The Preparation and Physiochemical Characterization of Tenebrio molitor Chitin Using Alcalase
Abstract
1. Introduction
2. Results and Discussions
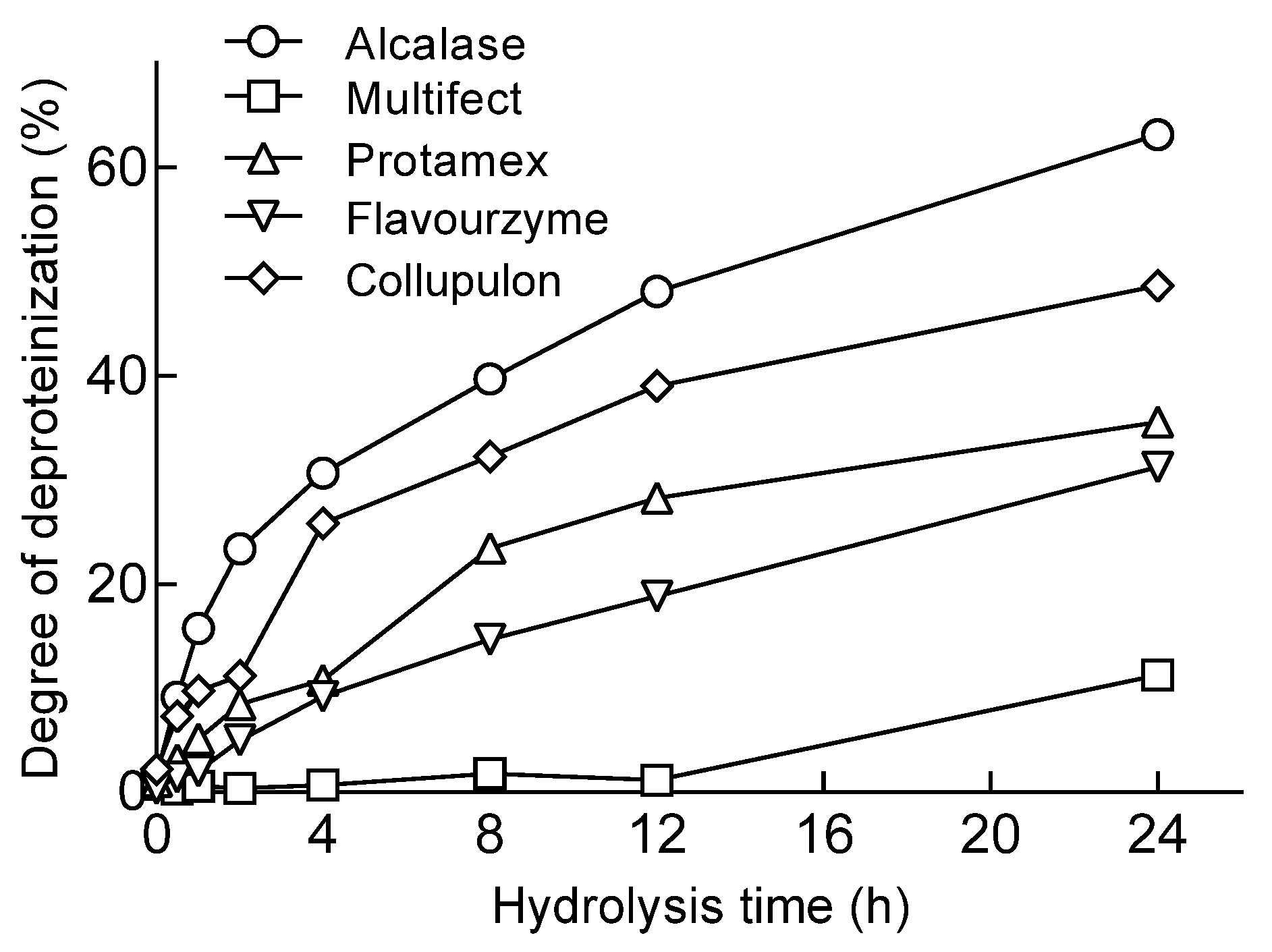
2.1. Protease Screening for Protein Removal from Mealworms
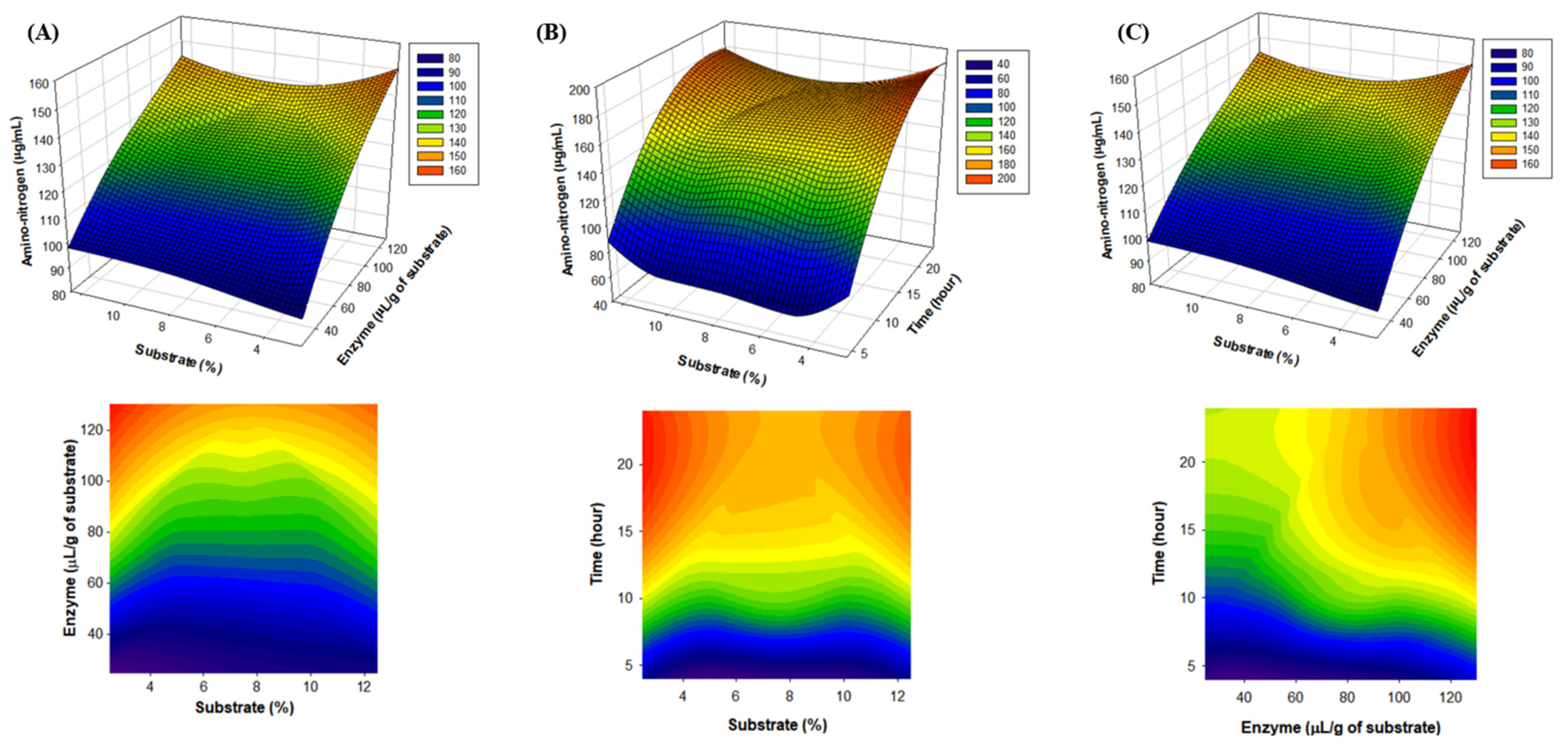
2.2. Establishment of Optimal Alcalase Hydrolysis Conditions for Mealworm Protein Removal by RSM
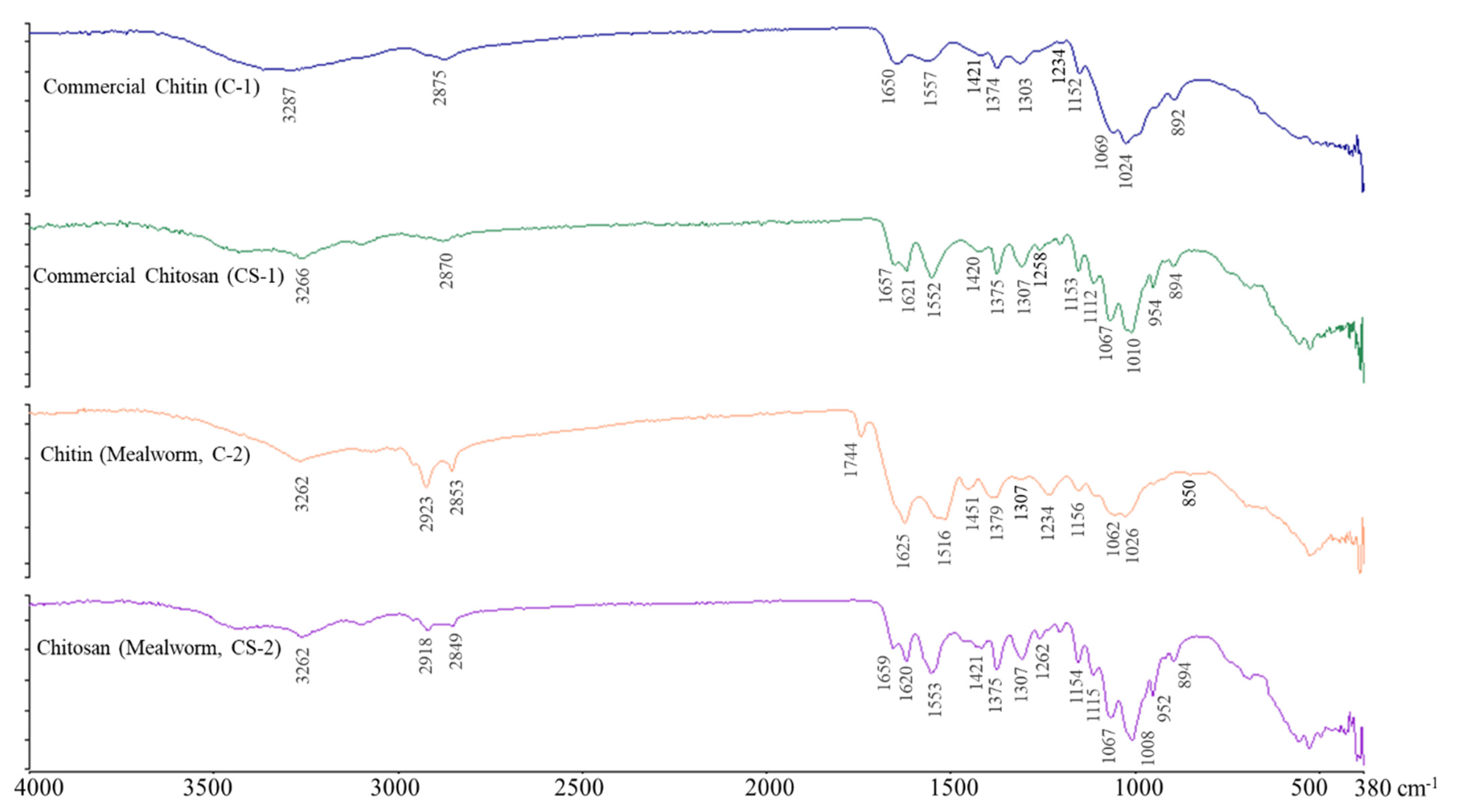
2.3. FT-IR Spectroscopy of Chitin and Chitosan
2.4. Thermodynamic Properties of Chitin and Chitosan Using DSC
2.5. Degree of Deacetylation (DDA) and Solubility of Chitosan
3. Materials and Methods
3.1. Materials
3.2. Mealworm Protein Hydrolysis and Deacetylation
3.3. Determination of Degree of Deproteinization (DP)
3.4. Optimization of Protein Removal Conditions by Alcalase by Response Surface Methodology (RSM)
3.5. α-Amino Nitrogen (AN) and Crude Protein Analysis
3.6. Solubility (%) of Chitosan Derived from Mealworm
3.7. FT-IR Spectroscopy of Chitin and Chitosan
3.8. Thermal Characterization of Chitin and Chitosan by Differential Scanning Calorimetry (DSC)
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jang, M.K.; Kong, B.G.; Jeong, Y.I.; Lee, C.H.; Nah, J.W. Physicochemical characterization of α-chitin, β-chitin, and γ-chitin separated from natural resources. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 3423–3432. [Google Scholar] [CrossRef]
- Islam, S.; Bhuiyan, M.R.; Islam, M. Chitin and chitosan: Structure, properties and applications in biomedical engineering. J. Polym. Environ. 2017, 25, 854–866. [Google Scholar] [CrossRef]
- Tarique, J.; Sapuan, S.; Aqil, N.; Farhan, A.; Faiz, J.; Shahrizan, S. A Comprehensive Review Based on Chitin and Chitosan Composites. In Composites from the Aquatic Environment; Springer: Singapore, 2023; pp. 15–66. [Google Scholar]
