Mechanical Force-Induced Color-Variable Luminescence of Carbon Dots in Boric Acid Matrix
Abstract
1. Introduction
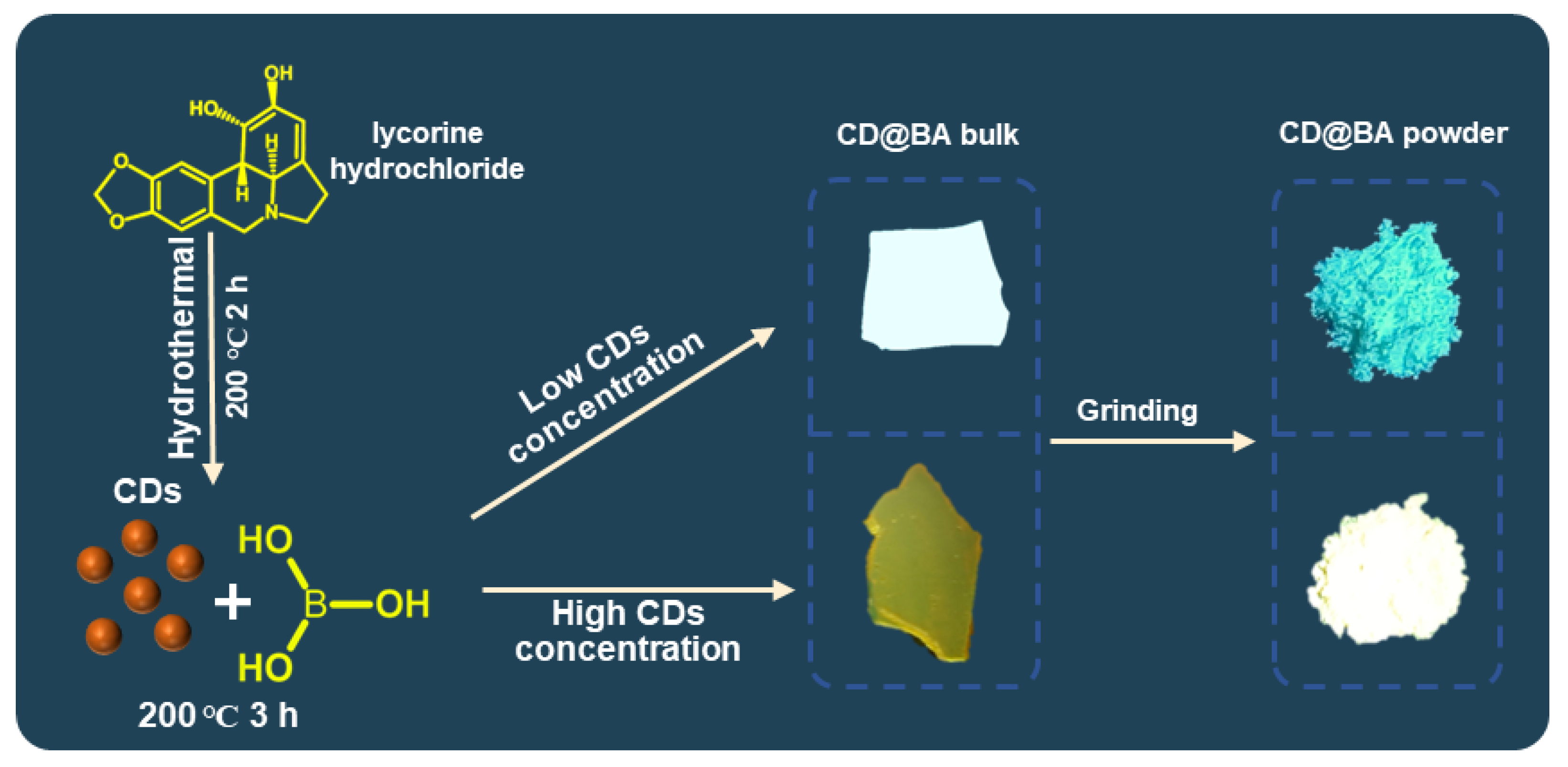
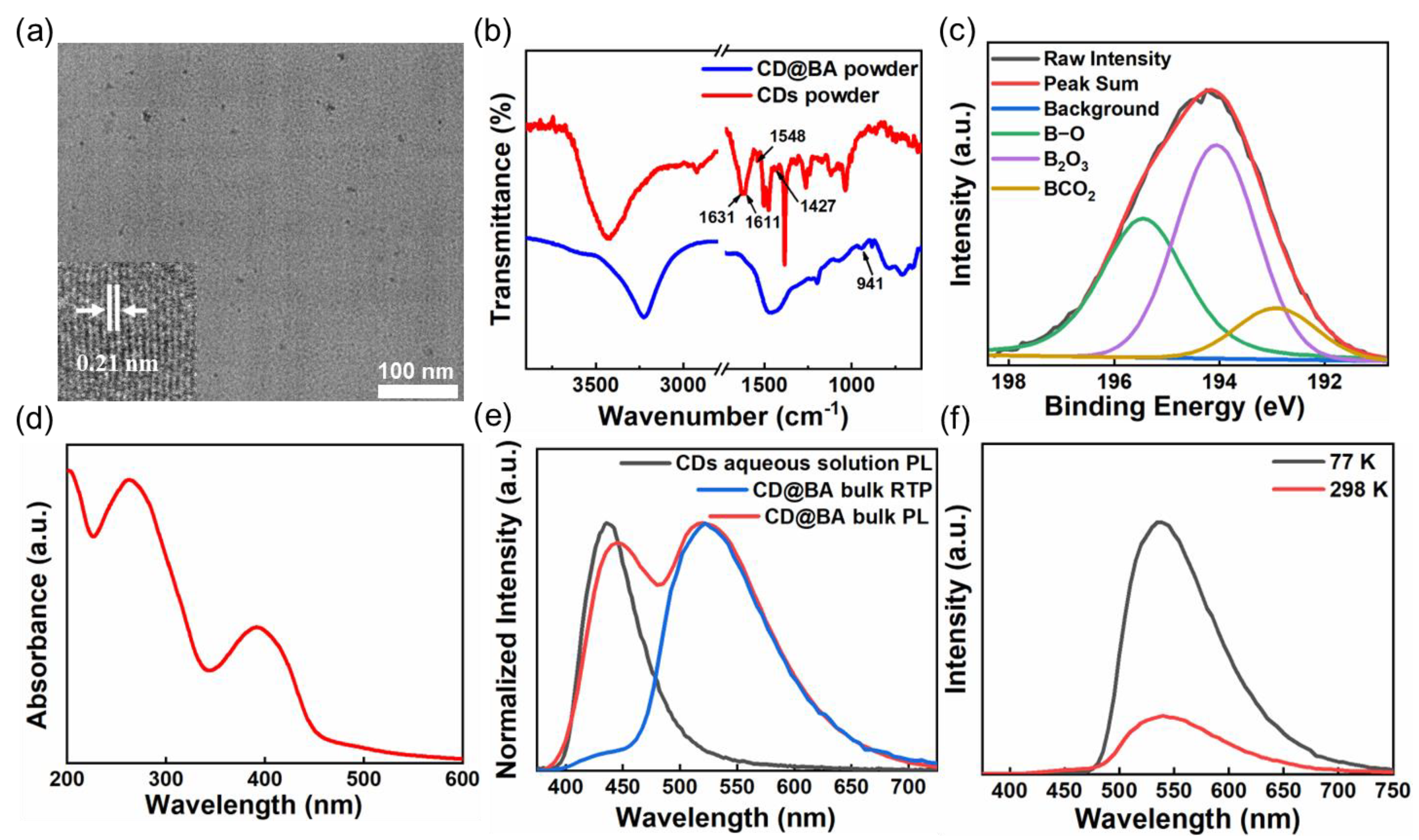
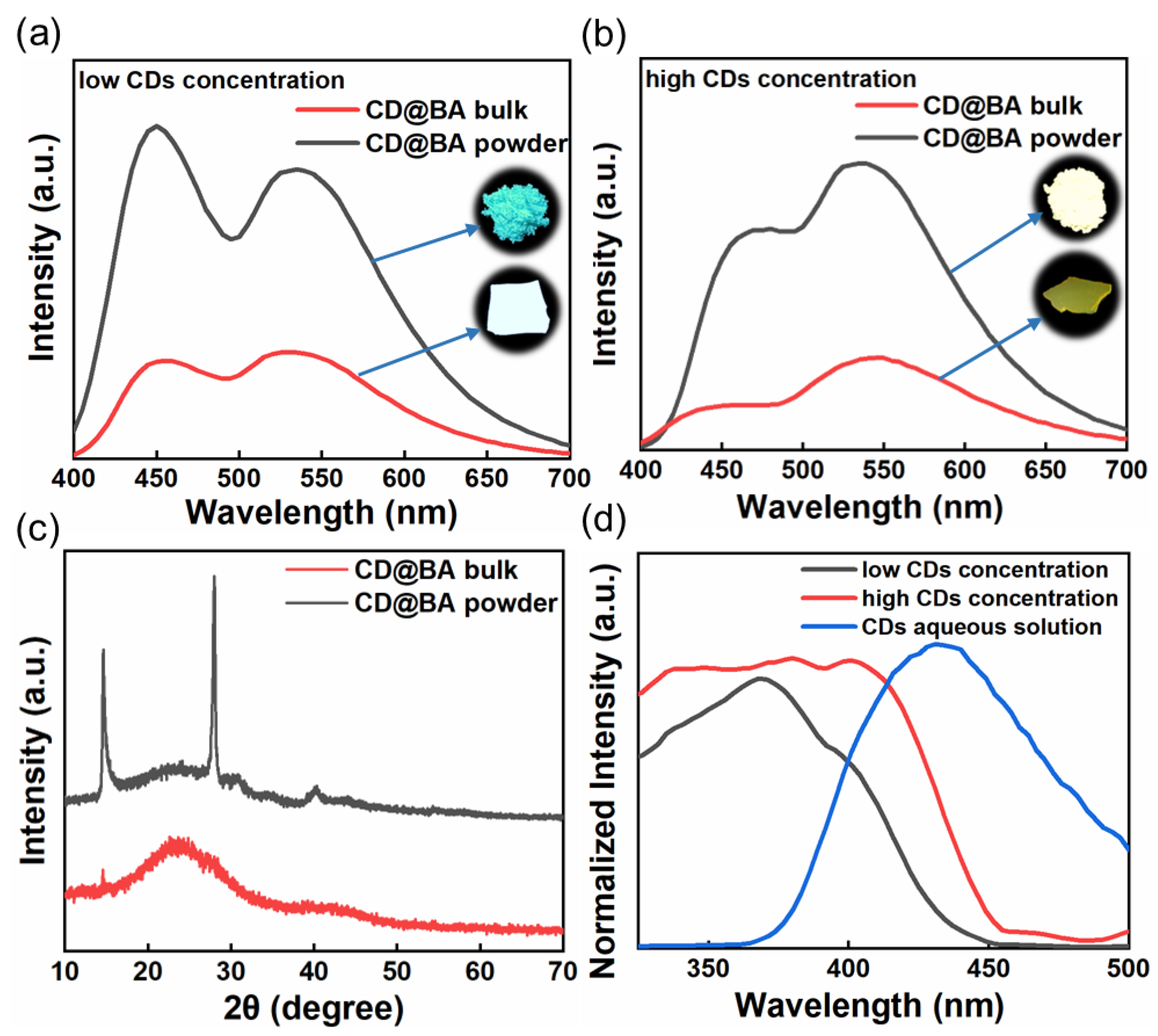
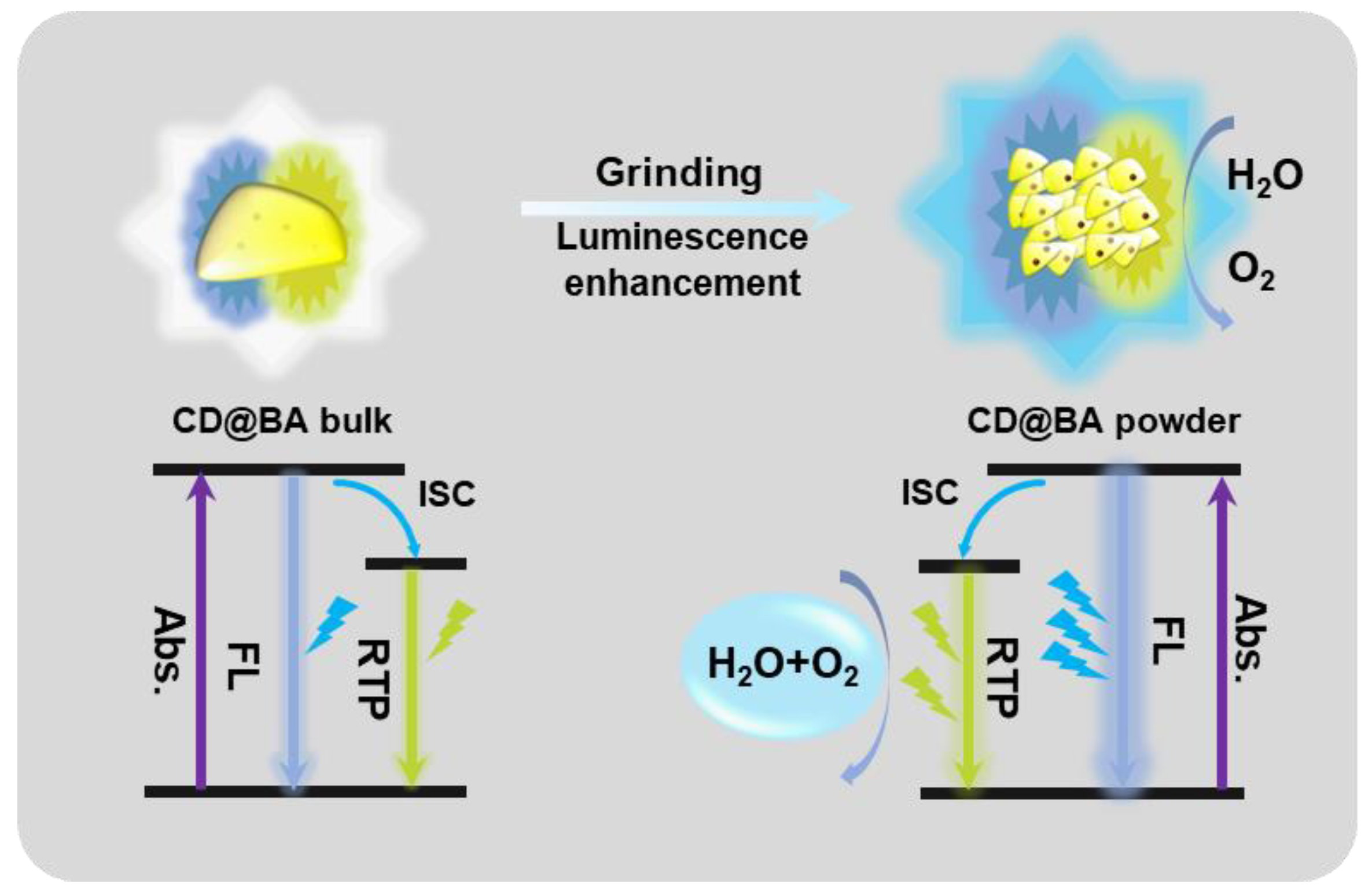
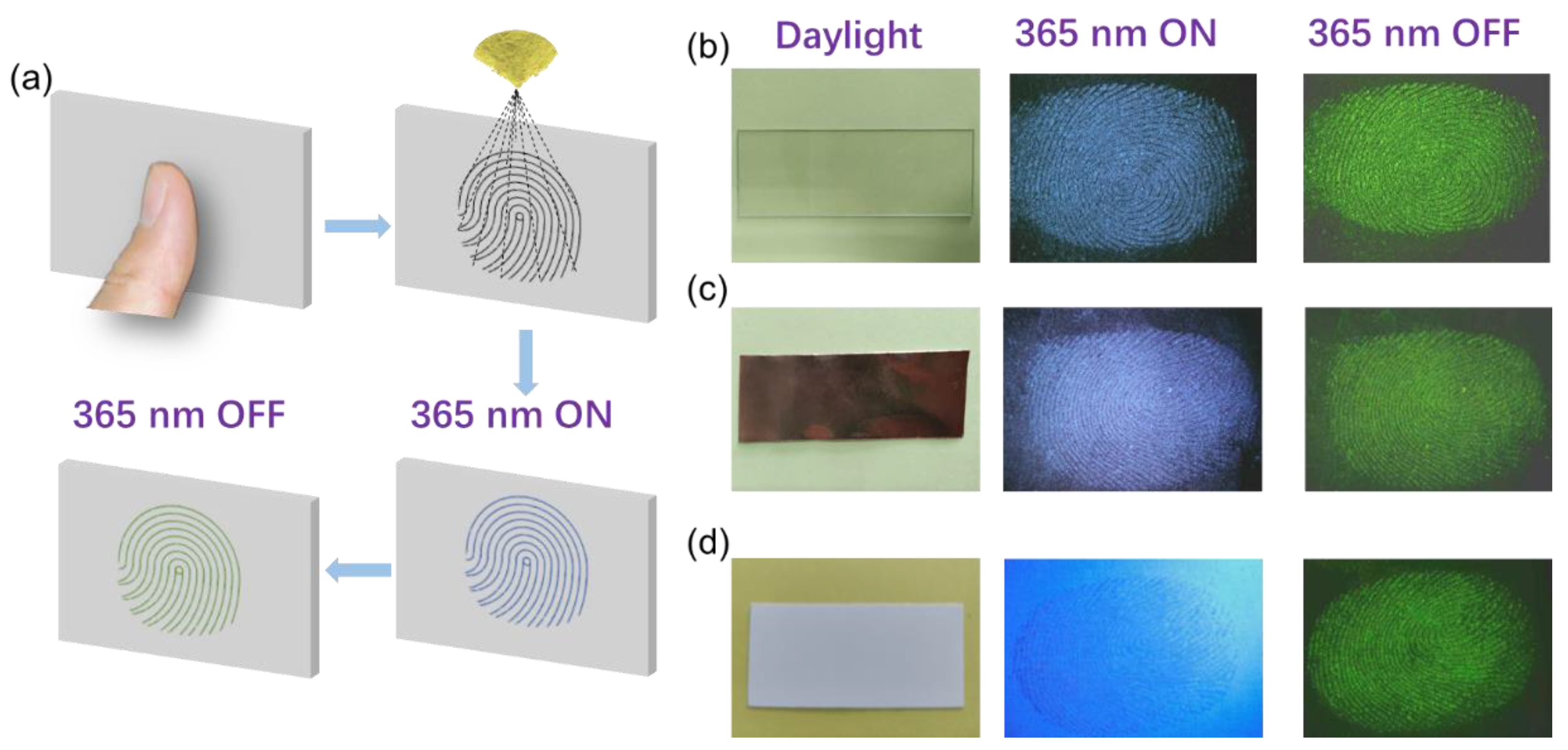
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Apparatus
3.3. Synthesis of CDs
3.4. Synthesis of CD@BA with Different Concentration of CDs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhao, W.; He, Z.; Tang, B.Z. Room-temperature phosphorescence from organic aggregates. Nat. Rev. Mater. 2020, 5, 869–885. [Google Scholar] [CrossRef]
- Gu, L.; Shi, H.; Bian, L.; Gu, M.; Ling, K.; Wang, X.; Ma, H.; Cai, S.; Ning, W.; Fu, L.; et al. Colour-tunable ultra-long organic phosphorescence of a single-component molecular crystal. Nat. Photonics. 2019, 13, 406–411. [Google Scholar] [CrossRef]
- Xiao, L.; Chen, Z.; Qu, B.; Luo, J.; Kong, S.; Gong, Q.; Kido, J. Recent progresses on materials for electrophosphorescent organic light-emitting devices. Adv. Mater. 2011, 23, 926–952. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, Y.; Wang, C.; Zheng, X.; Zheng, Y.; Gao, L.; Yang, C.; Li, Y.; Qu, L.; Zhao, Y. Color-Tunable Polymeric Long-Persistent Luminescence Based on Polyphosphazenes. Adv. Mater. 2020, 32, e1907355. [Google Scholar] [CrossRef] [PubMed]
- Bolton, O.; Lee, K.; Kim, H.J.; Lin, K.Y.; Kim, J. Activating efficient phosphorescence from purely organic materials by crystal design. Nat. Chem. 2011, 3, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Cheng, X.; Ma, Z.; Sijbesma, R.P.; Ma, Z. Polymer Mechanochromism from Force-Tuned Excited-State Intramolecular Proton Transfer. J. Am. Chem. Soc. 2022, 144, 9971–9979. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhang, H.; Xu, P.; Tian, Y.; Wang, C.; Xiang, S.; Boulatov, R.; Weng, W. A Mechanochemical Reaction Cascade for Controlling Load-Strengthening of a Mechanochromic Polymer. Angew. Chem. Int. Ed. Engl. 2020, 59, 21980–21985. [Google Scholar] [CrossRef]
- Tu, D.; Xu, C.-N.; Fujio, Y.; Yoshida, A. Mechanism of mechanical quenching and mechanoluminescence in phosphorescent CaZnOS:Cu. Light Sci. Appl. 2015, 4, e356. [Google Scholar] [CrossRef]
- Xue, P.; Ding, J.; Chen, P.; Wang, P.; Yao, B.; Sun, J.; Sun, J.; Lu, R. Mechanical force-induced luminescence enhancement and chromism of a nonplanar D–A phenothiazine derivative. J. Mater. Chem. C 2016, 4, 5275–5280. [Google Scholar] [CrossRef]
- Liu, H.; Gu, Y.; Dai, Y.; Wang, K.; Zhang, S.; Chen, G.; Zou, B.; Yang, B. Pressure-Induced Blue-Shifted and Enhanced Emission: A Cooperative Effect between Aggregation-Induced Emission and Energy-Transfer Suppression. J. Am. Chem. Soc. 2020, 142, 1153–1158. [Google Scholar] [CrossRef]
- Man, Z.; Lv, Z.; Xu, Z.; Liao, Q.; Liu, J.; Liu, Y.; Fu, L.; Liu, M.; Bai, S.; Fu, H. Highly Sensitive and Easily Recoverable Excitonic Piezochromic Fluorescent Materials for Haptic Sensors and Anti-Counterfeiting Applications. Adv. Funct. Mater. 2020, 30, 2000105. [Google Scholar] [CrossRef]
- Qi, Y.; Ding, N.; Wang, Z.; Xu, L.; Fang, Y. Mechanochromic Wide-Spectrum Luminescence Based on a Monoboron Complex. ACS Appl. Mater. Interfaces 2019, 11, 8676–8684. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.; Zhang, X.; Xu, B.; Zhou, X.; Ma, C.; Zhang, Y.; Liu, S.; Xu, J. Recent advances in organic mechanofluorochromic materials. Chem. Soc. Rev. 2012, 41, 3878–3896. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Li, M.; Yan, Y.; Lam, J.W.Y.; Zhang, Y.L.; Zhao, Y.S.; Wong, K.S.; Tang, B.Z. A tetraphenylethene-substituted pyridinium salt with multiple functionalities: Synthesis, stimuli-responsive emission, optical waveguide and specific mitochondrion imaging. J. Mater. Chem. C 2013, 1, 4640–4646. [Google Scholar] [CrossRef]
