Preparation of Polyoxometalate-Based Composite by Solidification of Highly Active Cobalt-Containing Polytungstate on Polymeric Ionic Liquid for the Efficient Isolation of Proteinase K
Abstract
1. Introduction
2. Results and Discussion
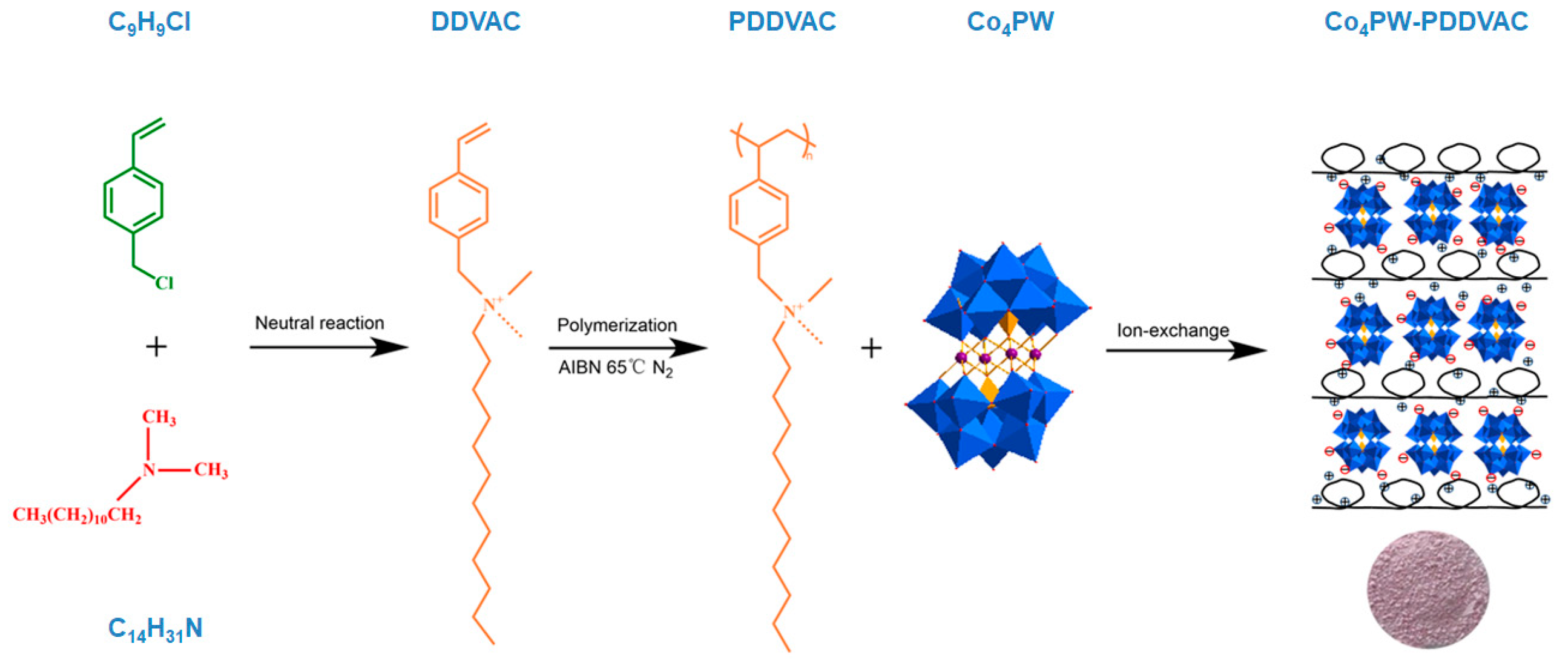
2.1. Fabrication and Characterization of the Co4PW–PDDVAC Composite
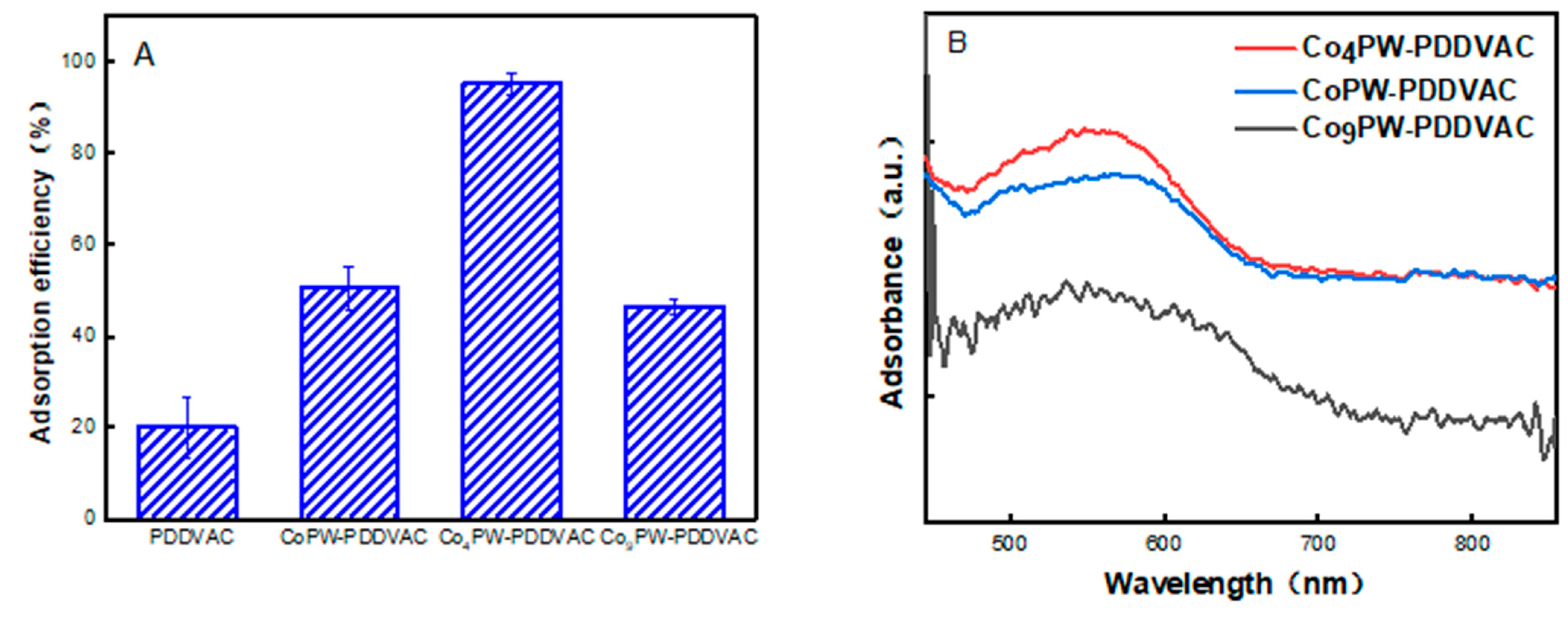
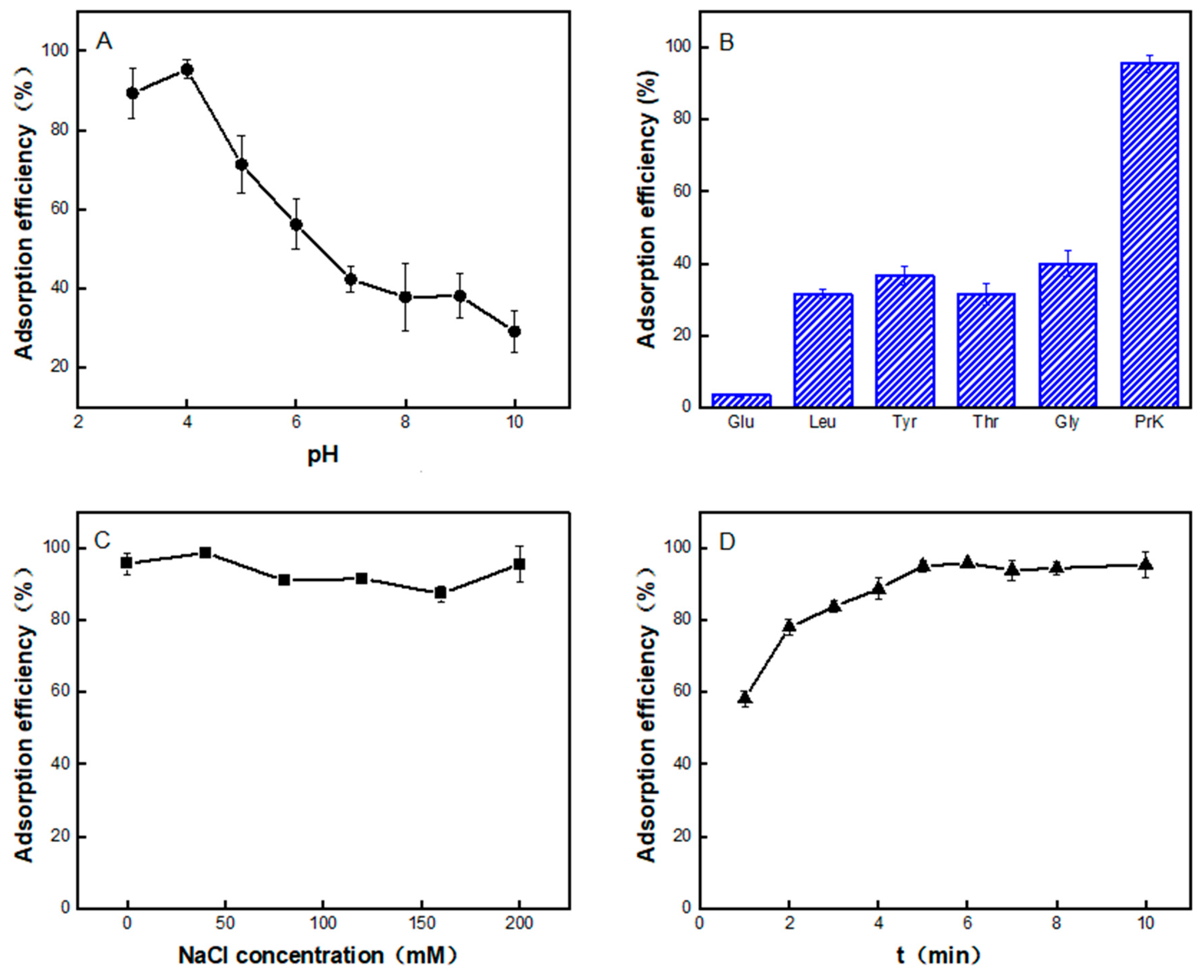
2.2. The Adsorption of PrK by the Co4PW–PDDVAC Composite
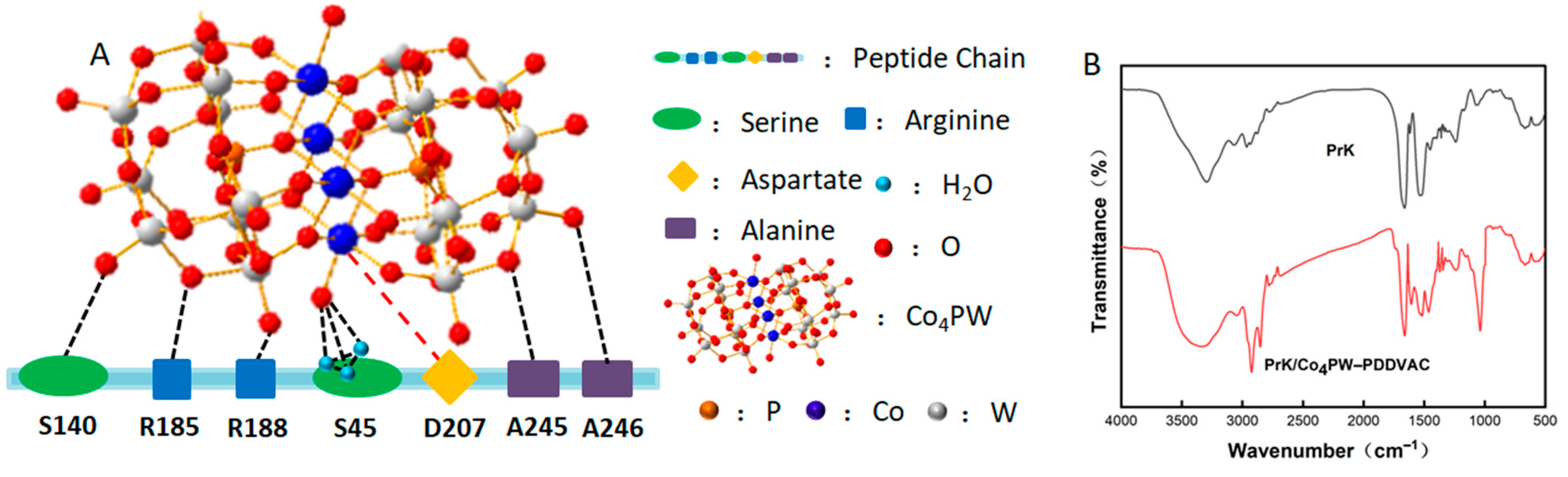
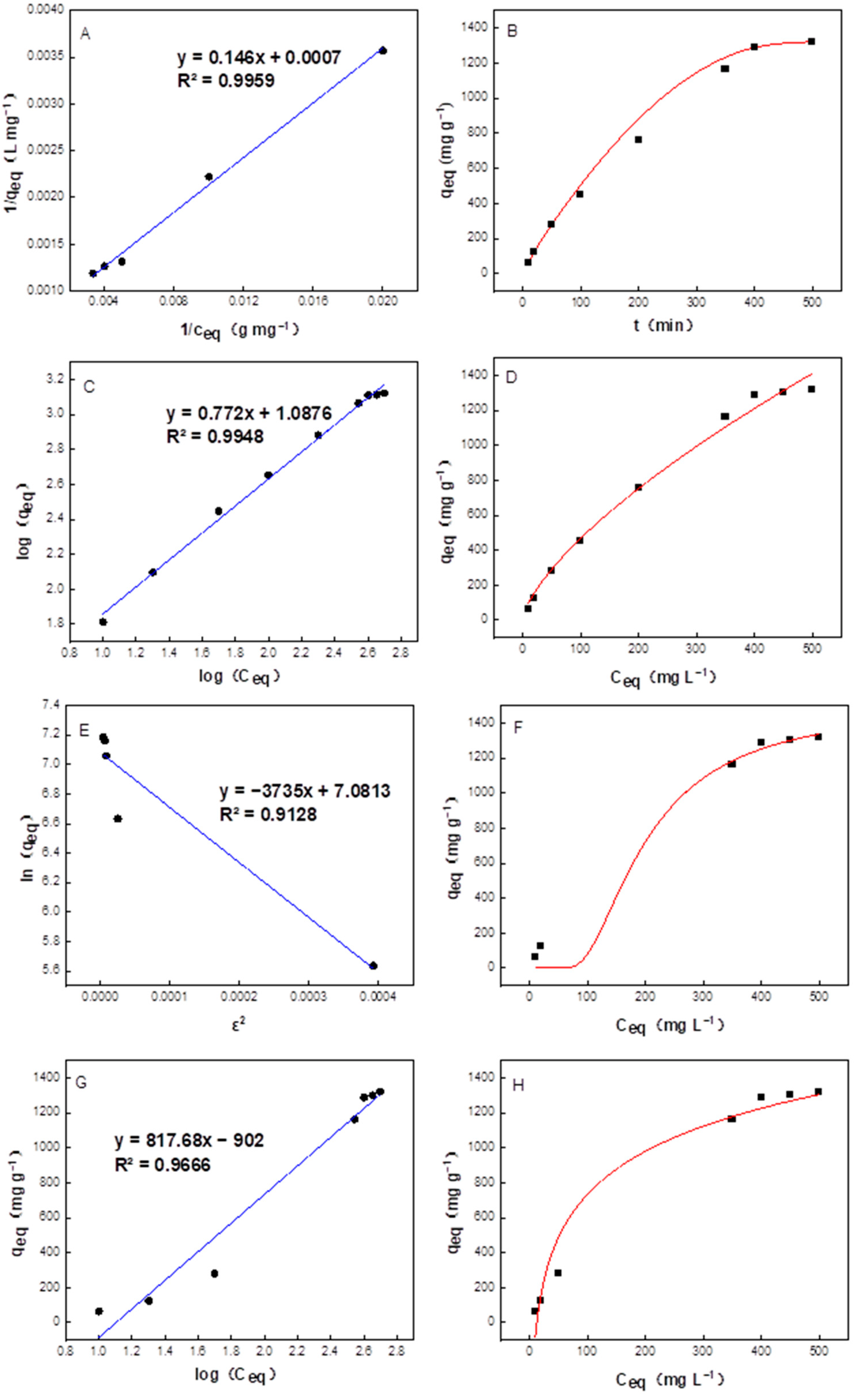
2.3. Thermodynamic Analysis of PrK Adsorption by the Co4PW–PDDVAC Composite
2.4. Kinetic Analysis of PrK Adsorption by the Co4PW–PDDVAC Composite
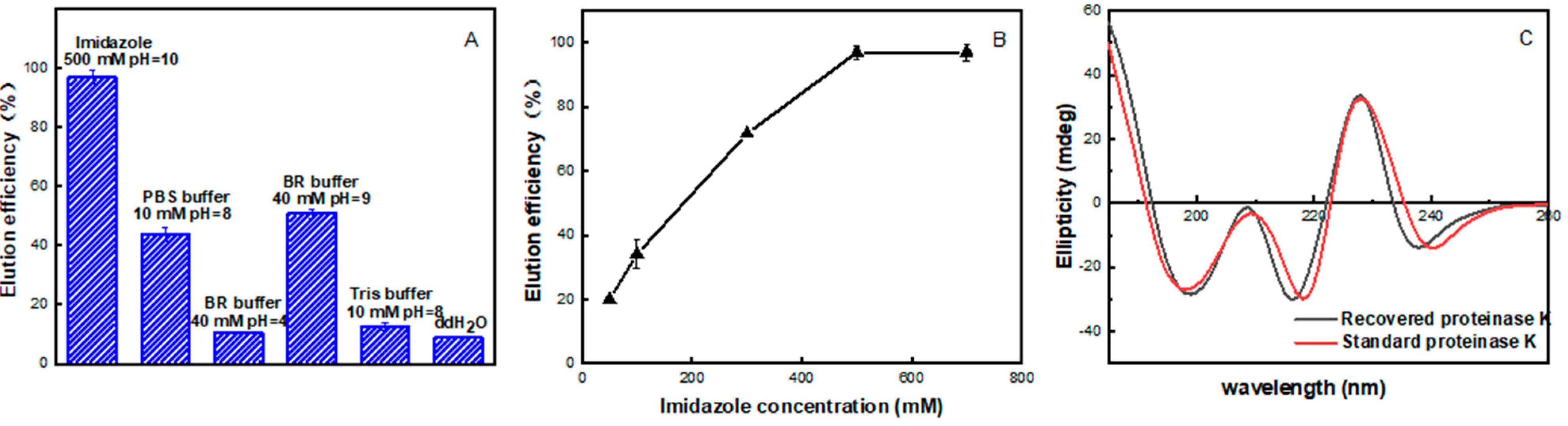
2.5. Recovery of Adsorbed PrK from the Co4PW–PDDVAC Composite
2.6. Reusability and Stability of the Co4PW–PDDVAC Composite
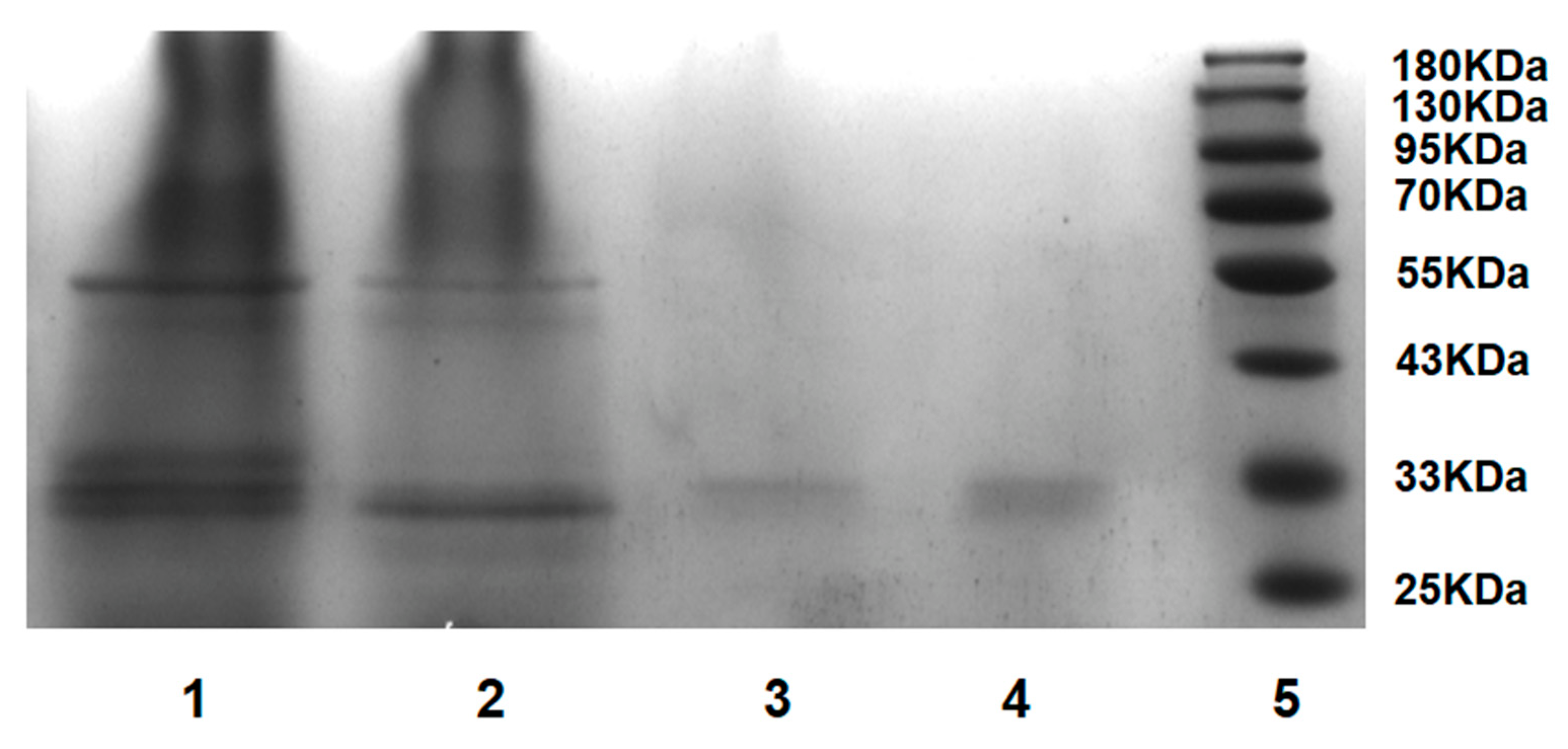
2.7. Isolation of PrK from Tritirachium album Limber
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Synthesis of Water-Soluble Polyoxometalate Co4PW
3.3. Preparation of Polymeric Ionic Liquid PPDVAC
3.4. Fabrication of the Co4PW–PDDVAC Composite
3.5. Characterization of the Co4PW–PDDVAC Composite
3.6. PrK Adsorption by the Co4PW–PDDVAC Composite
3.7. Determination of PrK Activity
3.8. Isolation of PrK from Tritirachium album Limber
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Colette, B. Rare Earth Polyoxometalates. Acc. Chem. Res. 2017, 50, 2205–2214. [Google Scholar] [CrossRef]
- Boubchir, K.I.; Kheddis, B.B.; Rabia, C.; Chebli, M.M.; Hamdi, M.; Silva, A.M.S. Recyclable Keggin Heteropolyacids as an Environmentally Benign Catalyst for the Synthesis of New 2-Benzoylamino-N-phenyl-benzamide Derivatives under Microwave Irradiations at Solvent-Free Conditions and the Evaluation of Biological Activity. Molecules 2018, 23, 8. [Google Scholar] [CrossRef] [PubMed]
- Izarova, N.V.; Pope, M.T.; Kortz, U. Noble Metals in Polyoxometalates. Angew. Chem. Int. Edit. 2012, 51, 9492–9510. [Google Scholar] [CrossRef] [PubMed]