- Khayrova, A.; Lopatin, S.; Varlamov, V. Obtaining chitin, chitosan and their melanin complexes from insects. Int. J. Biol. Macromol. 2021, 167, 1319–1328. [Google Scholar] [CrossRef]
- Jantzen da Silva Lucas, A.; Quadro Oreste, E.; Leao Gouveia Costa, H.; Martin Lopez, H.; Dias Medeiros Saad, C.; Prentice, C. Extraction, physicochemical characterization, and morphological properties of chitin and chitosan from cuticles of edible insects. Food Chem. 2021, 343, 128550. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Guo, N.; Sun, J.; Xue, C. Comprehensive utilization of shrimp waste based on biotechnological methods: A review. J. Clean. Prod. 2017, 143, 814–823. [Google Scholar] [CrossRef]
- ur Rehman, K.; Hollah, C.; Wiesotzki, K.; Heinz, V.; Aganovic, K.; ur Rehman, R.; Petrusan, J.-I.; Zheng, L.; Zhang, J.; Sohail, S. Insect-derived chitin and chitosan: A still unexploited resource for the edible insect sector. Sustainability 2023, 15, 4864. [Google Scholar] [CrossRef]
- Islam, N.; Hoque, M.; Taharat, S.F. Recent advances in extraction of chitin and chitosan. World J. Microb. Biotechnol. 2023, 39, 28. [Google Scholar] [CrossRef]
- Rass-Hansen, J.; Christensen, C.H.; Sehested, J.; Helveg, S.; Rostrup-Nielsen, J.R.; Dahl, S. Renewable hydrogen: Carbon formation on Ni and Ru catalysts during ethanol steam-reforming. Green Chem. 2007, 9, 1016–1021. [Google Scholar] [CrossRef]
- Jegannathan, K.R.; Nielsen, P.H. Environmental assessment of enzyme use in industrial production—A literature review. J. Clean. Prod. 2013, 42, 228–240. [Google Scholar] [CrossRef]
- Merzendorfer, H.; Zimoch, L. Chitin metabolism in insects: Structure, function and regulation of chitin synthases and chitinases. J. Exp. Biol. 2003, 206, 4393–4412. [Google Scholar] [CrossRef]
