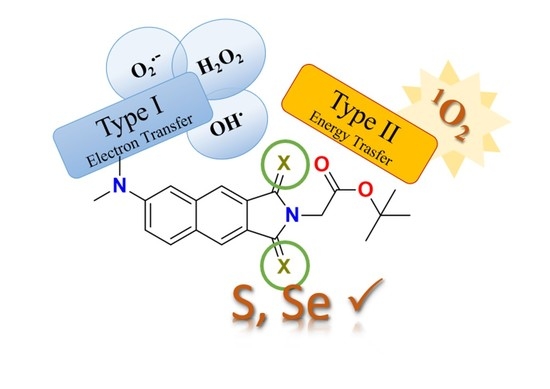
Sulphur- and Selenium-for-Oxygen Replacement as a Strategy to Obtain Dual Type I/Type II Photosensitizers for Photodynamic Therapy
Abstract
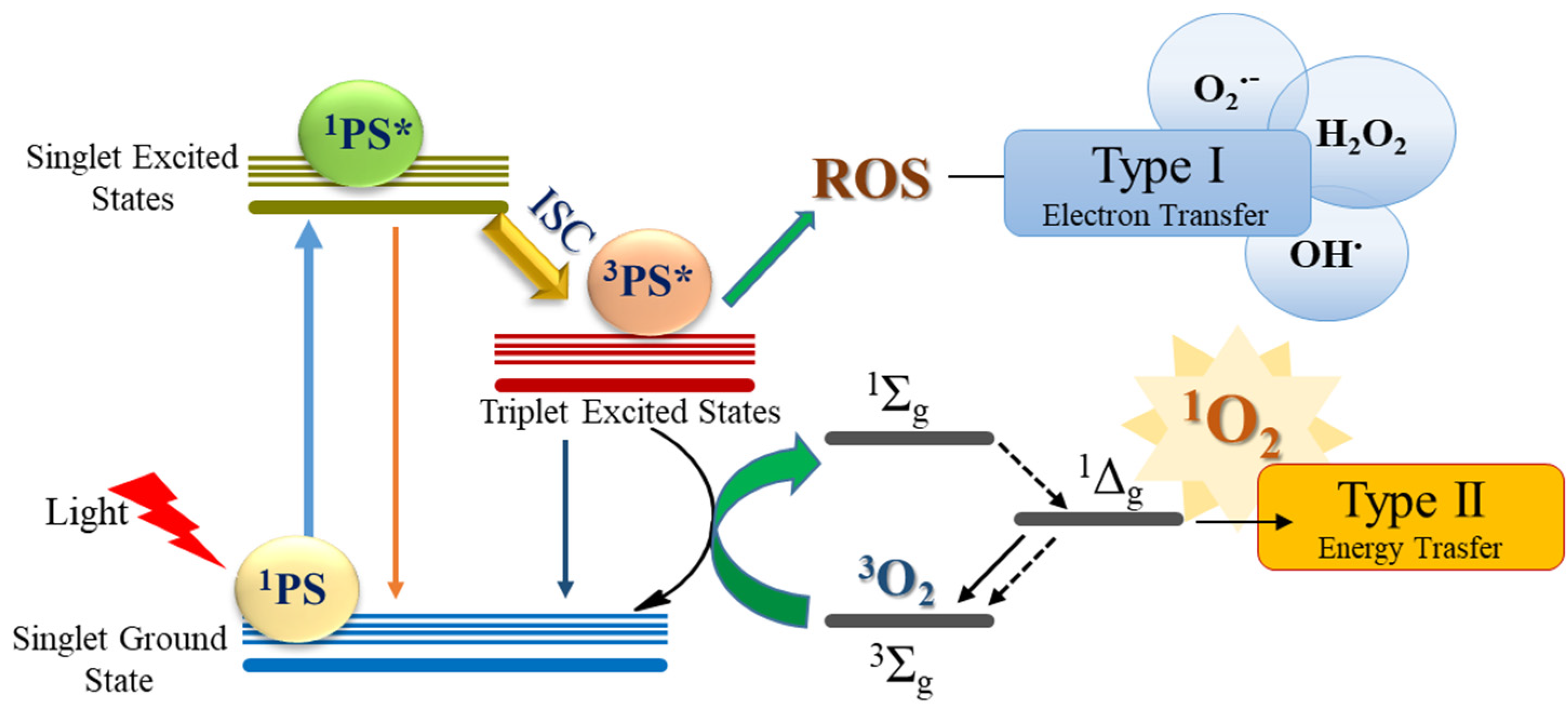
1. Introduction
2. Results and Discussion
3. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dąbrowski, J.M.; Pucelik, B.; Regiel-Futyra, A.; Brindell, M.; Mazuryk, O.; Kyzioł, A.; Stochel, G.; Macyk, W.; Arnaut, L.G. Engineering of Relevant Photodynamic Processes through Structural Modifications of Metallotetrapyrrolic Photosensitizers. Coord. Chem. Rev. 2016, 325, 67–101. [Google Scholar] [CrossRef]
- Yano, S.; Hirohara, S.; Obata, M.; Hagiya, Y.; Ogura, S.; Ikeda, A.; Kataoka, H.; Tanaka, M.; Joh, T. Current States and Future Views in Photodynamic Therapy. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 46–67. [Google Scholar] [CrossRef]
- MacDonald, I.J.; Dougherty, T.J. Basic Principles of Photodynamic Therapy. J. Porphyr. Phthalocyanines 2001, 05, 105–129. [Google Scholar] [CrossRef]
- Baptista, M.S.; Cadet, J.; di Mascio, P.; Ghogare, A.A.; Greer, A.; Hamblin, M.R.; Lorente, C.; Nunez, S.C.; Ribeiro, M.S.; Thomas, A.H.; et al. Type I and Type II Photosensitized Oxidation Reactions: Guidelines and Mechanistic Pathways. Photochem. Photobiol. 2017, 93, 912–919. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer: An Update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Dabrowski, J.M.; Arnaut, L.G. Photodynamic Therapy (PDT) of Cancer: From Local to Systemic Treatment. Photochem. Photobiol. Sci. 2015, 14, 1765–1780. [Google Scholar] [CrossRef]
- dos Santos, A.F.; de Almeida, D.R.Q.; Terra, L.F.; Baptista, M.S.; Labriola, L. Photodynamic Therapy in Cancer Treatment—An Update Review. J. Cancer Metastasis Treat. 2019, 2019, 25. [Google Scholar] [CrossRef]
- Kossodo, S.; LaMuraglia, G.M. Clinical Potential of Photodynamic Therapy in Cardiovascular Disorders. Am. J. Cardiovasc. Drugs 2001, 1, 15–21. [Google Scholar] [CrossRef]
- DeRosa, M. Photosensitized Singlet Oxygen and Its Applications. Coord. Chem. Rev. 2002, 233–234, 351–371. [Google Scholar] [CrossRef]
- Romero, O.C.; Straub, A.P.; Kohn, T.; Nguyen, T.H. Role of Temperature and Suwannee River Natural Organic Matter on Inactivation Kinetics of Rotavirus and Bacteriophage MS2 by Solar Irradiation. Environ. Sci. Technol. 2011, 45, 10385–10393. [Google Scholar] [CrossRef]
- Aroso, R.T.; Schaberle, F.A.; Arnaut, L.G.; Pereira, M.M. Photodynamic Disinfection and Its Role in Controlling Infectious Diseases. Photochem. Photobiol. Sci. 2021, 20, 1497–1545. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Cao, W.; Cao, J. Treatment of Rheumatoid Arthritis by Phototherapy: Advances and Perspectives. Nanoscale 2021, 13, 14591–14608. [Google Scholar] [CrossRef] [PubMed]
- Monfrecola, G.; Megna, M.; Rovati, C.; Arisi, M.; Rossi, M.; Calzavara-Pinton, I.; Fabbrocini, G.; Calzavara-Pinton, P. A Critical Reappraisal of Off-Label Use of Photodynamic Therapy for the Treatment of Non-Neoplastic Skin Conditions. Dermatology 2021, 237, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Stájer, A.; Kajári, S.; Gajdács, M.; Musah-Eroje, A.; Baráth, Z. Utility of Photodynamic Therapy in Dentistry: Current Concepts. Dent. J. 2020, 8, 43. [Google Scholar] [CrossRef]
