Abstract
Contemporary pharmacology dating back to the late 19th/early 20th centuries has benefitted largely from the incorporation of metal complexes. Various biological attributes have been successfully realized using metal/metal complex-based drugs. Among anticancer, antimicrobial, and antiviral applications, anticancer applications have extracted the maximum benefit from the metal complex, Cisplatin. The following review has compiled the various antiviral benefits harnessed through inputs from metal complexes. As a result of exploiting the pharmacological aspects of metal complexes, the anti-COVID-19 deliverables have been summarized. The challenges ahead, the gaps in this research area, the need to improvise incorporating nanoaspects in metal complexes, and the need to test metal complex-based drugs in clinical trials have been discussed and deliberated. The pandemic shook the entire world and claimed quite a percentage of the global population. Metal complex-based drugs are already established for their antiviral property with respect to enveloped viruses and extrapolating them for COVID-19 can be an effective way to manipulate drug resistance and mutant issues that the current anti-COVID-19 drugs are facing.
1. Introduction
Coronaviridae is a rather unique viral family, which has a significantly large RNA and a distinct morphology, as well as an extraordinary ability to pass from animals to humans. It is to be noted that three of the most transmissible and pathogenic coronaviruses from this family have breached the species barrier and produced lethal outbreaks, and unprecedented health emergencies since the 21st century began. Two human coronaviruses—severe acute respiratory syndrome coronavirus (SARS-CoV-1) and Middle Eastern respiratory syndrome coronavirus (MERS-CoV)—have caused nearly 10,000 cumulative cases with 10% and 34.4% fatality rates, respectively. SARS-CoV-2 originated from Wuhan in December 2019 [1]. Most countries were affected by this pandemic. It has swiftly spread worldwide, infecting almost 22 million people, and resulting in 770,000 fatalities, with a projected mortality rate of 3.6%.
Coronaviruses are classified into four genera: α-, β-, δ- and γ-coronaviruses. Mammals commonly contract moderate respiratory or gastrointestinal infections from Coronavirinae. Most BatCoV (coronavirus isolated from bats) are β-coronaviruses. β-coronaviruses include SARS-CoV-1 and -2 (subgroup 2b), MERS-CoV (subgroup 2c), and HCoV-OC43 and HCoV-HKU1 (subgroup 2a). HCoV-229E and HCoV-NL63 are α-coronaviruses (subgroup 1b) [2,3,4]. SARS-CoV-2 has a 29,891-nucleotide genome that encodes 9860 amino acids, four structural proteins, and sixteen non-structural proteins (NSPs). The 3-chymotrypsin-like protease (3CL-PR), RNA-dependent RNA polymerase (RdRp), and spike protein are potent and selective druggable targets [5,6,7]. NSP12 consists of an N-terminal NiRAN domain, an interface domain, and a C-terminal RdRp domain. It is essential for viral genome replication and mRNA synthesis [8,9,10]. RdRp has the conventional design of viral polymerases [11] with three subdomains: a fingers subdomain (residues Leu366 to Ala581 and Lys621 to Gly679), a palm subdomain (Thr582 to Pro620 and Thr680 to Gln815), and a thumb subdomain (His816 to Phe920) [8]. The palm subdomain’s active site has at least five conserved A-E motifs [8,12]. The catalytic residues Ser759, Asp760, and Asp761 are in motif C, while motif A has the divalent-cation–binding residue Asp618. The only confirmed authenticated treatment for SARS-CoV-2 infection is the nucleotide analogue pro-drug remdesivir, an RdRp inhibitor [13]. After witnessing the havoc COVID-19 caused, researchers worldwide have been frantically investigating many therapeutic options to find candidates that can combat coronavirus outbreaks.
Apart from the nucleotide analogue prodrug remdesivir, no other validated pharmaceutical treatment for SARS-CoV-2 infection or other human pathogenic coronaviruses is available. Remdesivir is the only FDA-approved emergency treatment. Remdesivir has not yet been tested in large-scale coronavirus clinical studies [14,15]. Molnupiravir, which is the first oral medicinal formulation for severe symptoms, has been studied in several clinical investigations [16,17,18]. The FDA and EMA are still investigating this formulation, but the UK has approved it. The first oral antiviral for COVID-19, Lagevrio (molnupiravir), was approved by MHRA. Despite recent headlines, molnupiravir appears to only work in early-stage COVID-19 patients. Its efficacy is minimal in hospitalized patients with advanced disease, which is foreseen as a limitation [19]. Vaccination works best for viral epidemics, as per history [20]. To stop the virus, many governments have accelerated vaccine licensing. SARS-CoV-2 vaccines were given to 22% of the world’s population by mid-June 2021 due to vigorous vaccination programs. The COVID-19 epidemic continues despite widespread public immunizations [21]. So, scientists worldwide are searching for novel SARS-CoV-2-fighting substances. Molecular knowledge of the virus is growing, and various therapeutic targets are being identified and defined. Drug repurposing, which is a system of using medications currently in clinical use for a different therapeutic indication, is a simple way to find active molecules to attack COVID-19. Tocilizumab, Chloroquine, and Remdesivir are interesting candidates for COVID-19 medication identified by drug repurposing. Metal-complex-based drugs may also be effective against COVID-19, according to Messori et al. [22,23]. It is interesting that none of the repurposed metal compounds have been put through clinical testing [24].
This review highlights the potential benefits that come from metal-complexes when used as antiviral compounds. The current status of applying metal-complex-based drugs as anti-COVID-19 therapeutics has been surveyed and the challenges and gaps as well as future recommendations exclusively listed and discussed.
2. Metal Complexes in Medical Applications
The various medicinal benefits reported from metal complexes are projected in Figure 1. Metals and metal complexes are being employed more in cancer treatment ever since their discovery [25,26,27,28,29]. Metals bound to N, O, and S form chelate rings that bind the metal more firmly than the non-chelate version. Proteins, enzymes, and DNA are electron-rich, but metal ions are electron-deficient; hence, metal ions interact with several key biological molecules [30]. Metallic complexes can bind large biological molecules better than metal-free organic substances, making them enzyme inhibitors [31]. Metal complexes can coordinate with non-metalloenzymes’ active sites and chelate metalloenzymes. Metals also catalyze reactive oxygen species (ROS) [32]. Main group elements and transition metals have been tested for anticancer properties [33,34,35].
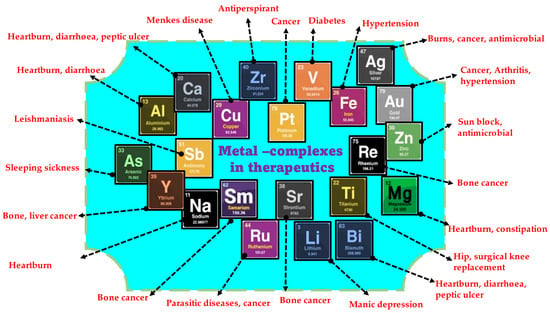
Figure 1.
Validated medicinal properties of various metal/metal complexes.
Platinum metal complexes, including cisplatin, carboplatin, and oxaliplatin are among the most active and extensively used cancer chemotherapeutic medicines [36,37,38]. In vivo, platinum compounds crosslink DNA, resulting in programmed cell death [39]. Due to primary and secondary resistance, these medications are only useful for sarcomas, small cell lung cancer (SCLC), ovarian cancer, lymphomas, and germ cell malignancies [40,41]. Hence, research groups create specific platinum complexes for cisplatin-resistant malignancies [42,43,44,45]. Metal coordination changes lipophilicity, which restricts cell entrance, and reduces side effects. Metal complexes have higher biological activity than free ligands [46]. Thiosemicarbazones and their metal complexes are used in medicinal chemistry and may suppress cancer cell activity [47,48]. Triapine (Vion Pharmaceuticals Inc., New Haven, CT, USA) inhibits DNA manufacture in leukemia L1210 cells by blocking ribonucleotide reductase [49].
Platinum-based drugs specifically target head and neck cancers. These heterocyclic thio-semicarbazone derivatives and their platinum and palladium complexes perform many pharmaceutical activities, including anti-tuberculosis [50], antibacterial [51], antitumor [52], antiprotozoal [53], antimalarial [54], antimicrobial [55], antiviral [56], antifungal [57], anticonvulsant [58], and anti-trypanosomal [59,60]. The metal complexes 5-substituted thiophene-2-carboxaldehydes yield thio-semicarbazones and their platinum(II) and palladium(II) complexes. In vitro antiviral and cytostatic/toxic actions of ligands and platinum and palladium complexes have been assessed. NAMI-A and KP1019 are potential ruthenium-based antitumor drugs [61]. These are a few notable mentions of the various biological applications, especially medical applications, enabled successfully by metal complexes. Figure 2 summarizes the various chemical properties of metal complexes that give them their biological attributes.
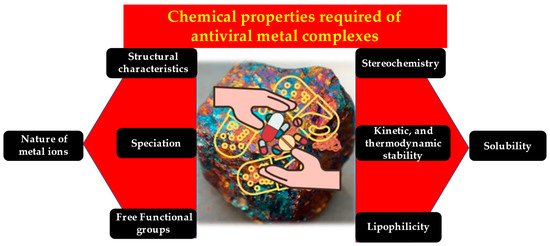
Figure 2.
