Penazaphilones J–L, Three New Hydrophilic Azaphilone Pigments from Penicillium sclerotiorum cib-411 and Their Anti-Inflammatory Activity
Abstract
1. Introduction
2. Results and Discussion
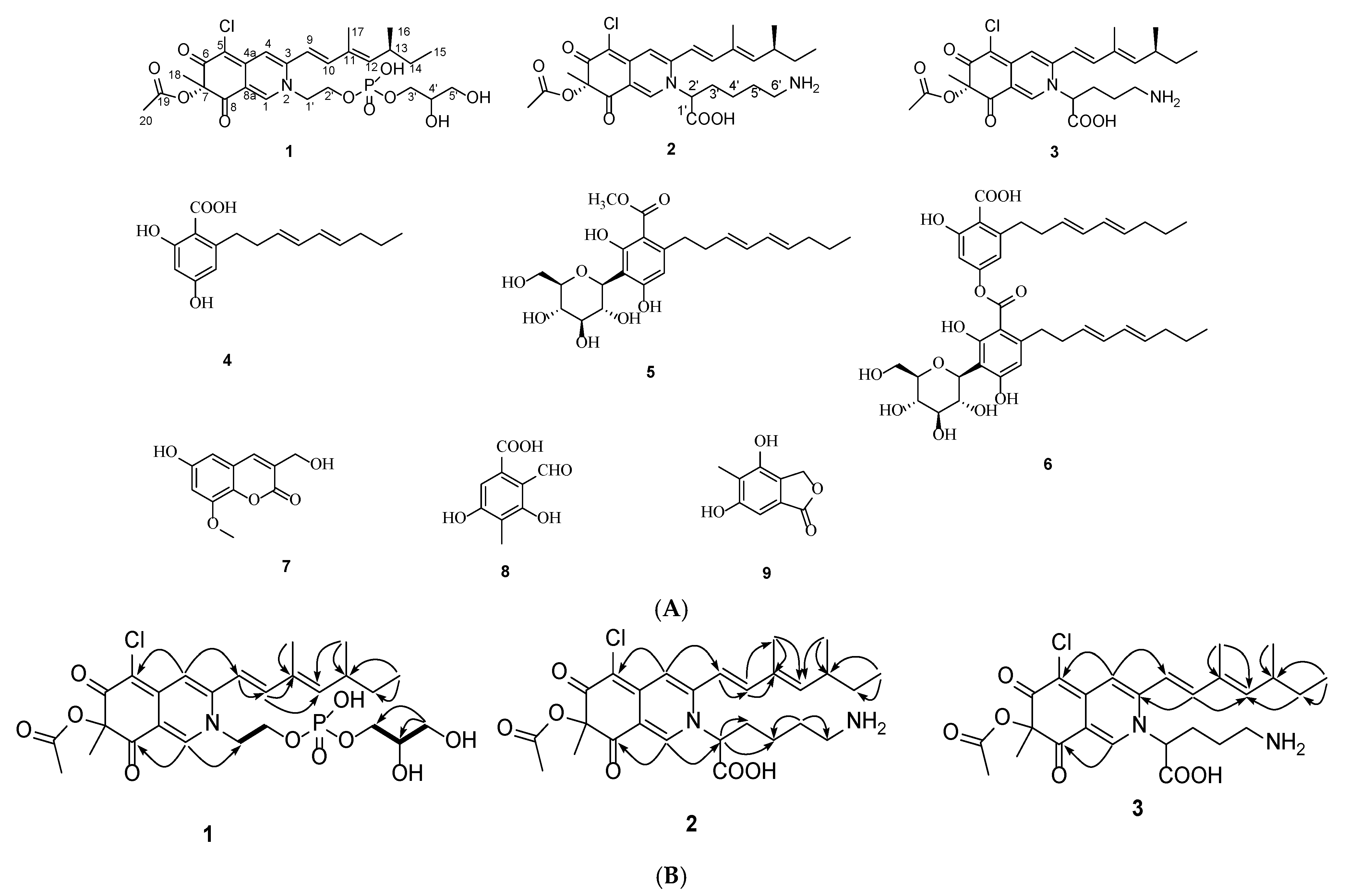
2.1. Structural Identification of Compounds 1–3
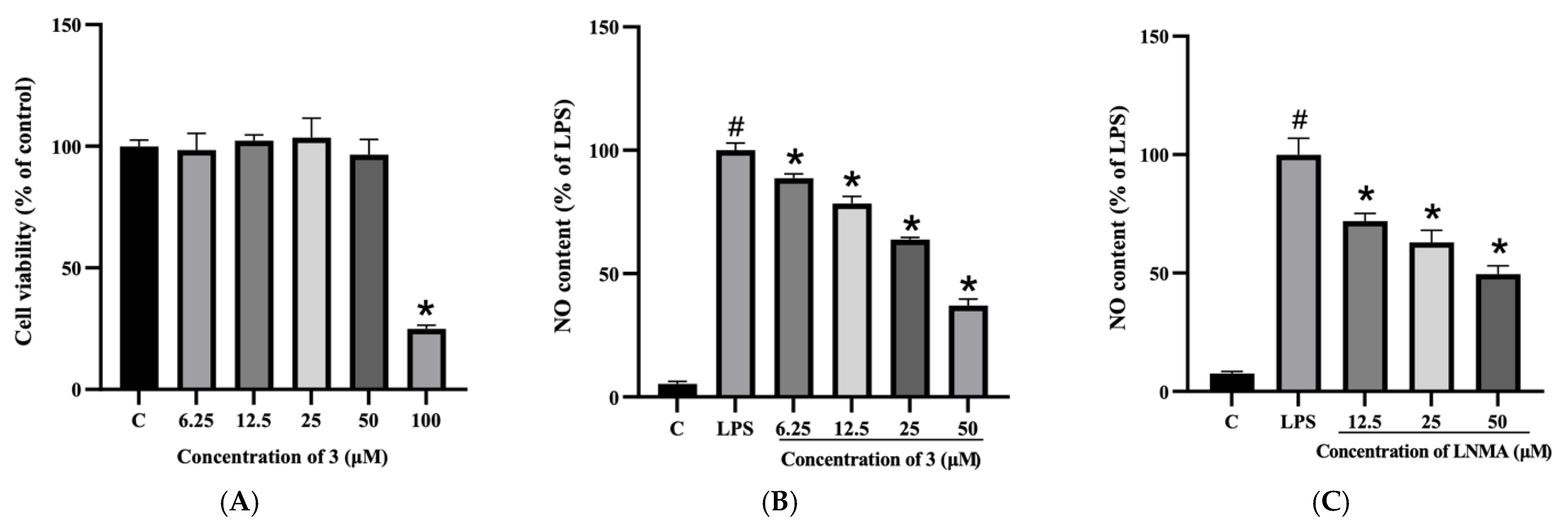
2.2. Effects of Compound 3 on the Viability of RAW264.7 Cells
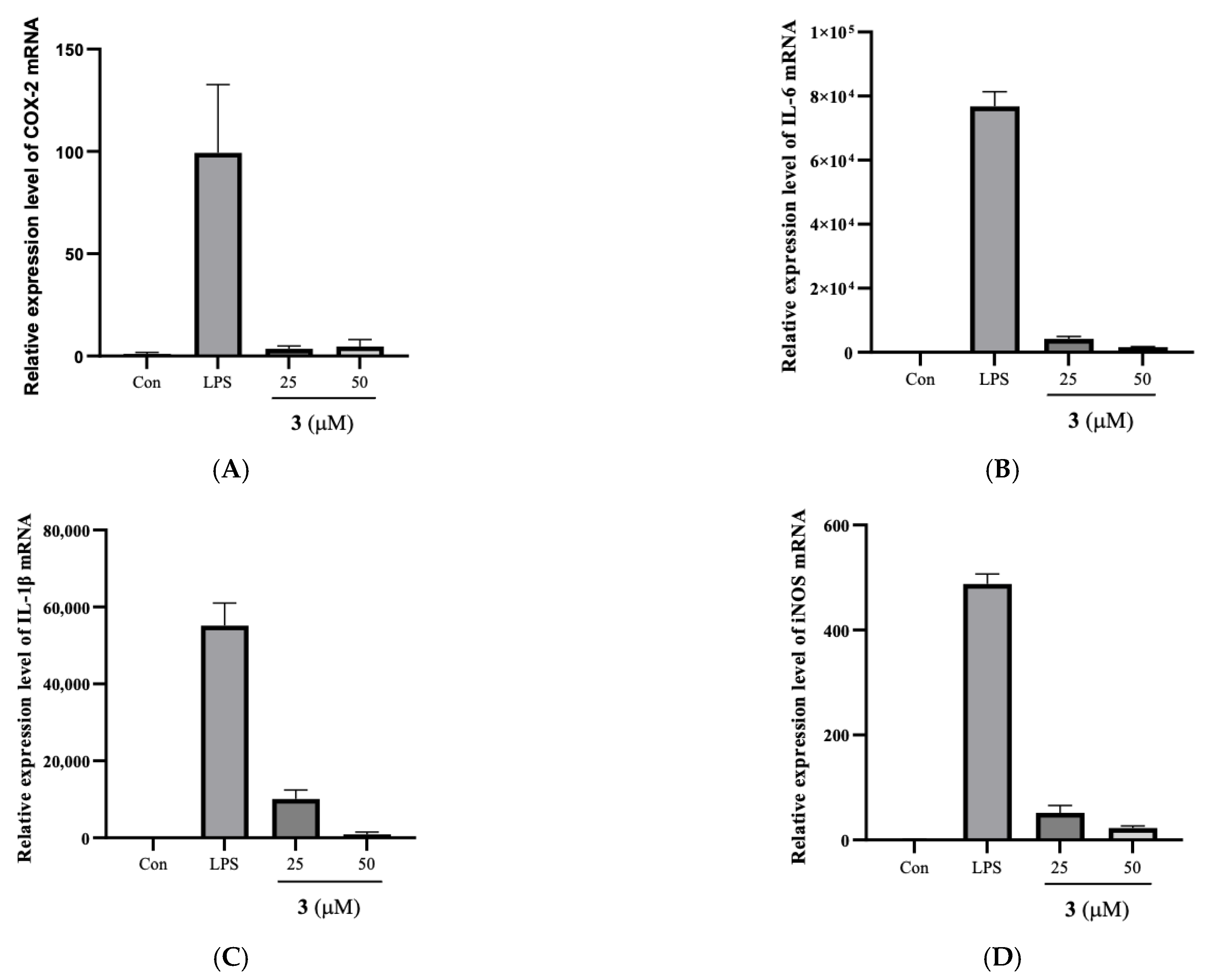
2.3. Compound 3 Reduced LPS-Induced over Expression of COX-2, IL-6, IL-1β, and iNOS
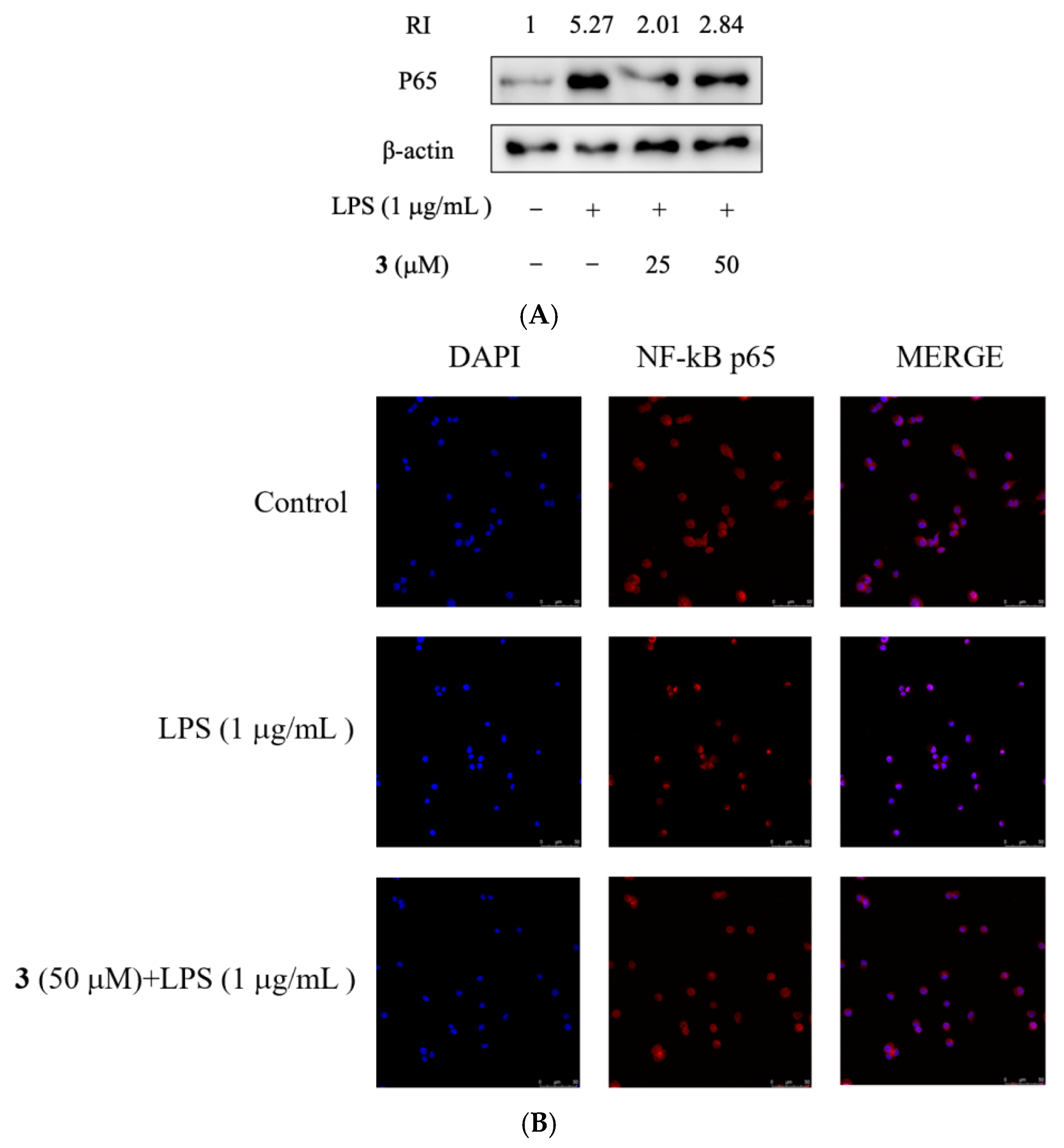
2.4. Effect of 3 on NF-κB Translocation
2.5. Compound 3 Suppressed NF-κB Activity Induced by LPS
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Strain and Fermentation Conditions
3.3. Extraction and Isolation
3.4. Structure Identification
3.4.1. Spectrometric Analyses
3.4.2. Penazaphilone J (1)
3.4.3. Penazaphilone K (2)
3.4.4. Penazaphilone L (3)
3.5. Anti-Inflammatory Activity Assays
3.5.1. Cell Line and Cell Culture
3.5.2. Cell Viability Assay
3.5.3. Measurement of Nitric Oxide (NO)
3.5.4. Quantitative RT-PCR
3.5.5. Western Blot
3.5.6. In Vitro Cell Uptake
3.5.7. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Misiek, M.; Hoffmeister, D. Fungal genetics, genomics, and secondary metabolites in pharmaceutical sciences. Planta Med. 2007, 73, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M.; Snader, K.M. The influence of natural products upon drug discovery. Nat. Prod. Rep. 2000, 17, 215–234. [Google Scholar] [CrossRef] [PubMed]
