Exploring Antimicrobial Features for New Imidazo[4,5-b]pyridine Derivatives Based on Experimental and Theoretical Study
Abstract
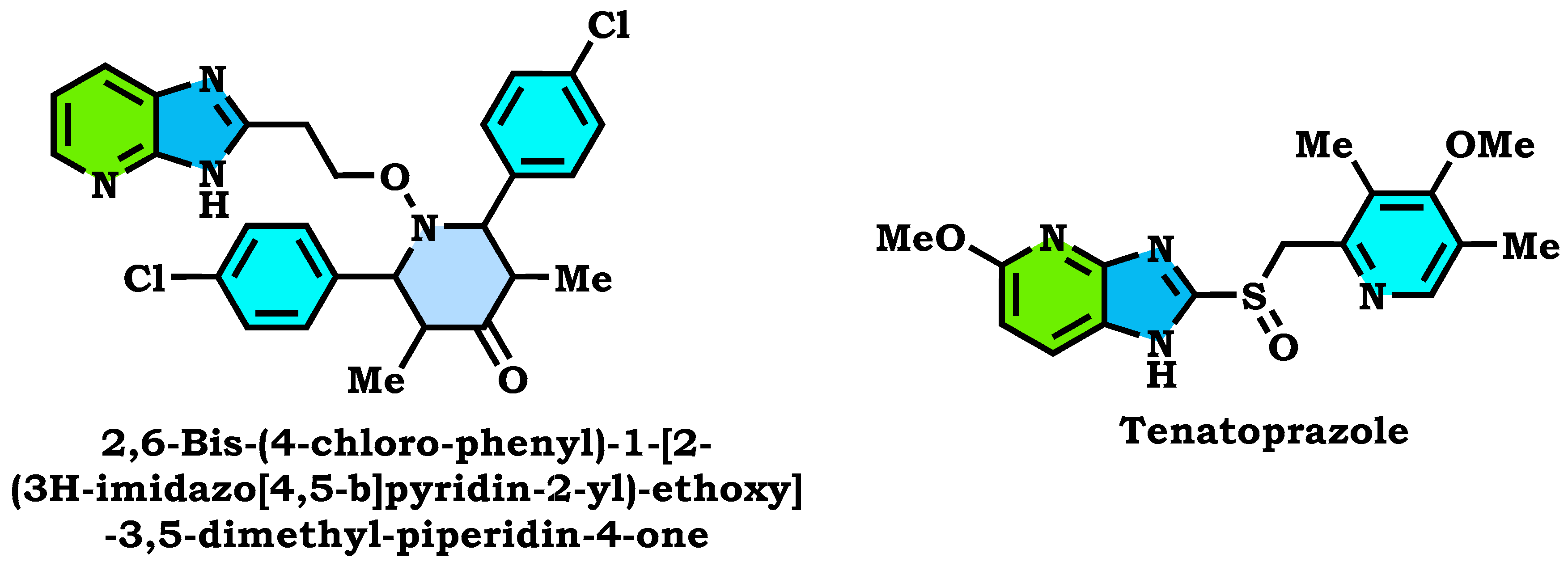
1. Introduction
2. Results and Discussion
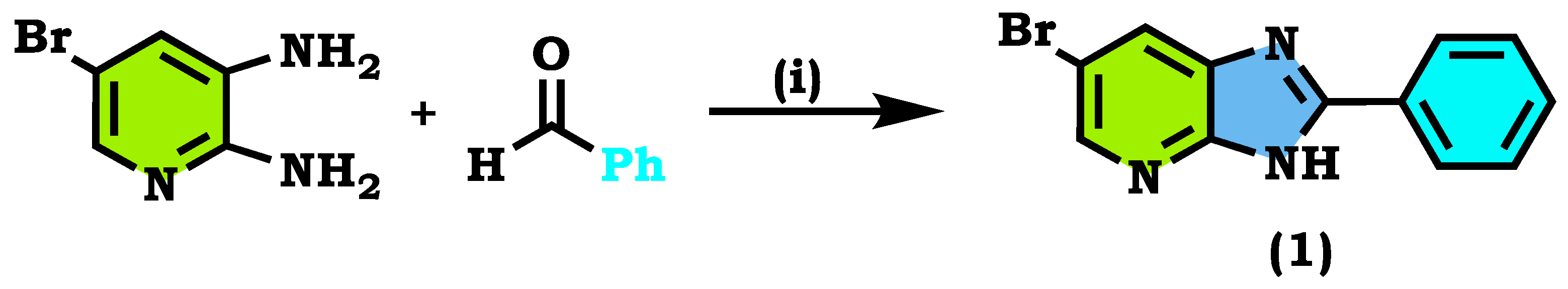
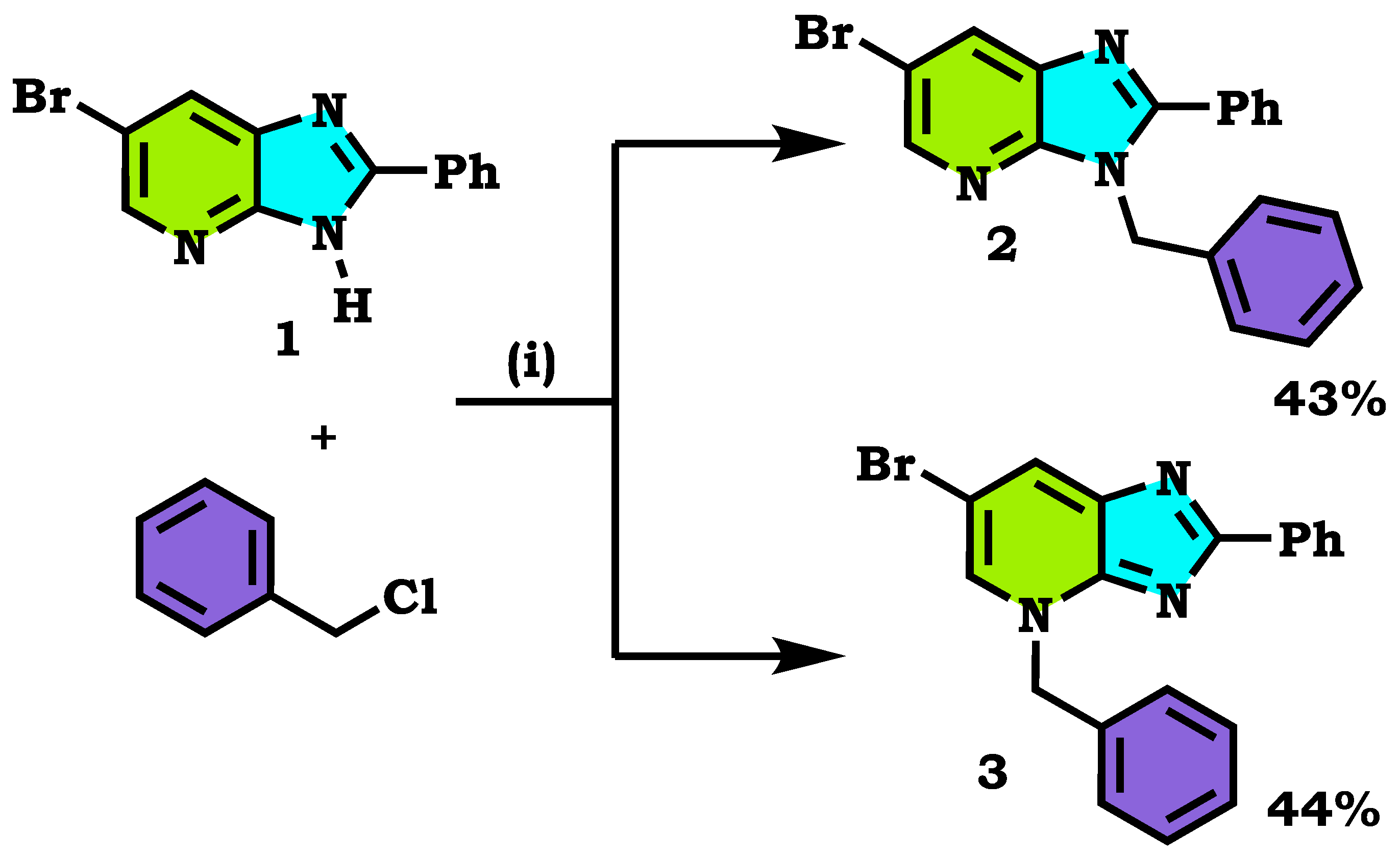
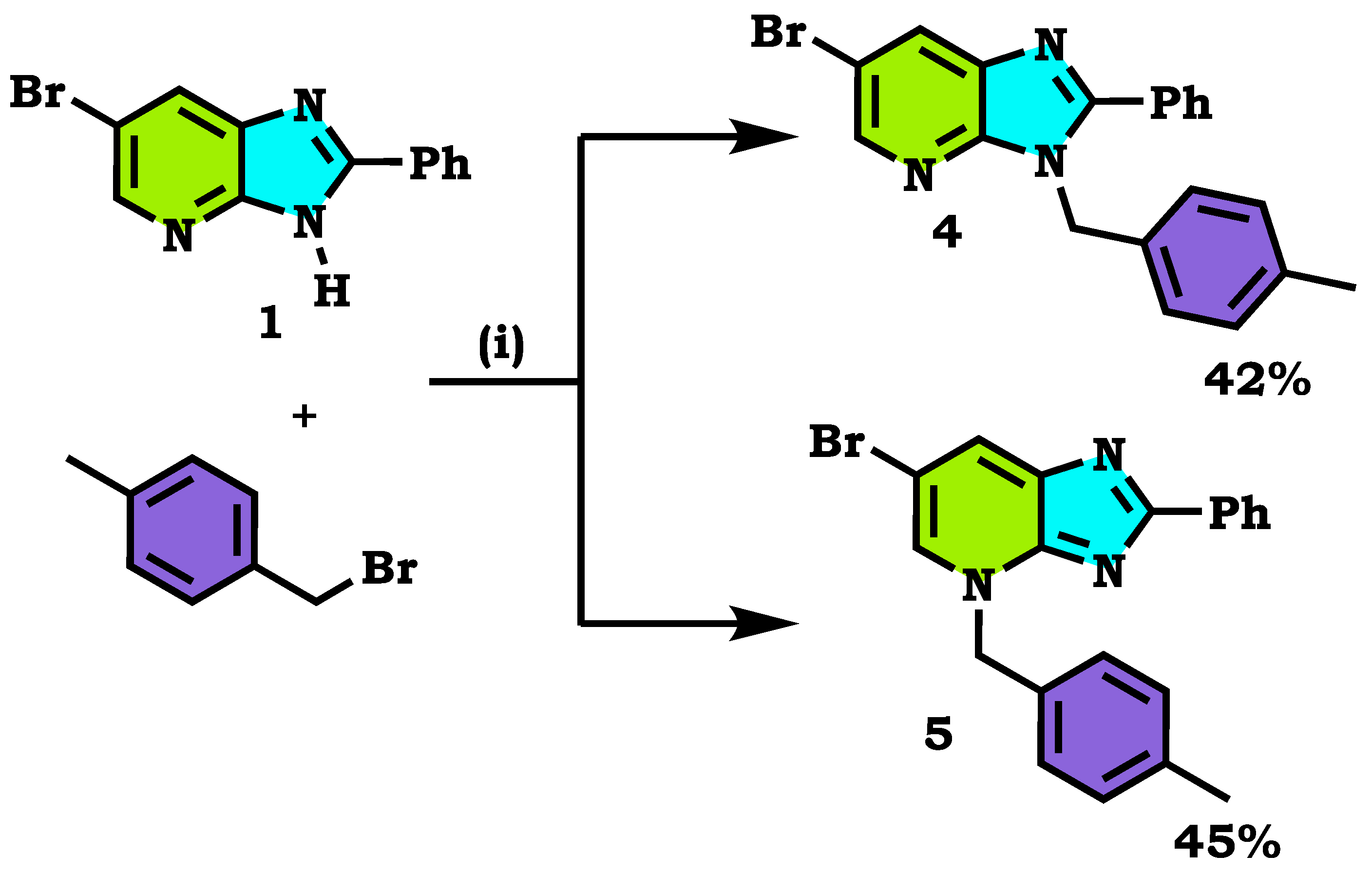
2.1. Chemistry
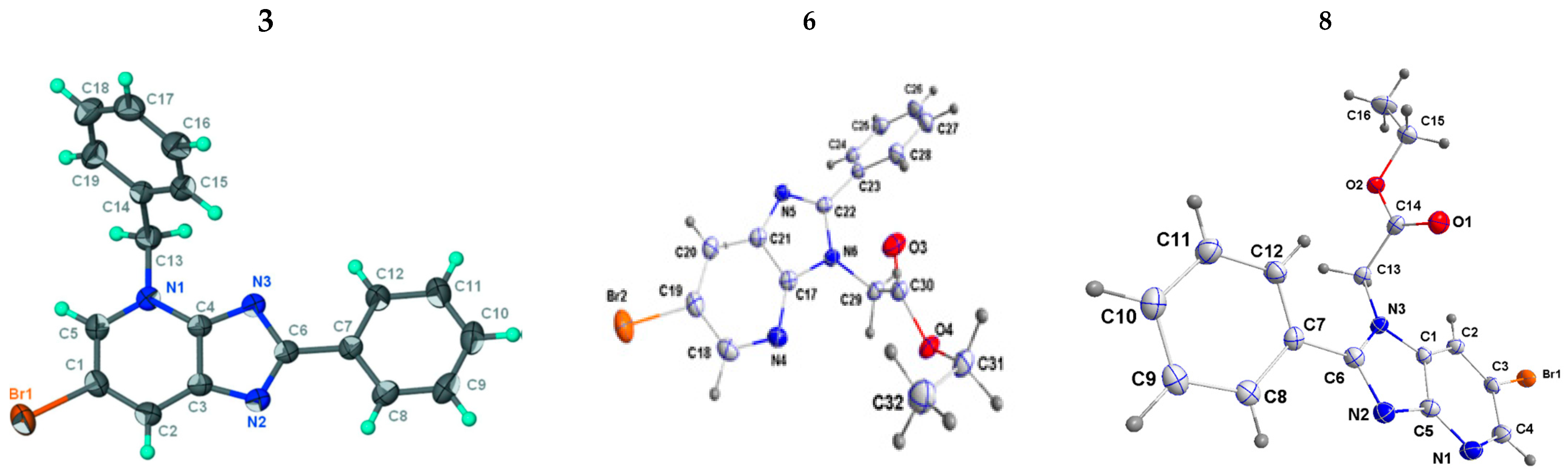
2.1.1. Crystallographic Data
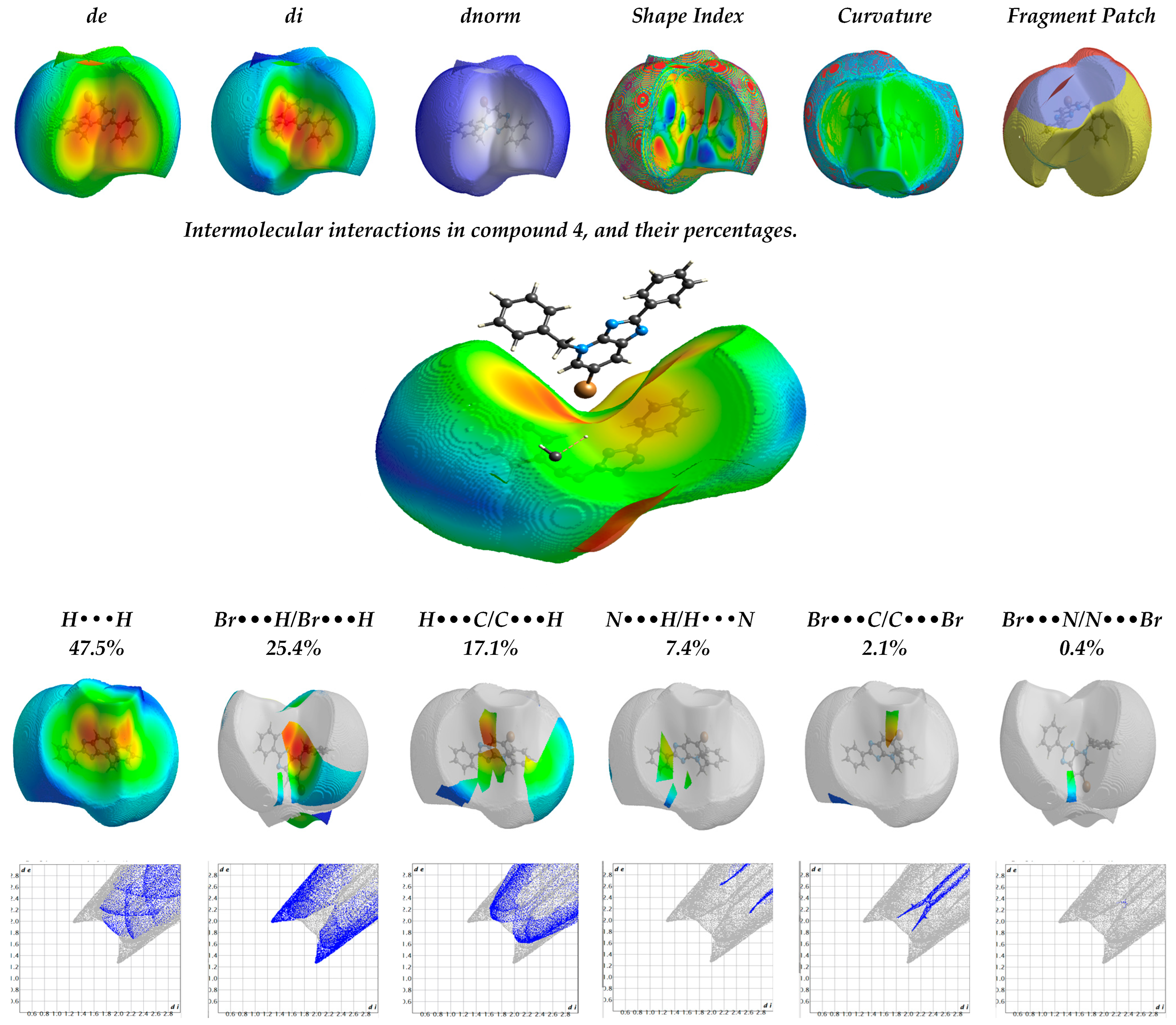
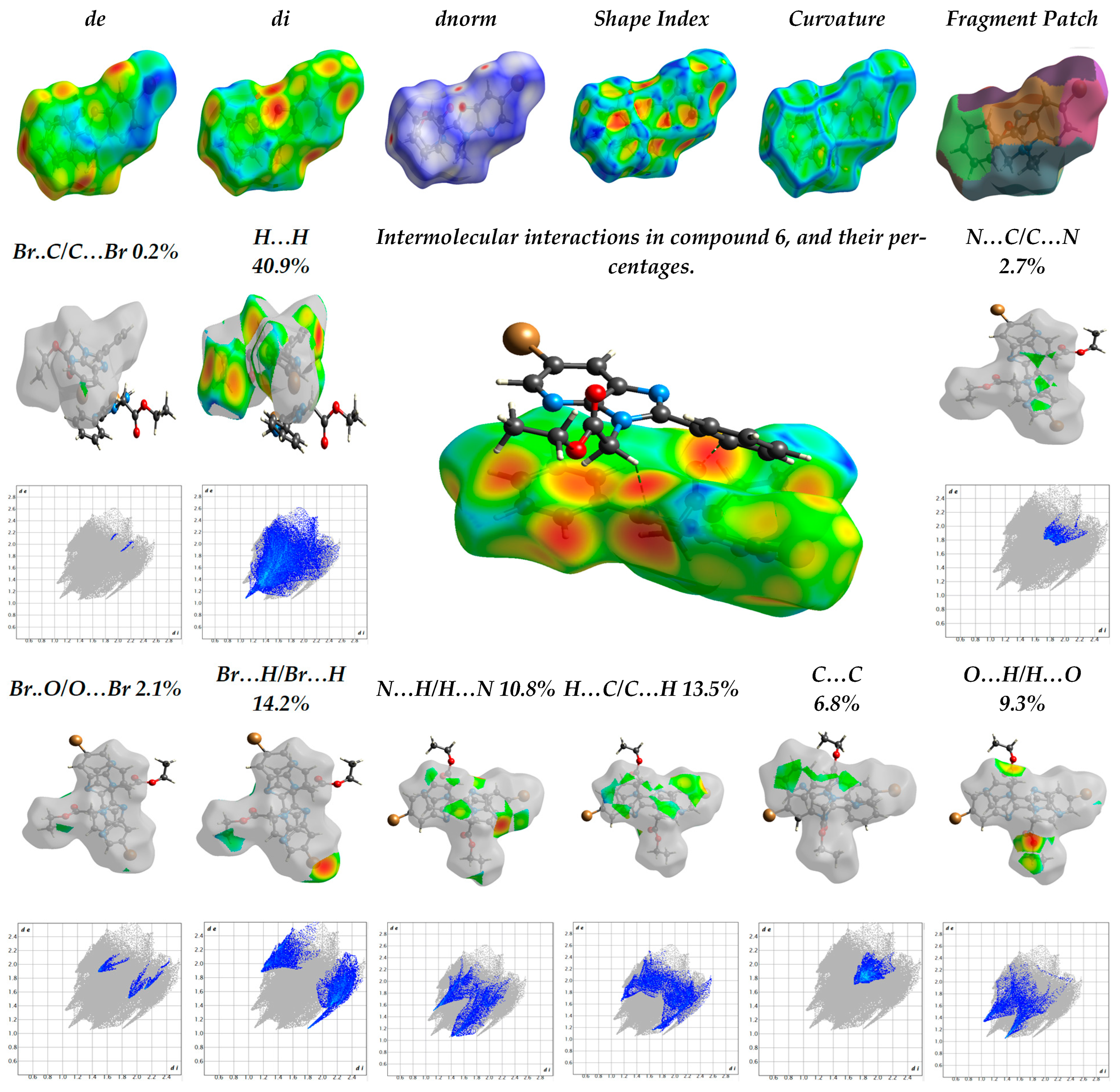
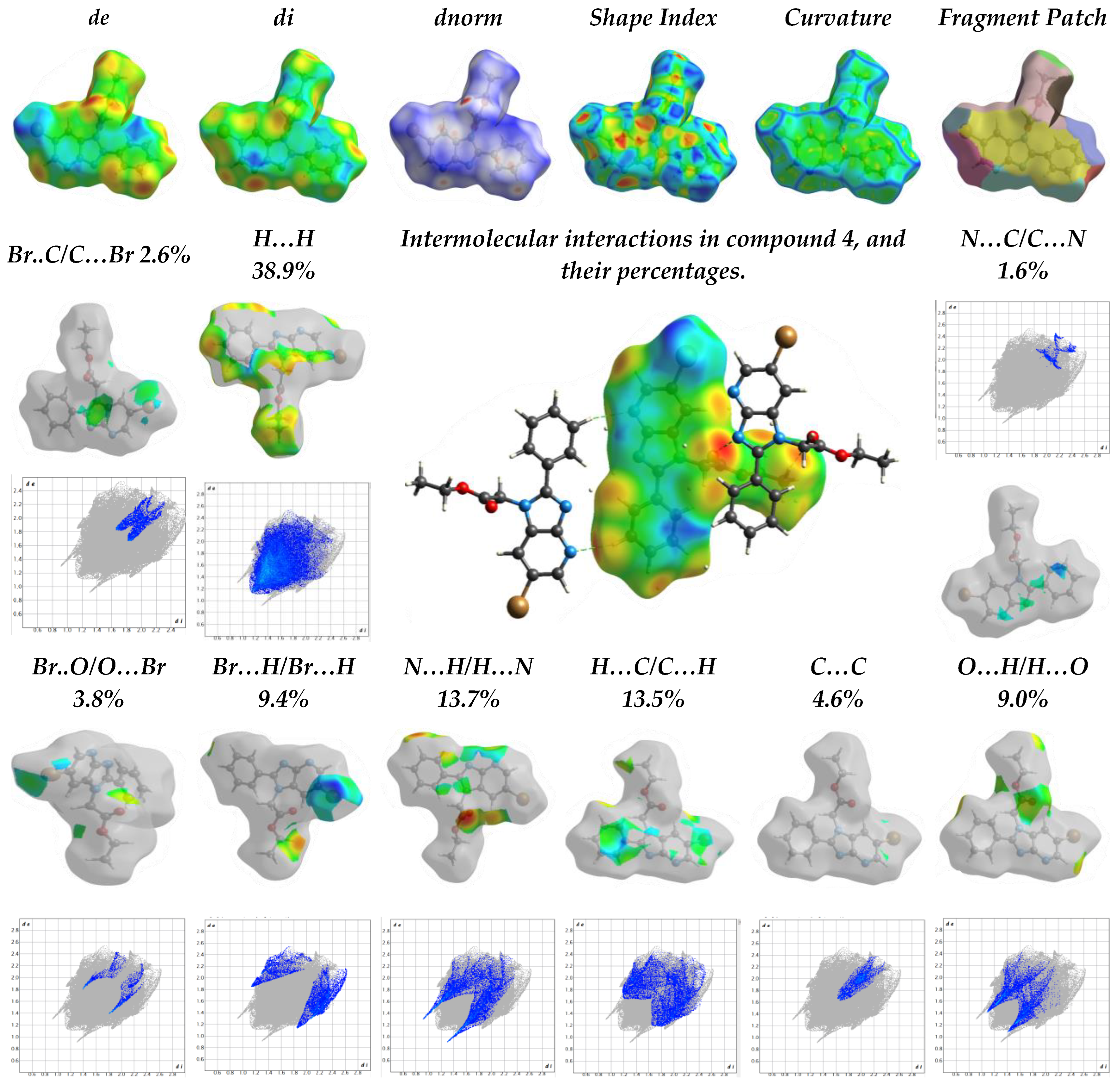
2.1.2. The Hirschfield Profile for Molecular Packing
2.2. Molecular Modelling Study
2.2.1. Tautomerization Structure and Optimization Geometry
2.2.2. Analysis of Frontier Molecular Orbitals’ FMOs and Electronic Reactivity Descriptors
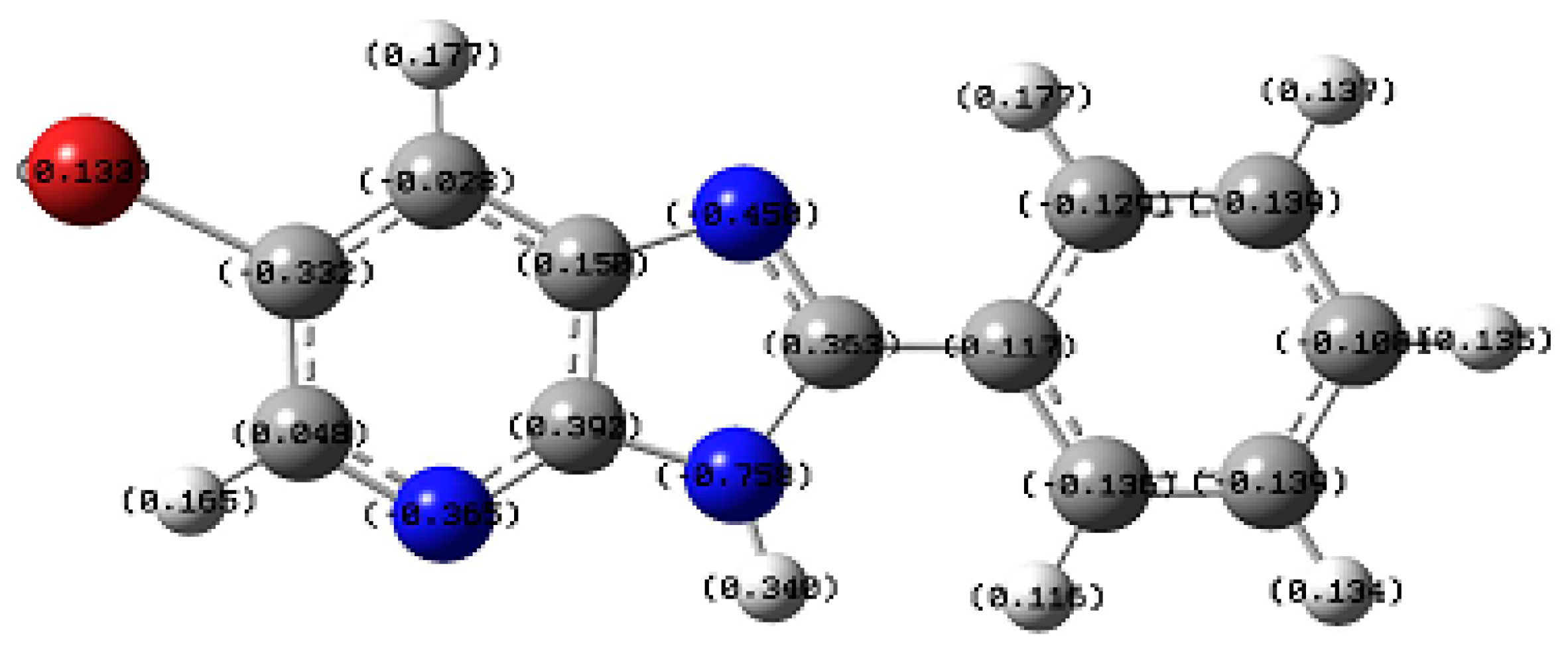
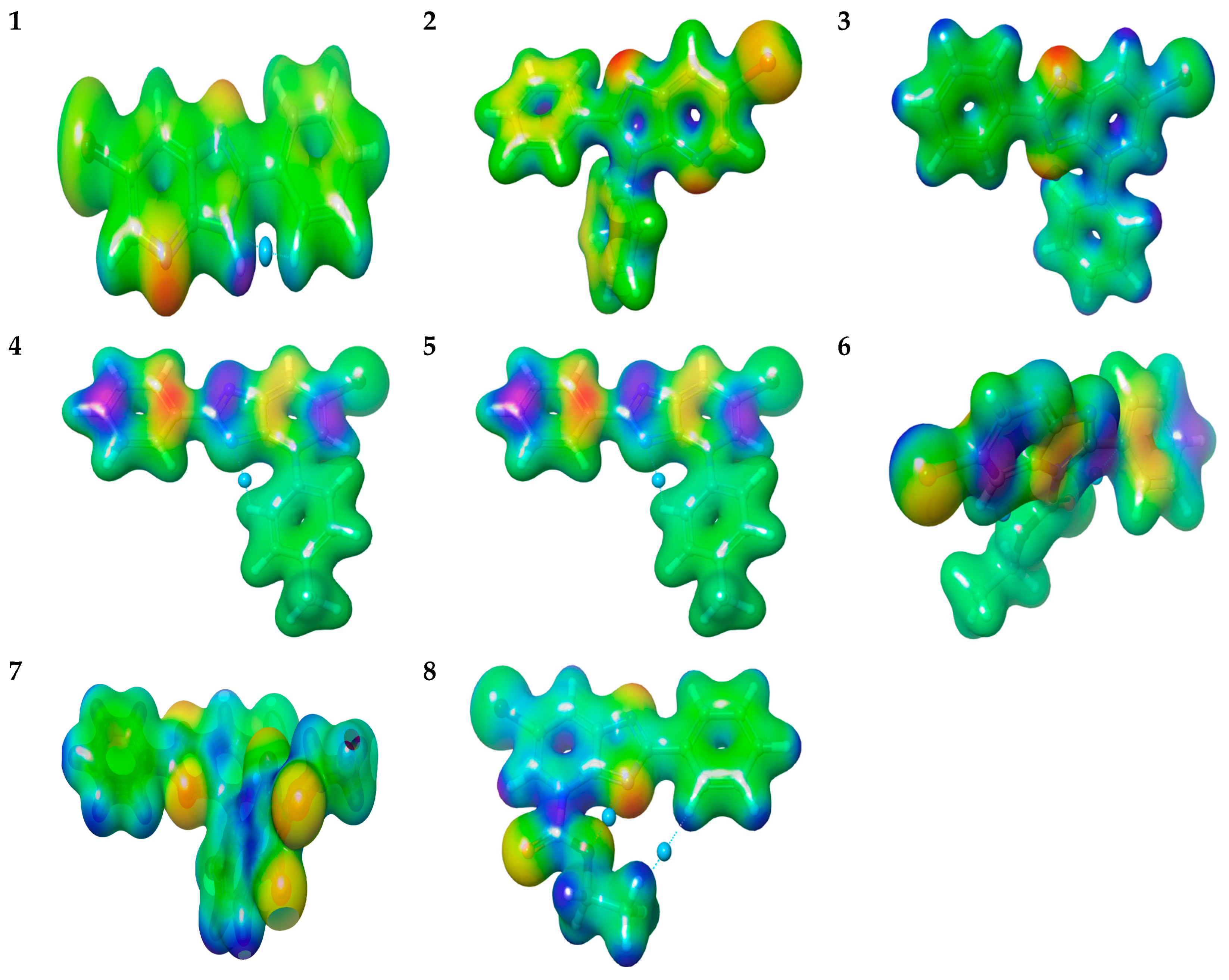
2.2.3. Molecular Electrostatic Potential “MEP” Fingerprint
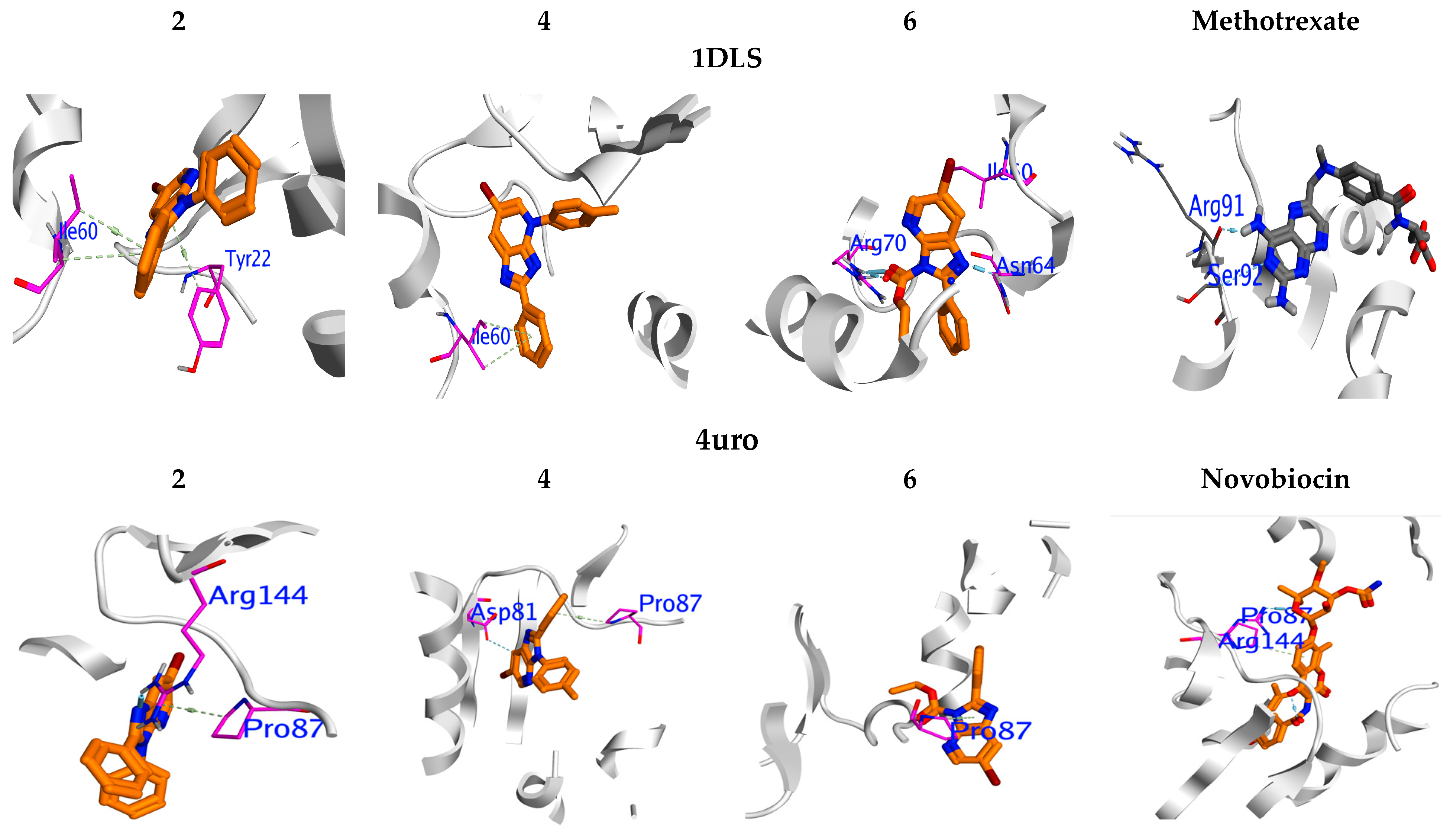
2.3. Molecular Docking Profile
2.4. Biological Activity
3. Materials and Methods
3.1. Chemistry
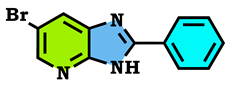
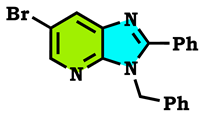

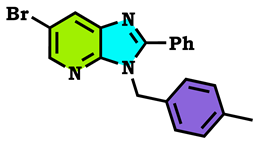
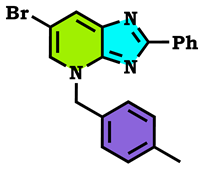



3.2. Theoretical Study
3.3. Biology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L.J. Anti-infective potential of natural products: How to develop a stronger in vitro ‘proof-of-concept’. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Bedos, J.P.; Leophonte, P. Expérience clinique du traitement par l’amoxicilline des pneumonies à pneumocoque de sensibilité diminuée à la pénicilline G. Médecine Mal. Infect. 1997, 27, 58–67. [Google Scholar] [CrossRef]
- Rodríguez-Sáiz, M.; Díez, B.; Barredo, J.L. Why did the Fleming strain fail in penicillin industry? Fungal Genet. Biol. 2005, 42, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Han, Y. Synergic effect of grape seed extract with amphotericin B against disseminated candidiasis due to Candida albicans. Phytomedicine 2007, 14, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Dash, N.; Chipem, F.A.S.; Swaminathan, R.; Krishnamoorthy, G. Hydrogen bond induced twisted intramolecular charge transfer in 2-(4′-N, N-dimethylaminophenyl) imidazo[4,5-b]pyridine. Chem. Phys. Lett. 2008, 460, 119–124. [Google Scholar] [CrossRef]
- Lukasik, P.M.; Elabar, S.; Lam, F.; Shao, H.; Liu, X.; Abbas, A.Y.; Wang, S. Synthesis and biological evaluation of imidazo[4,5-b]pyridine and 4-heteroaryl-pyrimidine derivatives as anti-cancer agents. Eur. J. Med. Chem. 2012, 57, 311–322. [Google Scholar] [CrossRef]
