Novel ICP-OES-Based Method for the Reliable Determination of the Total Content of 15 Elements in Yerba Mate Drinks along with the Determination of Caffeine and the In Vitro Bioaccessibility of the Compounds
Abstract
1. Introduction
2. Results and Discussion
2.1. Development of a Simplified Sample Preparation Procedure
2.1.1. Analytical Characteristic of ICP-OES versus Sample Preparation Procedure
2.1.2. Trueness of Results versus Sample Preparation Procedure
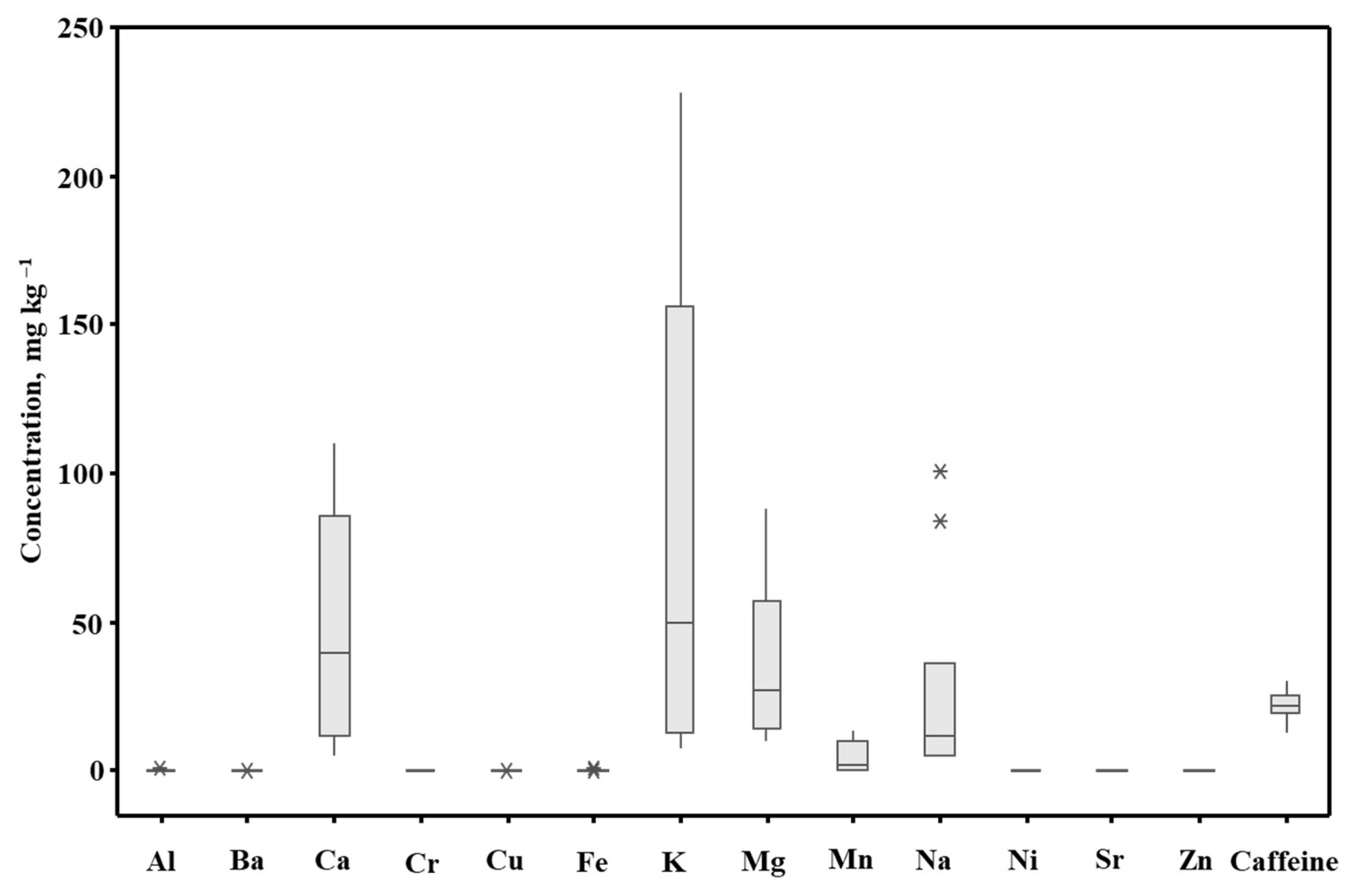
2.2. Application—Multielement Analysis of YM Drinks
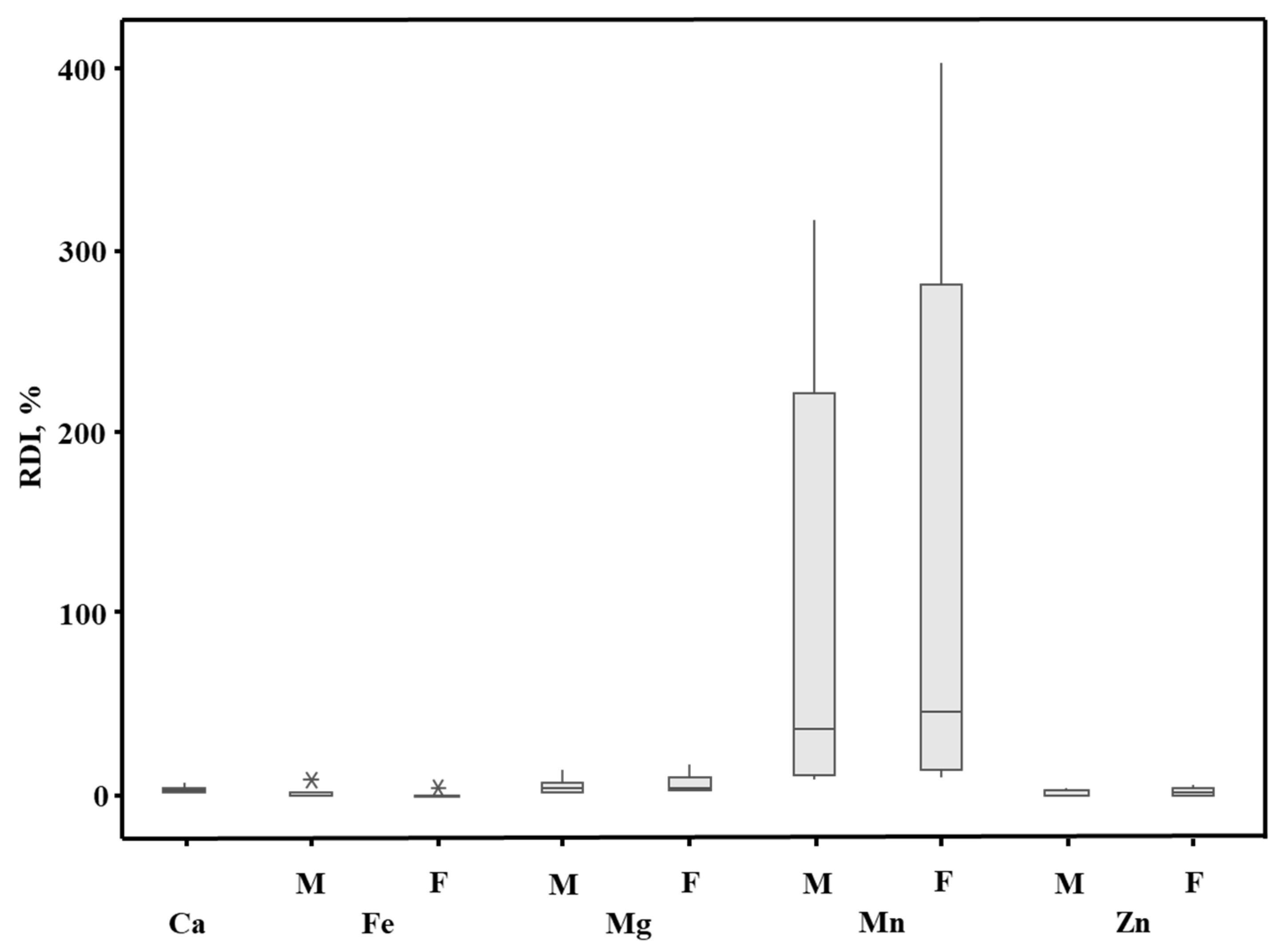
2.3. Bioaccessibility of Compounds from YM Drinks
3. Materials and Methods
3.1. Samples
3.2. Reagents and Solutions
3.3. Instrumentation
3.4. Sample Preparation Procedures Prior to Analysis
3.4.1. Total Content of Elements
3.4.2. Bioaccessible Fraction—In Vitro Gastrointestinal Digestion Procedure
3.4.3. Caffeine Content—Solvent Extraction and Detection
3.5. Spike-and-Recovery Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heck, C.I.; De Mejia, E.G. Yerba Mate Tea (Ilex paraguariensis): A Comprehensive Review on Chemistry, Health Implications, and Technological Considerations. J. Food Sci. 2007, 72, R138–R151. [Google Scholar] [CrossRef] [PubMed]
- Kujawska, M. Yerba Mate (Ilex paraguariensis) Beverage: Nutraceutical Ingredient or Conveyor for the Intake of Medicinal Plants? Evidence from Paraguayan Folk Medicine. Evid. Based Complement. Altern. Med. 2018, 2018, 6849317. [Google Scholar] [CrossRef] [PubMed]
- Bastos Markowicz, D.H.; Fornari, A.C.; de Queiroz, Y.S.; Soares, R.; Torres, E.A. The chlorogenic acid and caffeine content of yerba maté (Ilex paraguariensis) beverages. Acta Farm. Bonaer. 2005, 24, 91–95. [Google Scholar]
- Cordoba Bragança, V.L.; Melnikov, P.; Zanoni, L.Z. Trace Elements in Different Brands of Yerba Mate Tea. Biol. Trace Elem. Res. 2011, 144, 1197–1204. [Google Scholar] [CrossRef]
- Olivari, I.; Paz, S.; Gutiérrez, A.J.; González-Weller, D.; Hardisson, A.; Sagratini, G.; Rubio, C. Macroelement, trace element, and toxic metal levels in leaves and infusions of yerba mate (Ilex paraguariensis). Environ. Sci. Pollut. Res. 2020, 27, 21341–21352. [Google Scholar] [CrossRef] [PubMed]
- Tobaldini Frizon, C.N.; Perussello, C.A.; Alfredo, J.; Hoffmann-Ribani, R. Novel Beverages of Yerba-Mate and Soy: Bioactive Compounds and Functional Properties. Beverages 2018, 4, 21. [Google Scholar] [CrossRef]
- Samoggia, A.; Landuzzi, P.; Vicién, C.E. Market Expansion of Caffeine-Containing Products: Italian and Argentinian Yerba Mate Consumer Behavior and Health Perception. Int. J. Environ. Res. Public Health 2021, 18, 8117. [Google Scholar] [CrossRef]
- Burris, K.P.; Harte, F.M.; Davidson, P.M.; Stewart, C.N., Jr.; Zivanovic, S. Composition and bioactive properties of yerba mate (Ilex paraguariensis A. St.-Hill.: A review. Chil. J. Agric. Res. 2012, 72, 268–274. [Google Scholar] [CrossRef]
- Oellig, C.; Schunck, J.; Schwack, W. Determination of caffeine, theobromine and theophylline in Mate beer and Mate soft drinks by high-performance thin-layer chromatography. J. Chromatogr. 2018, 1533, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Pozebon, D.; Dressler, V.L.; Marcelo, M.C.A.; de Oliveira, T.C.; Ferrão, M.F. Toxic and nutrient elements in yerba mate (Ilex paraguariensis). Food Addit. Contam. Part B 2015, 8, 215–220. [Google Scholar] [CrossRef]