The unique chemical characteristics of metal complexes add up to their antiviral property.
4. Metal Complexes and Anti-COVID-19 Applications
Several chemicals are being tested, but the optimal treatment for COVID-19 is yet to emerge. Nevertheless, academic scientists worked with pharmaceutical companies to develop a variety of vaccines in 2020. These vaccinations protect healthy people but may not protect viral variations or immunocompromised patients. Hence, COVID-19 requires innovative treatments [103]. Researchers are developing and screening metal complexes to find novel therapeutics. Drug metal complexes apart from treating COVID-19 can additionally also provide other benefits. For instance, the body needs metal ions to make haemoglobin, zinc fingers regulate gene activities and recognize DNA [104,105], and zinc and copper are needed for growth and immune system development. Auranofin, a gold medication, may be a possibility for drug repurposing, although few other licensed metal complexes exist.
Computational approaches to assess chemical compound activity based on drug and target structure were deployed on vanadium compounds against COVID-19 [106]. Copper complexes containing chloroquine and hydroxychloroquine contain balanced polar and non-polar groups compared to the parent medication. Its structure allows metallodrug binding to ADP-ribose-1 monophosphatase enzyme’s active site of the virus. Inhibiting this enzyme prevents COVID-19 [107]. Another group reported in silico synthesis of Cu (II) and Co (II) thiazole-based ligand complexes and detailed their role as COVID-19 protein interactors using molecular docking [108]. Refat et al. validated the synthesis of binuclear Schiff base complexes (Zn[II], Cu[II], Co[II], and Ni[II]), and their biological activity was tested using Molecular docking methods. Ni(II) bound COVID-19 protease (6LU7) better in molecular docking experiments [109]. Rad et al. also presented a new COVID-19 treatment using simulations of Fe, Cr, and Ni transition metal-doped fullerenes–favipiravir complexes [110]. In another study, gibberellic acid (HGA), a plant hormone, was used to generate complexes with transition metals Cr(III), Ni(II), Hg(II), Zn(II), Co(II), Cd(II), Mn(II), and Cr(III). These new compounds were computationally tested for interaction with the COVID-19 active site 6LU7. Mn(II) had increased binding energy with the active site and was a putative 6LU7 inhibitor, representing probable anti-COVID-19 activity [111]. Omar and Ahamed synthesized tridentate Schiff base metal complexes of Zn(II), Cu(II), Mn(II), Ni(II), Fe(III), Cd(II), and Cr(II) (III) and screened for anti-COVID-19 activity using MOE2008 molecular docking experiments. They investigated the binding mechanisms of many metal complexes against COVID-19 major protease (SARS-CoV-2) combined with the inhibitor UAW247 (PDB ID: 6XBH). Cr(III) complex demonstrated low binding energy and stable interaction, pointing out strong COVID-19 antiviral activity [112].
Zn(II) boosts immunity and inhibits viral RNA-dependent RNA polymerase. Intracellular Zn(II) levels have been shown to influence DNA and RNA viruses, notably respiratory viruses like influenza, picornaviruses, and respiratory syncytial virus. Chloroquine and hydroxychloroquine work by introducing additional Zn(II) into cells as ionophores. Consequently, Zn(II) may directly block the SARS-CoV-2 replicative cycle [113]. Poupaert et al. also endorse Zn(II) for COVID-19 therapy. Quantum mechanics molecular simulations pointed out molecular Zn++ interactions. First- and second-generation macrolides like azithromycin (Zn++-antibiotic complex) apparently possess anti COVID-19 potential [114].
Despite metallodrugs’ growing therapeutic value, no meaningful measures have been taken to restrict their pandemic effectiveness. A few noteworthy initiatives exist. Marzo and Messori were the first to try metal-based pandemic medications. Auranofin was proposed against SARS-CoV-2 [115]. The researchers exploited the positive attributes of this compound, namely its biocompatibility (tolerance to the biological system), low toxicity, and multi-targeting. Two more experiments strengthened Marzo and Messori’s idea, Auranofin at low micromolar concentrations suppressed the virus by 95% after 48 h in human cells. It drastically inhibited human cell cytokine expression [116]. Gil-Moles and colleagues found that auranofin inhibited the spike protein’s interaction with ACE2’s active site. This is the virus’s main entry point into human cells. The chemical strongly inhibited SARS-CoV-1 and SARS-papain-like CoV-2’s protein (PLpro), a viral replication enzyme. This enzyme is the first to block this target protein experimentally [117].
Bismuth complexes have also been reported for their anti-COVID-19 potential. In 2007, researchers evaluated bismuth complexes’ capacity to attach to the 100-residue cysteine-rich metal-binding domain (NTPase/helicase), which plays a crucial role in SARS-CoV-2 life cycle [118]. Bismuth compounds inhibited SCV helicase enzymatic activity, paving the way for SARS-CoV-2 studies. In a 2020 study by Yuan et al. [119], ranitidine bismuth citrate was tested against COVID-19, in vitro and in vivo. Cell cultures and animals exhibited efficacy. In cell cultures, this combination protects against SARS-CoV-2 with low toxicity. This metal complex significantly decreases virus proliferation in Syrian hamsters, lowering the respiratory viral burden. Researchers also noted that the combination inhibited viral helicases in vitro, confirming prior studies on the enzyme’s usefulness as a pharmaceutical target [120] and bismuth complexes’ capacity to target it. However, these results have not been put through clinical trials. The one exception is a case study reported in the American Journal of Gastroenterology that indicated that a patient with exacerbated Crohn’s disease due to SARS-CoV-2 infection, who showed significant improvement [121]. His doctors prescribed bismuth subsalicylate 525 mg orally 2–4 times a day. Over six days, this treatment reduced diarrhoea, cough, appetite, energy, and virus-triggered symptoms. Figure 3 portrays the three-faceted anti-COVID-19 contribution that is made possible by metal-complexes.
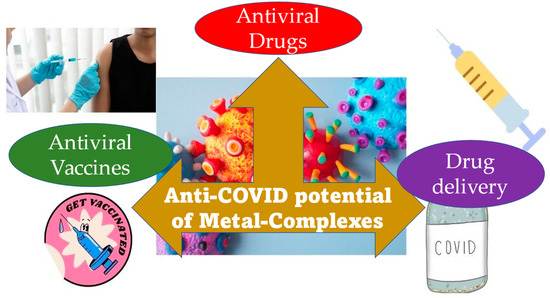
Figure 3.
Three modes of contribution from metal complexes towards the anti-COVID-19 activity.
Mechanism of Anti-COVID-19 Activity of Metal Complexes
Modern medicine has now paved the way to accommodate metallodrugs. The existing modes of action of pharmacological compounds with antibacterial and possible antiviral activity of metal complexes are discussed in this section. In a January 2020 review [122], researchers attempted to categorize metal complex drugs based on their action modes. Here we discuss mechanisms that we think are relevant to the anti-COVID-19 perspective, at this point. The first mechanism involves the covalent bonding of metallodrugs to biological targets [123]. Cisplatin and auranofin fall within this mechanistic category. These two medicinal compounds affect stereochemistry through their biological targets’ covalent connections. Biological processes are thus inhibited [124]. Auranofin blocks cell death oxidoreductive processes, activates the unfolded protein response (UPR), induces endoplasmic reticulum (ER) stress, and inhibits thioredoxin redox enzymes [125]. Thioredoxin transfers electrons to oxidative stress-protecting enzymes. Auranofin inhibits thioredoxin reductase by dislodging the gold complex’s ligands and forming a covalent connection with the active center’s cysteine residues [126]. Auranofin induces apoptosis by blocking these enzymes. Auranofin reduces cytokines and stimulates cellular immunity [127]. ER oxidative stress and UPR activation are responsible for coronavirus virulence, making this mechanism of action useful against coronaviruses [128]. According to the study, cells infected with this family of viruses may overexpress spike proteins. Other viral proteins activate UPR [129]. Inhibiting thioredoxin reductase and other redox enzymes may impair SARS-CoV-2 protein production [130]. Auranofin’s anti-inflammatory properties make it a potential COVID-19 drug. Systematic observation has shown that SARS-CoV-2 infection causes immediate respiratory inflammation and a cytokine storm with interleukin-6 (IL-6) upregulation [131]. Auranofin suppresses IL-6 signalling by inhibiting JAK1 and STAT3 phosphorylation, according to Nam-Hoon Kim and colleagues [132]. Inhibiting the inflammatory condition could save lives and lessen their virus-related mortality.