- Wang, C.; Xu, B.; Li, M.; Chi, Z.; Xie, Y.; Li, Q.; Li, Z. A stable tetraphenylethene derivative: Aggregation-induced emission, different crystalline polymorphs, and totally different mechanoluminescence properties. Mater. Horiz. 2016, 3, 220–225. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, B.; Yu, X.; Li, J.; Shang, J.; Yu, J. Carbon Dots in Porous Materials: Host-Guest Synergy for Enhanced Performance. Angew. Chem. Int. Ed. Engl. 2020, 59, 19390–19402. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, B.; Zhang, H.; Yu, J. Carbon Dots-in-Matrix Boosting Intriguing Luminescence Properties and Applications. Small 2019, 15, e1805504. [Google Scholar] [CrossRef]
- Tao, S.; Lu, S.; Geng, Y.; Zhu, S.; Redfern, S.A.T.; Song, Y.; Feng, T.; Xu, W.; Yang, B. Design of Metal-Free Polymer Carbon Dots: A New Class of Room-Temperature Phosphorescent Materials. Angew. Chem. Int. Ed. Engl. 2018, 57, 2393–2398. [Google Scholar] [CrossRef]
- Tan, J.; Li, Q.; Meng, S.; Li, Y.; Yang, J.; Ye, Y.; Tang, Z.; Qu, S.; Ren, X. Time-dependent phosphorescence colors from carbon dots for advanced dynamic information encryption. Adv. Mater. 2021, 33, 2006781. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, L.; Zheng, X.; Wang, Z.; Yang, C.; Tang, H.; Qu, L.; Li, Y.; Zhao, Y. Ultraviolet irradiation-responsive dynamic ultralong organic phosphorescence in polymeric systems. Nat. Commun. 2021, 12, 2297. [Google Scholar] [CrossRef]
