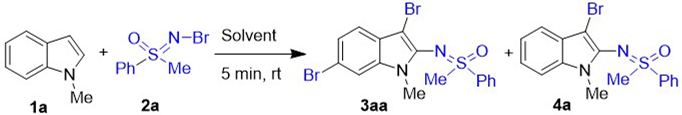
Abstract
A green one-pot 2,3,6-trifunctionalization of N-alkyl/aryl indoles was achieved by adding three equivalents of N-Br sulfoximine to the indole solution. A variety of 2-sulfoximidoyl-3,6-dibromo indoles were prepared with 38–94% yields using N-Br sulfoximines as both brominating and sulfoximinating reagents. Based on the results of controlled experiments, we propose that a radical substitution involving 3,6-dibromination and 2-sulfoximination occurs in the reaction process. This is first time that 2,3,6-trifunctionalization of indole in one pot has been achieved.
1. Introduction
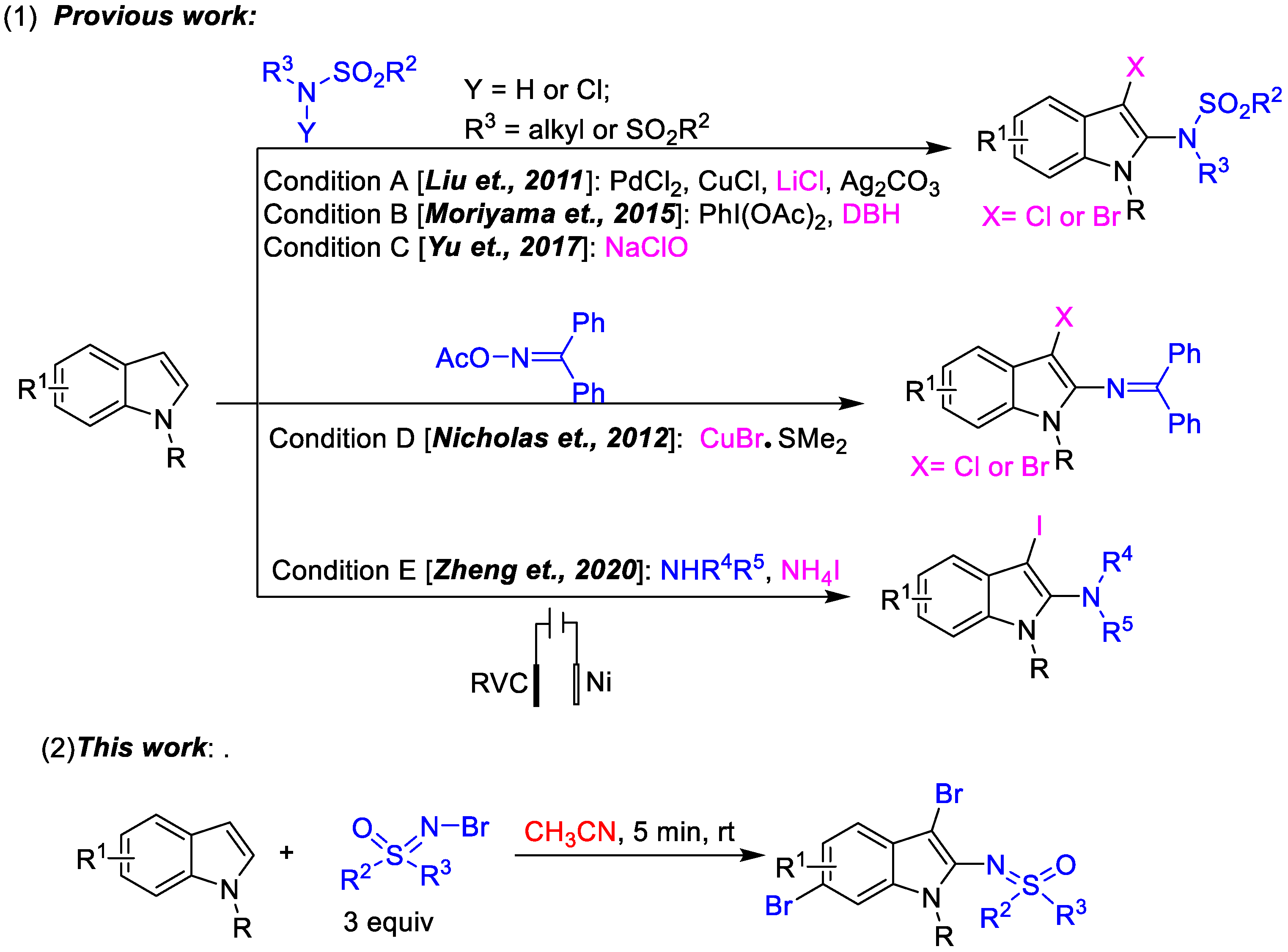
The indole moiety is always attractive because of its existence in a wide range of natural products and bioactive compounds as core structures [1,2,3,4,5,6]. Hence, the development of simple and green methods for the synthesis of multisubstituted indole derivatives is an important research area. Sulfoximine derivatives, a class of sulfur-containing compounds, have attracted considerable interest for their diverse applications in organic synthesis as building blocks [7,8], in asymmetric catalysis as ligands and chiral auxiliary [9,10,11] and in pharmacology as bioactive components [12,13,14]. A simple combination of indoles and sulfoximine maybe enable the facile synthesis of a series of potentially bioactive sulfoximidoyl indoles. The successful development of the new protocol will facilitate relative research based on sulfoximines and indoles. Normally, the direct functionalization, especially multifunctionalization, of indoles via C-H activation is an atom- and step-economical approach to expand the library of indoles [15,16,17,18,19,20,21,22,23,24,25,26]. Compared to the wildly known C2- and C3-functionalization of indoles [15,16,17,18,19,20,21], the practicality of transformation on other positions is decreased and more difficult. Due to the significant advances in the development of synthetic methodologies, various dual-functionalization strategies of indoles have been developed to introduce two functional groups at the common C2 and C3 positions of the indole core simultaneously [27,28,29,30,31,32]. Among them, effective one-step dehydrogenative aminohalogenation of indoles is always a research hotspot because of the further application of aminohalogenated indoles in the fields of organic synthesis and biology. Despite several excellent works having been reported in the last decade (Scheme 1) [33,34,35,36,37,38], the field of dehydrogenative aminohalogenation of indoles remains largely unexplored. The above studies, pioneering aminohalogenation of indoles, have included the aminohalogenation of N-R indoles using sulfonamides and its derivatives as aminating reagents [33,35,36], the copper-catalyzed aminobromination of indoles using CuBr as brominating reagent and active N-Br diphenylmethanimine as aminating reagent [34], the electrochemical aminoiodination of indoles using unactivated secondary amines [38]. Inspired by such dehydrogenative aminohalogenation of indoles, sulfoximines as a special aminating reagent entered our field of vision. As is known, due to our long-term research focus [39,40,41,42], the radical addition of olefins using N-X sulfoximines [43], and the curiosity about sulfoximine indoles, we wondered whether the active N-Br sulfoximines could be used as the aminating reagent or both brominating and aminating reagents to achieve the aminobromination of indoles. Fortunately, this was achieved, and 2,3,6-trifunctionalized indole derivatives were afforded accidentally in one step for the first time in the absence of any transition metal or additional additives (Scheme 1). Due to its step and atom economy, novelty, simplicity and practicality, this novel trifunctionalization of indoles represents important progress towards functionalized indoles.
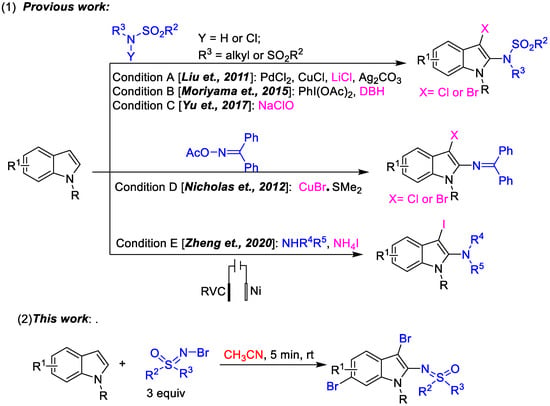
Scheme 1.
(1) The reported aminohologenation of indoles by using different aminating reagent [33,34,35,36,38]; (2) The green one-pot 2,3,6- trifunctinalization of indoles by using N-Br sulfoximines as both of brominating and aminating reagents.
2. Results and Discussion
2.1. Optimization
At the beginning of our investigation, the 5 mol% CuBr-catalyzed sulfoximidoylbromination of 1-methylindole (1a) using N-Br methyl phenyl sulfoximine (2a) as both brominating and aminating reagents was attempted in the presence of KOAc at room temperature (entry 1, Table S1). To our delight, the desired dual-functionalized product 4a was obtained with 29% yield as expected. However, the yield could not be improved more than 29% by varying copper salts, the amount of catalyst, temperature, or reaction time during further optimization (entries 2–10, Table S1). In the reaction process, the N-Br methyl phenyl sulfoximine (2a) was found to be consumed quickly. As such, the amount of N-Br methyl phenyl sulfoximine (2a) was evaluated, the trifunctionalized product 3aa was obtained suddenly with higher 76% isolated yield when the amount of 2a was increased threefold (entries 11–17, Table S1). According to the above results, the catalyst and base seem to have little effect on the reaction compared to the amount of 2a. Therefore, the reactions were tried in the absence of copper salts or cooperation of copper salts and KOAc, and 3aa was produced with excellent 90% or 88% yield, respectively (entries 18–19, Table S1). The model reaction with 1 equiv. 1-methylindole (1a) and 3 equiv. N-Br methyl phenyl sulfoximine (2a) in the absence of transition metal and base was reset (Table 1). The desired trifunctionalized product 3aa gave an excellent 94% isolated yield (entry 3, Table 1). Then, versatile solvents were evaluated in this simple protocol, and the desired product 3aa was afforded by all test solvents, except toluene, in 28–80% yields (Entries 4–8, Table 1). The lowest 28% yield was given by the protic solvent EtOH, and the cyclic ether solvents THF and 1,4-dioxane gave good 80% and 72% yields, respectively. The compound 3aa was also generated in moderate 64% yield using DCE as solvent. Finally, the optimum conditions of 2,3,6-trifunctionalization of indoles was chosen as 3 equiv. N-Br sulfoximines added to a solution of 1 equiv. indole derivatives (0.4 mmol) in 2 mL MeCN (entry 3, Table 1) with further stirring for 5 min at room temperature.

