Targeting Toxoplasma gondii ME49 TgAPN2: A Bioinformatics Approach for Antiparasitic Drug Discovery
Abstract
1. Introduction
2. Results and Discussion
2.1. Structure-Based Virtual Screening
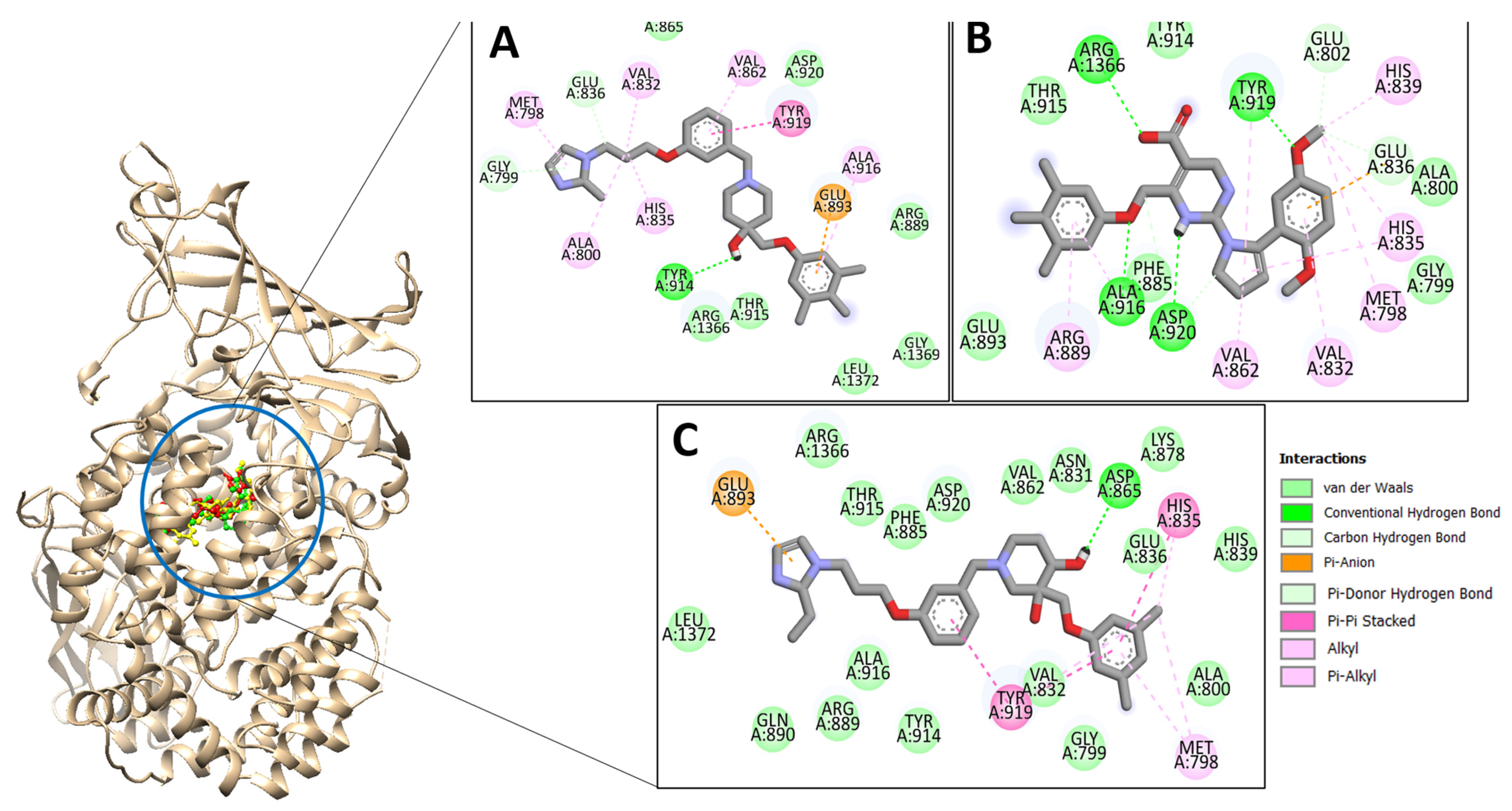
2.2. Top Three Compounds’ Docking Analysis
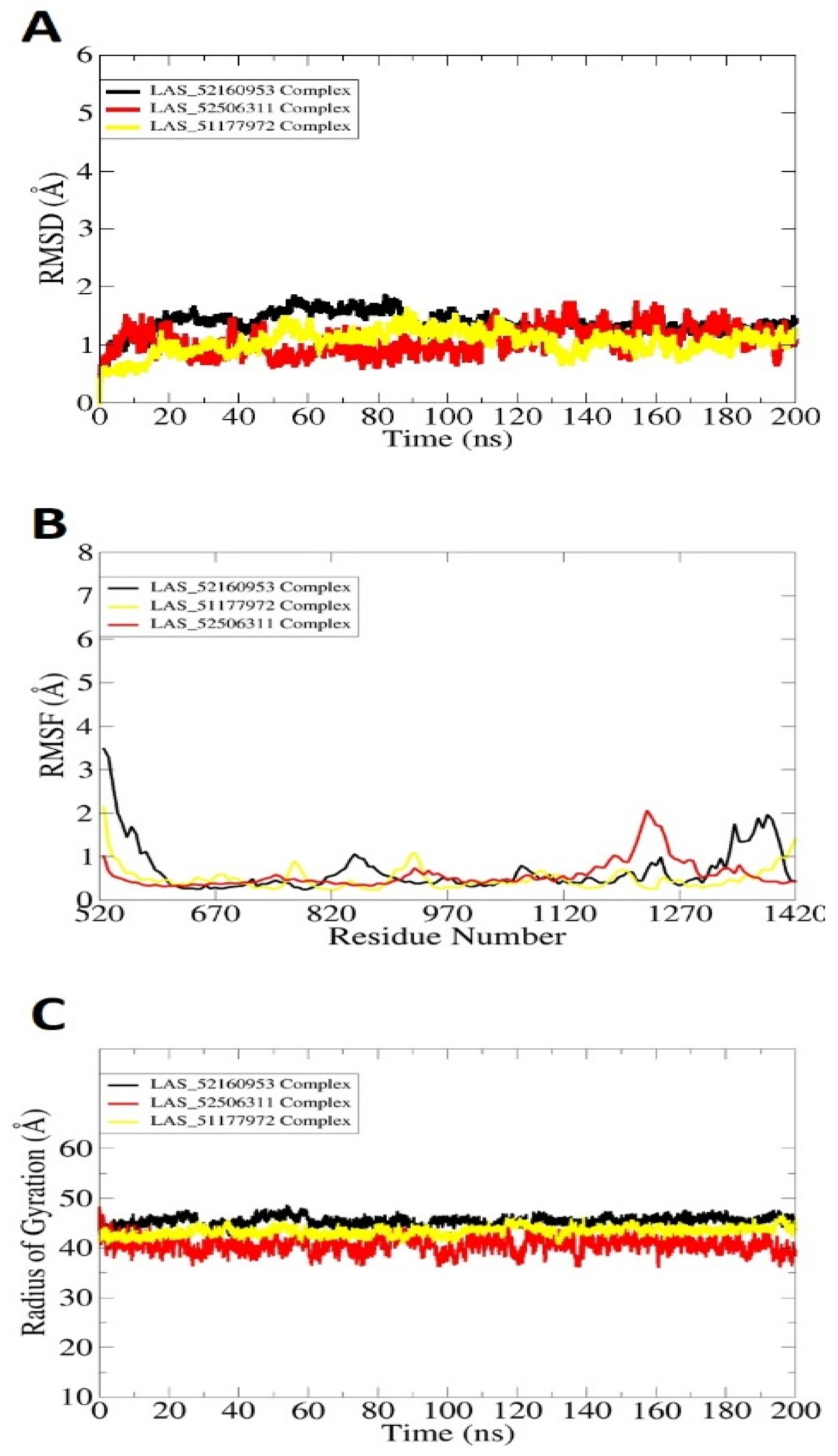
2.3. Molecular Dynamic Simulations
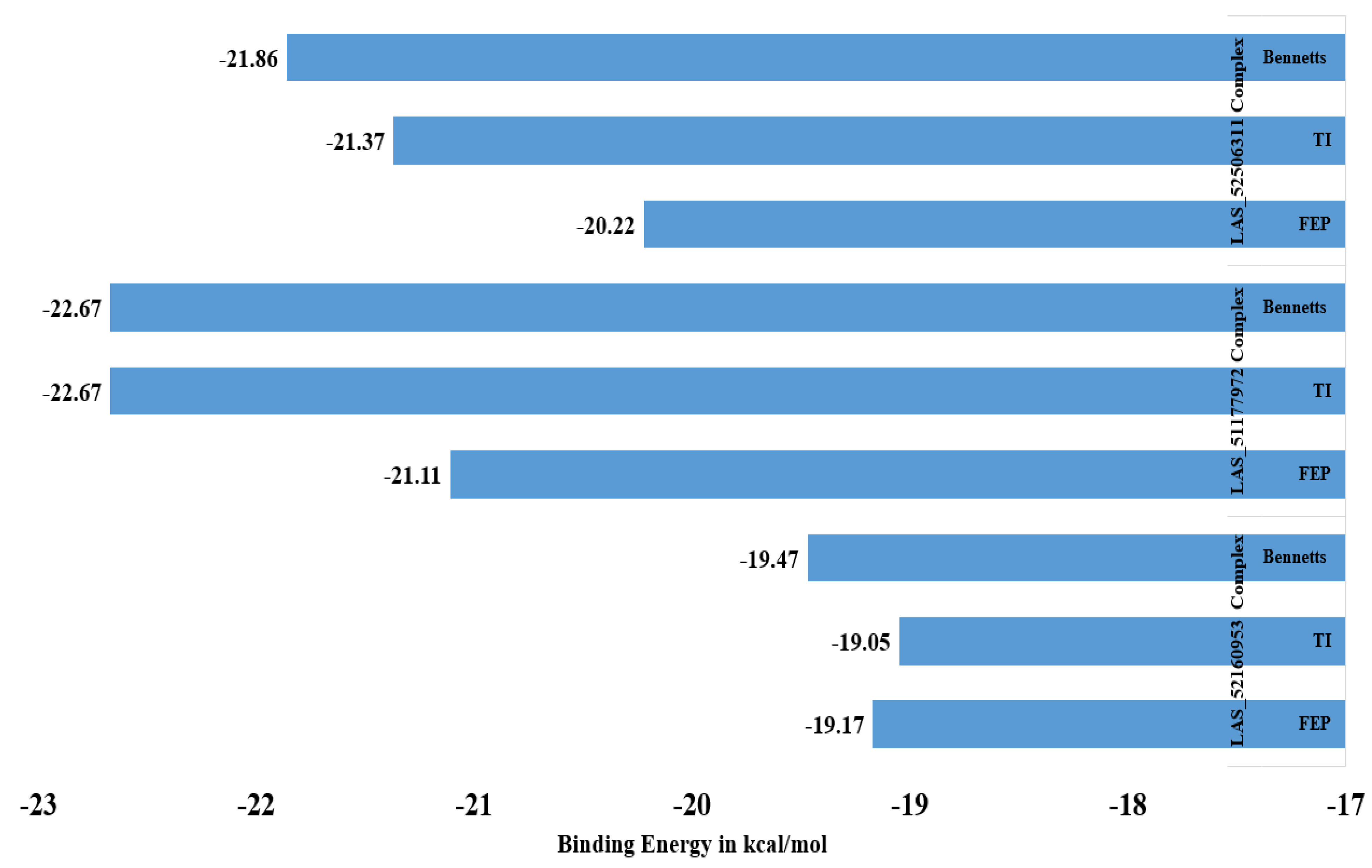
2.4. Binding Free Energy Calculations
2.5. WaterSwap Analysis
2.6. SwissADME Analysis
3. Materials and Methods
3.1. Preparation of Protein Structure and Asinex Library
3.2. Virtual Screening
3.3. Molecular Dynamic Simulation
3.4. MM/PBSA Binding Free Energy Calculations
3.5. Computational Pharmacokinetic Analysis
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chen, L.; Lin, Y.-L.; Peng, G.; Li, F. Structural basis for multifunctional roles of mammalian aminopeptidase N. Proc. Natl. Acad. Sci. USA 2012, 109, 17966–17971. [Google Scholar] [CrossRef] [PubMed]
- Marijanovic, E.M.; Swiderska, K.W.; Andersen, J.; Aschenbrenner, J.C.; Webb, C.T.; Drag, M.; Drinkwater, N.; McGowan, S. X-ray crystal structure and specificity of the Toxoplasma gondii ME49 TgAPN2. Biochem. J. 2020, 477, 3819–3832. [Google Scholar] [CrossRef] [PubMed]
- Drinkwater, N.; Lee, J.; Yang, W.; Malcolm, T.R.; McGowan, S. M1 Aminopeptidases as Drug Targets: Broad Applications or Therapeutic Niche? FEBS J. 2017, 284, 1473–1488. [Google Scholar] [CrossRef] [PubMed]
- Mathew, R.; Wunderlich, J.; Thivierge, K.; Cwiklinski, K.; Dumont, C.; Tilley, L.; Rohrbach, P.; Dalton, J.P. Biochemical and cellular characterisation of the Plasmodium falciparum M1 alanyl aminopeptidase (PfM1AAP) and M17 leucyl aminopeptidase (PfM17LAP). Sci. Rep. 2021, 11, 2854. [Google Scholar] [CrossRef] [PubMed]
- Bounaadja, L.; Schmitt, M.; Albrecht, S.; Mouray, E.; Tarnus, C.; Florent, I. Selective inhibition of PfA-M1, over PfA-M17, by an amino-benzosuberone derivative blocks malaria parasites development in vitro and in vivo. Malar. J. 2017, 16, 382. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.; Dubey, J. Toxoplasma gondii: Transmission, diagnosis and prevention. Clin. Microbiol. Infect. 2002, 8, 634–640. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Z.-D.; Huang, S.-Y.; Zhu, X.-Q. Diagnosis of toxoplasmosis and typing of Toxoplasma gondii. Parasites Vectors 2015, 8, 292. [Google Scholar] [CrossRef]
- Ali, A.; Naz, A.; Soares, S.C.; Bakhtiar, M.; Tiwari, S.; Hassan, S.S.; Hanan, F.; Ramos, R.; Pereira, U.; Barh, D.; et al. Pan-Genome Analysis of Human Gastric Pathogen, H. Pylori: Comparative Genomics and Pathogenomics Approaches to Identify Regions Associated with Pathogenicity and Prediction of Potential Core Therapeutic Targets. Biomed. Res. Int. 2015, 2015. [Google Scholar]