- Majewski Ulbrich, N.C.; do Prado, L.L.; Zimmer Barbosa, J.; Araujo, E.M.; Poggere, G.; Vargas Motta, A.C.; Prior, S.A.; Magri, E.; Young, S.D.; Broadley, M.R. Multi-elemental Analysis and Health Risk Assessment of Commercial Yerba Mate from Brazil. Biol. Trace Elem. Res. 2022, 200, 1455–1463. [Google Scholar] [CrossRef]
- Pereira, C.C.; Souza, A.O.; Oreste, E.O.; Cidade, M.J.A.; Cadore, S.; Ribeiro, A.S.; Vieira, M.A. Acid Decomposition of Yerba Mate (Ilex paraguariensis) Using a Reflux System for the Evaluation of Al, Ca, Cd, Cr, Cu, Fe, K, Mg, Mn, Na, Pb and Zn Contents by Atomic Spectrometric Techniques. J. Braz. Chem. Soc. 2016, 27, 685–693. [Google Scholar] [CrossRef]
- Janda, K.; Jakubczyk, K.; Łukomska, A.; Baranowska-Bosiacka, I.; Rębacz-Maron, E.; Dec, K.; Kochman, J.; Gutowska, I. Effect of the Yerba mate (Ilex paraguariensis) brewing method on the content of selected elements and antioxidant potential of infusions. Pol. J. Chem. Technol. 2020, 22, 54–60. [Google Scholar] [CrossRef]
- Szymczycha-Madeja, A.; Welna, M.; Pohl, P. Method validation for multi-elemental analysis of dialyzable and non-dialyzable fraction of coffee brews by F AAS and ICP OES: A bioaccessibility study. Food Anal. Meth. 2019, 12, 198–216. [Google Scholar] [CrossRef]
- Szymczycha-Madeja, A.; Welna, M.; Pohl, P. Simplified method of multi-elemental analysis of dialyzable fraction of tea infusions by FAAS and ICP OES. Biol. Trace Elem. Res. 2020, 195, 272–290. [Google Scholar] [CrossRef] [PubMed]
- Schmite, B.F.P.; Bitobrovec, A.; Hacke, A.C.M.; Pereira, R.P.; Weinert, P.L.; dos Anjos, V.E. In vitro bioaccessibility of Al, Cu, Cd, and Pb following simulated gastrointestinal digestion and total content of these metals in different Brazilian brands of yerba mate tea. Food Chem. 2019, 281, 285–293. [Google Scholar] [CrossRef]
- Marcelo, M.C.A.; Martins, C.A.; Pozebon, D.; Dressler, V.L.; Ferrão, M.F. Classification of yerbamate (Ilex paraguariensis) according to the country of origin based on element concentrations. Microchem. J. 2014, 117, 164–171. [Google Scholar] [CrossRef]
- Vargas Motta, A.C.; Zimmer Barbosa, J.; Magri, E.; Pedreira, G.Q.; Santin, D.; Prior, S.A.; Consalter, R.; Young, S.D.; Broadley, M.R.; Benedetti, E.L. Elemental composition of yerba mate (Ilex paraguariensis A.St.-Hil.) under low input systems of southern Brazil. Sci. Total Environ. 2020, 736, 139637. [Google Scholar] [CrossRef] [PubMed]
- Alice Teresa Valduga, A.T.; Gonçalves, I.L.; Ederlan Magri, E. Analysis of the Presence of Toxic Metals in Yerba Mate Samples: A Case Study from South Brazil. Water Air Soil Pollut. 2019, 230, 153–161. [Google Scholar] [CrossRef]
- Zimmer Barbosa, J.; Zambon, L.M.; Vargas Motta, A.C.; Wendling, I. Composition, hot-water solubility of elements and nutritional value of fruits and leaves of yerba mate. Ciênc. Agrotec. Lavras 2015, 39, 593–603. [Google Scholar] [CrossRef]
- Wróbel, K.; Wróbel, K.; Urbina, E.M.C. Determination of Total Aluminum, Chromium, Copper, Iron, Manganese, and Nickel and Their Fractions Leached to the Infusions of Black Tea, Green Tea, Hibiscus sabdariffa, and Ilex paraguariensis (Mate) by ETA-AAS. Biol. Trace Elem. Res. 2000, 78, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Konieczka, P.; Namieśnik, J. Quality Assurance and Quality Control in the Analytical Chemical Laboratory: A Practical Approach, 1st ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2009; pp. 16–22. [Google Scholar]