Table 1.
Exploring experimental conditions.
2.2. Extending the Scope of Indole Substrates
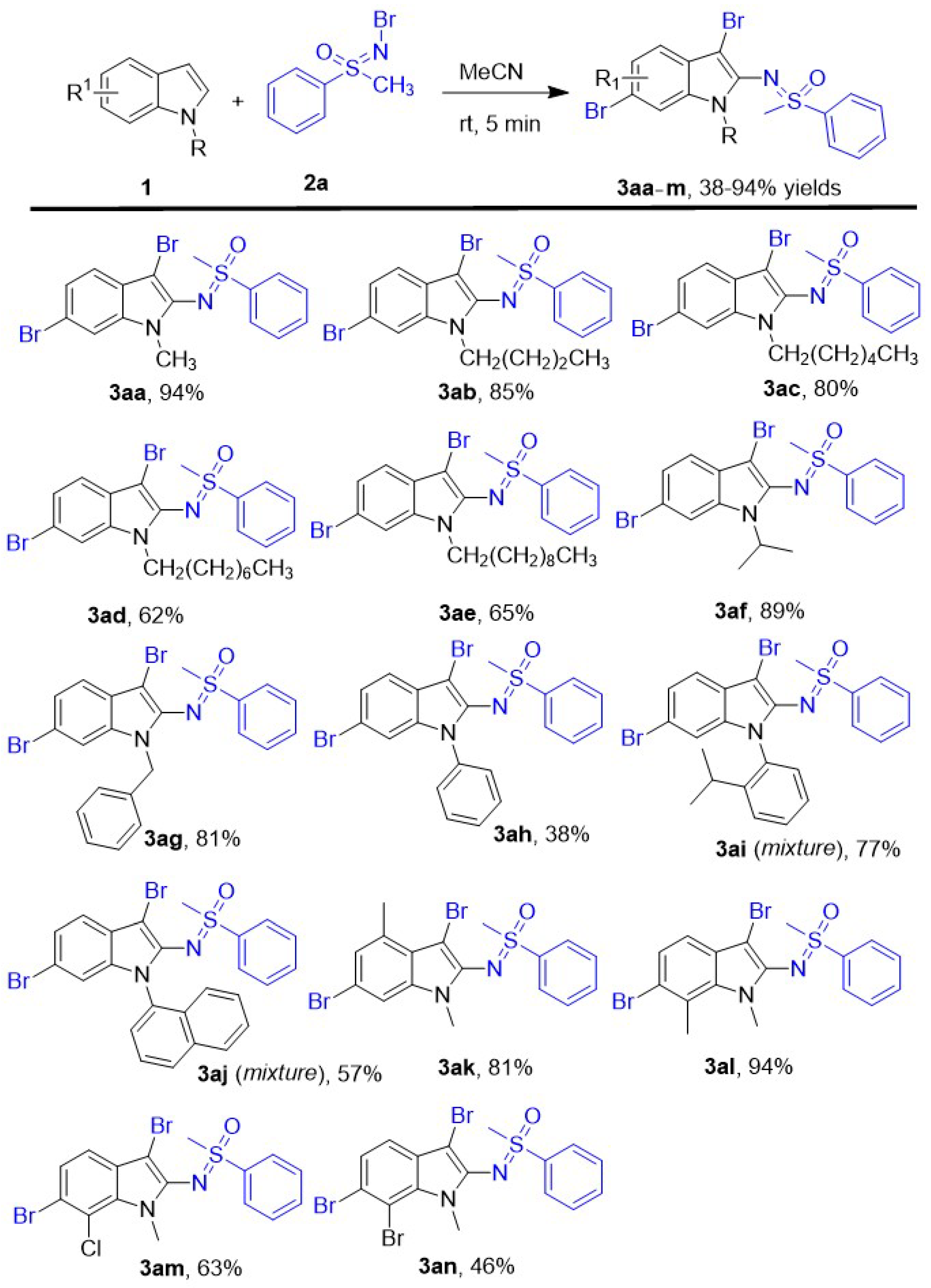
The substrate scope of indole derivatives was investigated in this new oxidative dibromo sulfoximination of N-R (R = alkyl or aryl) indoles using N-Br methyl phenyl sulfoximine as both of brominating and sulfoximinating reagents, and a series of corresponding 3,6-dibrom-2-sulfoximidoyl indoles were afforded in 38–94% yields under the optimal reaction conditions. The details of reaction were shown in Scheme 2. According to the results, the N-alkyl indoles including chain alkyl and benzyl groups have been investigated here, and all of them afforded the corresponding 2-sulfoximidoyl-3,6-dibromo indole derivatives 3aa-g in 62–94% yields. The lower yield was given by the indoles which has longer protecting alkyl chain of N atom. So the corresponding product 3aa was given in the best 94% yield by the N-CH3 indole, and N-octyl indole gave the product 3ad in worst 62% yield. The steric N-isopropyl indole was also performed smoothly in this new protocol to lead to compound 3af in good 89% yield, and the high yield showed the steric hindrance of N-protecting groups was affected this trifunctionalization of indole rarely. Furthermore, the N-benzyl indole which has an active methylene group was evaluated in this simple protocol and led to the product 3ag in good 81% yield. Besides different N- alkyl indoles, several N-aryl indoles have also been attempted as indole substrates here, and the desired products 3ah-j have been obtained as expectant in 38–77% yields. Among them, the best yield was given by the N-(2-isopropylphenyl) indole (3ai, 77%) which contained a big 2-isopropylphenyl as protecting group of N atom. Compared to the N-(2-isopropylphenyl) indole, the N-phenyl indole and N-naphthyl indole gave corresponding products 3ah and 3aj in decreased yields 38% and 57% yields respectively. Moreover, several substituted N-methyl indoles have been evaluated in this new transformation, too. When the 4- or 7- position of N-methyl indole was occupied by electron-donating methyl group, the corresponding products 3ak and 3al were given in good 81% and excellent 94% yields, respectively. Compared to the N-methyl indole, the 7-methyl group of N-methyl indole showed no obvious effect on the yield, and the 4-methyl group would make the yield slight decreased to 81%. When the 4-position of N-methyl indole was substituted by halogen Cl or Br, the reaction yields of the corresponding products 3am and 3an were decreased to 63% and 46%, respectively.
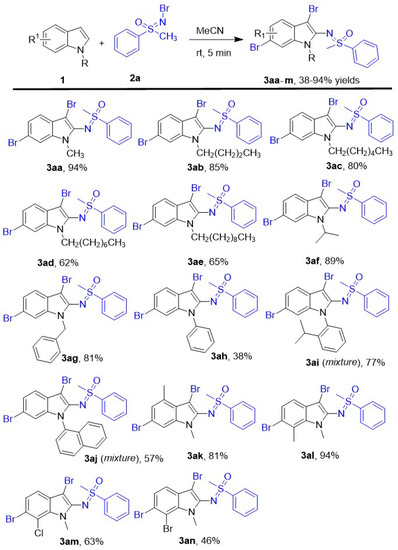
Scheme 2.
The scope of indoles derivative. Unless otherwise noted, the reactions were performed with Indoles 1 (0.4 mmol), N-Br methyl phenyl sulfoximine 2a (1.2 mmol), 2.0 mL MeCN, stirring 5 min at room temperature. All yields are isolated yields.