- Craig, L.H.; Christina, M.; Prosser, M. Homogeneous Catalysis by Transition Metal Oxygen Anion Clusters. Coord. Chem. Rev. 1995, 143, 407–455. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.X.; Yang, T.; Cai, Z.W.; Zheng, S.T. Four-Shell Polyoxometalates Featuring High-Nuclearity Ln26 Clusters: Structural Transformations of Nanoclusters into Frameworks Triggered by Transition-Metal Ions. Angew. Chem. Int. Ed. 2017, 56, 2664–2669. [Google Scholar] [CrossRef]
- Victor, W.D.; Walter, G.K. Metal Oxide Chemistry in Solution: The Early Transition Metal Polyoxoanions. Science 1985, 228, 533–541. [Google Scholar] [CrossRef]
- Yin, Q.; Tan, J.M.; Besson, C.; Geletii, Y.V.; Musaev, D.G.; Kuanetsov, A.E.; Luo, Z.; Hardcastle, K.I.; Hill, C.L. A Fast Soluble Carbon-Free Molecular Water Oxidation Catalyst Based on Abundant Metals. Science 2010, 328, 342–345. [Google Scholar] [CrossRef]
- Stracke, J.J.; Finke, R.G. Electrocatalytic Water Oxidation Beginning with the Cobalt Polyoxometalate [Co4(H2O)2(PW9O34)2]10-: Identification of Heterogeneous CoOx as the Dominant Catalyst. J. Am. Chem. Soc. 2011, 133, 14872–14875. [Google Scholar] [CrossRef]
- Nagaiah, T.C.; Gupata, D.; Adhikary, S.D.; Kafle, A.; Mandal, D. Tuning Polyoxometalate Composites with Carbonaceous Materials towards Oxygen Bifunctional Activity. J. Mater. Chem. A 2021, 9, 9228–9237. [Google Scholar] [CrossRef]
- Poole, F.; Shetty, P.H.; Poole, C.F. Organic Salts, Liquid at Room Temperature, as Mobile Phases in Liquid Chromatography. J. Chromatogr. A. 1986, 352, 407–425. [Google Scholar] [CrossRef]
- Krasovskiy, V.G.; Kapustin, J.I.; Gorbatsevich, O.B.; Glukhov, L.M.; Chernikova, E.A.; Koroteev, A.A.; Kustov, L.M. Properties of Dicationic Disiloxane Ionic Liquids. Molecules 2020, 25, 2949. [Google Scholar] [CrossRef]
- Imam, H.T.; Krasňan, V.; Rebros, M.; Marr, A.C. Applications of Ionic Liquids in Whole-Cell and Isolated Enzyme Biocatalysis. Molecules 2021, 26, 4791. [Google Scholar] [CrossRef]