The second mechanism of action of metal complexes against viruses, especially against SARS-CoV-2, is that of redox-active metal centers. Many metals or metal ions can be oxidized. Their oxidation states alter substituent dynamics and environmental chemical and biological processes [133,134]. Previous research has shown that the oxidation state and other intracellular variables affect virus life cycles [135]. Being intracellular parasites, viruses have several ways to exploit and damage cells [136]. Consequently, infected viruses shift the redox balance towards oxidation [137]. Respiratory viruses overproduce reactive oxygen species (ROS) and decrease glutathione, the cell’s main antioxidant. ROS and glutathione decrease and enhance viral replication pathways [138]. NOX oxidase, which has seven members, overproduces ROS during infection, with NOX2 being crucial to viral proliferation. Its absence reduces respiratory infection duration and severity [139]. NOX4 is an even better target. Lung epithelial cells overexpress NOX4 and produce ROS following infection [140]. ACE2, which is the principal receptor coronaviruses employ to enter cells, regulates NOX4 ROS generation [21]. ROS generation activates MAPKs and facilitates nuclear extraction of the filial ribonucleoprotein, allowing viral replication [141]. Thus, with SARS-CoV-2, overexpression of ROS and NOX2 has been linked. Damiano and her colleagues suggest reducing cell oxidative stress to reduce COVID-19 infection and safeguard high-risk patients [142]. In this direction metal complex-based drugs hold promise; however, their potential in this direction has not been proven.
The third mechanism is via photodynamic treatment (PDT) and photoactivated metal drug complexes. Due to their unique characteristics, metal complexes are ideal for such therapies [143]. Certain metals can absorb visible light and diatomically absorb two photons in the near-infrared region. Metals boost spin–orbit coupling, allowing the triplet state that produces simple oxygen. Finally, metal-containing compounds in PDT therapy are photostable. This mechanism is largely used effectively in treating cancer. Yet, it can also be applied to treat viruses [144]. PDT was first used against herpes genitalis in the early 1970s [145]. Since then, this approach has been tested against HPV, HIV, CMV, and others [146,147]. Enveloped viruses responded better to PDT, according to research reports. SARS-CoV-2, being an enveloped virus, was also envisioned to respond well [148,149]. This approach activates photosensitizers (PS) to produce ROS. PSs activate by absorbing photons at a specific wavelength. After a few nanoseconds of electron transport, the PS enters the triple state. ROS generation is the last stage before entering one of the two photochemical routes. Oxidative stress from ROS generation damages nucleic acids and microbial systems. The approach is advantageous since PS are non-toxic chemicals that only become toxic at the target spot following exposure to a specific wavelength of radiation [150]. To our knowledge, no clinical investigation has tested this approach for COVID-19 infection. We found important in vitro research evidence that suggests the strategy may work [151,152]. Nonetheless, it has been tested against such other coronaviruses as MERS-CoV. [153]. PDT’s ROS generation can inactivate SARS-structural CoV-2’s proteins, based on its structure. Photochemical inactivators, including riboflavin, curcumin, and chlorophyll derivatives inhibit coronaviruses, according to prior studies [154]. Mechanistically and administratively, this technique is interesting. Nebulization with a catheter for light supply is one way to treat SARS-CoV-2, which affects nasal and oropharyngeal epithelial cells [155,156]. In 2020, Dias suggested using this method to treat COVID-19 adjunctively [157]. Several researchers endorse this SARS-CoV-2 treatment despite its specificity and limited use against viral diseases [158].
SARS-high CoV-2’s mutation rate makes traditional antiviral medication design problematic. This virus modifies its protein targets often, leading to resistance to these drugs [159]. On the other hand, metal-based drugs do not act by interacting with specific molecular targets of the virus. Their activity lies in the virus’s general environment, an advantage that helps them to be less vulnerable to viral mutations and thereby to become a better pharmaceutical approach compared with the other options.
5. Challenges, Future Perspectives, and Conclusions
Metals and metal complexes, endowed with “magical” qualities, have long fascinated physicians and played a crucial part in contemporary pharmacology since the late 19th/early 20th century. Gold, bismuth, antimony, and mercury compounds have been successfully applied to treat infectious disorders such as tuberculosis, syphilis, and parasitic diseases [160,161]. Even arsenicals were often used in clinics. Due to legitimate concerns on their systemic toxicity and the arrival of new organic medications with greater pharmacological performance and reduced toxicity, these inorganic compounds were eventually abandoned. Yet, some inorganic medicines are still in clinical practice for a few particular applications where they provide valuable and irreplaceable efficacy with acceptable toxicity [162]. In spite of their systemic toxicity, platinum medications are still applied to nearly 50% of cancer chemotherapy procedures [163]. Given the seriousness of cancer, their toxicity may be acceptable. Nonetheless, antimony compounds for leishmaniasis, arsenic trioxide for promyelocytic leukemia, and bismuth compounds for Helicobacter pylori infections are noteworthy [163]. For the last four decades, interest in metal-based medications, because of the clinical success of cisplatin (first approved by the FDA in 1978), provoked and caught the attention of the international scientific community working in inorganic medicinal chemistry. The fact behind the spurred enthusiasm for metal-complex drugs is that they contain a wide variety of metal centers with unique chemical and reactivity properties arising from the metal’s electronic structure, coordination sphere, ligands, oxidation state, redox potential, etc. These chemical features cannot be reproduced by simple organic compounds. Metal complexes may directly inhibit enzymes, modify transcription factors, interact with a range of biomolecules through coordinative bonding, boost lipophilicity, affect cell membrane activities, interfere with the cell cycle, and more.
Cirri et al. [22] have proposed two strategies for identifying anti-COVID-19 metal complex-based drugs. The first strategy is through drug discovery from clinically proven metal-based medicine by repurposing clinically licensed metal-based medications for COVID-19 treatment. This is a simple way to find effective metallodrugs. The second strategy is through screening metal-based chemical libraries for drugs We recommend rigorous in vitro testing of vast and representative libraries of metal-based medicinal compounds against SARS-CoV-2 replication. Families of medicinally suitable metal-based medicines with tolerable toxicity should dominate investigational panels. Bismuth, ruthenium, and antimony compounds may be ideal due to their low toxicity.
In the course of this review, we identified a gap in the usage of the new generation state-of-the-art materials, such as fullerenes, graphene, and carbon nanotubes in the making of metal complexes. A recent study [149] based on the principle that singlet oxygen-generating compounds inactivate encapsulated viruses via photodynamic processes, demonstrated the use of Buckminsterfullerene (C60), a water-insoluble photosensitizer, to inactivate encapsulated viruses. VSV and Semliki Forest virus (SFV, Toga6iridae) were studied in this context. C60 produces much singlet oxygen and is photo-oxidatively inert. It is also easy to recycle from aqueous solutions. Hence, it may help to inactivate viruses in biological systems. Carbon materials have a lot to offer and hybridizing these with traditional metal complexes could greatly benefit this cause. The metal-complex aspect also lacks the nanofactor; few of these metal complexes involve nanometals. Nanotechnology had breached and broken through innumerable limitations of bulk materials. There is no doubt that more nanoaspects need to be incorporated into anti-COVID-19 prospects being sought from metal complex-based drugs.
Photodynamic therapy is one application that has greatly benefitted from metal complexes; yet, strangely, little has been done in this direction with respect to COVID-19 treatment. This is another gap that needs to be bridged. The other essential, crucial aspect lacking in the theme under discussion is that of clinical trials and testing. Mostly simulated/computational/in silico studies (with respect to anti-COVID-19 metal-complex applications) are reported. Few are done in vitro, and fewer still in vivo; clinical trials are almost nonexistent. Clinical trials are the actual green signal for any prescribed drug or treatment option.
The anti-COVID-19 options from metal complexes have been explored, and the current scenario with respect to the status quo of metallo drugs available and tested against SARS-CoV-2 has been reviewed and presented. The need for introducing and hybridizing conventional metal complexes with more new-age nanomaterials and the mandate for clinical trials for testing of the metallo complexes have been emphasized.