- Chung, Y.J.; Kim, J.; Park, C.B. Photonic Carbon Dots as an Emerging Nanoagent for Biomedical and Healthcare Applications. ACS Nano 2020, 14, 6470–6497. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhai, Y.; Li, Z.; Zhu, P.; Mao, S.; Zhu, C.; Du, D.; Belfiore, L.A.; Tang, J.; Lin, Y. Red carbon dots: Optical property regulations and applications. Mater. Today 2019, 30, 52–79. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, M.; Yang, M.; Yang, Q.; Zhang, Z.; Shi, J. Induction of long-lived room temperature phosphorescence of carbon dots by water in hydrogen-bonded matrices. Nat. Commun. 2018, 9, 734. [Google Scholar] [CrossRef] [PubMed]
- Buscema, M.; Island, J.O.; Groenendijk, D.J.; Blanter, S.I.; Steele, G.A.; van der Zant, H.S.; Castellanos-Gomez, A. Photocurrent generation with two-dimensional van der Waals semiconductors. Chem. Soc. Rev. 2015, 44, 3691–3718. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, F.; Li, X.; Li, Y.; Zhong, H.; Fan, L.; Yang, S. 53% efficient red emissive carbon quantum dots for high color rendering and stable warm white-light-emitting diodes. Adv. Mater. 2017, 29, 1702910. [Google Scholar] [CrossRef]
- Zhang, C.; Han, Y.; Lin, L.; Deng, N.; Chen, B.; Liu, Y. Development of Quantum Dots-Labeled Antibody Fluorescence Immunoassays for the Detection of Morphine. J. Agric. Food. Chem. 2017, 65, 1290–1295. [Google Scholar] [CrossRef]
- Yuan, F.; Wang, Y.-K.; Sharma, G.; Dong, Y.; Zheng, X.; Li, P.; Johnston, A.; Bappi, G.; Fan, J.Z.; Kung, H.; et al. Bright high-colour-purity deep-blue carbon dot light-emitting diodes via efficient edge amination. Nat. Photonics 2019, 14, 171–176. [Google Scholar] [CrossRef]
- Tan, J.; Zhang, J.; Li, W.; Zhang, L.; Yue, D. Synthesis of amphiphilic carbon quantum dots with phosphorescence properties and their multifunctional applications. J. Mater. Chem. C 2016, 4, 10146–10153. [Google Scholar] [CrossRef]
