1-Benzyl-5-bromo-3-hydrazonoindolin-2-ones as Novel Anticancer Agents: Synthesis, Biological Evaluation and Molecular Modeling Insights
Abstract
1. Introduction
2. Results and Discussion
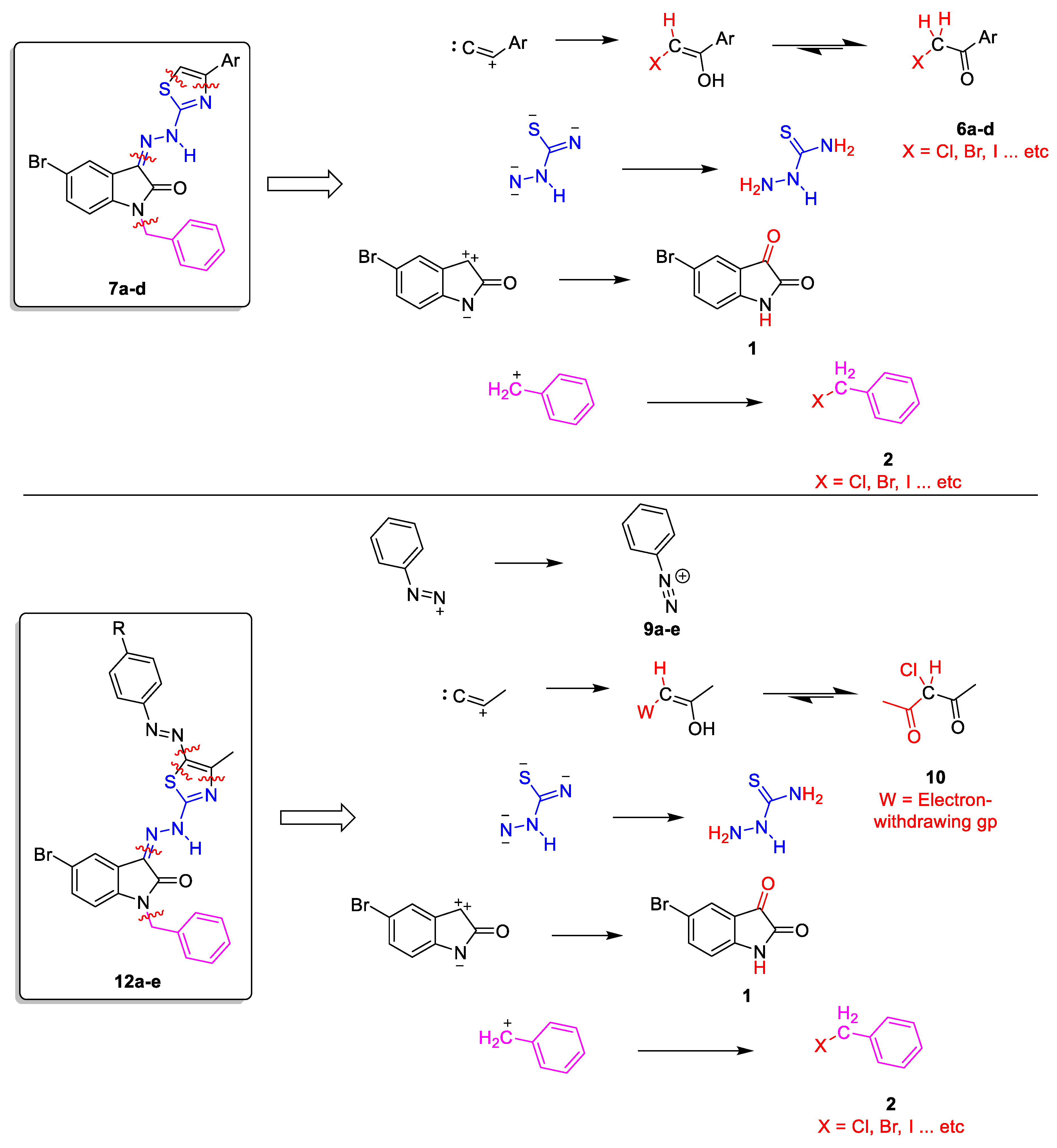
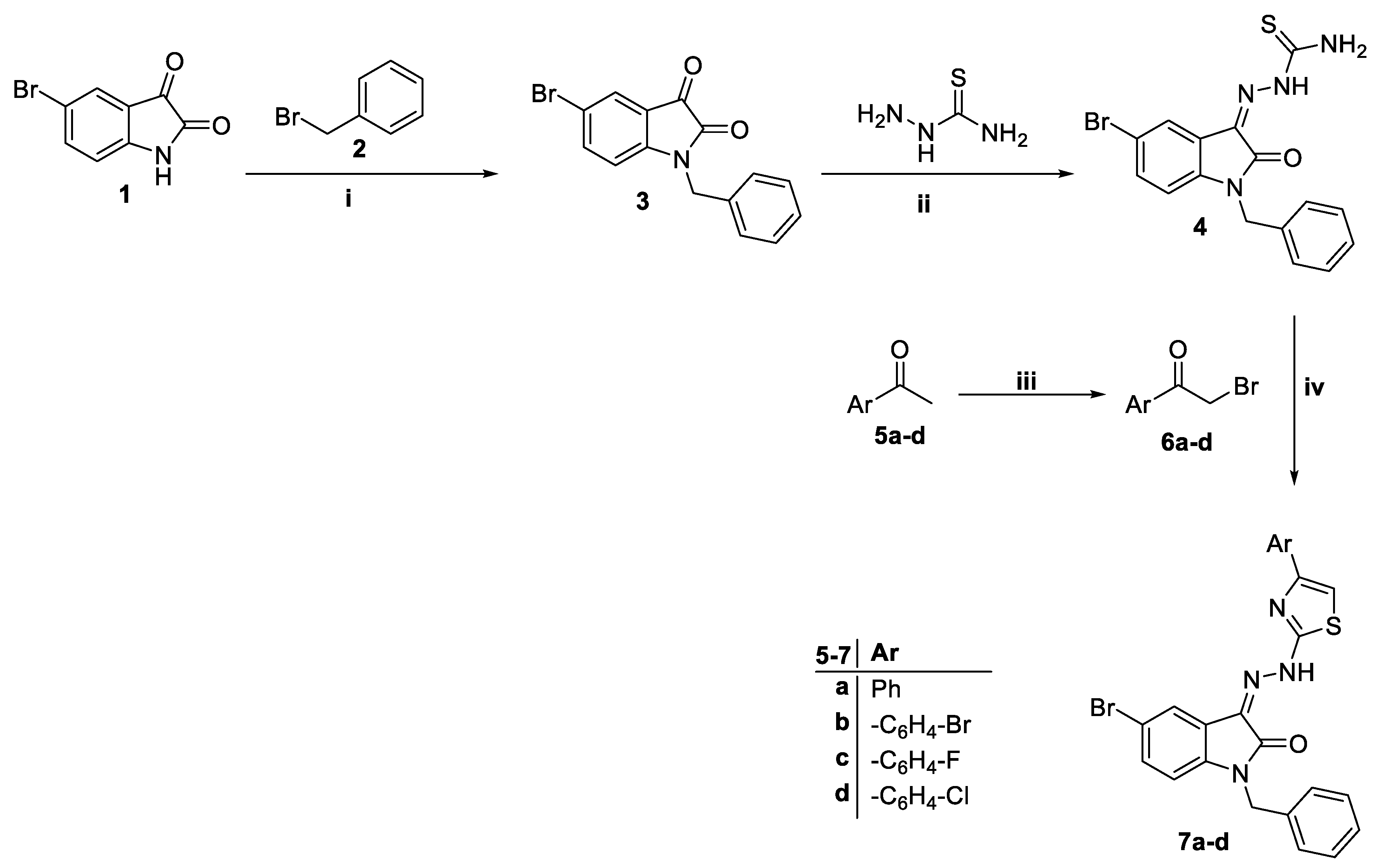
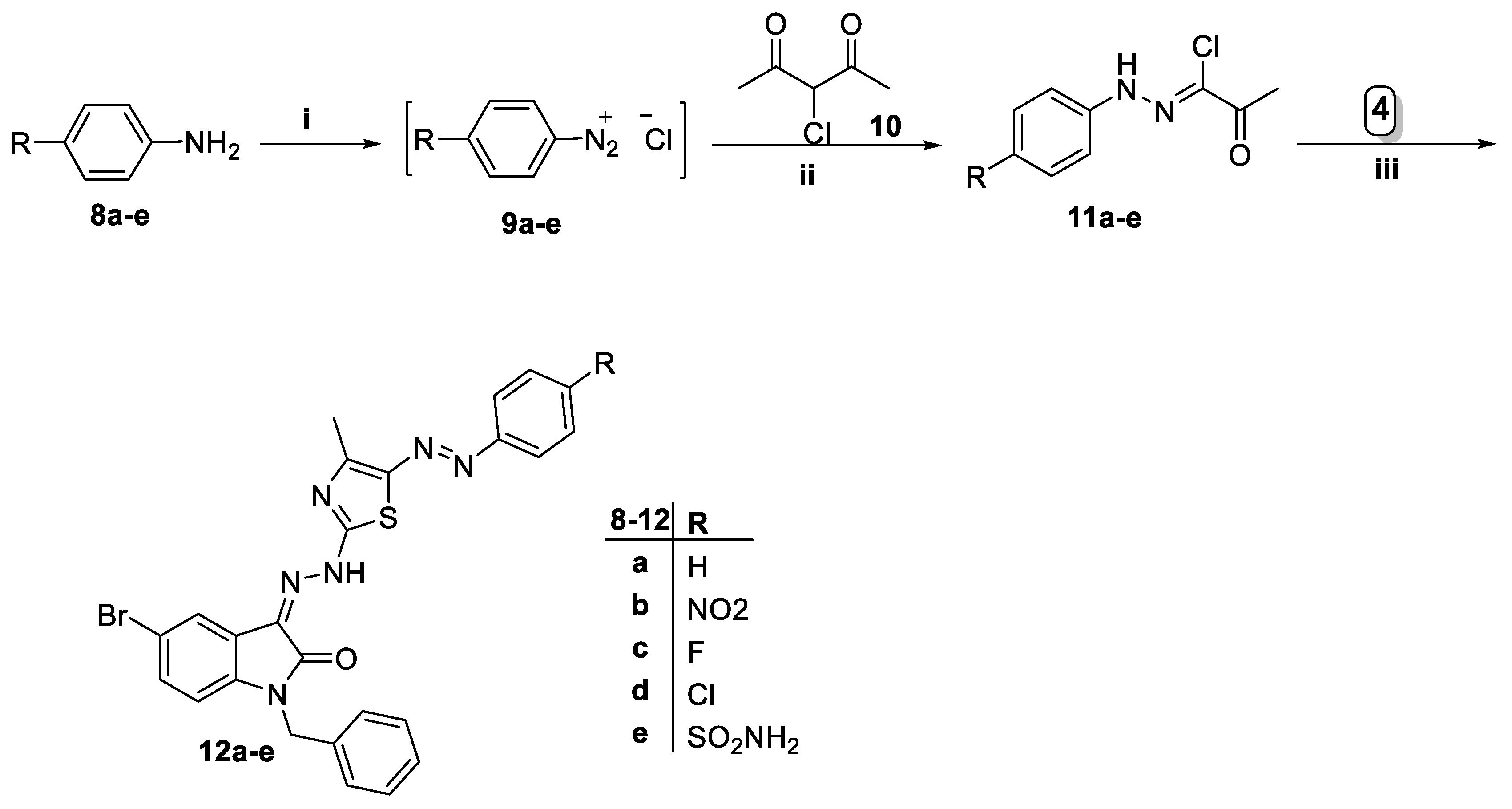
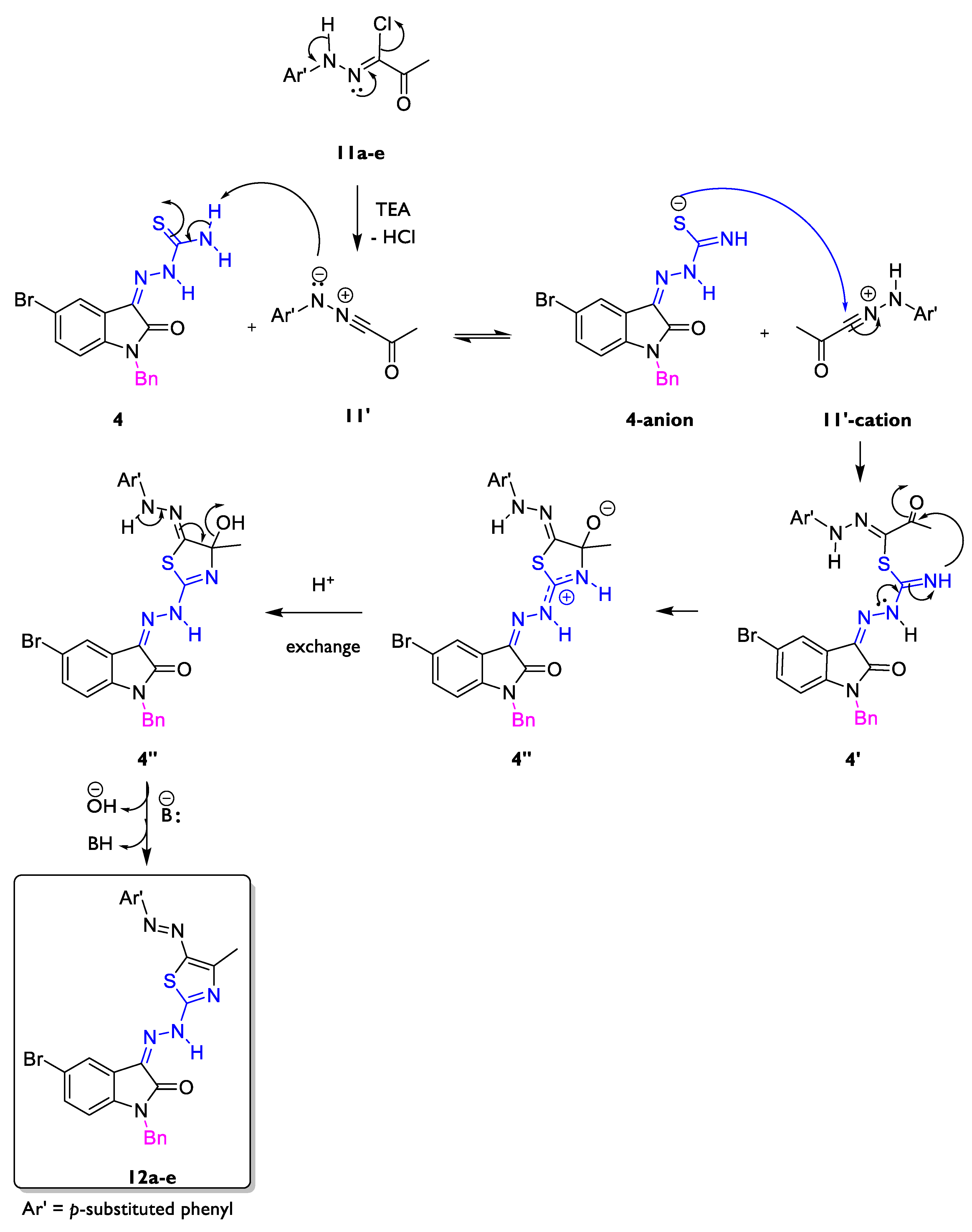
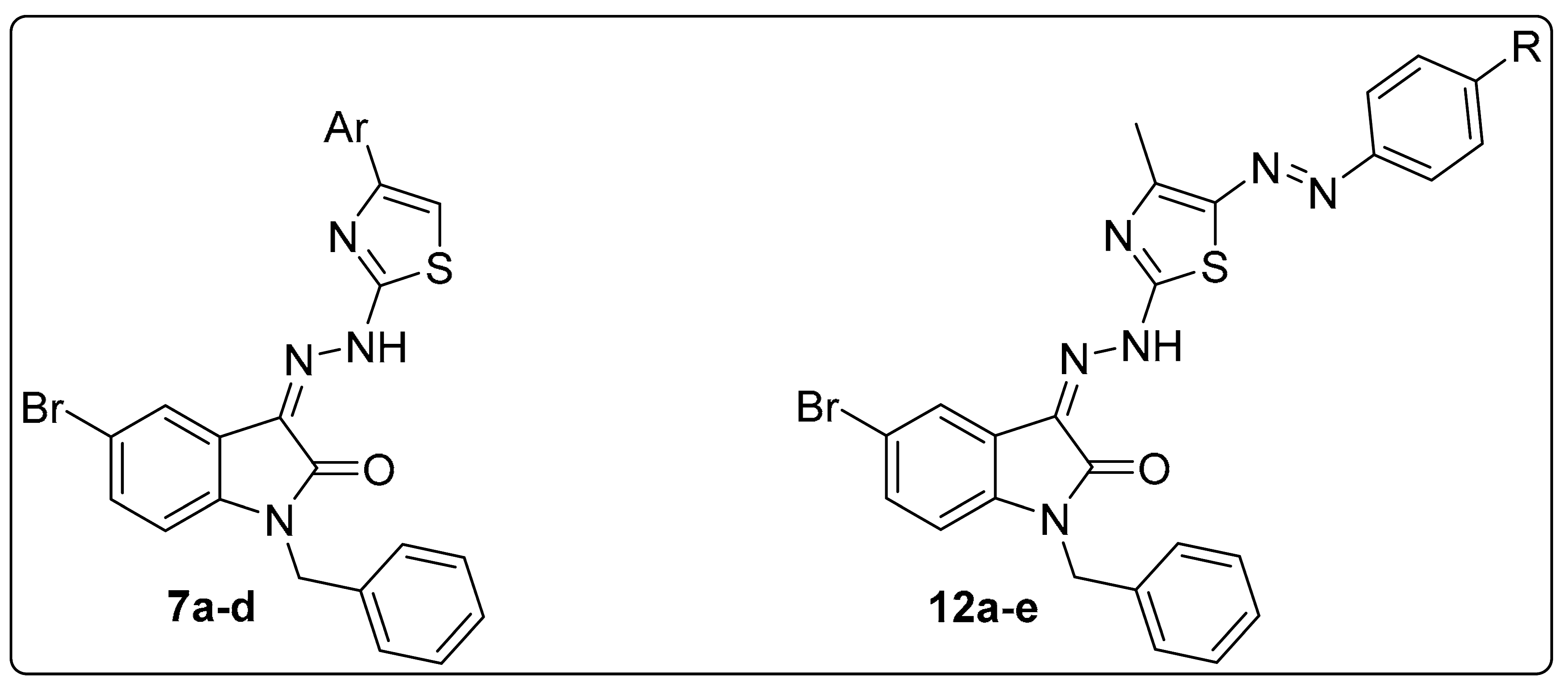
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. In Vitro Anti-Proliferative Activity
2.2.2. VEGFR-2 Inhibition
2.2.3. Apoptosis Induction
Effects on Activation of Proteolytic Caspases Cascade
Effects on Mitochondrial Apoptosis Pathway (Bcl-2 Family) Proteins
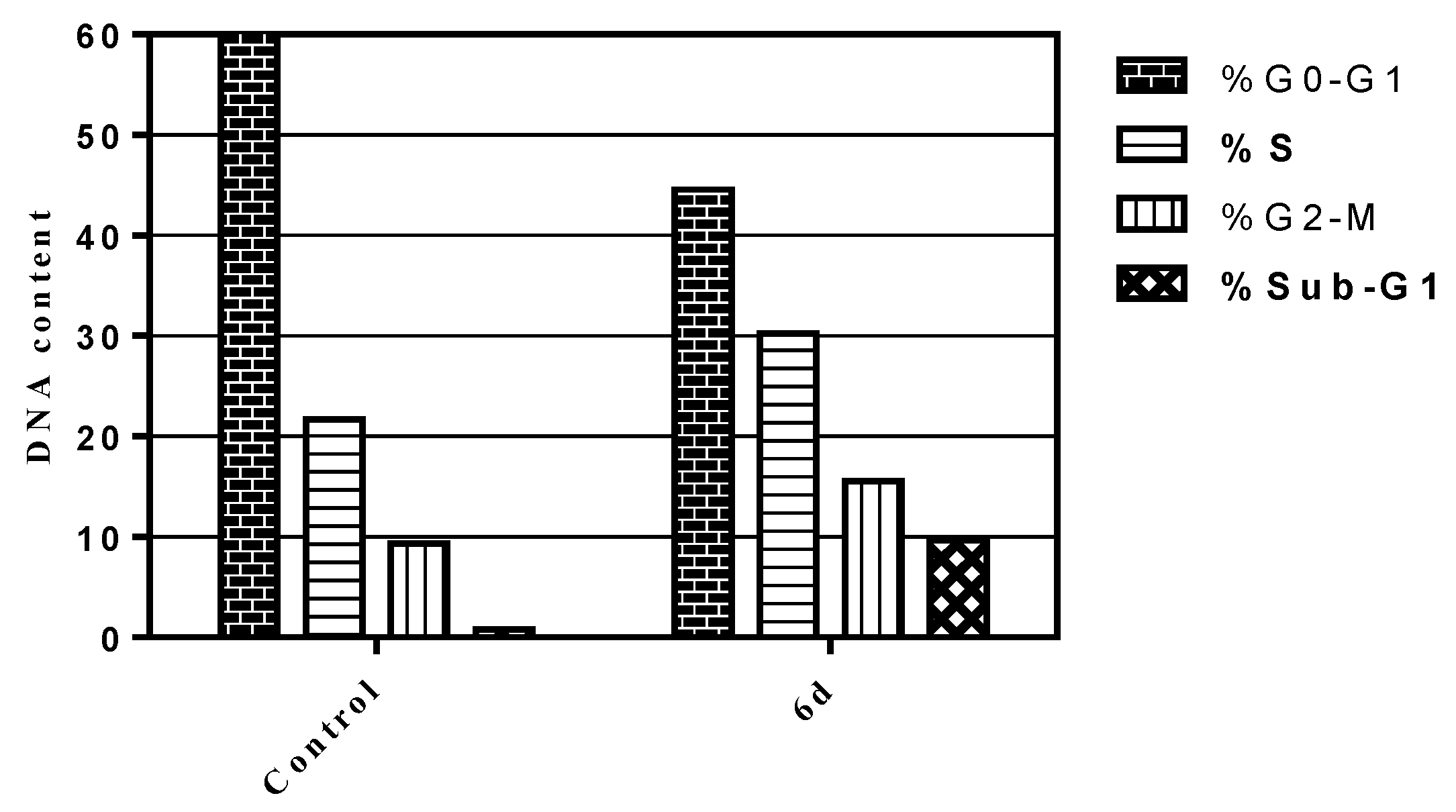
2.2.4. Cell Cycle Analysis
2.3. Molecular Modeling
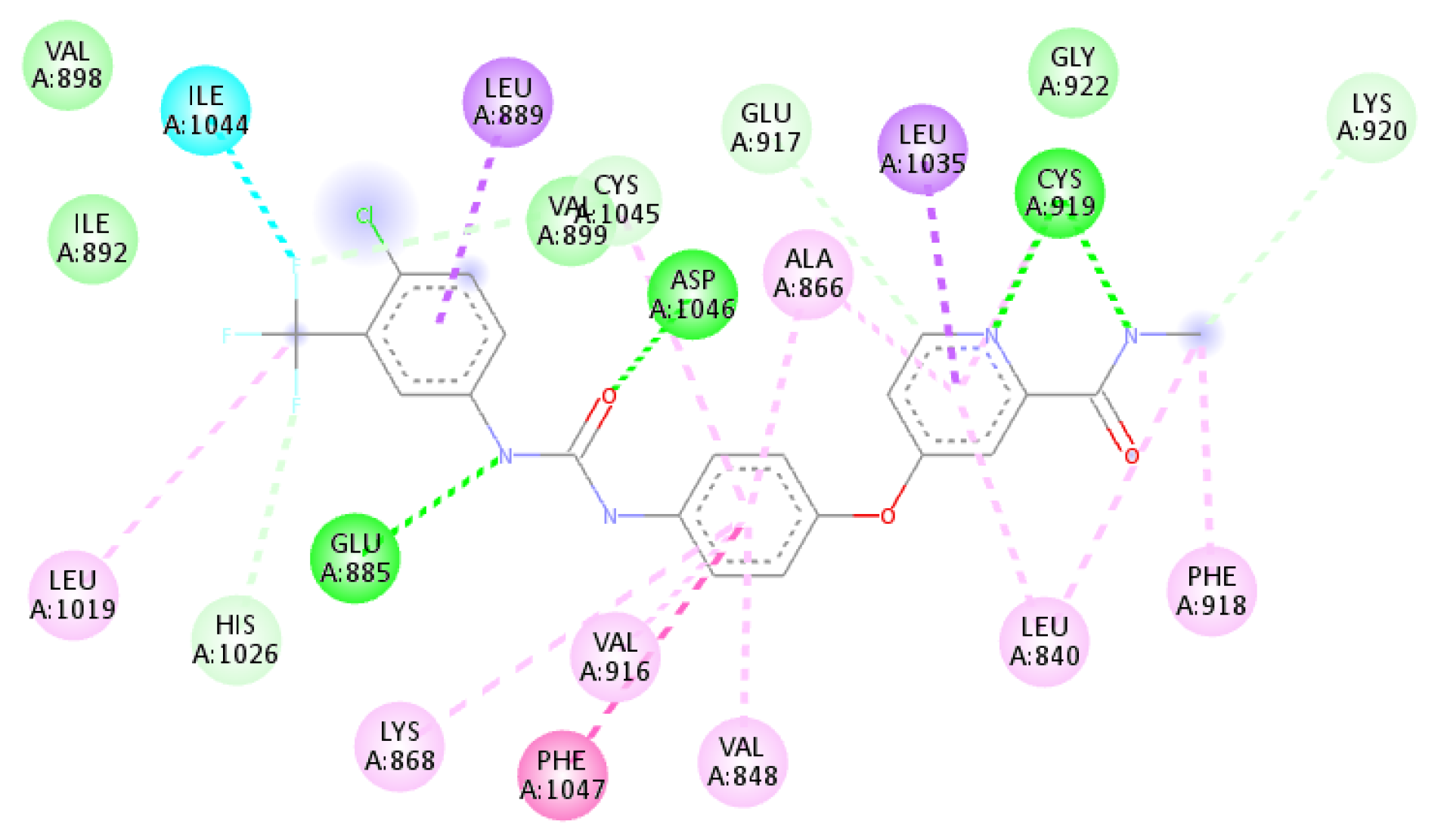
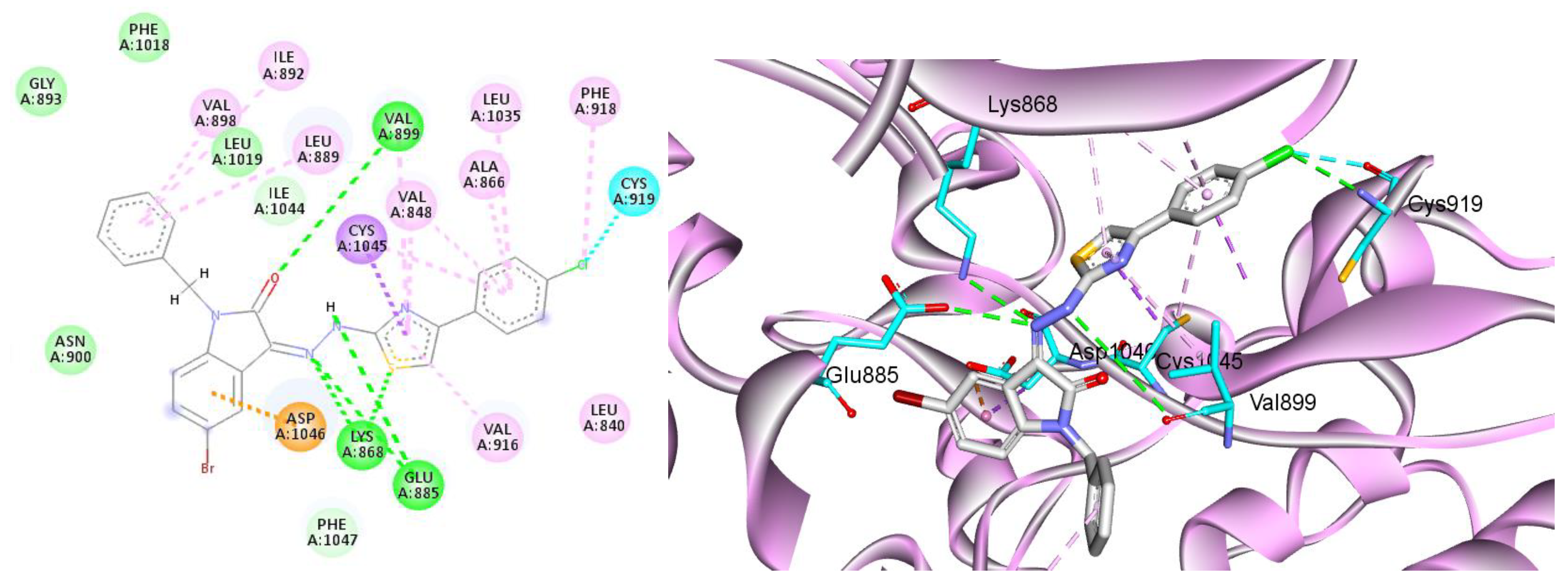
2.3.1. Docking Studies
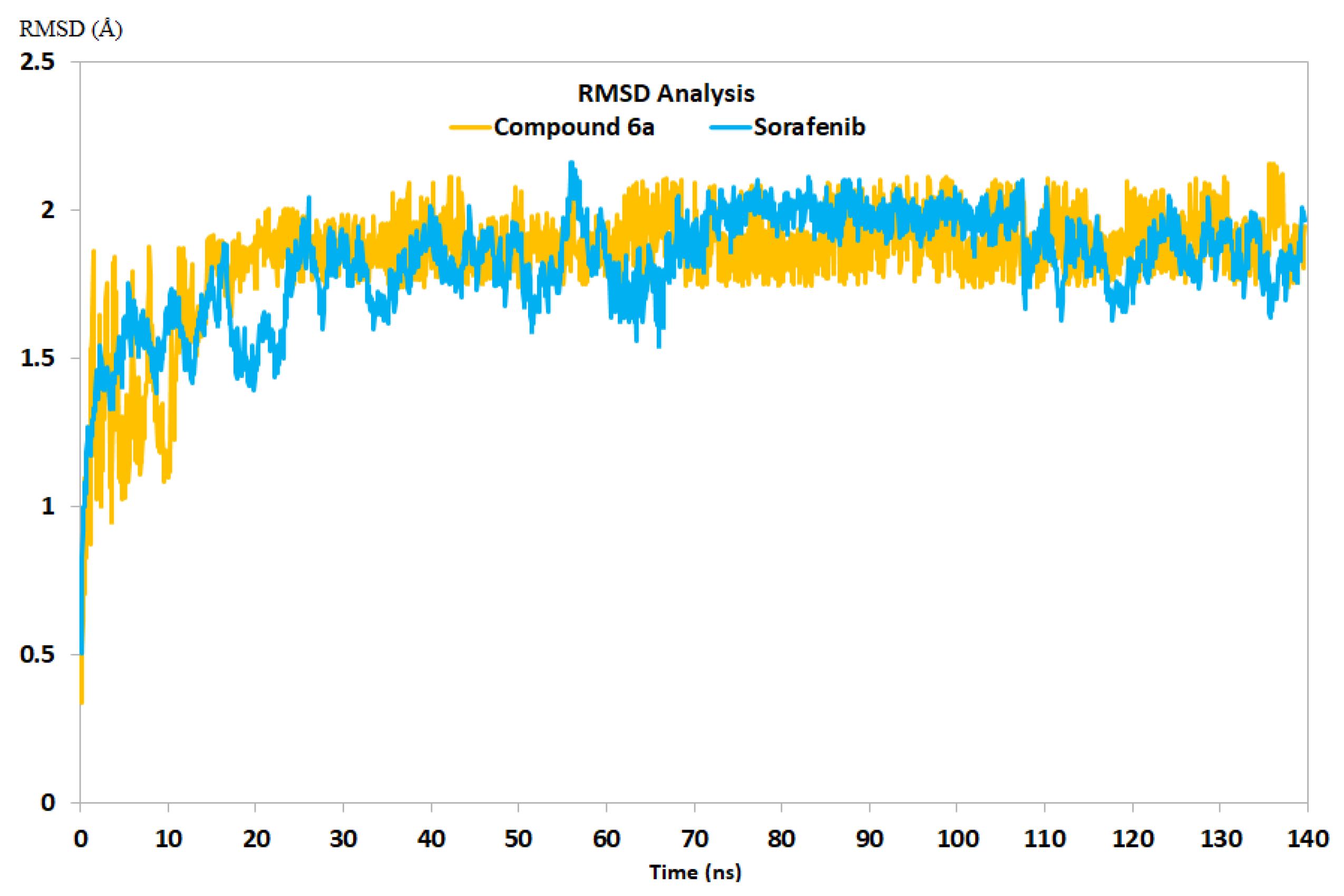
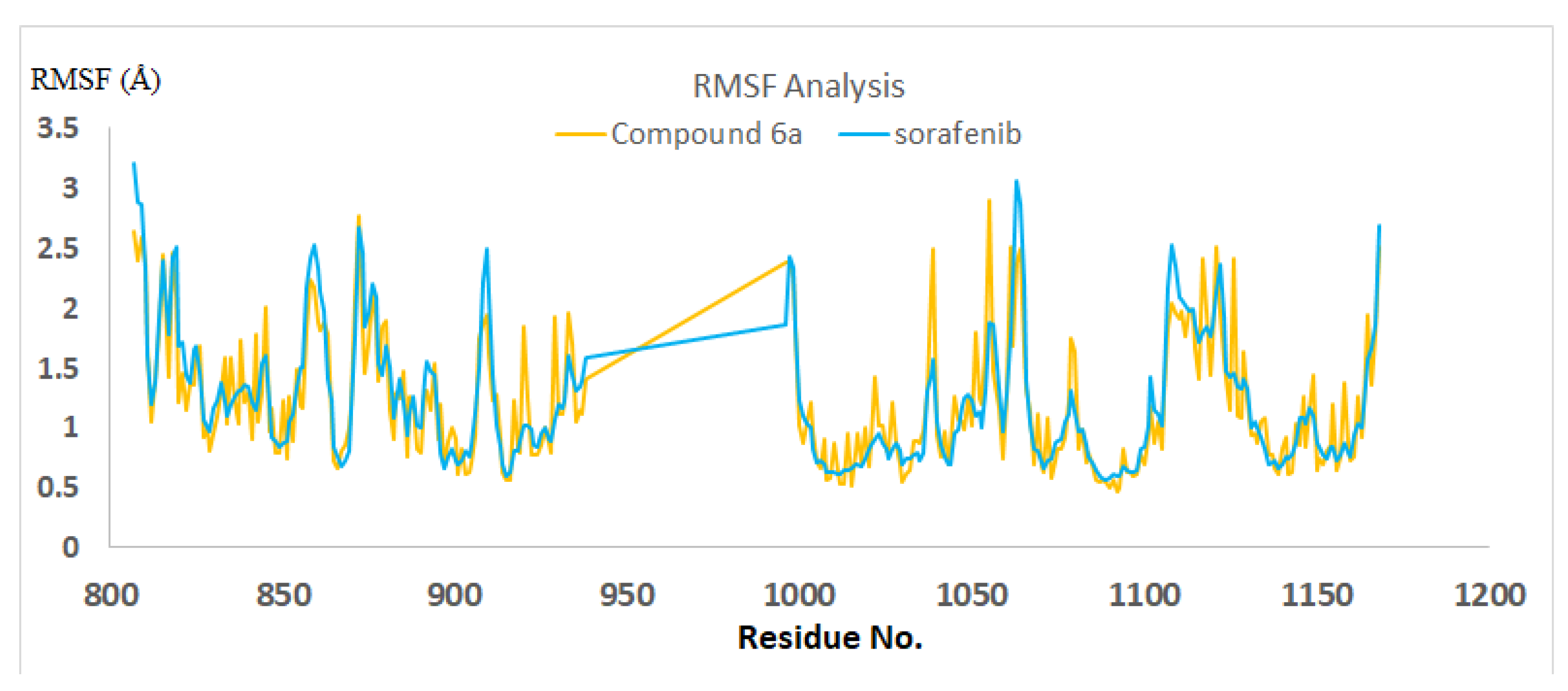
2.3.2. Molecular Dynamics
3. Conclusions
4. Experimental
4.1. Chemistry
4.1.1. General
4.1.2. 2-(1-Benzyl-5-bromo-2-oxoindolin-3-ylidene)hydrazine-1-carbothioamide 4
4.1.3. General Procedure for the Preparation of 1-benzyl-5-bromo-3-(2-(4-arylthiazol-2-yl)hydrazono)indolin-2-one 7a–d
4.1.4. General Procedure for Preparation of 1-benzyl-5-bromo-3-(2-(4-methyl-5-(aryldiazenyl)thiazol-2-yl)hydrazono)indolin-2-ones 12a–e
4.2. Pharmacological Assays
4.3. Molecular Modeling Studies
4.3.1. Docking Studies
4.3.2. Molecular Dynamics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Siegel, R.L.; Kimberly, D.M.; Hannah, E.F.; Jemal, A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Barreca, M.; Spanò, V.; Rocca, R.; Bivacqua, R.; Abel, A.-C.; Maruca, A.; Montalbano, A.; Raimondi, M.V.; Tarantelli, C.; Gaudio, E.; et al. Development of [1, 2] oxazoloisoindoles tubulin polymerization inhibitors: Further chemical modifications and potential therapeutic effects against lymphomas. Eur. J. Med. Chem. 2022, 243, 114744. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, X.; Yu, L.; Mao, M. Discovery of novel sulphonamide hybrids that inhibit LSD1 against bladder cancer cells. J. Enzym. Inhib. Med. Chem. 2022, 37, 866–875. [Google Scholar] [CrossRef]
- Labbozzetta, M.; Barreca, M.; Spanò, V.; Raimondi, M.V.; Poma, P.; Notarbartolo, M.; Barraja, P.; Alessandra, M. Novel insights on [1, 2] oxazolo [5, 4-e] isoindoles on multidrug resistant acute myeloid leukemia cell line. Drug. Dev. Res. 2022, 83, 1331–1341. [Google Scholar] [CrossRef]
- Sabt, A.; Eldehna, W.M.; Al-Warhi, T.; Alotaibi, O.J.; Elaasser, M.M.; Suliman, H.; Abdel-Aziz, H.A. Discovery of 3,6-disubstituted pyridazines as a novel class of anticancer agents targeting cyclin-dependent kinase 2: Synthesis, biological evaluation and in silico insights. J. Enzym. Inhib. Med. Chem. 2020, 35, 1616–1630. [Google Scholar] [CrossRef] [PubMed]
- Al-Warhi, T.; Al-Karmalawy, A.A.; Elmaaty, A.A.; Alshubramy, M.A.; Abdel-Motaal, M.; Majrashi, T.A.; Asem, M.; Nabil, A.; Eldehna, W.M.; Sharaky, M. Biological evaluation, docking studies, and in silico ADME prediction of some pyrimidine and pyridine derivatives as potential EGFRWT and EGFRT790M inhibitors. J. Enzym. Inhib. Med. Chem. 2023, 38, 176–191. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghulikah, H.A.; El-Sebaey, S.A.; Bass, A.K.A.; El-Zoghbi, M.S. New Pyrimidine-5-Carbonitriles as COX-2 Inhibitors: Design, Synthesis, Anticancer Screening, Molecular Docking, and In Silico ADME Profile Studies. Molecules 2022, 27, 7485. [Google Scholar] [CrossRef]
- Chao, M.-W.; Lin, T.E.; HuangFu, W.-C.; Chang, C.-D.; Tu, H.-J.; Chen, L.-C.; Yen, S.-C.; Sung, T.-Y.; Huang, W.-J.; Yang, C.-R.; et al. Identification of a dual TAOK1 and MAP4K5 inhibitor using a structure-based virtual screening approach. J. Enzym. Inhib. Med. Chem. 2020, 36, 98–108. [Google Scholar] [CrossRef]
