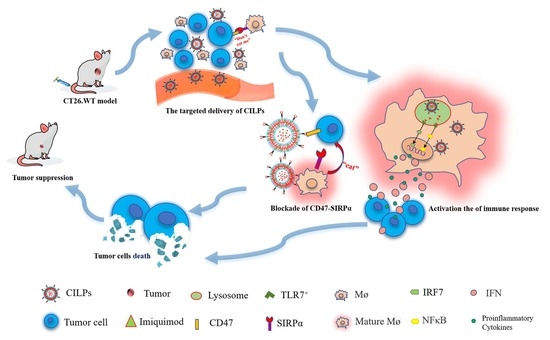
Liposome-Based Co-Immunotherapy with TLR Agonist and CD47-SIRPα Checkpoint Blockade for Efficient Treatment of Colon Cancer
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of LPs (Blank Liposomes), ILPs (Non-CD47-Targeted Imiquimod-Encapsulated Liposomes), CLPs (CD47 Targeted Liposomes), and CILPs (Coupled Fc-CV1 to Imiquimod (TLR7 agonist)-Loaded Liposomes)
2.2. In Vitro Cell Uptake Efficiency
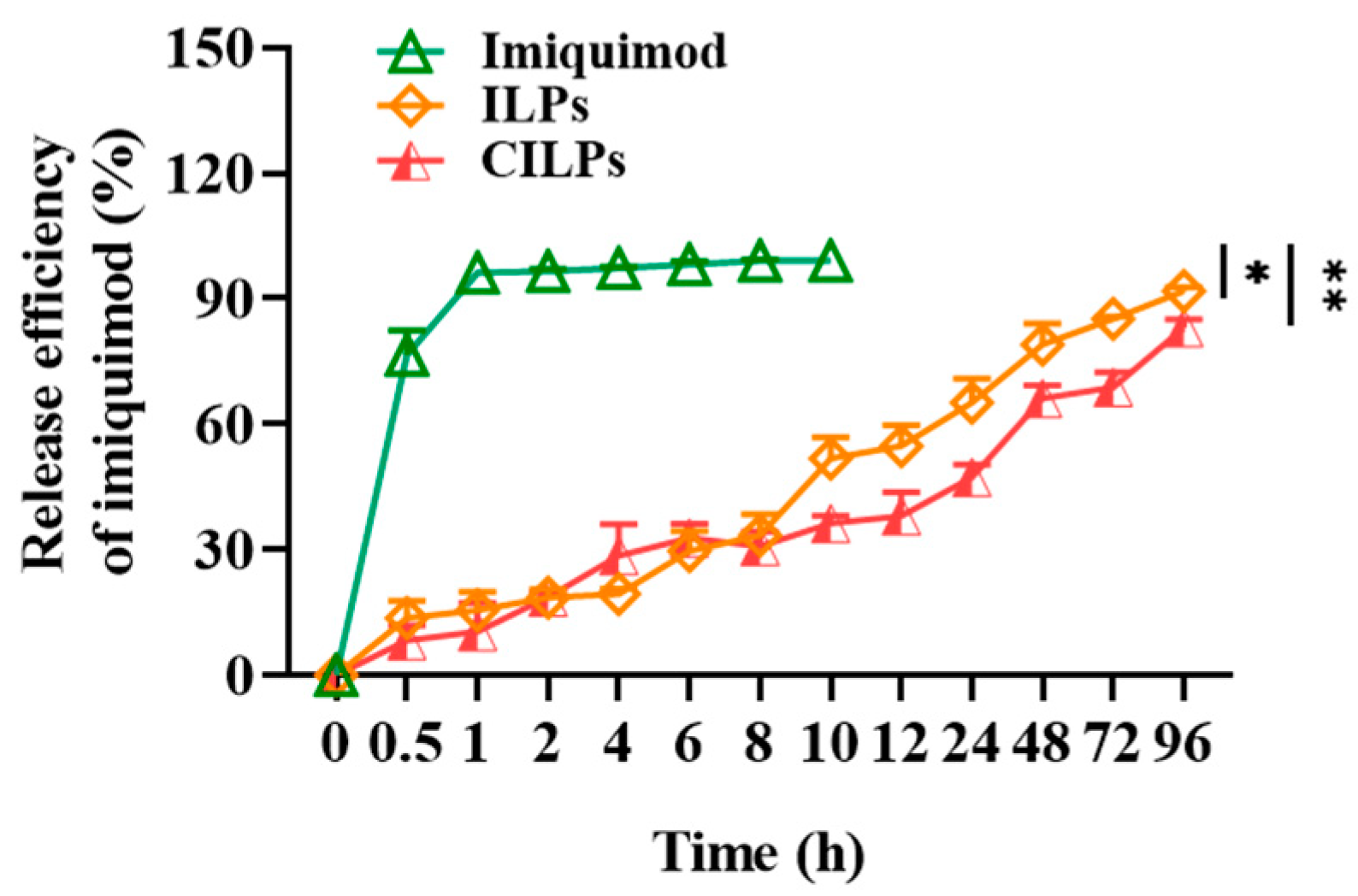
2.3. Release Curves of Liposomes
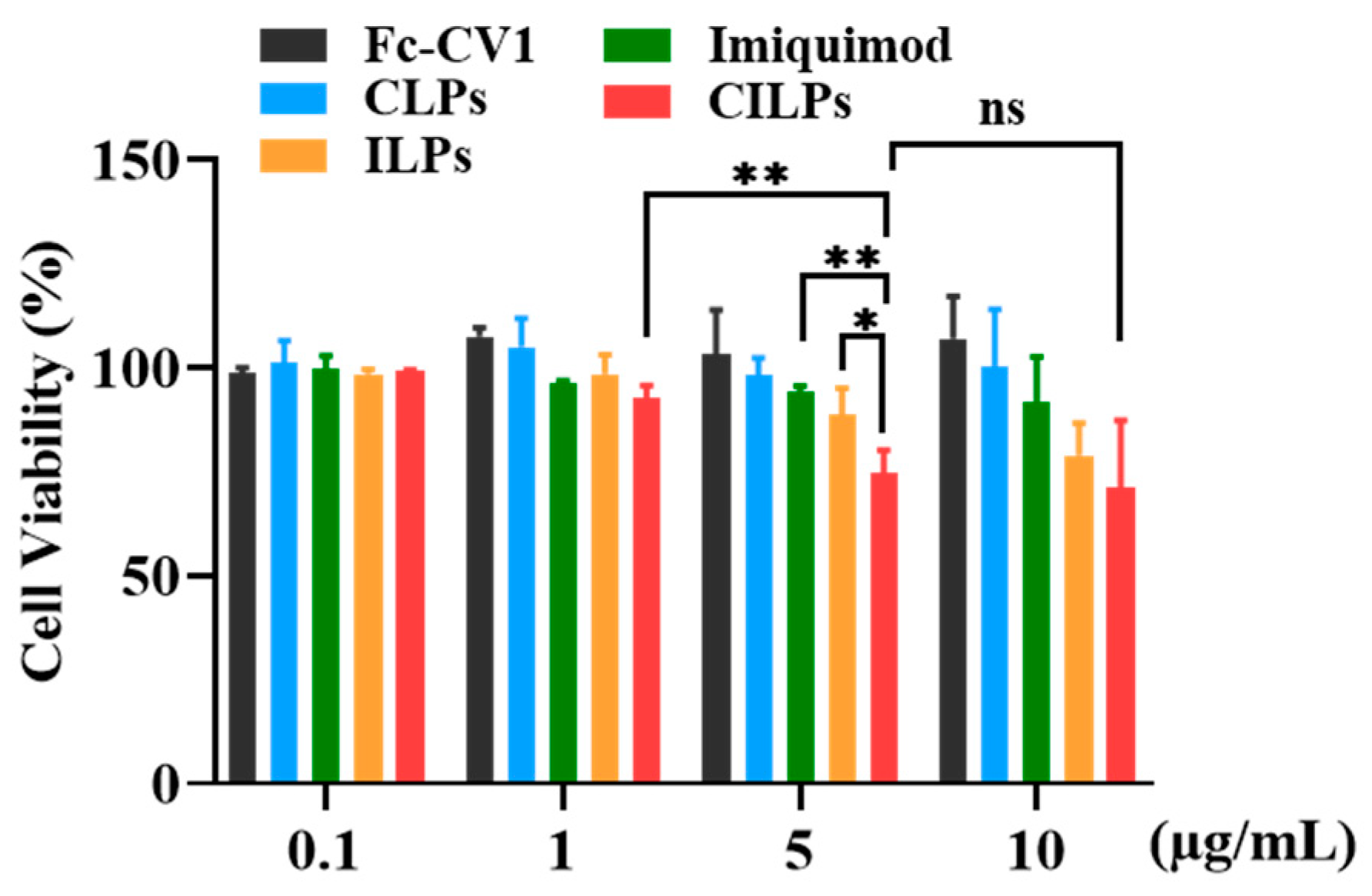
2.4. Cytotoxicity Study
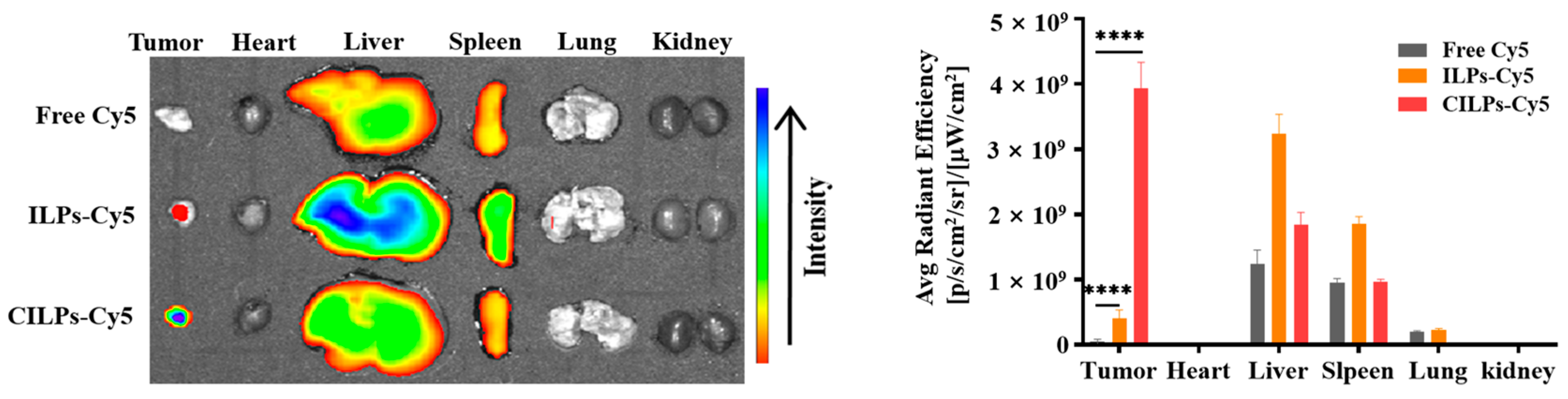
2.5. Biodistribution Study
2.6. In Vivo Tumor Inhibition Study
2.7. CILPs Increased T-Cell Infiltration and Secretion of IFNγ
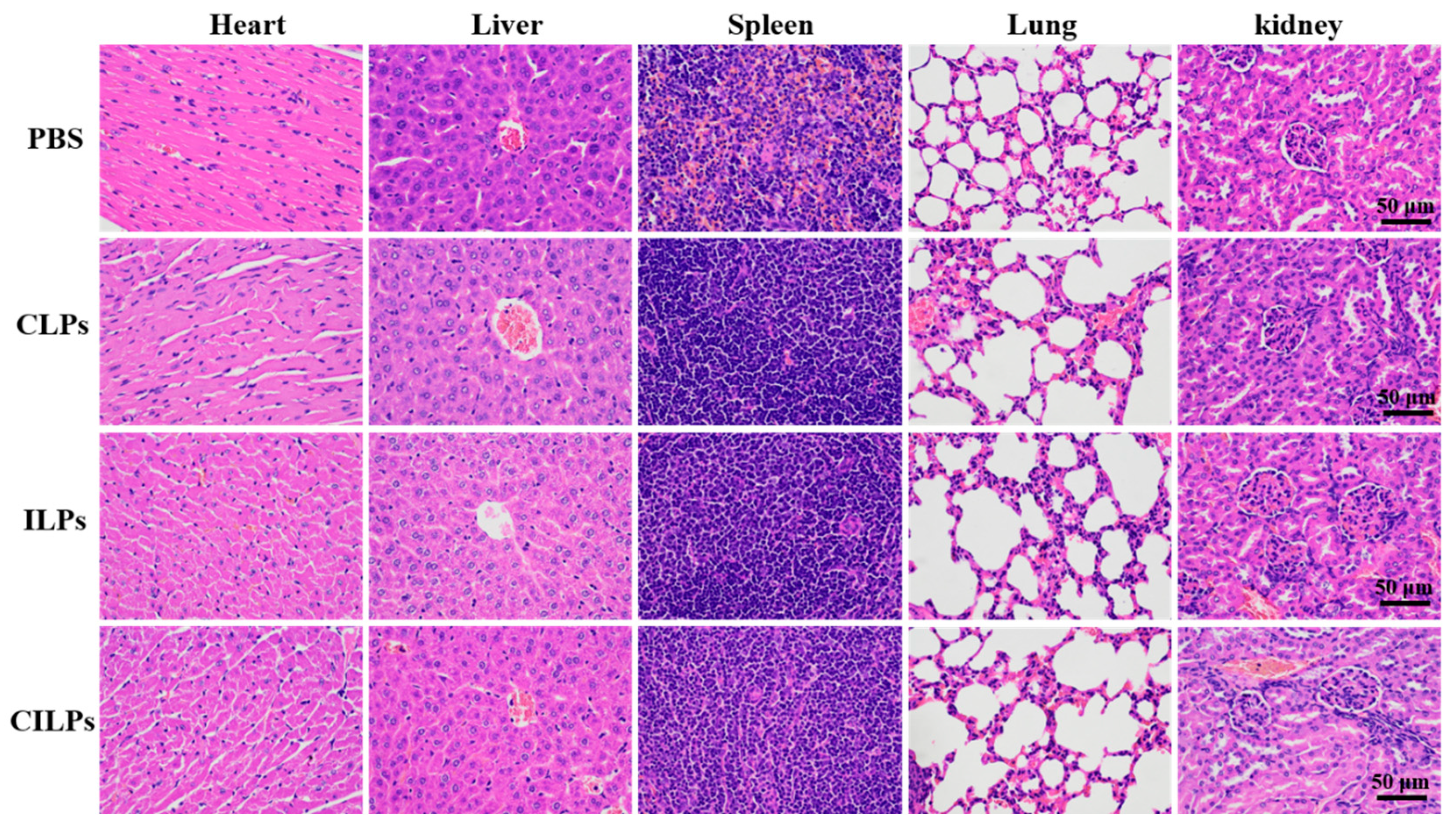
2.8. Biosecurity Assessment of CILPs
3. Materials and Methods
3.1. Materials
3.2. Preparation of Blank Liposomes
3.3. Preparation of CD47 Targeted Imiquimod-Encapsulated Liposomes
3.4. Characterization
3.5. In Vitro Cell Uptake Study
3.6. The Imiquimod Release Study
3.7. The Cytotoxicity of CILPs
3.8. Biodistribution Study
3.9. In Vivo Tumor Inhibition Study
3.10. Immunohistochemistry
3.11. Biosecurity Assessment of CILPs
3.12. Statistical Analysis
3.13. Animal Ethics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Carlson, R.D.; Flickinger, J.C., Jr.; Snook, A.E. Talkin’ Toxins: From Coley’s to Modern Cancer Immunotherapy. Toxins 2020, 12, 241. [Google Scholar] [CrossRef] [PubMed]