- Ghanem, N.M.; Farouk, F.; George, R.F.; Abbas, S.E.; El-Badry, O.M. Design and synthesis of novel imidazo[4,5-b]pyridine based compounds as potent anticancer agents with CDK9 inhibitory activity. Bioorganic Chem. 2018, 80, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Mylonas, S.; Mamalis, A. Synthesis and antitumor activity of new thiosemicarbazones of 2-acetylimidazo[4,5-b]pyridine. J. Heterocycl. Chem. 2005, 42, 1273–1281. [Google Scholar] [CrossRef]
- Temple, C.J. Antimitotic agents. Synthesis of imidazo[4,5-c]pyridin-6-ylcarbamates and imidazo[4,5-b]pyridin-5-ylcarbamates. J. Med. Chem. 1990, 33, 656–661. [Google Scholar]
- Bukowski, L.; Janowiec, M. 3-(2-Imidazo[4,5-b]pyridine) propionic acid and some of its derivatives with suspected tuberculostatic activity. Die Pharm. 1989, 44, 267–269. [Google Scholar] [CrossRef]
- Mantlo, N.B.; Kim, D.; Ondeyka, D.; Chang, R.S.; Kivlighn, S.D.; Siegl, P.K.; Greenlee, W.J. Imidazo[4,5-b]pyridine-based AT1/AT2 angiotensin II receptor antagonists. Bioorganic Med. Chem. Lett. 1994, 4, 17–22. [Google Scholar] [CrossRef]
- Nicolai, E.; Claude, S.; Teulon, J.M. New process for the synthesis of imidazo[4,5-b]pyridine derivatives as potent orally active thromboxane a2 receptor antagonists. J. Heterocycl. Chem. 1994, 31, 73–75. [Google Scholar] [CrossRef]
- Cappelli, A.; Pericot Mohr, G.L.; Giuliani, G.; Galeazzi, S.; Anzini, M.; Mennuni, L.; Ferrari, F.; Makovec, F.; Kleinrath, E.M.; Langer, T.; et al. Further studies on imidazo[4,5-b]pyridine AT1 angiotensin II receptor antagonists. Effects of the transformation of the 4-phenylquinoline backbone into 4-phenylisoquinolinone or 1-phenylindene scaffolds. J. Med. Chem. 2006, 49, 6451–6464. [Google Scholar] [CrossRef] [PubMed]
- Aridoss, G.; Balasubramanian, S.; Parthiban, P.; Kabilan, S. Synthesis and in vitro microbiological evaluation of imidazo (4,5-b) pyridinylethoxypiperidones. Eur. J. Med. Chem. 2006, 41, 268–275. [Google Scholar] [CrossRef]
- Domagala, F.; Ficheux, H. Pharmacokinetics of tenatoprazole, a novel proton pump inhibitor, in healthy male Caucasian volunteers. Gastroenterology 2003, 4, A231. [Google Scholar] [CrossRef]
- El Janati, A.; Ouzidan, Y.; Rodi, Y.K.; Chahdi, F.O.; Chraibi, M.; Benbrahim, K.F.; Cherif Alaoui, I.; El Hakmaoui, A.; Safi, M.; Akssira, M.; et al. Synthesis and antimicrobial activity of some quinoxaline derivatives. Moroc. J. Chem. 2021, 9, 346–353. [Google Scholar]
