A Novel Intelligent Indicator Film: Preparation, Characterization, and Application
Abstract
1. Introduction
2. Results and Discussion
2.1. Identification of ACN
2.2. PH Sensitivity of ACN
2.3. Antioxidant Activity of the Film-Forming Solutions
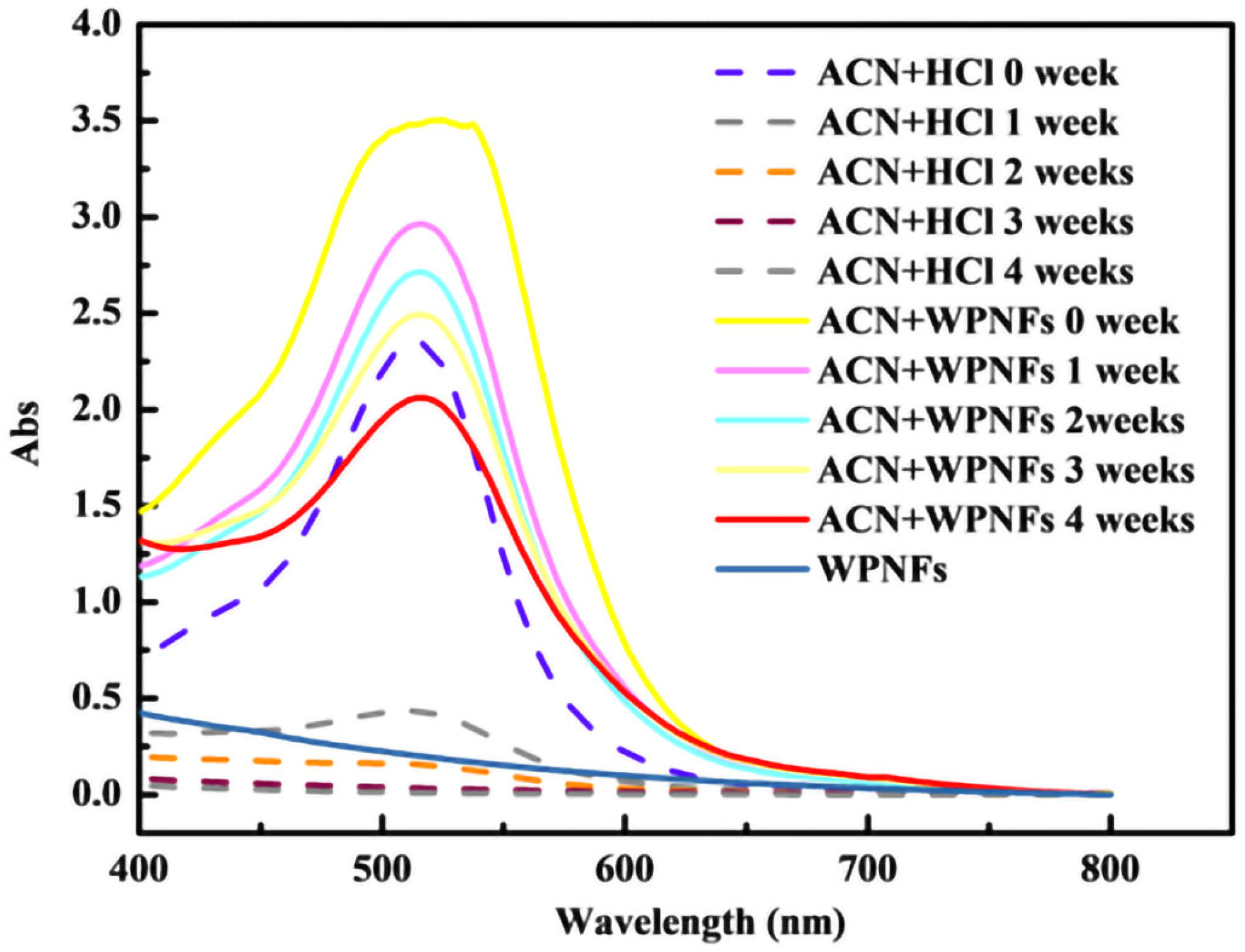
2.4. Color Protection of ACN by WPNFs
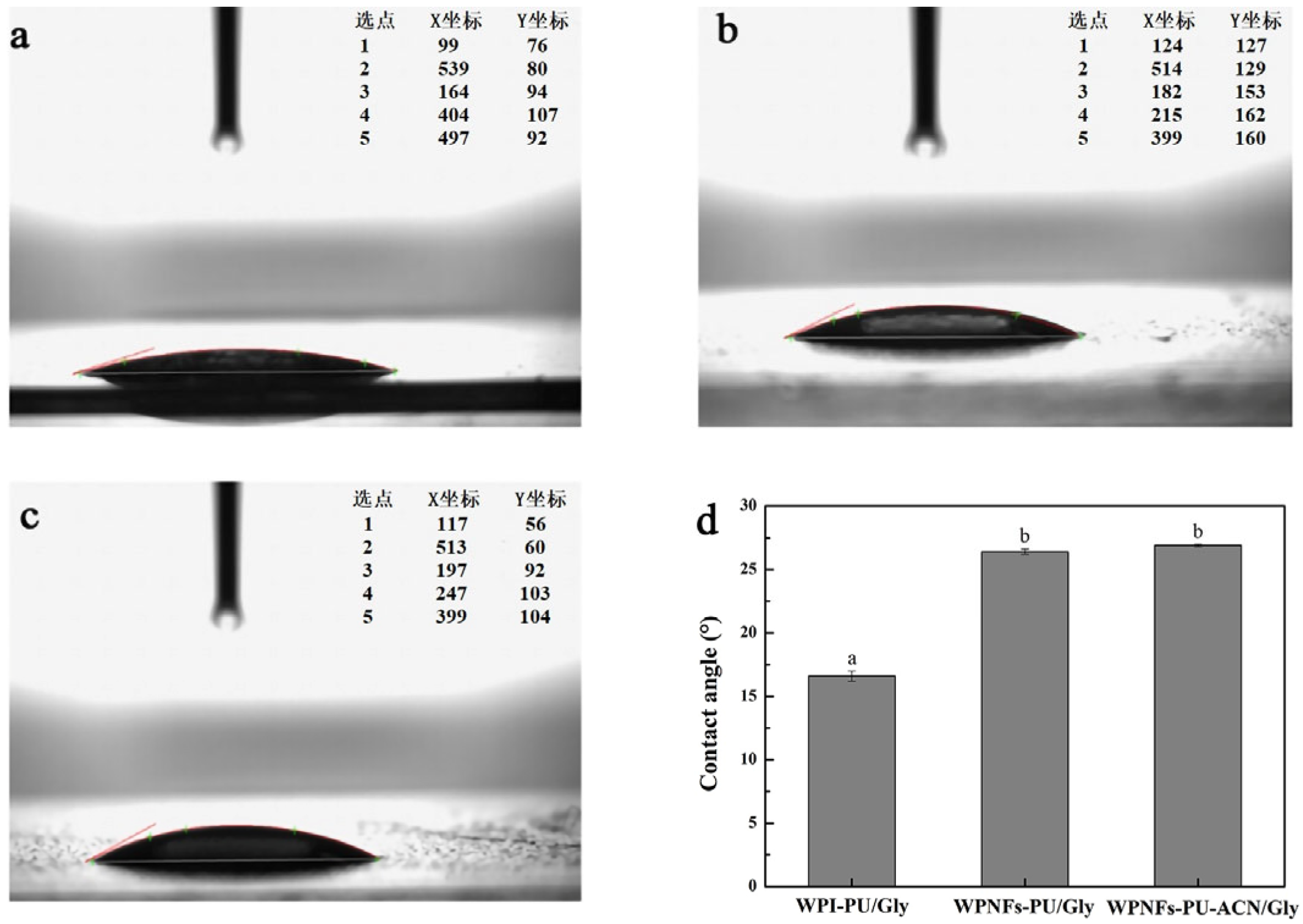
2.5. Water Contact Angle Analysis of the Edible Films
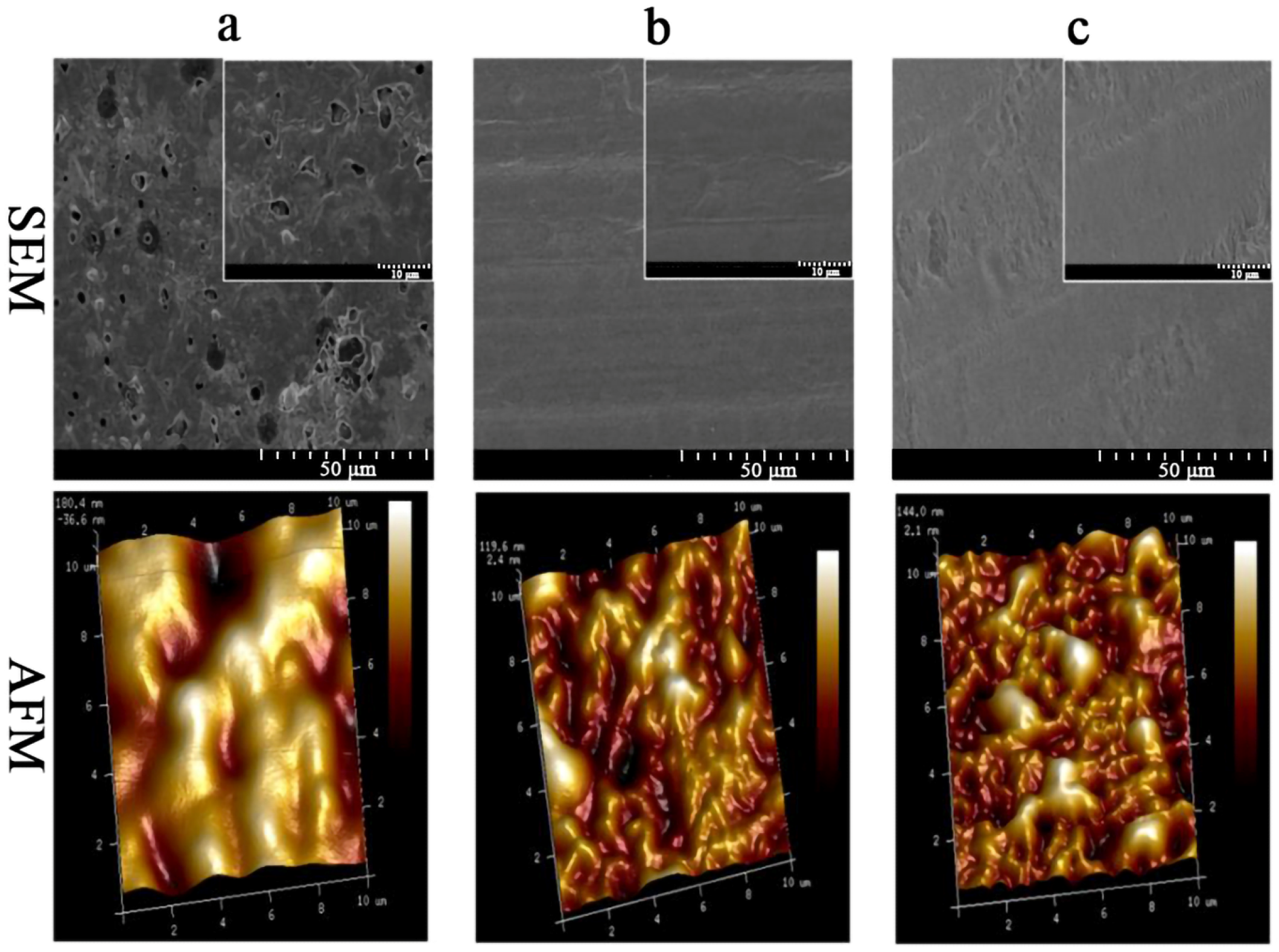
2.6. Microstructure of the Edible Films
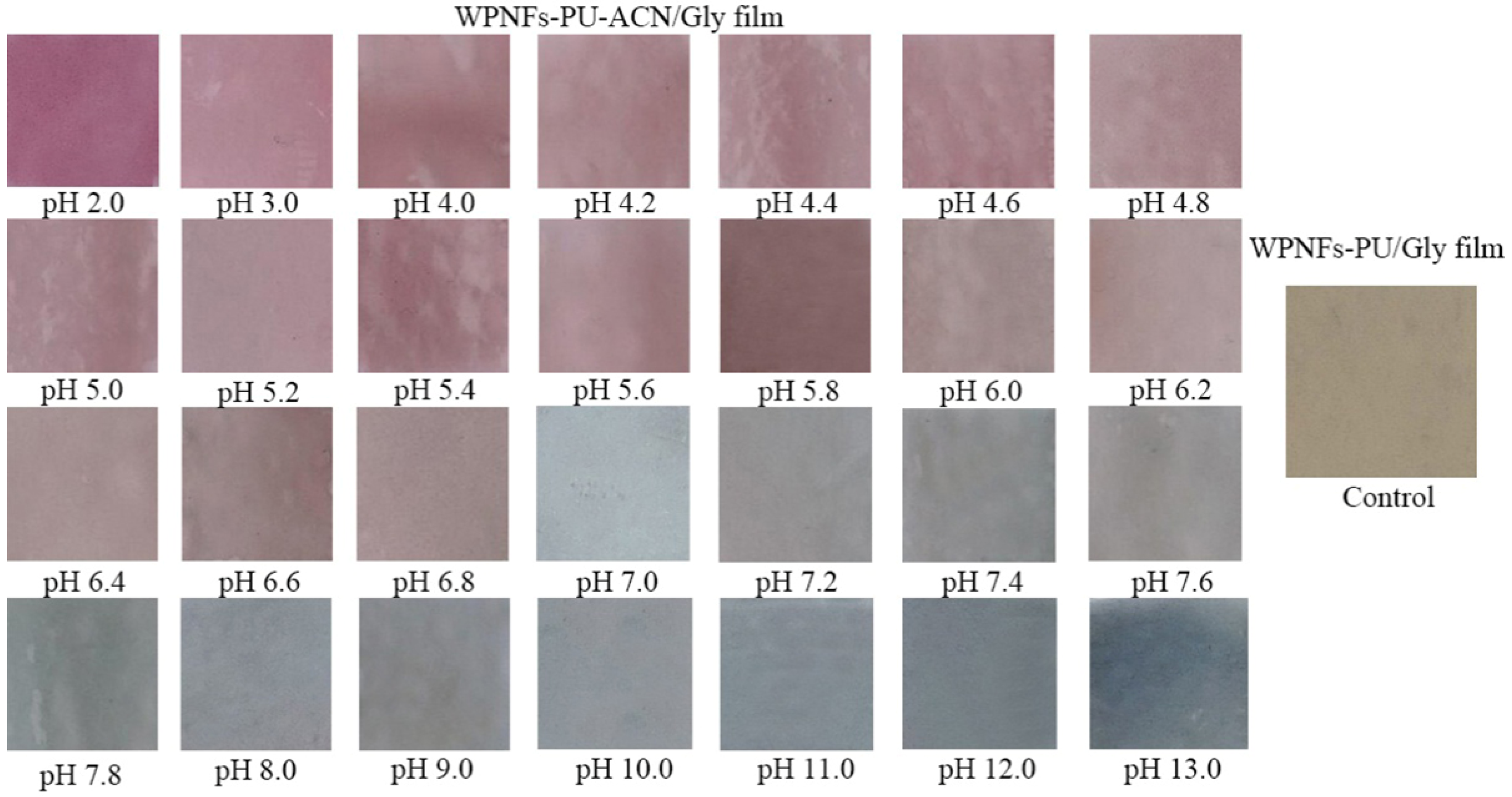
2.7. PH Sensitivity Analysis of Edible Films
2.8. Quality Assessment of Salmon
2.8.1. PH and TPA Changes of Salmon during Storage
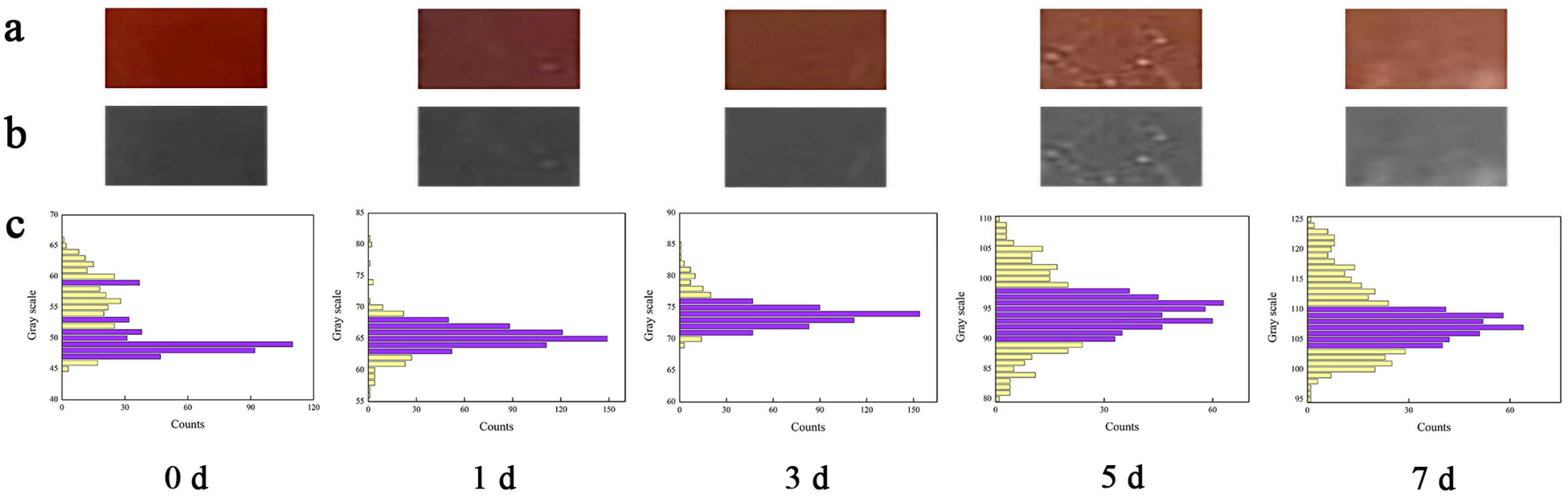
2.8.2. Color Response of WPNFs-PU-ACN/Gly Edible Film during Salmon Storage
3. Materials and Methods
3.1. Materials
3.2. Extraction and Characterization of ACN from Mulberry
3.2.1. Extraction of ACN
3.2.2. Purification of ACN
3.2.3. Identification of ACN
3.2.4. PH Sensitivity of ACN
3.3. Preparation of the Film-Forming Solutions
3.4. Characterization of Edible Film-Forming Solutions
3.4.1. Antioxidant Activity of the Film-Forming Solutions
3.4.2. Color Protection of WPNFs on ACN
3.5. Preparation of Edible Films
3.6. Functional Properties and Evaluation of Edible Films
3.6.1. Water Contact Angle of Edible Films
3.6.2. Scanning Electron Microscope (SEM) Analysis of Edible Films
3.6.3. Atomic Force Microscope (AFM) Analysis of Edible Films
3.6.4. PH Sensitivity of Edible Films
3.7. Quality Assessment of Salmon
3.7.1. Detection of Salmon Freshness
3.7.2. PH of Salmon
3.7.3. Determination of Texture Profile Analysis (TPA)
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohamed, S.A.A.; El-Sakhawy, M.; El-Sakhawy, M.A. Polysaccharides, Protein and Lipid -Based Natural Edible Films in Food Packaging: A Review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef]