- McGovern, O.L.; Rivera-Cuevas, Y.; Carruthers, V.B. Emerging Mechanisms of Endocytosis in Toxoplasma gondii. Life 2021, 11, 84. [Google Scholar] [CrossRef]
- Milne, G.; Webster, J.P.; Walker, M. Toxoplasma Gondii: An Underestimated Threat? Trends Parasitol. 2020, 36, 959–969. [Google Scholar] [CrossRef]
- Montazeri, M.; Mehrzadi, S.; Sharif, M.; Sarvi, S.; Tanzifi, A.; Aghayan, S.A.; Daryani, A. Drug Resistance in Toxoplasma gondii. Front. Microbiol. 2018, 9, 2587. [Google Scholar] [CrossRef]
- Macalino, S.J.Y.; Gosu, V.; Hong, S.; Choi, S. Role of computer-aided drug design in modern drug discovery. Arch. Pharm. Res. 2015, 38, 1686–1701. [Google Scholar] [CrossRef] [PubMed]
- Van Drie, J.H. Computer-aided drug design: The next 20 years. J. Comput. Aided Mol. Des. 2007, 21, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; MacKerell, A.D. Computer-Aided Drug Design Methods. In Antibiotics; Springer: Berlin/Heidelberg, Germany, 2017; pp. 85–106. [Google Scholar]
- Alamri, M.A.; Tariq, M.H.; Tahir ul Qamar, M.; Alabbas, A.B.; Alqahtani, S.M.; Ahmad, S. Discovery of Potential Phytochemicals as Inhibitors of TcdB, a Major Virulence Factors of Clostridioides Difficile. J. Biomol. Struct. Dyn. 2023, 1–9. [Google Scholar] [CrossRef]
- Altharawi, A.; Riadi, Y.; Qamar, M.T.U. An in Silico Quest for Next-Generation Antimalarial Drugs by Targeting Plasmodium Falciparum Hexose Transporter Protein: A Multi-Pronged Approach. J. Biomol. Struct. Dyn. 2023, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bechelane-Maia, E.H.; Assis, L.C.; Alves de Oliveira, T.; Marques da Silva, A.; Gutterres Taranto, A. Structure-based virtual screening: From classical to artificial intelligence. Front. Chem. 2020, 8, 343. [Google Scholar] [CrossRef]
- Meng, X.-Y.; Zhang, H.-X.; Mezei, M.; Cui, M. Molecular Docking: A Powerful Approach for Structure-Based Drug Discovery. Curr. Comput. Aided-Drug Des. 2011, 7, 146–157. [Google Scholar] [CrossRef]
- Huey, R.; Morris, G.M. Using AutoDock 4 with AutoDocktools: A Tutorial. Scripps Res. Inst. USA 2008, 54, 56. [Google Scholar]
- Hansson, T.; Oostenbrink, C.; van Gunsteren, W. Molecular Dynamics Simulations. Curr. Opin. Struct. Biol. 2002, 12, 190–196. [Google Scholar] [CrossRef]
- Qamar, M.T.U.; Ahmad, S.; Fatima, I.; Ahmad, F.; Shahid, F.; Naz, A.; Abbasi, S.W.; Khan, A.; Mirza, M.U.; Ashfaq, U.A.; et al. Designing multi-epitope vaccine against Staphylococcus aureus by employing subtractive proteomics, reverse vaccinology and immuno-informatics approaches. Comput. Biol. Med. 2021, 132, 104389. [Google Scholar] [CrossRef]