- Alanazi, M.M.; Elwan, A.; Alsaif, N.A.; Obaidullah, A.J.; Alkahtani, H.M.; Al-Mehizia, A.A.; Alsubaie, S.M.; Taghour, M.S.; Ibrahim, H.E. Discovery of new 3-methylquinoxalines as potential anti-cancer agents and apoptosis inducers targeting VEGFR-2: Design, synthesis, and in silico studies. J. Enzym. Inhib. Med. Chem. 2021, 36, 1732–1750. [Google Scholar] [CrossRef]
- Pralhad, T.; Madhusudan, S.; Rajendrakumar, K. Concept, mechanisms and therapeutics of angiogenesis in cancer and other diseases. J. Pharm. Pharmacol. 2003, 55, 1045–1053. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: A crucial target for anti-and pro-angiogenic therapies. Genes. Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef]
- Abhinand, C.S.; Raju, R.; Soumya, S.J.; Arya, P.S.; Sudhakaran, P.R. VEGF-A/VEGFR2 signaling network in endothelial cells relevant to angiogenesis. J. Cell. Commun. Signal. 2016, 10, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Cheke, R.S.; Patil, V.M.; Firke, S.D.; Ambhore, J.P.; Ansari, I.A.; Patel, H.M.; Shinde, S.D.; Pasupuleti, V.R.; Hassan, I.; Adnan, M.; et al. Therapeutic outcomes of isatin and its derivatives against multiple diseases: Recent developments in drug discovery. Pharmaceuticals 2022, 15, 272. [Google Scholar] [CrossRef] [PubMed]
- Guo, H. Isatin derivatives and their anti-bacterial activities. Eur. J. Med. Chem. 2018, 164, 678–688. [Google Scholar] [CrossRef] [PubMed]
- De Moraes Gomes, P.A.T.; Pena, L.J.; Leite, A.C.L. Isatin derivatives and their antiviral properties against arboviruses: A review. Mini Rev. Med. Chem. 2019, 19, 56–62. [Google Scholar] [CrossRef]
- Eldehna, W.M.; Fares, M.; Ibrahim, H.S.; Alsherbiny, M.A.; Aly, M.H.; Ghabbour, H.A.; Abdel-Aziz, H.A. Synthesis and cytotoxic activity of biphenylurea derivatives containing indolin-2-one moieties. Molecules 2016, 16, 762. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.M.S.; Herrmann, L.; Tsogoeva, S.B. Structural hybridization as a facile approach to new drug candidates. Bioorganic Med. Chem. Lett. 2020, 30, 127514. [Google Scholar] [CrossRef]
- Ding, Z.; Zhou, M.; Zeng, C. Recent advances in isatin hybrids as potential anticancer agents. Arch. Der. Pharm. 2020, 353, e1900367. [Google Scholar] [CrossRef]
- Hou, Y.; Shang, C.; Wang, H.; Jie, Y. Isatin–azole hybrids and their anticancer activities. Arch. Der. Pharm. 2020, 353, 1900272. [Google Scholar] [CrossRef]
- De Paiva, R.E.F.; Vieira, E.G.; da Silva, D.R.; Wegermann, C.A.; Ferreira, A.M.C. Anticancer compounds based on isatin-derivatives: Strategies to ameliorate selectivity and efficiency. Front. Mol. Biosci. 2021, 7, 627272. [Google Scholar] [CrossRef]
- Eldehna, W.M.; Altoukhy, A.; Mahrous, H.; Abdel-Aziz, H.A. Design, synthesis and QSAR study of certain isatin-pyridine hybrids as potential anti-proliferative agents. Eur. J. Med. Chem. 2015, 90, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Eldehna, W.M.; Al-Wabli, R.I.; Almutairi, M.S.; Keeton, A.B.; Piazza, G.A.; Abdel-Aziz, H.; Attia, M.I. Synthesis and biological evaluation of certain hydrazonoindolin-2-one derivatives as new potent anti-proliferative agents. J. Enzym. Inhib. Med. Chem. 2018, 33, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, Z. Coumarin-containing hybrids and their anticancer activities. Eur. J. Med. Chem. 2019, 181, 111587. [Google Scholar] [CrossRef]
- Eldehna, W.M.; El Hassab, M.A.; Abo-Ashour, M.F.; Al-Warhi, T.; Elaasser, M.M.; Safwat, N.A.; Suliman, H.; Ahmed, M.F.; Al-Rashood, S.T.; Abdel-Aziz, H.A.; et al. Development of isatin-thiazolo [3, 2-a] benzimidazole hybrids as novel CDK2 inhibitors with potent in vitro apoptotic anti-proliferative activity: Synthesis, biological and molecular dynamics investigations. Bioorganic. Chem. 2021, 110, 104748. [Google Scholar] [CrossRef] [PubMed]