- Tan, J.; Zou, R.; Zhang, J.; Li, W.; Zhang, L.; Yue, D. Large-scale synthesis of N-doped carbon quantum dots and their phosphorescence properties in a polyurethane matrix. Nanoscale 2016, 8, 4742–4747. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Tao, S.; Feng, T.; Zheng, C.; Zhu, S.; Yang, B. Carbonized Polymer Dots: A Brand New Perspective to Recognize Luminescent Carbon-Based Nanomaterials. J. Phys. Chem. Lett. 2019, 10, 5182–5188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.; Fan, Y.; Guo, X.; Zhou, L.; Lv, Y.; Lin, J. One-step microwave synthesis of N-doped hydroxyl-functionalized carbon dots with ultra-high fluorescence quantum yields. Nanoscale 2016, 8, 15281–15287. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yu, J.; Sui, L.; Zhu, S.; Tang, Z.; Yang, B.; Lu, S. Rational design of multi-color-emissive carbon dots in a single reaction system by hydrothermal. Adv. Sci. 2021, 8, 2001453. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhao, D.; Chen, X.; Wang, F.; Song, H.; Shen, D. Long lifetime pure organic phosphorescence based on water soluble carbon dots. Chem. Commun. 2013, 49, 5751–5753. [Google Scholar] [CrossRef]
- Li, Q.; Cheng, D.; Gu, H.; Yang, D.; Li, Y.; Meng, S.; Zhao, Y.; Tang, Z.; Zhang, Y.; Tan, J. Aggregation-induced Color Fine-tunable Carbon Dot Phosphorescence Covering from Green to Near-infrared for Advanced Information Encryption. Chem. Eng. J. 2023, 462, 142339. [Google Scholar] [CrossRef]
- Liu, Y.; Kang, X.; Xu, Y.; Li, Y.; Wang, S.; Wang, C.; Hu, W.; Wang, R.; Liu, J. Modulating the Carbonization Degree of Carbon Dots for Multicolor Afterglow Emission. ACS Appl. Mater. Interfaces 2022, 14, 22363–22371. [Google Scholar] [CrossRef]