- Papastavropoulou, K.; Xiao, J.; Proestos, C. Edible insects: Tendency or necessity (a review). eFood 2023, 4, e58. [Google Scholar] [CrossRef]
- Chung, M.Y.; Kwon, E.-Y.; Hwang, J.-S.; Goo, T.-W.; Yun, E.-Y. Pre-treatment conditions on the powder of Tenebrio molitor for using as a novel food ingredient. J. Sericult. Entomol. Sci. 2013, 51, 9–14. [Google Scholar]
- Lee, J.-H.; Lee, J.; Whang, J.; Nam, J.-S.; Han, H.-K.; Kim, S.-M.; Im, J.Y.; Choi, Y.; Kim, H.R.; Kim, S.-N. Changes in food composition of Tenebrio molitor by life stage. Korean J. Food Cook. Sci. 2016, 32, 656–663. [Google Scholar] [CrossRef]
- Hwang, S.-Y.; Choi, S.-K. Quality characteristics of muffins containing mealworm (Tenebrio molitor). Culin. Sci. Hosp. Res. 2015, 21, 104–115. [Google Scholar]
- Kim, S.; Kim, J.; Oh, H.; Kang, S.; Koo, H.; Kim, H.; Choi, H. Feed supplementation of yellow mealworms (Tenebrio molitor L.) improves blood characteristics and meat quality in broiler. Trends Agric. Life Sci. 2014, 49, 9–18. [Google Scholar]
- Song, Y.S.; Kim, M.W.; Moon, C.; Seo, D.J.; Han, Y.S.; Jo, Y.H.; Noh, M.Y.; Park, Y.K.; Kim, S.A.; Kim, Y.W. Extraction of chitin and chitosan from larval exuvium and whole body of edible mealworm, Tenebrio molitor. Entomol. Res. 2018, 48, 227–233. [Google Scholar] [CrossRef]
- Yu, M.-H.; Lee, H.-S.; Cho, H.-R.; Lee, S.-O. Enzymatic preparation and antioxidant activities of protein hydrolysates from Tenebrio molitor larvae (Mealworm). J. Korean Soc. Food Sci. Nutr. 2017, 46, 435–441. [Google Scholar] [CrossRef]
- Lee, H.-S.; Ryu, H.-J.; Song, H.-J.; Lee, S.-O. Enzymatic preparation and antioxidant activities of protein hydrolysates from Protaetia brevitarsis larvae. J. Korean Soc. Food Sci. Nutr. 2017, 46, 1164–1170. [Google Scholar]
