Anti-SARS-CoV-2 Activity and Cytotoxicity of Amaryllidaceae Alkaloids from Hymenocallis littoralis
Abstract
1. Introduction
2. Results and Discussion
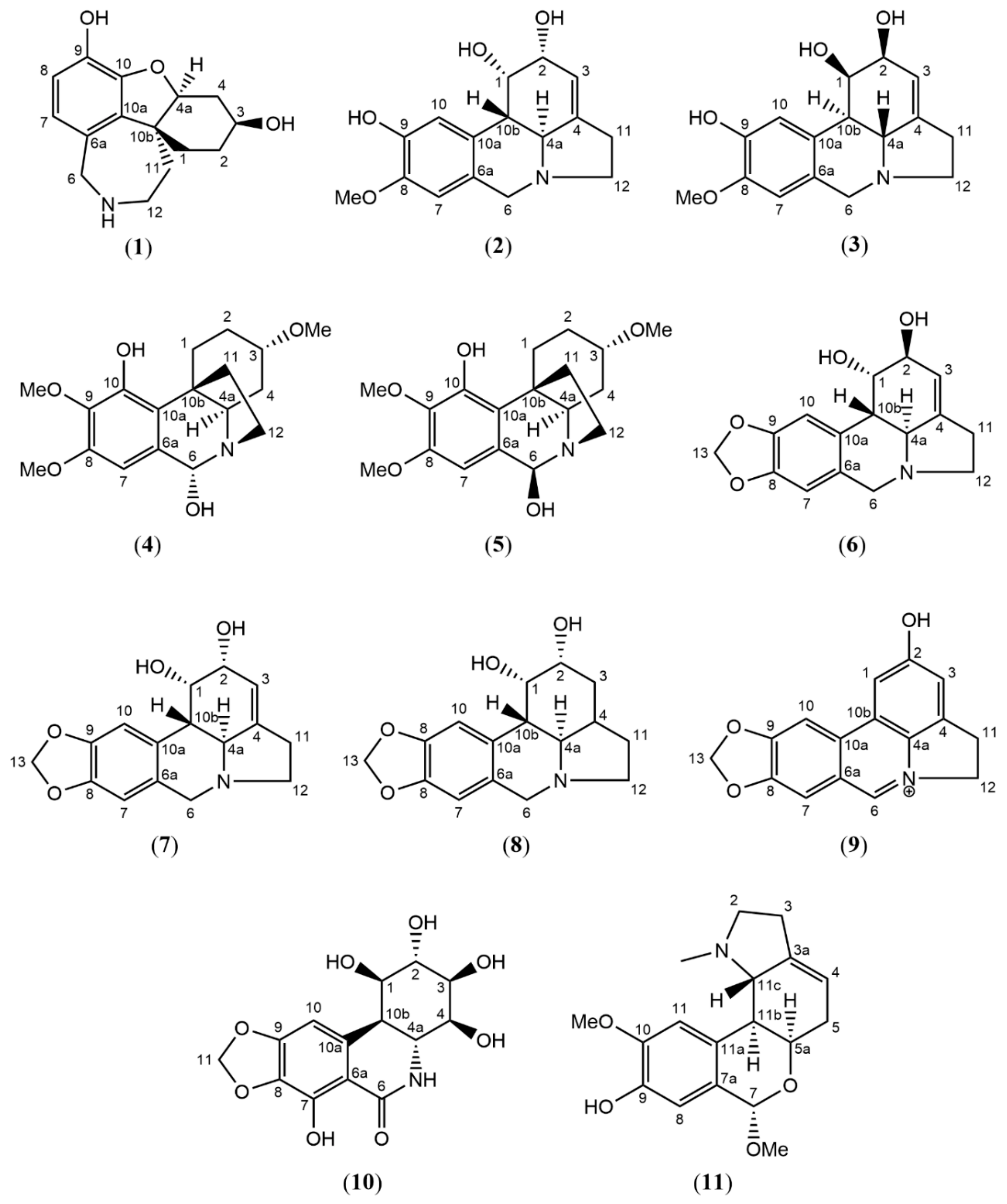
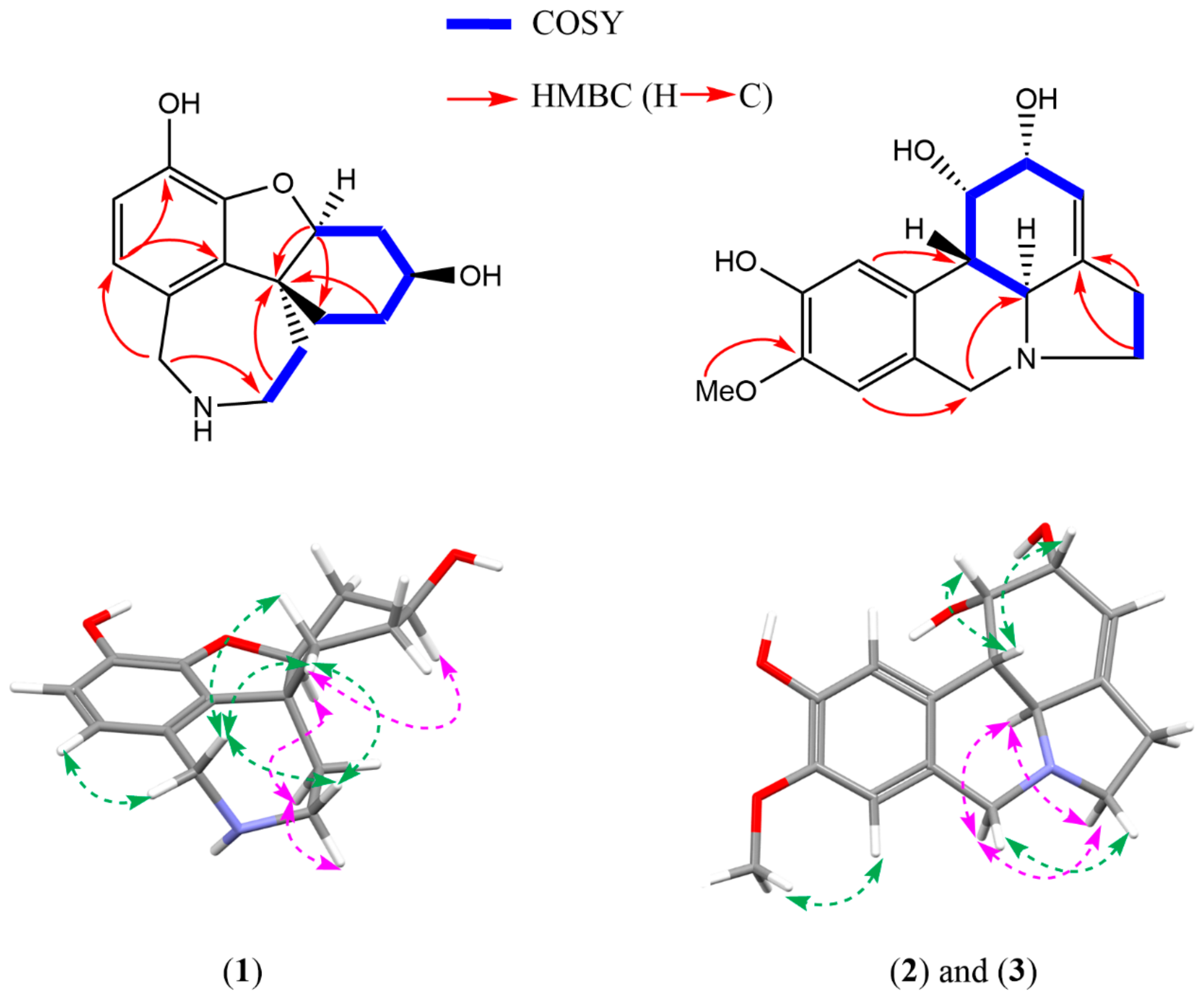
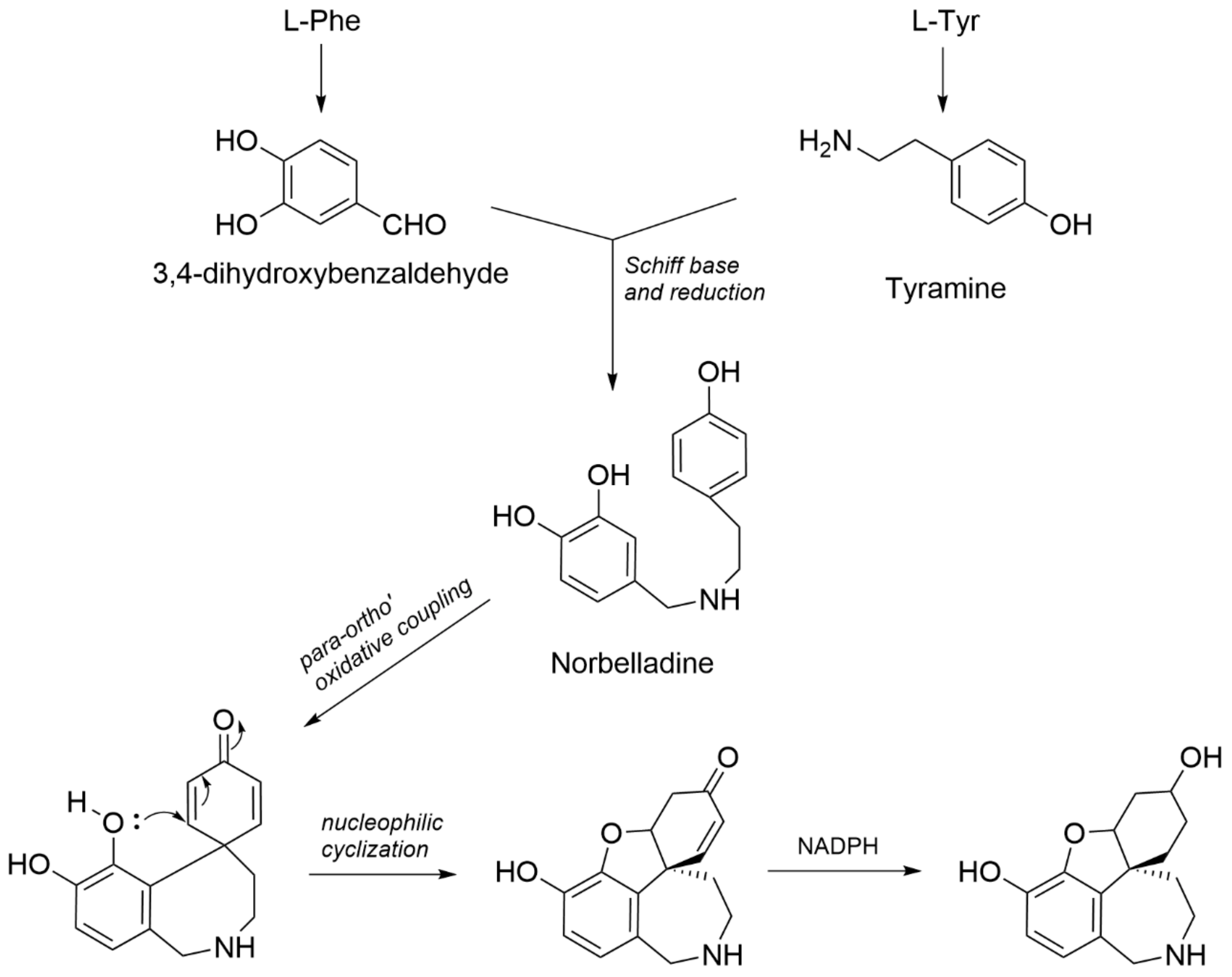
2.1. Structure Elucidation
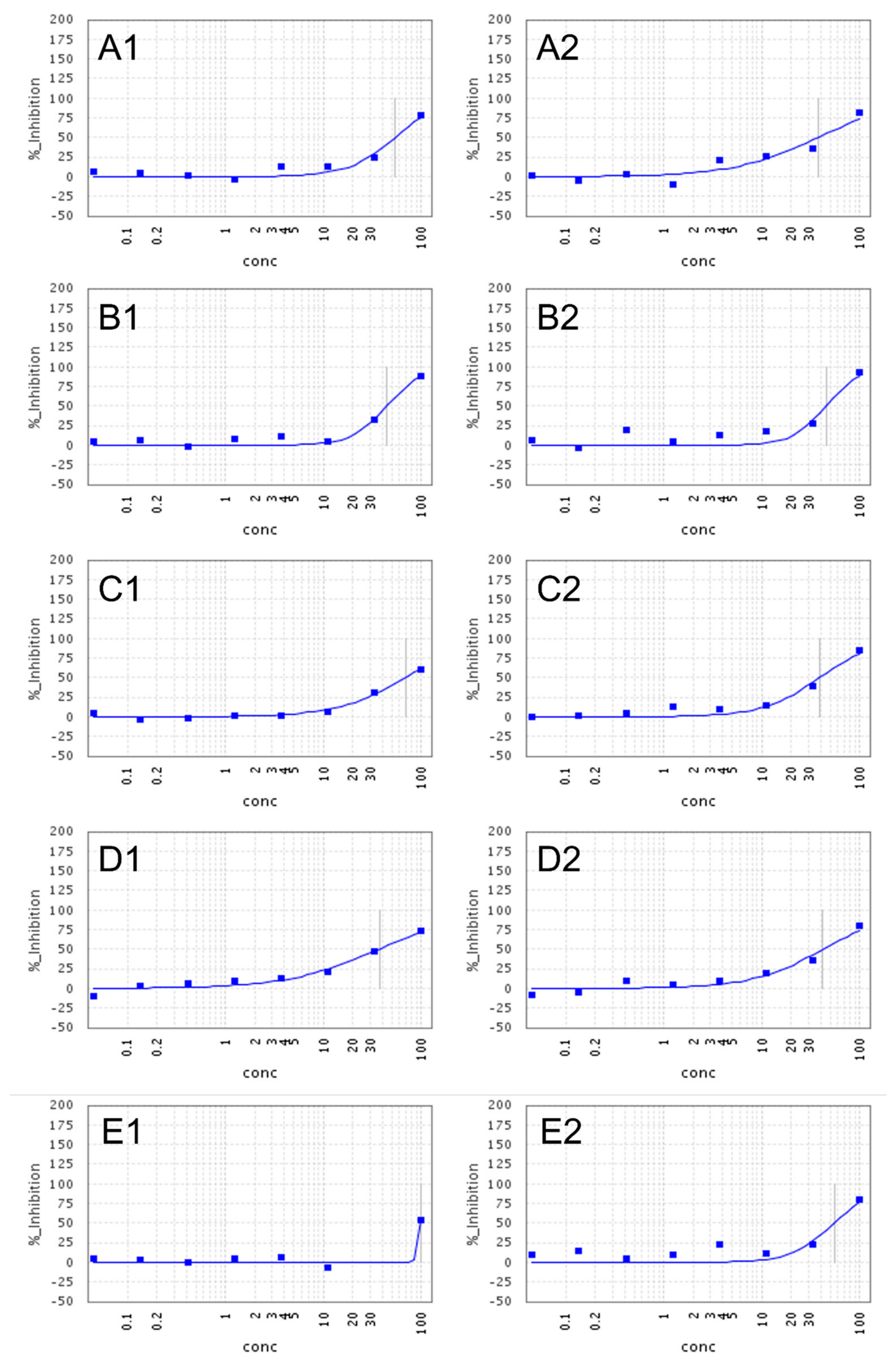
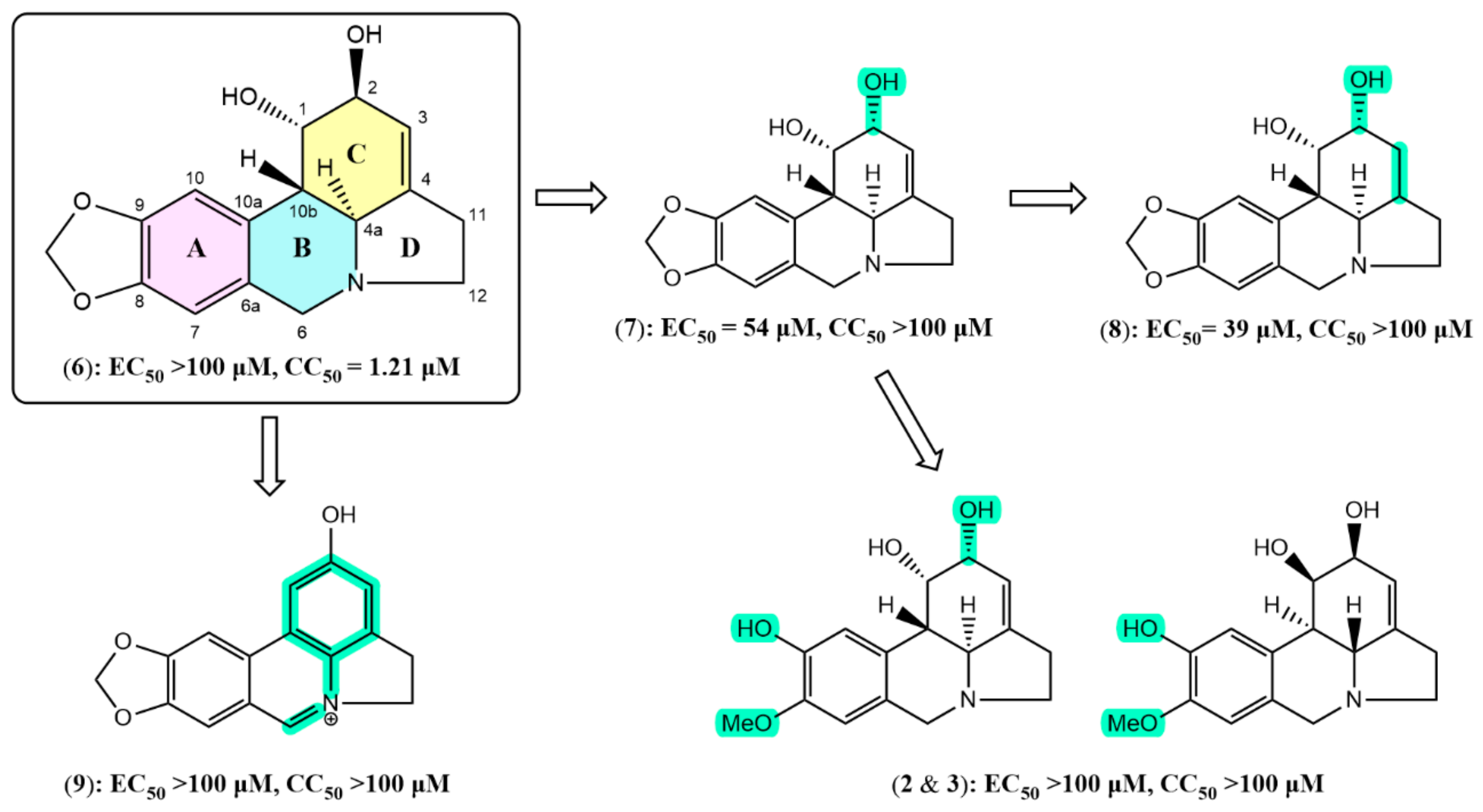
2.2. Anti-SARS-CoV-2 Activity and Cytotoxicity
3. Materials and Methods
