589. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khan, J.; Bin Dukhyil, A.A.; Alarousy, R.M.I.I.; Attah, E.I.; Sharma, T.; Khairnar, S.J.; Bendale, A.R. Chalcone Scaffolds, Bioprecursors of Flavonoids: Chemistry, Bioactivities, and Pharmacokinetics. Molecules 2021, 26, 7177. [Google Scholar] [CrossRef] [PubMed]
- Venturelli, S.; Burkard, M.; Biendl, M.; Lauer, U.M.; Frank, J.; Busch, C. Prenylated chalcones and flavonoids for the prevention and treatment of cancer. Nutrition 2016, 32, 1171–1178. [Google Scholar] [CrossRef]
- Liang, M.S.; Li, X.C.; Ouyang, X.J.; Xie, H.; Chen, D.F. Antioxidant Mechanisms of Echinatin and Licochalcone A. Molecules 2019, 24, 3. [Google Scholar] [CrossRef] [PubMed]
- Rammohan, A.; Reddy, J.S.; Sravya, G.; Rao, C.N.; Zyryanov, G.V. Chalcone synthesis, properties and medicinal applications: A review. Environ. Chem. Lett. 2020, 18, 433–458. [Google Scholar] [CrossRef]
- Azim, S.A.; Al-Hazmy, S.M.; Ebeid, E.M.; El-Daly, S.A. A new coumarin laser dye 3-(benzothiazol-2-yl)-7-hydroxycoumarin. Opt. Laser Technol. 2005, 37, 245–249. [Google Scholar] [CrossRef]
- Wang, C.; Li, Z. Molecular conformation and packing: Their critical roles in the emission performance of mechanochromic fluorescence materials. Mater. Chem. Front. 2017, 1, 2174–2194. [Google Scholar] [CrossRef]
- Danko, M.; Andics, A.; Kosa, C.; Hrdlovic, P.; Vegh, D. Spectral properties of chalcone containing triphenylamino structural unit in solution and in polymer matrices. Dye. Pigment. 2012, 92, 1257–1265. [Google Scholar] [CrossRef]
- Pannipara, M.; Asiri, A.M.; Alamry, K.A.; Arshad, M.N.; El-Daly, S.A. Spectroscopic Investigation, Effect of Solvent Polarity and Fluorescence Quenching of a New D-pi-A Type Chalcone Derivative. J. Fluoresc. 2014, 24, 1629–1638. [Google Scholar] [CrossRef]
- Zhou, B.; Jiang, P.X.; Lu, J.X.; Xing, C.G. Characterization of the Fluorescence Properties of 4-Dialkylaminochalcones and Investigation of the Cytotoxic Mechanism of Chalcones. Arch. Pharm. 2016, 349, 539–552. [Google Scholar] [CrossRef]
