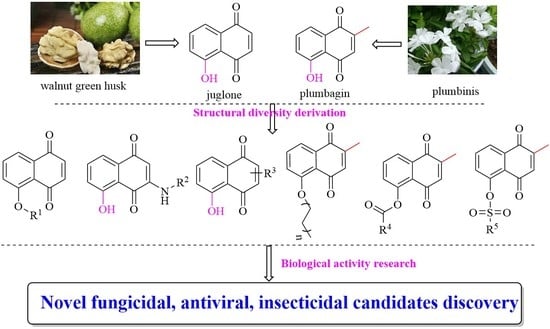
Natural Products for Pesticides Discovery: Structural Diversity Derivation and Biological Activities of Naphthoquinones Plumbagin and Juglone
Abstract
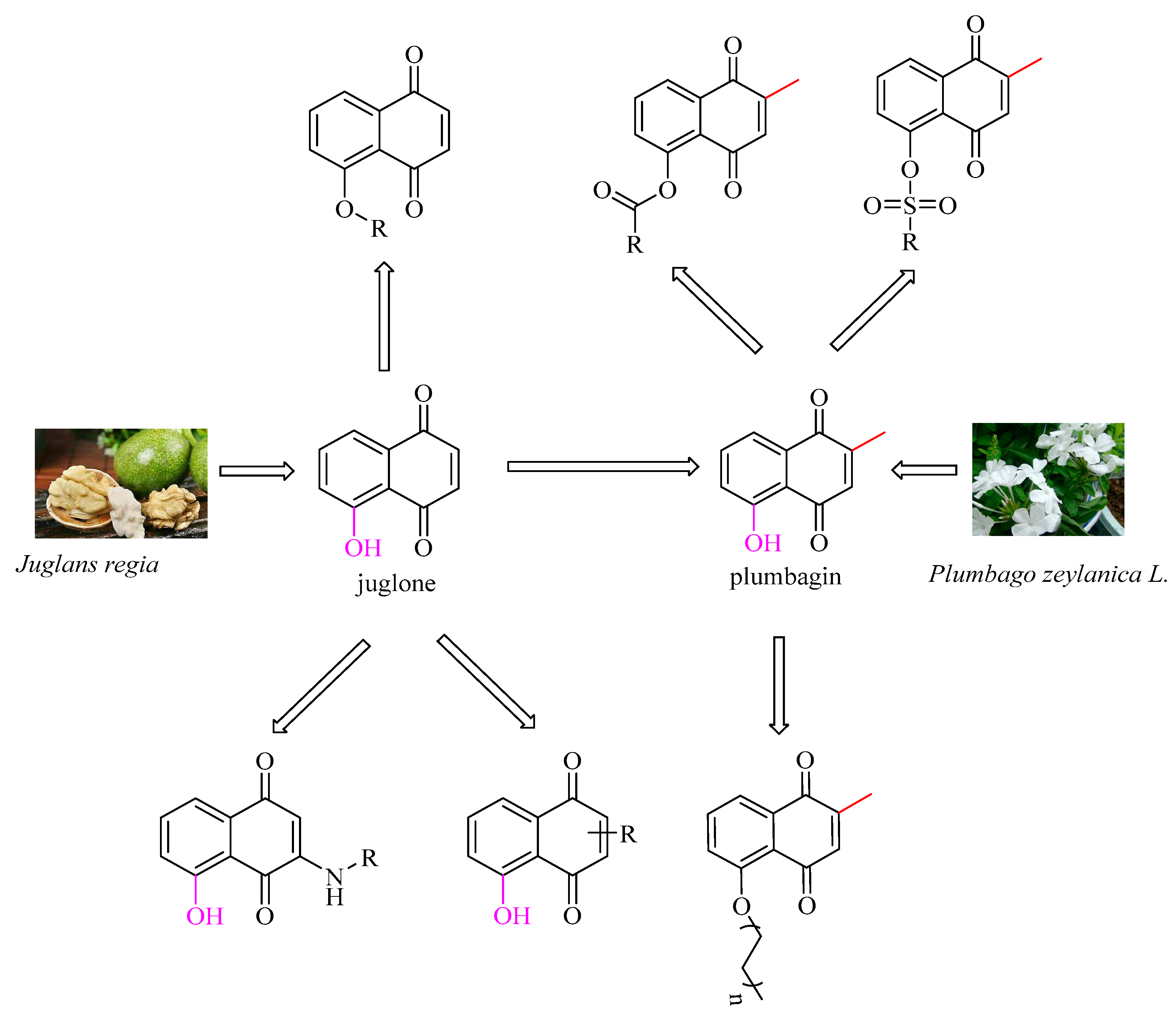
1. Introduction
2. Results and Discussion
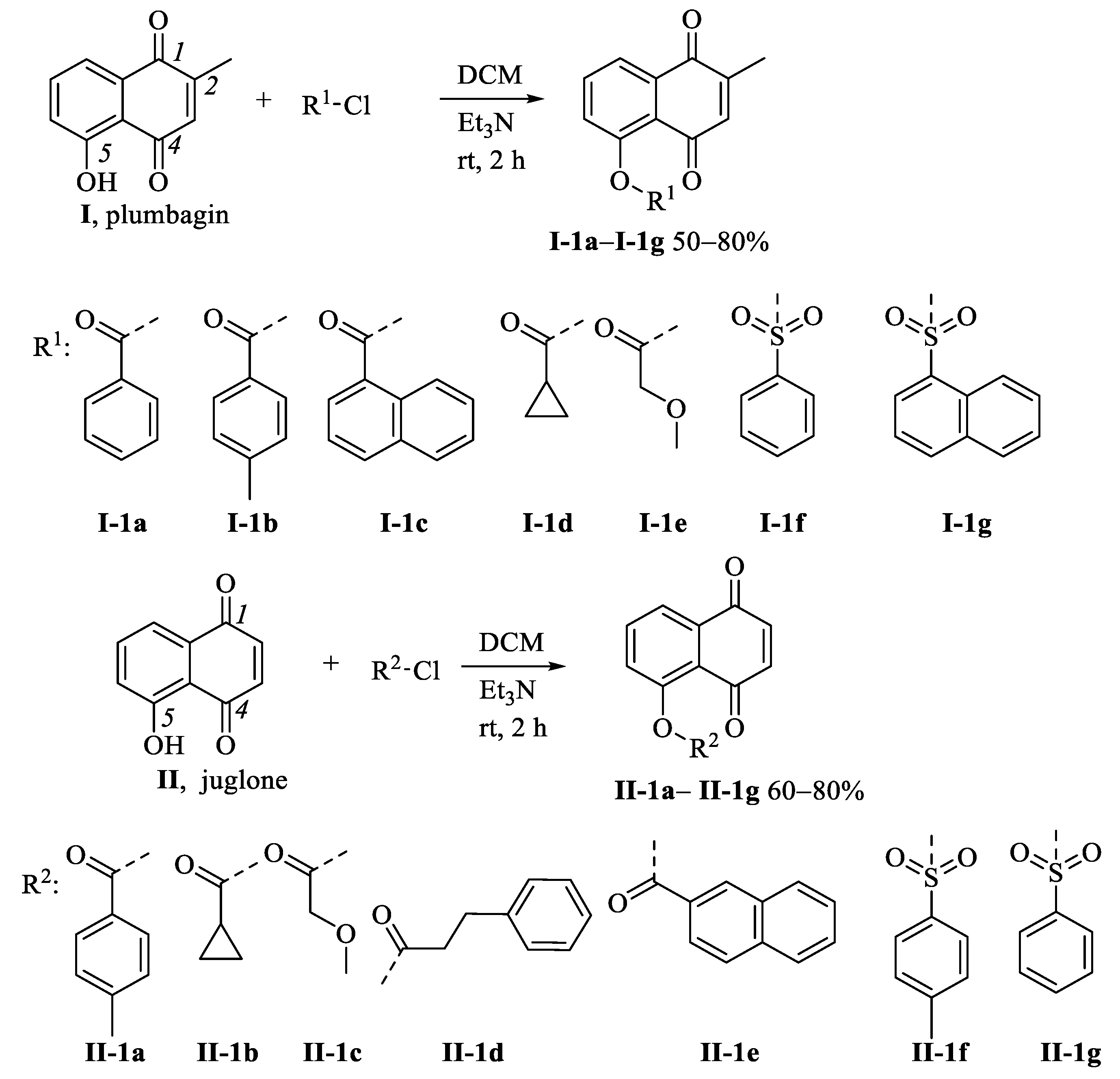
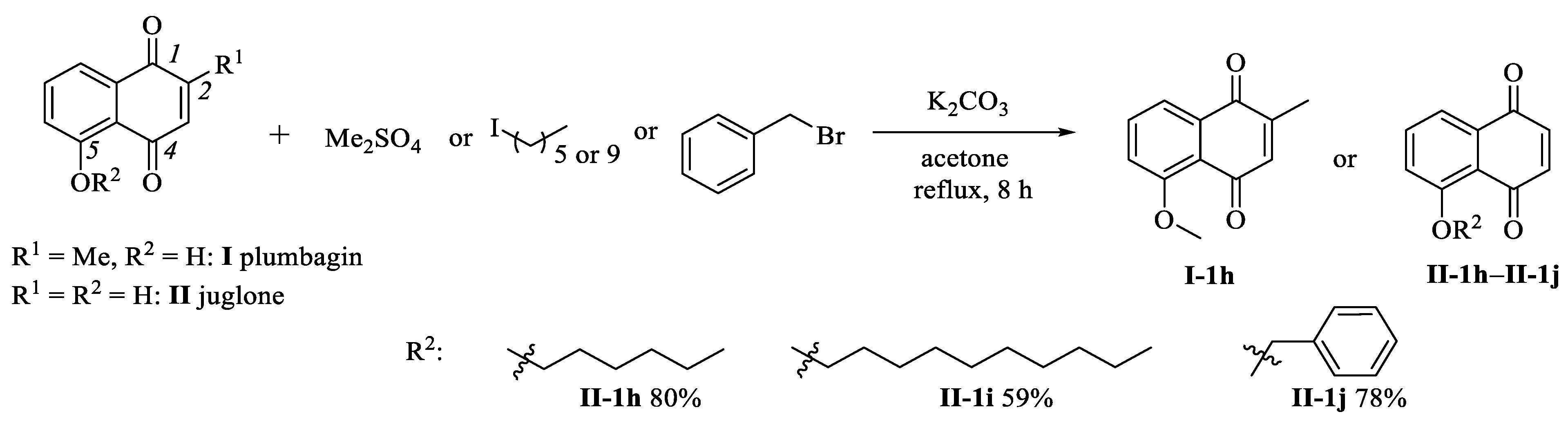
2.1. Chemistry
2.2. Fungicidal Activity Result and Structure-Activity Relationship (SAR)
2.3. Antiviral Activity Result and Structure-Activity Relationship (SAR)
2.4. Insecticidal Activity Result and Structure-Activity Relationship (SAR)
3. Materials and Methods
3.1. Synthetic Procedures
3.1.1. Chemicals
3.1.2. Instruments
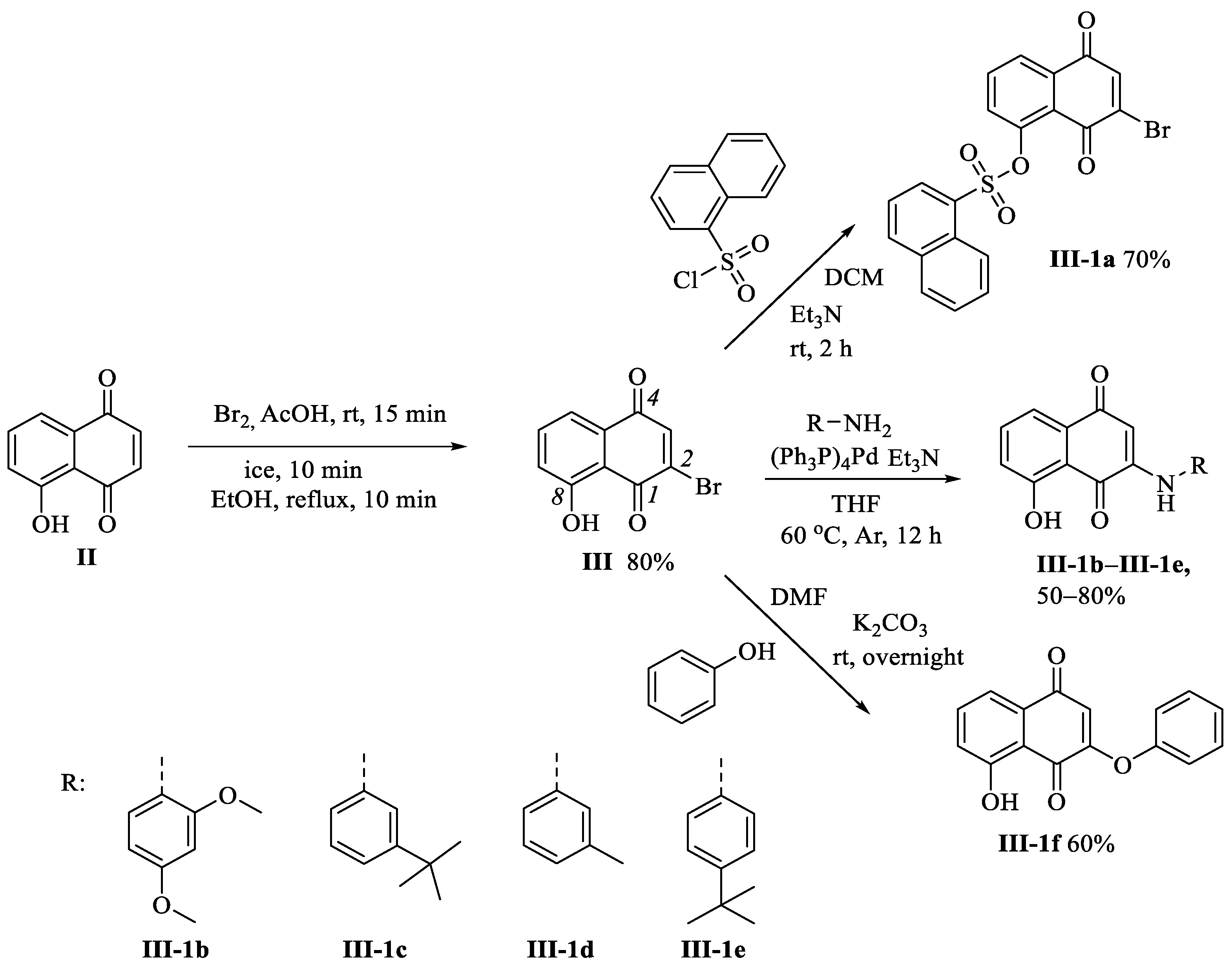
3.1.3. Preparation of 2-Bromo-8-hydroxynaphthalene-1,4-dione (III)
3.1.4. Preparation of Compounds I-1a–I-1g, II-1a–II-1g, III-1a
3.1.5. Preparation of Compounds I-1h and II-1h–II-1j
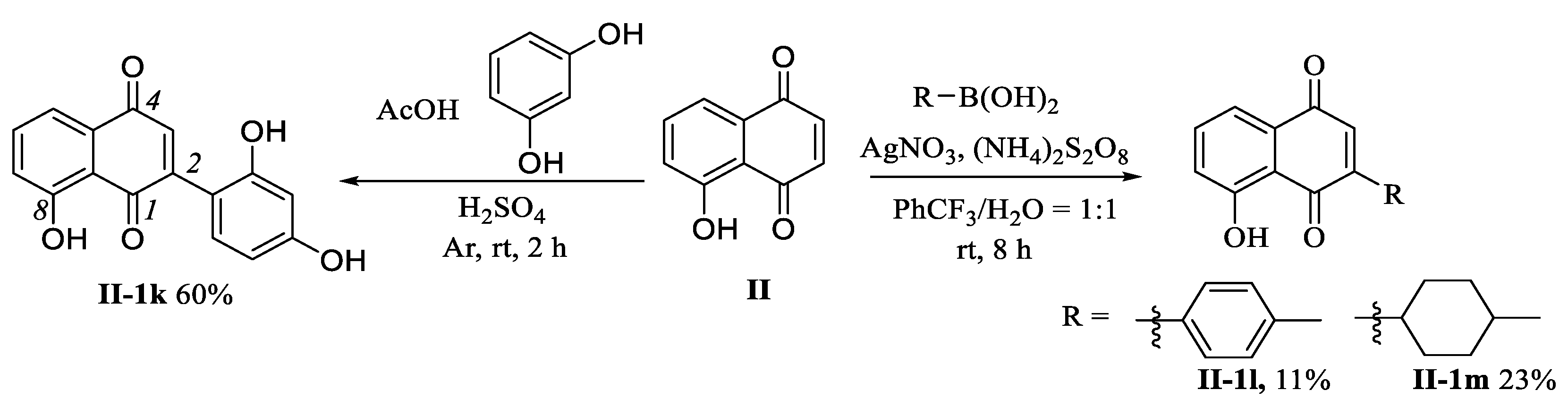
3.1.6. Preparation of 2-(2,4-Dihydroxyphenyl)-8-hydroxynaphthalene-1,4-dione (II-1k)
3.1.7. Preparation of Compounds II-1l and II-1m
3.1.8. Preparation of Compounds III-1b–III-1e
3.1.9. Preparation of Compound III-1f
3.2. Biological Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Godfray, H.C.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef]
- Lugtenberg, B. Principles of Plant-Microbe Interactions: Microbes for Sustainable Agriculture; Springer: Cham, Switzerland; Heidelberg, Germany; New York, NY, USA; Dordrecht, The Netherlands; London, UK, 2015; pp. 1–435. [Google Scholar]
- Dhaliwal, G.S.; Jindal, V.; Mohindru, B. Crop losses due to insect pests: Global and Indian scenario. Indian J. Entomol. 2015, 77, 165–168. [Google Scholar] [CrossRef]
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef] [PubMed]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdis. Toxicol. 2009, 2, 1–12. [Google Scholar]
- Jeschke, P. Progress of modern agricultural chemistry and future prospects. Pest Manag. Sci. 2016, 72, 433–455. [Google Scholar] [PubMed]
- Glare, T.; Caradus, J.; Gelernter, W.; Jackson, T.; Keyhani, N.; Köhl, J.; Marrone, P.; Morin, L.; Stewart, A. Have biopesticides come of age? Trends Biotechnol. 2012, 30, 250–258. [Google Scholar]
- Begg, G.S.; Cook, S.M.; Dye, R.; Ferrante, M.; Franck, P.; Lavigne, C.; Lövei, G.L.; Mansion-Vaquie, A.; Pell, J.K.; Petit, S.; et al. A functional overview of conservation biological control. Crop. Prot. 2017, 97, 145–158. [Google Scholar]