- Dufosse, L.; Fouillaud, M.; Caro, Y.; Mapari, S.A.S.; Sutthiwong, N. Filamentous fungi are large-scale producers of pigments and colorants for the food industry. Curr. Opin. Biotechnol. 2014, 26, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Lagashetti, A.C.; Dufosse, L.; Singh, S.K.; Singh, P.N. Fungal pigments and their prospects in different industries. Microorganisms 2019, 7, 604. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tian, Y.; Yang, S.X.; Zhang, Y.M.; Qin, J.C. Cytotoxic azaphilone alkaloids from Chaetomium globosum TY1. Bioorg. Med. Chem. Lett. 2013, 23, 2945–2947. [Google Scholar] [CrossRef]
- Shi, T.; Hou, X.-M.; Li, Z.-Y.; Cao, F.; Zhang, Y.-H.; Yu, J.-Y.; Zhao, D.-L.; Shao, C.-L.; Wang, C.-Y. Harzianumnones A and B: Two hydroxyanthraquinones from the coral-derived fungus Trichoderma harzianum. RSC Adv. 2018, 8, 27596–27601. [Google Scholar] [CrossRef]
- Bouhri, Y.; Askun, T.; Tunca, B.; Deniz, G.; Aksoy, S.A.; Mutlu, M. The orange-red pigment from Penicillium mallochii: Pigment production, optimization, and pigment efficacy against Glioblastoma cell lines. Biocatal. Agric. Biotechnol. 2020, 23, 101451. [Google Scholar] [CrossRef]
- Chen, M.; Shen, N.X.; Chen, Z.Q.; Zhang, F.M.; Chen, Y. Penicilones A–D, anti-MRSA azaphilones from the marine-derived fungus Penicillium janthinellum HK1-6. J. Nat. Prod. 2017, 80, 1081–1086. [Google Scholar] [CrossRef]
- Hsu, L.C.; Liang, Y.H.; Hsu, Y.W.; Kuo, Y.H.; Pan, T.M. Anti-inflammatory properties of yellow and orange pigments from Monascus purpureus NTU 568. J. Agric. Food Chem. 2013, 61, 2796–2802. [Google Scholar] [CrossRef]
- Hsu, Y.W.; Hsu, L.C.; Liang, Y.H.; Kuo, Y.H.; Pan, T.M. New bioactive orange pigments with yellow fluorescence from Monascus-fermented dioscorea. J. Agric. Food Chem. 2011, 59, 4512–4518. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, H.; Xue, D. Enhancement of antioxidant activity of Radix Puerariae and red yeast rice by mixed fermentation with Monascus purpureus. Food Chem. 2017, 226, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, C.; Tian, W.; Zhang, H.; Li, P.; Wang, J.; He, W. Theoretical and experimental investigation of the antioxidative activity of monascin. Food Funct. 2020, 11, 5915–5923. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.M.; Yang, S.X.; Qin, J.C. Azaphilones: Chemistry and Biology. Chem. Rev. 2013, 113, 4755–4811. [Google Scholar] [CrossRef] [PubMed]
- Mapari, S.A.S.; Thrane, U.; Meyer, A.S. Fungal polyketide azaphilone pigments as future natural food colorants? Trends Biotechnol. 2010, 28, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.R.; Sena, J.G.; Hoover, A.R.; King, J.B.; Ellis, T.K.; Powell, D.R.; Cichewicz, R.H. Chemical epigenetics alters the secondary metabolite composition of guttate excreted by an atlantic-forest-soil-derived Penicillium citreonigrum. J. Nat. Prod. 2010, 73, 942–948. [Google Scholar] [CrossRef]
- Kono, Y.; Takeuchi, S.; Daly, J.M. Isolation and structure of a new victoxinine derivative produced by Helminthosporium victoriae. Agric. Biol. Chem. 1983, 47, 2701–2702. [Google Scholar] [CrossRef]
- Tang, J.; Zhou, Z.; Yang, T.; Yao, C.; Wu, L.; Li, G. Azaphilone alkaloids with anti-inflammatory activity from fungus Penicillium sclerotiorum cib-411. J. Agric. Food Chem. 2019, 67, 2175–2182. [Google Scholar] [CrossRef]
- Bringmann, G.; Lang, G.; Steffens, S.; Günther, E.; Schaumann, K. Evariquinone, isoemericellin, and stromemycin from a sponge derived strain of the fungus Emericella variecolor. Phytochemistry 2003, 63, 437–443. [Google Scholar] [CrossRef]