- Sachdeva, T.; Milton, M.D. AIEE active novel red-emitting D-pi-A phenothiazine chalcones displaying large Stokes shift, solvatochromism and “turn-on” reversible mechanofluorochromism. Dye. Pigment. 2020, 181, 108539. [Google Scholar] [CrossRef]
- Wangngae, S.; Pewklang, T.; Chansaenpak, K.; Ganta, P.; Worakaensai, S.; Siwawannapong, K.; Kluaiphanngam, S.; Nantapong, N.; Lai, R.Y.; Kamkaew, A. A chalcone-based fluorescent responsive probe for selective detection of nitroreductase activity in bacteria. New J. Chem. 2021, 45, 11566–11573. [Google Scholar] [CrossRef]
- Krawczyk, P.; Pietrzak, M.; Janek, T.; Jedrzejewska, B.; Cysewski, P. Spectroscopic and nonlinear optical properties of new chalcone fluorescent probes for bioimaging applications: A theoretical and experimental study. J. Mol. Model. 2016, 22, 125. [Google Scholar] [CrossRef] [PubMed]
- Miriam, T.; Schwed, J.S.; Weizel, J.; Stark, H. Novel chalcone-based fluorescent human histamine H3 receptor ligands as pharmacological tools. Front. Syst. Neurosci. 2012, 6, 14. [Google Scholar] [CrossRef]
- Sirat, H.M.; Feng, Y.S.; Hazni, H.; Awang, K.; Ng, S.W. (E)-1-(2-Hydroxy-4,6-dimethoxyphenyl)-3-(4-methoxyphenyl)prop-2-en-1-one from Kaempferia rotunda Val. Acta Crystallogr. E 2010, 66, O2944-U1315. [Google Scholar] [CrossRef]
- Cheng, X.; Li, F.; Han, S.H.; Zhang, Y.F.; Jiao, C.J.; Wei, J.B.; Ye, K.Q.; Wang, Y.; Zhang, H.Y. Emission behaviors of unsymmetrical 1,3-diaryl-beta-diketones: A model perfectly disclosing the effect of molecular conformation on luminescence of organic solids. Sci. Rep. 2015, 5, 9140. [Google Scholar] [CrossRef]
- Sirat, H.M.; Jani, N.A.; Hazni, H.; Awang, K.; Ng, S.W. Flavokavain B from the rhizome of Alpinia mutica Roxb. Acta Crystallogr. E 2010, 66, O2866-U2623. [Google Scholar] [CrossRef]
- Zhang, X.L.; Zhang, Y.D.; Wang, T.; Guo, H.Y.; Liu, Q.M.; Su, H.X. Evaluation on Antioxidant Effect of Xanthohumol by Different Antioxidant Capacity Analytical Methods. J. Chem. 2014, 2014, 249485. [Google Scholar] [CrossRef]