- Baba, Y.F.; Misbahi, H.; Rodi, Y.K.; Ouzidan, Y.; Essassi, E.M.; Vincze, K.; Nové, M.; Gajdács, M.; Molnár, J.; Spengler, G.; et al. 2-oxo-1, 2-dihydroquinoline-4-carboxylic acid derivatives as potent modulators of ABCB1-related drug resistance of mouse T-lymphoma cells. Chem. Data Collect. 2020, 29, 100501. [Google Scholar] [CrossRef]
- Almalki, A.S.; Nazreen, S.; Malebari, A.M.; Ali, N.M.; Elhenawy, A.A.; Alghamdi, A.A.; Ahmad, A.; Alfaifi, S.Y.; Alsharif, M.A.; Alam, M.M. Synthesis and biological evaluation of 1, 2, 3-triazole tethered thymol-1, 3, 4-oxadiazole derivatives as anticancer and antimicrobial agents. Pharmaceuticals 2021, 14, 866. [Google Scholar] [CrossRef]
- Rodi, Y.K.; Baba, Y.F.; Mague, J.T.; Chraibi, M.; Benbrahim, K.F.; Chahdi, F.O.; Ouzidan, Y.; Essassi, E. Scientific Study & Research. Chem. Chem. Eng. Biotechnol. Food Ind. 2019, 20, 487–500. [Google Scholar]
- Lahmidi, S.; Anouar, E.; El Hamdaoui, L.; Ouzidan, Y.; Kaur, M.; Jasinski, J.P.; Sebbar, N.K.; Essassi, E.; El Moussaouiti, M.J. Synthesis, crystal structure, spectroscopic characterization, hirshfeld surface analysis, DFT calculations and antibacterial activity of ethyl 2-(4-vinylbenzyl)-2-(5-methyl-[1,2,4]triazolo[1,5-a]pyrimidin-7-yl)-3-(4-vinylphenyl)propanoate. Mol. Struct. 2019, 1191, 66–75. [Google Scholar] [CrossRef]
- Ellouz, M.; Sebbar, N.K.; Fichtali, I.; Ouzidan, Y.; Mennane, Z.; Charof, R.; Mague, J.T.; Urrutigoïty, M.; Essassi, E.M. Synthesis and antibacterial activity of new 1, 2, 3-triazolylmethyl-2 H-1, 4-benzothiazin-3 (4 H)-one derivatives. Chem. Cent. J. 2018, 12, 123. [Google Scholar] [CrossRef] [PubMed]
- Gangjee, A.; Zaware, N.; Raghavan, S.; Yang, J.; Thorpe, J.E.; Ihnat, M.A. N4-(3-Bromophenyl)-7-(substituted benzyl) pyrrolo [2, 3-d] pyrimidines as potent multiple receptor tyrosine kinase inhibitors: Design, synthesis, and in vivo evaluation. Bioorganic Med. Chem. 2012, 20, 2444–2454. [Google Scholar] [CrossRef] [PubMed]
- Vlasov, S.V.; Vlasova, O.D.; Severina, H.I.; Krolenko, K.Y.; Borysov, O.V.; Abu Sharkh AI, M.; Georgiyants, V.A. Design, Synthesis and In Vitro Antimicrobial Activity of 6-(1 H-Benzimidazol-2-yl)-3, 5-dimethyl-4-oxo-2-thio-3, 4-dihydrothieno [2, 3-d] pyrimidines. Sci. Pharm. 2021, 89, 49. [Google Scholar] [CrossRef]