3.1. General Experimental Procedure
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Computational Details
3.5. Anti-SARS-CoV-2 Assay
3.5.1. Cells and Virus
3.5.2. Antiviral Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Aniszewski, T. Alkaloids: Chemistry, Biology, Ecology, and Applications, 2nd ed.; Elsevier: Helsinki, Finland, 2015. [Google Scholar]
- Jin, Z. Amaryllidaceae and Sceletium Alkaloids. Nat. Prod. Rep. 2005, 22, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Takos, A.M.; Rook, F. Towards a Molecular Understanding of the Biosynthesis of Amaryllidaceae Alkaloids in Support of Their Expanding Medical Use. Int. J. Mol. Sci. 2013, 14, 11713–11741. [Google Scholar] [CrossRef] [PubMed]
- Marco, L.; Carreiras, C. Galanthamine, a Natural Product for the Treatment of Alzheimer’s Disease. Recent Pat. CNS Drug Discov. 2006, 1, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Berkov, S.; Osorio, E.; Viladomat, F.; Bastida, J. Chemodiversity, Chemotaxonomy and Chemoecology of Amaryllidaceae Alkaloids, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; Volume 83. [Google Scholar]
- National Plant Data Center, NRCS, USDA. The Plants Database: Hymenocallis littoralis (Jacq.) Salisb. Available online: https://www.itis.gov/servlet/SingleRpt/SingleRpt?search_topic=TSN&search_value=507014#null (accessed on 4 March 2023).