- Kolayli, S.; Ocak, M.; Kucuk, M.; Abbasoglu, R. Does caffeine bind to metal ions? Food Chem. 2013, 84, 383–388. [Google Scholar] [CrossRef]
- Otten, J.J.; Hellwig, J.P.; Meyers, L.D. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; The National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- WHO. World Health Organization. Trace Elements in Human Nutrition and Health; WHO Library: Geneva, Switzerland, 1996. [Google Scholar]
- SCHER—Scientific Committee on Health and Environmental Risks. Assessment of the Tolerable Daily Intake of Barium; SCHER: Brussels, Belgium, 2012. [Google Scholar]
- Pohl, P.; Bielawska-Pohl, A.; Dzimitrowicz, A.; Greda, K.; Jamroz, P.; Lesniewicz, A.; Szymczycha-Madeja, A.; Welna, M. Understanding element composition of medicinal plants used in herbalism-a case study by analytical atomic spectrometry. J. Pharmaceut. Biomed. 2018, 159, 262–271. [Google Scholar] [CrossRef] [PubMed]
| P1 | P2 | P3 | P4 | P5 | P6 | P7 | ||
|---|---|---|---|---|---|---|---|---|
| Al | a a | 40,190 | 40,883 | 42,130 | 41,619 | 40,230 | 43,033 | 42,479 |
| R2 b | 0.9999 | 0.9999 | 0.9999 | 0.9999 | 0.9999 | 0.9995 | 0.9999 | |
| range c | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | |
| RSD d | 0.54 | 0.67 | 0.10 | 0.22 | 0.92 | 0.80 | 0.88 | |
| LOD e | 2.0 (8.0) g | 1.5 (1.6) g | 1.1 (1.2) g | 2.6 (5.2) g | 2.4 (4.8) g | 2.0 (2.0) g | 1.5 (1.5) g | |
| LOQ f | 6.7 (27) g | 5.0 (5.4) g | 3.7 (4.0) g | 8.7 (17) g | 8.0 (16) g | 6.7 (6.7) g | 5.0 (5.0) g | |
| Ba | a a | 1,471,486 | 1,417,522 | 1,450,487 | 1,335,908 | 1,276,897 | 1,445,324 | 1,306,609 |
| R2 b | 0.9997 | 0.9993 | 0.9995 | 0.9992 | 0.9996 | 0.9994 | 0.9994 | |
| range c | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | |
| RSD d | 0.21 | 0.22 | 1.2 | 0.39 | 0.56 | 1.1 | 1.4 | |
| LOD e | 0.10 (0.40) g | 0.13 (0.14) g | 0.15 (0.16) g | 0.16 (0.32) g | 0.15 (0.30) g | 0.22 (0.22) g | 0.22 (0.22) g | |
| LOQ f | 0.33 (1.3) g | 0.43 (0.46) g | 0.50 (0.54) g | 0.53 (1.1) g | 0.50 (1.0)) g | 0.73 (0.73) g | 0.73 (0.73) g | |
| Ca | a a | 36,384 | 36,027 | 34,970 | 35,737 | 34,645 | 39,659 | 38,144 |
| R2 b | 0.9999 | 0.9999 | 0.9999 | 0.9999 | 0.9999 | 0.9996 | 0.9995 | |
| range c | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | |
| RSD d | 0.26 | 0.39 | 0.44 | 0.15 | 0.65 | 0.49 | 0.72 | |
| LOD e | 2.3 (9.2) g | 8.0 (8.6) g | 6.5 (7.0) g | 7.1 (14) g | 3.3 (6.6) g | 2.3 (2.3) g | 2.4 (2.4) g | |
| LOQ f | 7.7 (31) g | 27 (29) g | 22 (24) g | 24 (48) g | 11 (22) g | 7.7 (7.7) g | 8.0 (8.0) g | |
| Cd | a a | 36,772 | 35,485 | 35,686 | 36,685 | 34,301 | 37,687 | 37,452 |
| R2 b | 0.9999 | 0.9999 | 0.9999 | 0.9998 | 0.9999 | 0.9993 | 0.9998 | |
| range c | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | |
| RSD d | 0.37 | 0.41 | 0.74 | 0.10 | 0.32 | 0.56 | 0.55 | |
| LOD e | 0.39 (1.6) g | 0.48 (0.52) g | 0.46 (0.50) g | 0.49 (0.98) g | 0.43 (0.86) g | 0.27 (0.27) g | 0.60 (0.60) g | |
| LOQ f | 1.3 (5.2) g | 1.6 (1.7) g | 1.5 (1.6) g | 1.6 (3.2) g | 1.4 (2.8) g | 0.90 (0.90) g | 2.0 (2.0) g | |
| Cr | a a | 27,281 | 25,917 | 26,199 | 26,317 | 25,631 | 25,895 | 25,529 |
| R2 b | 0.9999 | 0.9999 | 0.9999 | 0.9999 | 0.9999 | 0.9996 | 0.9999 | |
| range c | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | |
| RSD d | 0.79 | 0.24 | 0.43 | 0.45 | 0.38 | 0.26 | 0.77 | |
| LOD e | 0.84 (3.4) g | 0.95 (1.0) g | 0.71 (0.80) g | 0.73 (1.5) g | 0.89 (1.8) g | 0.90 (0.90) g | 0.68 (0.68) g | |
| LOQ f | 2.8 (11) g | 3.2 (3.4) g | 2.4 (2.6) g | 2.4 (4.8) g | 3.0 (6.0) g | 3.0 (3.0) g | 2.3 (2.3) g | |