- Ali, S.; Ali, S.; Javed, S.O.; Shoukat, S.; Ahmad, S.; Ali, S.S.; Hussain, Z.; Waseem, M.; Rizwan, M.; Suleman, M. Proteome wide vaccine targets prioritization and designing of antigenic vaccine candidate to trigger the host immune response against the Mycoplasma genitalium infection. Microb. Pathog. 2021, 152, 104771. [Google Scholar] [CrossRef] [PubMed]
- Durrant, J.D.; McCammon, J.A. Molecular dynamics simulations and drug discovery. BMC Biol. 2011, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Raza, S.; Uddin, R.; Azam, S.S. Comparative subtractive proteomics based ranking for antibiotic targets against the dirtiest superbug: Acinetobacter baumannii. J. Mol. Graph. Model. 2018, 82, 74–92. [Google Scholar] [CrossRef]
- Sanober, G.; Ahmad, S.; Azam, S.S. Identification of plausible drug targets by investigating the druggable genome of MDR Staphylococcus epidermidis. Gene Rep. 2017, 7, 147–153. [Google Scholar] [CrossRef]
- Muneer, I.; Ahmad, S.; Naz, A.; Abbasi, S.W.; Alblihy, A.; Aloliqi, A.A.; Alkhayl, F.F.; Alrumaihi, F.; Ahmad, S.; El Bakri, Y. Discovery of Novel Inhibitors from Medicinal Plants for V-Domain Ig Suppressor of T-Cell Activation (VISTA). Front. Mol. Biosci. 2021, 8, 951. [Google Scholar] [CrossRef]
- Ahmad, S.; Waheed, Y.; Ismail, S.; Abbasi, S.W.; Najmi, M.H. A computational study to disclose potential drugs and vaccine ensemble for COVID-19 conundrum. J. Mol. Liq. 2020, 324, 114734. [Google Scholar] [CrossRef]
- Ehsan, N.; Ahmad, S.; Navid, A.; Azam, S.S. Identification of potential antibiotic targets in the proteome of multi-drug resistant Proteus mirabilis. Meta Gene 2018, 18, 167–173. [Google Scholar] [CrossRef]
- Genheden, S.; Ryde, U. The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin. Drug Discov. 2015, 10, 449–461. [Google Scholar] [CrossRef]
- Wang, E.; Sun, H.; Wang, J.; Wang, Z.; Liu, H.; Zhang, J.Z.H.; Hou, T. End-Point Binding Free Energy Calculation with MM/PBSA and MM/GBSA: Strategies and Applications in Drug Design. Chem. Rev. 2019, 119, 9478–9508. [Google Scholar] [CrossRef]
- El Bakri, Y.; Anouar, E.H.; Ahmad, S.; Nassar, A.A.; Taha, M.L.; Mague, J.T.; El Ghayati, L.; Essassi, E.M. Synthesis and Identification of Novel Potential Molecules Against COVID-19 Main Protease Through Structure-Guided Virtual Screening Approach. Appl. Biochem. Biotechnol. 2021, 193, 3602–3623. [Google Scholar] [CrossRef]
- Javed, N.; Ahmad, S.; Raza, S.; Azam, S.S. Subtractive Proteomics Supported with Rational Drug Design Approach Revealed ZINC23121280 as a Potent Lead Inhibitory Molecule for Multi-Drug Resistant Francisella Tularensis: Drug Designing for Multidrug-Resistant Francisella Tularensis. Proc. Pak. Acad. Sci. B Life Environ. Sci. 2021, 58, 1–42. [Google Scholar] [CrossRef]