Author Contributions
J.G. and M.M., preparation of the original draft and revisions; I.S., participated in the review and revisions, funding. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This article was supported by the KU Research Professor Program of Konkuk University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Santos-López, G.; Cortés-Hernández, P.; Vallejo-Ruiz, V.; Reyes-Leyva, J. SARS-CoV-2: Basic concepts, origin and treatment advances. Gac. Med. Mex. 2021, 157, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Leibowitz, J.L. The structure and functions of coronavirus genomic 3′ and 5′ ends. Virus Res. 2015, 206, 120–133. [Google Scholar] [CrossRef]
- Cui, J.; Li, F.; Shi, Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef]
- Song, Z.; Xu, Y.; Bao, L.; Zhang, L.; Yu, P.; Qu, Y.; Zhu, H.; Zhao, W.; Han, Y.; Qin, C. From SARS to MERS, Thrusting Coronaviruses into the Spotlight. Viruses 2019, 11, 59. [Google Scholar] [CrossRef]
- Chan, J.F.-W.; Kok, K.-H.; Zhu, Z.; Chu, H.; To, K.K.-W.; Yuan, S.; Yuen, K.-Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020, 9, 221–236. [Google Scholar] [CrossRef]
- Huang, J.; Song, W.; Huang, H.; Sun, Q. Pharmacological Therapeutics Targeting RNA-Dependent RNA Polymerase, Proteinase and Spike Protein: From Mechanistic Studies to Clinical Trials for COVID-19. J. Clin. Med. 2020, 9, 1131. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhou, Q.; Li, Y.; Garner, L.V.; Watkins, S.P.; Carter, L.J.; Smoot, J.; Gregg, A.C.; Daniels, A.D.; Jervey, S.; et al. Research and Development on Therapeutic Agents and Vaccines for COVID-19 and Related Human Coronavirus Diseases. ACS Cent. Sci. 2020, 6, 315–331. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yan, L.; Huang, Y.; Liu, F.; Zhao, Y.; Cao, L.; Wang, T.; Sun, Q.; Ming, Z.; Zhang, L.; et al. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science 2020, 368, 779–782. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.; Ruggiero, A.; Squeglia, F.; Maga, G.; Berisio, R. A Structural View of SARS-CoV-2 RNA Replication Machinery: RNA Synthesis, Proofreading and Final Capping. Cells 2020, 9, 1267. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.-M.; Wang, W.; Song, Z.-G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef]
- McDonald, S.M. RNA synthetic mechanisms employed by diverse families of RNA viruses. Wiley Interdiscip. Rev. RNA 2013, 4, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Hillen, H.S.; Kokic, G.; Farnung, L.; Dienemann, C.; Tegunov, D.; Cramer, P. Structure of replicating SARS-CoV-2 polymerase. Nature 2020, 584, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Artese, A.; Svicher, V.; Costa, G.; Salpini, R.; Di Maio, V.C.; Alkhatib, M.; Ambrosio, F.A.; Santoro, M.M.; Assaraf, Y.G.; Alcaro, S.; et al. Current status of antivirals and druggable targets of SARS CoV-2 and other human pathogenic coronaviruses. Drug Resist. Updat. 2020, 53, 100721. [Google Scholar] [CrossRef] [PubMed]
- FDA Approves First Treatment for COVID-19. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19 (accessed on 24 June 2021).
- Naveed, M.; Uddin, S.; Khan, M.K.; Khan, Z. Remdesivir for the treatment of COVID-19: A need for combined in vivo and in vitro studies to evaluate the efficacy. J. Pharm. Pract. 2021, 34, 343–346. [Google Scholar] [CrossRef]
- European Medicines Agency. COVID-19: EMA Starts Rolling Review of Molnupiravir. 2021. Available online: https://www.ema.europa.eu/en/news/covid-19-ema-starts-rolling-review-molnupiravir (accessed on 24 November 2021).
- COVID-19 Vaccine Tracker. Available online: https://vac-lshtm.shinyapps.io/ncov_vaccine_landscape/ (accessed on 24 June 2021).
- COVID-19 First In Human Study to Evaluate Safety, Toler Ability, and Pharmacokinetics of EIDD-2801 in Healthy Volunteers. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04392219?term=Molnupiravir&draw=2&rank=5 (accessed on 24 November 2021).
- Singh, A.; Singh, A.; Singh, R.; Misra, A. Molnupiravir in COVID-19: A systematic literature review. Diabetes Metab. Syndr. 2021, 15, 102329. [Google Scholar] [CrossRef]
- Taylor, M.W. Vaccines Against Viral Infections; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Ioannou, K.; Vlasiou, M.C. Metal-based complexes against SARS-CoV-2. Biometals 2022, 35, 639–652. [Google Scholar] [CrossRef]
- Cirri, D.; Pratesi, A.; Marzo, T.; Messori, L. Metallo therapeutics for COVID-19. Exploiting metal-based com pounds for the discovery of new antiviral drugs. Exp. Opin. Drug Discov. 2021, 16, 39–46. [Google Scholar] [CrossRef]
- De Paiva, R.E.F.; Neto, A.M.; Santos, I.A.; Jardim, A.C.G.; Corbi, P.P.; Bergamini, F.R.G. What is holding back the development of antiviral metallodrugs? A literature overview and implications for SARS-CoV-2 therapeutics and future viral outbreaks. Dalton Trans. 2020, 49, 16004–16033. [Google Scholar] [CrossRef]
- Karges, J.; Cohen, S.M. Metal complexes as antiviral agents for SARS-CoV-2. ChemBioChem 2021, 22, 2600–2607. [Google Scholar] [CrossRef]
- Rosenberg, B.; Vancamp, L.; Trosko, J.E.; Mansour, V.H. Platinum Compounds: A New Class of Potent Antitumour Agents. Nature 1969, 222, 385–386. [Google Scholar] [CrossRef]
- Orvig, C.; Abrams, M.J. Medicinal Inorganic Chemistry: Introduction. Chem. Rev. 1999, 99, 2201–2204. [Google Scholar] [CrossRef] [PubMed]
- Kovala-Demertzi, D.; Boccarelli, A.; Demertzis, M.; Coluccia, M. In vitroAntitumor Activity of 2-Acetyl Pyridine 4N-Ethyl Thiosemicarbazone and Its Platinum(II) and Palladium(II) Complexes. Chemotherapy 2007, 53, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, H.S.; Kojima, Y.; Yoshikawa, Y.; Kawabe, K.; Yasui, H. Antidiabetic vanadium(IV) and zinc(II) complexes. Coord. Chem. Rev. 2002, 226, 187–198. [Google Scholar] [CrossRef]
- Louie, A.Y.; Meade, T.J. Metal Complexes as Enzyme Inhibitors. Chem. Rev. 1999, 99, 2711–2734. [Google Scholar] [CrossRef]
- Kostova, I. Platinum Complexes as Anticancer Agents. Recent Patents Anti-Cancer Drug Discov. 2006, 1, 1–22. [Google Scholar] [CrossRef]
- Iakovidou, Z.; Papageorgiou, A.; Demertzis, A.M.; Mioglou, E.; Mourelatos, D.; Kotsis, A.; Yadav, P.N.; Kovala-Demertzi, D. Platinum(II) and palladium(II) complexes with 2-acetylpyridine thiosemicarbazone: Cytogenetic and antineoplastic effects. Anti-Cancer Drugs 2001, 12, 65–70. [Google Scholar] [CrossRef]
- Huang, R.; Wallqvist, A.; Covell, D.G. Anticancer metal compounds in NCI’s tumor-screening database: Putative mode of action. Biochem. Pharmacol. 2005, 69, 1009–1039. [Google Scholar] [CrossRef] [PubMed]
- Galanski, M.; Jakupec, M.; Keppler, B. Update of the Preclinical Situation of Anticancer Platinum Complexes: Novel Design Strategies and Innovative Analytical Approaches. Curr. Med. Chem. 2005, 12, 2075–2094. [Google Scholar] [CrossRef]
- Gielen, M.; Tiekink, E.R.T. Metallotherapeutic Drugs and Metal-Based Diagnostic Agents: The Use of Metals in Medicine; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Karki, S.S.; Thota, S.; Darj, S.Y.; Balzarini, J.; De Clercq, E. Synthesis, anticancer, and cytotoxic activities of some mononuclear Ru(II) compounds. Bioorg. Med. Chem. 2007, 15, 6632–6641. [Google Scholar] [CrossRef]
- Deegan, C.; Coyle, B.; McCann, M.; Devereux, M.; Egan, D.A. In vitro anti-tumour effect of 1,10-phenanthroline-5,6-dione (phendione), [Cu(phendione)3](ClO4)2·4H2O and [Ag(phendione)2]ClO4 using human epithelial cell lines. Chem. Interact. 2006, 164, 115–125. [Google Scholar] [CrossRef]