- Wang, S.Z.; Dong, G.Q.; Sheng, C.Q. Structural simplification of natural products. Chem. Rev. 2019, 119, 4180–4220. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar]
- Xu, H.; Zhang, K.; Lv, M.; Hao, M. Construction of cholesterol oxime ethers derivatives containing isoxazoline/isoxazole fragments and their agricultural bioactive properties/control efficiency. J. Agric. Food Chem. 2021, 69, 8098–8109. [Google Scholar] [CrossRef]
- Li, S.C.; Lv, M.; Sun, Z.Q.; Hao, M.; Xu, H. Optimization of osthole in the lactone ring: Structural elucidation, pesticidal activities, and control efficiency of osthole ester derivatives. J. Agric. Food Chem. 2021, 69, 6465–6474. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.B.; Li, T.Z.; Shan, X.J.; Lu, R.F.; Hao, M.; Lv, M.; Sun, Z.Q.; Xu, H. High value-added use of citrus industrial wastes in agriculture: Semisynthesis and anti-tobacco mosaic virus/insecticidal activities of ester derivatives of limonin modified in the B ring. J. Agric. Food Chem. 2020, 68, 12241–12251. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, N.; Kandwal, P.; Sharma, G.; Gambhir, L. Redox ticklers and beyond: Naphthoquinone repository in the spotlight against inflammation and associated maladies. Pharmacol. Res. 2022, 174, 105968. [Google Scholar] [CrossRef] [PubMed]
- Rani, R.; Sethi, K.; Kumar, S.; Varma, R.; Kumar, R. Natural naphthoquinones and their derivatives as potential drug molecules against trypanosome parasites. Chem. Biol. Drug Des. 2022, 100, 786–817. [Google Scholar] [CrossRef] [PubMed]
- Bao, N.; Ou, J.; Shi, W.; Li, N.; Chen, L.; Sun, J. Highly efficient synthesis and structure-activity relationships of a small library of substituted 1,4-naphthoquinones. Eur. J. Org. Chem. 2018, 2018, 2254–2258. [Google Scholar] [CrossRef]
- Guo, J.C.; Hao, Y.N.; Ji, X.F.; Wang, Z.W.; Liu, Y.X.; Ma, D.J.; Li, Y.Q.; Pang, H.L.; Ni, J.P.; Wang, Q.M. Optimization, structure-activity relationship and mode of action of nortopsentin analogues containing thiazole and oxazole moieties. J. Agric. Food Chem. 2019, 67, 10018–10031. [Google Scholar] [CrossRef]
- Zhang, M.J.; Ding, X.; Kang, J.; Gao, Y.Y.; Wang, Z.W.; Wang, Q.M. Marine natural product for pesticide candidate: Pulmonarin alkaloids as novel antiviral and anti-phytopathogenic-fungus agents. J. Agric. Food Chem. 2020, 68, 11350–11357. [Google Scholar] [CrossRef]