2.3. Extending the Scope of N-Br Sulfoximines Substrates
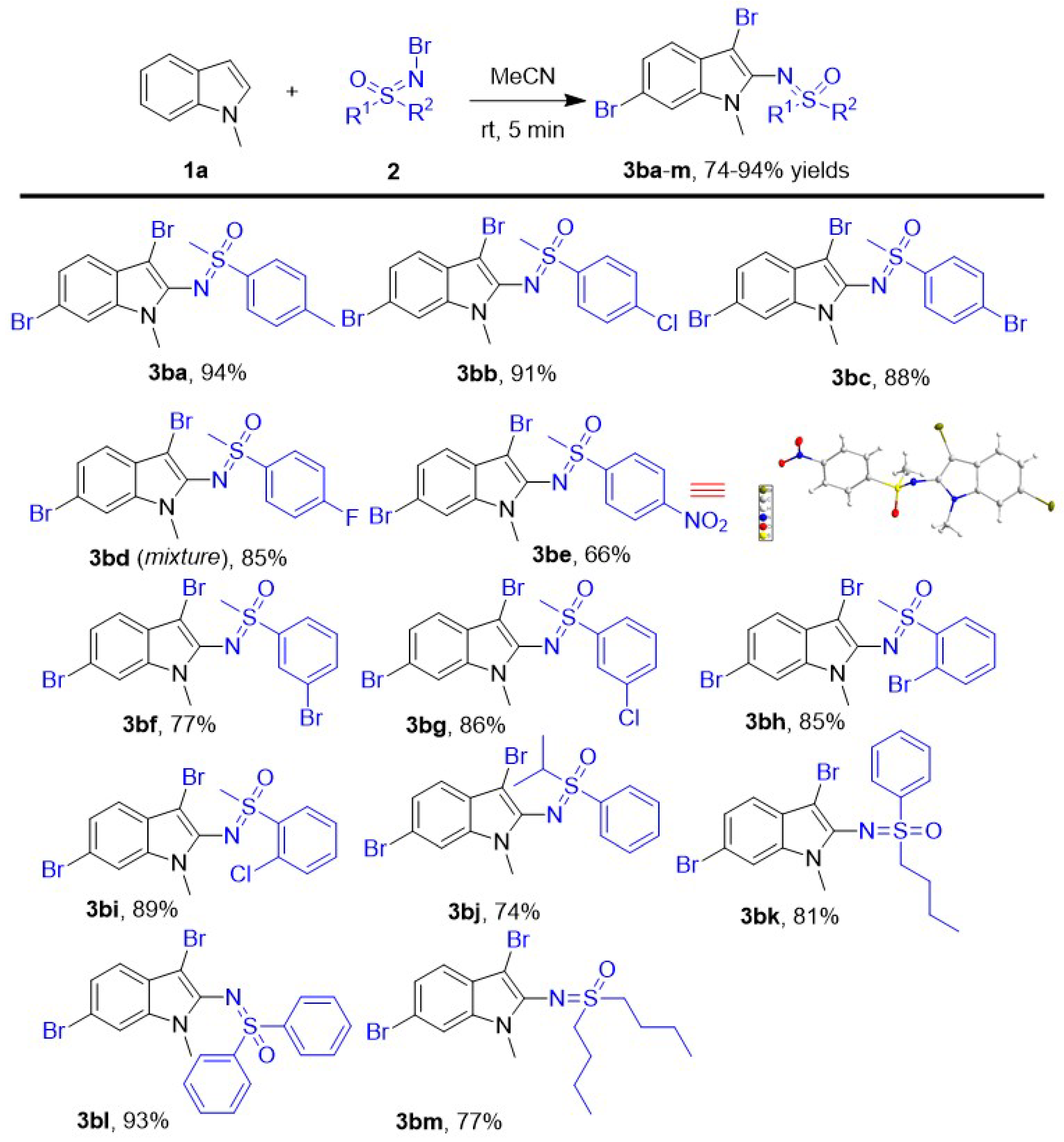
Subsequently, various N-Br sulfoximines have been implemented into this new tri-functionalization of N-methyl indoles as both of brominating and sulfoximinating reagents to evaluate the effect of different R1 or R2 group of N-Br sulfoximines (Scheme 3). First, versatile N-Br substituted phenyl methyl sulfoximines have been investigate into the dibromosulfoximination of N-methyl indole and all of them led to desired products 3ba–3bi smoothly in good–excellent yields (66–94%). For different N-Br para-substituted phenyl methyl sulfoximines, the electron-deficient substituents on the benzene ring led to decreased yields. Among them, the worst yield (3be, 66%) was given by the N-Br para-NO2 phenyl methyl sulfoximines, and the highest yield (3ba, 94%) was given by the para-CH3 phenyl methyl sulfoximines. When the N-Br para-halogenated phenyl methyl sulfoximines were investigated as substrate in this new protocol, all of them performed very well in the reaction, and the corresponding products 3bb-d were given in good–excellent 85–91% yields because of the electron withdrawal of halogen on the benzene ring. Compared to the N-Br para-bromo/chlorophenyl methyl sulfoximines, similar N-Br meta-bromo/cholorophenyl methyl sulfoximines were also achieved smoothly in this protocol and afforded corresponding products 3bf and 3bg smoothly in slightly lower 77% and 86% yields, respectively. Meanwhile, the 2-sulfoximidoyl-3,6-dibromo indoles 3bh and 3bi were obtained in good 85% and 89% yields using N-Br ortho-Bromo/cholorphenyl methyl sulfoximines as aminohalogenated substrates. The results of all tested N-Br halogenated phenyl methyl sulfoximines showed the electronic and steric effect has little influence in the yields. Subsequently, we varied the substituents of N-Br phenyl sulfoximines from steric isopropyl chain n-butyl to phenyl to evaluate the effect of substituent: the N-Br diphenyl sulfoximines led to the corresponding product 3bl in a similar 91% yield to N-Br phenyl methyl sulfoximines, and the steric N-Br isopropyl phenyl sulfoximine produced the compound 3bj in the lowest 74% yield. The N-Br n-butyl phenyl sulfoximine performed very well here and afforded the product 3bk in lower 81% yield compared to N-Br methyl phenyl sulfoximine. Besides N-Br phenyl sulfoximines, N-Br di-n-butyl sulfoximine also worked in this protocol and led to product 3bm in 77% yield.
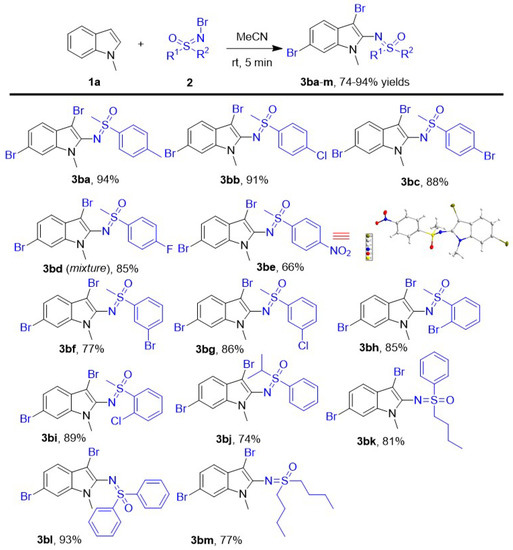
Scheme 3.
The scope of N-Br sulfoximines. Unless otherwise noted, the reactions were performed with Indoles 1a (0.4 mmol) and N-Br sulfoximines 2 (1.2 mmol) in 2.0 mL MeCN with stirring for 5 min at room temperature. All yields are isolated.
2.4. Control Reactions
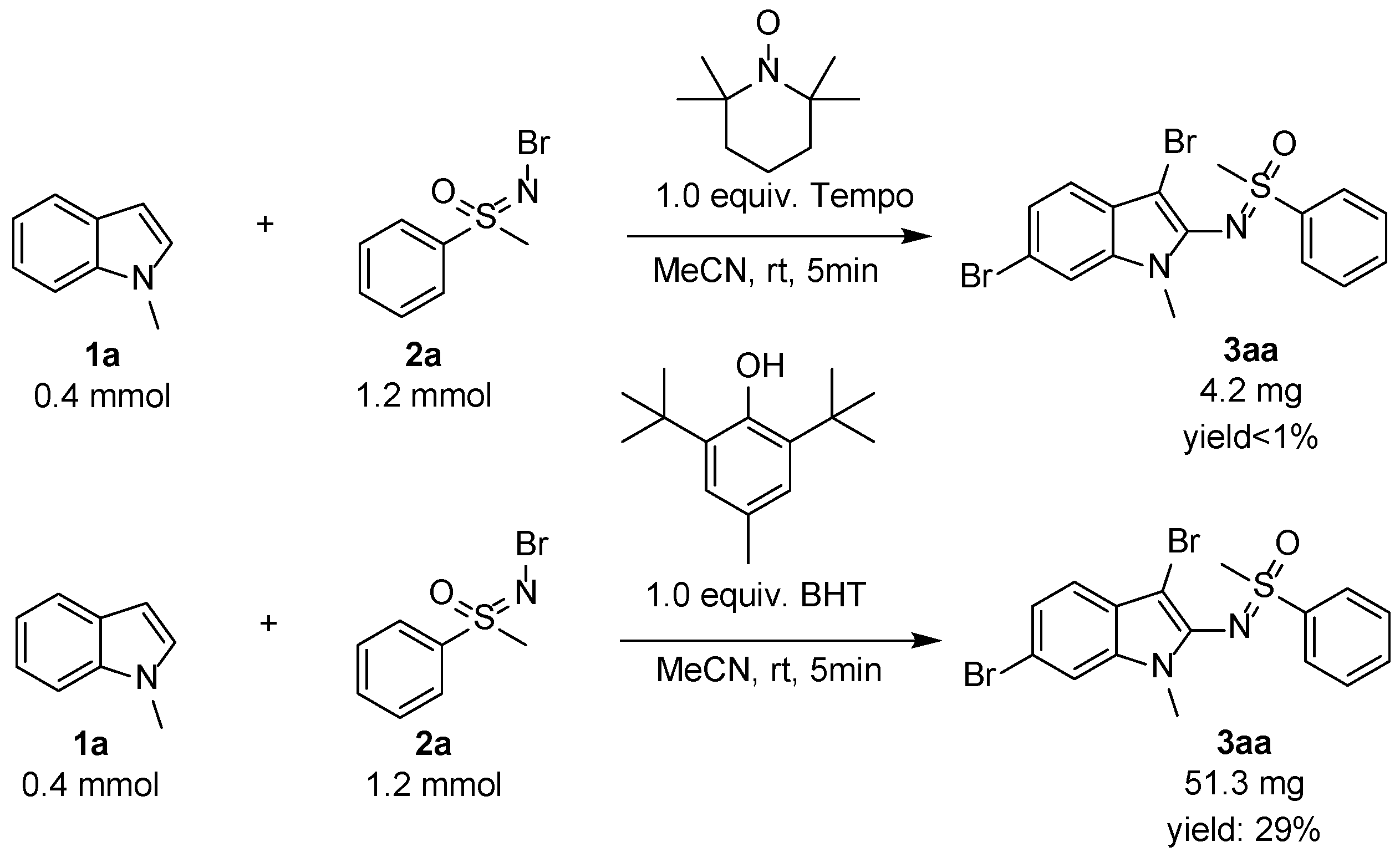
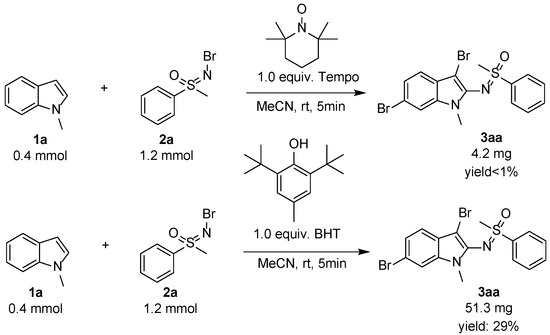
Due to the important synthetic significance and potential application of this useful new methodology, the mechanism was studied. The control reactions were assessed, and the results are shown in Scheme 4. Based on similar reported procedures [35,36,38], a radical process was proposed to have occurred, so 1.0 equiv. radical scavengers Tempo or BHT were added to the reaction mixture and led to the corresponding product 3aa in trace yield or low 29% yield from 94% yield as expected. Most of the starting materials were left in the reaction mixture. Combining the results in the optimization, we found only 2,3-disubstituted indole was given when the amount of N-Br sulfoximines was less than 1.5 equivalent, and the 2,3,6-trisubstituted product would be increased gradually with the increased N-Br sulfoximines, so the final step must be the radical substitution at the six-position of indoles. The radical 3,6-dibromo-2-sulfoximination of N-R (R = alkyl and Ar) indoles was proved in this new protocol.