- Jiang, K.; Wang, Y.; Lin, C.; Zheng, L.; Du, J.; Zhuang, Y.; Xie, R.; Li, Z.; Lin, H. Enabling robust and hour-level organic long persistent luminescence from carbon dots by covalent fixation. Light Sci. Appl. 2022, 11, 80. [Google Scholar] [CrossRef]
- Han, Z.; Li, P.; Deng, Y.; Li, H. Reversible and color-variable afterglow luminescence of carbon dots triggered by water for multi-level encryption and decryption. Chem. Eng. J. 2021, 415, 128999. [Google Scholar] [CrossRef]
- Li, Q.; Meng, S.; Li, Y.; Cheng, D.; Gu, H.; Zhao, Z.; Tang, Z.; Tan, J.; Qu, S. Surface ionization-induced tunable dynamic phosphorescence colors from carbon dots on paper for dynamic multimode encryption. Carbon 2022, 195, 191–198. [Google Scholar] [CrossRef]
- Zhao, J.-L.; Luo, Q.-Y.; Ruan, Q.; Chen, K.; Liu, C.; Redshaw, C.; Jin, Z. Red/Green Tunable-Emission Carbon Nanodots for Smart Visual Precision pH Sensing. Chem. Mater. 2021, 33, 6091–6098. [Google Scholar] [CrossRef]
- Navrotskaya, A.; Aleksandrova, D.; Chekini, M.; Yakavets, I.; Kheiri, S.; Krivoshapkina, E.; Kumacheva, E. Nanostructured Temperature Indicator for Cold Chain Logistics. ACS Nano 2022, 16, 8641–8650. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.-C.; Gou, S.-S.; Liu, K.-K.; Wu, W.-J.; Guo, C.-Z.; Lu, S.-Y.; Zang, J.-H.; Wu, X.-Y.; Lou, Q.; Dong, L.; et al. Ultralong and efficient phosphorescence from silica confined carbon nanodots in aqueous solution. Nano Today 2020, 34, 100900. [Google Scholar] [CrossRef]
- Li, Q.; Qin, Y.; Cheng, D.; Cheng, M.; Zhao, H.; Li, L.; Qu, S.; Tan, J.; Ding, J. Moist-Electric Generator with Efficient Output and Scalable Integration Based on Carbonized Polymer Dot and Liquid Metal Active Electrode. Adv. Funct. Mater. 2023, 33, 2211013. [Google Scholar] [CrossRef]