- Alberto, M.E.; Comuzzi, C.; Thandu, M.; Adamo, C.; Russo, N. 22π-Electrons [1.1.1.1.1] Pentaphyrin as a New Photosensitizing Agent for Water Disinfection: Experimental and Theoretical Characterization. Theor. Chem. Acc. 2016, 135, 29. [Google Scholar] [CrossRef]
- Bartolomeu, M.; Reis, S.; Fontes, M.; Neves, M.; Faustino, M.; Almeida, A. Photodynamic Action against Wastewater Microorganisms and Chemical Pollutants: An Effective Approach with Low Environmental Impact. Water 2017, 9, 630. [Google Scholar] [CrossRef]
- Semenova, O.; Kobzev, D.; Hovor, I.; Atrash, M.; Nakonechny, F.; Kulyk, O.; Bazylevich, A.; Gellerman, G.; Patsenker, L. Effect of Solubilizing Group on the Antibacterial Activity of Heptamethine Cyanine Photosensitizers. Pharmaceutics 2023, 15, 247. [Google Scholar] [CrossRef]
- Henderson, B.W.; Gollnick, S.O.; Snyder, J.W.; Busch, T.M.; Kousis, P.C.; Cheney, R.T.; Morgan, J. Choice of Oxygen-Conserving Treatment Regimen Determines the Inflammatory Response and Outcome of Photodynamic Therapy of Tumors. Cancer Res. 2004, 64, 2120–2126. [Google Scholar] [CrossRef]
- Falk-Mahapatra, R.; Gollnick, S.O. Photodynamic Therapy and Immunity: An Update. Photochem. Photobiol. 2020, 96, 550–559. [Google Scholar] [CrossRef]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic Therapy and Anti-Tumour Immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef]
- Gourdon, L.; Cariou, K.; Gasser, G. Phototherapeutic Anticancer Strategies with First-Row Transition Metal Complexes: A Critical Review. Chem. Soc. Rev. 2022, 51, 1167–1195. [Google Scholar] [CrossRef] [PubMed]
- Algorri, J.F.; Ochoa, M.; Roldán-Varona, P.; Rodríguez-Cobo, L.; López-Higuera, J.M. Photodynamic Therapy: A Compendium of Latest Reviews. Cancers 2021, 13, 4447. [Google Scholar] [CrossRef] [PubMed]
- Alberto, M.; Pirillo, J.; Russo, N.; Adamo, C. Theoretical Exploration of Type I/Type II Dual Photoreactivity of Promising Ru(II) Dyads for PDT Approach. Inorg. Chem. 2016, 55, 11185–11192. [Google Scholar] [CrossRef]
- Alberto, M.E.; Francés-Monerris, A. A Multiscale Free Energy Method Reveals an Unprecedented Photoactivation of a Bimetallic Os(II)–Pt(II) Dual Anticancer Agent. Phys. Chem. Chem. Phys. 2022, 24, 19584–19594. [Google Scholar] [CrossRef] [PubMed]
- Ponte, F.; Alberto, M.E.; De Simone, B.C.; Russo, N.; Sicilia, E. Photophysical Exploration of Dual-Approach PtII –BODIPY Conjugates: Theoretical Insights. Inorg. Chem. 2019, 58, 9882–9889. [Google Scholar] [CrossRef]
- Monro, S.; Colón, K.L.; Yin, H.; Roque, J.; Konda, P.; Gujar, S.; Thummel, R.P.; Lilge, L.; Cameron, C.G.; McFarland, S.A. Transition Metal Complexes and Photodynamic Therapy from a Tumor-Centered Approach: Challenges, Opportunities, and Highlights from the Development of TLD1433. Chem. Rev. 2019, 119, 797–828. [Google Scholar] [CrossRef]
- Roque, J.A.; Barrett, P.C.; Cole, H.D.; Lifshits, L.M.; Shi, G.; Monro, S.; von Dohlen, D.; Kim, S.; Russo, N.; Deep, G.; et al. Breaking the Barrier: An Osmium Photosensitizer with Unprecedented Hypoxic Phototoxicity for Real World Photodynamic Therapy. Chem. Sci. 2020, 11, 9784–9806. [Google Scholar] [CrossRef]
- Lameijer, L.N.; Ernst, D.; Hopkins, S.L.; Meijer, M.S.; Askes, S.H.C.; Le Dévédec, S.E.; Bonnet, S. A Red-Light-Activated Ruthenium-Caged NAMPT Inhibitor Remains Phototoxic in Hypoxic Cancer Cells. Angew. Chem. Int. Ed. 2017, 56, 11549–11553. [Google Scholar] [CrossRef]