- Yang, S.; Wang, T.N.; Zhou, Y.; Shi, L.; Lu, A.D.; Wang, Z.W. Discovery of cysteine and its derivatives as novel antiviral and antifungal agents. Molecules 2021, 26, 383. [Google Scholar] [CrossRef]
- Huang, S.S.; Zhu, B.B.; Wang, K.H.; Yu, M.; Wang, Z.W.; Li, Y.Q.; Liu, Y.X.; Zhang, P.L.; Li, S.J.; Li, Y.L.; et al. Design, synthesis, and insecticidal and fungicidal activities of quaternary ammonium salt derivatives of a triazolyphenyl isoxazoline insecticide. Pest Manag. Sci. 2022, 78, 2011–2021. [Google Scholar] [CrossRef]
- Ding, X.; Xu, Y.B.; Yan, L.L.; Chen, L.; Lu, Z.J.; Ge, C.Y.; Zhao, X.Y.; Wang, Z.W.; Lu, A.D.; Wang, Q.M. Marine sesquiterpenes for plant protection: Discovery of laurene sesquiterpenes and their derivatives as novel antiviral and antiphytopathogenic fungal agents. J. Agric. Food Chem. 2022, 70, 6006–6014. [Google Scholar] [CrossRef]
- Lamberth, C. Agrochemical lead optimization by scaffold hopping. Pest Manag. Sci. 2018, 74, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.; Chen, Y.L.; Shi, T.Z.; Wu, X.W.; Li, Q.X.; Hua, R.M. Synthesis and fungicidal activities of sanguinarine derivatives. Pestic. Biochem. Phys. 2018, 147, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Lamberth, C.; Jeanmart, S.; Luksch, T.; Plant, A. Current challenges and trends in the discovery of agrochemicals. Science 2013, 341, 742–746. [Google Scholar] [CrossRef]
- Guo, S.; He, F.; Song, B.; Wu, J. Future direction of agrochemical development for plant disease in China. Food Energy Secur. 2021, 10, e293. [Google Scholar] [CrossRef]
- Zhao, H.P.; Liu, Y.X.; Cui, Z.P.; Beattie, D.; Gu, Y.C.; Wang, Q.M. Design, synthesis, and biological activities of arylmethylamine substituted chlorotriazine and methylthiotriazine compounds. J. Agric. Food Chem. 2011, 59, 11711–11717. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.W.; Wei, P.; Wang, L.Z.; Wang, Q.M. Design, synthesis, and anti-tobacco mosaic virus (TMV) activity of phenanthroindolizidines and their analogues. J. Agric. Food Chem. 2012, 60, 10212–10219. [Google Scholar] [CrossRef]
- Gooding, G.V., Jr.; Hebert, T.T. A simple technique for purification of tobacco mosaic virus in large quantities. Phytopathology 1967, 57, 1285–1290. [Google Scholar]