- He, F.; Wang, B.; Zhao, J.; Zhao, X.P.; Yin, J. Influence of Tethered Ions on Electric Polarization and Electrorheological Property of Polymerized Ionic Liquids. Molecules 2020, 25, 2896. [Google Scholar] [CrossRef]
- Hallett, J.P.; Welton, T. Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef]
- Miao, L.; Duan, H.; Liu, M.; Lu, W.; Zhu, D.; Chen, T.; Li, L.; Gan, L. Poly(ionic liquid) Derived, N,S-Codoped Ultramicroporous Carbon Nanoparticles for Supercapacitors. Chem. Eng. J. 2017, 317, 651–659. [Google Scholar] [CrossRef]
- Qian, W.; Texter, J.; Yan, F. Frontiers in Poly(ionic liquid)s: Syntheses and Applications. Chem. Soc. Rev. 2017, 46, 1124–1159. [Google Scholar] [CrossRef]
- Xu, W.; Ledin, P.A.; Shevchenko, V.V.; TsukrukV, V. Architecture, Assembly, and Emerging Applications of Branched Functional Polyelectrolytes and Poly(ionic liquid)s. ACS Appl. Mater. Interfaces 2015, 7, 12570–12596. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Zhu, H.; Wang, W.J.; Li, B.G.; Zhu, S. Collectable and Recyclable Mussel-Inspired Poly(ionic liquid)-Based Sorbents for Ultrafast Water Treatment. ACS. Sustain. Chem. Eng. 2017, 5, 2829–2835. [Google Scholar] [CrossRef]
- Yuan, J.; Mecerreyes, D.; Antonietti, M. Poly(ionic liquid)s: An Update. Prog. Polym. Sci. 2013, 38, 1009–1036. [Google Scholar] [CrossRef]
- Hu, C.C.; Gao, Q.; Liu, S.; Chang, L.L.; Xia, K.S.; Han, B.; Zhou, C.G. Crosslinked Poly(ionic liquid) Anchored with Organic Probe as a New Promising Platform for Organic Solvent-free Recognition, Quantification, and Selective Removal of Heavy Metal Ion. Chem. Eng. J. 2018, 346, 458–465. [Google Scholar] [CrossRef]
- Chen, H.; Liang, Y.; Wang, M.; Lv, P.; Xuan, Y. Reverse ATRP of Ethyl Acrylate with Ionic Liquids as Reaction Medium. Chem. Eng. J. 2019, 147, 297–301. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Y.; Zhao, J.; Tong, Z.; Jin, S. Preparation of SA-g-(PAA-co-PDMC) Polyampholytic Superabsorbent Polymer and Its Application to the Anionic Dye Adsorption Removal From Effluents. Sep. Purif. Technol. 2017, 188, 329–340. [Google Scholar] [CrossRef]
