Optimization of the Brewing Conditions of Shanlan Rice Wine and Sterilization by Thermal and Intense Pulse Light
Abstract
1. Introduction
2. Results
2.1. Optimization of Fermentation Conditions of SRW
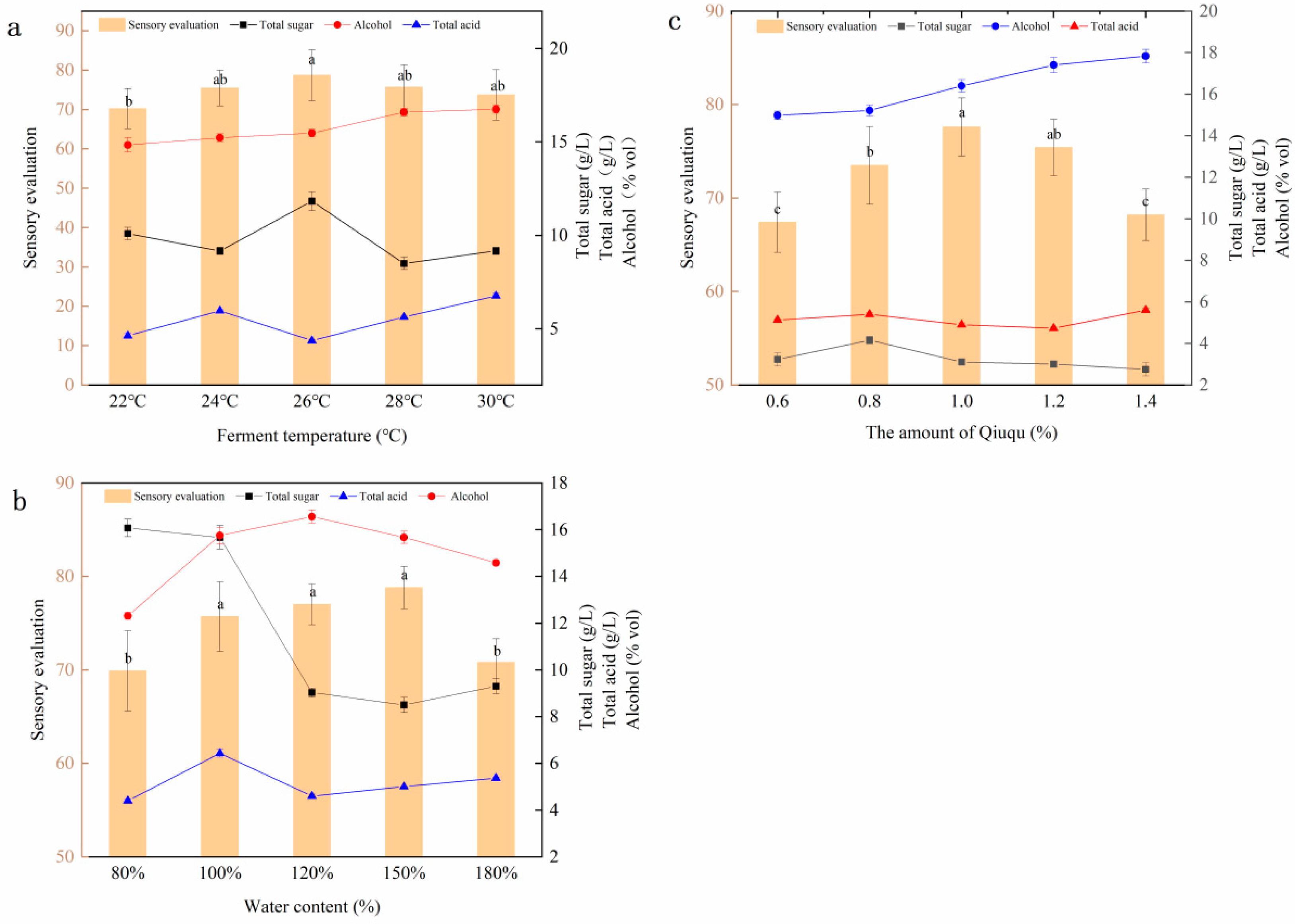
2.1.1. Single-Factor Experiment Results
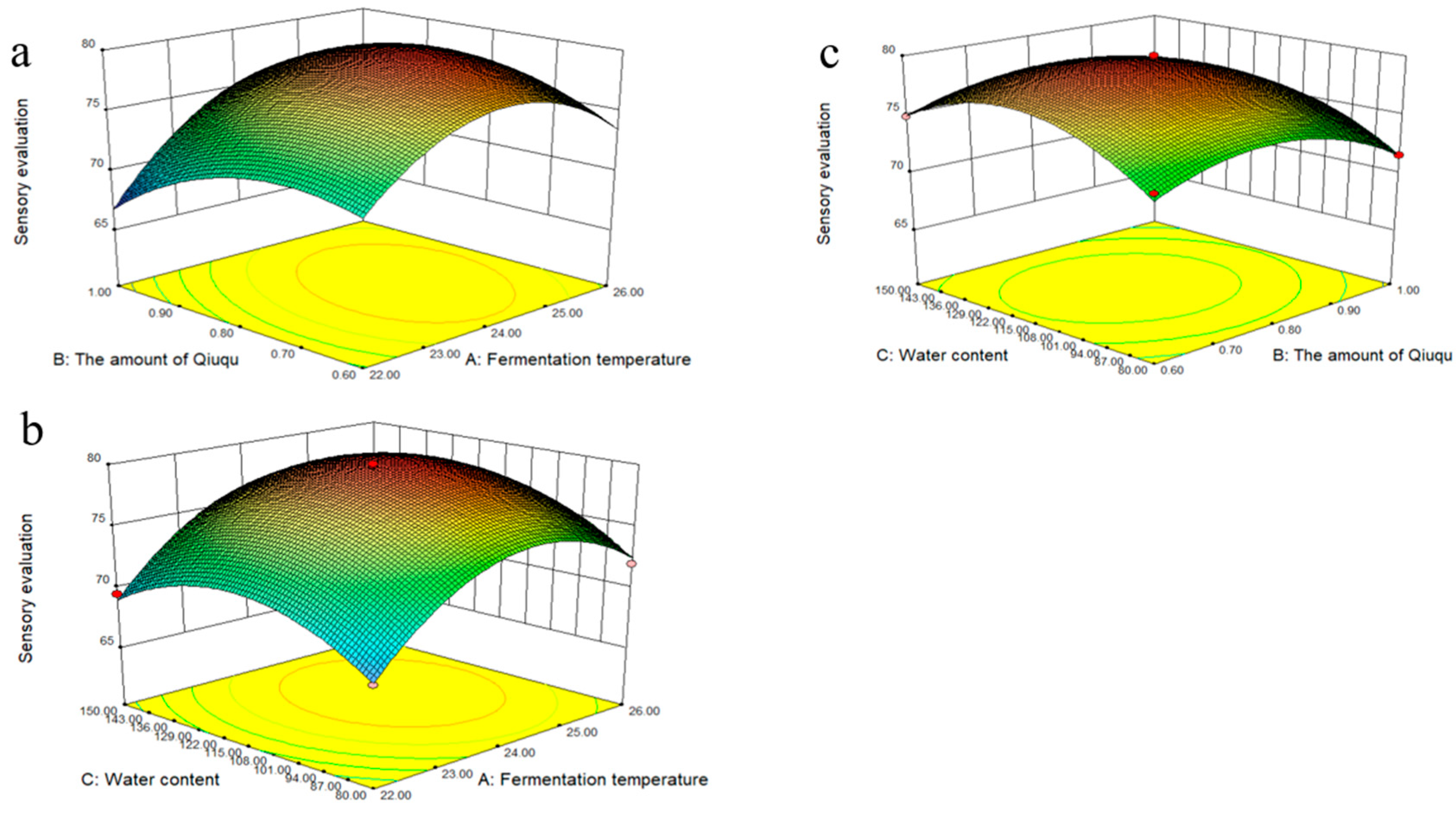
2.1.2. Response Surface Model Analysis of Sensory Evaluation
2.1.3. Response Surface Optimization of the Interaction between Various Factors
2.2. Effect of Sterilization Treatment on the Physicochemical Properties of SRW
2.3. Effects of Different Sterilization Treatments on the Free Amino Acids in SRW
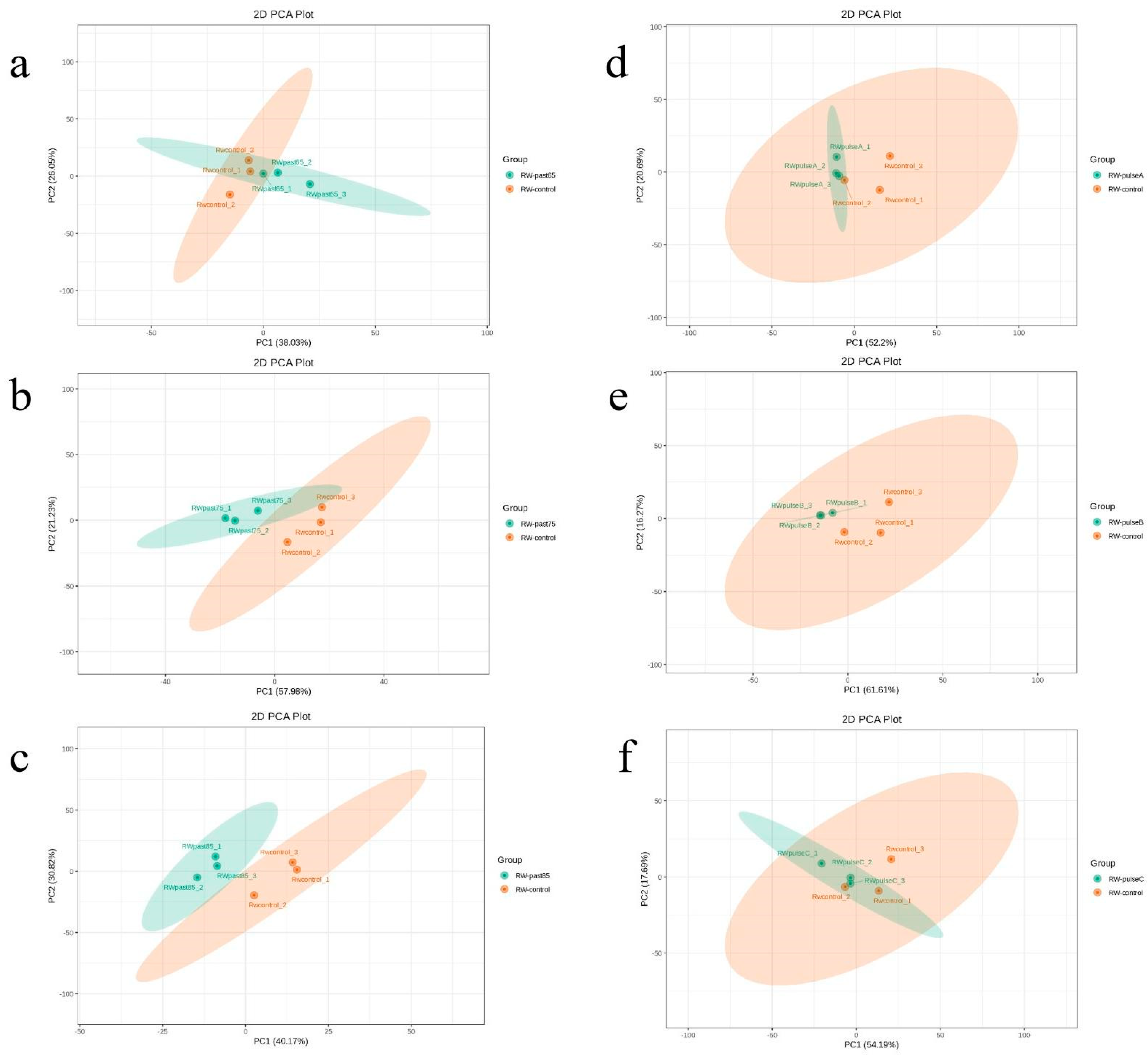
2.4. Difference of Flavor Metabolites before and after Sterilization by PCA
2.4.1. PCA
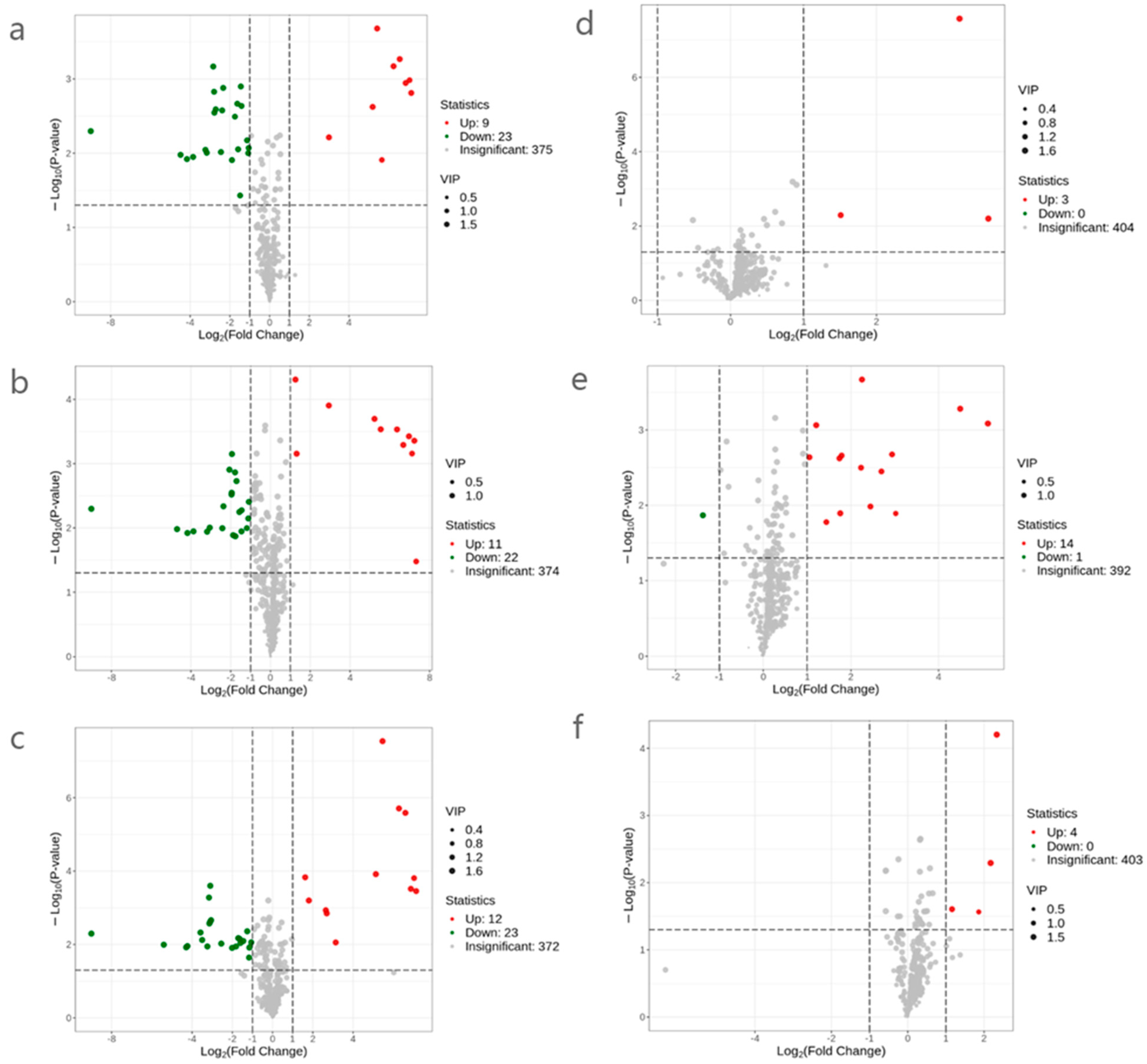
2.4.2. Screening and Analysis of Metabolites of Flavor Difference in SRW before and after Sterilization Treatment
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Qiuqu Preparation and SRW Brewing
3.3. Single-Factor and Response Surface Experiments
3.4. Sensory Evaluation Method of SRW
3.5. Sterilization of SRW
3.6. Determination of SRW’s Physicochemical Properties
3.7. Determination of Free Amino Acids in SRW
3.7.1. Sample Preparation and Extraction
3.7.2. UPLC Conditions
3.7.3. LC-MS/MS Analysis
3.8. Determination of Flavor Metabolites
3.8.1. Sample Preparation and Treatment
3.8.2. GC-MS Analysis
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yang, Y.; Xia, Y.; Wang, G.; Tao, L.; Yu, J.; Ai, L. Effects of boiling, ultra-high temperature and high hydrostatic pressure on free amino acids, flavor characteristics and sensory profiles in Chinese rice wine. Food Chem. 2019, 275, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Mosher, W.; Wiertzema, J.; Peng, P.; Min, M.; Cheng, Y.; An, J.; Ma, Y.; Fan, X.; Niemira, B.A.; et al. Effects of intense pulsed light and gamma irradiation on Bacillus cereus spores in mesquite pod flour. Food Chem. 2020, 344, 128675. [Google Scholar] [CrossRef] [PubMed]
- Ojha, K.S.; Tiwari, B.K.; O’Donnell, C.P. Chapter Six-Effect of Ultrasound Technology on Food and Nutritional Quality. Adv. Food Nutr. Res. 2018, 84, 207–240. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, R.A.H.; Mastwijk, H.C.; Berendsen, L.B.J.M.; Nederhoff, A.L.; Matser, A.M.; Van Boekel, M.A.J.S.; Groot, M.N.N. Moderate intensity Pulsed Electric Fields (PEF) as alternative mild preservation technology for fruit juice. Int. J. Food Microbiol. 2019, 298, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Song, B.-S.; Kim, M.J.; Moon, B.-G.; Go, S.-M.; Kim, J.-K.; Lee, Y.-J.; Park, J.-H. Effect of X-ray, gamma ray, and electron beam irradiation on the hygienic and physicochemical qualities of red pepper powder. LWT 2015, 63, 846–851. [Google Scholar] [CrossRef]