- Afrasiabi, Z.; Sinn, E.; Chen, J.; Ma, Y.; Rheingold, A.L.; Zakharov, L.N.; Rath, N.; Padhye, S. Appended 1,2-naphthoquinones as anticancer agents 1: Synthesis, structural, spectral and antitumor activities of ortho-naphthaquinone thiosemicarbazone and its transition metal complexes. Inorg. Chim. Acta 2004, 357, 271–278. [Google Scholar] [CrossRef]
- Alderden, R.A.; Hall, M.D.; Hambley, T. The Discovery and Development of Cisplatin. J. Chem. Educ. 2006, 83, 728–734. [Google Scholar] [CrossRef]
- Gomez, A.; Quiroga, C. Navarro Ranninger, Contribution to the SAR field of metallated and coordination complexes. Coord. Chem. Rev. 2004, 248, 119. [Google Scholar]
- Nath Yadav, P.; Demertzis, M.A.; Kovala-Demertzi, D.; Skoulika, S.; West, D.X. Palladium(II) Complex of the 5-Hydroxypyridine-2-carbaldehyde N(4)-ethylthiosemicarbazone: Synthesis and Characterization. Inorg. Chim. Acta 2003, 349, 30. [Google Scholar]
- Wong, E.; Giandomenico, C.M. Current Status of Platinum-Based Antitumor Drugs. Chem. Rev. 1999, 99, 2451–2466. [Google Scholar] [CrossRef]
- Hambley, T.W. The influence of structure on the activity and toxicity of Pt anti-cancer drugs. Coord. Chem. Rev. 1997, 166, 181–223. [Google Scholar] [CrossRef]
- Stordal, B.; Pavlakis, N.; Davey, R. Oxaliplatin for the treatment of cisplatin-resistant cancer: A systematic review. Cancer Treat. Rev. 2007, 33, 347–357. [Google Scholar] [CrossRef]
- Gojo, I.; Tidwell, M.L.; Greer, J.; Takebe, N.; Seiter, K.; Pochron, M.F.; Johnson, B.; Sznol, M.; Karp, J.E. Phase I and pharmacokinetic study of Triapine®, a potent ribonucleotide reductase inhibitor, in adults with advanced hematologic malignancies. Leuk. Res. 2007, 31, 1165–1173. [Google Scholar] [CrossRef]
- Quiroga, A.G.; Perez, J.M.; Lopez-Solera, I.; Montero, E.I.; Masaguer, J.R.; Alonso, C.; Navarro-Ranninger, C. Binuclear chlo-ro-bridged palladated and platinated complexes derived from p-isopropylbenzaldehyde thiosemicarbazone with cytotoxicity against cisplatin resistant tumor cell lines. J. Inorg. Biochem. 1998, 69, 275–281. [Google Scholar] [CrossRef]
- Rosu, T.; Pahontu, E.; Pasculescu, S.; Georgescu, R.; Stanica, N.; Curaj, A.; Popescu, A.; Leabu, M. Synthesis, characterization antibacterial and antiproliferative activity of novel Cu(II) and Pd(II) complexes with 2-hydroxy-8-R-tricyclo[7.3.1.0.2,7]tridecane-13-one thiosemicarbazone. Eur. J. Med. Chem. 2010, 45, 1627–1634. [Google Scholar] [CrossRef]
- Kovala-Demertzi, D.; Demertzis, M.A.; Filiou, E.; Pantazaki, A.A.; Yadav, P.N.; Miller, J.R.; Zheng, Y.; Kyriakidis, D.A. Platinum(II) and palladium(II) complexes with 2-acetyl pyridine 4N-ethyl thiosemicarbazone able to overcome the cis-platin resistance. Structure, antibacterial activity and DNA strand breakage. Biometals 2003, 16, 411–418. [Google Scholar] [CrossRef]
- Đilović, I.; Rubčić, M.; Vrdoljak, V.; Pavelić, S.K.; Kralj, M.; Piantanida, I.; Cindrić, M. Novel thiosemicarbazone derivatives as potential antitumor agents: Synthesis, physicochemical and structural properties, DNA interactions and antiproliferative activity. Bioorg. Med. Chem. 2008, 16, 5189–5198. [Google Scholar] [CrossRef] [PubMed]
- Finch, A.R.; Liu, M.-C.; Grill, S.P.; Rose, W.C.; Loomis, R.; Vasquez, K.M.; Cheng, Y.-C.; Sartorelli, A.C. Triapine (3-aminopyridine-2-carboxaldehyde- thiosemicarbazone): A potent inhibitor of ribonucleotide reductase activity with broad spectrum antitumor activity. Biochem. Pharmacol. 2000, 59, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Domagk, G.; Behnisch, R.; Mietzsch, F.; Schmidt, H. On a new class of compounds effective in vitro against tubercle bacilli. Naturwissenschaften 1946, 56, 315. [Google Scholar] [CrossRef]
- Kasuga, N.C.; Sekino, K.; Ishikawa, M.; Honda, A.; Yokoyama, M.; Nakano, S.; Shimada, N.; Koumo, C.; Nomiya, K. Synthesis, structural characterization and antimicrobial activities of 12 zinc(II) complexes with four thiosemicarbazone and two semi-carbazone ligands. J. Inorg. Biochem. 2003, 96, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Feun, L.; Modiano, M.; Lee, K.; Mao, J.; Marini, A.; Savaraj, N.; Plezia, P.; Almassian, B.; Colacino, E.; Fischer, J.; et al. Phase I and pharmacokinetic study of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP) using a single intravenous dose schedule. Cancer Chemother. Pharmacol. 2002, 50, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Bharti, N.; Husain, K.; Garza, M.G.; Cruz-Vega, E.D.; Castro-Garza, J.; Mata-Cardenas, B.D.; Naqvi, F.; Azam, A. Synthesis and in vitro antiprotozoal activity of 5-nitrothiophene-2-carboxaldehyde thiosemicarbazone derivatives. Bioorg. Med. Chem. Lett. 2002, 12, 3475–3478. [Google Scholar] [CrossRef]
- De Oliveira, R.B.; De Souza-Fagundes, E.M.; Soares, R.P.; Andrade, A.; Krettli, A.U.; Zani, C.L. Synthesis and antimalarial activity of semicarbazone and thiosemicarbazone derivatives. Eur. J. Med. Chem. 2008, 43, 1983–1988. [Google Scholar] [CrossRef]
- Abid, M.; Agarwal, S.M.; Azam, A. Synthesis and antiamoebic activity of metronidazole thiosemicarbazone analogues. Eur. J. Med. Chem. 2008, 43, 2035–2039. [Google Scholar] [CrossRef]
- Quenelle, D.C.; Keith, K.A.; Kern, E.R. In vitro and in vivo evaluation of isatin-beta-thiosemicarbazone and marboran against vaccinia and cowpox virus infections. Antivir. Res. 2006, 71, 24e30. [Google Scholar] [CrossRef]
- Vieites, M.L.; Otero, D.; Santos, J.; Toloza, R.; Figueroa, E.; Normbuena, C.; Olea Azar, G.; Aguirre, H.; Cerecetto, M.; Gonzalez, A.; et al. Gambino, Platinum(II) metal complexes as potential anti-Trypanosoma cruzi agents. J. Inorg. Biochem. 2008, 102, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Yogeeswari, P.; Sriram, D.; Jit, L.R.J.S.; Kumar, S.S.; Stables, J.P. Anticonvulsant and neurotoxicity evaluation of some 6-chlorobenzothiazolyl-2-thiosemicarbazones. Eur. J. Med. Chem. 2002, 37, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Vieites, M.; Otero, L.; Santos, D.; Olea-Azar, C.; Norambuena, E.; Aguirre, G.; Cerecetto, H.; González, M.; Kemmerling, U.; Morello, A.; et al. Platinum-based complexes of bioactive 3-(5-nitrofuryl)acroleine thiosemicarbazones showing anti-Trypanosoma cruzi activity. J. Inorg. Biochem. 2009, 103, 411–418. [Google Scholar] [CrossRef]
- Sodhi, R.K. Metal Complexes in Medicine: An Overview and Update from Drug Design Perspective. Cancer Ther. Oncol. Int. J. 2019, 14, 1–8. [Google Scholar] [CrossRef]
- Karaküçük-İyidoğan, A.; Taşdemir, D.; Oruç-Emre, E.E.; Balzarini, J. Novel platinum(II) and palla-dium(II) complexes of thiosemicarbazones derived from 5-substitutedthiophene-2-carboxaldehydes and their antiviral and cytotoxic activities. Eur. J. Med. Chem. 2011, 46, 5616–5624. [Google Scholar] [CrossRef]
- Diaz, R.S.; Shytaj, I.L.; Giron, L.B.; Obermaier, B.; Della Libera, E.; Galinskas, J.; Dias, D.; Hunter, J.; Janini, M.; Gossen, G.; et al. Potential impact of the antirheumatic agent auranofin on proviral HIV-1 DNA in individuals under intensified antiretroviral therapy: Results from a randomised clinical trial. Int. J. Antimicrobial. Agents 2019, 54, 592–600. [Google Scholar] [CrossRef]
- Savarino, A.; Shytaj, I.L. Chloroquine and beyond: Explor ing antirheumatic drugs to reduce immune hyperactivation in HIV/AIDS. Retrovirology 2015, 12, 51. [Google Scholar] [CrossRef]
- Blindauer, C.A.; Sigel, A.; Operschall, B.P.; Griesser, R.; Holy, A.; Sigel, A. Extent of Intramolecular π-stacks in Aqueous Solution in Mixed-Ligand Copper(II) Complexes Formed by Heteroaromatic Amines and the Anticancer and Antivirally Active 9-[2-(Phosphonomethoxy)Ethyl]Guanine (Pmeg). A Comparison with Related Acyclic Nucleotide Analogues. Polyhedron 2016, 103, 248–260. [Google Scholar]