- Alamri, M.A.; Ahmad, S.; Alqahtani, S.M.; Irfan, M.; Alabbas, A.B.; Qamar, M.T.U. Screening of marine natural products for potential inhibitors targeting biotin biosynthesis pathway in Mycobacterium tuberculosis. J. Biomol. Struct. Dyn. 2022, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Alamri, M.A.; Tahir ul Qamar, M.; Alabbas, A.B.; Alqahtani, S.M.; Alossaimi, M.A.; Azam, S.; Hashmi, M.H.; Rajoka, M.S.R. Molecular and Structural Analysis of Specific Mutations from Saudi Isolates of SARS-CoV-2 RNA-Dependent RNA Polymerase and Their Implications on Protein Structure and Drug–Protein Binding. Molecules 2022, 27, 6475. [Google Scholar] [CrossRef] [PubMed]
- Tahir ul Qamar, M.; Zhu, X.-T.; Chen, L.-L.; Alhussain, L.; Alshiekheid, M.A.; Theyab, A.; Algahtani, M. Target-Specific Machine Learning Scoring Function Improved Structure-Based Virtual Screening Performance for SARS-CoV-2 Drugs Development. Int. J. Mol. Sci. 2022, 23, 11003. [Google Scholar] [CrossRef]
- Alamri, M.A.; Mirza, M.U.; Adeel, M.M.; Ashfaq, U.A.; Qamar, M.T.U.; Shahid, F.; Ahmad, S.; Alatawi, E.A.; Albalawi, G.M.; Allemailem, K.S. Structural Elucidation of Rift Valley Fever Virus L Protein towards the Discovery of Its Potential Inhibitors. Pharmaceuticals 2022, 15, 659. [Google Scholar] [CrossRef]
- Ahmad, F.; Albutti, A.; Tariq, M.H.; Din, G.; Qamar, M.T.U.; Ahmad, S. Discovery of Potential Antiviral Compounds against Hendra Virus by Targeting Its Receptor-Binding Protein (G) Using Computational Approaches. Molecules 2022, 27, 554. [Google Scholar] [CrossRef]
- Maiorov, V.N.; Crippen, G.M. Significance of Root-Mean-Square Deviation in Comparing Three-dimensional Structures of Globular Proteins. J. Mol. Biol. 1994, 235, 625–634. [Google Scholar] [CrossRef]
- Ahmad, S.; Raza, S.; Uddin, R.; Azam, S.S. Binding mode analysis, dynamic simulation and binding free energy calculations of the MurF ligase from Acinetobacter baumannii. J. Mol. Graph. Model. 2017, 77, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Lobanov, M.Y.; Bogatyreva, N.S.; Galzitskaya, O.V. Radius of Gyration as an Indicator of Protein Structure Compactness. Mol. Biol. 2008, 42, 623–628. [Google Scholar] [CrossRef]
- Wahedi, H.M.; Ahmad, S.; Abbasi, S.W. Stilbene-based natural compounds as promising drug candidates against COVID-19. J. Biomol. Struct. Dyn. 2021, 39, 3225–3234. [Google Scholar] [CrossRef]