Scheme 4.
Control reactions and possible mechanism.
3. Chemistry
All solvents were obtained from commercial sources and used without further purification unless otherwise noted. The N-bromosulfoximines were prepared according to literature protocols [44,45]. All N-protected indoles were prepared according to literature protocols [46,47]. Other chemicals were obtained from Energy Chemical and Titan. 1H and 13C NMR spectra were recorded on a Bruker DRX-400 spectrometer using CDCl3 or DMSO-d6 as solvent and TMS as an internal standard. Mass spectra (API) were tested on an Agilent 6100 using liquid chromatography–mass spectrometry. Single-crystal X-ray diffraction was conducted in the X-ray and Spectral Center at Huazhong University of Science and Technology, Wuhan, China.
General Procedure (GP) for the Preparation of Products 3
N-bromosulfoximine 2 (1.2 mmol, 3 equiv.) was added to the solution of N-protected indole 1 (0.4 mmol) in MeCN (2 mL) in a 15 mL dry pressure tube equipped with a stirring bar. The reaction mixture was stirred at room temperature for another 5 min at room temperature. Then, the organic layer of the reaction mixture was removed under reduced pressure and the residue purified by column chromatography on a neutral alumina column using a mixture of petroleum ether and ethyl acetate as eluent to afford product 3.
- 3,6-Dibromo-2-(methyl phenylsulfoximidoyl)-1-methyl-1H-indole (3aa) According to the general procedure (GP) for the preparation of product 3, the 3,6-dibromo-2-(methyl phenylsulfoximidoyl)-1-methyl-1H-indole was prepared as black oil in 94% yield. 1H NMR (400 MHz, CDCl3) δ 8.25–8.20 (m, 2H), 7.73–7.68 (m, 1H), 7.66–7.60 (m, 2H), 7.37 (d, J = 1.6 Hz, 1H), 7.26 (s, 1H), 7.22 (dd, J = 8.4, 1.6 Hz, 1H), 3.71 (s, 3H), 3.26 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 139.2, 137.7, 135.0, 134.1, 129.7, 128.5, 125.6, 123.2, 119.2, 114.5, 112.1, 80.8, 44.6, 30.2.
- 3,6-Dibromo-2-(methyl phenylsulfoximidoyl)-1-butyl-1H-indole (3ab) According to the general procedure (GP) for the preparation of product 3, the 3,6-dibromo-2-(methyl phenylsulfoximidoyl)-1-butyl-1H-indole was prepared as black oil in 85% yield. 1H NMR (400 MHz, Chloroform-d) δ 8.25–8.18 (m, 2H), 7.73–7.66 (m, 1H), 7.65–7.58 (m, 2H), 7.37 (d, J = 1.7 Hz, 1H), 7.29–7.18 (m, 3H), 4.18 (dddd, J = 49.3, 14.5, 8.5, 6.7 Hz, 2H), 3.25 (s, 3H), 1.81–1.64 (m, 2H), 1.36 (dt, J = 14.9, 7.4 Hz, 2H), 0.93 (t, J = 7.4 Hz, 3H). 13C NMR (100 MHz, Chloroform-d) δ 139.6, 137.5, 134.2, 134.0, 129.6, 128.5, 125.8, 123.0, 119.2, 114.4, 112.3, 80.4, 44.7, 43.4, 31.9, 20.3, 13.9.
- 3,6-Dibromo-2-(methyl phenylsulfoximidoyl)-1-hexyl-1H-indole (3ac) According to the general procedure (GP) for the preparation of product 3, the 3,6-dibromo-2-(methyl phenylsulfoximidoyl)-1-hexyl-1H-indole was prepared as black oil in 80% yield. 1H NMR (400 MHz, DMSO-d6) δ 8.14–8.08 (m, 2H), 7.78–7.72 (m, 1H), 7.71–7.65 (m, 2H), 7.61 (d, J = 1.6 Hz, 1H), 7.17–7.10 (m, 2H), 4.19 (pt, J = 7.9, 4.0 Hz, 2H), 3.51 (s, 3H), 1.63 (qd, J = 10.3, 4.6 Hz, 2H), 1.31–1.19 (m, 6H), 0.87–0.78 (m, 3H). 13C NMR (100 MHz, CDCl3) δ 139.6, 138.3, 133.8, 133.5, 129.5, 127.7, 125.3, 122.4, 118.5, 113.1, 112.1, 77.8, 44.6, 42.5, 30.9, 29.1, 25.8, 22.1, 13.9. HR-MS(ESI), m/z (%): Calcd for C21H25Br2N2OS+ ([M+H]+): 511.0049, Found: 511.0052.
- 3,6-Dibromo-2-(methyl phenylsulfoximidoyl)-1-octyl-1H-indole (3ad) According to the general procedure (GP) for the preparation of product 3, the 3,6-dibromo-2-(methyl phenylsulfoximidoyl)-1-octyl-1H-indole was prepared as black oil in 62% yield. 1H NMR (400 MHz, CDCl3) δ 8.26–8.18 (m, 2H), 7.74–7.66 (m, 1H), 7.62 (dd, J = 8.3, 6.7 Hz, 2H), 7.36 (d, J = 1.6 Hz, 1H), 7.27 (d, J = 7.4 Hz, 1H), 7.20 (dd, J = 8.4, 1.6 Hz, 1H), 4.28–4.05 (m, 2H), 3.25 (s, 3H), 1.75 (h, J = 6.8 Hz, 2H), 1.36–1.20 (m, 10H), 0.86 (t, J = 6.7 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 139.4, 137.4, 134.1, 134.0, 129.6, 128.5, 125.7, 123.0, 119.2, 114.3, 112.2, 80.4, 44.6, 43.6, 31.9, 29.8, 29.4, 29.3, 27.0, 22.8, 14.3. Calcd for C23H29Br2N2OS+ (M): 539.0362, Found: 539.0355.
- 3,6-Dibromo-2-(methyl phenylsulfoximidoyl)-1-decyl-1H-indole (3ae) According to the general procedure (GP) for the preparation of product 3, the 3,6-dibromo-2-(methyl phenylsulfoximidoyl)-1-decyl-1H-indole was prepared as black oil in 65% yield. 1H NMR (400 MHz, CDCl3) δ 8.24–8.19 (m, 2H), 7.71–7.66 (m, 1H), 7.61 (dd, J = 8.4, 6.8 Hz, 2H), 7.36 (d, J = 1.7 Hz, 1H), 7.27 (s, 1H), 7.20 (dd, J = 8.4, 1.6 Hz, 1H), 4.28–4.04 (m, 2H), 3.24 (s, 3H), 1.75 (h, J = 7.4, 7.0 Hz, 2H), 1.37–1.19 (m, 14H), 0.87 (t, J = 6.8 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 139.6, 137.5, 134.2, 134.0, 129.6, 128.4, 125.8, 123.0, 119.2, 114.3, 112.2, 80.4, 44.7, 43.6, 32.0, 29.8, 29.7 (2C), 29.4 (2C), 27.0, 22.8, 14.2. HR-MS(ESI), m/z (%): Calcd for C25H33Br2N2OS+ ([M+H]+): 567.0675, Found: 567.0669.
- 3,6-Dibromo-2-(methyl phenylsulfoximidoyl)-1-isopropyl-1H-indole (3af) According to the general procedure (GP) for the preparation of product 3, the 3,6-dibromo-2-(methyl phenylsulfoximidoyl)-1-isopropyl-1H-indole was prepared as green oil in 89% yield. 1H NMR (400 MHz, CDCl3) δ 8.29–8.20 (m, 2H), 7.75–7.67 (m, 1H), 7.66–7.58 (m, 3H), 7.32–7.17 (m, 3H), 5.10 (p, J = 7.1 Hz, 1H), 3.23 (s, 3H), 1.64 (d, J = 7.0 Hz, 3H), 1.53 (d, J = 7.1 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 139.2, 136.8, 134.1, 132.6, 129.7, 128.5, 126.3, 122.8, 119.5, 114.2, 114.0, 81.2, 47.2, 44.1, 21.7, 21.2.