- El-Mawgoud, H.K.A.; Radwan, H.A.M.; Fouda, A.M.; El-Mariah, F.; Elhenawy, A.A.; Amr, A.E.; Almehizia, A.A.; Ghabbour, H.A.; El-Agrody, A.M. Synthesis, cytotoxic activity, crystal structure, DFT, molecular docking study of some heterocyclic compounds incorporating benzo [f] chromene moieties. J. Mol. Struct. 2022, 1260, 132829. [Google Scholar] [CrossRef]
- Okasha, R.M.; Fouda, A.M.; Bajaber, M.A.; Ghabbour, H.A.; Amr, A.E.G.E.; Naglah, A.M.; Almehizia, A.A.; Elhenawy, A.A.; El-Agrody, A.M. The Crystal Structure of 3-Amino-1-(4-Chlorophenyl)-9-Methoxy-1 H-Benzo [f] Chromene-2-Carbonitrile: Antimicrobial Activity and Docking Studies. Crystals 2022, 12, 982. [Google Scholar] [CrossRef]
- Kudin, K.N.; Burant, J.C.; Millam, J.M.; Iyengar, S.S.; Tomasi, J.; Barone, V.; Mennucci, B.; Cossi, M.; Scalmani, G.; Rega, N.; et al. Gaussian 03, Revision D.02; Gaussian, Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Bourichi, S.; Misbahi, H.; Kandri Rodi, Y.; Ouazzani Chahdi, F.; Essassi, E.M.; Gajdács, M.; Molnár, J.; Szabó, S.; Szalontai, B.; Spengler, G. In vitro evaluation of the multidrug resistance reversing activity of novel imidazo[4,5-b]pyridine derivatives. Anticancer Res. 2018, 38, 3999–4003. [Google Scholar] [CrossRef]
- Ouzidan, Y.; Rodi, Y.K.; Zouihri, H.; Essassi, E.M.; Ng, S.W. 3-Benzyl-6-bromo-2-(2-furyl)-3H-imidazo[4,5-b]pyridine. Acta Crystallogr. Sect. E Struct. Rep. Online 2010, 66, o1874. [Google Scholar] [CrossRef] [PubMed]
- Aziz, J.; Baladi, T.; Piguel, S. Direct Alkynylation of 3 H-Imidazo[4,5-b]pyridines Using gem-Dibromoalkenes as Alkynes Source. J. Org. Chem. 2016, 81, 4122–4133. [Google Scholar] [CrossRef] [PubMed]
- Doganc, F.; Alp, M.; Karabay, A.; Koç, A.; Eren, G.; Göker, H. Synthesis and Cytotoxicity of Some Imidazo[4,5-b]pyridine Derivatives and Their Regioselective N-Alkylation. ChemistrySelect 2021, 6, 1519–1525. [Google Scholar] [CrossRef]
- Zeinyeh, W.; Pilmé, J.; Radix, S.; Walchshofer, N. Regioselective N-alkylation of imidazo[4,5-b]pyridine-4-oxide derivatives: An experimental and DFT study. Tetrahedron Lett. 2009, 50, 1828–1833. [Google Scholar] [CrossRef]
- Zouitini, A.; Faizi MS, H.; Ouzidan, Y.; Ouazzani Chahdi, F.; Marrot, J.; Prim Dege, N.; Mashrai, A. Synthesis, crystal structure at 219 K and Hirshfeld surface analyses of 1, 4, 6-trimethylquinoxaline-2, 3 (1H, 4H)-dione monohydrate. Acta Crystallogr. Sect. E Crystallogr. Commun. 2020, 76, 1296–1301. [Google Scholar] [CrossRef] [PubMed]