- Jiang, K.; Wang, Y.; Cai, C.; Lin, H. Conversion of Carbon Dots from Fluorescence to Ultralong Room-Temperature Phosphorescence by Heating for Security Applications. Adv. Mater. 2018, 30, e1800783. [Google Scholar] [CrossRef]
- Liu, J.; Wang, N.; Yu, Y.; Yan, Y.; Zhang, H.; Li, J.; Yu, J. Carbon dots in zeolites: A new class of thermally activated delayed fluorescence materials with ultralong lifetimes. Sci. Adv. 2017, 3, e1603171. [Google Scholar] [CrossRef]
- Dong, X.; Wei, L.; Su, Y.; Li, Z.; Geng, H.; Yang, C.; Zhang, Y. Efficient long lifetime room temperature phosphorescence of carbon dots in a potash alum matrix. J. Mater. Chem. C 2015, 3, 2798–2801. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, J.; Pang, X.; Zhang, X.; Zhuang, J.; Zhang, H.; Hu, C.; Zheng, M.; Lei, B.; Liu, Y. Temperature-responsive conversion of thermally activated delayed fluorescence and room-temperature phosphorescence of carbon dots in silica. J. Mater. Chem. C 2020, 8, 5744–5751. [Google Scholar] [CrossRef]
- Feng, Q.; Xie, Z.; Zheng, M. Colour-tunable ultralong-lifetime room temperature phosphorescence with external heavy-atom effect in boron-doped carbon dots. Chem. Eng. J. 2021, 420, 127647. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.; Zheng, J.; Yang, Y.; Liu, X.; Xu, B. N, B-Codoping Induces High-Efficiency Solid-State Fluorescence and Dual Emission of Yellow/Orange Carbon Dots. ACS Sustain. Chem. Eng. 2021, 9, 2224–2236. [Google Scholar] [CrossRef]
- Li, W.; Zhou, W.; Zhou, Z.; Zhang, H.; Zhang, X.; Zhuang, J.; Liu, Y.; Lei, B.; Hu, C. A Universal Strategy for Activating the Multicolor Room-Temperature Afterglow of Carbon Dots in a Boric Acid Matrix. Angew. Chem. Int. Ed. Engl. 2019, 58, 7278–7283. [Google Scholar] [CrossRef]