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef]
- Chen, H.B.; Wang, J.J.; Cheng, Y.H.; Wang, C.S.; Liu, H.C.; Bian, H.G.; Pan, Y.R.; Sun, J.Y.; Han, W.W. Application of Pro-tein-Based Films and Coatings for Food Packaging: A Review. Polymers 2019, 11, 32. [Google Scholar] [CrossRef]
- Gao, Y.-Z.; Xu, H.-H.; Ju, T.-T.; Zhao, X.-H. The effect of limited proteolysis by different proteases on the formation of whey protein fibrils. J. Dairy Sci. 2013, 96, 7383–7392. [Google Scholar] [CrossRef]
- Luca, A. Schmitt Christophe. Globular plant protein aggregates for stabilization of food foams and emulsions. Trends Food Sci. Technol. 2017, 67, 248–259. [Google Scholar]
- Su, J.-F.; Huang, Z.; Yuan, X.-Y.; Wang, X.-Y.; Li, M. Structure and properties of carboxymethyl cellulose/soy protein isolate blend edible films crosslinked by Maillard reactions. Carbohydr. Polym. 2010, 79, 145–153. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Biendl, M. Physicochemical and antioxidant properties of biopolymer/candelilla wax emulsion films containing hop extract—A comparative study. Food Hydrocoll. 2016, 60, 384–392. [Google Scholar] [CrossRef]
- Pirsa, S.; Sani, I.K.; Mirtalebi, S.S. Nano-biocomposite based color sensors: Investigation of structure, function, and applications in intelligent food packaging. Food Packag. Shelf Life 2021, 31, 100789. [Google Scholar] [CrossRef]
- González, C.M.O.; Schelegueda, L.I.; Ruiz-Henestrosa, V.M.P.; Campos, C.A.; Basanta, M.F.; Gerschenson, L.N. Cassava Starch Films with Anthocyanins and Betalains from Agroindustrial by-Products: Their Use for Intelligent Label Development. Foods 2022, 11, 3361. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.Y.; Ren, Y.M.; Gu, Z.X.; Wang, L.J. Developing an intelligent film containing Vitis amurensis husk extracts: The effects of pH value of the film-forming solution. J. Clean. Prod. 2017, 166, 851–859. [Google Scholar] [CrossRef]