- Ouzidan, Y.; Kandri Rodi, Y.; Butcher, R.J.; Essassi, E.M.; El Ammari, L. 1-Nonyl-1H-benzimidazol-2 (3H)-one. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67, o283. [Google Scholar] [CrossRef]
- Henkelman, G.; Arnaldsson, A.; Jónsson, H. A fast robust algorithm Bader decomposition of charge density. Comput. Mater. Sci. 2003, 36, 354–360. [Google Scholar] [CrossRef]
- Koch, U.; Popelier, P. Characterization of C-H-O hydrogen bonds on the basis of the charge density. J. Phys. Chem. 1995, 99, 9747–9754. [Google Scholar] [CrossRef]
- Elsisi, D.M.; Ahmed, R.; Elhenawy, A.A.; Farag, A.A.; Ali, A.M.; Ammar, Y.A. Experimental and theoretical investigation for 6-Morpholinosulfonylquinoxalin-2 (1H)-one and its haydrazone derivate: Synthesis, characterization, tautomerization and antimicrobial evaluation. J. Mol. Struct. 2022, 1247, 131314. [Google Scholar] [CrossRef]
- Al-Harbi, L.M.; Al-Harbi, E.A.; Okasha, R.M.; El-Eisawy, R.A.; El-Nassag, M.A.; Mohamed, H.M.; Fouda, A.M.; Elhenawy, A.A.; Mora, A.; El-Agrody, A.M.; et al. Discovery of benzochromene derivatives first example with dual cytotoxic activity against the resistant cancer cell MCF-7/ADR and inhibitory effect of the P-glycoprotein expression levels. J. Enzym. Inhib. Med. Chem. 2023, 38, 2155814. [Google Scholar] [CrossRef]
- Ahmed, H.E.; Amer, A.; Senior, S.A.; Ihmaid, S.; Almalghrabi, M.; El Massry, A.M.; Sahar, A.; Elhenawy, A.A. Extensive Study of DFT-Quantum Calculations Based QSAR Modeling of Fused 1, 2, 4-Triazine Derivatives Revealed Potent CYP1A1 Inhibitors. J. Comput. Biophys. Chem. 2022, 21, 741–758. [Google Scholar] [CrossRef]
- Alsehli, M.H.; Al-Harbi, L.M.; Okasha, R.M.; Fouda, A.M.; Ghabbour, H.A.; Amr, A.E.-G.E.; Elhenawy, A.A.; El-Agrody, A.M. Synthesis, Cytotoxic Activity, Crystal Structure, DFT, Molecular Docking Study of β-Enaminonitrile Incorporating 1H-Benzo[f]-Chromene Moiety. Crystals 2023, 13, 24. [Google Scholar] [CrossRef]
- Lewis, W.S.; Cody, V.; Galitsky, N.; Luft, J.R.; Pangborn, W.; Chunduru, S.K.; Spencer, H.T.; Appleman, J.R.; Blakley, R.L. Methotrexate-resistant Variants of Human Dihydrofolate Reductase with Substitutions of Leucine 22: Kinetics, Crystallography, And Potential As Selectable Markers. J. Biol. Chem. 1995, 270, 5057–5064. [Google Scholar] [CrossRef]
- Lu, J.; Patel, S.; Sharma, N.; Soisson, S.M.; Kishii, R.; Takei, M.; Fukuda, Y.; Lumb, K.J.; Singh, S.B. Structures of kibdelomycin bound to Staphylococcus aureus GyrB and ParE showed a novel U-shaped binding mode. ACS Chem. Biol. 2014, 9, 2023–2031. [Google Scholar] [CrossRef]