- Nourian, A.; Khalili, H. Sofosbuvir as a potential option for the treatment of COVID-19. Acta Biomed. 2020, 91, 239–241. [Google Scholar]
- Carcelli, M.; Fisicaro, E.; Compari, C.; Contardi, L.; Rogolino, D.; Solinas, C.; Stevaert, A.; Naesens, L. Antiviral activity and metal ion-binding properties of some 2-hydroxy-3-methoxyphenyl acylhydrazones. Biometals 2017, 31, 81–89. [Google Scholar] [CrossRef]
- Nagaj, J.; Starosta, R.; Jezowska-Bojczuk, M. Acid–base characterization, coordination properties towards copper(II) ions and DNA interaction studies of ribavirin, an antiviral drug. J. Inorg. Biochem. 2015, 142, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Kirin, V.P.; Demkin, A.G.; Sukhikh, T.S.; Ilyicheva, T.N.; Maksakov, V.A. Cobalt complexes with biguanide deriva-tives—Synthesis, structure and antiviral activity. J. Mol. Struct. 2022, 1250, 131486. [Google Scholar] [CrossRef]
- Wanga, C.; Zhanga, R.; Weia, X.; Lva, M.; Jiang, Z. Metalloimmunology: The metal ion-controlled immunity. Adv. Immunol. 2020, 145, 187–241. [Google Scholar]
- Reed, S.G.; Orr, M.T.; Fox, C.B. Key roles of adjuvants in modern vaccines. Nat. Med. 2013, 19, 1597–1608. [Google Scholar] [CrossRef]
- Mohamed, G.G.; El-Sherif, A.A.; Saad, M.A.; El-Sawy, S.E.; Morgan, S.M. Mixed-ligand complex formation of tenoxicam drug with some transition metal ions in presence of valine: Synthesis, characterization, molecular docking, potentiometric and evaluation of the humeral immune response of calves. J. Mol. Liq. 2016, 223, 1311–1332. [Google Scholar] [CrossRef]
- El-Sonbati, A.; Diab, M.; Mohamed, G.; Saad, M.; Morgan, S.; El-Sawy, S. Polymer complexes. LXXVII. Synthesis, characterization, spectroscopic studies and immune response in cattle of quinoline polymer complexes. Appl. Organomet. Chem. 2019, 33, e4973. [Google Scholar] [CrossRef]
- Behzadi, M.; Vakili, B.; Ebrahiminezhad, A.; Nezafat, N. Iron nanoparticles as novel vaccine adjuvants. Eur. J. Pharm. Sci. 2021, 159, 105718. [Google Scholar] [CrossRef]
- Dykman, L.A. Gold nanoparticles for preparation of antibodies and vaccines against infectious diseases. Expert Rev. Vaccines 2020, 19, 465–477. [Google Scholar] [CrossRef]
- Dykman, L.A.; Khlebtsov, N.G. Immunological properties of gold nanoparticles. Chem. Sci. 2021, 8, 1719–1735. [Google Scholar] [CrossRef]
- Li, Y.; Jin, Q.; Ding, P.; Zhou, W.; Chai, Y.; Li, X.; Wang, Y.; Zhang, G.-P. Gold nanoparticles enhance immune responses in mice against recombinant classical swine fever virus E2 protein. Biotechnol. Lett. 2020, 42, 1169–1180. [Google Scholar] [CrossRef]
- Neto, L.M.M.; Kipnis, A.; Junqueira-Kipnis, A.P. Role of Metallic Nanoparticles in Vaccinology: Implications for Infectious Disease Vaccine Development. Front. Immunol. 2017, 8, 239. [Google Scholar] [CrossRef]
- Sengupta, A.; Azharuddin, M.; Al-Otaibi, N.; Hinkula, J. Efficacy and Immune Response Elicited by Gold Nanoparticle- Based Nanovaccines against Infectious Diseases. Vaccines 2022, 10, 505. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; Sun, S.; Chen, H.; Huang, J.; Du, P.; Dong, H.; Xu, X.; Mu, S.; Zhang, Z.; Guo, H. Golden-star nanoparticles as ad-juvant effectively promotes immune response to foot-and-mouth disease virus-like particles vaccine. Vaccines 2018, 36, 6752–6760. [Google Scholar] [CrossRef]
- Esquezaro, P.G.; Manzano, C.M.; Nakahata, D.H.; Santos, I.A.; Ruiz, U.E.; Santiago, M.B.; Silva, N.B.; Martins, C.H.; Pereira, D.H.; Bergamini, F.R.G.; et al. Synthesis, spectroscopic characterization and in vitro antibacterial and antiviral activities of novel silver(I) complexes with mafenide and ethyl-mafenide. J. Mol. Struct. 2021, 1246, 131261. [Google Scholar] [CrossRef]
- Maldonado, N.; Amo-Ochoa, P. The role of coordination compounds in virus research. Different approaches and trends. Dalton Trans. 2021, 50, 2310–2323. [Google Scholar] [CrossRef]
- Zoppi, C.; Messori, L.; Pratesi, A. ESI MS studies highlight the selective interaction of Auranofin with protein free thiols. Dalton Trans. 2020, 49, 5906–5913. [Google Scholar] [CrossRef]
- Kowalczyk, M.; Golonko, A.; Swisłocka, R.; Kalinowska, M.; Parcheta, M.; Swiergiel, A.; Lewandowski, W. Drug Design Strategies’ for the Treatment of Viral Disease. Plant Phenolic Compounds and Their Derivatives. Front. Pharmacol. 2021, 12, 709104. [Google Scholar] [CrossRef]
- Pettinari, C.; Pettinari, R.; Di Nicola, C.; Tombesi, A.; Scuri, S.; Marchetti, F. Antimicrobial MOFs. Coord. Chem. Rev. 2021, 446, 214121. [Google Scholar] [CrossRef]
- Niikura, K.; Matsunaga, T.; Suzuki, T.; Kobayashi, S.; Yamaguchi, H.; Orba, Y.; Kawaguchi, A.; Hasegawa, H.; Kajino, K.; Ninomiya, T.; et al. Gold Nanoparticles as a Vaccine Platform: Influence of Size and Shape on Immunological Responses in Vitro and in Vivo. ACS Nano 2013, 7, 3926–3938. [Google Scholar] [CrossRef]
- Staroverov, S.A.; Vidyasheva, I.V.; Gabalov, K.P.; Vasilenko, O.A.; Laskavyi, V.N.; Dykman, L.A. Immunostimulatory Effect of Gold Nanoparticles Conjugated with Transmissible Gastroenteritis Virus. Immunol. Microbiol. 2011, 151, 1350–1358. [Google Scholar] [CrossRef]
- Farfán-Castro, S.; García-Soto, M.J.; Comas-García, M.; Arévalo-Villalobos, J.-I.; Palestino, G.; González-Ortega, O.; Rosales Mendoza, S. Synthesis and immunogenicity assessment of a gold nanoparticle conjugate for the delivery of a peptide from SARS-CoV-2. Nanomedicine 2021, 34, 102372. [Google Scholar] [CrossRef]
- Sekimukai, H.; Iwata-Yoshikawa, N.; Fukushi, S.; Tani, H.; Kataoka, M.; Suzuki, T.; Hasegawa, H.; Niikura, K.; Arai, K.; Nagata, N. Gold nanoparticle-adiuvanted S protein induces a strong antigen-specific-related coronavirus infection, but fails to induce protective antibodies and limit eosinophilic infiltration in lungs. Microbiol. Immunol. 2020, 64, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Garrido, C.; Simpson, A.C.; Dahl, N.P.; Bresee, J.; Whitehead, D.C.; Lindsey, A.E.; Harris, T.L.; Smith, C.A.; Carter, C.J.; Feldheim, D.L.; et al. Gold nanoparticles to improve HIV drug delivery. Futur. Med. Chem. 2015, 7, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-S.; Hung, Y.-C.; Lin, W.-H.; Huang, G.S. Assessment of gold nanoparticles as a size-dependent vaccine carrier for enhancing the antibody response against synthetic foot-and-mouth disease virus peptide. Nanotechnology 2010, 21, 195101. [Google Scholar] [CrossRef]
- Zazo, H.; Colino, C.I.; Warzecha, K.T.; Hoss, M.; Gbureck, U.; Trautwein, C.; Tacke, F.; Lanao, J.M.; Bartneck, M. Gold Nanocarriers for Macrophage-Targeted Therapy of Human Immunodeficiency Virus. Macromol. Biosci. 2016, 17, 1600359. [Google Scholar] [CrossRef]
- Paul, A.M.; Shi, Y.; Acharya, D.; Douglas, J.R.; Cooley, A.; Anderson, J.F.; Huang, F.; Bai, F. Delivery of antiviral small inter-fering RNA with gold nanoparticles inhibits dengue virus infection in vitro. J. Gen. Virol. 2014, 95, 1712–1722. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sébrié, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous metal–organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 2009, 9, 172–178. [Google Scholar] [CrossRef]
- Yan, W.; Jain, A.; O’Carra, R.; Woodward, J.G.; Li, W.; Li, G.; Nath, A.; Mumper, R.J. Lipid nanoparticles with accessible nickel as a vaccine delivery system for single and multiple his-tagged HIV antigens. Res. Palliat. Care 2009, 1, 1–11. [Google Scholar]
- Zachar, O. Nanomedicine formulations for respiratory infections by inhalation delivery: Covid-19 and beyond. Med. Hypotheses 2022, 159, 110753. [Google Scholar] [CrossRef]
- Roome, T.; Razzak, A. Clinical implications of metals-based drug-delivery systems. In Metal Nanoparticles for Drug Delivery and Diagnostic Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 237–258. [Google Scholar]