- Eldehna, W.M.; Salem, R.; Elsayed, Z.M.; Al-Warhi, T.; Knany, H.R.; Ayyad, R.R.; Traiki, B.T.; Abdulla, M.-H.; Ahmad, R.; Abdel-Aziz, H.A.; et al. Development of novel benzofuran-isatin conjugates as potential antiproliferative agents with apoptosis inducing mechanism in Colon cancer. J. Enzym. Inhib. Med. Chem. 2021, 36, 1423–1434. [Google Scholar] [CrossRef]
- Eldehna, W.M.; Al-Rashood, S.T.; Al-Warhi, T.; Eskandrani, R.O.; Alharbi, A.; Ahmed, M.E.K. Novel oxindole/benzofuran hybrids as potential dual CDK2/GSK-3β inhibitors targeting breast cancer: Design, synthesis, biological evaluation, and in silico studies. J. Enzym. Inhib. Med. Chem. 2021, 36, 271–286. [Google Scholar] [CrossRef]
- Eldehna, W.M.; Almahli, H.; Al-Ansary, G.H.; Ghabbour, H.A.; Aly, M.H.; Ismael, O.E.; Al-Dhfyan, A.; Abdel-Aziz, H.A. Synthesis and in vitro anti-proliferative activity of some novel isatins conjugated with quinazoline/phthalazine hydrazines against triple-negative breast cancer MDA-MB-231 cells as apoptosis-inducing agents. J. Enzym. Inhib. Med. Chem. 2017, 32, 600–613. [Google Scholar] [CrossRef]
- Abdel-Aziz, H.A.; Eldehna, W.M.; Keeton, A.B.; Piazza, G.A.; Kadi, A.A.; Attwa, M.W.; Abdelhameed, A.S.; Attia, M.I. Isatin-benzoazine molecular hybrids as potential antiproliferative agents: Synthesis and in vitro pharmacological profiling. Drug Des. Dev. Ther. 2017, 11, 2333–2346. [Google Scholar] [CrossRef]
- Al-Warhi, T.; Abo-Ashour, M.F.; Almahli, H.; Alotaibi, O.J.; Al-Sanea, M.M.; Al-Ansary, G.H.; Ahmed, H.Y.; Elaasser, M.M.; Eldehna, W.M.; Abdel-Aziz, H.A. Novel [(N-alkyl-3-indolylmethylene) hydrazono] oxindoles arrest cell cycle and induce cell apoptosis by inhibiting CDK2 and Bcl-2: Synthesis, biological evaluation and in silico studies. J. Enzym. Inhib. Med. Chem. 2020, 35, 1300–1309. [Google Scholar] [CrossRef]
- Al-Warhi, T.; El Kerdawy, A.M.; Aljaeed, N.; Ismael, O.E.; Ayyad, R.R.; Eldehna, W.M.; Abdel-Aziz, H.A.; Al-Ansary, G.H. Synthesis, biological evaluation and in silico studies of certain oxindole–indole conjugates as anticancer CDK inhibitors. Molecules 2020, 25, 2031. [Google Scholar] [CrossRef]
- El-Naggar, M.; Eldehna, W.M.; Almahli, H.; Elgez, A.; Fares, M.; Elaasser, M.M.; Abdel-Aziz, H.A. Novel thiazolidinone/thiazolo [3, 2-a] benzimidazolone-isatinas apoptotic anti-proliferative agents towards breast cancer: One-pot synthesis and in vitro biological evaluation. Molecules 2018, 23, 1420. [Google Scholar] [CrossRef]
- Boda, S.; Nukala, S.K.; Ravinder, M. One-Pot Synthesis of Some New Isatin-1,2,4-oxadiazole Hybrids as VEGFR-2 Aiming Anticancer Agents. ChemistrySelect 2022, 7, e202200972. [Google Scholar] [CrossRef]
- Dhokne, P.; Sakla, A.P.; Shankaraiah, N. Structural insights of oxindole based kinase inhibitors as anticancer agents: Recent advances. Eur. J. Med. Chem. 2021, 216, 113334. [Google Scholar] [CrossRef]
- Seliem, I.A.; Panda, S.S.; Girgis, A.S.; Tran, Q.L.; Said, M.F.; Bekheit, M.S.; Abdelnaser, A.; Nasr, S.; Fayad, W.; Soliman, A.A.; et al. Development of Isatin-Based Schiff Bases Targeting VEGFR-2 Inhibition: Synthesis, Characterization, Antiproliferative Properties, and QSAR Studies. ChemMedChem 2022, 17, e202200164. [Google Scholar] [CrossRef] [PubMed]
- Srour, A.M.; Dawood, D.H.; Nossier, E.S.; El-Shiekh, R.A.; Mahmoud, A.E.; Hussien, A.G.; Omran, M.M.; Ali, M.M. Design, synthesis and molecular docking simulation of oxindole-based derivatives with dual VEGFR-2 and cholinesterase inhibitory activities. J. Mol. Struct. 2023, 1271, 134130. [Google Scholar] [CrossRef]