- Hao, J.; Gao, Y.; Xue, J.; Yang, Y.; Yin, J.; Wu, T.; Zhang, M. Phytochemicals, Pharmacological Effects and Molecular Mechanisms of Mulberry. Foods 2022, 11, 1170. [Google Scholar] [CrossRef]
- Chen, X.; Guan, Y.; Zeng, M.; Wang, Z.; Qin, F.; Chen, J.; He, Z. Effect of whey protein isolate and phenolic copigments in the thermal stability of mulberry anthocyanin extract at an acidic pH. Food Chem. 2022, 377, 132005. [Google Scholar] [CrossRef]
- Karaaslan, N.M.; Yaman, M. Determination of anthocyanins in cherry and cranberry by high-performance liquid chroma-tography-electrospray ionization-mass spectrometry. Eur. Food Res. Technol. 2016, 242, 127–135. [Google Scholar] [CrossRef]
- Cavalcanti, R.N.; Santos, D.T.; Meireles, M.A.A. Non-thermal stabilization mechanisms of anthocyanins in model and food systems—An overview. Food Res. Int. 2011, 44, 499–509. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Mi-gration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Jiao, Q.; Liu, Z.; Li, B.; Tian, B.; Zhang, N.; Liu, C.; Feng, Z.; Jiang, B. Development of Antioxidant and Stable Conjugated Linoleic Acid Pickering Emulsion with Protein Nanofibers by Microwave-Assisted Self-Assembly. Foods 2021, 10, 1892. [Google Scholar] [CrossRef] [PubMed]
- Tamura, S.; Yan, K.; Shimoda, H.; Murakami, N. Anthocyanins from Oryza sativa L. subsp. indica. Biochem. Syst. Ecol. 2010, 38, 438–440. [Google Scholar] [CrossRef]
- Chen, X.Q.; Nagao, N.; Itani, T.; Irifune, K. Anti-oxidative analysis, and identification and quantification of anthocyanin pigments in different coloured rice. Food Chem. 2012, 135, 2783–2788. [Google Scholar] [CrossRef]
- Lee, J.H. Identification and quantification of anthocyanins from the grains of black rice (Oryza sativa L.) varieties. Food Sci. Biotechnol. 2010, 19, 391–397. [Google Scholar] [CrossRef]
