Outstanding Electrochemical Performance of Ni-Rich Concentration-Gradient Cathode Material LiNi0.9Co0.083Mn0.017O2 for Lithium-Ion Batteries
Abstract
1. Introduction
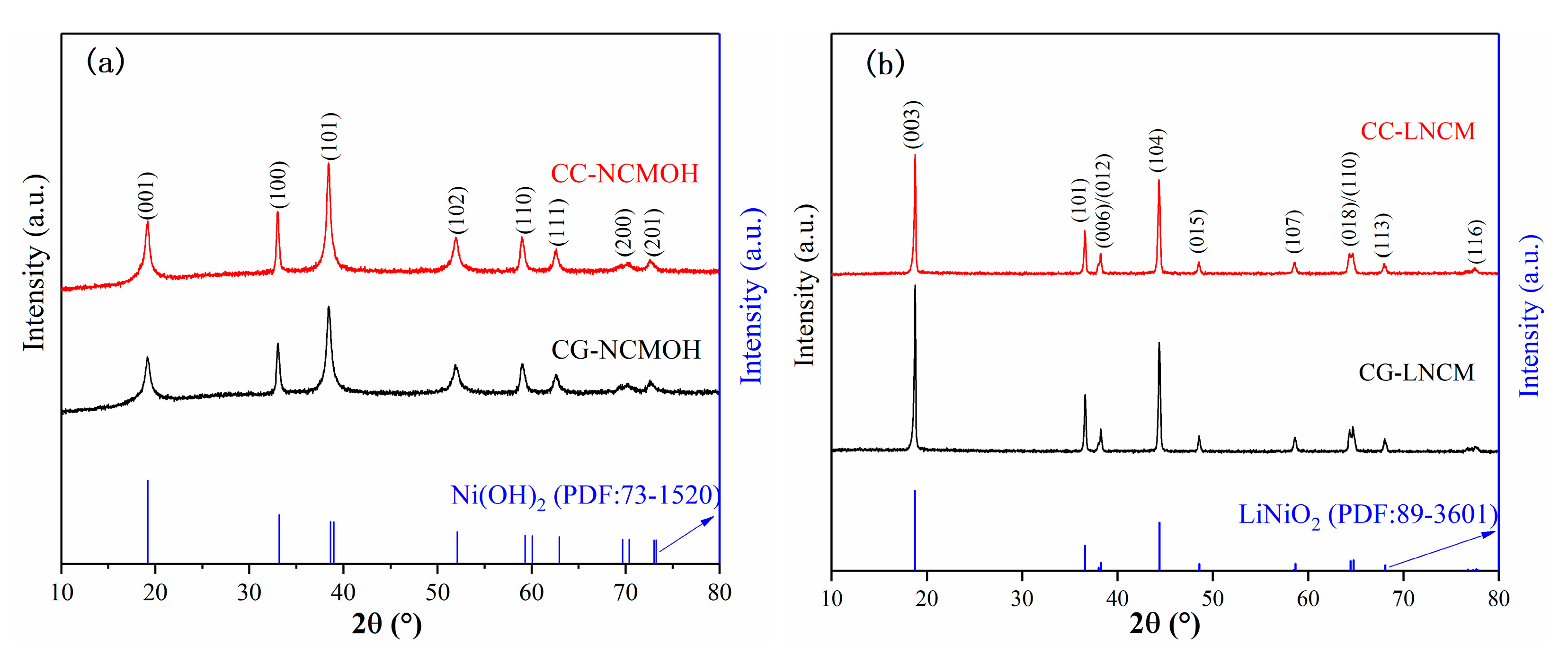
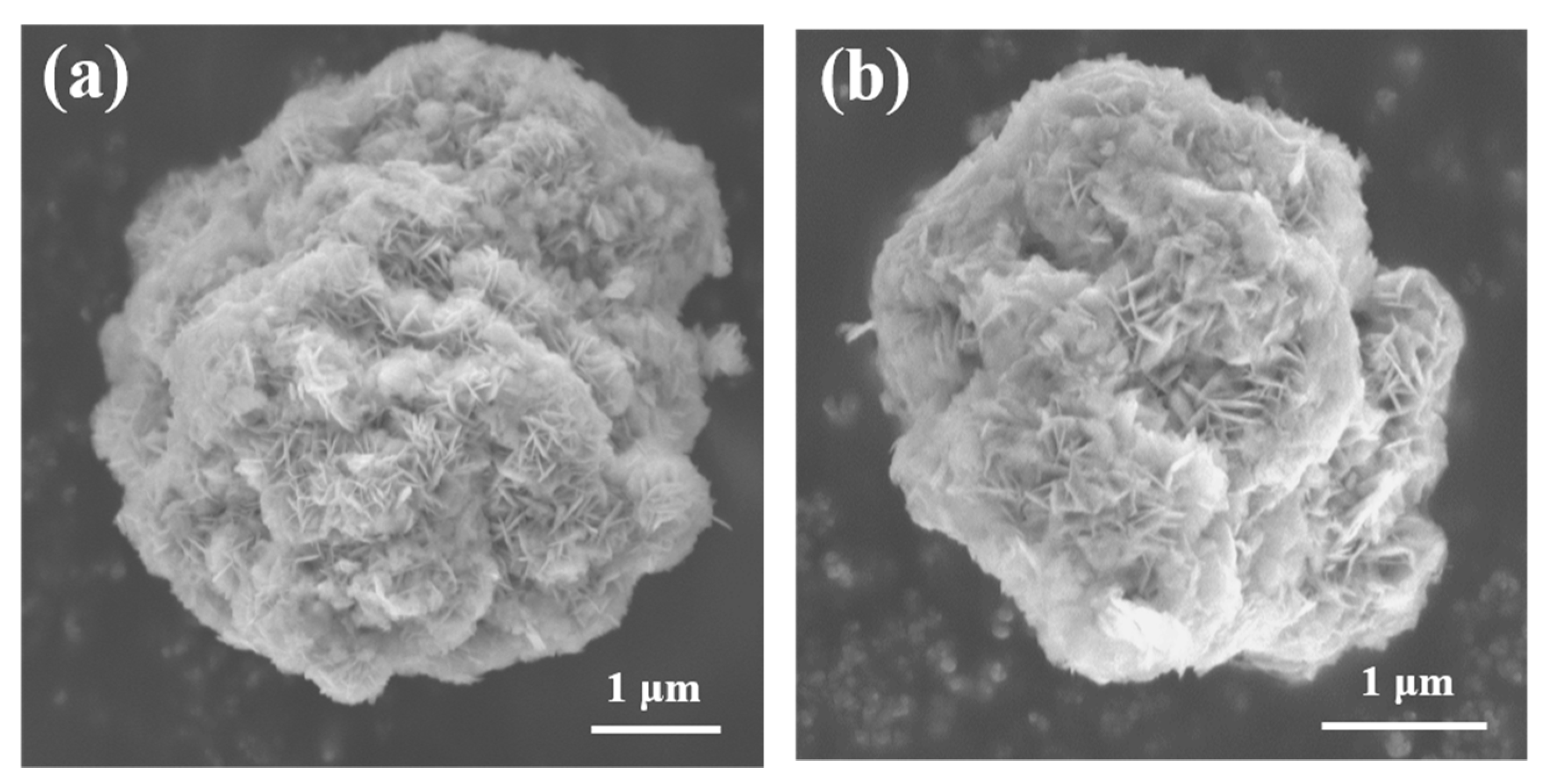
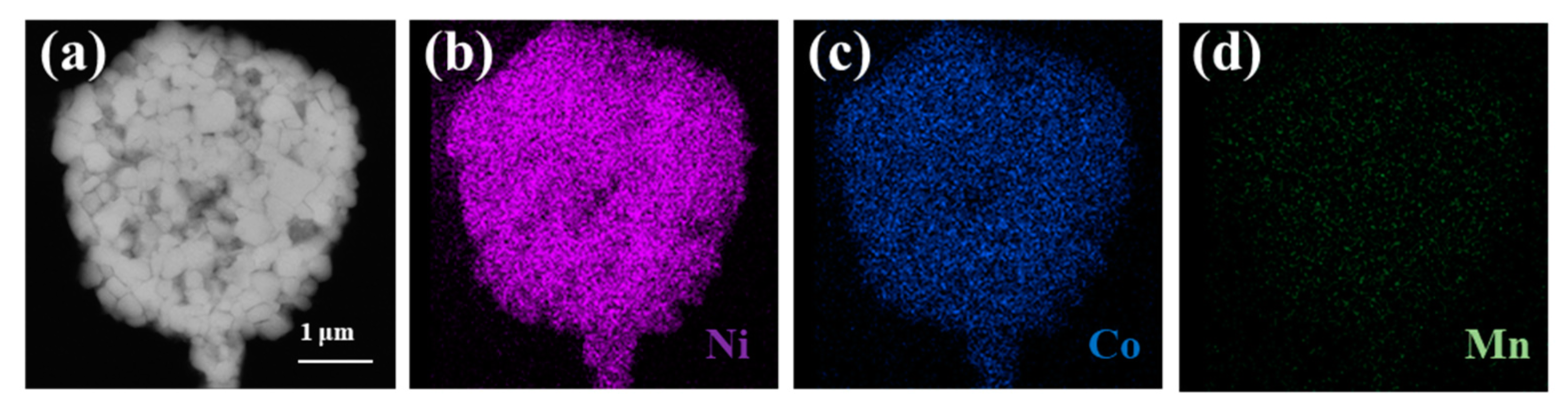
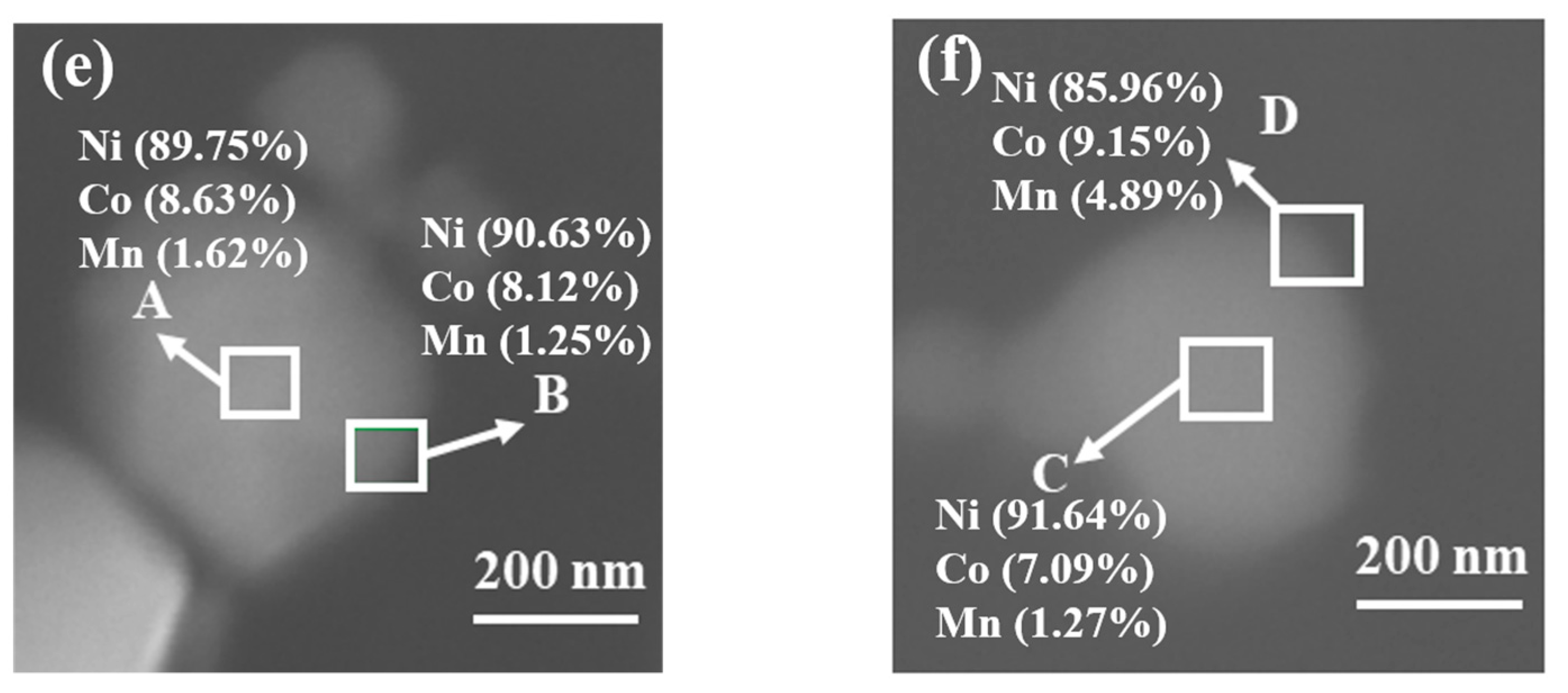
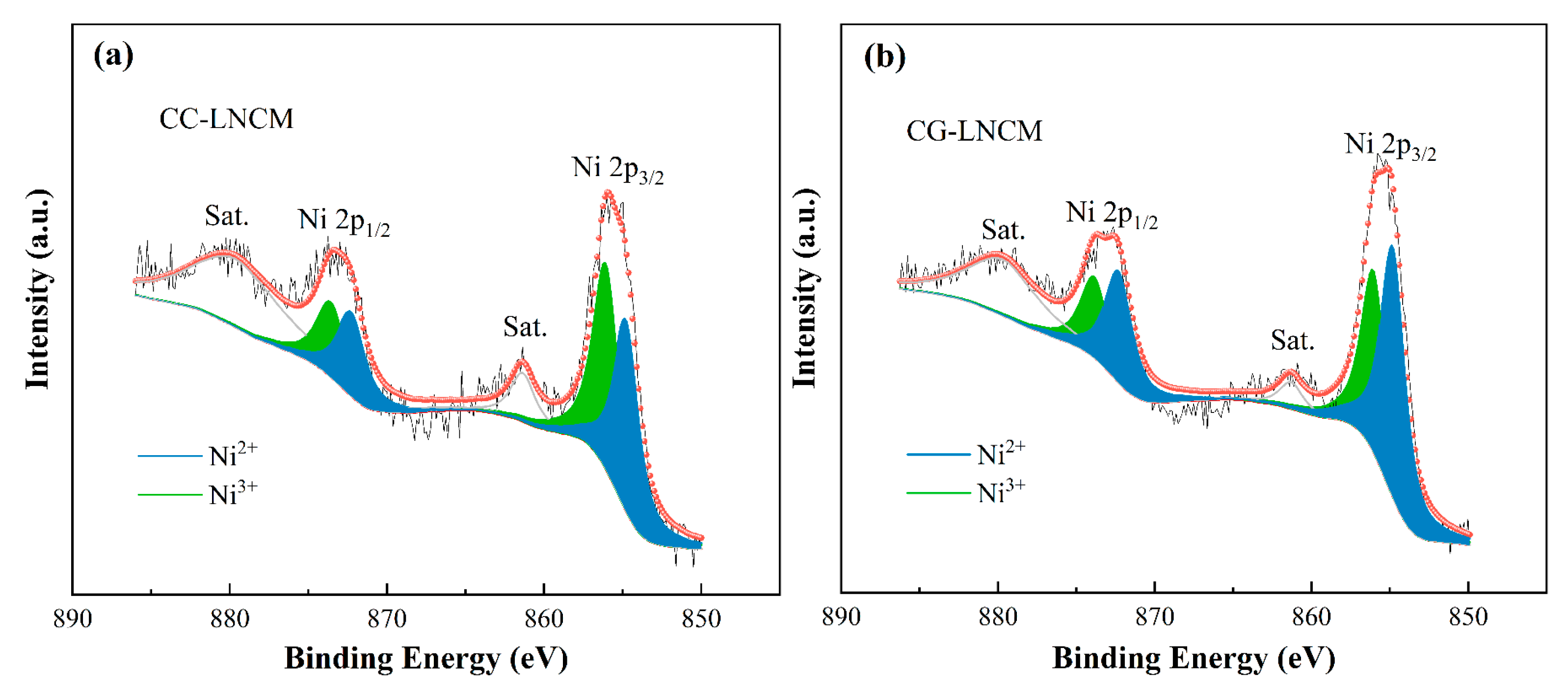
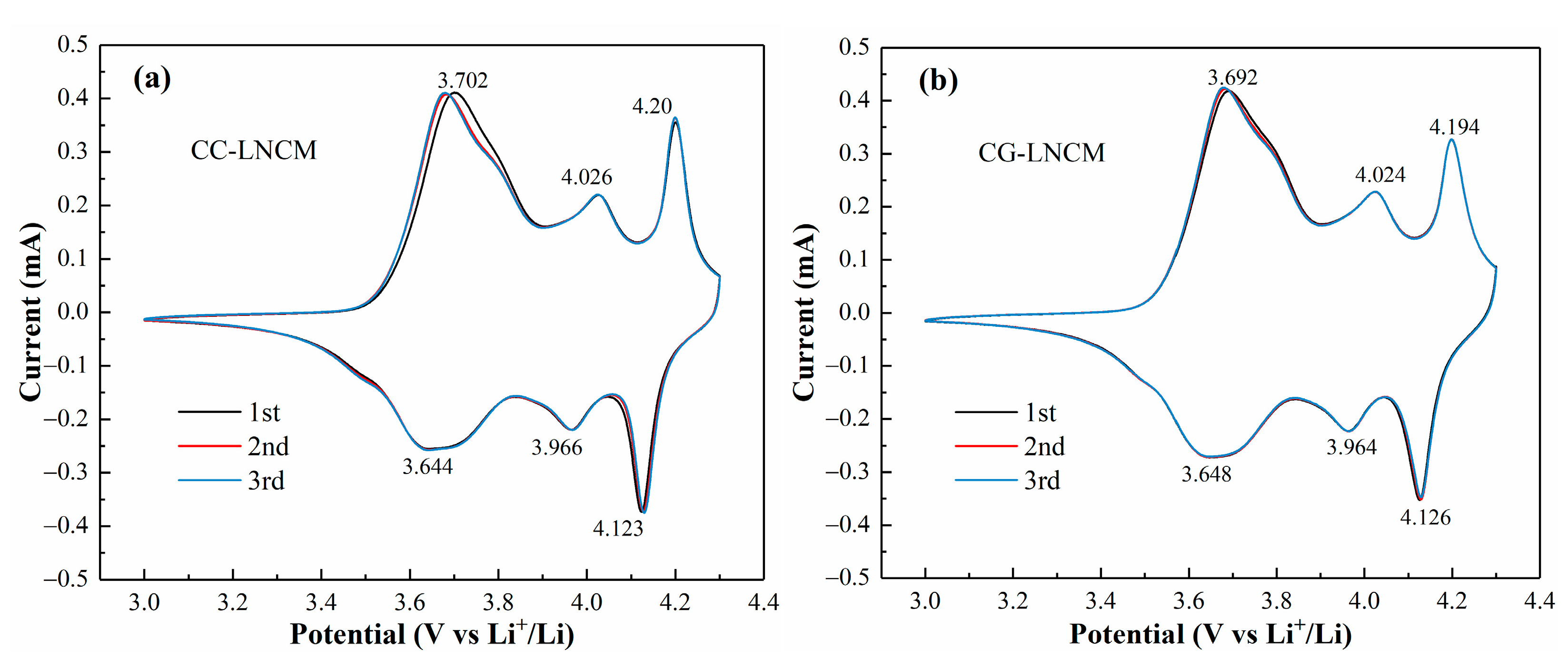
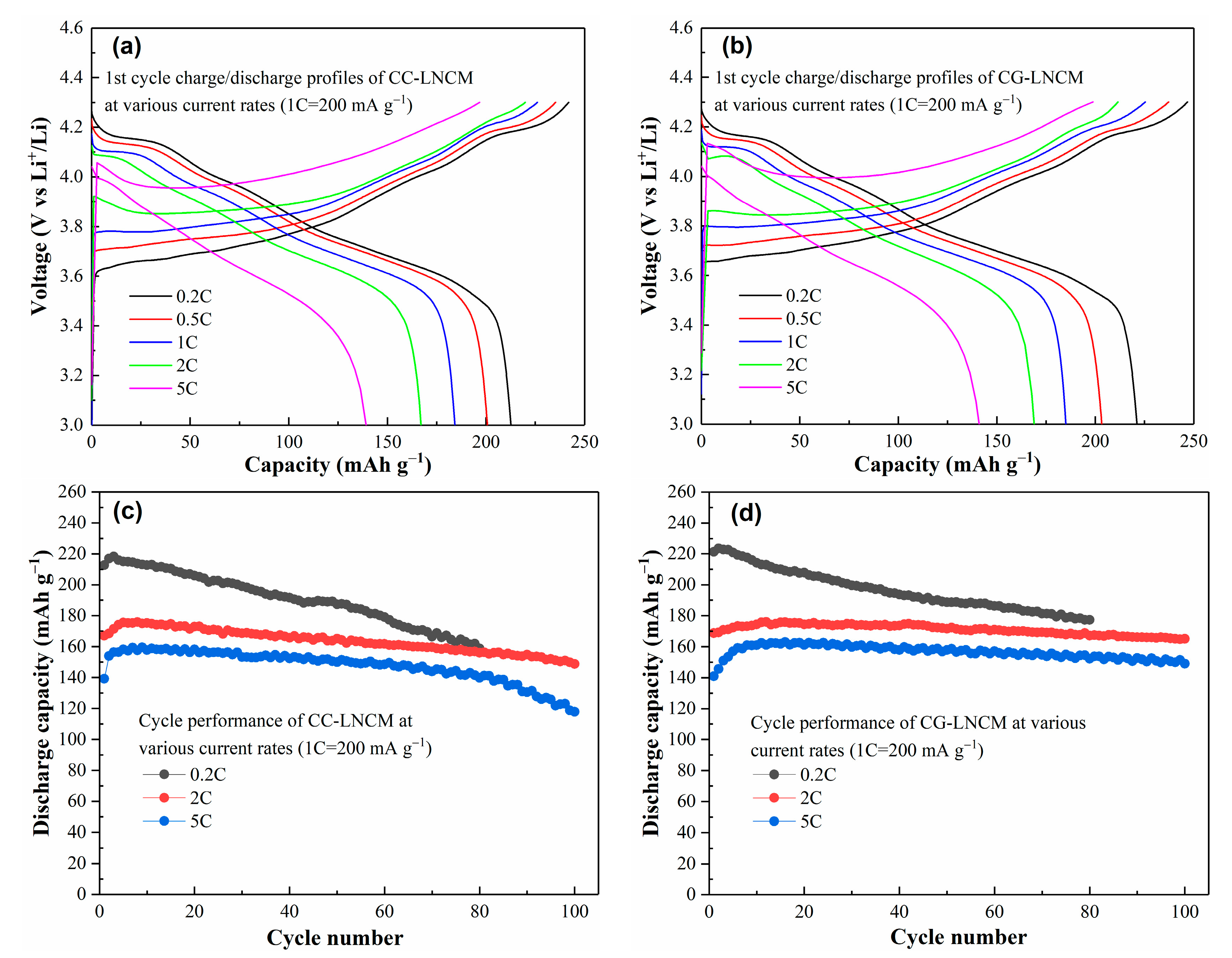
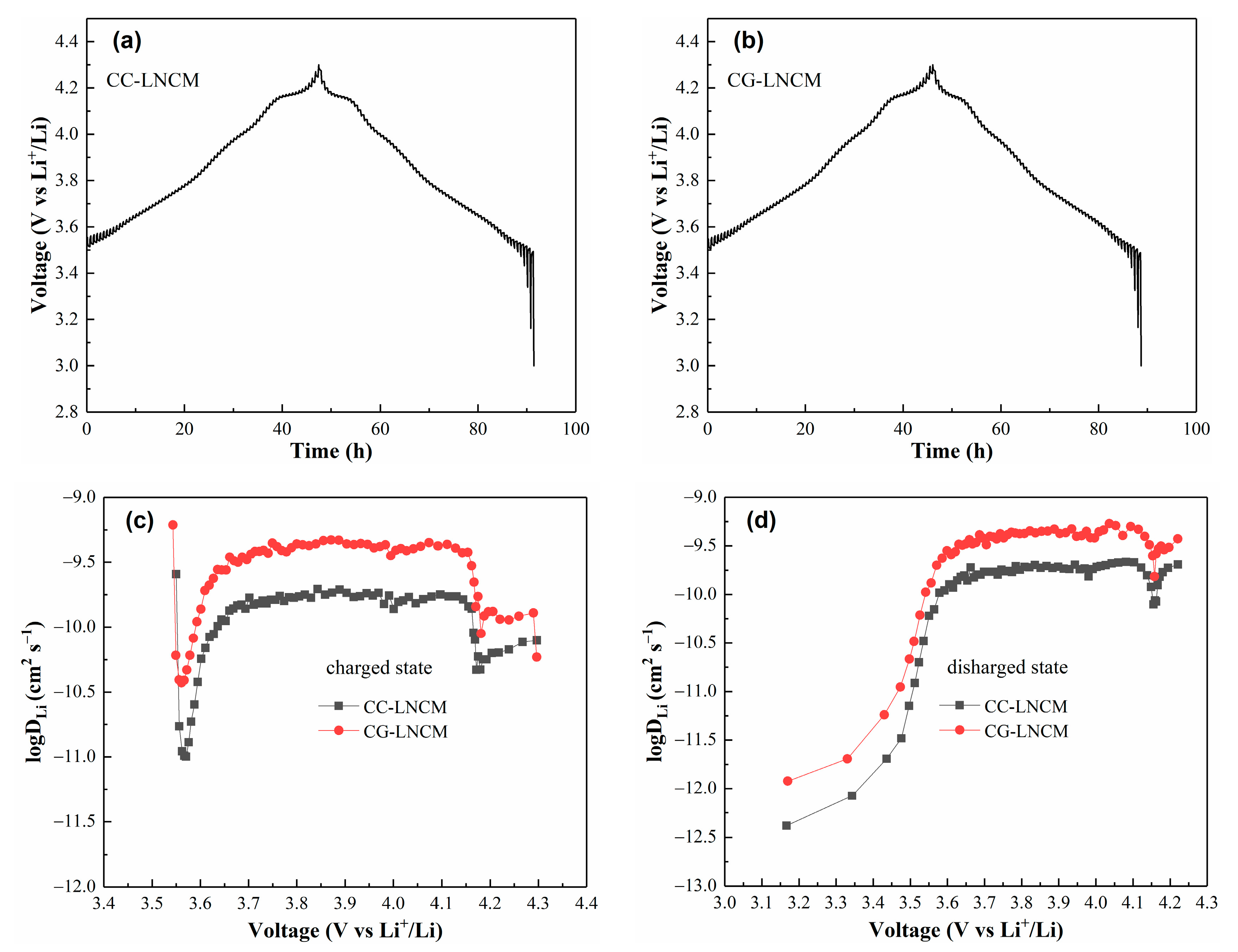
2. Results and Discussions
3. Materials and Methods
3.1. Synthesis of Materials
3.2. Material Characterizations
3.3. Electrochemical Properties Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359. [Google Scholar] [CrossRef] [PubMed]
- Palacin, M.R. Recent advances in rec hargeable battery materials: A chemist’s perspective. Chem. Soc. Rev. 2009, 38, 2565–2575. [Google Scholar] [CrossRef]
- Sun, Y.-K.; Myung, S.-T.; Park, B.-C.; Prakash, J.; Belharouak, I.; Amine, K. High-energy cathode material for long-life and safe lithium batteries. Nat. Mater. 2009, 8, 320. [Google Scholar] [CrossRef] [PubMed]
- Scrosati, B.; Hassoun, J.; Sun, Y.-K. Lithium-ion batteries. A look into the future. Energy Environ. Sci. 2011, 4, 3287–3295. [Google Scholar] [CrossRef]
- Zhao, J.; Hong, M.; Ju, Z.; Yan, X.; Gai, Y.; Liang, Z. Durable lithium metal anodes enabled by interfacial layers based on mechanically interlocked networks capable of energy dissipation. Angew. Chem. Int. Ed. Engl. 2022, 61, e202214386. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Yao, N.; Jiang, F.N.; Xie, J.; Sun, S.Y.; Chen, X.; Yuan, H.; Cheng, X.B.; Huang, J.Q.; Zhang, Q. Thermally stable polymer-rich solid electrolyte interphase for safe lithium metal pouch cells. Angew. Chem. Int. Ed. Engl. 2022, 61, e202214545. [Google Scholar] [CrossRef]