- Rosa, G.P.; Seca, A.M.L.; Barreto, M.D.; Silva, A.M.S.; Pinto, D.C.G.A. Chalcones and Flavanones Bearing Hydroxyl and/or Methoxyl Groups: Synthesis and Biological Assessments. Appl. Sci. 2019, 9, 2846. [Google Scholar] [CrossRef]
- Santos, C.M.M.; Silva, A.M.S. The Antioxidant Activity of Prenylflavonoids. Molecules 2020, 25, 696. [Google Scholar] [CrossRef]
- Dzoyem, J.P.; Nkuete, A.H.L.; Ngameni, B.; Eloff, J.N. Anti-inflammatory and anticholinesterase activity of six flavonoids isolated from Polygonum and Dorstenia species. Arch. Pharm. Res. 2017, 40, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Skrzypek, A.; Matysiak, J.; Karpinska, M.; Czarnecka, K.; Krecisz, P.; Stary, D.; Kukulowicz, J.; Paw, B.; Bajda, M.; Szymanski, P.; et al. Biological evaluation and molecular docking of novel 1,3,4-thiadiazole-resorcinol conjugates as multifunctional cholinesterases inhibitors. Bioorg. Chem. 2021, 107, 104617. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Jackson, W.R.; Choi, C.; Bergmark, W.R. Solvent Effects on Emission Yield and Lifetime for Coumarin Laser-Dyes—Requirements for a Rotatory Decay Mechanism. J. Phys. Chem. 1985, 89, 294–300. [Google Scholar] [CrossRef]
- Griffin, A.; Bond, A. CrysAlis(Pro): Facilitating variable temperature experiments. Acta Crystallogr. A 2011, 67, C277–C278. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Phase Annealing in Shelx-90—Direct Methods for Larger Structures. Acta Crystallogr. Sect. A 1990, 46, 467–473. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]


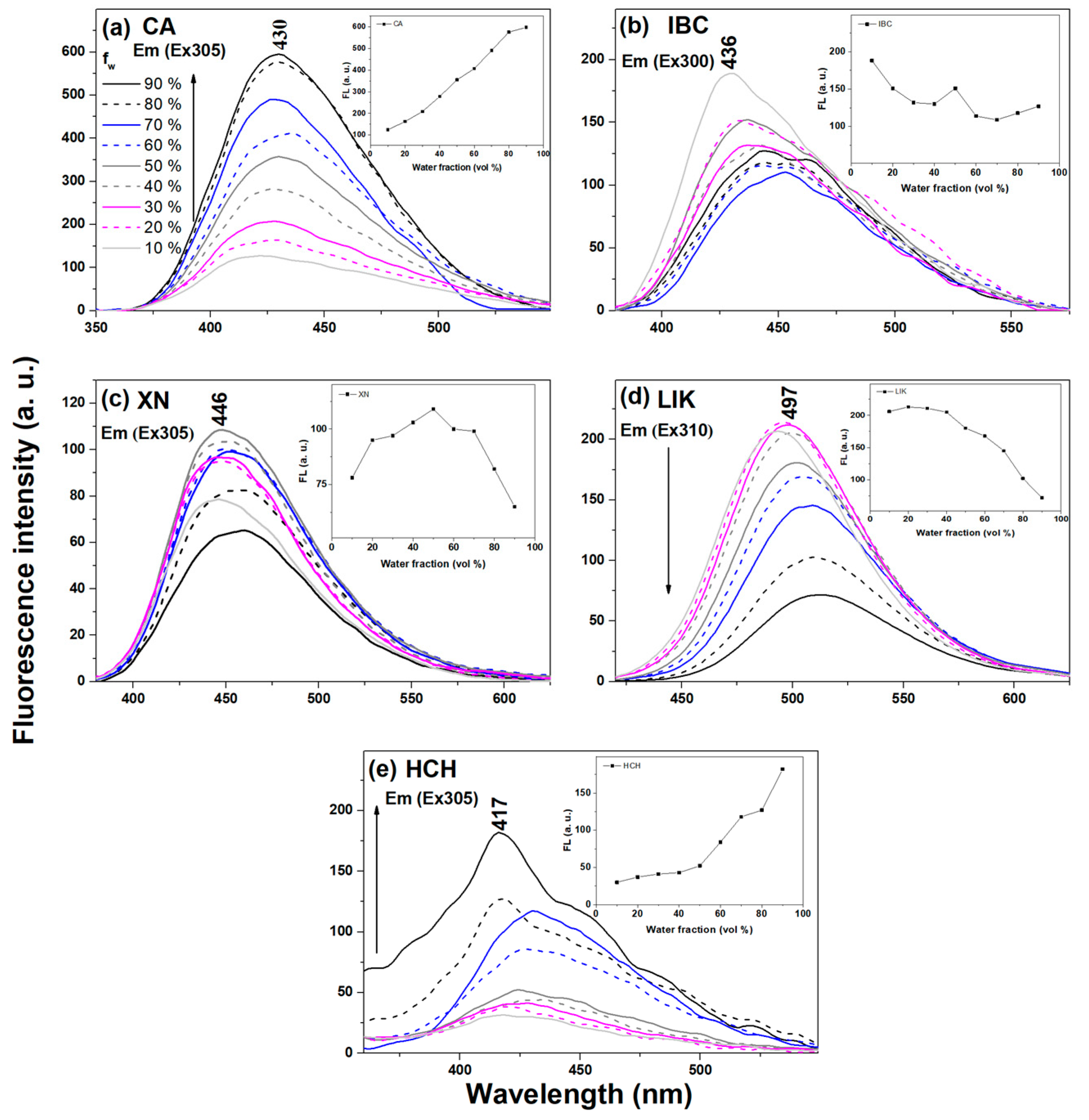

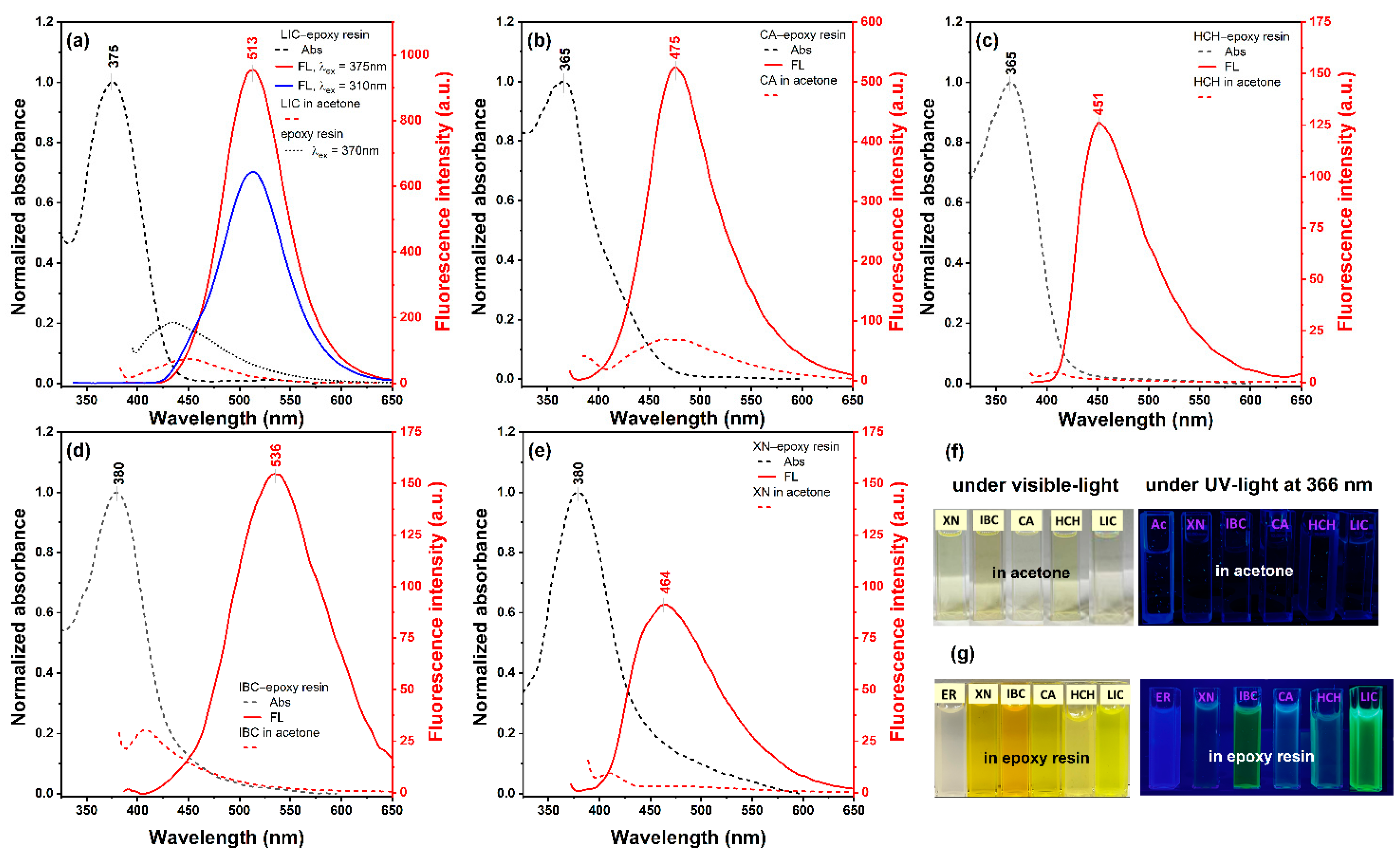
| Compound | Solvent | [nm] a | I b | F c | F [%] |
|---|---|---|---|---|---|
| LIC | Chloroform | 340 | 1217.45 | 0.017 | 1.67% |
| H2O | 360 | 4492.32 | 0.049 | 4.94% | |
| MeOH | 360 | 13,591.67 | 0.149 | 14.92% | |
| Ethylene glycol | 364 | 39,790.82 | 0.504 | 50.43% | |