- Pathak, A.; Pandey, V.; Pokharel, Y.R.; Devaraji, V.; Ali, A.; Haider, K.; Saad, S.; Dewangan, R.P.; Siddiqui, N.; Yar, M.S. Pharmacophore based drug design and synthesis of oxindole bearing hybrid as anticancer agents. Bioorganic Chem. 2021, 116, 105358. [Google Scholar] [CrossRef]
- Zou, Y. Benzofuran-isatin conjugates as potent VEGFR-2 and cancer cell growth inhibitors. J. Heterocycl. Chem. 2020, 57, 510–516. [Google Scholar] [CrossRef]
- Abdelgawad, M.A.; Hayallah, A.M.; Bukhari, S.N.A.; Musa, A.; Elmowafy, M.; Abdel-Rahman, H.M.; El-Gaber, A.; Mohammed, K. Design, Synthesis, Molecular Modeling, and Anticancer Evaluation of New VEGFR-2 Inhibitors Based on the Indolin-2-One Scaffold. Pharmaceuticals 2022, 15, 1416. [Google Scholar] [CrossRef]
- Yang, T.-H.; Lee, C.-I.; Huang, W.-H.; Lee, A.-R. Synthesis and evaluation of novel 2-pyrrolidone-fused (2-oxoindolin-3-ylidene) methylpyrrole derivatives as potential multi-target tyrosine kinase receptor inhibitors. Molecules 2017, 22, 913. [Google Scholar] [CrossRef]
- Al-Salem, H.S.; Arifuzzaman, M.; Issa, I.S.; Motiur Rahman, A.F.M. Isatin-hydrazones with multiple Receptor Tyrosine Kinases (RTKs) inhibitory activity and in-silico binding mechanism. Appl. Sci. 2021, 11, 3746. [Google Scholar] [CrossRef]
- Chang, Y.; Yuan, Y.; Zhang, Q.; Rong, Y.; Yang, Y.; Chi, M.; Liu, Z.; Zhang, Y.; Yu, P.; Teng, Y. Effects of an isatin derivative on tumor cell migration and angiogenesis. RSC Adv. 2020, 10, 1191–1197. [Google Scholar] [CrossRef]
- Kang, C.-M.; Liu, D.-Q.; Zhao, X.-H.; Dai, Y.-J.; Cheng, J.-G.; Lv, Y.-T. QSAR and molecular docking studies on oxindole derivatives as VEGFR-2 tyrosine kinase inhibitors. J. Recept. Signal. Transduct. 2015, 36, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.-H.; Lee, C.-I.; Huang, W.-H.; An-Rong, L. Structural optimization and evaluation of novel 2-pyrrolidone-fused (2-oxoindolin-3-ylidene) methylpyrrole derivatives as potential VEGFR-2/PDGFRβ inhibitors. Chem. Cent. J. 2017, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-P.; Liu, K.-L.; Li, X.-Y.; Lu, G.-Q.; Xue, W.-H.; Qian, X.-H.; Meng, F.-H. Design, synthesis, and in vitro and in vivo anti-angiogenesis study of a novel vascular endothelial growth factor receptor-2 (VEGFR-2) inhibitor based on 1,2,3-triazole scaffold. Eur. J. Med. Chem. 2020, 211, 113083. [Google Scholar] [CrossRef] [PubMed]
- Eldehna, W.M.; Fares, M.; Ibrahim, H.S.; Aly, M.H.; Zada, S.; Ali, M.M.; Abou-Seri, S.M.; Abdel-Aziz, H.A.; Abou El Ella, D.A. Indoline ureas as potential anti-hepatocellular carcinoma agents targeting VEGFR-2: Synthesis, in vitro biological evaluation and molecular docking. Eur. J. Med. Chem. 2015, 100, 89–97. [Google Scholar] [CrossRef]
- Mohamady, S.; Galal, M.; Eldehna, W.M.; Gutierrez, D.C.; Ibrahim, H.S.; Elmazar, M.M.; Ali, H.I. Dual Targeting of VEGFR2 and C-Met Kinases via the Design and Synthesis of Substituted 3-(Triazolo-thiadiazin-3-yl) indolin-2-one Derivatives as Angiogenesis Inhibitors. ACS Omega 2020, 5, 18872–18886. [Google Scholar] [CrossRef]
- Elkaeed, E.B.; Taghour, M.S.; Mahdy, H.A.; Eldehna, W.M.; El-Deeb, N.M.; Kenawy, A.M.; Alsfouk, B.A.; Dahab, M.A.; Metwaly, A.M.; Eissa, I.H.; et al. New quinoline and isatin derivatives as apoptotic VEGFR-2 inhibitors: Design, synthesis, anti-proliferative activity, docking, ADMET, toxicity, and MD simulation studies. J. Enzym. Inhib. Med. Chem. 2022, 37, 2191–2205. [Google Scholar] [CrossRef]
- Taghour, M.S.; Elkady, H.; Eldehna, W.M.; El-Deeb, N.M.; Kenawy, A.M.; Elkaeed, E.B.; Alsfouk, A.A.; Alesawy, M.S.; Metwaly, A.M.; Ibrahim, H.E. Design and synthesis of thiazolidine-2, 4-diones hybrids with 1,2-dihydroquinolones and 2-oxindoles as potential VEGFR-2 inhibitors: In-vitro anticancer evaluation and in-silico studies. J. Enzym. Inhib. Med. Chem. 2022, 37, 1903–1917. [Google Scholar] [CrossRef]