- Xu, J.; Kjer, J.; Sendker, J.; Wray, V.; Guan, H.; Edrada, R.; Müller, E.G.W.; Bayer, M.; Lin, W.; Wu, J.; et al. Cytosporones, coumarins, and an alkaloid from the endophytic fungus Pestalotiopsis sp. isolated from the Chinese mangrove plant Rhizophora mucronata. Bioorganic Med. Chem. 2009, 17, 7362–7367. [Google Scholar] [CrossRef]
- Proksa, B.; Uhrín, D.; Fuska, J.; Micháková, E. (−)-Mitorubrinol and phthaldehydic acids, new metabolites of Penicillium vermiculatum DANG. Collect. Czechoslov. Chem. Commun. 1992, 57, 408–414. [Google Scholar] [CrossRef]
- Ayer, W.A.; Racok, J.S. The metabolites of Talaromyces flavus: Part 1. Metabolites of the organic extract. Can. J. Chem. 1990, 68, 2085–2094. [Google Scholar] [CrossRef]
- Ning, F.; Liu, H.; Lash, G.E. The role of decidual macrophages during normal and pathological pregnancy. Am. J. Reprod. Immunol. 2016, 75, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Plowden, J.; Renshaw-Hoelscher, M.; Engleman, C.; Katz, J.; Sambhara, S. Innate immunity in aging: Impact on macrophage function. Aging Cell 2004, 3, 161–167. [Google Scholar] [CrossRef]
- An, J.; Chen, B.; Kang, X.; Zhang, R.; Guo, Y.; Zhao, J.; Yang, H. Neuroprotective effects of natural compounds on LPS-induced inflammatory responses in microglia. Am. J. Transl. Res. 2020, 12, 2353–2368. [Google Scholar] [PubMed]
- Huang, P.; Hong, J.; Mi, J.; Sun, B.; Zhang, J.; Li, C.; Yang, W. Polyphenols extracted from Enteromorpha clathrata alleviates inflammation in lipopolysaccharide-induced RAW 264.7 cells by inhibiting the MAPKs/NF-κB signaling pathways. J. Ethnopharmacol. 2022, 286, 114897. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Ahsan, H.; Zia, M.K.; Siddiqui, T.; Khan, F.H. Understanding oxidants and antioxidants: Classical team with new players. J. Food Biochem. 2020, 44, e13145. [Google Scholar] [CrossRef]