| Cu | a a | 39,753 | 38,458 | 38,405 | 39,048 | 38,388 | 38,091 | 37,257 |
| R2 b | 0.9999 | 0.9999 | 0.9999 | 0.9999 | 0.9999 | 0.9997 | 0.9999 | |
| range c | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | |
| RSD d | 0.69 | 0.55 | 0.77 | 0.46 | 0.54 | 0.65 | 1.1 | |
| LOD e | 0.54 (2.2) g | 1.2 (1.3) g | 0.73 (0.80) g | 1.4 (2.8) g | 0.71 (1.4) g | 0.81 (0.81) g | 0.78 (0.78) g | |
| LOQ f | 1.8 (7.2) g | 4.0 (4.3) g | 2.4 (2.6) g | 4.7 (9.4) g | 2.4 (4.8) g | 2.7 (2.7) g | 2.6 (2.6) g | |
| Fe | a a | 24,710 | 23,388 | 23,886 | 24,615 | 24,002 | 23,849 | 23,326 |
| R2 b | 0.9999 | 0.9999 | 0.9999 | 0.9999 | 0.9999 | 0.9994 | 0.9999 | |
| range c | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | |
| RSD d | 0.27 | 0.36 | 0.43 | 0.42 | 0.45 | 0.81 | 0.84 | |
| LOD e | 0.54 (2.2) g | 0.92 (1.0) g | 0.62 (0.70) g | 0.92 (1.8) g | 0.74 (1.5) g | 0.88 (0.88) g | 0.52 (0.52) g | |
| LOQ f | 1.8 (7.2) g | 3.1 (3.3) g | 2.1 (2.3) g | 3.1 (6.2) g | 2.5 (5.0) g | 2.9 (2.9) g | 1.7 (1.7) g | |
| K | a a | 71,023 | 74,757 | 76,594 | 80,209 | 78,507 | 85,054 | 87,271 |
| R2 b | 0.9989 | 0.9993 | 0.9990 | 0.9993 | 0.9993 | 0.9998 | 0.9996 | |
| range c | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | |
| RSD d | 0.69 | 0.93 | 0.79 | 0.28 | 1.7 | 0.63 | 0.95 | |
| LOD e | 2.0 (8.0) g | 1.8 (1.9) g | 2.3 (2.5) g | 1.9 (3.8) g | 2.0 (4.0) g | 2.9 (2.9) g | 1.3 (1.3) g | |
| LOQ f | 6.7 (27) g | 6.0 (6.5) g | 7.7 (8.3) g | 6.3 (13) g | 6.7 (13) g | 9.7 (9.7) g | 4.3 (4.3) g | |
| Mg | a a | 38,784 | 40,565 | 40,956 | 42,571 | 40,759 | 43,211 | 42,145 |
| R2 b | 0.9999 | 0.9999 | 0.9999 | 0.9999 | 0.9999 | 0.9997 | 0.9999 | |
| range c | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | |
| RSD d | 0.33 | 0.58 | 0.70 | 0.68 | 0.42 | 0.82 | 1.1 | |
| LOD e | 0.92 (3.7) g | 2.7 (2.9) g | 2.3 (2.5) g | 0.93 (1.9) g | 0.87 (1.7) g | 2.7 (2.7) g | 1.8 (1.8) g | |
| LOQ f | 3.1 (12) g | 9.0 (9.7) g | 7.7 (8.3) g | 3.1 (6.2) g | 2.9 (5.8) g | 9.0 (9.0) g | 6.0 (6.0) g | |
| Mn | a a | 262,146 | 256,897 | 260,614 | 260,614 | 215,639 | 253,719 | 258,973 |
| R2 b | 0.9999 | 0.9998 | 0.9999 | 0.9997 | 0.9999 | 0.9994 | 0.9999 | |
| range c | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | |
| RSD d | 1.6 | 2.2 | 1.7 | 0.94 | 2.6 | 0.91 | 2.8 | |
| LOD e | 0.10 (0.40) g | 0.33 (0.36) g | 0.19 (0.21) g | 0.15 (0.30) g | 0.10 (0.20) g | 0.35 (0.35) g | 0.14 (0.14) g | |
| LOQ f | 0.33 (1.3) g | 1.1 (1.2) g | 0.63 (0.68) g | 0.50 (1.0) g | 0.33 (0.66) g | 1.2 (1.2) g | 0.47 (0.47) g | |
| Na | a a | 196,714 | 217,996 | 210,142 | 222,108 | 194,598 | 218,742 | 217,587 |
| R2 b | 0.9990 | 0.9996 | 0.9994 | 0.9997 | 0.9998 | 0.9999 | 0.9999 | |
| range c | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | |
| RSD d | 0.29 | 0.58 | 0.50 | 0.19 | 1.2 | 0.91 | 1.5 | |
| LOD e | 2.5 (10) g | 4.9 (5.3) g | 2.5 (2.7) g | 0.66 (1.3) g | 1.3 (2.6) g | 4.3 (4.3) g | 0.87 (0.87) g | |
| LOQ f | 8.3 (33) g | 16 (17) g | 8.3 (9.0) g | 2.2 (4.4) g | 4.3 (8.6) g | 14 (14) g | 2.9 (2.9) g | |
| Ni | a a | 6636 | 6143 | 5924 | 6077 | 5983 | 6249 | 6350 |
| R2 b | 0.9999 | 0.9998 | 0.9999 | 0.9998 | 0.9999 | 0.9996 | 0.9998 | |
| range c | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | |
| RSD d | 0.57 | 0.26 | 0.58 | 0.28 | 0.84 | 1.3 | 0.87 | |
| LOD e | 2.8 (11) g | 4.1 (4.4) g | 2.9 (3.1) g | 3.1 (6.2) g | 2.3 (4.6) g | 3.4 (3.4) g | 3.4 (3.4) g | |