- Barbari, C.; Fontaine, T.; Parajuli, P.; Lamichhane, N.; Jakubski, S.; Lamichhane, P.; Deshmukh, R.R. Immunotherapies and Combination Strategies for Immuno-Oncology. Int. J. Mol. Sci. 2020, 21, 5009. [Google Scholar] [CrossRef]
- Hegde, P.S.; Chen, D.S. Top 10 Challenges in Cancer Immunotherapy. Immunity 2020, 52, 17–35. [Google Scholar] [CrossRef]
- Jain, K.K. Personalized Immuno-Oncology. Med. Princ. Pract. 2021, 30, 1–16. [Google Scholar] [CrossRef]
- Sanmamed, M.F.; Chen, L. A Paradigm Shift in Cancer Immunotherapy: From Enhancement to Normalization. Cell 2018, 175, 313–326. [Google Scholar] [CrossRef]
- Hussein, W.M.; Liu, T.Y.; Skwarczynski, M.; Toth, I. Toll-Like Receptor Agonists: A Patent Review (2011–2013). Expert Opin. Ther. Pat. 2014, 24, 453–470. [Google Scholar] [CrossRef]
- Chi, H.; Li, C.; Zhao, F.S.; Zhang, L.; Ng, T.B.; Jin, G.; Sha, O. Anti-Tumor Activity of Toll-Like Receptor 7 Agonists. Front. Pharmacol. 2017, 8, 304. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, S.; Li, H.; Wang, H.; Zhang, T.; Hutchinson, M.R.; Yin, H.; Wang, X. Small-Molecule Modulators of Toll-Like Receptors. Acc. Chem. Res. 2020, 53, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Le