- Qu, X.; Tang, Y.; Hua, S. Immunological approaches towards cancer and inflammation: A cross talk. Front. Immunol. 2018, 9, 563. [Google Scholar] [CrossRef]
- Rendra, E.; Riabov, V.; Mossel, D.M.; Sevastyanova, T.; Harmsen, M.C.; Kzhyshkowska, J. Reactive oxygen species (ROS) in macrophage activation and function in diabetes. Immunobiology 2019, 224, 242–253. [Google Scholar] [CrossRef]
- Papi, S.; Ahmadizar, F.; Hasanvand, A. The role of nitric oxide in inflammation and oxidative stress. Immunopathol. Persa 2019, 5, e08. [Google Scholar] [CrossRef]
- Saini, R.; Singh, S. Inducible nitric oxide synthase: An asset to neutrophils. J. Leukoc. Biol. 2019, 105, 49–61. [Google Scholar] [CrossRef]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Effect of high-fat diets on oxidative stress, cellular inflammatory response and cognitive function. Nutrients 2019, 11, 2579. [Google Scholar] [CrossRef] [PubMed]
- Mazzocchi, A.; De Cosmi, V.; Rise, P.; Milani, G.P.; Turolo, S.; Syren, M.-L.; Sala, A.; Agostoni, C. Bioactive compounds in edible oils and their role in oxidative stress and inflammation. Front. Physiol. 2021, 12, 659551. [Google Scholar] [CrossRef]
- Ren, D.; Wang, P.; Liu, C.; Wang, J.; Liu, X.; Liu, J.; Min, W. Hazelnut protein-derived peptide LDAPGHR shows anti-inflammatory activity on LPS-induced RAW264.7 macrophage. J. Funct. Foods 2018, 46, 449–455. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, S.; Cheng, X.; Lu, C.; Tao, W.; Zhang, Y.; William, B.C.; Cao, X.; Yi, S.; Liu, Y.; et al. Euphorbia factor L2 alleviates lipopolysaccharide-induced acute lung injury and inflammation in mice through the suppression of NF-κB activation. Biochem. Pharmacol. 