- Li, R.; Liu, Z.; Jiang, F.; Zhao, Y.; Yang, G.Y.; Hong, L. Enhancement of Thermal Stability of PrK by Biocompatible Cholinium-Based Ionic Liquids Phys. Chem. Chem. Phys. 2022, 24, 13057–13065. [Google Scholar] [CrossRef] [PubMed]
- Jany, K.D.; Lederer, G.; Mayer, B. Amino Acid Sequence of PrK from the Mold Tritirachium Album Limber. FEBS. Letters 1986, 199, 139–144. [Google Scholar] [CrossRef]
- Betzel, C.; Gourinath, S.; Kumar, P. Structure of a Serine Protease PrK from Tritirachium Album Limber at 0.98 Å Resolution. Biochemistry 2001, 40, 3080–3088. [Google Scholar] [CrossRef]
- Pähler, A.; Banerjee, A.; Dattagupta, J.K.; Fujiwara, T.; Lindner, K.; Pal, G.P.; Suck, D.; Weber, G.; Saenger, W. Three-Dimensional Structure of Fungal Proteinase K Reveals Similarity to Bacterial Subtilisin. EMBO J. 1984, 3, 1311–1314. [Google Scholar] [CrossRef]
- Li, Q.H.; Qi, W.; Li, W.; Sun, H.; Bu, W.; Wu, L. A Highly Transparent and Luminescent Hybrid Based on the Copolymerization of Surfactant-Encapsulated Polyoxometalate and Methyl Methacrylate. Adv. Mater. 2005, 17, 2688–2692. [Google Scholar] [CrossRef]
- Yang, H.W.; Bai, L.J.; Wei, D.L.; Yang, L.X.; Wang, W.X.; Chen, H.; Niu, Y.Z.; Xue, Z.X. Ionic Self-assembly of Poly(ionic liquid)-Polyoxometalate Hybrids for Selective Adsorption of Anionic Dyes. Chem. Eng. J. 2019, 358, 850–859. [Google Scholar] [CrossRef]
- Roy, S.C.D.; Mourad, M.; Rijneveld-Ockers, T.M. Synthesis and Characterization of Large Surface Hexagonal. Langmuir 2017, 23, 339–401. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.V.; Neimark, A.P.; Olivier, J.; Rodriguez-Reinoso, F.; Rouquerol, J.S.W.; Sing, K. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure. Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Sun, Y.L.; Wang, B.L.; Yang, N.; Tantai, X.W.; Xiao, X.M.; Dou, H.Z.; Zhang, L.H.; Jiang, B.; Wang, D.Q. Synthesis of RGO-Supported Molybdenum Carbide (Mo2C-RGO) for Hydrogen Evolution Reaction under the Function of Poly(Ionic Liquid). Ind. Eng. Chem. Res. 2019, 58, 8996–9005. [Google Scholar] [CrossRef]