- Hahn, T.; Tafi, E.; Paul, A.; Salvia, R.; Falabella, P.; Zibek, S. Current state of chitin purification and chitosan production from insects. J. Chem. Technol. Biotechnol. 2020, 95, 2775–2795. [Google Scholar] [CrossRef]
- Ibitoye, E.B.; Lokman, I.H.; Hezmee, M.N.M.; Goh, Y.M.; Zuki, A.B.Z.; Jimoh, A.A. Extraction and physicochemical characterization of chitin and chitosan isolated from house cricket. Biomed. Mater. 2018, 13, 025009. [Google Scholar] [CrossRef]
- Campana-Filho, S.P.; Britto, D.d.; Curti, E.; Abreu, F.R.; Cardoso, M.B.; Battisti, M.V.; Sim, P.C.; Goy, R.C.; Signini, R.; Lavall, R.L. Extraction, structures and properties of alpha-and beta-chitin. Quím. Nova 2007, 30, 644–650. [Google Scholar] [CrossRef]
- Liu, S.; Sun, J.; Yu, L.; Zhang, C.; Bi, J.; Zhu, F.; Qu, M.; Jiang, C.; Yang, Q. Extraction and characterization of chitin from the beetle Holotrichia parallela Motschulsky. Molecules 2012, 17, 4604–4611. [Google Scholar] [CrossRef]
- Lim, S.H.; Hudson, S.M. Synthesis and antimicrobial activity of a water-soluble chitosan derivative with a fiber-reactive group. Carbohydr. Res. 2004, 339, 313–319. [Google Scholar] [CrossRef]
- Song, C.; Yu, H.; Zhang, M.; Yang, Y.; Zhang, G. Physicochemical properties and antioxidant activity of chitosan from the blowfly Chrysomya megacephala larvae. Int. J. Biol. Macromol. 2013, 60, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Vino, A.B.; Ramasamy, P.; Shanmugam, V.; Shanmugam, A. Extraction, characterization and in vitro antioxidative potential of chitosan and sulfated chitosan from Cuttlebone of Sepia aculeata Orbigny, 1848. Asian Pac. J. Trop. Biomed. 2012, 2, S334–S341. [Google Scholar] [CrossRef]