- 3,6-Dibromo-2-(methyl phenylsulfoximidoyl)-1-benzyl-1H-indole (3ag) According to the general procedure (GP) for the preparation of product 3, the 3,6-dibromo-2-(methyl phenylsulfoximidoyl)-1-benzyl-1H-indole was prepared as white solid in 81% yield. M.p.: 162.5–163.4 °C. 1H NMR (400 MHz, DMSO-d6) δ 7.97–7.90 (m, 2H), 7.74–7.67 (m, 1H), 7.61 (dd, J = 8.4, 7.1 Hz, 2H), 7.55 (d, J = 1.5 Hz, 1H), 7.31 (dd, J = 8.1, 6.6 Hz, 2H), 7.27–7.21 (m, 1H), 7.18–7.09 (m, 4H), 5.58–5.40 (m, 2H), 3.50 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 139.2, 138.9, 138.1, 133.8, 133.6, 129.5, 128.6, 127.7, 127.2, 126.9, 125.6, 122.8, 118.6, 113.2, 112.4, 77.7, 45.6, 44.7. HR-MS(ESI), m/z (%): Calcd for C22H19Br2N2OS+ ([M+H]+): 516.9579, Found: 516.9587.
- 3,6-Dibromo-2-(methyl phenylsulfoximidoyl)-1-phenyl-1H-indole (3ah) According to the general procedure (GP) for the preparation of product 3, the 3,6-dibromo-2-(methyl phenylsulfoximidoyl)-1-phenyl-1H-indole was prepared as yellow oil in 38% yield. 1H NMR (400 MHz, CDCl3) δ 7.78–7.73 (m, 2H), 7.60–7.49 (m, 3H), 7.49–7.38 (m, 5H), 7.31 (d, J = 8.3 Hz, 1H), 7.26–7.24 (m, 1H), 7.24–7.21 (m, 1H), 3.07 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 139.6, 137.9, 136.8, 135.0, 133.7, 129.5, 129.4, 129.3, 128.5, 128.1 (2C), 126.1, 123.9, 119.4, 115.0, 112.8, 82.3, 44.9. HR-MS(ESI), m/z (%): Calcd for C21H17Br2N2OS+ ([M+H]+): 502.9423, Found: 502.9429.
- 3,6-Dibromo-2-(methyl phenylsulfoximidoyl)-1-(2-isopropylphenyl)-1H-indole (3ai) According to the general procedure (GP) for the preparation of product 3, the 3,6-dibromo-2-(methyl phenylsulfoximidoyl)-1-(2-isopropylphenyl)-1H-indole was prepared as black oil in 77% yield. 1H NMR (400 MHz, CDCl3) δ 7.79–7.71 (m, 1H), 7.58–7.46 (m, 4H), 7.45–7.20 (m, 5H), 7.16–7.09 (m, 1H), 6.94 (dd, J = 16.6, 1.7 Hz, 1H), 3.10 (d, J = 33.4 Hz, 3H), 1.15 (dd, J = 13.2, 6.8 Hz, 3H), 0.99 (dd, J = 6.9, 4.2 Hz, 3H). 13C NMR (100 MHz, DMSO-d6) δ 149.2, 148.4, 140.2 (2C), 139.1, 138.9, 135.8 (2C), 134.6, 134.2, 133.6, 133.5, 130.2, 129.6 (2C), 129.4, 129.3, 128.3, 128.0, 127.2, 126.8, 126.6, 126.5, 126.3, 126.1, 123.7, 123.6, 119.3, 119.1, 114.8, 114.7, 112.7 (2C), 80.4, 80.1, 45.7, 44.8, 28.3, 28.1, 24.8, 24.6, 23.4, 23.0. HR-MS(ESI), m/z (%): Calcd for C24H23Br2N2OS+ ([M+H]+): 544.9892, Found: 544.9900.
- 3,6-Dibromo-2-(methyl phenylsulfoximidoyl)-1-(naphthalen-1-yl)-1H-indole (3aj) According to the general procedure (GP) for the preparation of product 3, the 3,6-dibromo-2-(methyl phenylsulfoximidoyl)-1-(naphthalen-1-yl)-1H-indole was prepared as black oil in 57% yield. 1H NMR (400 MHz, CDCl3) δ 8.04–7.93 (m, 2H), 7.60 (dd, J = 8.3, 7.2 Hz, 1H), 7.55–7.32 (m, 7H), 7.30–7.14 (m, 4H), 6.87 (dd, J = 3.2, 1.7 Hz, 1H), 2.98 (d, J = 12.5 Hz, 3H). 13C NMR (100 MHz, DMSO-d6) δ 139.8 (2C), 139.4, 138.9, 136.1, 136.0, 134.5 (2C), 133.5, 133.4 (2C), 131.6, 131.5, 129.4 (2C), 129.2, 129.1, 128.4, 128.3, 128.1, 128.0, 127.9, 127.4, 127.3, 126.8, 126.7, 126.3 (2C), 125.7, 125.4, 123.9, 123.8, 123.3, 119.4 (2C), 115.0 (2C), 113.1, 113.0, 82.1, 81.1, 45.1, 44.9.
- 3,6-Dibromo-2-(methyl phenylsulfoximidoyl)-1,4-dimethyl-1H-indole (3ak) According to the general procedure (GP) for the preparation of product 3, the 3,6-dibromo-2-(methyl phenylsulfoximidoyl)-1,4-dimethyl-1H-indole was prepared as yellow oil in 80% yield. 1H NMR (400 MHz, CDCl3) δ 8.30–8.23 (m, 2H), 7.74–7.67 (m, 1H), 7.67–7.60 (m, 2H), 7.23 (d, J = 1.7 Hz, 1H), 6.96 (dd, J = 1.9, 1.0 Hz, 1H), 3.68 (s, 3H), 3.23 (s, 3H), 2.75 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 139.2, 137.3, 135.2, 134.1, 131.2, 129.6, 128.5, 124.7, 122.8, 114.1, 110.3, 81.0, 44.1, 30.4, 19.3.
- 3,6-Dibromo-2-(methyl phenylsulfoximidoyl)-1,7-dimethyl-1H-indole (3al) According to the general procedure (GP) for the preparation of product 3, the 3,6-dibromo-2-(methyl phenylsulfoximidoyl)-1,7-dimethyl-1H-indole was prepared as black solid in 94% yield. M.p.: 122.6–124.2 °C. 1H NMR (400 MHz, CDCl3) δ 8.27–8.18 (m, 2H), 7.73–7.65 (m, 1H), 7.62 (dd, J = 8.3, 6.7 Hz, 2H), 7.29 (d, J = 8.4 Hz, 1H), 7.10 (d, J = 8.4 Hz, 1H), 3.96 (s, 3H), 3.23 (s, 3H), 2.81 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 139.3, 138.3, 134.2, 134.0, 129.6, 128.5, 126.8, 124.8, 120.7, 119.4, 116.9, 81.6, 44.3, 34.0, 19.0. HR-MS(ESI), m/z (%): Calcd for C17H17Br2N2OS+ ([M+H]+): 454.9423, Found:454.9426.
- 3,6-Dibromo-2-(methyl phenylsulfoximidoyl)-7-chloro-1-methyl-1H-indole (3am) According to the general procedure (GP) for the preparation of product 3, the 3,6-dibromo-2-(methyl phenylsulfoximidoyl)-7-chloro-1-methyl-1H-indole was prepared as yellow oil in 63% yield. 1H NMR (400 MHz, CDCl3) δ 8.24–8.18 (m, 2H), 7.73–7.67 (m, 1H), 7.63 (ddt, J = 8.3, 6.8, 1.4 Hz, 2H), 7.31 (d, J = 8.4 Hz, 1H), 7.15 (d, J = 8.4 Hz, 1H), 4.08 (s, 3H), 3.26 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 139.2, 139.1, 134.2, 131.0, 129.7, 128.4, 128.3, 124.9, 117.5, 116.7, 116.2, 81.3, 44.7, 33.4.
- 3,6,7-Tribromo-2-(methyl phenylsulfoximidoyl)-1-methyl-1H-indole (3an) According to the general procedure (GP) for the preparation of product 3, the 3,6,7-tribromo-2-(methyl phenylsulfoximidoyl)-1-methyl-1H-indole was prepared as black oil in 46% yield. 1H NMR (400 MHz, DMSO-d6) δ 8.13–8.08 (m, 2H), 7.78–7.72 (m, 1H), 7.71–7.65 (m, 2H), 7.36 (d, J = 8.4 Hz, 1H), 7.12 (d, J = 8.3 Hz, 1H), 4.02 (s, 3H), 3.56 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 140.3, 139.2, 133.9, 131.5, 129.5, 127.9, 127.7, 124.7, 117.8, 117.6, 105.3, 79.0, 44.4, 33.0.
- 3,6-Dibromo-2-(methyl p-tolylsulfoximidoyl)-1-methyl-1H-indole (3ba) According to the general procedure (GP) for the preparation of product 3, the 3,6-dibromo-2-(methyl p-tolylsulfoximidoyl)-1-methyl-1H-indole was prepared as purple oil in 84% yield. 1H NMR (400 MHz, CDCl3) δ 8.13–8.05 (m, 2H), 7.42 (d, J = 8.0 Hz, 2H), 7.36 (d, J = 1.7 Hz, 1H), 7.28 (s, 1H), 7.22 (dd, J = 8.4, 1.6 Hz, 1H), 3.71 (s, 3H), 3.24 (s, 3H), 2.48 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 145.2, 137.9, 136.1, 135.0, 130.3, 128.5, 125.6, 123.1, 119.2, 114.5, 112.1, 80.8, 44.7, 30.2, 21.8. HR-MS(ESI), m/z (%): Calcd for C17H17Br2N2OS+ ([M+H]+): 454.9423, Found: 454.9438.