- Redondo, E.; Tsai, W.Y.; Daffos, B.; Taberna, P.L.; Simon, P.; Goikolea, E.; Mysyk, R. Outstanding Room-temperature Capacitance of Biomass-derived Microporous Carbons in Ionic Liquid Electrolyte. Electrochem. Commun. 2017, 79, 5–8. [Google Scholar] [CrossRef]
- Guo, P.F.; Zhang, D.D.; Guo, Z.Y. PEGylated Titanate Nanosheets: Hydrophilic Monolayers with a Superior Capacity for the Selective Isolation of Immunoglobulin G. Nanoscale 2018, 10, 12535–12542. [Google Scholar] [CrossRef]
- Maasoumeh, J.; Hossein, K.; Abdolreza, R. A cobalt Schiff Base Complex on TiO2 Nanoparticles as an Effective Synergistic Nanocatalyst for Aerobic C-H Oxidation. RSC. Adv. 2016, 30, 25034–25046. [Google Scholar] [CrossRef]
- Acela, L.B.; Alfredo, G.; Gilles, B. Nickel-Containing Polyoxotungstates Based on [PW9O34]9− and [PW10O39]13− Keggin Lacunary Anions Supported on Al2O3 for Dibenzothiophene Hydrodesulfurization Application. ACS Catal. 2019, 9, 6711–6727. [Google Scholar] [CrossRef]
- Ritu, T.; Dubey, V.K.; Jagannadham, M.V. Effect of Alkyl alcohols on Partially Unfolded State of PrK: Differential Stability of α-helix and β-sheet Rich Regions of the Enzyme. Biochimie 2019, 91, 951–960. [Google Scholar] [CrossRef]
- Joscha, B.; Elias, T.; Nadiia, I.; Gumerova, G.G.; Alexander, P.R.; Annette, R. Speciation of Transition-Metal-Substituted Keggin-Type Silicotungstates Affected by the Co-crystallization Conditions with PrK. Inorg. Chem. 2021, 60, 15096–15100. [Google Scholar] [CrossRef]
- Raghu, A.V.; Gadaginamath, G.S.; Mallikarjuna, N.N.; Aminabhavi, T.M. Synthesis and Characterization of Novel Polyureas Based on Benzimidazoline-2-one and Benzimidazoline-2-thione Hard Segments. J. Appl. Polym. Sci. 2006, 100, 576–583. [Google Scholar] [CrossRef]