| LOQ f | 9.3 (37) g | 14 (15) g | 9.7 (10) g | 10 (20) g | 7.7 (15) g | 11 (11) g | 11 (11) g | |
| Pb | a a | 1854 | 1823 | 1817 | 1816 | 1823 | 1865 | 1837 |
| R2 b | 0.9999 | 0.9999 | 0.9999 | 0.9998 | 0.9999 | 0.9994 | 0.9998 | |
| range c | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | |
| RSD d | 0.28 | 1.2 | 1.2 | 1.4 | 1.5 | 1.2 | 0.42 | |
| LOD e | 8.6 (34) g | 9.4 (10) g | 7.9 (8.5) g | 8.2 (16) g | 6.7 (13) g | 11 (11) g | 10 (10) g | |
| LOQ f | 29 (116) g | 31 (33) g | 26 (28) g | 27 (54) g | 22 (44) g | 37 (37) g | 33 (33) g | |
| Sr | a a | 4,158,828 | 3,956,137 | 3,817,574 | 3,877,645 | 3,704,165 | 3,776,872 | 3,751,271 |
| R2 b | 0.9995 | 0.9997 | 0.9995 | 0.9997 | 0.9992 | 0.9998 | 0.9997 | |
| range c | 0–4 | 0–4 | 0–4 | 0–4 | 0–4 | 0–4 | 0–4 | |
| RSD d | 0.31 | 0.24 | 0.86 | 0.29 | 3.0 | 1.0 | 1.7 | |
| LOD e | 0.10 (0.40) g | 0.11 (0.12) g | 0.10 (0.11) g | 0.10 (0.20) g | 0.10 (0.20) g | 0.15 (0.15) g | 0.10 (0.10) g | |
| LOQ f | 0.33 (1.3) g | 0.37 (0.40)) g | 0.33 (0.36) g | 0.33 (0.64) g | 0.33 (0.64)) g | 0.50 (0.50) g | 0.33 (0.33) g | |
| Zn | a a | 31,116 | 30,613 | 30,822 | 31,726 | 30,422 | 35,284 | 34,036 |
| R2 b | 0.9999 | 0.9999 | 0.9999 | 0.9998 | 0.9999 | 0.9991 | 0.9998 | |
| range c | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | 0–10 | |
| RSD d | 0.13 | 0.73 | 0.41 | 0.39 | 0.80 | 0.34 | 0.96 | |
| LOD e | 0.63 (2.5) g | 0.80 (0.86) g | 0.43 (0.46) g | 0.61 (1.2) g | 0.61 (1.2) g | 0.82 (0.82) g | 0.77 (0.77) g | |
| LOQ f | 2.1 (8.4) g | 2.7 (2.9) g | 1.4 (1.5) g | 2.0 (4.0) g | 2.0 (4.0) g | 2.7 (2.7) g | 2.6 (2.6) g | |
| P1 | P2 | P3 | P4 | P5 | P6 | P7 | ||
|---|---|---|---|---|---|---|---|---|
| Al | Ct a | 0.073 ± 0.001 | 0.070 ± 0.002 | 0.073 ± 0.001 | 0.070 ± 0.002 | 0.072 ± 0.001 | 0.069 ± 0.003 | 0.071 ± 0.002 |
| Fcalculated b | 4.00 | 1.00 | 4.00 | 1.00 | 9.00 | 4.00 | ||
| ∣tcalculated∣ c | 2.324 | 0.000 | 2.324 | 1.225 | 2.191 | 1.549 | ||
| Ba | Ct a | 0.082 ± 0.001 | 0.075 ± 0.001 | 0.080 ± 0.001 | 0.072 ± 0.003 | 0.079 ± 0.001 | 0.070 ± 0.003 | 0.072 ± 0.002 |
| Fcalculated b | 1.00 | 1.00 | 9.00 | 4.00 | 9.00 | 4.00 | ||
| ∣tcalculated∣ c | 8.573 | 2.327 | 5.477 | 2.324 | 6.573 | 7.746 | ||
| Ca | Ct a | 89.7 ± 0.2 | 88.2 ± 0.8 | 91.0 ± 0.4 | 85.1 ± 1.0 | 88.0 ± 0.7 | 73.7 ± 1.6 | 82.1 ± 1.3 |
| Fcalculated b | 16.00 | 6.25 | 25.00 | 12.25 | 64.00 | 42.25 | ||
| ∣tcalculated∣ c | 3.151 | 2.573 | 6.379 d | 4.045 | 14.033 d | 8.172 d | ||
| Cd | Ct a | <1.6 e | <0.52 e | <0.50 e | <0.98 e | <0.86 e | <0.27 e | <0.60 e |
| Cr | Ct a | <3.4 e | <1.0 e | <0.80 e | <1.5 e | <1.8 e | <0.90 e | <0.68 e |
| Cu | Ct a | <2.2 e | <1.3 e | <0.80 e | <2.8 e | <1.4 e | <0.81 e | <0.78 e |
| Fe | Ct a | <2.2 e | 0.012 ± 0.001 | 0.014 ± 0.001 | <1.8 e | <1.5 e | 0.011 ± 0.002 | 0.012 ± 0.001 |
| K | Ct a | 12.5 ± 0.2 | 12.0 ± 0.2 | 12.5 ± 0.1 | 11.6 ± 0.1 | 11.9 ± 0.1 | 11.1 ± 0.2 | 11.8 ± 0.1 |
| Fcalculated b | 1.00 | 4.00 | 4.00 | 4.00 | 1.00 | 4.00 | ||
| ∣tcalculated∣ c | 3.062 | 0.000 | 6.971 | 4.648 | 8.573 | 5.422 | ||
| Mg | Ct a | 14.7 ± 0.1 | 14.2 ± 0.2 | 14.8 ± 0.1 | 14.3 ± 0.2 | 14.7 ± 0.1 | 14.1 ± 0.3 | 14.3 ± 0.2 |
| Fcalculated b | 4.00 | 1.00 | 4.00 | 1.00 | 9.00 | 4.00 | ||
| ∣tcalculated∣ c | 3.873 | 1.225 | 3.098 | 0.000 | 3.286 | 3.098 | ||
| Mn | Ct a | 0.400 ± 0.003 | 0.374 ± 0.005 | 0.392 ± 0.002 | 0.365 ± 0.005 | 0.385 ± 0.004 | 0.367 ± 0.004 | 0.377 ± 0.002 |
| Fcalculated b | 2.78 | 1.78 | 2.78 | 1.78 | 1.78 | 2.25 | ||