| 1:9 (90% H2O) | 362 | 6399.15 | 0.070 | 7.01% | |
| 5:5 (50% H2O) | 362 | 13,691.85 | 0.151 | 15.14% | |
| 9:1 (10% H2O) | 362 | 13,124.63 | 0.144 | 14.36% | |
| CA | Chloroform | 323 | 221.22 | 0.003 | 0.32% |
| H2O | 337 | 907.41 | 0.011 | 1.07% | |
| MeOH | 322 | 143.06 | 0.002 | 0.18% | |
| Ethylene glycol | 328 | 504.93 | 0.007 | 0.71% | |
| 1:9 (90% H2O) | 332 | 487.63 | 0.006 | 0.58% | |
| 5:5 (50% H2O) | 328 | 195.08 | 0.002 | 0.24% | |
| 9:1 (10% H2O) | 323 | 229.40 | 0.003 | 0.28% | |
| IBC | Chloroform | 347 | 489.47 | 0.007 | 0.66% |
| H2O | 351 | 93.26 | 0.001 | 0.11% | |
| MeOH | 347 | 335.02 | 0.004 | 0.38% | |
| Ethylene glycol | 353 | 761.5321 | 0.010 | 1.00% | |
| 1:9 (90% H2O) | 353 | 156.8975 | 0.002 | 0.18% | |
| 5:5 (50% H2O) | 353 | 223.9067 | 0.003 | 0.25% | |
| 9:1 (10% H2O) | 350 | 298.9348 | 0.003 | 0.34% | |
| XN | Chloroform | 346 | 117.50 | 0.002 | 0.16% |
| H2O | 348 | 332.82 | 0.004 | 0.40% | |
| MeOH | 348 | 64.58 | 0.001 | 0.07% | |
| Ethylene glycol | 353 | 339.84 | 0.004 | 0.44% | |
| 1:9 (90% H2O) | 355 | 220.20 | 0.002 | 0.25% | |
| 5:5 (50% H2O) | 351 | 147.85 | 0.002 | 0.17% | |
| 9:1 (10% H2O) | 351 | 147.02 | 0.002 | 0.17% | |
| HCH | Chloroform | 345 | 31.30 | 0.000 | 0.04% |
| H2O | 350 | 764.23 | 0.009 | 0.86% | |
| MeOH | 343 | 40.77 | 0.000 | 0.05% | |
| Ethylene glycol | 348 | 397.70 | 0.005 | 0.53% | |
| 1:9 (90% H2O) | 348 | 180.46 | 0.002 | 0.21% | |
| 5:5 (50% H2O) | 348 | 190.35 | 0.002 | 0.22% | |
| 9:1 (10% H2O) | 344 | 168.18 | 0.002 | 0.19% |
| HCH | ||||
| D─H···A | D─H (Å) | H···A (Å) | Angle (°) | Symmetry |
| O2A─H2A···O1B | 0.820 | 1.717 | 148.29 | x, y, z |
| O2B─H2B···O1B | 0.818 | 1.724 | 148.05 | x, y, z |
| IBC | ||||
| D─H···A | D─H (Å) | H···A (Å) | Angle (°) | Symmetry |
| O2─H2···O1 | 0.819 | 1.823 | 146.55 | x, y, z |
| O3─H3···O1 | 0.820 | 1.990 | 157.88 | x, y, z |
| Samples Dissolved in DMSO | DPPH Radical Scavenging (%) after 30 min |
| CA | 2.09 ± 0.05 |