Mercier, I.; Poujol, D.; Sanlaville, A.; Sisirak, V.; Gobert, M.; Durand, I.; Dubois, B.; Treilleux, I.; Marvel, J.; Vlach, J.; et al. Tumor Promotion by Intratumoral Plasmacytoid Dendritic Cells Is Reversed by Tlr7 Ligand Treatment. Cancer Res. 2013, 73, 4629–4640. [Google Scholar] [CrossRef]
- Ma, F.; Zhang, J.; Zhang, J.; Zhang, C. The Tlr7 Agonists Imiquimod and Gardiquimod Improve Dc-Based Immunotherapy for Melanoma in Mice. Cell. Mol. Immunol. 2010, 7, 381–388. [Google Scholar] [CrossRef]
- Billan, S.; Kaidar-Person, O.; Gil, Z. Treatment after Progression in the Era of Immunotherapy. Lancet Oncol. 2020, 21, e463–e476. [Google Scholar] [CrossRef] [PubMed]
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune Checkpoint Inhibitors: Recent Progress and Potential Biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Chan, H.L.; Chen, P. Immune Checkpoint Inhibitors: Basics and Challenges. Curr. Med. Chem. 2019, 26, 3009–3025. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Yan, B.; Tian, X.; Liu, Q.; Jin, J.; Shi, J.; Hou, Y. Cd47/Sirpalpha Pathway Mediates Cancer Immune Escape and Immunotherapy. Int. J. Biol. Sci. 2021, 17, 3281–3287. [Google Scholar] [CrossRef]
- Swoboda, D.M.; Sallman, D.A. The Promise of Macrophage Directed Checkpoint Inhibitors in Myeloid Malignancies. Best Practice and Research. Clin. Haematol. 2020, 33, 101221. [Google Scholar]