2018, 155, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. Toll-like receptors in the pathogenesis of neuroinflammation. J. Neuroimmunol. 2019, 332, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Guo, S.; Yang, C.; Yang, J.; Chen, Y.; Shaukat, A.; Zhao, G.; Wu, H.; Deng, G. Barbaloin protects against lipopolysaccharide (LPS)-induced acute lung injury by inhibiting the ROS-mediated PI3K/AKT/NF-κB pathway. Int. Immunopharmacol. 2018, 64, 140–150. [Google Scholar] [CrossRef]
- Meng, L.; Li, L.; Lu, S.; Li, K.; Su, Z.; Wang, Y.; Fan, X.; Li, X.; Zhao, G. The protective effect of dexmedetomidine on LPS-induced acute lung injury through the HMGB1-mediated TLR4/NF-κB and PI3K/Akt/mTOR pathways. Mol. Immunol. 2018, 94, 7–17. [Google Scholar] [CrossRef]
- Omura, S.; Tanaka, H.; Matsuzaki, K.; Ikeda, H.; Masuma, R. Isochromophilones I and II, novel inhibitors against gp120-CD4 binding from Penicillium sp. J. Antibiot. 1993, 46, 1908–1911. [Google Scholar] [CrossRef]
- Nam, J.Y.; Kim, H.K.; Kwon, J.Y.; Han, M.Y.; Son, K.H.; Lee, U.C.; Choi, J.D.; Kwon, B.M. 8-O-Methylsclerotiorinamine, antagonist of the Grb2-SH2 domain, isolated from Penicillium multicolor. J. Nat. Prod. 2000, 63, 1303–1305. [Google Scholar] [CrossRef]
- Lucas, E.M.F.; de Castro, M.C.M.; Takahashi, J.A. Antimicrobial properties of sclerotiorin, isocHromophilone VI and pencolide, metabolites from a Brazilian cerrado isolate of Penicillium sclerotiorum van Beyma. Braz. J. Microbiol. 2007, 38, 785–789. [Google Scholar] [CrossRef]
- Chidananda, C.; Sattur, A.P. Sclerotiorin, a novel inhibitor of lipoxygenase from Penicillium frequentans. J. Agric. Food Chem. 2007, 55, 2879–2883. [Google Scholar] [CrossRef] [PubMed]