- Kaneda, I.; Kubo, F.; Sakurai, H. Antioxidative Compounds in the Extracts of Black Rice Brans. J. Health Sci. 2006, 52, 495–511. [Google Scholar] [CrossRef]
- Zhang, M.W.; Zhang, R.F.; Zhang, F.X.; Liu, R.H. Phenolic Profiles and Antioxidant Activity of Black Rice Bran of Different Commercially Available Varieties. J. Agric. Food Chem. 2010, 58, 7580–7587. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.H.; Qin, P.Y.; Zhang, Y.; Cui, S.H.; Ren, G.X. Identification of anthocyanins isolated from black rice (Oryza sativa L.) and their degradation kinetics. Food Res. Int. 2013, 50, 691–697. [Google Scholar] [CrossRef]
- Zepon, K.M.; Martins, M.M.; Marques, M.S.; Heckler, J.M.; Morisso, F.D.; Moreira, M.G.; Ziulkoski, A.L.; Kanis, L.A. Smart wound dressing based on kappa-carrageenan/locust bean gum/cranberry extract for monitoring bacterial infections. Carbohyd. Polym. 2019, 206, 362–370. [Google Scholar] [CrossRef]
- Borkowski, T.; Szymusiak, H.; Gliszczyńska-Swigło, A.; Tyrakowska, B. The effect of 3-O-β-glucosylation on structural trans-formations of anthocyanidins. Food Res. Int. 2005, 38, 1031–1037. [Google Scholar] [CrossRef]
- Zhai, X.D.; Shi, J.Y.; Zou, X.B.; Wang, S.; Jiang, C.P.; Zhang, J.J.; Huang, X.W.; Zhang, W.; Holmes, M. Novel colorimetric films based on starch/polyvinyl alcohol incorporated with roselle anthocyanins for fish freshness monitoring. Food Hydrocoll. 2017, 69, 308–317. [Google Scholar] [CrossRef]
- Ma, Q.Y.; Liang, T.Q.; Cao, L.L.; Wang, L.J. Intelligent poly (vinyl alcohol)-chitosan nanoparticles-mulberry extracts films capable of monitoring pH variations. Int. J. Biol. Macromol. 2018, 108, 576–584. [Google Scholar] [CrossRef]
- Obón, J.M.; Díaz-García, M.C.; Castellar, M.R. Red fruit juice quality and authenticity control by HPLC. J. Food Compos. Anal. 2011, 24, 760–771. [Google Scholar] [CrossRef]