- Humayun, F.; Khan, A.; Ahmad, S.; Yuchen, W.; Wei, G.; Nizam-Uddin, N.; Hussain, Z.; Khan, W.; Zaman, N.; Rizwan, M.; et al. Abrogation of SARS-CoV-2 Interaction with Host (NRP1) Neuropilin-1 Receptor through High-Affinity Marine Natural Compounds to Curtail the Infectivity: A Structural-Dynamics Data. Comput. Biol. Med. 2021, 141, 104714. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Irfan, M.; Hameed, A.R.; Ullah, A.; Abideen, S.A.; Ahmad, S.; Haq, M.U.; El Bakri, Y.; Al-Harbi, A.I.; Ali, M. Vaccinomics to design a multi-epitope-based vaccine against monkeypox virus using surface-associated proteins. J. Biomol. Struct. Dyn. 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Ranaghan, K.E.; Azam, S.S. Combating tigecycline resistant Acinetobacter baumannii: A leap forward towards multi-epitope based vaccine discovery. Eur. J. Pharm. Sci. 2019, 132, 1–17. [Google Scholar] [CrossRef]
- Ain, Q.U.; Ahmad, S.; Azam, S.S. Subtractive proteomics and immunoinformatics revealed novel B-cell derived T-cell epitopes against Yersinia enterocolitica: An etiological agent of Yersiniosis. Microb. Pathog. 2018, 125, 336–348. [Google Scholar] [CrossRef]
- Van De Waterbeemd, H.; Gifford, E. ADMET in Silico Modelling: Towards Prediction Paradise? Nat. Rev. Drug Discov. 2003, 2, 192–204. [Google Scholar] [CrossRef]
- Assenov, Y.; Ramírez, F.; Schelhorn, S.-E.; Lengauer, T.; Albrecht, M. Computing topological parameters of biological networks. Bioinformatics 2007, 24, 282–284. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Egan, W.J.; Merz, K.M.; Baldwin, J.J. Prediction of Drug Absorption Using Multivariate Statistics. J. Med. Chem. 2000, 43, 3867–3877. [Google Scholar] [CrossRef]
- Whitty, A. Growing PAINS in academic drug discovery. Futur. Med. Chem. 2011, 3, 797–801. [Google Scholar] [CrossRef]
- Sussman, J.L.; Lin, D.; Jiang, J.; Manning, N.O.; Prilusky, J.; Ritter, O.; Abola, E.E. Protein Data Bank (PDB): Database of Three-Dimensional Structural Information of Biological Macromolecules. Acta Crystallogr. Sect. D Biol. Crystallogr. 1998, 54, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Cheng, X.; Islam, M.S.; Huang, L.; Rui, H.; Zhu, A.; Lee, H.S.; Qi, Y.; Han, W.; Vanommeslaeghe, K.; et al. CHARMM-GUI PDB Manipulator for Advanced Modeling and Simulations of Proteins Containing Nonstandard Residues. Adv. Protein Chem. Struct. Biol. 2014, 96, 235–265. [Google Scholar] [CrossRef] [PubMed]
- Anandakrishnan, R.; Aguilar, B.; Onufriev, A.V. H++ 3.0: Automating pK prediction and the preparation of biomolecular structures for atomistic molecular modeling and simulations. Nucleic Acids Res. 2012, 40, W537–W541. [Google Scholar] [CrossRef] [PubMed]
- Kaliappan, S.; Bombay, I.I.T. UCSF Chimera-Overview. 2018. Available online: http://doer.col.org/handle/123456789/9120 (accessed on 15 November 2022).