- Miao, Y.; Hynan, P.; von Jouanne, A.; Yokochi, A. Current Li-ion battery technologies in electric vehicles and opportunities for advancements. Energies 2019, 12, 1074. [Google Scholar] [CrossRef]
- Shao, Y.; Huang, B.; Liu, Q.; Liao, S. Preparation and modification of Ni-Co-Mn ternary cathode materials. Prog. Chem. 2018, 30, 410–419. [Google Scholar] [CrossRef]
- Bai, X.; Ban, L.; Zhuang, W. Research progress on coating and doping modification of nickel rich ternary cathode materials. J. Inorg. Mater. 2020, 35, 972–986. [Google Scholar] [CrossRef]
- Liu, L.; Li, M.; Chu, L.; Jiang, B.; Lin, R.; Zhu, X.; Cao, G. Layered ternary metal oxides: Performance degradation mechanisms as cathodes, and design strategies for high-performance batteries. Prog. Mater. Sci. 2020, 111, 100655. [Google Scholar] [CrossRef]
- Song, L.; Du, J.; Xiao, Z.; Jiang, P.; Cao, Z.; Zhu, H. Research progress on the surface of high-nickel nickel-cobalt-manganese ternary cathode materials: A mini review. Front. Chem. 2020, 8, 761. [Google Scholar] [CrossRef]
- Choi, J.U.; Voronina, N.; Sun, Y.-K.; Myung, S.-T. Recent progress and perspective of advanced high-energy Co-less Ni-rich cathodes for Li-ion batteries: Yesterday, today, and tomorrow. Adv. Energy Mater. 2020, 10, 2002027. [Google Scholar] [CrossRef]
- Xu, R.; Xu, W.; Wang, J.; Liu, F.; Sun, W.; Yang, Y. A review on regenerating materials from spent lithium-ion batteries. Molecules 2022, 27, 2285. [Google Scholar] [CrossRef]
- Wang, X.; Ding, Y.-L.; Deng, Y.-P.; Chen, Z. Ni-rich/Co-poor layered cathode for automotive Li-ion batteries: Promises and challenges. Adv. Energy Mater. 2020, 10, 1903864. [Google Scholar] [CrossRef]