- Raghu, A.V.; Jeong, H.M.; Kim, J.H.; Lee, Y.R.; Cho, Y.B.; Sirsalmath, K. Synthesis and Characterization of Novel Polyurethanes Based on 4-{(4-Hydroxyphenyl)iminomethyl}phenol. Macromol. Res. 2008, 16, 194–199. [Google Scholar] [CrossRef]
- Cornelly, V.V.; Sorel, M.; Harry, G.; Dries, B.A.B.; Karin, B.M.; Alphons, G.J.V. FTIR Spectra of Whey and Casein Hydrolysates in Relation to Their Functional Properties. J. Agric. Food. Chem. 2002, 50, 6943–6950. [Google Scholar] [CrossRef]
- Breibeck, J.; Bijelic, A.; Rompel, A. Transition Metal-Substituted Keggin Polyoxotungstates Enabling Covalent Attachment to PrK upon Co-crystallization. Chem. Commun. 2019, 55, 11519–11522. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.D.; Hu, L.L.; Chen, Q.; Chen, X.W.; Wang, J.H. Selective Adsorption of Hemoglobin with Polyoxometalate-Derived Hybrid by Solidification of Super-Lacunary Phosphotungstate Polyoxoanions. Talanta 2019, 159, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Xie, A.; Dai, J.; Cui, J.; Lang, J.; Wei, M.; Dai, X.; Li, C.; Yan, Y. Novel Graphene Oxide-Confined Nanospace Directed Synthesis of Glucose-Based Porous Carbon Nanosheets with Enhanced Adsorption Performance. ACS Sustain. Chem. Eng. 2017, 5, 11566–11576. [Google Scholar] [CrossRef]
- Chang, Y.; Ren, C.; Yang, Q.; Zhang, Z.; Dong, L.; Chen, X.; Xue, D. Preparation and Characterization of Hexadecyl Functionalized Magnetic Silica Nanoparticles and its Application in Rhodamine 6G removal. Appl. Surf. Sci. 2011, 257, 8610–8616. [Google Scholar] [CrossRef]
- Fakhri, A.; Adami, S. Adsorption and Thermodynamic Study of Cephalosporins Antibiotics from Aqueous Solution onto MgO Nanoparticles. J. Taiwan. Inst. Chem. E 2013, 45, 1001–1006. [Google Scholar] [CrossRef]