- Arai, N.; Shiomi, K.; Tomoda, H.; Tabata, N.; Yang, D.J.; Masuma, R.; Kawakubo, T.; Omura, S. Isochromophilones III–VI, inhibitors of acyl-CoA: Cholesterol acyltransferase produced by Penicillium multicolor FO-3216. J. Antibiot. 1995, 48, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; He, Y.; Zhou, J.; Shen, T.; Hu, W. Immunomodulatory effects of wheat bran arabinoxylan on RAW264.7 macrophages via the NF-κB signaling pathway using RNA-seq analysis. Food Res. Int. 2021, 140, 110067. [Google Scholar] [CrossRef]

| No. | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | |
| 1 | 8.24 (1H, s) | 144.6 | 8.19 (1H, s) | 143.7 | 8.21 (1H, s) | 143.7 |
| 3 | 148.3 | 148.2 | 148.1 | |||
| 4 | 7.18 (1H, s) | 112.6 | 7.18 (1H, s) | 112.6 | 7.19 (1H, s) | 112.6 |
| 4a | 151.9 | 151.3 | 151.2 | |||
| 5 | 101.3 | 101.3 | 101.4 | |||
| 6 | 185.6 | 185.5 | 185.6 | |||
| 7 | 86.2 | 86.2 | 86.2 | |||
| 8 | 194.9 | 195.0 | 195.0 | |||
| 8a | 116.4 | 116.6 | 116.6 | |||
| 9 | 6.58 (1H, d, 15.5) | 117.1 | 6.47 (1H, d, 15.5) | 116.6 | 6.48 (1H, d, 15.3) | 116.4 |
| 10 | 7.11 (1H, d, 15.4) | 146.8 | 7.13 (1H, d, 15.4) | 146.6 | 7.15 (1H, d, 15.4) | 146.8 |
| 11 | 134.0 | 133.8 | 133.9 | |||
| 12 | 5.78 (1H, d, 9.7) | 148.8 | 5.80 (1H, d, 9.7) | 149.1 | 5.81 (1H, d, 9.8) | 149.1 |
| 13 | 2.54 (1H, m) | 36.2 | 2.55 (1H, m) | 36.2 | 2.55 (1H, m) | 36.2 |
| 14 | 1.45 (1H, m) 1.37 (1H, m) | 31.1 | 1.46 (1H, m) 1.36 (1H, m) | 31.1 | 1.46 (1H, m) 1.38 (1H, m) | 31.1 |
| 15 | 0.90 (3H, t, 7.4) | 12.4 | 0.90 (3H, t, 7.4) | 12.3 | 0.90 (3H, t, 7.3) | 12.4 |
| 16 | 1.03 (3H, d, 6.6) | 20.6 | 1.04 (3H, d, 6.6) | 20.6 | 1.04 (3H, d, 6.6) | 20.5 |
| 17 | 1.94 (3H, s) | 12.8 | 1.93 (3H, s) | 12.8 | 1.95 (3H, s) | 12.8 |