- Dallakyan, S.; Olson, A.J. Small-Molecule Library Screening by Docking with PyRx. In Chemical Biology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 243–250. [Google Scholar]
- Halgren, T. A Merck Molecular Force Field. J. Comput. Chem. 1996, 17, 490–519. [Google Scholar] [CrossRef]
- Horoiwa, S.; Yokoi, T.; Masumoto, S.; Minami, S.; Ishizuka, C.; Kishikawa, H.; Ozaki, S.; Kitsuda, S.; Nakagawa, Y.; Miyagawa, H. Structure-based virtual screening for insect ecdysone receptor ligands using MM/PBSA. Bioorg. Med. Chem. 2019, 27, 1065–1075. [Google Scholar] [CrossRef]
- Lionta, E.; Spyrou, G.; Vassilatis, D.K.; Cournia, Z. Structure-Based Virtual Screening for Drug Discovery: Principles, Applications and Recent Advances. Curr. Top. Med. Chem. 2014, 14, 1923–1938. [Google Scholar] [CrossRef]
- Shaker, B.; Ahmad, S.; Lee, J.; Jung, C.; Na, D. In silico methods and tools for drug discovery. Comput. Biol. Med. 2021, 137, 104851. [Google Scholar] [CrossRef]
- Case, D.A.; Belfon, K.; Ben-Shalom, I.; Brozell, S.R.; Cerutti, D.; Cheatham, T.; Cruzeiro, V.W.D.; Darden, T.; Duke, R.E.; Giambasu, G.; et al. Amber 2020, University of California, San Fransisco. J. Amer. Chem. Soc. 2020, 142, 3823–3835. [Google Scholar]
- Case, D.A.; Babin, V.; Berryman, J.T.; Betz, R.M.; Cai, Q.; Cerutti, D.S.; Cheatham, T.E., III; Darden, T.A.; Duke, R.E.; Gohlke, H.; et al. The FF14SB Force Field. Amber 2014, 14, 29–31. [Google Scholar]
- Dickson, C.J.; Rosso, L.; Betz, R.M.; Walker, R.C.; Gould, I.R. GAFFlipid: A General Amber Force Field for the accurate molecular dynamics simulation of phospholipid. Soft Matter 2012, 8, 9617–9627. [Google Scholar] [CrossRef]
- He, X.; Liu, S.; Lee, T.-S.; Ji, B.; Man, V.H.; York, D.M.; Wang, J. Fast, Accurate, and Reliable Protocols for Routine Calculations of Protein–Ligand Binding Affinities in Drug Design Projects Using AMBER GPU-TI with ff14SB/GAFF. ACS Omega 2020, 5, 4611–4619. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, W.; Kollman, P.A.; Case, D.A. Antechamber: An Accessory Software Package for Molecular Mechanical Calculations. J. Am. Chem. Soc. 2001, 222, U403. [Google Scholar]
- Kräutler, V.; Van Gunsteren, W.F.; Hünenberger, P.H. A Fast SHAKE Algorithm to Solve Distance Constraint Equations for Small Molecules in Molecular Dynamics Simulations. J. Comput. Chem. 2001, 22, 501–508. [Google Scholar] [CrossRef]
- Izaguirre, J.A.; Catarello, D.P.; Wozniak, J.M.; Skeel, R.D. Langevin stabilization of molecular dynamics. J. Chem. Phys. 2001, 114, 2090–2098. [Google Scholar] [CrossRef]
- Roe, D.R.; Cheatham III, T.E. PTRAJ and CPPTRAJ: Software for Processing and Analysis of Molecular Dynamics Trajectory Data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef]
- Turner, P.J. XMGRACE, Version 5.1. 19; Center Coastal Land-Margin Research, Oregon Graduate Institute of Science and Technology: Beaverton, OR, USA, 2005. [Google Scholar]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Genheden, S.; Kuhn, O.; Mikulskis, P.; Hoffmann, D.; Ryde, U. The Normal-Mode Entropy in the MM/GBSA Method: Effect of System Truncation, Buffer Region, and Dielectric Constant. J. Chem. Inf. Model. 2012, 52, 2079–2088. [Google Scholar] [CrossRef]
- Miller, B.R.; McGee, T.D.; Swails, J.M.; Homeyer, N.; Gohlke, H.; Roitberg, A.E. MMPBSA.py: An Efficient Program for End-State Free Energy Calculations. J. Chem. Theory Comput. 2012, 8, 3314–3321. [Google Scholar] [CrossRef]