- Lentz, R.W.; Colton, M.D.; Mitra, S.S.; Messersmith, W.A. Innate Immune Checkpoint Inhibitors: The Next Breakthrough in Medical Oncology? Mol. Cancer Ther. 2021, 20, 961–974. [Google Scholar] [CrossRef]
- Jiang, P.; Lagenaur, C.F.; Narayanan, V. Integrin-Associated Protein Is a Ligand for the P84 Neural Adhesion Molecule. J. Biol. Chem. 1999, 274, 559–562. [Google Scholar] [CrossRef]
- Seiffert, M.; Cant, C.; Chen, Z.; Rappold, I.; Brugger, W.; Kanz, L.; Brown, E.J.; Ullrich, A.; Buhring, H.J. Human Signal-Regulatory Protein Is Expressed on Normal, but Not on Subsets of Leukemic Myeloid Cells and Mediates Cellular Adhesion Involving Its Counterreceptor Cd47. Blood 1999, 94, 3633–3643. [Google Scholar] [CrossRef]
- Matlung, H.L.; Szilagyi, K.; Barclay, N.A.; van den Berg, T.K. The Cd47-Sirpalpha Signaling Axis as an Innate Immune Checkpoint in Cancer. Immunol. Rev. 2017, 276, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Oldenborg, P.A.; Zheleznyak, A.; Fang, Y.F.; Lagenaur, C.F.; Gresham, H.D.; Lindberg, F.P. Role of Cd47 as a Marker of Self on Red Blood Cells. Science 2000, 288, 2051–2054. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Gao, J.; Fu, Y.; Hua, P.; Jing, Y.; Cai, M.; Wang, H.; Tong, T. The Role of Cd47-Sirpalpha Immune Checkpoint in Tumor Immune Evasion and Innate Immunotherapy. Life Sci. 2021, 273, 119150. [Google Scholar] [CrossRef]