- Delezuk, J.A.d.M.; Cardoso, M.B.; Domard, A.; Campana-Filho, S.P. Ultrasound-assisted deacetylation of beta-chitin: Influence of processing parameters. Polym. Int. 2011, 60, 903–909. [Google Scholar] [CrossRef]
- Cestari, A.R.; Vieira, E.F.; Tavares, A.M.; Bruns, R.E. The removal of the indigo carmine dye from aqueous solutions using cross-linked chitosan: Evaluation of adsorption thermodynamics using a full factorial design. J. Hazard. Mater. 2008, 153, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Soon, C.Y.; Tee, Y.B.; Tan, C.H.; Rosnita, A.T.; Khalina, A. Extraction and physicochemical characterization of chitin and chitosan from Zophobas morio larvae in varying sodium hydroxide concentration. Int. J. Biol. Macromol. 2018, 108, 135–142. [Google Scholar] [CrossRef]
- Kim, M.W.; Song, Y.S.; Han, Y.S.; Jo, Y.H.; Choi, M.H.; Park, Y.K.; Kang, S.H.; Kim, S.A.; Choi, C.; Jung, W.J. Production of chitin and chitosan from the exoskeleton of adult two-spotted field crickets (Gryllus bimaculatus). Entomol. Res. 2017, 47, 279–285. [Google Scholar] [CrossRef]
- Bellich, B.; D’Agostino, I.; Semeraro, S.; Gamini, A.; Cesàro, A. “The good, the bad and the ugly” of chitosans. Mar. Drugs 2016, 14, 99. [Google Scholar] [CrossRef]
- Kubota, N.; Eguchi, Y. Facile preparation of water-soluble N-acetylated chitosan and molecular weight dependence of its water-solubility. Polym. J. 1997, 29, 123–127. [Google Scholar] [CrossRef]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Baek, H.; Cadwallader, K. Enzymatic hydrolysis of crayfish processing by-products. J. Food Sci. 1995, 60, 929–935. [Google Scholar] [CrossRef]