- Sahakyan, H. Improving virtual screening results with MM/GBSA and MM/PBSA rescoring. J. Comput. Mol. Des. 2021, 35, 731–736. [Google Scholar] [CrossRef]
- Duan, L.; Liu, X.; Zhang, J.Z. Interaction Entropy: A New Paradigm for Highly Efficient and Reliable Computation of Protein–Ligand Binding Free Energy. J. Am. Chem. Soc. 2016, 138, 5722–5728. [Google Scholar] [CrossRef] [PubMed]
- Woods, C.J.; Malaisree, M.; Michel, J.; Long, B.; McIntosh-Smith, S.; Mulholland, A.J. Rapid decomposition and visualisation of protein–ligand binding free energies by residue and by water. Faraday Discuss. 2014, 169, 477–499. [Google Scholar] [CrossRef] [PubMed]
- Woods, C.J.; Malaisree, M.; Hannongbua, S.; Mulholland, A.J. A water-swap reaction coordinate for the calculation of absolute protein–ligand binding free energies. J. Chem. Phys. 2011, 134, 054114. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
| Ligand | Structure | IUPAC Name | Binding Affinity |
|---|---|---|---|
| LAS_52160953 | 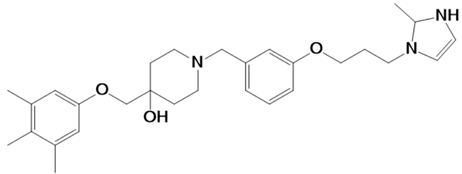 | 1-(3-(3-(2-methyl-2,3-dihydro-1H-imidazol-1-yl)propoxy)benzyl)-4-((3,4,5-trimethylphenoxy)methyl)piperidin-4-ol | −8.6 |
| LAS_51177972 | 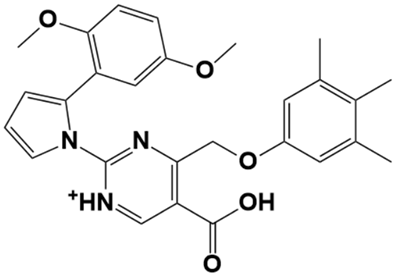 | 5-carboxy-2-(2-(2,5-dimethoxyphenyl)-1H-pyrrol-1-yl)-4-((3,4,5-trimethylphenoxy)methyl)pyrimidin-1-ium | −8.5 |
| LAS_52506311 | 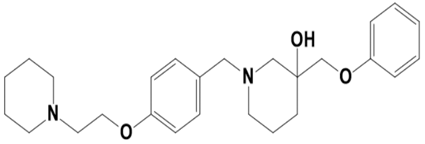 | 3-(phenoxymethyl)-1-(4-(2-(piperidin-1-yl)ethoxy)benzyl)piperidin-3-ol | −8.3 |
| LAS_52160943 | 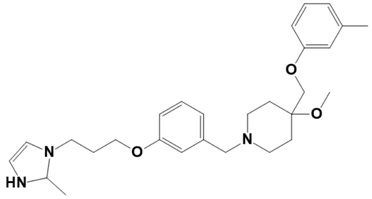 | 4-methoxy-1-(3-(3-(2-methyl-2,3-dihydro-1H-imidazol-1-yl)propoxy)benzyl)-4-((m-tolyloxy)methyl)piperidine | −7.8 |
| LAS_52157607 | 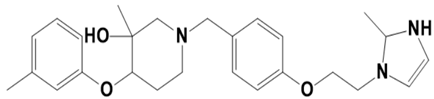 | 3-methyl-1-(4-(2-(2-methyl-2,3-dihydro-1H-imidazol-1-yl)ethoxy)benzyl)-4-(m-tolyloxy)piperidin-3-ol | −7.7 |
| LAS_52160863 | 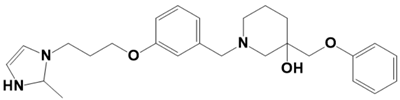 | 1-(3-(3-(2-methyl-2,3-dihydro-1H-imidazol-1-yl)propoxy)benzyl)-3-(phenoxymethyl)piperidin-3-ol | −7.7 |
| LAS_52171211 | 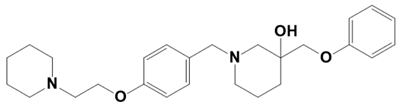 | 3-(phenoxymethyl)-1-(4-(2-(piperidin-1-yl)ethoxy)benzyl)piperidin-3-ol | −7.6 |
| LAS_52506188 | 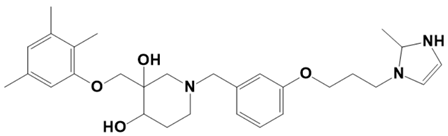 | 1-(3-(3-(2-methyl-2,3-dihydro-1H-imidazol-1-yl)propoxy)benzyl)-3-((2,3,5-trimethylphenoxy)methyl)piperidine-3,4-diol | −7.4 |
| LAS_52157615 | 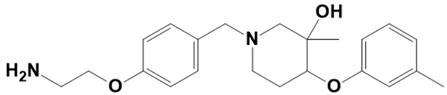 | 1-(4-(2-aminoethoxy)benzyl)-3-methyl-4-(m-tolyloxy)piperidin-3-ol | −7.1 |
| LAS_32135590 | 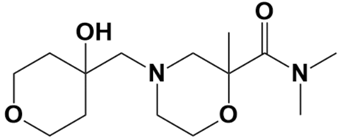 | 4-((4-hydroxytetrahydro-2H-pyran-4-yl)methyl)-N,N,2-trimethylmorpholine-2-carboxamide | −6.1 |
| Energy Parameter | LAS_52160953 Complex | LAS_51177972 Complex | LAS_52506311 Complex |
|---|---|---|---|
| MM-GBSA | |||
| van der Waals Energy | −36.89 | −33.60 | −36.02 |
| Electrostatic Energy | −14.23 | −12.78 | −14.85 |
| Delta Gas Phase Energy | −51.12 | −46.38 | −50.87 |
| Delta Solvation Energy | 11.67 | 10.19 | 11.47 |
| Net Energy | −39.45 | −36.19 | −39.4 |
| MM-PBSA | |||
| van der Waals Energy | −36.89 | −33.60 | −36.02 |
| Electrostatic Energy | −14.23 | −12.78 | −14.85 |
| Delta Gas Phase Energy | −51.12 | −46.38 | −50.87 |
| Delta Solvation Energy | 12.55 | 11.15 | 13.75 |
| Net Energy | −38.57 | −35.23 | −37.12 |
| Physicochemical Properties | LAS_52160953 | LAS_51177972 | LAS_52506311 |
|---|---|---|---|
| Formula | C29H41N3O3 | C27H29N3O5+ | C26H36N2O3 |