- Jiang, M.; Danilov, D.L.; Eichel, R.-A.; Notten, P.H.L. A review of degradation mechanisms and recent achievements for Ni-rich cathode-based Li-ion batteries. Adv. Energy Mater. 2021, 11, 2103005. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.; Cha, H.; Yoon, M.; Park, M.; Cho, J. Prospect and reality of Ni-rich cathode for commercialization. Adv. Energy Mater. 2018, 8, 1702028. [Google Scholar] [CrossRef]
- Butt, A.; Ali, G.; Kubra, K.T.; Sharif, R.; Salman, A.; Bashir, M.; Jamil, S. Recent advances in enhanced performance of Ni-rich cathode materials for Li-ion batteries: A review. Energy Technol. 2022, 10, 2100775. [Google Scholar] [CrossRef]
- Liao, C.; Li, F.; Liu, J. Challenges and modification strategies of Ni-rich cathode materials operating at high-voltage. Nanomaterials 2022, 12, 1888. [Google Scholar] [CrossRef]
- Yin, S.; Deng, W.; Chen, J.; Gao, X.; Zou, G.; Hou, H.; Ji, X. Fundamental and solutions of microcrack in Ni-rich layered oxide cathode materials of lithium-ion batteries. Nano Energy 2021, 83, 105854. [Google Scholar] [CrossRef]
- Ahaliabadeh, Z.; Kong, X.; Fedorovskaya, E.; Kallio, T. Extensive comparison of doping and coating strategies for Ni-rich positive electrode materials. J. Power Sources 2022, 540, 231633. [Google Scholar] [CrossRef]
- Tao, J.; Mu, A.; Geng, S.; Xiao, H.; Zhang, L.; Huang, Q. Influences of direction and magnitude of Mg2+ doping concentration gradient on the performance of full concentration gradient cathode material. J. Solid State Electrochem. 2021, 25, 1959–1974. [Google Scholar] [CrossRef]
- Li, Q.; Li, Z.; Wu, S.; Wang, Z.; Liu, X.; Li, W.; Li, N.; Wang, J.; Zhuang, W. Utilizing diverse functions of zirconium to enhance the electrochemical performance of Ni-rich layered cathode materials. ACS Appl. Energy Mater. 2020, 3, 11741–11751. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, K.; Wang, B. Coating strategies of Ni-rich layered cathode in LIBs. Chem. Res. Chin. Univ. 2021, 42, 1514–1529. [Google Scholar] [CrossRef]