- Yin, H.; Deng, Y.; He, Y.; Dong, J.; Lu, J.; Chang, Z. A preliminary study of the quality attributes of a cloudy wheat beer treated by flash pasteurization. J. Inst. Brew. 2017, 123, 366–372. [Google Scholar] [CrossRef]
- Mahendran, R.; Ramanan, K.R.; Barba, F.J.; Lorenzo, J.M.; López-Fernández, O.; Munekata, P.E.S.; Tiwari, B.K. Recent advances in the application of pulsed light processing for improving food safety and increasing shelf life. Trends Food Sci. Technol. 2019, 88, 67–79. [Google Scholar] [CrossRef]
- John, D.; Ramaswamy, H.S. Pulsed light technology to enhance food safety and quality: A mini-review. Curr. Opin. Food Sci. 2018, 23, 70–79. [Google Scholar] [CrossRef]
- Basak, S.; Mahale, S.; Chakraborty, S. Changes in quality attributes of pulsed light and thermally treated mixed fruit beverages during refrigerated storage (4 °C) condition. Innov. Food Sci. Emerg. Technol. 2022, 78, 103025. [Google Scholar] [CrossRef]
- Shen, D.; Labreche, F.; Wu, C.; Fan, G.; Li, T.; Shi, H.; Ye, C. Preparation and aroma analysis of flavonoid-rich ginkgo seeds fermented using rice wine starter. Food Biosci. 2021, 44, 101459. [Google Scholar] [CrossRef]
- Tian, T.; Yang, H.; Yang, F.; Li, B.; Sun, J.; Wu, D.; Lu, J. Optimization of fermentation conditions and comparison of flavor compounds for three fermented greengage wines. LWT 2018, 89, 542–550. [Google Scholar] [CrossRef]
- Singh, A.; Kocher, G.S. Standardization of seed and peel infused Syzygium cumini -wine fermentation using response surface methodology. LWT 2020, 134, 109994. [Google Scholar] [CrossRef]
- Jin, T.-Y.; Saravanakumar, K.; Wang, M.-H. Effect of different sterilization methods on physicochemical and microbiological properties of rice wine. Beni-Suef University. J. Basic Appl. Sci. 2018, 7, 487–491. [Google Scholar] [CrossRef]
- Ha, S.-J.; Kim, Y.-J.; Oh, S.-W. Effect of high hydrostatic pressure (HHP) treatment on chemical and microbiological properties of Makgeolli. J. Korean Soc. Appl. Biol. Chem. 2013, 56, 325–329. [Google Scholar] [CrossRef]
- Wang, P.; Mao, J.; Meng, X.; Li, X.; Liu, Y.; Feng, H. Changes in flavour characteristics and bacterial diversity during the traditional fermentation of Chinese rice wines from Shaoxing region. Food Control 2014, 44, 58–63. [Google Scholar] [CrossRef]