- Jaume, B.; Strahil, B.; Laura, T. Chemical and Biological Aspects of Amaryllidaceae Alkaloids. Recent Adv. Pharm. Sci. 2011, 3, 66–100. [Google Scholar]
- Singh, G.; Saxena, R.K. Chemistry and Medicinal Properties of Hymenocallis littoralis. Int. J. Sci. Res. 2017, 6, 2016–2018. [Google Scholar]
- Ding, Y.; Qu, D.; Zhang, K.M.; Cang, X.X.; Kou, Z.N.; Xiao, W.; Zhu, J.B. Phytochemical and Biological Investigations of Amaryllidaceae Alkaloids: A Review. J. Asian Nat. Prod. Res. 2017, 19, 53–100. [Google Scholar] [CrossRef]
- Chen, N.; Ji, Y.; Zhang, W.; Xu, Y.; Yan, X.; Sun, Y.; Song, H.; Xu, C.; Cai, L.; Zheng, H.; et al. Chemical Constituents from Hymenocallis littoralis. Lett. Org. Chem. 2016, 13, 536–539. [Google Scholar] [CrossRef]
- Ji, Y.B. Research Progress on Chemical Compositions of Hymenocallis littoralis. In Medicine Sciences and Bioengineering; CRC Press: Boca Raton, FL, USA, 2015; pp. 759–762. [Google Scholar]
- Lin, L.Z.; Hu, S.F.; Chai, H.B.; Pengsuparp, T.; Pezzuto, J.M.; Cordell, G.A.; Ruangrungsi, N. Lycorine Alkaloids from Hymenocallis littoralis. Phytochemistry 1995, 40, 1295–1298. [Google Scholar] [CrossRef]
- Ma, W.; Wang, S.; Wang, Y.; Zeng, J.; Xu, J.; He, X. Antiproliferative Amaryllidaceae Alkaloids from the Bulbs of Hymenocallis littoralis (Jacq.) Salisb. Phytochemistry 2022, 197, 113112. [Google Scholar] [CrossRef]
- Le, N.T.H.; Vermeyen, T.; Aerts, R.; Herrebout, W.A.; Pieters, L.; Tuenter, E. Epimeric Mixture Analysis and Absolute Configuration Determination Using an Integrated Spectroscopic and Computational Approach—A Case Study of Two Epimers of 6-Hydroxyhippeastidine from Hymenocallis littoralis. Molecules 2023, 28, 214. [Google Scholar] [CrossRef]
- Ingrassia, L.; Lefranc, F.; Mathieu, V.; Darro, F. Amaryllidaceae Isocarbostyril Alkaloids and Their Derivatives as Promising Antitumor Agents. Transl. Oncol. 2008, 1, 1–13. [Google Scholar] [CrossRef]
- Fürst, R. Narciclasine–an Amaryllidaceae Alkaloid with Potent Antitumor and Anti-Inflammatory Properties. Planta Med. 2016, 82, 1389–1394. [Google Scholar] [CrossRef] [PubMed]
- Ndibwami, A. A General Synthesis of Phenanthridinone Alkaloids. Synlett 2007, 2007, 1940–1945. [Google Scholar] [CrossRef]
- Ji, Y.B.; Chen, N.; Zhu, H.W.; Ling, N.; Li, W.L.; Song, D.X.; Gao, S.Y.; Zhang, W.C.; Ma, N.N. Alkaloids from Beach Spider Lily (Hymenocallis littoralis) Induce Apoptosis of HepG-2 Cells by the Fas-Signaling Pathway. Asian Pac. J. Cancer Prev. 2014, 15, 9319–9325. [Google Scholar] [CrossRef] [PubMed]
- Nair, J.J.; van Staden, J. Antiviral Alkaloid Principles of the Plant Family Amaryllidaceae. Phytomedicine 2022, 108, 154480. [Google Scholar] [CrossRef]
- Majnooni, M.B.; Fakhri, S.; Bahrami, G.; Naseri, M.; Farzaei, M.H.; Echeverría, J. Alkaloids as Potential Phytochemicals against SARS-CoV-2: Approaches to the Associated Pivotal Mechanisms. Evid. Based Complement. Altern. Med. 2021, 2021, 6632623. [Google Scholar] [CrossRef]
- Raman, K.; Rajagopal, K.; Islam, F.; Dhawan, M.; Mitra, S.; Aparna, B.; Varakumar, P.; Byran, G.; Choudhary, O.P.; Emran, T. Bin. Role of Natural Products towards the SARS-CoV-2: A Critical Review. Ann. Med. Surg. 2022, 80, 104062. [Google Scholar] [CrossRef]