- Li, S.; Wu, M.; Kang, Y.; Zheng, H.W.; Zheng, X.J.; Fang, D.C.; Jin, L.P. Grinding-Triggered Single Crystal-to-Single Crystal Transformation of a Zinc(II) Complex: Mechanochromic Luminescence and Aggregation-Induced Emission Properties. Inorg. Chem. 2019, 58, 4626–4633. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhao, Z.; Meng, S.; Li, Y.; Zhao, Y.; Zhang, B.; Tang, Z.; Tan, J.; Qu, S. Ultra-strong phosphorescence with 48% quantum yield from grinding treated thermal annealed carbon dots and boric acid composite. SmartMat 2021, 3, 260–268. [Google Scholar] [CrossRef]
- Huang, L.; Liu, L.; Li, X.; Hu, H.; Chen, M.; Yang, Q.; Ma, Z.; Jia, X. Crystal-state photochromism and dual-mode mechanochromism of an organic molecule with fluorescence, room-temperature phosphorescence, and delayed fluorescence. Angew. Chem. Int. Ed. 2019, 58, 16445–16450. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, S.; Cheng, D.; Gu, H.; Li, Y.; Qin, Y.; Tan, J.; Li, Q. Mechanical Force-Induced Color-Variable Luminescence of Carbon Dots in Boric Acid Matrix. Molecules 2023, 28, 3388. https://doi.org/10.3390/molecules28083388
Meng S, Cheng D, Gu H, Li Y, Qin Y, Tan J, Li Q. Mechanical Force-Induced Color-Variable Luminescence of Carbon Dots in Boric Acid Matrix. Molecules. 2023; 28(8):3388. https://doi.org/10.3390/molecules28083388
Chicago/Turabian StyleMeng, Shuai, Dengke Cheng, Hailing Gu, Yuchen Li, Yukun Qin, Jing Tan, and Qijun Li. 2023. "Mechanical Force-Induced Color-Variable Luminescence of Carbon Dots in Boric Acid Matrix" Molecules 28, no. 8: 3388. https://doi.org/10.3390/molecules28083388
APA StyleMeng, S., Cheng, D., Gu, H., Li, Y., Qin, Y., Tan, J., & Li, Q. (2023). Mechanical Force-Induced Color-Variable Luminescence of Carbon Dots in Boric Acid Matrix. Molecules, 28(8), 3388. https://doi.org/10.3390/molecules28083388