- Xie, G.-F.; Yang, D.-D.; Liu, X.-Q.; Cheng, X.-X.; Rui, H.-F.; Zhou, H.-J. Correlation between the amino acid content in rice wine and protein content in glutinous rice. J. Inst. Brew. 2016, 122, 162–167. [Google Scholar] [CrossRef]
- Zhang, C.L.; Hao, S.; Xiao, Z.L.; Zhi, G.H.; Wei, X.L.; Dong, L.D. Microbial communities and amino acids during the fermentation of Wuyi Hong Qu Huangjiu. LWT 2020, 130, 109743. [Google Scholar] [CrossRef]
- Gao, H.; Wang, W.; Xu, D.; Wang, P.; Zhao, Y.; Mazza, G.; Zhang, X. Taste-active indicators and their correlation with antioxidant ability during the Monascus rice vinegar solid-state fermentation process. J. Food Compos. Anal. 2021, 104, 104133. [Google Scholar] [CrossRef]
- Bhagat, B.; Chakraborty, S. Potential of pulsed light treatment to pasteurize pomegranate juice: Microbial safety, enzyme inactivation, and phytochemical retention. LWT 2022, 159, 113215. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, C.; Gao, X.; Kang, Y.; Huang, M.; Wu, J.; Liu, Y.; Zhang, J.; Li, H.; Zhang, Y. Characterization of key aroma compounds in Huangjiu from northern China by sensory-directed flavor analysis. Food Res. Int. 2020, 134, 109238. [Google Scholar] [CrossRef]
- Jin, Z.; Cai, G.; Wu, C.; Hu, Z.; Xu, X.; Xie, G.; Wu, D.; Lu, J. Profiling the key metabolites produced during the modern brewing process of Chinese rice wine. Food Res. Int. 2021, 139, 109955. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Xia, Y.J.; Hu, W.Y.; Tao, L.; Liu, H.D.; Xie, C.L.; Bai, W.D.; Ai, L.Z. Soaking induced discrepancies in oenological properties, flavor profiles, microbial community and sensory characteristic of Huangjiu (Chinese rice wine). LWT 2020, 139, 110575, Prepublish. [Google Scholar] [CrossRef]
- Yang, Y.; Ai, L.; Mu, Z.; Liu, H.; Yan, X.; Ni, L.; Zhang, H.; Xia, Y. Flavor compounds with high odor activity values (OAV > 1) dominate the aroma of aged Chinese rice wine (Huangjiu) by molecular association. Food Chem. 2022, 383, 132370. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Sun, F.; Wang, W.; Liu, Y.; Wang, J.; Sun, J.; Mu, J.; Gao, Z. Effects of spontaneous fermentation on the microorganisms diversity and volatile compounds during ‘Marselan’ from grape to wine. LWT 2020, 134, 110193. [Google Scholar] [CrossRef]
- Chen, S.; Xu, Y. The Influence of Yeast Strains on the Volatile Flavour Compounds of Chinese Rice Wine. J. Inst. Brew. 2010, 116, 190–196. [Google Scholar] [CrossRef]