- Mustapha, S.; Shuaib, D.T.; Ndamitso, M.M.; Etsuyankpa, M.B.; Sumaila, A.; Mohammed, U.M.; Nasirudeen, M.B. Adsorption Isotherm, Kinetic and Thermodynamic Studies for the Removal of Pb(II), Cd(II), Zn(II) and Cu(II) Ions from Aqueous Solutions Using Albizia Lebbeck Pods. Appl. Water Sci. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Fan, S.; Tang, J.; Wang, Y.; Li, H.; Zhang, H.; Tang, J.; Wang, Z.; Li, X. Biochar Prepared from Co-pyrolysis of Municipal Sewage Sludge and Tea Waste for the Adsorption of Methylene Blue from Aqueous Solutions: Kinetics, Isotherm, Thermodynamic and Mechanism. J. Mol. Liq. 2016, 20, 432–441. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, Z. Application of Dubinin–Radushkevich Isotherm Model at the Solid/Solution Interface: A Theoretical Analysis. J. Mol. Liq. 2019, 277, 646–648. [Google Scholar] [CrossRef]
- Piccin, J.S.; Dotto, G.L.; Pinto, L.A.A. Adsorption Isotherms and Thermochemical Data of FDandC RED N°40 Binding by Chitosan. Braz. J. Chem. Eng. 2011, 28, 295–304. [Google Scholar] [CrossRef]
- Low, M.J.D. Kinetics of Chemisorption of Gases on Solids. Chem. Rev. 1960, 60, 267–312. [Google Scholar] [CrossRef]
- Ebeling, W.; Hennrich, N.; Klockow, M.; Metz, H.; Orth, H.D.; Lang, H. PrK from Tritirachium Album Limber. Eur. J. Biochem. 1974, 47, 91–97. [Google Scholar] [CrossRef]
- Kunitz, M. Crystalline Soybean Trypsin Inhibitor. Gen. Physiol. Biophys. 1947, 30, 291–310. [Google Scholar] [CrossRef]


| T (K) | Kd (L g−1) | ΔS0 (J mol−1) | ΔH0 (KJ mol−1) | ΔG0 (KJ mol−1) | |
|---|---|---|---|---|---|
| PrK | 288 | 6899.85 | −98.48 | −49.62 | −21.26 |
| 293 | 5005.98 | −20.77 | |||
| 303 | 2868.55 | −19.78 | |||
| 318 | 909.48 | −18.30 | |||
| 323 | 796.75 | −17.81 |
| Parameters | Linear | Nonlinear |
|---|---|---|
| Langmuir model | ||
| bL (L mg−1) | 0.004794 | 0.002180 |
| Qm (mg g−1) | 1428 | 2628 |
| R2 | 0.9959 | 0.9951 |
| Freundlich model | ||
| n | 1.295 | 1.457 |
| KF (mg g−1) | 12.23 | 19.81 |
| R2 | 0.9948 | 0.9899 |
| Dubinin–Radushkevich model | ||
| β (mol2 KJ−2) | 3735 | 0.004795 |
| Qm (mg g−1) | 1190 | 1503 |
| R2 | 0.9128 | 0.9882 |
| Temkin model | ||
| KT (L/mg) | 0.06310 | 0.07886 |
| BT (KJ mol−1) | 3.300 | 6.977 |
| R2 | 0.9666 | 0.9666 |
| Parameters | Linear | Nonlinear |
|---|---|---|