- Habeeb, A.F. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal. Biochem. 1966, 14, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Baxter, A.; Dillon, M.; Taylor, K.D.A.; Roberts, G.A.F. Improved method for i.r. determination of the degree of n-acetylation of chitosan. Int. J. Biol. Macromol. 1992, 14, 166–169. [Google Scholar] [CrossRef] [PubMed]
| RSM Result | Actual Result | ||||||
|---|---|---|---|---|---|---|---|
| Substrate (g) | Enzyme (μL) | Time (h) | Amino-Nitrogen (mg/g of Sample) | Substrate (g) | Enzyme (μL) | Time (h) | Amino-Nitrogen (mg/g of Sample) |
| 7.5 | 80 | 24 | 163.17 | 7.5 | 80 | 24 | 162.99 |
| Sample | Exotherm (°C) | Endotherm (°C) | ||||||
|---|---|---|---|---|---|---|---|---|
| Ts1 (°C) | Tmax1 (°C) | Te1 (°C) | ΔH1 (J/g) | Ts2 (°C) | Tmax2 (°C) | Te2 (°C) | ΔH2 (J/g) | |
| commercial chitin | 140.77 | 143.29 | 157.37 | −116.6 | 349.90 | 385.40 | 397.15 | 17.848 |
| mealworm chitin | 132.82 | 153.76 | 179.76 | −91.08 | 325.22 | 359.82 | 394.48 | 24.075 |
| commercial chitosan | 140.77 | 143.65 | 165.68 | −183.1 | 281.20 | 306.47 | 331.38 | 104.7 |
| mealworm chitosan | 141.13 | 147.63 | 197.81 | −179.9 | 301.06 | 337.52 | 371.82 | 181.7 |
| Mealworm Chitosan | Commercial Chitosan | |
|---|---|---|
| Degree of deacetylation (%) | 37.34 ± 4.35 | 57.58 ± 7.88 |
| Solubility (%) | 45.94 ± 15.33 | 61.34 ± 15.96 |
| Run Order | Coded Variables | Real Variables | Amino-Nitrogen (mg/g of Sample) | ||||
|---|---|---|---|---|---|---|---|
| X | Y | Z | X Substrate Concentration (%) | Y Enzyme Concentration (%) | Z Time (h) | ||
| 1 | −1.00000 | −1.00000 | −1.00000 | 5 | 50 | 6 | 83.46 ± 0.33 |
| 2 | 0.00000 | −1.68179 | 0.00000 | 7.5 | 25 | 8 | 94.93 ± 4.35 |
| 3 | 1.0000 | −1.0000 | −1.00000 | 10 | 50 | 6 | 83.49 ± 0.06 |
| 4 | 1.68179 | 0.00000 | 0.00000 | 12.5 | 80 | 8 | 122.03 ± 0.94 |