- Zhao, C.; Su, W.; Mu, Y.C.; Jiang, L.; Mu, Y. Correlations between microbiota with physicochemical properties and volatile flavor components in black glutinous rice wine fermentation. Food Res. Int. 2020, 138, 109800. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, B.; Xu, Y. Regulating yeast flavor metabolism by controlling saccharification reaction rate in simultaneous saccharification and fermentation of Chinese Maotai-flavor liquor. Int. J. Food Microbiol. 2015, 200, 39–46. [Google Scholar] [CrossRef]
- Xu, S.; Ma, Z.; Chen, Y.; Li, J.; Jiang, H.; Qu, T.; Zhang, W.; Li, C.; Liu, S. Characterization of the flavor and nutritional value of coconut water vinegar based on metabolomics. Food Chem. 2022, 369, 130872. [Google Scholar] [CrossRef]
- Li, L.; Ma, X.W.; Zhan, R.L. Profiling of volatile fragrant components in a mini-core collection of mango germplasms from seven countries. PLoS ONE 2017, 12, e0187487. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Li, S. Volatile characteristics of 50 peaches and nectarines evaluated by HP–SPME with GC–MS. Food Chem. 2009, 116, 356–364. [Google Scholar] [CrossRef]


| Run | Factor 1 (X1) | Factor 2 (X2) | Factor 3 (X3) | Response | |||
|---|---|---|---|---|---|---|---|
| Fermentation Temperature (°C) | The Amount of Qiuqu (%) | Water Content (%) | Sensory Evaluation (Scores) | Total Acid Content (g/L) | Alcohol Content (% (v/v)) | Total Sugar Content (g/L) | |
| 1 | 24 | 1.00 | 150.00 | 72 | 4.48 | 12.83 | 17.76 |
| 2 | 24 | 0.80 | 115.00 | 79 | 5.23 | 14.03 | 15.08 |
| 3 | 26 | 0.80 | 80.00 | 72 | 6.04 | 15.61 | 16.21 |
| 4 | 26 | 1.00 | 115.00 | 75.3 | 5.54 | 9.90 | 14.80 |
| 5 | 22 | 1.00 | 115.00 | 66.9 | 4.58 | 13.72 | 15.36 |
| 6 | 22 | 0.80 | 80.00 | 68 | 4.92 | 17.00 | 16.07 |
| 7 | 24 | 0.60 | 80.00 | 73.8 | 5.58 | 17.19 | 14.66 |
| 8 | 24 | 0.80 | 115.00 | 79.9 | 5.23 | 14.40 | 15.36 |
| 9 | 24 | 0.80 | 115.00 | 79.4 | 4.97 | 14.86 | 14.10 |
| 10 | 26 | 0.60 | 115.00 | 73.6 | 4.63 | 14.95 | 17.06 |
| 11 | 24 | 1.00 | 80.00 | 71.6 | 5.32 | 16.11 | 14.52 |
| 12 | 22 | 0.80 | 150.00 | 69.5 | 4.26 | 13.73 | 15.51 |
| 13 | 22 | 0.60 | 115.00 | 71.3 | 4.23 | 8.77 | 13.67 |
| 14 | 24 | 0.60 | 150.00 | 74.9 | 4.60 | 14.61 | 13.96 |
| 15 | 24 | 0.80 | 115.00 | 79 | 4.83 | 14.12 | 14.80 |
| 16 | 24 | 0.80 | 115.00 | 80 | 5.02 | 14.53 | 14.66 |
| 17 | 26 | 0.80 | 150.00 | 74.7 | 4.11 | 13.21 | 14.94 |
| Model | p < 0.0001 | ||||||
| R2 (%) | 98.99 | ||||||
| R2adj (%) | 97.70 | ||||||
| R2pre (%) | 88.58 | ||||||
| Lack of fit | not significant |
| Parameters | Rw-Control | Rw-past65 | Rw-past75 | Rw-past85 | Rw-pulseA | Rw-pulseB | Rw-pulseC |
|---|---|---|---|---|---|---|---|
| Ethanol (20 °C)/(%, v/v) | 17.53 ± 0.025 a | 16.83 ± 0.034 b | 16.91 ± 0.054 b | 15.33 ± 0.025 e | 16.38 ± 0.054 c | 16.31 ± 0.033 cd | 16.21 ± 0.034 d |