- Maduray, K.; Parboosing, R. Metal Nanoparticles: A Promising Treatment for Viral and Arboviral Infections. Biol. Trace Element Res. 2020, 199, 3159–3176. [Google Scholar] [CrossRef]
- Tortella, G.; Rubilar, O.; Fincheira, P.; Pieretti, J.; Duran, P.; Lourenço, I.; Seabra, A. Bactericidal and Virucidal Activities of Biogenic Metal-Based Nanoparticles: Advances and Perspectives. Antibiotics 2021, 10, 783. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Ingle, A.P.; Gupta, I.; Brandelli, A. Bioactivity of noble metal nanoparticles decorated with biopolymers and their application in drug delivery. Int. J. Pharm. 2015, 496, 159–172. [Google Scholar] [CrossRef]
- Yang, J.; Yue, L.; Yang, Z.; Miao, Y.; Ouyang, R.; Hu, Y. Metal-Based Nanomaterials: Work as Drugs and Carriers against Viral Infections. Nanomaterials 2021, 11, 2129. [Google Scholar] [CrossRef]
- Bibi, S.; Urrehman, S.; Khalid, L.; Yaseen, M.; Khan, A.Q.; Jia, R. Metal doped fullerene complexes as promising drug delivery materials against COVID-19. Chem. Pap. 2021, 75, 6487–6497. [Google Scholar] [CrossRef]
- Fischer, N.O.; Blanchette, C.D.; Chromy, B.A.; Kuhn, E.A.; Segelke, B.W.; Corzett, M.; Bench, G.; Mason, P.W.; Hoeprich, P.D. Immobilization of His-Tagged Proteins on Nickel-Chelating Nanolipoprotein Particles. Bioconjugate Chem. 2009, 20, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Halimi, V.; Daci, A.; Stojanovska, S.; Panovska-Stavridis, I.; Stevanovic, M.; Filipce, V.; Grozdanova, A. Current regulatory approaches for accessing potential COVID-19 therapies. J. Pharm. Policy Pract. 2020, 13, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Naureen, B.; Miana, G.; Shahid, K.; Asghar, M.; Tanveer, S.; Sarwar, A. Iron (III) and zinc (II) monodentate Schiff base metal complexes: Synthesis, characterisation and biological activities. J. Mol. Struct. 2021, 1231, 129946. [Google Scholar] [CrossRef]
- Tripathi, K. A review–can metal ions be incorporated into drugs? Asian J. Res. Chem. 2009, 2, 14–18. [Google Scholar]
- Vlasiou, M.C.; Pafti, K.S. Screening possible drug molecules for Covid-19. The example of vanadium (III/IV/V) complex molecules with computational chemistry and molecular docking. Comput. Toxicol. 2021, 18, 100157. [Google Scholar] [CrossRef]
- Ali, A.; Sepay, N.; Afzal, M.; Alarifi, A.; Shahid, M.; Ahmad, M. Molecular designing, crystal structure determination and in silico screening of copper(II) complexes bearing 8-hydroxyquinoline derivatives as anti-COVID-19. Bioorg. Chem. 2021, 110, 104772. [Google Scholar] [CrossRef]
- Almalki, S.A.; Bawazeer, T.M.; Asghar, B.; Alharbi, A.; Aljohani, M.M.; Khalifa, M.E.; El-Metwaly, N. Synthesis and characterization of new thiazole-based Co (II) and Cu (II) complexes; therapeutic function of thiazole towards COVID-19 in comparing to current antivirals in treatment protocol. J. Mol. Struct. 2021, 2021, 130961. [Google Scholar] [CrossRef] [PubMed]
- Refat, M.S.; Gaber, A.; Alsanie, W.F. Utilization and simulation of innovative new binuclear Co (ii), Ni (ii), Cu (ii), and Zn (ii) diimine Schiff base complexes in sterilization and coronavirus resistance (Covid-19). Open Chem. 2021, 19, 772–784. [Google Scholar] [CrossRef]
- Rad, A.S.; Ardjmand, M.; Esfahani, M.R.; Khodashenas, B. DFT calculations towards the geometry optimization, electronic structure, infrared spectroscopy and UV–vis analyses of Favipiravir adsorption on the first-row transition metals doped fullerenes; a new strategy for COVID-19 therapy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 247, 119082. [Google Scholar] [CrossRef] [PubMed]
- Refat, M.S.; Altalhi, T.; Bakare, S.B.; Al-Hazmi, G.H.; Alam, K. New Cr(III), Mn(II), Fe(III), Co(II), Ni(II), Zn(II), Cd(II), and Hg(II) Gibberellate Complexes: Synthesis, Structure, and Inhibitory Activity Against COVID-19 Protease. Russ. J. Gen. Chem. 2021, 91, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.G.; Omar, M.M.; Ahmed, Y.M. Metal complexes of tridentate schiff base: Synthesis, characterization, biological activity and molecular docking studies with COVID-19 protein receptor. J. Inorg. Gen. Chem. 2021, 647, 2201–2218. [Google Scholar] [CrossRef] [PubMed]
- Hecel, A.; Ostrowska, M.; Stokowa-Sołtys, K.; Wątły, J.; Dudek, D.; Miller, A.; Potocki, S.; Matera-Witkiewicz, A.; Dominguez-Martin, A.; Kozłowski, H.; et al. Zinc (II)—The overlooked éminence grise of chloroquine’s fight against COVID-19? Pharmaceuticals 2020, 13, 228. [Google Scholar] [CrossRef] [PubMed]
- Poupaert, J.H.; Aguida, B.; Hountondji, C. Study of the interaction of zinc cation with azithromycin and its significance in the COVID-19 treatment: A molecular approach. Open Biochem. J. 2020, 14, 33–40. [Google Scholar] [CrossRef]
- Marzo, T.; Messori, L. (A role for metal-based drugs in fghting COVID-19 infection? The case of auranofn. ACS Med. Chem. Lett. 2020, 11, 1067–1068. [Google Scholar] [CrossRef]
- Rothan, H.A.; Stone, S.; Natekar, J.; Kumari, P.; Arora, K.; Kumar, M. The FDA-approved gold drug auranofin inhibits novel coronavirus (SARS-COV-2) replication and attenuates inflammation in human cells. Virology 2020, 547, 7–11. [Google Scholar] [CrossRef]
- Gil-Moles, M.; Basu, U.; Büssing, R.; Hofmeister, H.; Türck, S.; Varchmin, A.; Ott, I. Gold metallodrugs to target coronavirus proteins: Inhibitory effects on the spike-ACE2 interaction and PLpro protease activity by auranofn and gold organometallics. Chem 2020, 26, 15140–15144. [Google Scholar] [CrossRef]
- Yang, N.; Tanner, J.A.; Zheng, B.-J.; Watt, R.M.; He, M.-L.; Lu, L.-Y.; Jiang, J.-Q.; Shum, K.-T.; Lin, Y.-P.; Wong, K.-L.; et al. Bismuth complexes inhibit the SARS coronavirus. Angew. Chem. Int. Ed. 2007, 46, 6464–6468. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Wang, R.; Chan, J.F.-W.; Zhang, A.J.; Cheng, T.; Chik, K.K.-H.; Ye, Z.-W.; Wang, S.; Lee, A.C.-Y.; Jin, L.; et al. Metallodrug ranitidine bismuth cit rate suppresses SARS-CoV-2 replication and relieves virus-associated pneumonia in Syrian hamsters. Nat. Microbiol. 2020, 11, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
- Frick, D.N. Helicases as antiviral drug targets. Drug News Perspect. 2003, 16, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.C.; Wolf, C.H.; Rubin, D.T. Temporal Improvement of a COVID-19-Positive Crohn’s Disease Patient Treated With Bismuth Subsalicylate. Am. J. Gastroenterol. 2020, 115, 1298. [Google Scholar] [CrossRef] [PubMed]
- Boros, E.; Dyson, P.J.; Gasser, G. Classification of metal-based drugs according to their mechanisms of action. Chem 2020, 6, 41–60. [Google Scholar] [CrossRef]
- Riccardi, L.; Genna, V.; De Vivo, M. Metal–ligand interactions in drug design. Nat. Rev. Chem. 2018, 2, 100–112. [Google Scholar] [CrossRef]
- Fuertes, M.A.; Castilla, J.; Alonso, C.; Pérez, J.M. Cisplatin biochemical mechanism of action: From cytotoxic ity to induction of cell death through interconnections between apoptotic and necrotic pathways. Curr. Med. Chem. 2003, 10, 257–266. [Google Scholar] [CrossRef]
- Harbut, M.B.; Vilchèze, C.; Luo, X.; Hensler, M.E.; Guo, H.; Yang, B.; Chatterjee, A.K.; Nizet, V.; Jacobs, W.R.; Schultz, P.G.; et al. Auranofn exerts broad-spectrum bactericidal activities by targeting thiol-redox homeostasis. Proc. Natl. Acad. Sci. USA 2015, 112, 4453–4458. [Google Scholar] [CrossRef]
- Roder, C.; Thomson, M.J. Auranofin: Repurposing an Old Drug for a Golden New Age. Drugs R&D 2015, 15, 13–20. [Google Scholar] [CrossRef]
- Walz, D.T.; DiMartino, M.J.; Griswold, D.E.; Intoccia, A.P.; Flanagan, T.L. Biologic actions and pharmacokinetic studies of auranofn. Am. J. Med. 1983, 75, 90–108. [Google Scholar] [CrossRef]