| 5 | 1.00000 | 1.00000 | −1.00000 | 10 | 100 | 6 | 99.45 ± 3.48 |
| 6 | 0.00000 | 0.00000 | 1.68179 | 7.5 | 80 | 24 | 162.99 ± 4.68 |
| 7 | 0.00000 | 0.00000 | 0.00000 | 7.5 | 80 | 8 | 118.61 ± 0.63 |
| 8 | 0.00000 | 0.00000 | 0.00000 | 7.5 | 80 | 8 | 119.66 ± 1.60 |
| 9 | 1.00000 | 1.00000 | 1.00000 | 10 | 100 | 12 | 148.87 ± 3.34 |
| 10 | 0.00000 | 0.00000 | 0.00000 | 7.5 | 80 | 8 | 122.48 ± 1.77 |
| 11 | −1.00000 | −1.00000 | −1.00000 | 5 | 50 | 6 | 78.42 ± 0.78 |
| 12 | 0.00000 | 0.00000 | 0.00000 | 7.5 | 80 | 8 | 121.15 ± 3.01 |
| 13 | −1.68179 | 0.00000 | 0.00000 | 2.5 | 80 | 8 | 132.79 ± 1.38 |
| 14 | 0.00000 | 0.00000 | 0.00000 | 7.5 | 80 | 8 | 116.00 ± 2.08 |
| 15 | −1.00000 | 1.00000 | 1.00000 | 5 | 100 | 12 | 157.99 ± 0.92 |
| 16 | 0.00000 | 0.00000 | 0.00000 | 7.5 | 80 | 8 | 120.07 ± 0.20 |
| 17 | 0.00000 | 0.00000 | −1.68179 | 7.5 | 80 | 4 | 65.57 ± 1.74 |
| 18 | 0.00000 | 0.00000 | 0.00000 | 7.5 | 80 | 8 | 121.79 ± 3.02 |
| 19 | −1.00000 | −1.00000 | 1.00000 | 5 | 50 | 12 | 127.91 ± 1.14 |
| 20 | 1.00000 | −1.00000 | −1.00000 | 10 | 50 | 6 | 83.45 ± 5.49 |
| 21 | 0.00000 | 0.00000 | 0.00000 | 7.5 | 80 | 8 | 122.96 ± 2.51 |
| 22 | 0.00000 | −1.68179 | 0.00000 | 7.5 | 25 | 8 | 97.14 ± 1.94 |
| 23 | 1.68179 | 0.00000 | 0.00000 | 12.5 | 80 | 8 | 130.52 ± 1.02 |
| 24 | 0.00000 | 0.00000 | 0.00000 | 7.5 | 80 | 8 | 129.01 ± 0.79 |
| 25 | 0.00000 | 0.00000 | 1.68179 | 7.5 | 80 | 24 | 162.01 ± 2.17 |
| 26 | 0.00000 | 0.00000 | −1.68179 | 7.5 | 80 | 4 | 67.89 ± 3.08 |
| 27 | 0.00000 | 0.00000 | 0.00000 | 7.5 | 80 | 8 | 120.45 ± 1.74 |
| 28 | −1.00000 | 1.00000 | −1.00000 | 5 | 100 | 6 | 94.96 ± 0.41 |
| 29 | 1.00000 | 1.00000 | −1.00000 | 10 | 100 | 6 | 99.25 ± 0.57 |
| 30 | 0.00000 | 1.68179 | 0.00000 | 7.5 | 130 | 8 | 136.45 ± 4.17 |