- Hayat, S.M.G.; Bianconi, V.; Pirro, M.; Jaafari, M.R.; Hatamipour, M.; Sahebkar, A. Cd47: Role in the Immune System and Application to Cancer Therapy. Cell. Oncol. 2020, 43, 19–30. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, Y.; Gao, P.; Yao, Z. Targeting Cd47: The Achievements and Concerns of Current Studies on Cancer Immunotherapy. J. Thorac. Dis. 2017, 9, E168–E174. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, Q.; Xiao, W.; Zhao, Y.; Pi, J.; Xu, H.; Zhao, H.; Xu, J.; Evans, C.E.; Jin, H. Advances in Anti-Tumor Treatments Targeting the Cd47/Sirpalpha Axis. Front. Immunol. 2020, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Pu, Y.; Cron, K.; Deng, L.; Kline, J.; Frazier, W.A.; Xu, H.; Peng, H.; Fu, Y.X.; Xu, M.M. Cd47 Blockade Triggers T Cell-Mediated Destruction of Immunogenic Tumors. Nat. Med. 2015, 21, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Weiskopf, K.; Ring, A.M.; Ho, C.C.; Volkmer, J.P.; Levin, A.M.; Volkmer, A.K.; Ozkan, E.; Fernhoff, N.B.; van de Rijn, M.; Weissman, I.L.; et al. Engineered Sirpalpha Variants as Immunotherapeutic Adjuvants to Anticancer Antibodies. Science 2013, 341, 88–91. [Google Scholar] [CrossRef]
- Xiong, Z.; Ohlfest, J.R. Topical Imiquimod Has Therapeutic and Immunomodulatory Effects against Intracranial Tumors. J. Immunother. 2011, 34, 264–269. [Google Scholar] [CrossRef]