| ∣tcalculated∣ c | 7.723 | 2.771 | 10.397 | 5.196 | 11.432 | 11.049 | ||
| Na | Ct a | 25.0 ± 0.2 | 24.8 ± 0.4 | 24.9 ± 0.2 | 22.9 ± 0.5 | 25.0 ± 0.4 | 23.1 ± 0.7 | 23.6 ± 0.2 |
| Fcalculated b | 4.00 | 1.00 | 6.25 | 4.00 | 12.25 | 1.00 | ||
| ∣tcalculated∣ c | 0.775 | 0.612 | 6.754 | 0.000 | 4.520 | 8.573 | ||
| Ni | Cta | <11 e | <4.4 e | <3.1 e | <6.2 e | <4.6 e | <3.4 e | <3.4 e |
| Pb | Cta | <34 e | <10 e | <8.5 e | <16 e | <13 e | <11 e | <10 e |
| Sr | Ct a | 0.480 ± 0.002 | 0.453 ± 0.004 | 0.474 ± 0.002 | 0.458 ± 0.005 | 0.460 ± 0.003 | 0.437 ± 0.003 | 0.438 ± 0.002 |
| Fcalculated b | 1.78 | 1.00 | 2.78 | 1.00 | 1.00 | 1.00 | ||
| ∣tcalculated∣ c | 9.353 | 2.449 | 6.535 | 8.165 | 17.555 | 17.146 | ||
| Zn | Ct a | 0.016 ± 0.001 | 0.014 ± 0.001 | 0.016 ± 0.001 | 0.012 ± 0.001 | 0.015 ± 0.001 | 0.010 ± 0.001 | 0.012 ± 0.001 |
| Fcalculated b | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||
| ∣tcalculated∣ c | 2.449 | 0.000 | 4.899 | 1.225 | 7.348 | 4.899 | ||
| Concentration a, mg kg−1 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| YM1 | YM2 | YM3 | BS4 | YM5 | YM6 | YM7 | YM8 | YM9 | YM10 | YM11 | Min.–Max. | Mean b | |
| Al | 0.111 (0.93) | 0.251 (0.39) | 0.210 (0.95) | 0.070 (2.9) | 0.073 (1.4) | 1.26 (0.95) | 0.526 (0.76) | 0.596 (0.36) | 0.459 (0.21) | 0.072 (2.7) | 0.347 (1.3) | 0.070–1.26 | 0.237 |
| Ba | 0.094 (1.2) | 0.066 (1.7) | 0.047 (2.2) | 0.029 (3.5) | 0.080 (1.2) | 0.328 (1.8) | 0.176 (0.66) | 0.163 (0.73) | 0.099 (1.2) | 0.080 (1.4) | 0.114 (1.0) | 0.029–0.328 | 0.095 |
| Ca | 10.3 (1.2) | 24.4 (1.2) | 4.85 (2.1) | 85.8 (2.7) | 91.0 (0.44) | 57.8 (1.3) | 39.6 (0.76) | 18.9 (2.6) | 11.9 (0.84) | 110 (0.91) | 41.2 (0.97) | 4.85–110 | 30.6 |
| Cd | <0.50 c | <0.50 c | <0.50 c | <0.50 c | <0.50 c | <0.50 c | <0.50 c | <0.50 c | <0.50 c | <0.50 c | <0.50 c | --- | --- |
| Cr | <0.80 c | <0.80 c | <0.80 c | <0.80 c | <0.80 c | 0.025 (4.0) | 0.010 (10) | 0.014 (7.1) | 0.011 (9.1) | <0.80 c | 0.011 (9.1) | 0.010–0.025 | 0.013 |
| Cu | <0.80 c | <0.80 c | 0.028 (3.6) | <0.80 c | <0.80 c | 0.026 (3.8) | 0.089 (2.2) | 0.042 (2.4) | 0.013 (7.7) | <0.80 c | 0.033 (3.0) | 0.013–0.089 | 0.032 |
| Fe | 0.072 (2.8) | 0.071 (3.3) | 0.018 (5.6) | 0.050 (3.1) | 0.014 (7.1) | 1.12 (0.63) | 0.302 (0.21) | 0.146 (1.9) | 0.072 (1.9) | 0.054 (3.7) | 0.128 (1.1) | 0.014–1.12 | 0.085 |
| K | 10.2 (2.9) | 41.8 (0.96) | 31.6 (2.8) | 49.6 (2.4) | 12.5 (0.80) | 640 (1.4) | 152 (2.0) | 229 (4.4) | 108 (0.93) | 7.30 (0.82) | 157 (0.64) | 7.30–640 | 118 |
| Mg | 10.4 (1.9) | 18.3 (1.1) | 10.5 (1.9) | 27.3 (3.7) | 14.8 (0.68) | 88.8 (1.1) | 47.4 (0.84) | 70.0 (4.3) | 42.2 (0.94) | 15.4 (1.3) | 57.2 (0.70) | 10.4–88.8 | 28.2 |
| Mn | 0.853 (2.5) | 1.95 (0.54) | 1.43 (0.76) | 0.374 (1.4) | 0.392 (0.51) | 11.7 (1.1) | 6.59 (1.0) | 13.9 (0.86) | 8.90 (0.89) | 0.465 (2.3) | 10.0 (0.56) | 0.374–11.7 | 2.40 |
| Na | 12.0 (1.7) | 5.40 (1.9) | 8.90 (4.5) | 101 (3.0) | 24.9 (0.80) | 6.20 (4.8) | 5.30 (1.9) | 84.1 (4.8) | 5.30 (1.9) | 22.6 (2.7) | 36.1 (0.28) | 5.30–101 | 16.0 |
| Ni | 0.060 (3.3) | 0.080 (1.7) | <3.1 c | 0.081 (3.7) | <3.1 c | 0.134 (2.2) | 0.091 (0.22) | 0.090 (1.1) | 0.050 (2.0) | 0.058 (3.4) | 0.099 (5.1) | 0.050–0.134 | 0.076 |
| Pb | <8.5 c | <8.5 c | <8.5 c | <8.5 c | <8.5 c | <8.5 c | <8.5 c | <8.5 c | <8.5 c | <8.5 c | <8.5 c | --- | --- |
| Sr | 0.192 (1.6) | 0.428 (0.47) | 0.053 (1.9) | 0.424 (0.26) | 0.474 (0.42) | 0.267 (0.44) | 0.155 (0.65) | 0.098 (1.0) | 0.053 (1.9) | 0.462 (0.22) | 0.167 (0.60) | 0.053–0.474 | 0.194 |