- Li, S.Z.; Wang, D.M.; Jiao, S.M. Pesticide Experiment Methods-Fungicide Sector; Li, S.Z., Ed.; Agriculture Press of China: Beijing, China, 1991; pp. 93–94. [Google Scholar]
- Leberman, R. Isolation of plant viruses by means of simple coacervates. Virology 1966, 30, 341–347. [Google Scholar] [CrossRef]
- Fraenkel Conrat, H.; Williams, R.C. Reconstitution of active tobacco mosaic virus fromits inactive protein and nucleic acid components. Proc. Natl. Acad. Sci. USA 1955, 41, 690–698. [Google Scholar] [CrossRef]
- Ni, W.J.; Li, C.J.; Liu, Y.X.; Song, H.J.; Wang, L.Z.; Song, H.B.; Wang, Q.M. Various bioactivity and relationship of structure−activity of matrine analogues. J. Agric. Food Chem. 2017, 65, 2039–2047. [Google Scholar] [CrossRef]
- Yu, X.L.; Liu, Y.X.; Li, Y.Q.; Wang, Q.M. Design, synthesis, acaricidal/insecticidal activity, and structure−activity relationship studies of novel oxazolines containing sulfone/sulfoxide groups based on the sulfonylurea receptor protein-binding site. J. Agric. Food Chem. 2016, 64, 3034–3040. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.X.; Song, H.J.; Li, Y.Q.; Wang, Q.M. Additive effects on the improvement of insecticidal activity: Design, synthesis, and insecticidal activity of novel pymetrozine derivatives. Bioorg. Med. Chem. 2016, 24, 391–402. [Google Scholar] [CrossRef]
| Compd | Fungicidal Activities (%) at 50 μg/mL | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F.O | C.H | P.P | R.C | B.M | W.A | F.M | A.S | F.G | P.I | P.C | S.S | B.C | R.S | |
| I | 89 ± 2 | 100 | 85 ± 3 | 37 ± 3 | 73 ± 3 | 83 ± 3 | 90 ± 3 | 31 ± 2 | 78 ± 3 | 78 ± 2 | 100 | 97 ± 2 | 83 ± 2 | 100 |
| I-1a | 41 ± 3 | 40 ± 4 | 50 ± 3 | 64 ± 4 | 49 ± 3 | 50 ± 4 | 56 ± 3 | 31 ± 4 | 44 ± 4 | 33 ± 4 | 42 ± 3 | 34 ± 4 | 61 ± 1 | 76 ± 3 |
| I-1b | 57 ± 3 | 33 ± 2 | 36 ± 1 | 58 ± 3 | 61 ± 4 | 52 ± 3 | 49 ± 4 | 25 ± 1 | 36 ± 1 | 44 ± 1 | 36 ± 5 | 17 ± 1 | 22 ± 1 | 46 ± 1 |
| I-1c | 30 ± 1 | 27 ± 1 | 46 ± 2 | 64 ± 3 | 26 ± 2 | 43 ± 1 | 37 ± 2 | 6 ± 1 | 50 ± 2 | 33 ± 2 | 22 ± 1 | 28 ± 2 | 46 ± 2 | 83 ± 1 |
| I-1d | 46 ± 2 | 43 ± 2 | 14 ± 1 | 70 ± 1 | 53 ± 1 | 47 ± 1 | 56 ± 1 | 38 ± 2 | 50 ± 2 | 67 ± 2 | 44 ± 2 | 35 ± 1 | 54 ± 3 | 79 ± 2 |
| I-1e | 96 ± 2 | 100 | 97 ± 2 | 97 ± 2 | 78 ± 2 | 79 ± 2 | 92 ± 2 | 50 ± 1 | 53 ± 2 | 78 ± 2 | 67 ± 2 | 44 ± 2 | 85 ± 2 | 100 |