| Total sugar(g/L) | 18.21 ± 0.500 c | 20.79 ± 0.995 b | 20.44 ± 0.495 b | 22.08 ± 0.318 a | 17.97 ± 0.518 c | 17.82 ± 0.317 c | 17.70 ± 0.110 c |
| Total acid(g/L) | 5.29 ± 0.042 a | 5.13 ± 0.078 bc | 5.23 ± 0.000 ab | 5.10 ± 0.042 cd | 4.94 ± 0.062 e | 4.88 ± 0.047 e | 5.00 ± 0.039 de |
| pH | 4.35 ± 0.005 c | 4.44 ± 0.009 b | 4.36 ± 0.009 c | 4.46 ± 0.009 b | 4.54 ± 0.009 a | 4.52 ± 0.005 a | 4.53 ± 0.009 a |
| Amino Acid (μg/mL) | Rw-Conrtol | Rw-past65 | Rw-Past75 | Rw-past85 | Rw-pulseA | Rw-pulseB | Rw-pulseC |
|---|---|---|---|---|---|---|---|
| His | 16.39 ± 0.51 b | 17.45 ± 0.22 a | 16.21 ± 0.2 b | 16.46 ± 0.3 b | 16.32 ± 0.21 b | 15.37 ± 0.19 c | 14.52 ± 0.39 c |
| Hyp | 2.57 ± 0.1 a | 2.65 ± 0.21 a | 2.48 ± 0.14 a | 2.51 ± 0.14 a | 2.63 ± 0.19 a | 2.35 ± 0.11 a | 2.48 ± 0.19 a |
| Arg | 76.24 ± 0.53 c | 79.39 ± 0.16 a | 64.5 ± 0.27 f | 72.38 ± 0.27 d | 78.44 ± 0.07 b | 68.51 ± 0.07 e | 64.39 ± 0.4 f |
| Asn | 131.56 ± 0.21 a | 129.36 ± 0.22 b | 108.61 ± 0.25 g | 118.53 ± 0.24 e | 124.57 ± 0.16 c | 122.42 ± 0.22 d | 109.31 ± 0.08 f |
| Gln | 309.77 ± 0.82 b | 329.47 ± 0.23 a | 261.35 ± 0.08 f | 243.66 ± 0.26 g | 296.71 ± 0.26 d | 303.54 ± 0.33 c | 286.21 ± 0.01 e |
| Ser | 71.5 ± 0.16 a | 69.29 ± 0.19 b | 60.22 ± 0.09 f | 64.6 ± 0.08 d | 65.36 ± 0.19 c | 63.36 ± 0.53 e | 58.33 ± 0.36 g |
| Gly | 134.07 ± 0.7 c | 142.06 ± 0.22 a | 121.33 ± 0.28 f | 130.45 ± 0.4 d | 135.46 ± 0.17 b | 130.62 ± 0.29 d | 129.49 ± 0.39 e |
| Asp | 56.27 ± 0.25 b | 62.76 ± 0.18 a | 50.4 ± 0.15 e | 47.44 ± 0.29 f | 54.45 ± 0.21 c | 52.32 ± 0.21 d | 45.16 ± 0.23 g |
| Glu | 138.37 ± 0.12 b | 152.67 ± 0.16 a | 120.34 ± 0.26 f | 126.32 ± 0.1 e | 133.17 ± 0.12 d | 138.39 ± 0.49 b | 118.33 ± 0.35 g |
| Thr | 38.58 ± 0.19 b | 41.52 ± 0.24 a | 34.61 ± 0.23 e | 35.55 ± 0.27 d | 41.45 ± 0.28 a | 36.49 ± 0.41 c | 33.24 ± 0.15 f |
| Ala | 348.35 ± 0.24 b | 370.23 ± 0.17 a | 296.52 ± 0.32 g | 308.56 ± 0.1 e | 336.27 ± 0.18 d | 346.43 ± 0.38 c | 299.69 ± 0.26 f |
| Pro | 286.65 ± 0.34 b | 301.65 ± 0.34 a | 240.17 ± 0.12 g | 258.53 ± 0.38 e | 277.65 ± 0.13 d | 280.33 ± 0.16 c | 250.64 ± 0.11 f |
| Lys | 20.74 ± 0.21 b | 24.57 ± 0.34 a | 15.52 ± 0.16 d | 15.67 ± 0.24 d | 18.69 ± 0.23 c | 18.8 ± 0.15 c | 13.39 ± 0.32 e |
| Met | 21.2 ± 0.14 b | 21.82 ± 0.15 a | 17.5 ± 0.33 e | 19.41 ± 0.18 c | 19.42 ± 0.13 c | 17.34 ± 0.14 e | 18.5 ± 0.3 d |
| Tyr | 94.17 ± 0.14 b | 97.52 ± 0.31 a | 87.44 ± 0.2 d | 94.43 ± 0.22 b | 91.44 ± 0.41 c | 81.2 ± 0.2 e | 91.86 ± 0.19 c |
| Val | 64.49 ± 0.24 b | 69.51 ± 0.33 a | 52.52 ± 0.19 g | 56.61 ± 0.22 e | 62.73 ± 0.19 d | 63.28 ± 0.25 c | 55.96 ± 0.16 f |