- Loftus, L.M.; Al-Afyouni, K.F.; Turro, C. New Ru II Scaffold for Photoinduced Ligand Release with Red Light in the Photodynamic Therapy (PDT) Window. Chem. Eur. J. 2018, 24, 11550–11553. [Google Scholar] [CrossRef]
- Roque, J.A., III; Cole, H.D.; Barrett, P.C.; Lifshits, L.M.; Hodges, R.O.; Kim, S.; Deep, G.; Francés-Monerris, A.; Alberto, M.E.; Cameron, C.G.; et al. Intraligand Excited States Turn a Ruthenium Oligothiophene Complex into a Light-Triggered Ubertoxin with Anticancer Effects in Extreme Hypoxia. J. Am. Chem. Soc. 2022, 144, 8317–8336. [Google Scholar] [CrossRef]
- El-Sayed, M.A. Triplet State. Its Radiative and Nonradiative Properties. Acc. Chem. Res. 1968, 1, 8–16. [Google Scholar] [CrossRef]
- Banfi, S.; Caruso, E.; Caprioli, S.; Mazzagatti, L.; Canti, G.; Ravizza, R.; Gariboldi, M.; Monti, E. Photodynamic Effects of Porphyrin and Chlorin Photosensitizers in Human Colon Adenocarcinoma Cells. Bioorg. Med. Chem. 2004, 12, 4853–4860. [Google Scholar] [CrossRef] [PubMed]
- Alberto, M.E.; Marino, T.; Quartarolo, A.D.; Russo, N. Photophysical Origin of the Reduced Photodynamic Therapy Activity of Temocene Compared to Foscan®: Insights from Theory. Phys. Chem. Chem. Phys. 2013, 15, 16167. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Xu, Q.; Wang, W.; Shao, J.; Huang, W.; Dong, X. Type I Photosensitizers Revitalizing Photodynamic Oncotherapy. Small 2021, 17, 2006742. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhuang, Z.; Zhao, Z.; Tang, B.Z. Type I AIE Photosensitizers: Mechanism and Application. VIEW 2022, 3, 20200121. [Google Scholar] [CrossRef]
- Ignarro, L.J.; Freeman, B.A. Nitric Oxide Biology and Pathobiology; Ignarro, L., Ed.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Herzberg, G. Spectra of Diatomic Molecules, 2nd ed.; Van Nostrand Reinhold: New York, NY, USA, 1950. [Google Scholar]
- Alberto, M.E.; de Simone, B.C.; Liuzzi, S.; Marino, T.; Russo, N.; Toscano, M. Iodine Substituted Phosphorus Corrole Complexes as Possible Photosensitizers in Photodynamic Therapy: Insights from Theory. J. Comput. Chem. 2020, 41, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-H.; Tang, X.-F.; Chang, X.-P.; Zhang, T.-S.; Xie, B.-B.; Cui, G. Mechanistic Photophysics of Tellurium-Substituted Uracils: Insights from Multistate Complete-Active-Space Second-Order Perturbation Calculations. J. Phys. Chem. A 2021, 125, 8816–8826. [Google Scholar] [CrossRef]
- Jena, S.; Tulsiyan, K.D.; Kumari, A.; Das, R.; Biswal, H.S. Thiolumazines as Heavy-Atom-Free Photosensitizers for Applications in Daylight Photodynamic Therapy: Insights from Ultrafast Excited-State Dynamics. J. Phys. Chem. B 2022, 126, 6083–6094. [Google Scholar] [CrossRef]
- Farrell, K.M.; Brister, M.M.; Pittelkow, M.; Sølling, T.I.; Crespo-Hernández, C.E. Heavy-Atom-Substituted Nucleobases in Photodynamic Applications: Substitution of Sulfur with Selenium in 6-Thioguanine Induces a Remarkable Increase in the Rate of Triplet Decay in 6-Selenoguanine. J. Am. Chem. Soc. 2018, 140, 11214–11218. [Google Scholar] [CrossRef]
- Fang, Y.-G.; Valverde, D.; Mai, S.; Canuto, S.; Borin, A.C.; Cui, G.; González, L. Excited-State Properties and Relaxation Pathways of Selenium-Substituted Guanine Nucleobase in Aqueous Solution and DNA Duplex. J. Phys. Chem. B 2021, 125, 1778–1789. [Google Scholar] [CrossRef]