- Alghamdi, H.; Nazreen, S.; Elhenawy, A.A.; Abdelbaset, M. Experimental and theoretical investigation of new 1, 3, 4-oxadiazole based dioxovanadium (VO+ 2) complexes and their in-vitro antimicrobial potency. Mater. Express. 2021, 11, 888–903. [Google Scholar] [CrossRef]
- Domyati, D.; Zabin, S.A.; Elhenawy, A.A.; Abdelbaset, M. Preparation, Antimicrobial Activity and Docking Study of Vanadium Mixed Ligand Complexes Containing 4-Amino-5-hydrazinyl-4 H-1, 2, 4-triazole-3-thiol and Aminophenol Derivatives. Processes 2021, 9, 1008. [Google Scholar] [CrossRef]
- Alzahrani, A.S.; Shareefa, S.N.; Elhenawy, A.A.; Neamatallah, T.; Alam, M.M. Synthesis, Biological Evaluation, and Molecular Docking of New Benzimidazole-1, 2, 3-Triazole Hybrids as Antibacterial and Antitumor Agents. Polycycl. Aromat. Compd. 2022, 1–12. [Google Scholar] [CrossRef]
- Alzahrani, H.A.; Alam, M.M.; Elhenawy, A.A.; Malebari, A.M.; Nazreen, S. Synthesis, antiproliferative, docking and DFT studies of benzimidazole derivatives as EGFR inhibitors. J. Mol. Struct. 2022, 1253, 132265. [Google Scholar] [CrossRef]
- Sameeh, M.Y.; Khowdiary, M.M.; Nassar, H.S.; Abdelall, M.M.; Suliman, A.; Elhenawy, A.A. Discovery Potent of Thiazolidinedione Derivatives as Antioxidant, α-Amylase Inhibitor, and Antidiabetic Agent. Biomedicines 2021, 10, 24. [Google Scholar] [CrossRef]
- Albalawi, F.; Mohammed, A.A.; El-Nassag, R.; El-Eisawy, A.; Alderhami, B.M.; Mohamed, I.; Fouda, A.M.; Afifi, T.H.; Elhenawy, A.A.; Mora, A.; et al. Synthesis of 9-Hydroxy-1 H-Benzo [f] chromene Derivatives with Effective Cytotoxic Activity on MCF7/ADR, P-Glycoprotein Inhibitors, Cell Cycle Arrest and Apoptosis Effects. Int. J. Mol. Sci. 2022, 24, 49. [Google Scholar] [CrossRef]
- Lehninger, A.; Cox, N. Principles of Biochemistry; World Publishers: New York, NY, USA, 1993; Volume 58. [Google Scholar]
- Chermette, H.; Cox, N. Chemical reactivity indexes in density functional theory. J. Comput. Chem. 1999, 20, 129–154. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098. [Google Scholar]
- Sadiki, M.; Balouiri, M.; Barkai, H.; Maataoui, H.; Ibnsoud, S.; Elabed, S. Synergistic antibacterial effect of Myrtus communis and Thymus vulgaris essential oils fractional inhibitory concentration index. Int. J. Pharm. Pharm. Sci. 2014, 6, 121–124. [Google Scholar]
- Wang, X.-L.; Wan, K.; Zhou, C.-H. Synthesis of novel sulfanilamide-derived 1, 2, 3-triazoles and their evaluation for antibacterial and antifungal activities. Eur. J. Med. Chem. 2010, 45, 4631–4639. [Google Scholar]