- Terasawa, N.; Saotome, A.; Tachimura, Y.; Mochizuki, A.; Ono, H.; Takenaka, M.; Murata, M. Identification and some prop-erties of anthocyanin isolated from zuiki, stalk of Colocasia esculenta. J. Agric. Food Chem. 2007, 55, 4154–4159. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jiao, Q.; Wang, L.; Zhang, Y.; Jiang, B.; Li, D.; Feng, Z.; Liu, C. Preparation and evaluation of a novel high internal phase Pickering emulsion based on whey protein isolate nanofibrils derived by hydrothermal method. Food Hydrocoll. 2021, 123, 107180. [Google Scholar] [CrossRef]
- Zhang, W.N.; He, J.J.; Pan, Q.H.; Han, F.L.; Duan, C.Q. Separation and Character Analysis of Anthocyanins from Mulberry (Morus alba L.) Pomace. Czech J. Food Sci. 2011, 29, 268–726. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Whey protein edible films modified with almond and walnut oils. Food Hydrocoll. 2016, 52, 78–86. [Google Scholar] [CrossRef]
- Vogler, E.A. Structure and reactivity of water at biomaterial surfaces. Adv. Colloid Interface Sci. 1998, 74, 69–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.N.; Yu, H.L.; Tian, B.; Jiang, B.; Xu, J.; Li, D.M.; Feng, Z.B.; Liu, C.H. Novel Edible Coating with Antioxidant and Antimicrobial Activities Based on Whey Protein Isolate Nanofibrils and Carvacrol and Its Application on Fresh-Cut Cheese. Coatings 2019, 9, 583. [Google Scholar] [CrossRef]
- Yan, J.; Zhongsu, M.; Haiyue, Z.; Huijuan, B. Gas permeability and microstructure of edible films. Sci. Tech-Nology Food Ind. 2012, 33, 364–368. [Google Scholar]
- Bonilla, J.; Atarés, L.; Vargas, M.; Chiralt, A. Edible films and coatings to prevent the detrimental effect of oxygen on food quality: Possibilities and limitations. J. Food Eng. 2012, 110, 208–213. [Google Scholar] [CrossRef]
- Choi, I.; Lee, J.Y.; Lacroix, M.; Han, J. Intelligent pH indicator film composed of agar/potato starch and anthocyanin extracts from purple sweet potato. Food Chem. 2017, 218, 122–128. [Google Scholar] [CrossRef]