| I-1f | 20 ± 2 | 30 ± 2 | 19 ± 1 | 50 ± 2 | 25 ± 1 | 36 ± 2 | 36 ± 2 | 6 ± 1 | 27 ± 2 | 11 ± 1 | 6 ± 1 | 34 ± 1 | 47 ± 2 | 22 ± 2 |
| I-1g | 26 ± 2 | 23 ± 2 | 50 ± 1 | 57 ± 3 | 23 ± 1 | 34 ± 2 | 48 ± 2 | 37 ± 2 | 27 ± 2 | 33 ± 1 | 22 ± 1 | 34 ± 2 | 52 ± 3 | 22 ± 1 |
| I-1h | 64 ± 2 | 80 ± 2 | 18 ± 1 | 74 ± 4 | 52 ± 2 | 60 ± 3 | 80 ± 3 | 31 ± 2 | 50 ± 1 | 55 ± 2 | 61 ± 2 | 29 ± 2 | 54 ± 2 | 60 ± 3 |
| II | 25 ± 1 | 20 ± 1 | 16 ± 1 | 23 ± 1 | 25 ± 1 | 37 ± 1 | 43 ± 1 | 43 ± 2 | 16 ± 1 | 33 ± 1 | 22 ± 1 | 17 ± 1 | 30 ± 1 | 37 ± 2 |
| II-1a | 66 ± 2 | 100 | 63 ± 2 | 37 ± 2 | 70 ± 3 | 75 ± 2 | 80 ± 1 | 25 ± 1 | 44 ± 2 | 33 ± 2 | 63 ± 3 | 32 ± 2 | 39 ± 2 | 63 ± 2 |
| II-1b | 62 ± 3 | 70 ± 2 | 29 ± 2 | 57 ± 2 | 54 ± 3 | 44 ± 2 | 65 ± 3 | 56 ± 1 | 67 ± 2 | 66 ± 2 | 75 ± 2 | 48 ± 1 | 32 ± 2 | 87 ± 4 |
| II-1c | 67 ± 2 | 73 ± 2 | 44 ± 1 | 82 ± 3 | 78 ± 3 | 58 ± 3 | 61 ± 2 | 56 ± 2 | 64 ± 2 | 89 ± 2 | 77 ± 3 | 62 ± 3 | 28 ± 1 | 69 ± 2 |
| II-1d | 42 ± 2 | 50 ± 1 | 36 ± 1 | 61 ± 3 | 41 ± 2 | 55 ± 2 | 61 ± 3 | 18 ± 1 | 27 ± 1 | 55 ± 1 | 77 ± 2 | 39 ± 2 | 37 ± 2 | 72 ± 1 |
| II-1e | 75 ± 2 | 83 ± 2 | 48 ± 2 | 71 ± 3 | 60 ± 2 | 58 ± 1 | 63 ± 2 | 18 ± 1 | 33 ± 2 | 44 ± 2 | 66 ± 2 | 29 ± 1 | 54 ± 2 | 66 ± 3 |
| II-1f | 26 ± 1 | 23 ± 1 | 22 ± 1 | 16 ± 1 | 21 ± 2 | 37 ± 2 | 36 ± 3 | 31 ± 2 | 19 ± 1 | 22 ± 1 | 13 ± 1 | 11 ± 1 | 17 ± 2 | 30 ± 2 |
| II-1g | 26 ± 1 | 33 ± 2 | 8 ± 1 | 78 ± 4 | 37 ± 2 | 43 ± 2 | 51 ± 2 | 43 ± 3 | 25 ± 2 | 33 ± 1 | 38 ± 2 | 34 ± 1 | 32 ± 2 | 51 ± 3 |
| II-1h | 26 ± 1 | 26 ± 1 | 89 ± 2 | 50 ± 2 | 25 ± 1 | 37 ± 2 | 29 ± 3 | 6 ± 1 | 22 ± 2 | 33 ± 2 | 27 ± 2 | 46 ± 2 | 21 ± 1 | 27 ± 1 |
| II-1i | 9 ± 1 | 13 ± 1 | 29 ± 1 | 23 ± 2 | 10 ± 1 | 36 ± 2 | 22 ± 2 | 18 ± 1 | 11 ± 1 | 11 ± 1 | 19 ± 1 | 34 ± 2 | 21 ± 2 | 30 ± 2 |
| II-1j | 23 ± 1 | 30 ± 2 | 29 ± 2 | 71 ± 2 | 23 ± 2 | 37 ± 3 | 17 ± 1 | 12 ± 1 | 27 ± 2 | 22 ± 2 | 22 ± 3 | 72 ± 3 | 50 ± 4 | 48 ± 4 |
| II-1k | 44 ± 2 | 53 ± 3 | 78 ± 2 | 37 ± 2 | 43 ± 3 | 51 ± 2 | 58 ± 1 | 31 ± 2 | 41 ± 1 | 44 ± 2 | 75 ± 2 | 46 ± 2 | 45 ± 3 | 60 ± 4 |
| II-1l | 32 ± 2 | 46 ± 2 | 85 ± 2 | 37 ± 1 | 37 ± 2 | 55 ± 3 | 56 ± 3 | 43 ± 2 | 44 ± 2 | 22 ± 1 | 22 ± 1 | 61 ± 2 | 43 ± 3 | 71 ± 2 |
| II-1m | 19 ± 2 | 40 ± 1 | 90 ± 2 | 34 ± 2 | 51 ± 2 | 60 ± 3 | 53 ± 3 | 25 ± 2 | 41 ± 1 | 78 ± 2 | 19 ± 1 | 54 ± 2 | 56 ± 2 | 68 ± 3 |
| III | 12 ± 1 | 20 ± 2 | 26 ± 1 | 53 ± 2 | 25 ± 1 | 32 ± 1 | 34 ± 3 | 12 ± 1 | 17 ± 1 | 11 ± 1 | 13 ± 1 | 23 ± 2 | 21 ± 2 | 38 ± 2 |