- Kamath, P.; Darwin, E.; Arora, H.; Nouri, K. A Review on Imiquimod Therapy and Discussion on Optimal Management of Basal Cell Carcinomas. Clin. Drug Investig. 2018, 38, 883–899. [Google Scholar] [CrossRef]
- Liu, Y.; Castro Bravo, K.M.; Liu, J. Targeted Liposomal Drug Delivery: A Nanoscience and Biophysical Perspective. Nanoscale Horiz. 2021, 6, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Farjadian, F.; Ghasemi, A.; Gohari, O.; Roointan, A.; Karimi, M.; Hamblin, M.R. Nanopharmaceuticals and Nanomedicines Currently on the Market: Challenges and Opportunities. Nanomedicine 2019, 14, 93–126. [Google Scholar] [CrossRef]
- Zhou, X.; Jiao, L.; Qian, Y.; Dong, Q.; Sun, Y.; Zheng, W.; Zhao, W.; Zhai, W.; Qiu, L.; Wu, Y.; et al. Repositioning Azelnidipine as a Dual Inhibitor Targeting Cd47/Sirpalpha and Tigit/Pvr Pathways for Cancer Immuno-Therapy. Biomolecules 2021, 11, 706. [Google Scholar] [CrossRef]
- Ni, K.; Luo, T.; Culbert, A.; Kaufmann, M.; Jiang, X.; Lin, W. Nanoscale Metal-Organic Framework Co-Delivers Tlr-7 Agonists and Anti-Cd47 Antibodies to Modulate Macrophages and Orchestrate Cancer Immunotherapy. J. Am. Chem. Soc. 2020, 142, 12579–12584. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, L.; Liang, C.; Wang, C.; Peng, R.; Liu, Z. Photothermal Therapy with Immune-Adjuvant Nanoparticles Together with Checkpoint Blockade for Effective Cancer Immunotherapy. Nat. Commun. 