- Wang, X.; Ruan, X.; Du, C.-F.; Yu, H. Developments in surface/interface engineering of Ni-rich layered cathode materials. Chem. Rec. 2022, 22, e202200119. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ge, W.; Li, W.; Wang, F.; Liu, W.; Qu, M.Z.; Peng, G. Facile fabrication of ethoxy-functional polysiloxane wrapped LiNi0.6Co0.2Mn0.2O2 cathode with improved cycling performance for rechargeable Li-ion battery. ACS Appl. Mater. Interfaces 2016, 8, 18439–18449. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Zhang, S.; Di, A.; Deng, W.; Zou, G.; Hou, H.; Ji, X. Challenges and strategies towards single-crystalline Ni-rich layered cathodes. Adv. Energy Mater. 2022, 12, 2201510. [Google Scholar] [CrossRef]
- Pang, P.; Tan, X.; Wang, Z.; Cai, Z.; Nan, J.; Xing, Z.; Li, H. Crack-free single-crystal LiNi0.83Co0.10Mn0.07O2 as cycling/thermal stable cathode materials for high-voltage lithium-ion batteries. Electrochim. Acta 2021, 365, 137380. [Google Scholar] [CrossRef]
- Lee, S.-H.; Sim, S.-J.; Jin, B.-S.; Kim, H.-S. High performance well-developed single crystal LiNi0.91Co0.06Mn0.03O2 cathode via LiCl-NaCl flux method. Mater. Lett. 2020, 270, 127615. [Google Scholar] [CrossRef]
- Ran, Q.; Zhao, H.; Hu, Y.; Hao, S.; Liu, J.; Li, H.; Liu, X. Enhancing surface stability of LiNi0.8Co0.1Mn0.1O2 cathode with hybrid core-shell nanostructure induced by high-valent titanium ions for Li-ion batteries at high cut-off voltage. J. Alloys Compd. 2020, 834, 155099. [Google Scholar] [CrossRef]