| III-1a | 23 ± 2 | 30 ± 2 | 31 ± 2 | 78 ± 2 | 39 ± 3 | 37 ± 3 | 58 ± 4 | 18 ± 1 | 22 ± 2 | 22 ± 1 | 27 ± 2 | 11 ± 1 | 37 ± 2 | 21 ± 1 |
| III-1b | 17 ± 1 | 23 ± 2 | 36 ± 2 | 27 ± 1 | 29 ± 2 | 34 ± 3 | 7 ± 1 | 12 ± 1 | 22 ± 2 | 22 ± 1 | 22 ± 3 | 34 ± 3 | 52 ± 3 | 53 ± 4 |
| III-1c | 14 ± 1 | 20 ± 1 | 30 ± 3 | 21 ± 2 | 17 ± 1 | 34 ± 2 | 29 ± 2 | 6 ± 2 | 17 ± 2 | 11 ± 2 | 11 ± 2 | 23 ± 1 | 26 ± 2 | 30 ± 3 |
| III-1d | 16 ± 1 | 80 ± 2 | 22 ± 2 | 23 ± 2 | 29 ± 2 | 34 ± 3 | 36 ± 3 | 12 ± 1 | 11 ± 1 | 11 ± 1 | 5 ± 1 | 23 ± 2 | 43 ± 2 | 22 ± 3 |
| III-1e | 9 ± 1 | 17 ± 2 | 33 ± 2 | 23 ± 2 | 15 ± 1 | 29 ± 2 | 24 ± 1 | 25 ± 1 | 13 ± 2 | 11 ± 2 | 11 ± 1 | 17 ± 1 | 30 ± 2 | 22 ± 2 |
| III-1f | 37 ± 2 | 40 ± 2 | 68 ± 3 | 23 ± 3 | 43 ± 1 | 43 ± 2 | 61 ± 3 | 12 ± 1 | 33 ± 2 | 55 ± 2 | 61 ± 2 | 23 ± 1 | 52 ± 2 | 68 ± 3 |
| Chlorothalonil b | 95 ± 2 | 19 ± 1 | 98 ± 2 | 98 ± 1 | 97 ± 1 | 98 ± 1 | 83 ± 3 | 38 ± 2 | 100 | 73 ± 2 | 88 ± 2 | 100 | 92 ± 2 | 100 |
| Carbendazim b | 100 | 28 ± 1 | 98 ± 2 | 98 ± 1 | 97 ± 1 | 98 ± 2 | 90 ± 3 | 13 ± 1 | 100 | 48 ± 2 | 44 ± 1 | 100 | 42 ± 1 | 100 |
| Pyrimethanil b | 19 ± 1 | 83 ± 2 | 71 ± 3 | 84 ± 2 | 28 ± 1 | 21 ± 1 | 27 ± 1 | 88 ± 2 | 59 ± 1 | 79 ± 3 | 100 | 100 | 100 | 100 |
| Compd. | Regression Equation | r2 | Protection Effect EC50 (µg/mL) |
|---|---|---|---|
| I | y = 2.71 + 1.99x | 0.9808 | 17.70 |
| I-1e | y = 2.64 + 2.29x | 0.9987 | 11.35 |
| II-1a | y = 2.78 + 2.18x | 0.9861 | 12.56 |
| Pyrimethanil | y = 2.78 + 1.84x | 0.9885 | 19.17 |
| Compd. | Concn (μg/mL) | Inactive Effect (%) | Curative Effect (%) | Protective Effect (%) |
|---|---|---|---|---|
| I | 500 | 13 ± 3 | – | – |
| I-1a | 500 | 15 ± 2 | – | – |
| I-1b | 500 | 26 ± 3 | – | – |
| I-1c | 500 | 4 ± 1 | – | – |
| I-1d | 500 | 24 ± 1 | – | – |
| I-1e | 500 | 10 ± 1 | – | – |
| I-1f | 500 | 43 ± 4 | 37 ± 2 | 36 ± 2 |
| 100 | 7 ± 1 | 9 ± 2 | 0 | |
| I-1g | 500 | 19 ± 4 | – | – |
| I-1h | 500 | 14 ± 3 | – | – |
| II | 500 | 18 ± 3 | – | – |
| II-1a | 500 | 16 ± 2 | – | – |
| II-1b | 500 | 14 ± 4 | – | – |
| II-1c | 500 | 16 ± 4 | – | – |
| II-1d | 500 | 0 | – | – |
| II-1e | 500 | 18 ± 1 | – | – |
| II-1f | 500 | 44 ± 2 | 36 ± 2 | 39 ± 3 |
| 100 | 5 ± 1 | 8 ± 1 | 10 ± 2 | |
| II-1g | 500 | 37 ± 5 | – | – |
| II-1h | 500 | 0 | – | – |
| II-1i | 500 | 22 ± 2 | ||