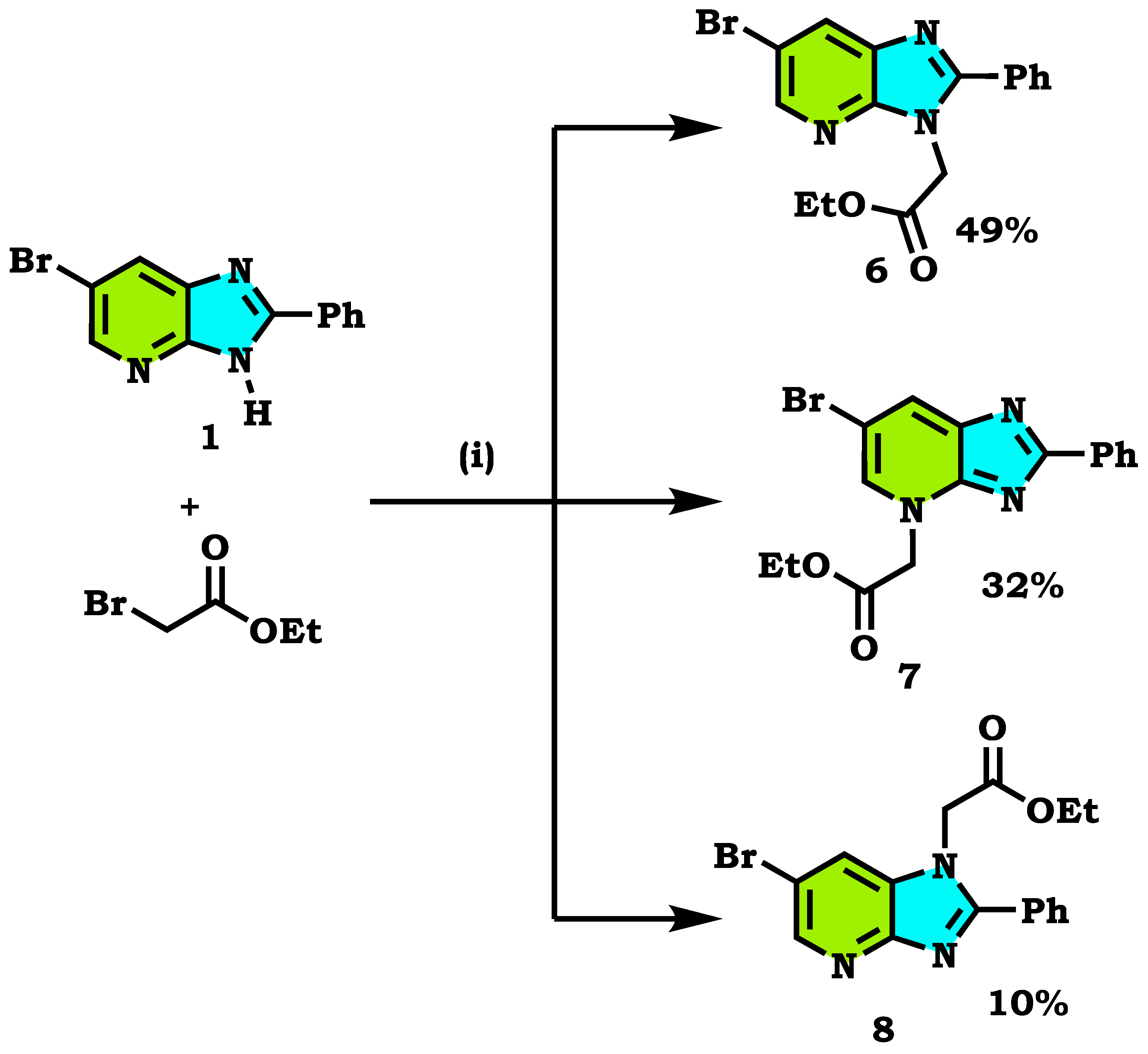


| Compound Alkylated at Third Position | Energy E3 (Kcal/mol.) | Compound Alkylated at Fourth Position | Energy E4 (Kcal/mol.) | Compound Alkylated at First Position | Energy E1 (Kcal/mol.) |
|---|---|---|---|---|---|
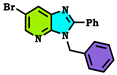 | −298.122 Kcal/mol. | 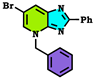 | −263.047 Kcal/mol. | T | ND |
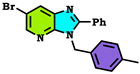 | −294.781 Kcal/mol. |  | −260.099 Kcal/mol. | T | ND |
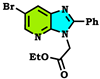 | −297.763 Kcal/mol. | 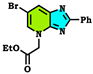 | −262.738 Kcal/mol. | 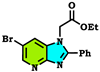 | −263.756 Kcal/mol. |
| HOMO | LUMO | Δε | η | S | IP | μ | χ | ω | ΔNmax | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | −0.225 | −0.053 | 0.173 | 0.086 | 11.563 | 0.225 | −0.095 | −0.139 | 0.292 | −0.803 |
| 2 | −0.216 | −0.082 | 0.135 | 0.067 | 14.819 | 0.139 | −0.115 | −0.149 | 0.403 | −1.104 |
| 3 | −0.224 | −0.073 | 0.151 | 0.075 | 12.806 | 0.159 | −0.110 | −0.148 | 0.345 | −0.935 |
| 4 | −0.228 | −0.054 | 0.174 | 0.087 | 11.480 | 0.228 | −0.097 | −0.141 | 0.299 | −0.810 |
| 5 | −0.237 | −0.060 | 0.177 | 0.089 | 11.271 | 0.237 | −0.104 | −0.148 | 0.322 | −0.836 |
| 6 | −0.220 | −0.115 | 0.105 | 0.053 | 18.993 | 0.148 | −0.141 | −0.167 | 0.616 | −1.590 |
| 7 | −0.223 | −0.101 | 0.122 | 0.061 | 16.442 | 0.169 | −0.131 | −0.161 | 0.510 | −1.329 |
| 8 | −0.222 | −0.080 | 0.142 | 0.071 | 14.081 | 0.148 | −0.115 | −0.151 | 0.398 | −1.065 |
| ΔE | rmsd | H.B | EInt. | E_ele | LE | Ki | ΔE | rmsd | H.B | EInt. | E_ele | LE | Ki | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | −5.45 | 1.34 | 36.07 | −16.26 | −10.64 | −11.83 | 2.47 | −5.978 | 1.396 | 34.336 | −13.795 | −8.124 | −11.829 | 2.471 |
| 2 | −7.00 | 1.95 | 38.57 | −22.93 | −9.34 | −7.24 | 1.98 | −6.272 | 1.243 | 74.826 | −21.907 | −9.019 | −7.242 | 1.980 |
| 3 | −6.52 | 1.58 | 81.36 | −18.78 | −7.49 | −4.67 | 1.54 | −5.858 | 1.081 | 36.965 | −15.246 | −8.351 | −4.666 | 1.540 |
| 4 | −8.44 | 1.46 | 32.85 | −25.31 | −8.15 | −6.14 | 1.81 | −6.020 | 1.030 | 81.941 | −18.905 | −9.377 | −8.654 | 2.158 |
| 5 | −6.67 | 1.88 | 44.24 | −17.35 | −9.50 | −8.65 | 2.16 | −5.762 | 1.374 | 44.248 | −21.344 | −9.142 | −5.655 | 1.733 |
| 6 | −7.86 | 1.29 | 18.08 | −24.58 | −7.07 | −4.19 | 0.94 | −6.116 | 3.687 | 55.830 | −18.595 | −8.311 | −6.928 | 1.936 |