- Christy, M.P.; Uekusa, Y.; Gerwick, L.; Gerwick, W.H. Natural Products with Potential to Treat RNA Virus Pathogens Including SARS-CoV-2. J. Nat. Prod. 2021, 84, 161–182. [Google Scholar] [CrossRef]
- Chakravarti, R.; Singh, R.; Ghosh, A.; Dey, D.; Sharma, P.; Velayutham, R.; Roy, S.; Ghosh, D. A Review on Potential of Natural Products in the Management of COVID-19. RSC Adv. 2021, 11, 16711–16735. [Google Scholar] [CrossRef]
- Van Breemen, R.B.; Muchiri, R.N.; Bates, T.A.; Weinstein, J.B.; Leier, H.C.; Farley, S.; Tafesse, F.G. Cannabinoids Block Cellular Entry of SARS-CoV-2 and the Emerging Variants. J. Nat. Prod. 2022, 85, 176–184. [Google Scholar] [CrossRef]
- Welsch, J.T.; Smalley, T.B.; Matlack, J.K.; Avalon, N.E.; Binning, J.M.; Johnson, M.P.; Allcock, A.L.; Baker, B.J.; Tuaimenals, B.-H. Merosesquiterpenes from the Irish Deep-Sea Soft Coral Duva Florida with Bioactivity against Cervical Cancer Cell Lines. J. Nat. Prod. 2022, 86, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Cordell, G.A. The Alkaloids: Chemistry and Biology, Volume 63, 1st ed.; Academic Press: Chicago, IL, USA, 2006. [Google Scholar]
- Cedrón, J.C.; Del Arco-Aguilar, M.; Estévez-Braun, A.; Ravelo, Á.G. Chapter 1—Chemistry and Biology of Pancratium Alkaloids, 1st ed.; Academic Press: Cambridge, MA, USA, 2010; Volume 68, pp. 1–37. [Google Scholar]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach; John Wiley & Sons: Hoboken, NJ, USA, 2002. [Google Scholar]
- Marcarino, M.O.; Cicetti, S.; Zanardi, M.M.; Sarotti, A.M. A Critical Review on the Use of DP4+ in the Structural Elucidation of Natural Products: The Good, the Bad and the Ugly. A Practical Guide. Nat. Prod. Rep. 2022, 39, 58–76. [Google Scholar] [CrossRef] [PubMed]
- Grimblat, N.; Gavín, J.A.; Hernández Daranas, A.; Sarotti, A.M. Combining the Power of J Coupling and DP4 Analysis on Stereochemical Assignments: The J-DP4 Methods. Org. Lett. 2019, 21, 4003–4007. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Zhang, Q.Y.; Li, X.D.; Xiong, J.; Xiao, S.Q.; Wang, Z.; Zhang, Z.R.; Deng, C.L.; Yang, X.L.; Wei, H.P.; et al. Gemcitabine, Lycorine and Oxysophoridine Inhibit Novel Coronavirus (SARS-CoV-2) in Cell Culture. Emerg. Microbes Infect. 2020, 9, 1170–1173. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.H.; Min, J.S.; Jeon, S.; Lee, J.; Kim, S.; Park, T.; Park, D.; Jang, M.S.; Park, C.M.; Song, J.H.; et al. Lycorine, a Non-Nucleoside RNA Dependent RNA Polymerase Inhibitor, as Potential Treatment for Emerging Coronavirus Infections. Phytomedicine 2021, 86, 153440. [Google Scholar] [CrossRef]
- Ren, P.; Shang, W.; Yin, W.; Ge, H.; Wang, L.; Zhang, X.; Li, B.; Li, H.; Xu, Y.; Xu, E.H.; et al. A Multi-Targeting Drug Design Strategy for Identifying Potent Anti-SARS-CoV-2 Inhibitors. Acta Pharmacol. Sin. 2022, 43, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Long, L.; Xiaojuan, X.; Peifu, F.; Mao, Y.; Jing, L. Lycorine: A Prospective Natural Lead for Anticancer Drug Discovery. Biomed. Pharmacother. 2018, 107, 615–624. [Google Scholar] [CrossRef]
- Nair, J.J.; Van Staden, J. Cytotoxicity Studies of Lycorine Alkaloids of the Amaryllidaceae. Nat. Prod. Commun. 2014, 9, 1193–1210. [Google Scholar] [CrossRef]