- Taghour, M.S.; Elkady, H.; Eldehna, W.M.; El-Deeb, N.; Kenawy, A.M.; Elkaeed, E.B.; Alsfouk, B.A.; Alesawy, M.S.; Husein, D.Z.; Metwaly, A.M.; et al. Design, synthesis, anti-proliferative evaluation, docking, and MD simulations studies of new thiazolidine-2, 4-diones targeting VEGFR-2 and apoptosis pathway. PLoS ONE 2022, 17, e0272362. [Google Scholar] [CrossRef]
- Abdelsalam, E.A.; El-Hafeez, A.A.A.; Eldehna, W.M.; El Hassab, M.A.; Marzouk, H.M.M.; Elaasser, M.M.; Taleb, N.A.A.; Amin, K.M.; Abdel-Aziz, H.A.; Ghosh, P.; et al. Discovery of novel thiazolyl-pyrazolines as dual EGFR and VEGFR-2 inhibitors endowed with in vitro antitumor activity towards non-small lung cancer. J. Enzym. Inhib. Med. Chem. 2022, 37, 2265–2282. [Google Scholar] [CrossRef]
- King, L.C.; Ostrum, G.K. Selective Bromination with Copper(II) Bromide 1. J. Org. Chem. 1964, 29, 3459–3461. [Google Scholar] [CrossRef]
- Al-Sanea, M.M.; Al-Ansary, G.H.; Elsayed, Z.M.; Maklad, R.M.; Elkaeed, E.B.; Abdelgawad, M.A.; Bukhari, S.N.A.; Abdel-Aziz, M.M.; Suliman, H.; Eldehna, W.M. Development of 3-methyl/3-(morpholinomethyl)benzofuran derivatives as novel antitumor agents towards non-small cell lung cancer cells. J. Enzym. Inhib. Med. Chem. 2021, 36, 987–999. [Google Scholar] [CrossRef] [PubMed]
- Eldehna, W.M.; Maklad, R.M.; Almahli, H.; Al-Warhi, T.; Elkaeed, E.B.; Abourehab, M.A.S.; Abdel-Aziz, H.A.; El Kerdawy, A.M. Identification of 3-(piperazinylmethyl) benzofuran derivatives as novel type II CDK2 inhibitors: Design, synthesis, biological evaluation, and in silico insights. J. Enzym. Inhib. Med. Chem. 2022, 37, 1227–1240. [Google Scholar] [CrossRef] [PubMed]
- Maklad, R.M.; AbdelHafez, E.-S.M.; Abdelhamid, D.; Aly, O.M. Tubulin inhibitors: Discovery of a new scaffold targeting extra-binding residues within the colchicine site through anchoring substituents properly adapted to their pocket by a semi-flexible linker. Bioorganic. Chem. 2020, 99, 103767. [Google Scholar] [CrossRef]
- Ismail, R.S.; Abou-Seri, S.M.; Eldehna, W.M.; Ismail, N.S.; Elgazwi, S.M.; Ghabbour, H.A.; Ahmed, M.S.; Halaweish, F.T.; El Ella, D.A.A. Novel series of 6-(2-substitutedacetamido)-4-anilinoquinazolines as EGFR-ERK signal transduction inhibitors in MCF-7 breast cancer cells. Eur. J. Med. Chem. 2018, 155, 782–796. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.-L. Hard soft acids bases (HSAB) principle and organic chemistry. Chem. Rev. 1975, 75, 1–20. [Google Scholar] [CrossRef]
- Aly, O.M.; Beshr, E.A.; Maklad, R.M.; Mustafa, M.; Gamal-Eldeen, A.M. Synthesis, Cytotoxicity, Docking Study, and Tubulin Polymerization Inhibitory Activity of Novel 1-(3,4-Dimethoxyphenyl)-5-(3,4,5-trimethoxyphenyl)-1 H -1,2,4-triazole-3-carboxanilides: 1-(3,4-Dimethoxyphenyl)-5-(3,4,5-trimethoxyphenyl)-1H-1,2,4-triazole-3-carboxanilides. Arch. Der. Pharm. 2014, 347, 658–667. [Google Scholar] [CrossRef]
- Elasasy, M.E.A.; Elnaggar, D.H.; Hafez, N.A.A.; Azab, M.E.; Amr, A.E.; Omran, M.M.; Mohamed, A.M. Synthesis and Antiproliferative Activity of Novel Hydrazono Thiazolidene and Thiazole Derivatives Bearing Rhodanine Moiety. Russ. J. Gen. Chem. 2021, 91, 915–925. [Google Scholar] [CrossRef]
- Abdelhamid, A.O.; Sayed, A.R.; Zaki, Y.H. Reaction of Hydrazonoyl Halides 511: A Facile Synthesis of 5-Arylthiazoles and Triazolino [4,3-a]pyrimidines as Antimicrobial Agents. Phosphorus Sulfur Silicon Relat. Elements 2007, 182, 1447–1457. [Google Scholar] [CrossRef]
- Phillips, R.R. The Japp-Klingemann Reaction. In Organic Reactions; John Wiley & Sons, Inc., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 143–178. [Google Scholar] [CrossRef]