| 31 | −1.00000 | −1.00000 | 1.00000 | 5 | 50 | 12 | 130.30 ± 2.66 |
| 32 | −1.68179 | 0.00000 | 0.00000 | 2.5 | 80 | 8 | 127.57 ± 1.31 |
| 33 | 1.00000 | 1.00000 | 1.00000 | 10 | 100 | 12 | 155.39 ± 1.99 |
| 34 | 0.00000 | 0.00000 | 0.00000 | 7.5 | 80 | 8 | 119.53 ± 2.70 |
| 35 | 0.00000 | 1.68179 | 0.00000 | 7.5 | 130 | 8 | 139.70 ± 2.11 |
| 36 | 1.00000 | −1.00000 | 1.00000 | 10 | 50 | 12 | 132.45 ± 4.34 |
| 37 | 1.00000 | −1.00000 | 1.00000 | 10 | 50 | 12 | 131.45 ± 2.71 |
| 38 | −1.00000 | 1.00000 | −1.00000 | 5 | 100 | 6 | 94.92 ± 2.38 |
| 39 | 0.00000 | 0.00000 | 0.00000 | 7.5 | 80 | 8 | 125.42 ± 1.43 |
| 40 | −1.00000 | 1.00000 | 1.00000 | 5 | 100 | 12 | 159.73 ± 1.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Kim, H.; Ahn, Y.; Hong, K.-B.; Kim, I.-W.; Choi, R.-Y.; Suh, H.J.; Han, S.H. The Preparation and Physiochemical Characterization of Tenebrio molitor Chitin Using Alcalase. Molecules 2023, 28, 3254. https://doi.org/10.3390/molecules28073254
Kim H, Kim H, Ahn Y, Hong K-B, Kim I-W, Choi R-Y, Suh HJ, Han SH. The Preparation and Physiochemical Characterization of Tenebrio molitor Chitin Using Alcalase. Molecules. 2023; 28(7):3254. https://doi.org/10.3390/molecules28073254
Chicago/Turabian StyleKim, Hyemi, Hyeongyeong Kim, Yejin Ahn, Ki-Bae Hong, In-Woo Kim, Ra-Yeong Choi, Hyung Joo Suh, and Sung Hee Han. 2023. "The Preparation and Physiochemical Characterization of Tenebrio molitor Chitin Using Alcalase" Molecules 28, no. 7: 3254. https://doi.org/10.3390/molecules28073254
APA StyleKim, H., Kim, H., Ahn, Y., Hong, K.-B., Kim, I.-W., Choi, R.-Y., Suh, H. J., & Han, S. H. (2023). The Preparation and Physiochemical Characterization of Tenebrio molitor Chitin Using Alcalase. Molecules, 28(7), 3254. https://doi.org/10.3390/molecules28073254