- Sun, Y.K.; Myung, S.T.; Kim, M.H.; Prakash, J.; Amine, K. Synthesis and characterization of Li[(Ni0.8Co0.1Mn0.1)(0.8)(Ni0.5Mn0.5)(0.2)]O2 with the microscale core-shell structure as the positive electrode material for lithium batteries. J. Am. Chem. Soc. 2005, 127, 13411–13418. [Google Scholar] [CrossRef]
- Lim, B.-B.; Myung, S.-T.; Yoon, C.S.; Sun, Y.-K. Comparative Study of Ni-rich layered cathodes for rechargeable lithium batteries: Li[Ni0.85Co0.11Al0.04]O2 and Li[Ni0.84Co0.06Mn0.09Al0.01]O2 with two-step full concentration gradients. ACS Energy Lett. 2016, 1, 283–289. [Google Scholar] [CrossRef]
- Park, G.-T.; Ryu, H.-H.; Noh, T.-C.; Kang, G.-C.; Sun, Y.-K. Microstructure-optimized concentration-gradient NCM cathode for long-life Li-ion batteries. Mater. Today 2022, 52, 9–18. [Google Scholar] [CrossRef]
- Park, N.Y.; Ryu, H.H.; Park, G.T.; Noh, T.C.; Sun, Y.K. Optimized Ni-rich NCMA cathode for electric vehicle batteries. Adv. Energy Mater. 2021, 11, 200377. [Google Scholar] [CrossRef]
- Hou, P.; Zhang, H.; Zi, Z.; Zhang, L.; Xu, X. Core-shell and concentration-gradient cathodes prepared via co-precipitation reaction for advanced lithium-ion batteries. J. Mater. Chem. A 2017, 5, 4254–4279. [Google Scholar] [CrossRef]
- Tsai, S.-Y.; Fung, K.-Z. Synthesis routes on electrochemical behavior of Co-free layered LiNi0.5Mn0.5O2 cathode for Li-ion batteries. Molecules 2023, 28, 794. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, Z.; Zhang, Y.; Hu, H.; Teng, X.; Wang, D.; Gao, P.; Zhu, Y. Full-gradient structured LiNi0.8Co0.1Mn0.1O2 cathode material with improved rate and cycle performance for lithium ion batteries. Electrochim. Acta 2019, 309, 74–85. [Google Scholar] [CrossRef]
- Tan, Z.; Li, Y.; Xi, X.; Yang, J.; Xu, Y.; Xiong, Y.; Wang, S.; Liu, S.; Zheng, J. Lattice engineering to alleviate microcrack of LiNi0.9Co0.05Mn0.05O2 cathode for optimization their Li+ storage functionalities. Electrochim. Acta 2022, 401, 139482. [Google Scholar] [CrossRef]