- 3,6-Dibromo-2-(methyl 4-chlorophenylsulfoximidoyl)-1-methyl-1H-indole (3bb) According to the general procedure (GP) for the preparation of product 3, the 3,6-dibromo-2-(methyl 4-chlorophenylsulfoximidoyl)-1-methyl-1H-indole was prepared as purple oil in 91% yield. 1H NMR (400 MHz, CDCl3) δ 8.18–8.12 (m, 2H), 7.62–7.56 (m, 2H), 7.36 (d, J = 1.6 Hz, 1H), 7.29–7.19 (m, 2H), 3.69 (s, 3H), 3.25 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 141.0, 137.7, 137.3, 135.0, 130.0, 129.9, 125.5, 123.3, 119.2, 114.7, 112.2, 80.7, 44.7, 30.2. HR-MS(ESI), m/z (%): Calcd for C16H14Br2ClN2OS+ ([M+H]+): 474.8877, Found: 474.8897.
- 3,6-Dibromo-2-(methyl 4-bromophenylsulfoximidoyl)-1-methyl-1H-indole (3bc) According to the general procedure (GP) for the preparation of product 3, the 3,6-dibromo-2-(methyl 4-bromophenylsulfoximidoyl)-1-methyl-1H-indole was prepared as purple oil in 88% yield. 1H NMR (400 MHz, DMSO-d6) δ 8.05–8.00 (m, 2H), 7.91–7.86 (m, 2H), 7.60 (d, J = 1.6 Hz, 1H), 7.16 (dd, J = 8.3, 1.6 Hz, 1H), 7.12 (d, J = 8.4 Hz, 1H), 3.68 (s, 3H), 3.57 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 138.8, 138.4, 134.2, 132.5, 129.9, 127.9, 125.1, 122.5, 118.4, 113.2, 112.2, 78.0, 44.3, 29.7.
- 3,6-Dibromo-2-(methyl 4-fluorophenylsulfoximidoyl)-1-methyl-1H-indole (mixture 3bd) According to the general procedure (GP) for the preparation of product 3, the 3,6-dibromo-2-(methyl 4-fluorophenylsulfoximidoyl)-1-methyl-1H-indole and 3,6-dibromo-2-(methyl 4-fluorophenylsulfoximidoyl)-1-methyl-1H-indole were prepared as orange solid in about 85% yield (the ratio of trisubstituted product to disubstituted product was about 3.5: 1). 1H NMR (400 MHz, CDCl3, main tri-substituted product) δ 8.24 (dd, J = 9.0, 5.0 Hz, 2H), 7.37 (d, J = 1.6 Hz, 1H),7.32–7.26 (m, 4H), 7.24–7.21 (m, 1H), 3.71 (3H), 3.26 (3H); 13C NMR (100 MHz, CDCl3) (main tri-substituted product) δ 166.2 (d, J = 255.4), 137.5, 135.0, 131.5 (d, J = 9.7 Hz), 125.5, 123.2, 119.2, 117.9, 117.0 (d, J = 22.6 Hz), 114.6, 112.1, 80.8, 44.7, 30.2.
- 3,6-Dibromo-2-(methyl 4-nitrophenylsulfoximidoyl)-1-methyl-1H-indole (3be) According to the general procedure (GP) for the preparation of product 3, the 3,6-dibromo-2-(methyl 4-nitrophenylsulfoximidoyl)-1-methyl-1H-indole was prepared as orange solid in 66% yield. M.p.: 157.3–158.1 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.46 (d, J = 8.8 Hz, 2H), 8.36 (d, J = 8.7 Hz, 2H), 7.60 (d, J = 1.7 Hz, 1H), 7.19–7.07 (m, 2H), 3.69 (d, J = 7.3 Hz, 6H). 13C NMR (100 MHz, DMSO-d6) δ 150.4, 145.5, 137.9, 134.2, 129.5, 125.1, 124.6, 122.6, 118.5, 113.3, 112.3, 78.1, 44.0, 29.7.
- 3,6-Dibromo-2-(methyl 3-bromophenylsulfoximidoyl)-1-methyl-1H-indole (3bf) According to the general procedure (GP) for the preparation of product 3, the 3,6-dibromo-2-(methyl 3-bromophenylsulfoximidoyl)-1-methyl-1H-indole was prepared as black oil in 77% yield. 1H NMR (400 MHz, CDCl3) δ 8.37 (t, J = 1.8 Hz, 1H), 8.14 (ddd, J = 8.0, 1.9, 1.0 Hz, 1H), 7.81 (ddd, J = 8.0, 1.9, 1.0 Hz, 1H), 7.49 (t, J = 7.9 Hz, 1H), 7.36 (dd, J = 1.6, 0.5 Hz, 1H), 7.28 (d, J = 0.5 Hz, 1H), 7.22 (dd, J = 8.4, 1.6 Hz, 1H), 3.70 (s, 3H), 3.28 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 141.1, 137.2, 137.1, 135.0, 131.4, 131.1, 127.1, 125.5, 123.5, 123.3, 119.3, 114.7, 112.2, 80.8, 44.8, 30.2.
- 3,6-Dibromo-2-(methyl 3-chlorophenylsulfoximidoyl)-1-methyl-1H-indole (3bg) According to the general procedure (GP) for the preparation of product 3, the 3,6-dibromo-2-(methyl 3-chlorophenylsulfoximidoyl)-1-methyl-1H-indole was prepared as black oil in 86% yield. 1H NMR (400 MHz, CDCl3) δ 8.21 (t, J = 1.9 Hz, 1H), 8.09 (ddd, J = 7.9, 1.8, 1.1 Hz, 1H), 7.65 (ddd, J = 8.1, 2.0, 1.1 Hz, 1H), 7.55 (t, J = 8.0 Hz, 1H), 7.36 (dd, J = 1.6, 0.5 Hz, 1H), 7.27 (d, J = 0.5 Hz, 1H), 7.21 (dd, J = 8.4, 1.6 Hz, 1H), 3.69 (s, 3H), 3.28 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 141.1, 137.1, 135.8, 135.0, 134.3, 130.9, 128.6, 126.6, 125.5, 123.2, 119.3, 114.7, 112.1, 80.7, 44.7, 30.2.
- 3,6-Dibromo-2-(methyl 2-bromophenylsulfoximidoyl)-1-methyl-1H-indole (3bh) According to the general procedure (GP) for the preparation of product 3, the 3,6-dibromo-2-(methyl 2-bromophenylsulfoximidoyl)-1-methyl-1H-indole was prepared as black oil in 85% yield. 1H NMR (400 MHz, CDCl3) δ 8.47 (dd, J = 8.0, 1.7 Hz, 1H), 7.75 (dd, J = 7.9, 1.4 Hz, 1H), 7.55 (td, J = 7.7, 1.3 Hz, 1H), 7.48 (td, J = 7.6, 1.8 Hz, 1H), 7.33–7.31 (m, 1H), 7.22–7.16 (m, 2H), 3.66 (s, 3H), 3.57 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 139.3, 137.4, 135.9, 134.9, 134.7, 132.8, 128.4, 125.9, 123.0, 120.6, 119.2, 114.3, 111.8, 78.3, 43.7, 29.9.
- 3,6-Dibromo-2-(methyl 2-chlorophenylsulfoximidoyl)-1-methyl-1H-indole (3bi) According to the general procedure (GP) for the preparation of product 3, the 3,6-dibromo-2-(methyl 2-chlorophenylsulfoximidoyl)-1-methyl-1H-indole was prepared as black oil in 89% yield. 1H NMR (400 MHz, DMSO-d6) δ 8.28 (dd, J = 7.9, 1.6 Hz, 1H), 7.75–7.60 (m, 3H), 7.58 (d, J = 1.7 Hz, 1H), 7.14 (dd, J = 8.3, 1.7 Hz, 1H), 7.06 (d, J = 8.3 Hz, 1H), 3.74 (s, 3H), 3.66 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 138.2, 137.3, 135.4, 134.0, 132.1, 132.0, 131.0, 128.2, 125.2, 122.5, 118.4, 113.1, 112.1, 76.5, 43.9, 29.5. HR-MS(ESI), m/z (%): Calcd for C16H14Br2ClN2OS+: 474.8877, Found: 474.8863.
- 3,6-Dibromo-2-(isopropyl phenylsulfoximidoyl)-1-methyl-1H-indole (3bj) According to the general procedure (GP) for the preparation of product 3, the 3,6-dibromo-2-(isopropyl phenylsulfoximidoyl)-1-methyl-1H-indole was prepared as green oil in 74% yield 1H NMR (400 MHz, CDCl3) δ 8.01–7.95 (m, 2H), 7.64–7.59 (m, 1H), 7.56–7.50 (m, 2H), 7.29–7.27 (m, 1H), 7.15 (dd, J = 1.8, 1.1 Hz, 2H), 3.69 (s, 3H), 3.58 (p, J = 6.8 Hz, 1H), 1.45 (d, J = 6.7 Hz, 3H), 1.31 (d, J = 6.8 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 138.5, 136.1, 134.7, 133.8, 129.8, 129.4, 126.0, 122.9, 118.8, 113.8, 111.7, 78.7, 58.2, 30.2, 16.8, 16.0. HR-MS(ESI), m/z (%): Calcd for C18H19Br2N2OS+ ([M+H]+): 468.9579, Found: 468.9588.