- Valverde, D.; Mai, S.; Canuto, S.; Borin, A.C.; González, L. Ultrafast Intersystem Crossing Dynamics of 6-Selenoguanine in Water. JACS Au 2022, 2, 1699–1711. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Rodríguez, L.A.; Crespo-Hernández, C.E. Thionated Organic Compounds as Emerging Heavy-Atom-Free Photodynamic Therapy Agents. Chem. Sci. 2020, 11, 11113–11123. [Google Scholar] [CrossRef] [PubMed]
- Salon, J.; Gan, J.; Abdur, R.; Liu, H.; Huang, Z. Synthesis of 6-Se-Guanosine RNAs for Structural Study. Org. Lett. 2013, 15, 3934–3937. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.E.A.; Sheng, J.; Zhang, W.; Huang, Z. High Fidelity of Base Pairing by 2-Selenothymidine in DNA. J. Am. Chem. Soc. 2010, 132, 2120–2121. [Google Scholar] [CrossRef]
- Faustino, I.; Curutchet, C.; Luque, F.J.; Orozco, M. The DNA-Forming Properties of 6-Selenoguanine. Phys. Chem. Chem. Phys. 2014, 16, 1101–1110. [Google Scholar] [CrossRef]
- Nguyen, V.-N.; Qi, S.; Kim, S.; Kwon, N.; Kim, G.; Yim, Y.; Park, S.; Yoon, J. An Emerging Molecular Design Approach to Heavy-Atom-Free Photosensitizers for Enhanced Photodynamic Therapy under Hypoxia. J. Am. Chem. Soc. 2019, 141, 16243–16248. [Google Scholar] [CrossRef]
- Tang, J.; Wang, L.; Loredo, A.; Cole, C.; Xiao, H. Single-Atom Replacement as a General Approach towards Visible-Light/near-Infrared Heavy-Atom-Free Photosensitizers for Photodynamic Therapy. Chem. Sci. 2020, 11, 6701–6708. [Google Scholar] [CrossRef]
- Ortiz-Rodríguez, L.A.; Hoehn, S.J.; Loredo, A.; Wang, L.; Xiao, H.; Crespo-Hernández, C.E. Electronic Relaxation Pathways in Heavy-Atom-Free Photosensitizers Absorbing Near-Infrared Radiation and Exhibiting High Yields of Singlet Oxygen Generation. J. Am. Chem. Soc. 2021, 143, 2676–2681. [Google Scholar] [CrossRef]
- Alberto, M.E.; de Simone, B.C.; Marino, T.; Toscano, M.; Russo, N. Chalcogen Effects in the Photophysical Properties of Dimethylamino-1,8-Naphthalimide Dyes Revealed by DFT Investigation. J. Phys. Chem. A 2022, 126, 5167–5172. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian, 16, Revision C.1; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef] [PubMed]
- Hirata, S.; Head-Gordon, M. Time-Dependent Density Functional Theory within the Tamm–Dancoff Approximation. Chem. Phys. Lett. 1999, 314, 291–299. [Google Scholar] [CrossRef]
- Peach, M.J.G.; Williamson, M.J.; Tozer, D.J. Influence of Triplet Instabilities in TDDFT. J. Chem. Theory. Comput. 2011, 7, 3578–3585. [Google Scholar] [CrossRef] [PubMed]
- Alberto, M.E.; de Simone, B.C.; Mazzone, G.; Sicilia, E.; Russo, N. The Heavy Atom Effect on Zn(II) Phthalocyanine Derivatives: A Theoretical Exploration of the Photophysical Properties. Phys. Chem. Chem. Phys. 2015, 17, 23595–23601. [Google Scholar] [CrossRef] [PubMed]
- Ruud, K.; Schimmelpfennig, B.; Ågren, H. Internal and External Heavy-Atom Effects on Phosphorescence Radiative Lifetimes Calculated Using a Mean-Field Spin–Orbit Hamiltonian. Chem. Phys. Lett. 1999, 310, 215–221. [Google Scholar] [CrossRef]
- Dalton, a Molecular Electronic Structure Program, Release 2011-07-20 (2011). Available online: http://daltonprogram.org (accessed on 9 February 2023).