| Ile | 38.6 ± 0.19 b | 40.31 ± 0.11 a | 31.44 ± 0.27 f | 34.59 ± 0.07 d | 37.61 ± 0.3 c | 38.34 ± 0.42 b | 33.54 ± 0.2 e |
| Leu | 114.69 ± 0.12 b | 118.55 ± 0.26 a | 95.47 ± 0.14 g | 103.31 ± 0.11 e | 111.3 ± 0.32 d | 111.86 ± 0.09 c | 101.5 ± 0.27 f |
| Phe | 82.08 ± 0.15 b | 83.66 ± 0.16 a | 73.54 ± 1.73 c | 84.38 ± 0.23 a | 82.48 ± 0.08 b | 74.23 ± 0.19 c | 81.58 ± 0.11 b |
| Try | 16.24 ± 0.18 a | 16.51 ± 0.22 a | 14.5 ± 0.16 c | 15.82 ± 0.19 b | 14.53 ± 0.3 c | 11.5 ± 0.4 d | 14.61 ± 0.1 c |
| GABA | 82.37 ± 0.46 b | 84.62 ± 0.31 a | 69.47 ± 0.15 d | 66.38 ± 0.21 e | 75.3 ± 0.28 c | 75.37 ± 0.24 c | 62.34 ± 0.19 f |
| Umani | 194.64 ± 0.37 b | 215.43 ± 0.3 a | 170.74 ± 0.38 f | 173.75 ± 0.26 e | 187.63 ± 0.3 d | 190.71 ± 0.68 c | 163.5 ± 0.29 g |
| Bitter | 523.65 ± 1.02 b | 547.48 ± 0.95 a | 451.15 ± 2.21 g | 493.65 ± 1.45 d | 513.55 ± 0.31 c | 483.09 ± 1.02 e | 471.36 ± 1 f |
| Sweet | 864.34 ± 0.7 b | 907.7 ± 1.12 a | 738.23 ± 0.6 g | 784.05 ± 1.02 e | 836.79 ± 0.48 d | 840.43 ± 0.95 c | 759.13 ± 0.74 f |
| Total | 2144.92 ± 3.02 b | 2255.57 ± 1.82 a | 1834.16 ± 2.57 g | 1915.56 ± 3.3 e | 2075.98 ± 1.08 c | 2052.06 ± 2.86 d | 1885.09 ± 1.73 f |
| Class | Relative Peak Area | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | Rw-past65 | Rw-past75 | Rw-past85 | Rw-pulseA | Rw-pulseB | Rw-pulseC | ||
| Esters | Isobutyric acid, 2-methyl phenyl ester | 3064.29 ± 212.338 | 4021.68 ± 630.041 | 5252.79 ± 307.514 | 10,691.62 ± 579.114 | 3214.03 ± 53.711 | 4392.76 ± 386.596 | 3476.62 ± 293.656 |
| p-Tolyl isobutyrate | 12,028.53 ± 205.325 | 17,559.02 ± 1077.723 | 29,911.44 ± 1007.936 | 77,672.37 ± 4326.788 | 16,982.43 ± 949.484 | 23,225.27 ± 1166.487 | 15,345.01 ± 1647.744 | |
| Isopentyl acetate | 732,535.5 ± 73,436.897 | 268,613.25 ± 62,438.254 | 224,228.23 ± 36,969.183 | 224,757.95 ± 4961.607 | 653,504.86 ± 23,454.302 | 602,725.46 ± 16,781.934 | 700,803.69 ± 47,184.793 | |
| (E,E)-2,6,10-Dodecatrienoic acid, 3,7,11-trimethyl-, methyl ester | 13,613.64 ± 3988.728 | 4177.8 ± 115.78 | 6463.12 ± 2022.43 | 4647.21 ± 86.049 | 14,803.81 ± 588.338 | 18,140.12 ± 1476.511 | 14,469.59 ± 545.758 | |
| (E)-9-Tetradecen-1-ol, acetate | 17,606.21 ± 5413.779 | 5924.86 ± 969.367 | 8907.79 ± 2279.311 | 6641.17 ± 601.302 | 19391.19 ± 421.39 | 23,789.08 ± 1184.703 | 19,636.87 ± 723.008 | |
| (E)-3, 7-dimethyl octyl-2, 6-dienyl 2-methyl butyrate | 51,110.66 ± 4382.248 | 7797.53 ± 838.508 | 13,219.06 ± 1108.526 | 5950.76 ± 810.254 | 52,802.61 ± 5542.946 | 85,948.53 ± 18,498.518 | 50,599.68 ± 2327.055 | |