| Zn | 0.034 (6.5) | 0.032 (4.0) | 0.057 (2.3) | 0.031 (4.2) | 0.016 (6.2) | 0.716 (1.1) | 0.464 (1.0) | 0.519 (0.21) | 0.316 (0.70) | 0.015 (10) | 0.384 (0.60) | 0.015–0.519 | 0.100 |
| Caffeine d | 30.3 (0.33) | 22.7 (0.44) | 22.6 (2.2) | 19.7 (1.3) | 19.6 (2.5) | 20.3 (2.3) | 12.5 (0.80) | 29.2 (1.4) | 19.3 (1.0) | 25.2 (0.40) | 22.1 (0.90) | 12.5–30.3 | 21.8 |
| Caffeine e | 30 | 20 | 21 | 20 | 20 | ND | 20 | ND | 20 | 25 | 20 | --- | --- |
| YM1 | YM2 | YM3 | YM4 | YM5 | YM6 | YM7 | YM8 | YM9 | YM10 | YM11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | |||||||||||
| D: dialyzate b | 0.048 (4.2) | 0.096 (3.2) | 0.079 (5.1) | 0.040 (2.5) | 0.040 (2.5) | 0.481 (1.2) | 0.191 (1.6) | 0.218 (5.0) | 0.153 (5.2) | 0.026 (3.8) | 0.131 (1.5) |
| ND: nondialyzate c | 0.067 (1.5) | 0.176 (0.57) | 0.155 (0.65) | 0.042 (2.4) | 0.048 (4.2) | 0.796 (1.3) | 0.357 (0.28) | 0.407 (0.74) | 0.314 (0.64) | 0.049 (3.1) | 0.234 (4.2) |
| Agreement d | 104 | 108 | 111 | 117 | 121 | 101 | 104 | 105 | 102 | 104 | 105 |
| Bioaccessibility e | 43.2 | 38.2 | 37.6 | 57.1 | 54.8 | 38.2 | 36.3 | 36.6 | 33.3 | 36.1 | 37.8 |
| Ba | |||||||||||
| D: dialyzate b | 0.049 (2.0) | 0.03 (2.9) | 0.025 (4.0) | 0.018 (5.6) | 0.049 (2.0) | 0.202 (0.50) | 0.099 (2.0) | 0.092 (2.2) | 0.051 (2.0) | 0.048 (2.1) | 0.066 (1.5) |
| ND: nondialyzate c | 0.041 (2.4) | 0.029 (3.4) | 0.019 (5.3) | 0.011 (9.1) | 0.040 (2.5) | 0.148 (1.4) | 0.072 (1.4) | 0.076 (1.3) | 0.044 (2.3) | 0.034 (2.9) | 0.050 (2.0) |
| Agreement d | 95.7 | 97.0 | 93.6 | 100 | 111 | 107 | 97.2 | 103 | 96.0 | 102 | 102 |
| Bioaccessibility e | 52.1 | 53.0 | 53.2 | 62.1 | 61.3 | 61.6 | 56.3 | 56.4 | 51.5 | 60.0 | 57.9 |
| Ca | |||||||||||
| D: dialyzate b | 5.20 (1.5) | 12.5 (4.0) | 2.94 (0.34) | 45.2 (0.22) | 41.8 (0.48) | 32.9 (4.3) | 22.1 (0.45) | 10.0 (4.7) | 6.41 (1.7) | 55.3 (2.0) | 20.4 (0.49) |
| ND: nondialyzate c | 4.57 (3.3) | 13.4 (0.75) | 1.62 (1.9) | 49.1 (0.20) | 46.2 (0.43) | 29.2 (2.7) | 21.0 (2.9) | 9.18 (4.0) | 5.40 (1.7) | 58.6 (0.51) | 22.0 (3.6) |
| Agreement d | 94.9 | 106 | 94.0 | 110 | 96.7 | 107 | 109 | 101 | 99.2 | 104 | 103 |
| Bioaccessibility e | 50.5 | 51.2 | 60.6 | 52.7 | 45.9 | 59.6 | 55.8 | 52.9 | 53.9 | 50.3 | 49.5 |
| Fe | |||||||||||
| D: dialyzate b | 0.035 (2.9) | 0.028 (3.6) | 0.006 (17) | 0.015 (6.7) | 0.005 (20) | 0.576 (1.4) | 0.109 (0.92) | 0.051 (2.0) | 0.030 (3.3) | 0.026 (3.2) | 0.057 (1.8) |
| ND: nondialyzate c | 0.035 (2.9) | 0.046 (2.2) | 0.013 (7.7) | 0.034 (2.9) | 0.011 (9.1) | 0.601 (1.5) | 0.211 (0.95) | 0.088 (2.3) | 0.041 (2.1) | 0.027 (3.1) | 0.076 (2.6) |
| Agreement d | 97.2 | 104 | 106 | 98.0 | 114 | 105 | 106 | 95.2 | 98.6 | 98.4 | 104 |
| Bioaccessibility e | 48.8 | 39.5 | 33.3 | 30.4 | 32.9 | 51.4 | 36.1 | 34.9 | 42.4 | 48.4 | 44.5 |
| Mg | |||||||||||
| D: dialyzate b | 4.41 (0.68) | 8.35 (3.1) | 4.47 (4.5) | 13.4 (1.5) | 7.03 (1.6) | 47.5 (2.5) | 25.6 (0.39) | 34.0 (3.2) | 20.0 (3.0) | 7.90 (0.76) | 26.8 (1.5) |
| ND: nondialyzate c | 5.65 (0.68) | 9.72 (0.31) | 5.75 (1.4) | 14.9 (0.67) | 8.17 (0.49) | 42.8 (2.1) | 25.0 (0.40) | 35.6 (1.1) | 22.4 (0.45) | 8.25 (0.73) | 28.5 (0.70) |
| Agreement d | 97.1 | 98.9 | 97.1 | 104 | 103 | 102 | 107 | 99.4 | 100 | 105 | 96.7 |
| Bioaccessibility e | 42.4 | 45.6 | 42.6 | 49.1 | 47.5 | 53.5 | 54.0 | 48.6 | 47.4 | 51.3 | 46.9 |
| Mn | |||||||||||
| D: dialyzate b | 0.338 (1.5) | 0.837 (3.7) | 0.594 (3.2) | 0.178 (2.8) | 0.185 (1.1) | 5.80 (3.8) | 3.04 (0.33) | 6.60 (4.1) | 3.89 (4.1) | 0.223 (4.0) | 4.62 (1.7) |