| Molecular weight | 479.65 g/mol | 475.54 g/mol | 424.58 g/mol |
| Num. heavy atoms | 35 | 35 | 31 |
| Num. arom. heavy atoms | 12 | 23 | 12 |
| Fraction Csp3 | 0.52 | 0.22 | 0.54 |
| Num. rotatable bonds | 10 | 8 | 9 |
| Num. H-bond acceptors | 4 | 5 | 5 |
| Num. H-bond donors | 2 | 3 | 1 |
| Molar Refractivity | 153.33 | 134.40 | 132.19 |
| TPSA | 57.20 Å2 | 98.20 Å2 | 45.17 Å2 |
| Lipophilicity | |||
| Log Po/w (iLOGP) | 4.71 | 3.60 | 4.50 |
| Log Po/w (XLOGP3) | 5.22 | 4.66 | 3.97 |
| Log Po/w (WLOGP) | 3.21 | 3.84 | 3.04 |
| Log Po/w (MLOGP) | 2.83 | 2.47 | 2.72 |
| Log Po/w (SILICOS-IT) | 4.79 | 5.03 | 4.29 |
| Consensus Log Po/w | 4.15 | 3.92 | 3.70 |
| Water Solubility | |||
| Log S (ESOL) | −5.70 | −5.68 | −4.67 |
| Solubility | 9.66 × 10−4 mg/mL; 2.01 × 10−6 mol/L | 9.88 × 10−4 mg/mL; 2.08 × 10−6 mol/L | 9.16 × 10−3 mg/mL; 2.16 × 10−5 mol/L |
| Class | Moderately soluble | Moderately soluble | Moderately soluble |
| Log S (Ali) | −6.17 | −6.45 | −4.62 |
| Solubility | 3.25 × 10−4 mg/mL; 6.77 × 10−7 mol/L | 1.69 × 10−4 mg/mL; 3.56 × 10−7 mol/L | 1.02 × 10−2 mg/mL; 2.40 × 10−5 mol/L |
| Class | Poorly soluble | Poorly soluble | Moderately soluble |
| Log S (SILICOS-IT) | −7.47 | −8.38 | −6.78 |
| Solubility | 1.63 × 10−5 mg/mL; 3.39 × 10−8 mol/L | 2.00 × 10−6 mg/mL; 4.21 × 10−9 mol/L | 7.11 × 10−5 mg/mL; 1.67 × 10−7 mol/L |
| Class | Poorly soluble | Poorly soluble | Poorly soluble |
| Pharmacokinetics | |||
| GI absorption | High | High | High |
| BBB permeant | Yes | No | Yes |
| P-gp substrate | Yes | No | Yes |
| CYP1A2 inhibitor | No | No | No |
| CYP2C19 inhibitor | No | Yes | No |
| CYP2C9 inhibitor | No | Yes | No |
| CYP2D6 inhibitor | Yes | Yes | Yes |
| CYP3A4 inhibitor | Yes | Yes | Yes |
| Log Kp (skin permeation) | −5.52 cm/s | −5.89 cm/s | −6.07 cm/s |
| Druglikeness | |||
| Lipinski | Yes; 0 violation | Yes; 0 violation | Yes; 0 violation |
| Ghose | No; 2 violations: MR > 130, #atoms > 70 | No; 1 violation: MR > 130 | No; 1 violation: MR > 130 |
| Veber | Yes | Yes | Yes |
| Egan | Yes | Yes | Yes |
| Muegge | No; 1 violation: XLOGP3 > 5 | Yes | Yes |
| Bioavailability Score | 0.55 | 0.55 | 0.55 |
| Medicinal Chemistry | |||
| PAINS | 0 alert | 0 alert | 0 alert |
| Brenk | 0 alert | No; 3 violations: MW > 350, Rotors > 7, XLOGP3 > 3.5 | No; 3 violations: MW > 350, Rotors > 7, XLOGP3 > 3.5 |
| Lead-likeness | No; 3 violations: MW > 350, Rotors > 7, XLOGP3 > 3.5 | No; 1 violations: MW > 350 | No; 1 violations: MW > 350 |
| Synthetic accessibility | 4.87 | 4.76 | 4.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altharawi, A. Targeting Toxoplasma gondii ME49 TgAPN2: A Bioinformatics Approach for Antiparasitic Drug Discovery. Molecules 2023, 28, 3186. https://doi.org/10.3390/molecules28073186
Altharawi A. Targeting Toxoplasma gondii ME49 TgAPN2: A Bioinformatics Approach for Antiparasitic Drug Discovery. Molecules. 2023; 28(7):3186. https://doi.org/10.3390/molecules28073186
Chicago/Turabian StyleAltharawi, Ali. 2023. "Targeting Toxoplasma gondii ME49 TgAPN2: A Bioinformatics Approach for Antiparasitic Drug Discovery" Molecules 28, no. 7: 3186. https://doi.org/10.3390/molecules28073186
APA StyleAltharawi, A. (2023). Targeting Toxoplasma gondii ME49 TgAPN2: A Bioinformatics Approach for Antiparasitic Drug Discovery. Molecules, 28(7), 3186. https://doi.org/10.3390/molecules28073186