- Ji, Y.B.; Yang, S.L.; Zhang, X.L.; Liu, Y.J. Review on the Structure Modification of Lycorine. IOP Conf. Ser. Earth Environ. Sci. 2017, 100, 012052. [Google Scholar] [CrossRef]
- Wang, P.; Li, L.F.; Wang, Q.Y.; Shang, L.Q.; Shi, P.Y.; Yin, Z. Anti-Dengue-Virus Activity and Structure-Activity Relationship Studies of Lycorine Derivatives. ChemMedChem 2014, 9, 1522–1533. [Google Scholar] [CrossRef]
- Grimblat, N.; Zanardi, M.M.; Sarotti, A.M. Beyond DP4: An Improved Probability for the Stereochemical Assignment of Isomeric Compounds Using Quantum Chemical Calculations of NMR Shifts. J. Org. Chem. 2015, 80, 12526–12534. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Cao, Y.; Song, H.; Wang, X.; Yao, S. Calculations of Optical Rotation: Influence of Molecular Structure. J. Serb. Chem. Soc. 2012, 77, 56–63. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Cheeseman, J.R.; Frisch, M.J. Calculation of Optical Rotation Using Density Functional Theory. J. Phys. Chem. A 2001, 105, 5356–5371. [Google Scholar] [CrossRef]
- Bally, T.; Rablen, P.R. Quantum-Chemical Simulation of 1H NMR Spectra. 2. Comparison of DFT-Based Procedures for Computing Proton-Proton Coupling Constants in Organic Molecules. J. Org. Chem. 2011, 76, 4818–4830. [Google Scholar] [CrossRef] [PubMed]
- Boudewijns, R.; Thibaut, H.J.; Kaptein SJ, F.; Li, R.; Vergote, V.; Seldeslachts, L.; Van Weyenbergh, J.; De Keyzer, C.; Bervoets, L.; Sharma, S.; et al. STAT2 Signaling Restricts Viral Dissemination but Drives Severe Pneumonia in SARS-CoV-2 Infected Hamsters. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Ivens, T.; Van Den Eynde, C.; Van Acker, K.; Nijs, E.; Dams, G.; Bettens, E.; Ohagen, A.; Pauwels, R.; Hertogs, K. Development of a Homogeneous Screening Assay for Automated Detection of Antiviral Agents Active against Severe Acute Respiratory Syndrome-Associated Coronavirus. J. Virol. Methods 2005, 129, 56–63. [Google Scholar] [CrossRef]
- Jochmans, D.; Leyssen, P.; Neyts, J. A Novel Method for High-Throughput Screening to Quantify Antiviral Activity against Viruses That Induce Limited CPE. J. Virol. Methods 2012, 183, 176–179. [Google Scholar] [CrossRef] [PubMed]
| Experimental | Calculated | |||
|---|---|---|---|---|
| Position | δC, Type | δH (J in Hz) | δC | δH |
| 1 | 24.4, CH2 | 1.67 m | 25.1 | 1.53 |
| 1 | 24.4, CH2 | 1.85 * | 25.1 | 2.11 |
| 2 | 26.9, CH2 | 1.81 * | 26.9 | 2.23 |
| 2 | 26.9, CH2 | 1.45 m | 26.9 | 1.15 |
| 3 | 64.7, CH | 4.09 m | 64.2 | 3.91 |
| 4 | 31.2, CH2 | 2.00 * | 29.2 | 2.08 |
| 4 | 31.2, CH2 | 2.32 m | 29.2 | 2.33 |
| 4a | 88.9, CH | 4.31 t (3.2) | 90.2 | 4.50 |
| 6 | 52.7, CH2 | 3.83 d (15.0) | 50.3 | 3.71 |
| 6 | 52.7, CH2 | 3.97 d (15.0) | 50.3 | 3.95 |
| 6a | 128.9, C | - | 135.1 | - |
| 7 | 120.6, CH | 6.51 d (8.0) | 120.0 | 6.52 |
| 8 | 114.9, CH | 6.55 d (8.0) | 112.2 | 6.57 |
| 9 | 141.1, C | - | 140.5 | - |
| 10 | 146.0, C | - | 144.5 | - |
| 10a | 135.5, C | - | 134.5 | - |
| 10b | 47.2, C | - | 49.2 | - |
| 11 | 36.5, CH2 | 1.75 * | 39.7 | 1.71 |
| 11 | 36.5, CH2 | 1.98 * | 39.7 | 1.83 |
| 12 | 46.5, CH2 | 3.26 m | 44.4 | 3.20 |
| 12 | 46.5, CH2 | 3.19 m | 44.4 | 3.19 |
| CMAE | 1.8 | 0.13 | ||
| Max. outlier | 6.2 | 0.42 | ||
| Compound 1 | Compounds 2 and 3 | |||
|---|---|---|---|---|