| ND: nondialyzate c | 0.500 (0,20) | 1.12 (0.89) | 0.847 (1.8) | 0.218 (0.46) | 0.234 (0.85) | 6.45 (2.2) | 3.47 (0.86) | 7.92 (1.8) | 5.00 (1.2) | 0.255 (0.39) | 5.62 (0.89) |
| Agreement d | 98.2 | 100 | 101 | 106 | 107 | 105 | 98.8 | 104 | 99.9 | 103 | 102 |
| Bioaccessibility e | 39.6 | 42.9 | 41.5 | 47.6 | 47.2 | 49.6 | 46.1 | 47.5 | 43.7 | 48.0 | 46.2 |
| Sr | |||||||||||
| D: dialyzate b | 0.109 (0.92) | 0.232 (3.9) | 0.035 (2.9) | 0.228 (2.6) | 0.271 (2.6) | 0.169 (3.6) | 0.094 (1.1) | 0.064 (3.1) | 0.034 (2.9) | 0.258 (2.7) | 0.109 (1.8) |
| ND: nondialyzate c | 0.089 (2.2) | 0.215 (0.47) | 0.019 (5.3) | 0.199 (1.0) | 0.248 (0.80) | 0.109 (2.8) | 0.066 (4.5) | 0.039 (5.1) | 0.017 (5.9) | 0.220 (1.8) | 0.073 (2.7) |
| Agreement d | 103 | 104 | 102 | 101 | 110 | 104 | 103 | 105 | 96.2 | 104 | 92.4 |
| Bioaccessibility e | 56.8 | 54.2 | 66.0 | 53.8 | 57.2 | 63.6 | 60.6 | 65.3 | 64.2 | 55.8 | 55.3 |
| Zn | |||||||||||
| D: dialyzate b | 0.017 (5.9) | 0.019 (10) | 0.031 (3.2) | 0.017 (5.9) | 0.009 (11) | 0.401 (2.0) | 0.241 (0.83) | 0.282 (3.9) | 0.154 (3.2) | 0.007 (14) | 0.191 (0.52) |
| ND: nondialyzate c | 0.020 (5.0) | 0.018 (5.6) | 0.029 (3.4) | 0.019 (5.3) | 0.008 (12) | 0.357 (2.5) | 0.253 (2.0) | 0.248 (4.8) | 0.172 (3.5) | 0.010 (10) | 0.210 (1.4) |
| Agreement d | 109 | 116 | 105 | 116 | 106 | 106 | 107 | 102 | 103 | 113 | 104 |
| Bioaccessibility e | 50.0 | 59.4 | 54.4 | 54.8 | 50.8 | 56.0 | 51.9 | 54.3 | 48.7 | 42.4 | 49.7 |
| Caffeine | |||||||||||
| D: dialyzate b | 13.9 (0.72) | 10.9 (0.92) | 11.4 (1.8) | 9.35 (0.43) | 9.16 (6.2) | 10.5 (1.9) | 5.57 (1.4) | 13.6 (0.74) | 9.49 (0.84) | 13.9 (1.4) | 10.4 (1.9) |
| ND: nondialyzate c | 17.1 (0.58) | 13.0 (0.77) | 12.4 (0.81) | 10.0 (1.0) | 10.4 (5.7) | 13.1 (0.76) | 6.73 (0.15) | 17.0 (0.59) | 10.6 (1.9) | 14.6 (0.68) | 12.1 (0.83) |
| Agreement d | 102 | 105 | 106 | 97.9 | 99.8 | 102 | 98.5 | 101 | 104 | 113 | 102 |
| Bioaccessibility e | 45.9 | 48.0 | 50.4 | 47.5 | 46.7 | 51.7 | 44.6 | 46.6 | 49.2 | 55.2 | 41.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Welna, M.; Szymczycha-Madeja, A.; Pohl, P. Novel ICP-OES-Based Method for the Reliable Determination of the Total Content of 15 Elements in Yerba Mate Drinks along with the Determination of Caffeine and the In Vitro Bioaccessibility of the Compounds. Molecules 2023, 28, 3374. https://doi.org/10.3390/molecules28083374
Welna M, Szymczycha-Madeja A, Pohl P. Novel ICP-OES-Based Method for the Reliable Determination of the Total Content of 15 Elements in Yerba Mate Drinks along with the Determination of Caffeine and the In Vitro Bioaccessibility of the Compounds. Molecules. 2023; 28(8):3374. https://doi.org/10.3390/molecules28083374
Chicago/Turabian StyleWelna, Maja, Anna Szymczycha-Madeja, and Pawel Pohl. 2023. "Novel ICP-OES-Based Method for the Reliable Determination of the Total Content of 15 Elements in Yerba Mate Drinks along with the Determination of Caffeine and the In Vitro Bioaccessibility of the Compounds" Molecules 28, no. 8: 3374. https://doi.org/10.3390/molecules28083374
APA StyleWelna, M., Szymczycha-Madeja, A., & Pohl, P. (2023). Novel ICP-OES-Based Method for the Reliable Determination of the Total Content of 15 Elements in Yerba Mate Drinks along with the Determination of Caffeine and the In Vitro Bioaccessibility of the Compounds. Molecules, 28(8), 3374. https://doi.org/10.3390/molecules28083374