| II-1j | 500 | 25 ± 3 | – | – |
| II-1k | 500 | 28 ± 3 | – | – |
| II-1l | 500 | 35 ± 4 | – | – |
| II-1m | 500 | 32 ± 4 | – | – |
| III | 500 | 24 ± 4 | – | – |
| III-1a | 500 | 8 ± 3 | – | – |
| III-1b | 500 | 39 ± 3 | – | – |
| 100 | 6 ± 1 | – | – | |
| III-1c | 500 | 17 ± 4 | – | – |
| III-1d | 500 | 35 ± 3 | – | – |
| 100 | 3 ± 1 | – | – | |
| III-1e | 500 | 26 ± 1 | – | – |
| III-1f | 500 | 31 ± 1 | – | – |
| Ribavirin b | 500 | 38 ± 2 | 36 ± 2 | 40 ± 3 |
| 100 | 11 ± 1 | 13 ± 1 | 11 ± 2 |
| Compd. | Insecticidal Activities (%)/ Concn (μg/mL) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M.S | H.A | S.F | O.N | C.P | A.C | T.C | P.X | ||||||
| 600 | 200 | 600 | 600 | 600 | 10 | 600 | 600 | 200 | 600 | 200 | 100 | 10 | |
| I | 100 | 50 ± 0 | 35 ± 5 | 30 ± 10 | 40 ± 0 | 60 ± 0 | 0 | 0 | – | 90 ± 10 | 60 ± 10 | – | – |
| I-1a | 0 | – | 0 | 0 | 0 | 0 | 0 | 50 ± 0 | – | 70 ± 10 | – | – | – |
| I-1b | 50 ± 10 | – | 20 ± 0 | 20 ± 0 | 0 | 0 | 0 | 0 | – | 30 ± 0 | – | – | – |
| I-1c | 40 ± 10 | – | 15 ± 5 | 10 ± 0 | 20 ± 0 | 0 | 0 | 0 | – | 80 ± 10 | – | – | – |
| I-1d | 30 ± 10 | – | 10 ± 0 | 0 | 0 | 0 | 0 | 0 | – | 0 | – | – | – |
| I-1e | 10 ± 0 | – | 0 | 0 | 0 | 0 | 0 | 0 | – | 55 ± 5 | – | – | – |
| I-1f | 10 ± 0 | – | 10 ± 0 | 10 ± 0 | 15 ± 5 | 0 | 0 | 0 | – | 70 ± 10 | – | – | – |
| I-1g | 20 ± 10 | – | 25 ± 5 | 0 | 0 | 0 | 0 | 0 | – | 85 ± 5 | 65 ± 5 | – | – |
| I-1h | 0 | – | 0 | 0 | 0 | 0 | 0 | 100 | 80 ± 10 | 80 ± 0 | – | – | – |
| II | 50 ± 10 | – | 15 ± 5 | 0 | 0 | 0 | 0 | 0 | – | 85 ± 5 | 55 ± 5 | – | – |
| II-1a | 10 ± 0 | – | 0 | 0 | 15 ± 5 | 0 | 0 | 0 | – | 90 ± 0 | 70 ± 10 | – | – |
| II-1b | 0 | – | 0 | 0 | 0 | 0 | 40 ± 10 | 75 ± 5 | 0 | 100 | 70 ± 0 | – | – |
| II-1c | 30 ± 0 | – | 0 | 0 | 0 | 0 | 0 | 0 | – | 0 | – | – | – |
| II-1d | 0 | – | 0 | 0 | 0 | 0 | 0 | 0 | – | 100 | 100 | 85 ± 5 | 60 ± 0 |
| II-1e | 0 | – | 0 | 0 | 0 | 0 | 0 | 75 ± 5 | 0 | 80 ± 10 | 45 ± 5 | – | – |
| II-1f | 30 ± 0 | – | 15 ± 5 | 10 ± 0 | 25 ± 5 | 0 | 50 ± 0 | 0 | – | 70 ± 0 | – | – | – |
| II-1g | 0 | – | 0 | 0 | 10 ± 0 | 0 | 0 | 0 | – | 75 ± 5 | – | – | – |
| II-1h | 30 ± 0 | – | 20 ± 10 | 20 ± 0 | 40 ± 0 | 0 | 0 | 70 ± 10 | 0 | 100 | 85 ± 5 | 50 ± 0 | – |
| II-1i | 20 ± 10 | – | 0 | 0 | 0 | 0 | 0 | 0 | – | 80 ± 10 | 50 ± 10 | – | – |
| II-1j | 20 ± 10 | – | 0 | 0 | 0 | 0 | 0 | 100 | 65 ± 5 | 90 ± 0 | 65 ± 5 | – | – |