- Ji, S.; Ge, J.; Escudero, D.; Wang, Z.; Zhao, J.; Jacquemin, D. Molecular Structure–Intersystem Crossing Relationship of Heavy-Atom-Free BODIPY Triplet Photosensitizers. J. Org. Chem. 2015, 80, 5958–5963. [Google Scholar] [CrossRef]
- Brémond, É.; Alberto, M.E.; Russo, N.; Ricci, G.; Ciofini, I.; Adamo, C. Photophysical Properties of NIR-Emitting Fluorescence Probes: Insights from TD-DFT. Phys. Chem. Chem. Phys. 2013, 15, 10019. [Google Scholar] [CrossRef]
- Alberto, M.E.; Marino, T.; Russo, N.; Sicilia, E.; Toscano, M. The Performance of Density Functional Based Methods in the Description of Selected Biological Systems and Processes. Phys. Chem. Chem. Phys. 2012, 14, 14943. [Google Scholar] [CrossRef]
- de Simone, B.C.; Marino, T.; Prejanò, M.; Russo, N. Can Fused Thiophene–Pyrrole-Containing Rings Act as Possible New Electrochromic Dyes? A Computational Prediction. Theor. Chem. Acc. 2016, 135, 238. [Google Scholar] [CrossRef]
- Li, Y.; Prejanò, M.; Toscano, M.; Russo, N. Oenin/Syringic Acid Copigmentation: Insights From a Theoretical Study. Front. Chem. 2019, 7, 579. [Google Scholar] [CrossRef] [PubMed]
- Marian, C.M. Spin-Orbit Coupling and Intersystem Crossing in Molecules. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 187–203. [Google Scholar] [CrossRef]
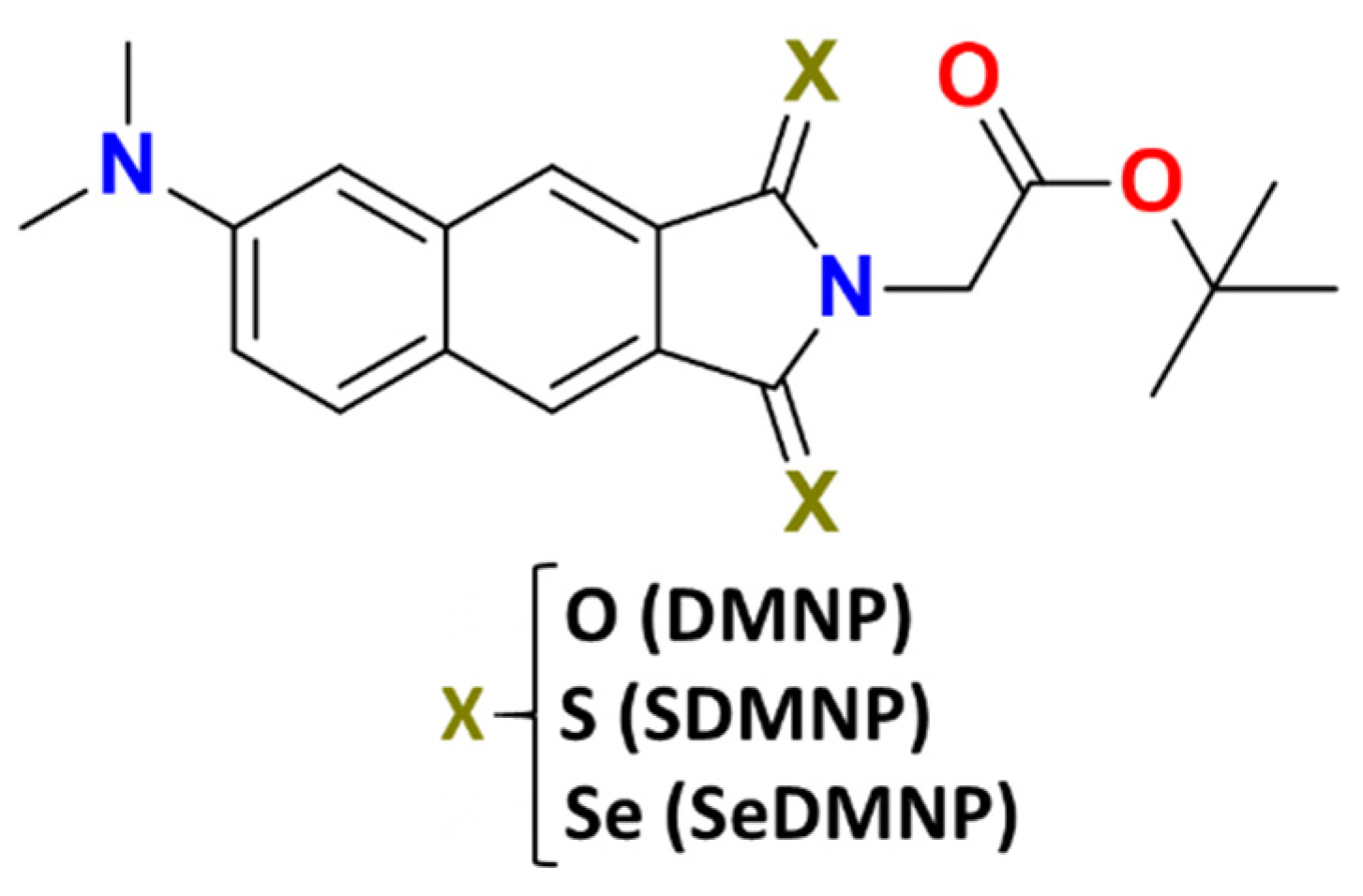
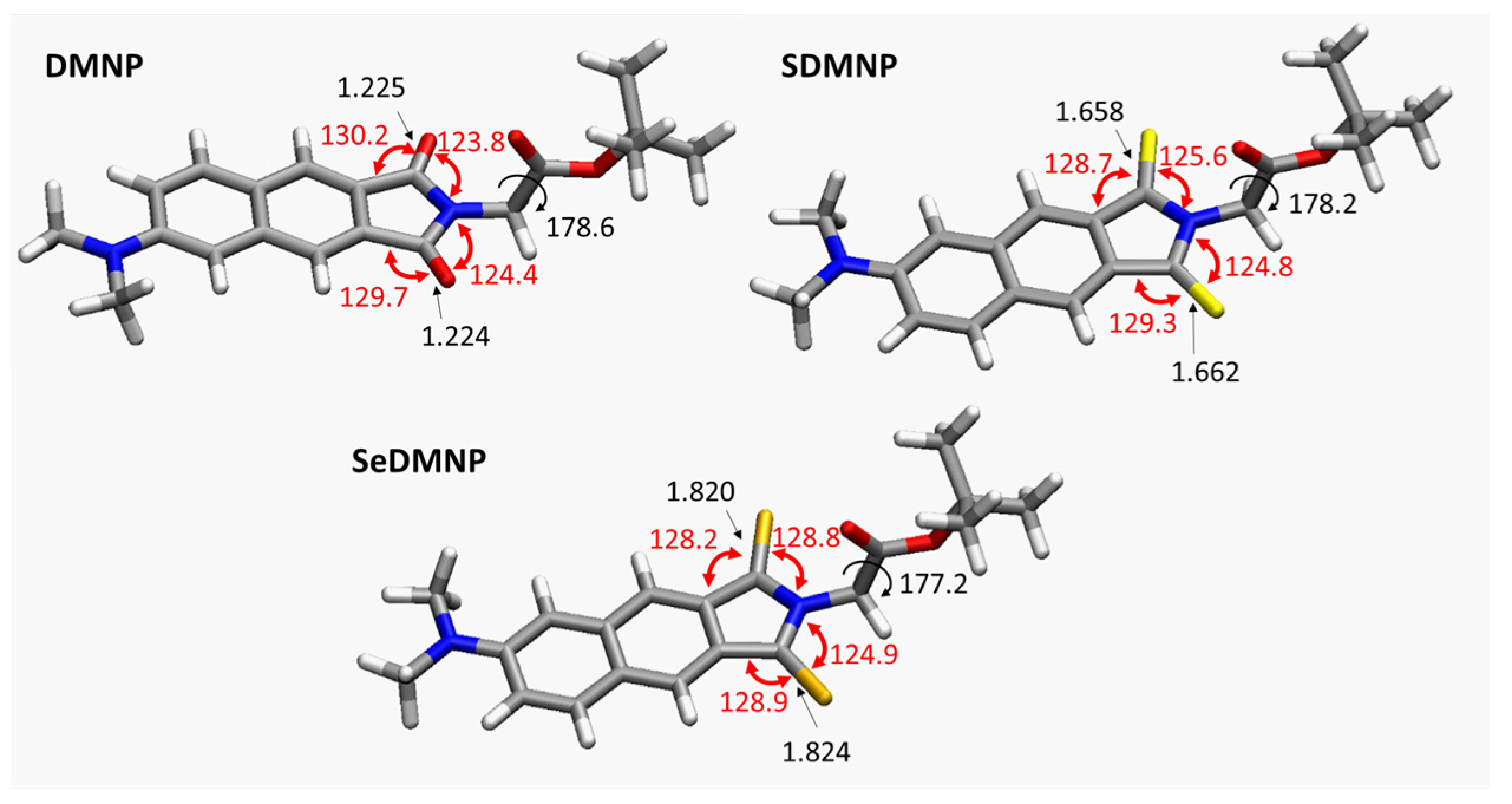

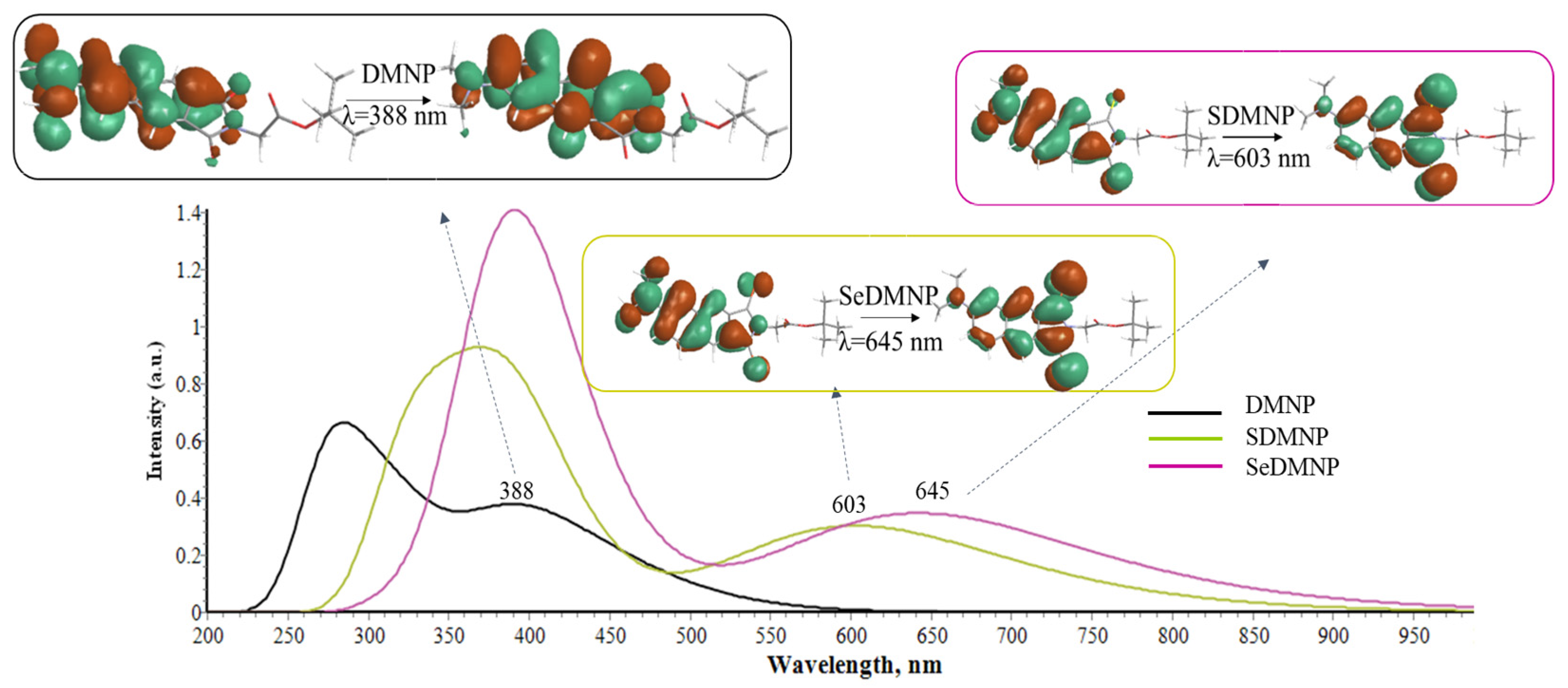
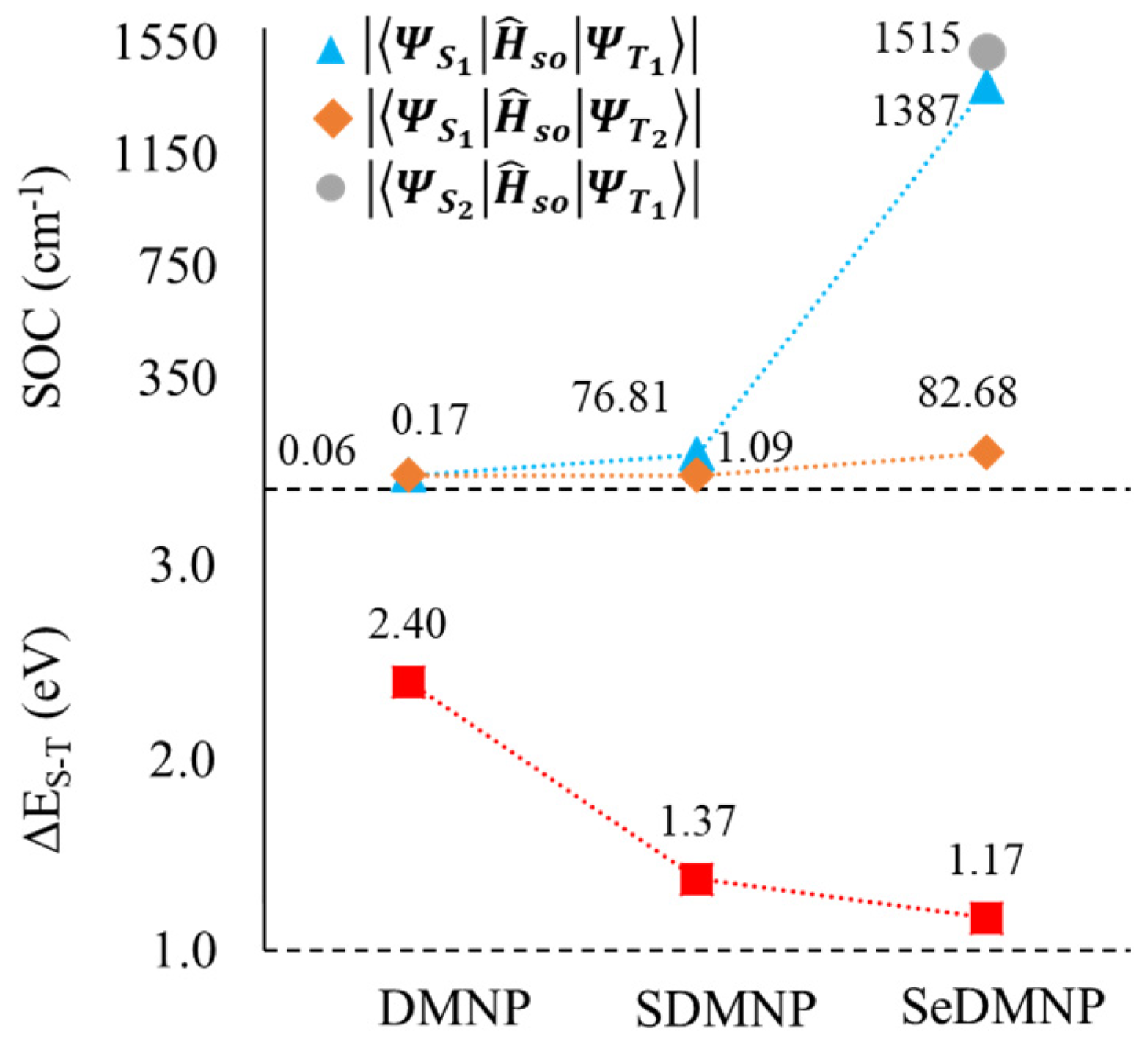