| Diastereomer | Probability (%) | Diastereomer | Probability (%) | JH-4a/H-10b |
| SRR | 26.78 | SRRR | 0 | 10.69 |
| SRS | 0 | SRRS | 61.05 | 5.52 |
| SSR | 73.22 | SRSR | 0 | 7.36 |
| SSS | 0 | SRSS | 0 | 10.56 |
| SSRR | 0 | 10.25 | ||
| SSRS | 10.15 | 5.78 | ||
| SSSR | 0 | 7.99 | ||
| SSSS | 28.80 | 10.85 | ||
| Compound 1 | Compounds 2 and 3 | |||
|---|---|---|---|---|
| Experimental | −63.6 | −8.0 | ||
| B3LYP/6-31++G(d,p)// 6-311++G(3df,2dp) | SSR RRS | −48.8 48.7 | SRSS RSRR | −230.4 230.6 |
| B3LYP/6-31++G(d,p)// aug-cc-pVTZ | SSR RRS | −46.8 46.6 | SRSS RSRR | −227.7 227.9 |
| In CD3OD | In (CD3)2SO | |||||
|---|---|---|---|---|---|---|
| Experimental | Calculated | Experimental | ||||
| Position | Δc, Type | δH (J in Hz) | δC | δH | δC | δH |
| 1 | 71.6, CH | 4.49 s | 75.1 | 3.94 | 70.8, CH | 4.21 s |
| 2 | 72.9, CH | 4.19 * | 71.2 | 4.31 | 72.2, CH | 3.96 s |
| 3 | 119.9, CH | 5.59 s | 126.7 | 5.62 | 118.9, CH | 5.35 s |
| 4 | n.o. | - | - | 142.4, C | - | |
| 4a | 62.6, CH | 3.06 d (11.0) | 59.9 | 2.96 | 61.4, CH | 2.58 d (10.6) |
| 6α | 57.0, CH2 | 4.19 * | 56.4 | 4.03 | 57.0, CH2 | 3.99 d (13.8) |
| 6β | 57.0, CH2 | 3.71 d (14.1) | 56.4 | 3.57 | 57.0, CH2 | 3.30 d (13.8) |
| 6a | 127.2, C | - | 129.3 | - | 127.5, C | - |
| 7 | 111.7, CH | 6.76 s | 107.8 | 6.73 | 111.6, CH | 6.63 s |
| 8 | 147.8, C | - | 145.2 | - | 146.1, C | - |
| 9 | 146.8, C | - | 145.4 | - | 145.2, C | - |
| 10 | 112.7, CH | 6.88 s | 114.2 | 7.07 | 112.4, CH | 6.70 s |
| 10a | 128.8, C | - | 128.5 | - | 129.1, C | - |
| 10b | 40.5, CH | 2.76 d (11.1) | 41.6 | 3.05 | 40.2, CH | 2.49 * |
| 11 | 29.3, CH2 | 2.68 * (2H) | 31.3 | 2.70 | 28.5, CH2 | 2.46 * (2H) |
| 12α | 54.8, CH2 | 3.40 * | 53.1 | 3.38 | 53.9, CH2 | 3.20 t (8.2) |
| 12β | 54.8, CH2 | 2.67 * | 53.1 | 2.61 | 53.9, CH2 | 2.19 q (8.4) |
| 8-OMe | 56.5, CH3 | 3.84 s | 54.5 | 3.97 | 56.2, CH3 | 3.71 s |
| CMAE | 2.3 | 0.13 | ||||
| Max. outlier | 6.8 | 0.55 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, N.-T.-H.; De Jonghe, S.; Erven, K.; Vermeyen, T.; Baldé, A.M.; Herrebout, W.A.; Neyts, J.; Pannecouque, C.; Pieters, L.; Tuenter, E. Anti-SARS-CoV-2 Activity and Cytotoxicity of Amaryllidaceae Alkaloids from Hymenocallis littoralis. Molecules 2023, 28, 3222. https://doi.org/10.3390/molecules28073222
Le N-T-H, De Jonghe S, Erven K, Vermeyen T, Baldé AM, Herrebout WA, Neyts J, Pannecouque C, Pieters L, Tuenter E. Anti-SARS-CoV-2 Activity and Cytotoxicity of Amaryllidaceae Alkaloids from Hymenocallis littoralis. Molecules. 2023; 28(7):3222. https://doi.org/10.3390/molecules28073222
Chicago/Turabian StyleLe, Ngoc-Thao-Hien, Steven De Jonghe, Kristien Erven, Tom Vermeyen, Aliou M. Baldé, Wouter A. Herrebout, Johan Neyts, Christophe Pannecouque, Luc Pieters, and Emmy Tuenter. 2023. "Anti-SARS-CoV-2 Activity and Cytotoxicity of Amaryllidaceae Alkaloids from Hymenocallis littoralis" Molecules 28, no. 7: 3222. https://doi.org/10.3390/molecules28073222
APA StyleLe, N.-T.-H., De Jonghe, S., Erven, K., Vermeyen, T., Baldé, A. M., Herrebout, W. A., Neyts, J., Pannecouque, C., Pieters, L., & Tuenter, E. (2023). Anti-SARS-CoV-2 Activity and Cytotoxicity of Amaryllidaceae Alkaloids from Hymenocallis littoralis. Molecules, 28(7), 3222. https://doi.org/10.3390/molecules28073222