| II-1k | 20 ± 0 | – | 0 | 0 | 0 | 0 | 0 | 0 | – | 60 ± 10 | – | – | – |
| II-1l | 10 ± 0 | – | 15 ± 5 | 15 ± 5 | 30 ± 10 | 0 | 0 | 0 | – | 75 ± 5 | – | – | – |
| II-1m | 15 ± 5 | – | 0 | 15 ± 5 | 10 ± 0 | 0 | 0 | 0 | – | 60 ± 10 | – | – | – |
| III | 30 ± 10 | – | 0 | 15 ± 5 | 0 | 0 | 0 | 0 | – | 40 ± 10 | – | – | – |
| III-1a | 10 ± 0 | – | 0 | 0 | 0 | 0 | 0 | 0 | – | 70 ± 10 | – | – | – |
| III-1b | 20 ± 10 | – | 15 ± 5 | 15 ± 5 | 0 | 0 | 0 | 0 | – | 80 ± 10 | 40 ± 10 | – | – |
| III-1c | 0 | – | 0 | 0 | 0 | 0 | 45 ± 5 | 60 ± 0 | – | 100 | 100 | 70 ± 0 | 30 ± 0 |
| III-1d | 30 ± 10 | – | 0 | 15 ± 5 | 0 | 0 | 0 | 50 ± 0 | – | 100 | 75 ± 5 | – | – |
| III-1e | 30 ± 0 | – | 10 ± 0 | 0 | 0 | 0 | 0 | 0 | – | 75 ± 5 | – | – | – |
| III-1f | 0 | – | 0 | 0 | 0 | 40 ± 10 | 0 | 0 | – | 85 ± 5 | 50 ± 0 | – | – |
| Matrine b | 80 ± 10 | 45 ± 5 | 45 ± 5 | 50 ± 0 | 35 ± 5 | 25 ± 5 | 35 ± 5 | 55 ± 5 | 50 ± 0 | 35 ± 5 | 30 ± 0 | 25 ± 5 | 15 ± 5 |
| Hexaflumuron b | 100 | 100 | 100 | 100 | 45 ± 5 | 55 ± 5 | 45 ± 5 | 100 | 75 ± 5 | 100 | 95 ± 5 | 85 ± 5 | 55 ± 5 |
| Rotenone b | 100 | 35 ± 5 | 50 ± 10 | 55 ± 5 | 95 ± 5 | 100 | 100 | 35 ± 5 | 50 ± 0 | 100 | 35 ± 5 | 20 ± 0 | 20 ± 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Wang, B.; Ma, H.; Wang, Z.; Liu, Y.; Wang, Q. Natural Products for Pesticides Discovery: Structural Diversity Derivation and Biological Activities of Naphthoquinones Plumbagin and Juglone. Molecules 2023, 28, 3328. https://doi.org/10.3390/molecules28083328
Wang K, Wang B, Ma H, Wang Z, Liu Y, Wang Q. Natural Products for Pesticides Discovery: Structural Diversity Derivation and Biological Activities of Naphthoquinones Plumbagin and Juglone. Molecules. 2023; 28(8):3328. https://doi.org/10.3390/molecules28083328
Chicago/Turabian StyleWang, Kaihua, Beibei Wang, Henan Ma, Ziwen Wang, Yuxiu Liu, and Qingmin Wang. 2023. "Natural Products for Pesticides Discovery: Structural Diversity Derivation and Biological Activities of Naphthoquinones Plumbagin and Juglone" Molecules 28, no. 8: 3328. https://doi.org/10.3390/molecules28083328
APA StyleWang, K., Wang, B., Ma, H., Wang, Z., Liu, Y., & Wang, Q. (2023). Natural Products for Pesticides Discovery: Structural Diversity Derivation and Biological Activities of Naphthoquinones Plumbagin and Juglone. Molecules, 28(8), 3328. https://doi.org/10.3390/molecules28083328