- Kurek, M.; Garofulić, I.E.; Bakić, M.T.; Ščetar, M.; Uzelac, V.D.; Galić, K. Development and evaluation of a novel antioxidant and pH indicator film based on chitosan and food waste sources of antioxidants. Food Hydrocoll. 2018, 84, 238–246. [Google Scholar] [CrossRef]
- Liang, T.Q.; Sun, G.H.; Le Cao, L.; Li, J.; Wang, L.J. A pH and NH3 sensing intelligent film based on Artemisia sphaerocephala Krasch. gum and red cabbage anthocyanins anchored by carboxymethyl cellulose sodium added as a host complex. Food Hydrocoll. 2018, 87, 858–868. [Google Scholar] [CrossRef]
- Huang, J.M.; Chen, B.W.; Zeng, Q.H.; Liu, Y.; Liu, H.Q.; Zhao, Y.; Wang, J.J. Application of the curcumin-mediated photo-dynamic inactivation for preserving the storage quality of salmon contaminated with L. monocytogenes. Food Chem. 2011, 359, 129974. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Guo, X.; Ji, M.; Wu, J.; Zhu, W.; Wang, J.; Cheng, C.; Chen, L.; Zhang, Q. Preservative effects of fish gelatin coating enriched with CUR/betaCD emulsion on grass carp (Ctenopharyngodon idellus) fillets during storage at 4 °C. Food Chem. 2019, 272, 643–652. [Google Scholar] [CrossRef]
- Shen, D.; Zhang, S.; Ming, W.; He, W.; Zhang, G.; Xie, Z. Development of a new machine vision algorithm to estimate potato’s shape and size based on support vector machine. J. Food Process Eng. 2022, 45, e13974. [Google Scholar] [CrossRef]
- Doi, R. Hue-shifting for accurate and precise quantification of biochemical substances using diagnostic test strips. Color. Technol. 2021, 137, 530–543. [Google Scholar] [CrossRef]
- Huang, J.; Chen, M.; Zhou, Y.; Li, Y.; Hu, Y. Functional characteristics improvement by structural modification of hy-droxypropyl methylcellulose modified polyvinyl alcohol films incorporating roselle anthocyanins for shrimp freshness moni-toring. Int. J. Biol. Macromol. 2020, 162, 1250–1261. [Google Scholar] [CrossRef]