- Farghaly, M.; Abdel-Wahab, B.F.; Ahmed, E.M. Synthesis, antiviral and antimicrobial screening of some new 2-oxoindoline derivatives. Chem. Heterocycl. Comp. 2009, 45, 539–544. [Google Scholar] [CrossRef]
- Sanad, S.M.H.; Mekky, A.E.M.; Said, A.Y.; Elneairy, M.A.A. Pyridine-2(1H)-thiones: Versatile precursors for one-pot synthesis of new Nicotinonitrile-Thiazole hybrids. J. Heterocyclic. Chem. 2021, 58, 1461–1471. [Google Scholar] [CrossRef]
- Al-Hussain, S.A.; Alshehrei, F.; Zaki, M.E.A.; Harras, M.F.; Farghaly, T.A.; Muhammad, Z.A. Fluorinated hydrazonoyl chlorides as precursors for synthesis of antimicrobial azoles. J. Heterocyclic. Chem. 2020, 58, 589–602. [Google Scholar] [CrossRef]
- Voronin, V.V.; Ledovskaya, M.S.; Gordeev, E.G.; Rodygin, K.S.; Ananikov, V.P. [3+2]-Cycloaddition of in Situ Generated Nitrile Imines and Acetylene for Assembling of 1,3-Disubstituted Pyrazoles with Quantitative Deuterium Labeling. J. Org. Chem. 2018, 83, 3819–3828. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Bhat, S.V.; Prabhath, M.R.R.; Molino, A.; Nauha, E.; Wilson, D.J.D.; Moses, J.E. Synthesis of 4-Triazol-3-imines via Selective Stepwise Cycloaddition of Nitrile Imines with Organo-cyanamides. Org. Lett. 2018, 20, 4263–4266. [Google Scholar] [CrossRef]
- Konkel, M.J.; Lagu, B.; Boteju, L.W.; Jimenez, H.; Noble, S.; Walker, M.W.; Chandrasena, G.; Blackburn, T.P.; Nikam, S.S.; Wright, J.L.; et al. 3-arylimino-2-indolones are potent and selective galanin GAL3 receptor antagonists. J. Med. Chem. 2006, 49, 3757–3758. [Google Scholar] [CrossRef] [PubMed]
- Wegermann, C.A.; Monzani, E.; Casella, L.; Ribeiro, M.A.; Bruzeguini, C.E.; Vilcachagua, J.D.; Costa, L.A.S.; Ferreira, A.M.D.C. Unveiling geometrical isomers and tautomers of isatin-hydrazones by NMR spectroscopy. J. Mol. Struct. 2021, 1250, 131633. [Google Scholar] [CrossRef]
- Jakusová, K.; Donovalová, J.; Gáplovský, M.; Cigáň, M.; Stankovičová, H.; Self-association, A.G. Self-association, tautomerism and E–Z isomerization of isatin–phenylsemicarbazones–spectral study and theoretical calculations. J. Phys. Org. Chem. 2013, 26, 805–813. [Google Scholar] [CrossRef]
- Abdel-Aziz, H.A.; Ghabbour, H.A.; Eldehna, W.M.; Qabeel, M.M. Synthesis, crystal structure, and biological activity of cis/trans amide rotomers of (Z)-N′-(2-Oxoindolin-3-ylidene) formohydrazide. J. Chem. 2014, 2014, 760434. [Google Scholar] [CrossRef]
- Sabt, A.; Eldehna, W.M.; Ibrahim, T.M.; Bekhit, A.A.; Rasha, Z.B. New antileishmanial quinoline linked isatin derivatives targeting DHFR-TS and PTR1: Design, synthesis, and molecular modeling studies. Eur. J. Med. Chem. 2023, 246, 114959. [Google Scholar] [CrossRef]
- Pfeffer, C.M.; Singh, A.T. Apoptosis: A target for anticancer therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef]
- Hu, W.; Kavanagh, J.J. Anticancer therapy targeting the apoptotic pathway. Lancet Oncol. 2003, 4, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Qadir, M.I.; Perveen, N.; Ahmad, B.; Saleem, U.; Irshad, T. Inhibitors of apoptotic proteins: New targets for anticancer therapy. Chem. Biol. Drug. Des. 2013, 82, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.C. Apoptosis-based therapies. Nat. Rev. Drug. Discov. 2002, 1, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Yaochen, L.; Guojun, Z. Cell cycle regulation and anticancer drug discovery. Cancer Biol. Med. 2017, 14, 348. [Google Scholar]
- Eastman, A. Cell cycle checkpoints and their impact on anticancer therapeutic strategies. J. Cell. Biochem. 2004, 91, 223–231. [Google Scholar] [CrossRef]
- Källblad, P.; Mancera, R.L.; Nikolay, P. Assessment of multiple binding modes in ligand−protein docking. J. Med. Chem. 2004, 47, 3334–3337. [Google Scholar] [CrossRef]