- Li, G.; Zhang, Z.; Wang, R.; Huang, Z.; Zuo, Z.; Zhou, H. Effect of trace Al surface doping on the structure, surface chemistry and low temperature performance of LiNi0.5Co0.2Mn0.3O2 cathode. Electrochim. Acta 2016, 212, 399–407. [Google Scholar] [CrossRef]
- Zhou, H.; Zhou, F.; Shi, S.; Yang, W.; Song, Z. Influence of working temperature on the electrochemical characteristics of Al2O3-coated LiNi0.8Co0.1Mn0.1O2 cathode materials for Li-ion batteries. J. Alloys Compd. 2020, 847, 156412. [Google Scholar] [CrossRef]
- Lian, K.K.; Kirk, D.W.; Thorpe, S.J. Investigation of a “two-state” tafel phenomenon for the oxygen evolution reaction on an amorphous Ni-Co alloy. J. Electrochem. Soc. 1995, 142, 3704. [Google Scholar] [CrossRef]
- Mansour, A.N. Characterization of NiO by XPS. Surf. Sci. Spectra 1994, 3, 231–238. [Google Scholar] [CrossRef]
- Mansour, A.N. Characterization of LiNiO2 by XPS. Surf. Sci. Spectra 1996, 3, 279–286. [Google Scholar] [CrossRef]
- Kosova, N.V.; Devyatkina, E.T.; Kaichev, V.V. Optimization of Ni2+/Ni3+ ratio in layered Li(Ni,Mn,Co)O2 cathodes for better electrochemistry. J. Power Sources 2007, 174, 965–969. [Google Scholar] [CrossRef]
- Yang, J.; Xia, Y. Suppressing the phase transition of the layered Ni-rich oxide cathode during high-voltage cycling by introducing low-content Li2MnO3. ACS Appl. Mater. Interfaces 2016, 8, 1297–1308. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-W.; Zhang, X.-J.; Si, J.-J.; Yang, J.; Sun, X.-Y. TiO2-coated LiNi0.9Co0.08Al0.02O2 cathode materials with enhanced cycle performance for Li-ion batteries. Rare Met. 2021, 40, 1719–1726. [Google Scholar] [CrossRef]
- Huang, J.; Duan, J.; Du, K.; Cao, Y.; Peng, Z.; Hu, G. Enhanced cycling performance of LiNi0.9Co0.08Al0.02O2 via Co-rich surface. JOM 2020, 72, 738–744. [Google Scholar] [CrossRef]