- 3,6-Dibromo-2-(butyl phenylsulfoximidoyl)-1-methyl-1H-indole (3bk) According to the general procedure (GP) for the preparation of product 3, the 3,6-dibromo-2-(butyl phenylsulfoximidoyl)-1-methyl-1H-indole was prepared as black oil in 81% yield. 1H NMR (400 MHz, CDCl3) δ 8.15–8.08 (m, 2H), 7.69–7.63 (m, 1H), 7.58 (dd, J = 8.4, 6.8 Hz, 2H), 7.33 (d, J = 1.6 Hz, 1H), 7.24 (d, J = 3.4 Hz, 1H), 7.18 (dd, J = 8.4, 1.6 Hz, 1H), 3.70 (s, 3H), 3.39 (dddd, J = 54.7, 14.0, 11.2, 5.1 Hz, 2H), 1.76–1.53 (m, 2H), 1.38–1.27 (m, 2H), 0.84 (t, J = 7.3 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 138.0, 137.7, 134.8, 133.9, 129.5, 129.0, 125.7, 123.0, 119.0, 114.2, 111.9, 80.2, 56.7, 30.2, 25.3, 21.5, 13.6.
- 3,6-Dibromo-2-(diphenylsulfoximidoyl)-1-methyl-1H-indole (3bl) According to the general procedure (GP) for the preparation of product 3, the 3,6-dibromo-2-(diphenylsulfoximidoyl)-1-methyl-1H-indole was prepared as black oil in 93% yield. 1H NMR (400 MHz, CDCl3) δ 8.13–8.09 (m, 4H), 7.58–7.46 (m, 6H), 7.30–7.27 (m, 1H), 7.19–7.12 (m, 2H), 3.75 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 140.3, 137.8, 134.9, 133.4, 129.5, 128.5, 125.9, 123.0, 119.1, 114.2, 111.9, 80.5, 30.6. HR-MS(ESI), m/z (%): Calcd for C21H17Br2N2OS+ ([M+H]+): 502.9423, Found: 502.9429.
- 3,6-Dibromo-2-(dibutylsulfoximidoyl)-1-methyl-1H-indole (3bm) According to the general procedure (GP) for the preparation of product 3, the mixture of 3,6-dibromo-2-(dibutylsulfoximidoyl)-1-methyl-1H-indole and 2,6-dibromo-3-(dibutylsulfoximidoyl)-1-methyl-1H-indole was prepared as black oil in 77% yield. 1H NMR (400 MHz, CDCl3) δ 7.34 (d, J = 1.6 Hz, 1H), 7.27 (s, 1H), 7.20 (dd, J = 8.4, 1.6 Hz, 1H), 3.66 (d, J = 18.4 Hz, 3H), 3.33–3.14 (m, 4H), 1.99–1.75 (m, 4H), 1.47 (h, J = 7.4 Hz, 4H), 0.95 (t, J = 7.4 Hz, 6H). 13C NMR (for main product) (100 MHz, CDCl3) δ 138.1, 134.8, 125.5, 123.0, 119.0, 114.3, 112.0, 80.8, 52.8, 30.07, 25.1, 21.9, 13.7.
4. Conclusions
In conclusion, a green 3,6-dibromo-2-sulfoximination of N-R (R = alkyl, benzyl and aryl) indoles using N-Br sulfoximines as both brominating and sulfoximinating reagents was developed, and a total of 27 products were prepared in 38–94% yields. This protocol introduces two bromo and one sulfoximidoyl group on indole moiety with absolute atom and step economy by stirring the reaction mixture for 5 min in CH3CN at room temperature. This method is compatible with a wide range of substrates and affords most of the products in >60% yields. It also adheres to the principles of green chemistry. A range of potential bioactive sulfoximidoyl indoles with two good-leaving-group Br was prepared via this novel trifunctionalization of indoles in good to excellent yields. According to the control reactions, this is a radical bromosulfoximidoyl process involving Br radical and sulfoximidoyl radical generated by N-Br sulfoximine in the presence of N-R (R = alkyl, benzyl or aryl) indoles. This new green synthesis of N-methyl-3,6-dibromo-2-sulfoximidoyl indoles will facilitate the understanding of transition-metal-free trifunctionalization of indoles and the development of research based on sulfoximidoyl indoles. Additionally, the successful application of N-X sulfoximines as both sulfoximinating and halogenating reagents would further promote the development of sulfoximination and halogenation. Organic syntheses or biochemistry based on indoles or sulfoximines will be greatly developed. In the near future, we will test the bioactivity of this new sulfoximidoyl indoles as pesticide, silkworm medicine, and medicine. We will also continue to expand the library of sulfoximidoyl indoles through the functionalization of the 3- or 6-position C-Br bond.
5. Patents
One Chinese invention patent (CN113121403B) resulting from this work is reported in this manuscript [48].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28083380/s1, including general procedure (GP) for the preparation of N-bromosulfoximines, general procedure (GP) for the preparation of N-aryl indole, general procedure (GP) for the preparation of N-alkyl indole, general procedure (GP) for the preparation of various N-methyl indole and additional optimization (Table S1. Characterization data of substrates, X-ray crystallography of product 3be, and 1H and 13C NMR spectra of substrates and products 3); Table S1: The additional optimization of bromosulfoximidation of N-Me indole; Table S2: Crystal data and structure refinement; References [49,50,51] are cited in the supplementary materials.
Author Contributions
Conceptualization, X.Y.C., S.Z. and C.Y.; methodology, Y.T. (Yaonan Tang), X.X. and M.H.; data curation, Y.T. (Yaonan Tang), X.X., Y.T. (Yisong Tang), X.Y.C. and S.Z.; writing—original draft preparation, X.Y.C. and Y.T. (Yaonan Tang); writing—review and editing, X.Y.C., Y.T. (Yisong Tang), S.Z. and C.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant 32271543.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the Supplementary Materials.
Acknowledgments
The authors are grateful to Long Wang from Huazhong University of Science and Technology for some good advice.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Somei, M.; Yamada, F. Simple indole alkaloids and those with a non-rearranged monoterpenoid unit. Nat. Prod. Rep. 2005, 22, 73–103. [Google Scholar] [CrossRef] [PubMed]
- Crich, D.; Banerjee, A. Chemistry of the Hexahydropyrrolo[2,3-b]indoles: Configuration, Conformation, Reactivity, and Applications in Synthesis. Acc. Chem. Res. 2007, 40, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Cai, X.-H.; Liu, Y.-P.; Li, Y.; Wang, Y.-Y.; Luo, X.-D.; Melodinines, A.−G. Monoterpenoid Indole Alkaloids from Melodinus henryi. J. Nat. Prod. 2010, 73, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Kochanowska-Karamyan, A.J.; Hamann, M.T. Marine Indole Alkaloids: Potential New Drug Leads for the Control of Depression and Anxiety. Chem. Rev. 2010, 110, 4489–4497. [Google Scholar] [CrossRef]
- Zuo, Z.; Xie, W.; Ma, D. Total Synthesis and Absolute Stereochemical Assignment of (−)-Communesin F. J. Am. Chem. Soc. 2010, 132, 13226–13228. [Google Scholar] [CrossRef]
- Stempel, E.; Gaich, T. Cyclohepta[b]indoles: A Privileged Structure Motif in Natural Products and Drug Design. Acc. Chem. Res. 2016, 49, 2390–2402. [Google Scholar] [CrossRef]
- Wiezorek, S.; Lamers, P.; Bolm, C. Conversion and degradation pathways of sulfoximines. Chem. Soc. Rev. 2019, 48, 5408–5423. [Google Scholar] [CrossRef]
- Zheng, W.; Chen, X.; Chen, F.; He, Z.; Zeng, Q. Syntheses and Transformations of Sulfoximines. Chem. Rec. 2021, 21, 396–416. [Google Scholar] [CrossRef]
- Reetz, M.T.; Bondarev, O.G.; Gais, H.-J.; Bolm, C. BINOL-derived N-phosphino sulfoximines as ligands for asymmetric catalysis. Tetrahedron Lett. 2005, 46, 5643–5646. [Google Scholar] [CrossRef]
- Lu, S.-M.; Bolm, C. Highly Enantioselective Synthesis of Optically Active Ketones by Iridium-Catalyzed Asymmetric Hydrogenation. Angew. Chem. Int. Ed. 2008, 47, 8920–8923. [Google Scholar] [CrossRef]
- Frings, M.; Thomé, I.; Schiffers, I.; Pan, F.; Bolm, C. Catalytic, Asymmetric Synthesis of Phosphonic γ-(Hydroxyalkyl)butenolides with Contiguous Quaternary and Tertiary Stereogenic Centers. Chem. Eur. J. 2014, 20, 1691–1700. [Google Scholar] [CrossRef]
- Lücking, U. Sulfoximines: A Neglected Opportunity in Medicinal Chemistry. Angew. Chem. Int. Ed. 2013, 52, 9399–9408. [Google Scholar] [CrossRef]
- Han, Y.; Xing, K.; Zhang, J.; Tong, T.; Shi, Y.; Cao, H.; Yu, H.; Zhang, Y.; Liu, D.; Zhao, L. Application of sulfoximines in medicinal chemistry from 2013 to 2020. Eur. J. Med. Chem. 2021, 209, 112885. [Google Scholar] [CrossRef]