| Ethyl octanoate | 1,949,717.16 ± 336,693.747 | 87,137.31 ± 19,088.494 | 75,100.86 ± 16,436.007 | 45,943.67 ± 17,635.828 | 1,896,875.12 ± 40,543.238 | 1,885,887.78 ± 39,710.295 | 2,090,866.82 ± 36,486.562 | |
| Butyric acid hexyl ester | 156,814.59 ± 28,451.425 | 8,762.96 ± 1,075.5 | 8,678.03 ± 967.986 | 7,991.14 ± 826.559 | 151,275.06 ± 4,584.326 | 150,082.6 ± 4103.551 | 165,890.7 ± 3696.6 | |
| Pentanoic acid, 4-methyl-methyl ester | 29,881.13 ± 3168.636 | 13,648.63 ± 1157.526 | 13,129.85 ± 4741.83 | 12,470.63 ± 1413.552 | 25,590.93 ± 2968.793 | 28,316.2 ± 7503.432 | 25,375.05 ± 4840.742 | |
| Resorcinol monoacetate | 8748.93 ± 964.15 | 2835.26 ± 545.963 | 4046.45 ± 372.45 | 3934.37 ± 119.828 | 8296.5 ± 239.1 | 9026.14 ± 516.486 | 8241.96 ± 957.017 | |
| Octyl butyrate | 257,667.55 ± 39,485.494 | 27,468.92 ± 4380.501 | 31,012.32 ± 1347.491 | 22,783.38 ± 7640.48 | 256,052.98 ± 7341.198 | 299,970.59 ± 6593.387 | 265,885.75 ± 16,229.577 | |
| Total | 3,232,788.19 | 447,947.22 | 419,949.94 | 50,942.57 | 3,098,789.52 | 3,131,504.53 | 3,360,591.74 | |
| Alcohols | 2,6-Dimethyl-1-nonen-3-yn-5-ol | 4399.45 ± 776.729 | 4959.92 ± 567.986 | 6977 ± 631.921 | 13,534.5 ± 807.302 | 5389.07 ± 1160.899 | 5586.05 ± 519.566 | 5091.27 ± 211.157 |
| 2-Ethyl-1-dodecanol | 59,375.02 ± 5554.812 | 86,45.81 ± 1189.908 | 15,278.39 ± 1610.646 | 6733.12 ± 1159.443 | 58,705.96 ± 4355.568 | 98,319.92 ± 21,247.89 | 57,452.67 ± 3117.81 | |
| (1α, 2α, 3α)-2-methyl-3-(1-methylethenyl)-Cyclohexanol | 132,292.58 ± 15,869.088 | 44,087.29 ± 2911.496 | 48,112.85 ± 1153.987 | 42,473.48 ± 4229.971 | 142,957.67 ± 2669.479 | 138,343.46 ± 5552.434 | 146,039.16 ± 5189.607 | |
| (Z)-3-nonyl-1-alcohol | 855.88 ± 877.617 | 113,375.52 ± 6817.513 | 118,909.62 ± 6030.446 | 112,044.97 ± 3506.555 | 2119.61 ± 566.665 | 6952.1 ± 1740.731 | 3102.97 ± 534.152 | |
| 1,3-Dioxolane-2,2-diethanol | 4628.89 ± 570.668 | ND | ND | ND | 4548.44 ± 233.204 | 5949.89 ± 441.628 | 4315.01 ± 151.088 | |
| Total | 201,551.82 | 171,068.54 | 189,277.86 | 174,786.07 | 213,720.75 | 255,151.42 | 216,001.08 | |
| Ketones | 1-Hepten-3-one | 349,577.97 ± 39,562.94 | 131,182.08 ± 26,877.715 | 118,109.34 ± 12,500.191 | 115,758.5 ± 3226.809 | 315,648.35 ± 28,105.849 | 299,848.98 ± 16,709.773 | 344,682.67 ± 18,267.568 |
| 3,4-Hexanedione, 2,2,5-trimethyl- | 6867.29 ± 2424.017 | 4476.04 ± 552.765 | 4126.52 ± 818.708 | 7201.94 ± 1724.076 | 19,556.12 ± 2959.43 | 32,710.9 ± 1738.292 | 15,332.5 ± 301.281 | |
| 4-phenyl-2-butanone | 3161.09 ± 306.501 | 3675.17 ± 476.072 | 3130.46 ± 214.618 | 4501.33 ± 671.979 | 5915.59 ± 381.984 | 10,682.54 ± 1619.663 | 4731.09 ± 121.618 | |