- Jiang, B.; Zhong, S.; Yu, H.; Chen, P.; Li, B.; Li, D.; Liu, C.; Feng, Z. Covalent and Noncovalent Complexation of Phosvitin and Gallic Acid: Effects on Protein Functionality and In Vitro Digestion Properties. J. Agric. Food Chem. 2022, 70, 11715–11726. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Wang, L.L.; Zhu, M.S.H.; Wu, S.; Wang, X.J.; Li, D.M.; Liu, C.H.; Feng, Z.B.; Tian, B. Separation, structural charac-teristics and biological activity of lactic acid bacteria exopolysaccharides separated by aqueous two-phase system. LWT-Food Sci. Technol. 2021, 147, 111617. [Google Scholar] [CrossRef]
- Wang, Q.N.; Liu, W.H.; Tian, B.; Li, D.M.; Liu, C.H.; Jiang, B.; Feng, Z.B. Preparation and Characterization of Coating Based on Protein Nanofibers and Polyphenol and Application for Salted Duck Egg Yolks. Foods 2020, 9, 449. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, X.J.; Wang, L.L.; Wu, S.; Li, D.M.; Liu, C.H.; Feng, Z.B. Fabrication and Characterization of a Micro-emulsion Stabilized by Integrated Phosvitin and Gallic Acid. J. Agric. Food Chem. 2020, 68, 5437–5447. [Google Scholar] [CrossRef]
- Naghdi, S.; Rezaei, M.; Abdollahi, M. A starch-based pH-sensing and ammonia detector film containing betacyanin of pa-perflower for application in intelligent packaging of fish. Int. J. Biol. Macromol. 2021, 191, 161–170. [Google Scholar] [CrossRef]
- Ereira, V.A.; de Arruda, I.N.Q.; Stefani, R. Active chitosan/PVA films with anthocyanins from Brassica oleraceae (Red Cabbage) as Time–Temperature Indicators for application in intelligent food packaging. Food Hydrocoll. 2015, 43, 180–188. [Google Scholar]