- Fung, T.S.; Liu, D.X. Coronavirus infection, ER stress, apoptosis and innate immunity. Front. Microbiol. 2014, 5, 296. [Google Scholar] [CrossRef] [PubMed]
- Siu, K.-L.; Chan, C.-P.; Kok, K.-H.; Woo, P.C.-Y.; Jin, D.-Y. Comparative analysis of the activation of unfolded protein response by spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus HKU1. Cell Biosci. 2014, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Rothan, H.A.; Kumar, M. Role of endoplasmic reticulum associated proteins in favivirus replication and assembly complexes. Pathogens 2019, 8, 148. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.-H.; Lee, M.-Y.; Park, S.-J.; Choi, J.-S.; Oh, M.-K.; Kim, I.-S. Auranofn blocks interleukin-6 signalling by inhibiting phosphorylation of JAK1 and STAT3. Immunology 2007, 122, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Carter, E.D. Oxidation-reduction reactions of metal ions. Environ. Health Perspect. 1995, 103, 17–19. [Google Scholar] [CrossRef]
- Nencioni, L.; Sgarbanti, R.; Amatore, D.; Checconi, P.; Celestino, I.; Limongi, D.; Anticoli, S.; Palamara, A.T.; Garaci, E. Intracellular Redox Signaling as Therapeutic Target for Novel Antiviral Strategy. Curr. Pharm. Des. 2011, 17, 3898–3904. [Google Scholar] [CrossRef]
- Khomich, O.A.; Kochetkov, S.N.; Bartosch, B.; Ivanov, A.V. Redox Biology of Respiratory Viral Infections. Viruses 2018, 10, 392. [Google Scholar] [CrossRef]
- Mahalingam, S.; Meanger, J.; Foster, P.S.; Lidbury, B.A. The viral manipulation of the host cellular and immune envi ronments to enhance propagation and survival: A focus on RNA viruses. J. Leukoc. Biol. 2002, 72, 429–439. [Google Scholar] [CrossRef]
- Gullberg, R.C.; Steel, J.J.; Moon, S.; Soltani, E.; Geiss, B.J. Oxidative stress influences positive strand RNA virus genome synthesis and capping. Virology 2015, 475, 219–229. [Google Scholar] [CrossRef]
- Chen, K.-K.; Minakuchi, M.; Wuputra, K.; Ku, C.-C.; Pan, J.-B.; Kuo, K.-K.; Lin, Y.-C.; Saito, S.; Lin, C.-S.; Yokoyama, K.K. Redox control in the pathophysiology of infuenza virus infection. BMC Microbiol. 2020, 20, 214. [Google Scholar] [CrossRef] [PubMed]
- Vlahos, R.; Sambas, J.; Bozinovski, S.; Broughton, B.R.S.; Drum Mond, G.R.; Selemidis, S. Inhibition of NOX2 oxi dase activity ameliorates infuenza A virus-induced lung infammation. PLoS Pathog. 2011, 7, e1001271. [Google Scholar] [CrossRef]
- Amatore, D.; Sgarbanti, R.; Aquilano, K.; Baldelli, S.; Limongi, D.; Civitelli, L.; Nencioni, L.; Garaci, E.; Ciriolo, M.R.; Palamara, A.T. Influenza virus replication in lung epithelial cells depends on redox-sensitive pathways activated byNOX4-derivedROS. Cell Microbiol. 2014, 17, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Nencioni, L.; De Chiara, G.; Sgarbanti, R.; Amatore, D.; Aquilano, K.; Marcocci, M.E.; Serafino, A.; Torcia, M.; Cozzolino, F.; Ciriolo, M.R.; et al. Bcl-2 Expression and p38MAPK Activity in Cells Infected with Influenza A Virus. J. Biol. Chem. 2009, 284, 16004–16015. [Google Scholar] [CrossRef] [PubMed]
- Damiano, S.; Sozio, C.; La Rosa, G.; Santillo, M. NOX-Dependent Signaling Dysregulation in Severe COVID-19: Clues to Effective Treatments. Front. Cell. Infect. Microbiol. 2020, 10, 608435. [Google Scholar] [CrossRef]
- McKenzie, L.K.; Bryant, H.E.; Weinstein, J.A. Transition metal complexes as photosensitisers in one- and two-photon photodynamic therapy. Coord. Chem. Rev. 2019, 379, 2–29. [Google Scholar] [CrossRef]
- Wiehe, A.; O’Brien, J.M.; Senge, M.O. Trends and targets in antiviral phototherapy. Photochem. Photobiol. Sci. 2019, 18, 2565–2612. [Google Scholar] [CrossRef]
- Dai, T.; Huang, Y.-Y.; Hamblin, M.R. Photodynamic therapy for localized infections—State of the Art. Photodiagnosis Photodyn. Ther. 2009, 6, 170–188. [Google Scholar] [CrossRef]
- Ichimura, H.; Yamaguchi, S.; Kojima, A.; Tanaka, T.; Niiya, K.; Takemori, M.; Hasegawa, K.; Nishimura, R. Eradication and reinfection of human papillomavirus after photodynamic therapy for cervical intraepithelial Neoplasia. Int. J. Clin. Oncol. 2003, 8, 322–325. [Google Scholar] [CrossRef]
- Tardivo, J.P.; Del Giglio, A.; Paschoal, L.H.; Baptista, M.S.; Baptista, M.S. New Photodynamic Therapy Protocol to Treat AIDS-Related Kaposi’s Sarcoma. Photomed. Laser Surg. 2006, 24, 528–531. [Google Scholar] [CrossRef]
- Käsermann, F.; Kempf, C. Photodynamic inactivation of enveloped viruses by buckminsterfullerene. Antiviral. Res. 1997, 34, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.; Benvenuto, D.; Giovanetti, M.; Angeletti, S.; Ciccozzi, M.; Pascarella, S. Sars-CoV-2 envelope and mem brane proteins: Structural diferences linked to virus char acteristics? Biomed. Res. Int. 2020, 2020, e4389089. [Google Scholar] [CrossRef]
- Allison, R.R.; Moghissi, K. Photodynamic therapy (PDT): PDT mechanisms. Clin. Endosc. 2013, 46, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Svyatchenko, V.A.; Nikonov, S.D.; Mayorov, A.P.; Gelfond, M.L.; Loktev, V.B. Antiviral photodynamic therapy: Inactivation and inhibition of SARS-CoV-2 in vitro using methylene blue and radachlorin. Photodiagnosis Photodyn. Ther. 2021, 33, 102112. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-Y.; Gu, M.; Leung, J.-K.; Li, X.; Yuan, Y.; Shen, C.; Wang, L.; Zhao, E.; Chen, S. A Membrane-Targeting Photosensitizer with Aggregation-Induced Emission Characteristics for Highly Efficient Photodynamic Combat of Human Coronaviruses. Small 2021, 17, 2101770. [Google Scholar] [CrossRef] [PubMed]
- Keil, S.D.; Bowen, R.; Marschner, S. Inactivation of Middle East respiratory syndrome coronavirus (MERS-CoV) in plasma products using a riboflavin-based and ultraviolet light-based photochemical treatment. Transfusion 2016, 56, 2948–2952. [Google Scholar] [CrossRef] [PubMed]
- Ruane, P.H.; Edrich, R.; Gampp, D.; Keil, S.D.; Leonard, R.L.; Goodrich, R.P. Photochemical inactivation of selected viruses and bacteria in platelet concentrates using riboflavin and light. Transfusion 2004, 44, 877–885. [Google Scholar] [CrossRef]
- Blanco, K.C.; Inada, N.M.; Carbinatto, F.M.; Giusti, A.L.; Bagnato, V.S. Treatment of recurrent pharyngotonsillitis by photodynamic therapy. Photodiagnosis Photodyn. Ther. 2017, 18, 138–139. [Google Scholar] [CrossRef]
- Kassab, G.; Gerald, M.C.; Inada, N.M.; Achilles, A.E.; Guerra, V.G.; Bagnato, V.S. Nebulization as a tool for photosen sitizer de-livery to the respiratory tract. J. Biophoton. 2019, 12, e201800189. [Google Scholar] [CrossRef]
- Dias, L.D.; Blanco, K.C.; Bagnato, V.S. COVID-19: Beyond the virus. The use of photodynamic therapy for the treat ment of infections in the respiratory tract. Photodiagnosis Photodyn. Ther. 2020, 31, 101804. [Google Scholar] [CrossRef]
- Moghissi, K.; Dixon, K.; Gibbins, S. Does PDT have potential in treating COVID 19 patients? Photodiagnosis Photodyn. Ther. 2020, 31, 101889. [Google Scholar] [CrossRef]
- Ivan Lozada, M.; Daniela Torres, L.; Maria Bolaño, R.; Luis Moscote, S. High mutation rate in SARS-CoV-2: Will it hit us the same way forever? J. Infect. Dis. Epidemiol. 2020, 6, 371–384. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Barry, N.P.E.; Sadler, P.J. Exploration of the medical periodic table: Towards new targets. Chem. Commun. 2013, 49, 5106–5131. [Google Scholar] [CrossRef]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef]
- Lengfelder, E.; Hofmann, W.-K.; Nowak, A.D. Impact of arsenic trioxide in the treatment of acute promyelocytic leukemia. Leukemia 2011, 26, 433–442. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