| 2,2,6-trimethyl-cyclohexanone | 76,828.05 ± 6918.019 | 40,305 ± 8873.589 | 36,174.55 ± 2929.82 | 40,554.89 ± 3659.857 | 76,779.21 ± 2724.664 | 82,392.47 ± 6402.953 | 73,773.66 ± 8257.353 | |
| Total | 436,434.40 | 179,638.29 | 161,540.87 | 168,016.66 | 417,899.27 | 425,634.89 | 438,519.92 | |
| Phenols | 3-methyl-phenol | 1664.79 ± 293.884 | 2639.11 ± 1060.4 | 2149.54 ± 572.866 | 2390.39 ± 661.082 | 19,278.33 ± 2542.569 | 58,085.63 ± 2950.302 | 8369.55 ± 144.182 |
| p-Cresol | 3059.76 ± 183.622 | 3952.55 ± 1599.361 | 3910.41 ± 290.461 | 3312.75 ± 400.071 | 26,949.13 ± 224.902 | 68,871.51 ± 2677.255 | 13,764.45 ± 1407.483 | |
| Total | 4724.55 | 6591.66 | 6059.95 | 5703.14 | 46,227.46 | 126,957.14 | 22,134.00 | |
| Acids | alpha-cyclopentyl-Benzeneacetic Acid | 730.98 ± 659.929 | 36,857.73 ± 7172.521 | 117,134 ± 37,731.265 | 47,504.99 ± 20,360.309 | 960.12 ± 885.407 | 579.68 ± 496.721 | ND |
| Total | 730.98 | 36,857.73 | 117,134.00 | 47,504.99 | 960.12 | 579.68 | ND | |
| Aldehydes | (2E,4Z)-2,4-Decadienal | 11,583.35 ± 2596.763 | 5398.78 ± 434.088 | 8227.6 ± 345.334 | 18,593.62 ± 192.731 | 18,232.51 ± 3852.31 | 15,761.81 ± 1774.755 | 14,633.17 ± 906.357 |
| 7-methyl-3-methylene-6-octenal | 4859.97 ± 4396.688 | 562,199.41 ± 35,086.469 | 606,166.83 ± 24,301.727 | 568,750.45 ± 21,586.876 | 6901.12 ± 1117.369 | 31,490.25 ± 5610.622 | 10,932.13 ± 1283.039 | |
| Benzaldehyde | 233,259.01 ± 39,542.699 | 258,615.46 ± 29,804.367 | 234,231.83 ± 3959.265 | 298,238.85 ± 8346.082 | 380,206.41 ± 32,780.122 | 538,673.48 ± 24,656.753 | 343,914.71 ± 49,818.112 | |
| Total | 249,702.33 | 826,213.65 | 848,626.26 | 885,582.92 | 405,340.04 | 585,925.54 | 369,480.01 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Zhang, Y.; Zhong, Q. Optimization of the Brewing Conditions of Shanlan Rice Wine and Sterilization by Thermal and Intense Pulse Light. Molecules 2023, 28, 3183. https://doi.org/10.3390/molecules28073183
Wu X, Zhang Y, Zhong Q. Optimization of the Brewing Conditions of Shanlan Rice Wine and Sterilization by Thermal and Intense Pulse Light. Molecules. 2023; 28(7):3183. https://doi.org/10.3390/molecules28073183
Chicago/Turabian StyleWu, Xiaoqian, Yunzhu Zhang, and Qiuping Zhong. 2023. "Optimization of the Brewing Conditions of Shanlan Rice Wine and Sterilization by Thermal and Intense Pulse Light" Molecules 28, no. 7: 3183. https://doi.org/10.3390/molecules28073183
APA StyleWu, X., Zhang, Y., & Zhong, Q. (2023). Optimization of the Brewing Conditions of Shanlan Rice Wine and Sterilization by Thermal and Intense Pulse Light. Molecules, 28(7), 3183. https://doi.org/10.3390/molecules28073183