| IBC | 2.36 ± 0.07 |
| XN | 7.99 ± 0.05 |
| LIC | 29.24 ± 0.70 |
| HCH | 2.09 ± 0.03 |
| (+)-catechin | 94.14 ± 0.44 |
| Samples Dissolved in MeOH | DPPH Radical Scavenging (%) after 30 min |
| CA | 0.79 ± 0.07 |
| IBC | 0.61 ± 0.01 |
| XN | 4.34 ± 0.05 |
| LIC | 26.50 ± 0.92 |
| HCH | 0.30 ± 0.06 |
| Samples Dissolved in MeOH | DPPH Radical Scavenging (%) after 60 min |
| CA | 1.33 ± 0.14 |
| IBC | 1.03 ± 0.06 |
| XN | 6.72 ± 0.30 |
| LIC | 29.60 ± 0.98 |
| HCH | 0.35 ± 0.05 |
| Sample | AChE IC50 [µM] Mean ± SD | BuChE IC50 [µM] Mean ± SD |
|---|---|---|
| CA | 67.87 ± 0.01 | 75.29 ± 0.41 |
| IBC | 35.48 ± 0.01 | 43.05 ± 0.16 |
| XN | 40.81 ± 0.04 | 65.79 ± 0.34 |
| LIC | 23.41 ± 0.02 | 42.28 ± 0.06 |
| HCH | >100 | 76.54 ± 3.03 |
| Neostygmine | 0.02 ± 0.01 | 0.03 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budziak-Wieczorek, I.; Kamiński, D.; Skrzypek, A.; Ciołek, A.; Skrzypek, T.; Janik-Zabrotowicz, E.; Arczewska, M. Naturally Occurring Chalcones with Aggregation-Induced Emission Enhancement Characteristics. Molecules 2023, 28, 3412. https://doi.org/10.3390/molecules28083412
Budziak-Wieczorek I, Kamiński D, Skrzypek A, Ciołek A, Skrzypek T, Janik-Zabrotowicz E, Arczewska M. Naturally Occurring Chalcones with Aggregation-Induced Emission Enhancement Characteristics. Molecules. 2023; 28(8):3412. https://doi.org/10.3390/molecules28083412
Chicago/Turabian StyleBudziak-Wieczorek, Iwona, Daniel Kamiński, Alicja Skrzypek, Anna Ciołek, Tomasz Skrzypek, Ewa Janik-Zabrotowicz, and Marta Arczewska. 2023. "Naturally Occurring Chalcones with Aggregation-Induced Emission Enhancement Characteristics" Molecules 28, no. 8: 3412. https://doi.org/10.3390/molecules28083412
APA StyleBudziak-Wieczorek, I., Kamiński, D., Skrzypek, A., Ciołek, A., Skrzypek, T., Janik-Zabrotowicz, E., & Arczewska, M. (2023). Naturally Occurring Chalcones with Aggregation-Induced Emission Enhancement Characteristics. Molecules, 28(8), 3412. https://doi.org/10.3390/molecules28083412