- Mo, Y.; Guo, L.; Jin, H.; Du, B.; Cao, B.; Chen, Y.; Li, D.; Chen, Y. Building nickel-rich cathodes with large concentration gradient for high performance lithium-ion batteries. J. Power Sources 2020, 468, 228405. [Google Scholar] [CrossRef]
- Zhang, C.; Wan, J.; Li, Y.; Zheng, S.; Zhou, K.; Wang, D.; Wang, D.; Hong, C.; Gong, Z.; Yang, Y. Restraining the polarization increase of Ni-rich and low-Co cathodes upon cycling by Al-doping. J. Mater. Chem. A 2020, 8, 6893–6901. [Google Scholar] [CrossRef]
- Su, Y.; Chen, G.; Chen, L.; Shi, Q.; Lv, Z.; Lu, Y.; Bao, L.; Li, N.; Chen, S.; Wu, F. Roles of fast-ion conductor LiTaO3 modifying Ni-rich cathode material for Li-ion batteries. Chemsuschem 2021, 14, 1955–1961. [Google Scholar] [CrossRef]
- Qian, R.; Liu, Y.; Cheng, T.; Li, P.; Chen, R.; Lyu, Y.; Guo, B. Enhanced surface chemical and structural stability of Ni-rich cathode materials by synchronous lithium-ion conductor coating for lithium-ion batteries. ACS Appl. Mater. Interfaces 2020, 12, 13813–13823. [Google Scholar] [CrossRef]
- Hao, S.; Zhang, D.; Li, Y.; Xi, X.; Wang, S.; Li, X.; Shen, X.; Liu, S.; Zheng, J. Multifunctionality of cerium decoration in enhancing the cycling stability and rate capability of a nickel-rich layered oxide cathode. Nanoscale 2021, 13, 20213–20224. [Google Scholar] [CrossRef]
- Park, J.-S.; Hong, Y.J.; Kim, J.H.; Kang, Y.C. Carbon-templated strategy toward the synthesis of dense and yolk-shell multi-component transition metal oxide cathode microspheres for high-performance Li ion batteries. J. Power Sources 2020, 461, 15. [Google Scholar] [CrossRef]