| Cpds | VEA (S0) | VIP (S0) | VEA (T1) | VIP (T1) |
|---|---|---|---|---|
| DMNP | −2.48 | 5.42 | −4.66 | 3.56 |
| SDMNP | −3.51 | 5.43 | −4.83 | 4.23 |
| SeDMNP | −3.71 | 5.42 | −4.84 | 4.39 |
| O2 | −3.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prejanò, M.; Alberto, M.E.; De Simone, B.C.; Marino, T.; Toscano, M.; Russo, N. Sulphur- and Selenium-for-Oxygen Replacement as a Strategy to Obtain Dual Type I/Type II Photosensitizers for Photodynamic Therapy. Molecules 2023, 28, 3153. https://doi.org/10.3390/molecules28073153
Prejanò M, Alberto ME, De Simone BC, Marino T, Toscano M, Russo N. Sulphur- and Selenium-for-Oxygen Replacement as a Strategy to Obtain Dual Type I/Type II Photosensitizers for Photodynamic Therapy. Molecules. 2023; 28(7):3153. https://doi.org/10.3390/molecules28073153
Chicago/Turabian StylePrejanò, Mario, Marta Erminia Alberto, Bruna Clara De Simone, Tiziana Marino, Marirosa Toscano, and Nino Russo. 2023. "Sulphur- and Selenium-for-Oxygen Replacement as a Strategy to Obtain Dual Type I/Type II Photosensitizers for Photodynamic Therapy" Molecules 28, no. 7: 3153. https://doi.org/10.3390/molecules28073153
APA StylePrejanò, M., Alberto, M. E., De Simone, B. C., Marino, T., Toscano, M., & Russo, N. (2023). Sulphur- and Selenium-for-Oxygen Replacement as a Strategy to Obtain Dual Type I/Type II Photosensitizers for Photodynamic Therapy. Molecules, 28(7), 3153. https://doi.org/10.3390/molecules28073153