- Tripathi, S.; Srivastava, G.; Ashok, S. Molecular dynamics simulation and free energy landscape methods in probing L215H, L217R and L225M βI-tubulin mutations causing paclitaxel resistance in cancer cells. Biochem. Biophys. Res. Commun. 2016, 476, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Karki, S.S.; Bahaduria, V.S.; Rana, V.; Kumar, S.; Subbaro, P.G.; Das, U.; Balzarini, J.; De Clercq, E.; Dimmock, J.R. 1-Arylmethyl-2, 3-dioxo-2, 3-dihydroindole thiosemicarbazones as leads for developing cytotoxins and anticonvulsants. J. Enzym. Inhib. Med. Chem. 2008, 24, 537–544. [Google Scholar] [CrossRef]
- Da Costa, D.P.; de Castro, A.C.; da Silva, G.A.; Lima-Junior, C.G.; de Andrade Júnior, F.P.; de Oliveira Lima, E.; Vaz, B.G.; da Silva, L.C. Microwave-assisted synthesis and antimicrobial activity of novel spiro 1, 3, 4-thiadiazolines from isatin derivatives. J. Heterocycl. Chem. 2021, 58, 766–776. [Google Scholar] [CrossRef]
- Eldehna, W.M.; Abo-Ashour, M.F.; Al-Warhi, T.; Al-Rashood, S.T.; Alharbi, A.; Ayyad, R.R.; Al-Khayal, K.; Abdulla, M.; Abdel-Aziz, H.A.; Ahmad, R.; et al. Development of 2-oxindolin-3-ylidene-indole-3-carbohydrazide derivatives as novel apoptotic and anti-proliferative agents towards colorectal cancer cells. J. Enzym. Inhib. Med. Chem. 2021, 36, 320–329. [Google Scholar] [CrossRef]
- Abou-Seri, S.M.; Eldehna, W.M.; Ali, M.M.; El Ella, D.A.A. 1-Piperazinylphthalazines as potential VEGFR-2 inhibitors and anticancer agents: Synthesis and in vitro biological evaluation. Eur. J. Med. Chem. 2016, 107, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, H.A.; Eldehna, W.M.; Fares, M.; Al-Rashood, S.T.A.; Al-Rashood, K.A.; Abdel-Aziz, M.M.; Soliman, D.H. Synthesis, biological evaluation and 2D-QSAR study of halophenyl bis-hydrazones as antimicrobial and antitubercular agents. Int. J. Mol. Sci. 2015, 16, 8719–8743. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Khalifa, M.M.; Al-Karmalawy, A.A.; Elkaeed, E.B.; Nafie, M.S.; Tantawy, M.A.; Eissa, I.H.; Mahdy, H.A. Topo II inhibition and DNA intercalation by new phthalazine-based derivatives as potent anticancer agents: Design, synthesis, anti-proliferative, docking, and in vivo studies. J. Enzym. Inhib. Med. Chem. 2021, 37, 299–314. [Google Scholar] [CrossRef]
- Hagras, M.; El Deeb, M.A.; Elzahabi, H.S.A.; Elkaeed, E.B.; Mehany, A.B.M.; Eissa, I.H. Discovery of new quinolines as potent colchicine binding site inhibitors: Design, synthesis, docking studies, and anti-proliferative evaluation. J. Enzym. Inhib. Med. Chem. 2021, 36, 640–658. [Google Scholar] [CrossRef]
- Nafie, M.S.; Boraei, A.T. Exploration of novel VEGFR2 tyrosine kinase inhibitors via design and synthesis of new alkylated indolyl-triazole Schiff bases for targeting breast cancer. Bioorganic Chem. 2022, 122, 105708. [Google Scholar] [CrossRef]
- Rayes, E.; Samir, M.; El Enany, G.; Ali, I.A.I.; Ibrahim, W.; Mohamed, S.; Nafie, M.S. Synthesis of novel phthalazinedione-based derivatives with promising cytotoxic, anti-bacterial, and molecular docking studies as vegfr2 inhibitors. ACS Omega 2022, 7, 26800–26811. [Google Scholar] [CrossRef]
- Eldehna, W.M.; Abo-Ashour, M.F.; Ibrahim, H.S.; Al-Ansary, G.H.; Ghabbour, H.A.; Elaasser, M.M.; Ahmed, H.Y.A.; Safwat, N.A. Novel [(3-indolylmethylene) hydrazono] indolin-2-ones as apoptotic anti-proliferative agents: Design, synthesis and in vitro biological evaluation. J. Enzym. Inhib. Med. Chem. 2018, 33, 686–700. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]
- AW, S.; van Aalten, D.M.F. PRODRG: A tool for high-throughput crystallography of protein-ligand complexes. Acta Cryst. D. Biol. Cryst. 2004, 60, 1355–1363. [Google Scholar]