2016, 7, 13193. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Long, Y.; Guo, R.; Liu, X.; Tang, X.; Rao, J.; Yin, S.; Zhang, Z.; Li, M.; He, Q. Multifunctional Polymeric Micelle-Based Chemo-Immunotherapy with Immune Checkpoint Blockade for Efficient Treatment of Orthotopic and Metastatic Breast Cancer. Acta Pharm. Sin. B 2019, 9, 819–831. [Google Scholar] [CrossRef]
- Huang, S.W.; Wang, S.T.; Chang, S.H.; Chuang, K.C.; Wang, H.Y.; Kao, J.K.; Liang, S.M.; Wu, C.Y.; Kao, S.H.; Chen, Y.J.; et al. Imiquimod Exerts Antitumor Effects by Inducing Immunogenic Cell Death and Is Enhanced by the Glycolytic Inhibitor 2-Deoxyglucose. J. Investig. Dermatol. 2020, 140, 1771–1783.e6. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; He, X.; Zhang, Z.; Zhao, Y.; Feng, W. Metabolism of Nanomaterials in Vivo: Blood Circulation and Organ Clearance. Acc. Chem. Res. 2013, 46, 761–769. [Google Scholar] [CrossRef]
- Akhtari, J.; Rezayat, S.M.; Teymouri, M.; Alavizadeh, S.H.; Gheybi, F.; Badiee, A.; Jaafari, M.R. Targeting, Bio Distributive and Tumor Growth Inhibiting Characterization of Anti-Her2 Affibody Coupling to Liposomal Doxorubicin Using Balb/C Mice Bearing Tubo Tumors. Int. J. Pharm. 2016, 505, 89–95. [Google Scholar] [CrossRef]
- Woodle, M.C.; Papahadjopoulos, D. Liposome Preparation and Size Characterization. Methods Enzymol. 1989, 171, 193–217. [Google Scholar] [PubMed]