- Sun, J.; Jiang, H.; Wu, H.; Tong, C.; Pang, J.; Wu, C. Multifunctional bionanocomposite films based on konjac gluco-mannan/chitosan with nano-ZnO and mulberry anthocyanin extract for active food packaging. Food Hydrocoll. 2020, 107, 105942. [Google Scholar] [CrossRef]
- Wang, X.C.; Yong, H.M.; Gao, L.; Li, L.L.; Jin, M.J.; Liu, J. Preparation and characterization of antioxidant and pH-sensitive films based on chitosan and black soybean seed coat extract. Food Hydrocoll. 2019, 89, 56–66. [Google Scholar] [CrossRef]
- Yu, D.; Xu, Y.; Jiang, Q.; Yang, F.; Xia, W. Freshness assessment of grass carp (Ctenopharyngodon idellus) fillets during stroage at 4 °C by physicochemical, microbiological and sensorial evaluations. J. Food Saf. 2017, 37, e12305. [Google Scholar] [CrossRef]
- Barraza, F.A.A.; León, R.A.Q.; Álvarez, P.X.L. Kinetics of protein and textural changes in Atlantic salmon under frozen storage. Food Chem. 2015, 182, 120–127. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, B.; Chen, P.; Guo, J.; Yu, H.; Zhong, S.; Li, D.; Liu, C.; Feng, Z.; Jiang, B. A Novel Intelligent Indicator Film: Preparation, Characterization, and Application. Molecules 2023, 28, 3384. https://doi.org/10.3390/molecules28083384
Han B, Chen P, Guo J, Yu H, Zhong S, Li D, Liu C, Feng Z, Jiang B. A Novel Intelligent Indicator Film: Preparation, Characterization, and Application. Molecules. 2023; 28(8):3384. https://doi.org/10.3390/molecules28083384
Chicago/Turabian StyleHan, Bing, Peifeng Chen, Jiaxuan Guo, Hongliang Yu, Shaojing Zhong, Dongmei Li, Chunhong Liu, Zhibiao Feng, and Bin Jiang. 2023. "A Novel Intelligent Indicator Film: Preparation, Characterization, and Application" Molecules 28, no. 8: 3384. https://doi.org/10.3390/molecules28083384
APA StyleHan, B., Chen, P., Guo, J., Yu, H., Zhong, S., Li, D., Liu, C., Feng, Z., & Jiang, B. (2023). A Novel Intelligent Indicator Film: Preparation, Characterization, and Application. Molecules, 28(8), 3384. https://doi.org/10.3390/molecules28083384