- Hassab, M.A.E.; Fares, M.; Amin, M.K.A.H.; Al-Rashood, S.T.; Alharbi, A.; Eskandrani, R.O.; Alkahtani, H.M.; Eldehna, W.M. Toward the identification of potential α-ketoamide covalent inhibitors for SARS-CoV-2 main protease: Fragment-based drug design and MM-PBSA calculations. Processes 2021, 9, 1004. [Google Scholar] [CrossRef]
- El Hassab, M.A.; Shoun, A.A.; Al-Rashood, S.T.; Al-Warhi, T.; Eldehna, W.M. Identification of a new potential SARS-COV-2 RNA-dependent RNA polymerase inhibitor via combining fragment-based drug design, docking, molecular dynamics, and MM-PBSA calculations. Front. Chem. 2020, 8, 915. [Google Scholar] [CrossRef] [PubMed]
- El Hassab, M.A.; Ibrahim, T.M.; Al-Rashood, S.T.; Alharbi, A.; Eskandrani, R.O.; Eldehna, W.M. In silico identification of novel SARS-COV-2 2’-O-methyltransferase (nsp16) inhibitors: Structure-based virtual screening, molecular dynamics simulation and MM-PBSA approaches. J. Enzyme Inhib. Med. Chem. 2021, 36, 727–736. [Google Scholar] [CrossRef] [PubMed]
- El Hassab, M.A.; Ibrahim, T.M.; Shoun, A.A.; Al-Rashood, S.T.; Alkahtani, H.M.; Alharbi, A.; Eskandrani, R.O.; Eldehna, W.M. In silico identification of potential SARS COV-2 2′-O-methyltransferase inhibitor: Fragment-based screening approach and MM-PBSA calculations. RSC Adv. 2021, 11, 16026–16033. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; Van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Parrinello, M.; Rahman, A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]

| Comd. | Ar/R | IC50 (µM) a | |
|---|---|---|---|
| MCF-7 | A-549 | ||
| 7a | C6H5 | 19.53 ± 1.05 | NA b |
| 7b | 4-Br-C6H4 | NA b | NA b |
| 7c | 4-F-C6H4 | 7.17 ± 0.94 | 20.43 ± 1.51 |
| 7d | 4-Cl-C6H4 | 2.93 ± 0.47 | 9.57 ± 0.62 |
| 12a | H | 39.53 ± 2.02 | 61.76 ± 4.01 |
| 12b | NO2 | NA b | NA b |
| 12c | F | 27.65 ± 2.39 | 12.20 ± 1.54 |
| 12d | Cl | 13.92 ± 1.21 | 29.45 ± 2.06 |
| 12e | SO2NH2 | NA b | NA b |
| Dox. | - | 4.30 ± 0.84 | 6.4 ± 0.79 |
| Compound | VEGFR-2 IC50 (μM) a |
|---|---|
| 7c | 0.728 |
| 7d | 0.503 |
| Sorafenib | 0.112 |
| Comp. | Caspase-3 IU/mL | Caspase-9 IU/mL | Bax IU/mL | Bcl-2 IU/mL | Bax/Bcl-2 Ratio |
|---|---|---|---|---|---|
| Control | 0.0156 | 0.0111 | 1.3 | 3.137 | 0.4144 |
| 7d | 0.2862 *** | 0.1853 ** | 102.9 *** | 0.6449 ** | 159.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Warhi, T.; Almahli, H.; Maklad, R.M.; Elsayed, Z.M.; El Hassab, M.A.; Alotaibi, O.J.; Aljaeed, N.; Ayyad, R.R.; Ghabour, H.A.; Eldehna, W.M.; et al. 1-Benzyl-5-bromo-3-hydrazonoindolin-2-ones as Novel Anticancer Agents: Synthesis, Biological Evaluation and Molecular Modeling Insights. Molecules 2023, 28, 3203. https://doi.org/10.3390/molecules28073203
Al-Warhi T, Almahli H, Maklad RM, Elsayed ZM, El Hassab MA, Alotaibi OJ, Aljaeed N, Ayyad RR, Ghabour HA, Eldehna WM, et al. 1-Benzyl-5-bromo-3-hydrazonoindolin-2-ones as Novel Anticancer Agents: Synthesis, Biological Evaluation and Molecular Modeling Insights. Molecules. 2023; 28(7):3203. https://doi.org/10.3390/molecules28073203
Chicago/Turabian StyleAl-Warhi, Tarfah, Hadia Almahli, Raed M. Maklad, Zainab M. Elsayed, Mahmoud A. El Hassab, Ohoud J. Alotaibi, Nada Aljaeed, Rezk R. Ayyad, Hazem A. Ghabour, Wagdy M. Eldehna, and et al. 2023. "1-Benzyl-5-bromo-3-hydrazonoindolin-2-ones as Novel Anticancer Agents: Synthesis, Biological Evaluation and Molecular Modeling Insights" Molecules 28, no. 7: 3203. https://doi.org/10.3390/molecules28073203
APA StyleAl-Warhi, T., Almahli, H., Maklad, R. M., Elsayed, Z. M., El Hassab, M. A., Alotaibi, O. J., Aljaeed, N., Ayyad, R. R., Ghabour, H. A., Eldehna, W. M., & El-Ashrey, M. K. (2023). 1-Benzyl-5-bromo-3-hydrazonoindolin-2-ones as Novel Anticancer Agents: Synthesis, Biological Evaluation and Molecular Modeling Insights. Molecules, 28(7), 3203. https://doi.org/10.3390/molecules28073203