- Karpel-Massler, G.; Kast, R.E.; Siegelin, M.D.; Dwucet, A.; Schneider, E.; Westhoff, M.A.; Wirtz, C.; Chen, X.Y.; Halatsch, M.E.; Bolm, C. Anti-glioma Activity of Dapsone and Its Enhancement by Synthetic Chemical Modification. Neurochem. Res. 2017, 42, 3382–3389. [Google Scholar] [CrossRef]
- Lane, B.S.; Brown, M.A.; Sames, D. Direct Palladium-Catalyzed C-2 and C-3 Arylation of Indoles: A Mechanistic Rationale for Regioselectivity. J. Am. Chem. Soc. 2005, 127, 8050–8057. [Google Scholar] [CrossRef]
- Seregin, I.V.; Gevorgyan, V. Direct transition metal-catalyzed functionalization of heteroaromatic compounds. Chem. Soc. Rev. 2007, 36, 1173–1193. [Google Scholar] [CrossRef]
- Lebrasseur, N.; Larrosa, I. Room Temperature and Phosphine Free Palladium Catalyzed Direct C-2 Arylation of Indoles. J. Am. Chem. Soc. 2008, 130, 2926–2927. [Google Scholar] [CrossRef]
- Légaré, M.-A.; Courtemanche, M.-A.; Rochette, É.; Fontaine, F.-G. Metal-free catalytic C-H bond activation and borylation of heteroarenes. Science 2015, 349, 513–516. [Google Scholar] [CrossRef]
- Toutov, A.A.; Liu, W.-B.; Betz, K.N.; Fedorov, A.; Stoltz, B.M.; Grubbs, R.H. Silylation of C–H bonds in aromatic heterocycles by an Earth-abundant metal catalyst. Nature 2015, 518, 80–84. [Google Scholar] [CrossRef]
- Inoue, F.; Saito, T.; Semba, K.; Nakao, Y. C3-Selective alkenylation of N-acylindoles with unactivated internal alkynes by cooperative nickel/aluminium catalysis. Chem. Commun. 2017, 53, 4497–4500. [Google Scholar] [CrossRef]
- Leitch, J.A.; Bhonoah, Y.; Frost, C.G. Beyond C2 and C3: Transition-Metal-Catalyzed C–H Functionalization of Indole. ACS Catal. 2017, 7, 5618–5627. [Google Scholar] [CrossRef]
- Leitch, J.A.; McMullin, C.L.; Mahon, M.F.; Bhonoah, Y.; Frost, C.G. Remote C6-Selective Ruthenium-Catalyzed C–H Alkylation of Indole Derivatives via σ-Activation. ACS Catal. 2017, 7, 2616–2623. [Google Scholar] [CrossRef]
- Yamaguchi, M. C-H Functionalization of Indoles at the C7 Position. J. Synth. Org. Chem Jpn. 2017, 75, 671–672. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, P.; Wang, D.; Wang, M.; Yuan, Y.; Shi, Z. PIII-Chelation-Assisted Indole C7-Arylation, Olefination, Methylation, and Acylation with Carboxylic Acids/Anhydrides by Rhodium Catalysis. Angew. Chem. Int. Ed. 2019, 58, 1504–1508. [Google Scholar] [CrossRef]
- Choi, I.; Mueller, V.; Ackermann, L. Ruthenium(II)-carboxylate-catalyzed C4/C6–H dual alkylations of indoles. Tetrahedron Lett. 2021, 72, 153064–153070. [Google Scholar] [CrossRef]
- Huang, W.-J.; Ma, Y.-Y.; Liu, L.-X.; Wu, B.; Jiang, G.-F.; Zhou, Y.-G. Chiral Phosphoric Acid-Catalyzed C6 Functionalization of 2,3-Disubstituted Indoles for Synthesis of Heterotriarylmethanes. Org. Lett. 2021, 23, 2393–2398. [Google Scholar] [CrossRef]
- Kawasaki, T.; Nonaka, Y.; Matsumura, K.; Monai, M.; Sakamoto, M. An Efficient Synthetic Method for 2-Methoxy-L,2-dihydro-3H-indol-3-ones. Synth. Commun. 1999, 29, 3251–3261. [Google Scholar] [CrossRef]
- Konstantinova, L.S.; Rakitin, O.A.; Rees, C.W. A one-step synthesis of fused pentathiepins. Chem. Commun. 2002, 11, 1204–1205. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, Q.Y.; Liu, J.; Wu, P.; Yi, H.; Lei, A. A trans diacyloxylation of indoles. Chem. Commun. 2012, 48, 3239–3241. [Google Scholar] [CrossRef]
- Jiang, X.; Li, G.; Yu, C. Synthesis of N-aryl-3-(arylimino)-3H-indol-2-amines via hypervalent iodine promoted oxidative diamination of indoles. Tetrahedron Lett. 2018, 59, 1506–1510. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, H.; Yu, S. NaClO-Promoted Atroposelective Couplings of 3-Substituted Indoles with Amino Acid Derivatives. Org. Lett. 2019, 21, 4754–4758. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Shi, X.; Sun, W.; Zhao, J.; Zhu, Y.-P.; Liu, L.; Zhu, B. Palladium-Catalyzed C-2 and C-3 Dual C–H Functionalization of Indoles: Synthesis of Fluorinated Isocryptolepine Analogues. Org. Lett. 2020, 22, 4097–4102. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Gao, P.; Shen, Y.-W.; Liang, Y.-M. Palladium-/Copper-Catalyzed Regioselective Amination and Chloroamination of Indoles. Org. Lett. 2011, 13, 4196–4199. [Google Scholar] [CrossRef]
- John, A.; Nicholas, K.M. Copper-Mediated Multiple C–H Functionalization of Aromatic N-Heterocycles: Bromoamination of Indoles and Pyrroles. Organometallics 2012, 31, 7914–7920. [Google Scholar] [CrossRef]
- Moriyama, K.; Ishida, K.; Togo, H. Egioselective Csp2–H dual functionalization of indoles using hypervalent iodine(III): Bromo-amination via 1,3-migration of imides on indolyl(phenyl)iodonium imides. Chem. Commun. 2015, 51, 2273–2276. [Google Scholar] [CrossRef]
- Liu, X.; Tong, K.; Zhang, A.H.; Tan, R.X.; Yu, S. Metal-free chloroamidation of indoles with sulfonamides and NaClO. Org. Chem. Front. 2017, 4, 1354–1357. [Google Scholar] [CrossRef]
- Tu, D.; Luo, J.; Jiang, C. Copper-mediated domino C–H iodination and nitration of indoles. Chem. Commun. 2018, 54, 2514–2517. [Google Scholar] [CrossRef]
- Lei, N.; Shen, Y.; Li, Y.; Tao, P.; Yang, L.; Su, Z.; Zheng, K. Electrochemical Iodoamination of Indoles Using Unactivated Amines. Org. Lett. 2020, 22, 9184–9189. [Google Scholar] [CrossRef]
- Chen, X.Y.; Wang, L.; Frings, M.; Bolm, C. Copper-Catalyzed N-Alkynylations of Sulfoximines with Bromoacetylenes. Org. Lett. 2014, 16, 3796–3799. [Google Scholar] [CrossRef]
- Chen, X.Y.; Bohmann, R.A.; Wang, L.; Dong, S.; Räuber, C.; Bolm, C. Palladium/Copper-Cocatalyzed Oxidative Amidobrominations of Alkenes. Chem. Eur. J. 2015, 21, 10330–10333. [Google Scholar] [CrossRef]
- Yuan, M.; Chen, X.; Lin, S. Synthesis of the Functionalized Enamine. Prog. Chem. 2018, 30, 1082–1096. [Google Scholar]
- Chen, X.Y.; Zhang, L.; Tang, Y.; Yuan, S.; Zhu, B.; Chen, G.; Cheng, X. Green H2O-Promoted Solvent-Free Synthesis of Enaminocarbonyl Compounds with High Stereoselectivity from Electron-Deficient Terminal Alkynes. Synlett 2020, 31, 878–882. [Google Scholar] [CrossRef]
- Akasaka, T.; Furukawa, N.; Oae, S. Sulfoximidoyl radical. Homolytic addition of n-halosulfoximides to olefins. Tetrahedron Lett. 1979, 20, 2035–2038. [Google Scholar] [CrossRef]
- Xie, Y.; Zhou, B.; Zhou, S.; Zhou, S.; Wei, W.; Liu, J.; Zhan, Y.; Cheng, D.; Chen, M.; Li, Y.; et al. Sulfimine-Promoted Fast O Transfer: One-step Synthesis of Sulfoximine from Sulfide. ChemistrySelect 2017, 2, 1620–1624. [Google Scholar] [CrossRef]
- Bohnen, C.; Bolm, C. N-Trifluoromethylthiolated Sulfoximines. Org. Lett. 2015, 17, 3011–3013. [Google Scholar] [CrossRef]
- Hong, X.; Tan, Q.; Liu, B.; Xu, B. Isocyanide-Induced Activation of Copper Sulfate: Direct Access to Functionalized Heteroarene Sulfonic Esters. Angew. Chem. Int. Ed. 2017, 56, 3961–3965. [Google Scholar] [CrossRef]
- Antilla, J.C.; Klapars, A.; Buchwald, S.L. The Copper-Catalyzed N-Arylation of Indoles. J. Am. Chem. Soc. 2002, 124, 11684–11688. [Google Scholar] [CrossRef]
- Chen, X.Y.; Tang, Y.N.; Chen, Y.; Sun, C.Y.; Tang, Y.S. The One-Step Preparation of Trisubstituted Indoles. China Patent CN113121403B, 30 December 2022. [Google Scholar]
- Wang, C.; Shi, P.; Bolm, C. Visible light-promoted NH-halogenation of sulfoximines with dichloromethane or dibromomethane. Org. Chem. Front. 2021, 8, 2919–2923. [Google Scholar] [CrossRef]
- Banjare, S.K.; Nanda, T.; Pati, B.V.; Adhikari, G.K.D.; Dutta, J.; Ravikumar, P.C. Breaking the Trend: Insight into Unforeseen Reactivity of Alkynes in Cobalt-Catalyzed Weak Chelation-Assisted Regioselective C(4)–H Functionalization of 3-Pivaloyl Indole. ACS Catalysis 2021, 11, 11579–11587. [Google Scholar] [CrossRef]
- Das, D.; Bhutia, Z.T.; Chatterjee, A.; Banerjee, M. Mechanochemical Pd(II)-Catalyzed Direct and C-2-Selective Arylation of Indoles. J. Org. Chem. 2019, 84, 10764–10774. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).