| Pseudo-first-order | ||
| qeq (mg g−1) | 247.0 | 474.0 |
| K1 (min−1) | 0.4521 | 0.8240 |
| R2 | 0.9944 | 0.9370 |
| Pseudo-second-order | ||
| qeq (mg g−1) | 512.0 | 526.0 |
| K2 (g mg−1 min−1) | 0.003151 | 0.002543 |
| R2 | 0.9980 | 0.9720 |
| Intraparticle | ||
| K3 (mg g−1 min−1/2) | 78.70 | 78.70 |
| C (mg g−1) | 265.0 | 265.0 |
| R2 | 0.7761 | 0.7443 |
| Elovich | ||
| α | 3402 | 4338 |
| β | 0.01200 | 0.01250 |
| R2 | 0.9070 | 0.8800 |
| Commercial Procedure [51] | This Work | |
|---|---|---|
| Isolation technique | Ammonium sulfate precipitate + Sephadex G-75 chromatography | Co4PW–PDDVAC-based solid-phase adsorption |
| Purification time | 2–3 d | 35 min (adsorption 5 min + elution 30 min) |
| Protein yield | 2% | 79.1% |
| Activity yield | 28% | 68.1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Chu, N.; Chen, X. Preparation of Polyoxometalate-Based Composite by Solidification of Highly Active Cobalt-Containing Polytungstate on Polymeric Ionic Liquid for the Efficient Isolation of Proteinase K. Molecules 2023, 28, 3307. https://doi.org/10.3390/molecules28083307
Yang J, Chu N, Chen X. Preparation of Polyoxometalate-Based Composite by Solidification of Highly Active Cobalt-Containing Polytungstate on Polymeric Ionic Liquid for the Efficient Isolation of Proteinase K. Molecules. 2023; 28(8):3307. https://doi.org/10.3390/molecules28083307
Chicago/Turabian StyleYang, Jiaxuan, Ning Chu, and Xuwei Chen. 2023. "Preparation of Polyoxometalate-Based Composite by Solidification of Highly Active Cobalt-Containing Polytungstate on Polymeric Ionic Liquid for the Efficient Isolation of Proteinase K" Molecules 28, no. 8: 3307. https://doi.org/10.3390/molecules28083307
APA StyleYang, J., Chu, N., & Chen, X. (2023). Preparation of Polyoxometalate-Based Composite by Solidification of Highly Active Cobalt-Containing Polytungstate on Polymeric Ionic Liquid for the Efficient Isolation of Proteinase K. Molecules, 28(8), 3307. https://doi.org/10.3390/molecules28083307