| 7 | −6.35 | 1.12 | 52.20 | −22.09 | −10.31 | −5.66 | 1.73 | −6.391 | 2.993 | 49.144 | −14.564 | −8.344 | −4.769 | 1.562 |
| 8 | −6.53 | 1.71 | 35.38 | −14.32 | −8.29 | −1.17 | 1.94 | −6.822 | 1.409 | 36.965 | −18.855 | −8.942 | −1.168 | 0.155 |
| Ref. | −7.85 | 1.68 | −203.76 | −27.82 | −10.09 | −6.58 | 1.88 | −7.240 | 1.372 | 25.581 | −21.869 | −9.444 | −6.138 | 1.815 |
| Strains | B. cereus | E. coli | |
|---|---|---|---|
| Products | |||
| (4) | + | - | |
| (6) | + | + | |
| Concentration mg/mL | 4 | 6 | ||
|---|---|---|---|---|
| B. cereus | E. coli | B. cereus | E. coli | |
| 5 | - | + | - | - |
| 2.5 | - | + | - | - |
| 1.25 | - | + | - | + |
| 0.625 | - | + | - | + |
| 0.312 | - | + | - | + |
| 0.15 | - | + | + | + |
| 0.07 | - | + | + | + |
| 0.03 | + | + | + | + |
| 0.01 | + | + | + | + |
| 0.005 | + | + | + | + |
| 0.0025 | + | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hjouji, M.-y.; Almehdi, A.M.; Elmsellem, H.; Seqqat, Y.; Ouzidan, Y.; Tebbaa, M.; Lfakir, N.A.; Kandri Rodi, Y.; Chahdi, F.O.; Chraibi, M.; et al. Exploring Antimicrobial Features for New Imidazo[4,5-b]pyridine Derivatives Based on Experimental and Theoretical Study. Molecules 2023, 28, 3197. https://doi.org/10.3390/molecules28073197
Hjouji M-y, Almehdi AM, Elmsellem H, Seqqat Y, Ouzidan Y, Tebbaa M, Lfakir NA, Kandri Rodi Y, Chahdi FO, Chraibi M, et al. Exploring Antimicrobial Features for New Imidazo[4,5-b]pyridine Derivatives Based on Experimental and Theoretical Study. Molecules. 2023; 28(7):3197. https://doi.org/10.3390/molecules28073197
Chicago/Turabian StyleHjouji, Mohammed-yassin, Ahmed M. Almehdi, Hicham Elmsellem, Yousra Seqqat, Younes Ouzidan, Mohamed Tebbaa, Noura Ait Lfakir, Youssef Kandri Rodi, Fouad Ouazzani Chahdi, Marwa Chraibi, and et al. 2023. "Exploring Antimicrobial Features for New Imidazo[4,5-b]pyridine Derivatives Based on Experimental and Theoretical Study" Molecules 28, no. 7: 3197. https://doi.org/10.3390/molecules28073197
APA StyleHjouji, M.-y., Almehdi, A. M., Elmsellem, H., Seqqat, Y., Ouzidan, Y., Tebbaa, M., Lfakir, N. A., Kandri Rodi, Y., Chahdi, F. O., Chraibi, M., Fikri Benbrahim, K., Al-Omar, M. A., Almehizia, A. A., Naglah, A. M., El-Mowafi, S. A., & Elhenawy, A. A. (2023). Exploring Antimicrobial Features for New Imidazo[4,5-b]pyridine Derivatives Based on Experimental and Theoretical Study. Molecules, 28(7), 3197. https://doi.org/10.3390/molecules28073197