- Yang, X.; Tang, Y.; Shang, G.; Wu, J.; Lai, Y.; Li, J.; Qu, Y.; Zhang, Z. Enhanced cyclability and high-rate capability of LiNi0.88Co0.095Mn0.025O2 cathodes by homogeneous Al3+ doping. ACS Appl. Mater. Interfaces 2019, 11, 32015–32024. [Google Scholar] [CrossRef]
- Weppner, W.; Huggins, R.A. Determination of the kinetic parameters of mixed-conducting flectrodes and application to the system Li3Sb. J. Electrochem. Soc. 1977, 124, 1569. [Google Scholar] [CrossRef]
- Chen, Q.; Qiao, X.; Wang, Y.; Zhang, T.; Peng, C.; Yin, W.; Liu, L. Electrochemical performance of Li3−xNaxV2(PO4)3/C composite cathode materials for lithium ion batteries. J. Power Sources 2012, 201, 267–273. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Guo, Y.; Chen, Y.; Gao, N.; Sun, R.; Lu, Y.; Chen, Q. Outstanding Electrochemical Performance of Ni-Rich Concentration-Gradient Cathode Material LiNi0.9Co0.083Mn0.017O2 for Lithium-Ion Batteries. Molecules 2023, 28, 3347. https://doi.org/10.3390/molecules28083347
Li H, Guo Y, Chen Y, Gao N, Sun R, Lu Y, Chen Q. Outstanding Electrochemical Performance of Ni-Rich Concentration-Gradient Cathode Material LiNi0.9Co0.083Mn0.017O2 for Lithium-Ion Batteries. Molecules. 2023; 28(8):3347. https://doi.org/10.3390/molecules28083347
Chicago/Turabian StyleLi, Hechen, Yiwen Guo, Yuanhua Chen, Nengshuang Gao, Ruicong Sun, Yachun Lu, and Quanqi Chen. 2023. "Outstanding Electrochemical Performance of Ni-Rich Concentration-Gradient Cathode Material LiNi0.9Co0.083Mn0.017O2 for Lithium-Ion Batteries" Molecules 28, no. 8: 3347. https://doi.org/10.3390/molecules28083347
APA StyleLi, H., Guo, Y., Chen, Y., Gao, N., Sun, R., Lu, Y., & Chen, Q. (2023). Outstanding Electrochemical Performance of Ni-Rich Concentration-Gradient Cathode Material LiNi0.9Co0.083Mn0.017O2 for Lithium-Ion Batteries. Molecules, 28(8), 3347. https://doi.org/10.3390/molecules28083347