| 18 | 1.52 (3H, s) | 23.8 | 1.50 (3H, s) | 23.8 | 1.50 (3H, s) | 23.8 |
| 19 | 171.6 | 171.6 | 171.6 | |||
| 20 | 2.12 (3H, s) | 20.2 | 2.12 (3H, s) | 20.2 | 2.12 (3H, s) | 20.2 |
| 1′ | 4.42 (2H, m) | 55.6 | 8.11 (1H, s) | 164.8 | 8.09 (1H, s) | 164.7 |
| 2′ | 4.16 (2H, m) | 64.5 | 3.67 (1H, m) | 55.2 | 3.68 (1H, m) | 55.0 |
| 3′ | 3.79 (2H, m) | 67.8 | 1.83 (2H, m) | 31.6 | 1.35 (1H, m) 1.29 (1H, m) | 28.8 |
| 4′ | 3.71 (1H, m) | 72.5 | 1.46 (1H, m) 1.36 (1H, m) | 23.0 | 1.88 (2H, m) | 27.1 |
| 5′ | 3.52 (2H, m) | 63.7 | 1.83 (2H, m) | 30.6 | 4.21 (2H, m) | 54.9 |
| 6′ | 4.16 (2H, m) | 55.4 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Hu, Y.; Yang, T.; Qian, X.; Hu, W.; Li, G. Penazaphilones J–L, Three New Hydrophilic Azaphilone Pigments from Penicillium sclerotiorum cib-411 and Their Anti-Inflammatory Activity. Molecules 2023, 28, 3146. https://doi.org/10.3390/molecules28073146
Zhang X, Hu Y, Yang T, Qian X, Hu W, Li G. Penazaphilones J–L, Three New Hydrophilic Azaphilone Pigments from Penicillium sclerotiorum cib-411 and Their Anti-Inflammatory Activity. Molecules. 2023; 28(7):3146. https://doi.org/10.3390/molecules28073146
Chicago/Turabian StyleZhang, Xia, Yeye Hu, Tao Yang, Xueqing Qian, Weicheng Hu, and Guoyou Li. 2023. "Penazaphilones J–L, Three New Hydrophilic Azaphilone Pigments from Penicillium sclerotiorum cib-411 and Their Anti-Inflammatory Activity" Molecules 28, no. 7: 3146. https://doi.org/10.3390/molecules28073146
APA StyleZhang, X., Hu, Y., Yang, T., Qian, X., Hu, W., & Li, G. (2023). Penazaphilones J–L, Three New Hydrophilic Azaphilone Pigments from Penicillium sclerotiorum cib-411 and Their Anti-Inflammatory Activity. Molecules, 28(7), 3146. https://doi.org/10.3390/molecules28073146