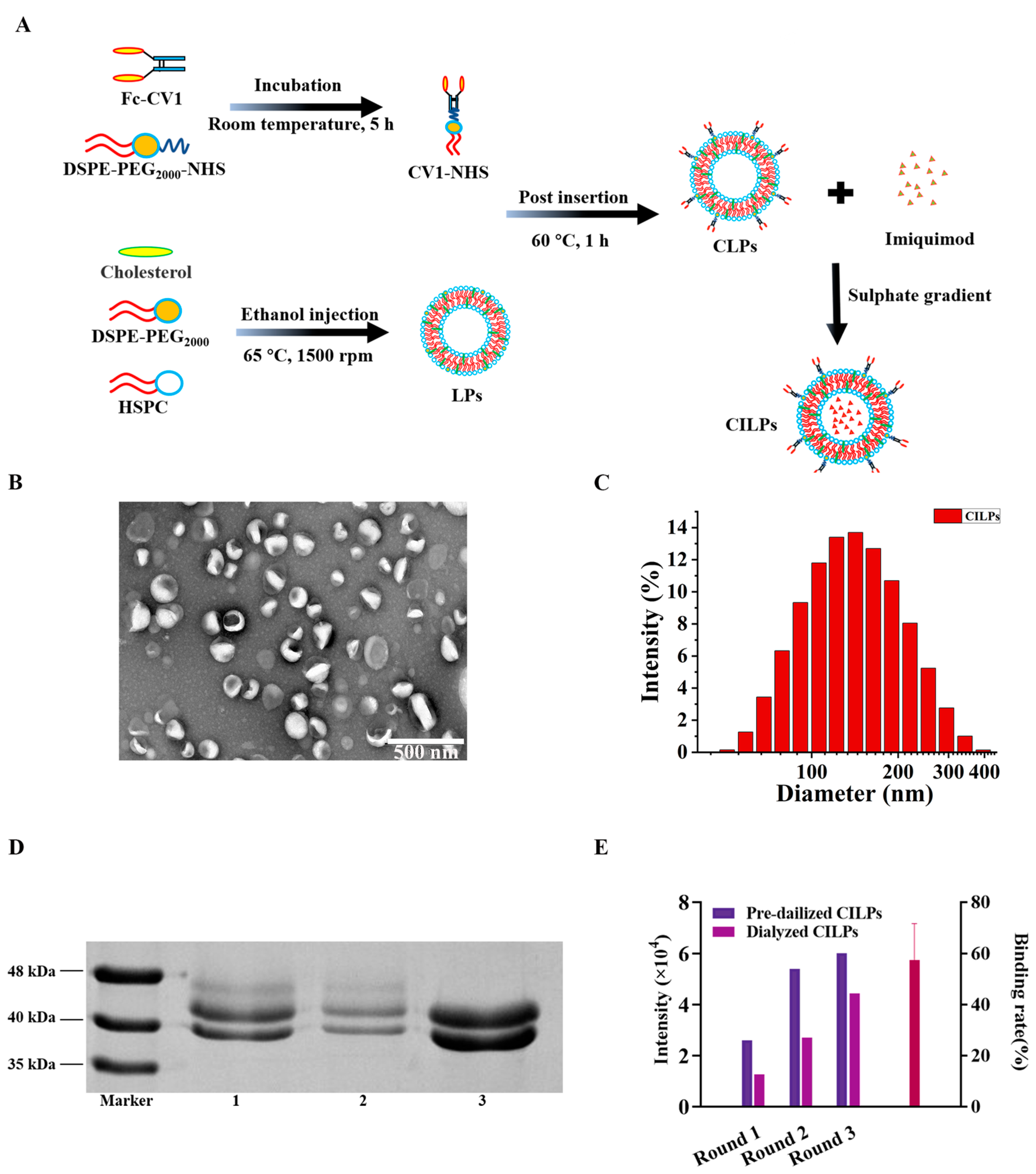



| Sample | Diameters (nm) | PDI | Zeta-Potential (mV) | EE (%) |
|---|---|---|---|---|
| LPs | 111.567 ± 0.655 | 0.095 ± 0.005 | −2.231 ± 0.167 | / |
| CLPs | 117.467 ± 0.340 | 0.120 ± 0.018 | −2.492 ± 0.033 | / |
| ILPs | 120.233 ± 0.776 | 0.125 ± 0.005 | −2.383 ± 0.070 | 81.205 ± 8.102 |
| CILPs | 130.033 ± 0.974 | 0.144 ± 0.011 | −2.075 ± 0.070 | 85.443 ± 4.108 |
| Group | Tumor Weight (g) | TGI |
|---|---|---|
| PBS | 1.246 ± 0.335 | \ |
| LPs | 0.926 ± 0.150 | 28.319% |
| imiquimod | 0.697 ± 0.212 | 44.061% |
| Fc-CV1 | 0.799 ± 0.192 | 35.875% |
| ILPs | 0.643 ± 0.282 | 48.395% |
| CLPs | 0.846 ± 0.252 | 32.103% |
| CILPs | 0.195 ± 0.054 | 84.350% |
| Groups | ALT (10–96 U/L) | AST (36–235 U/L) | BUN (10–35 mg/dL) | CREA (11–85 umol/L) |
|---|---|---|---|---|
| PBS | 46.75 ± 1.75 | 262.42 ± 12.42 | 5.42 ± 0.42 | 51.34 ± 1.34 |
| ILPs | 52.44 ± 2.44 | 209.58 ± 9.58 | 7.06 ± 1.06 | 46.10 ± 1.10 |
| CLPs | 78.27 ± 3.27 | 208.49 ± 8.49 | 23.12 ± 3.12 | 39.57 ± 1.57 |
| CILPs | 53.36 ± 3.36 | 232.91 ± 12.91 | 8.00 ± 1.00 | 44.87 ± 4.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, R.; Chu, X.; Zhang, J.; Fu, R.; Feng, C.; Jia, D.; Wang, R.; Yan, H.; Li, G.; Li, J. Liposome-Based Co-Immunotherapy with TLR Agonist and CD47-SIRPα Checkpoint Blockade for Efficient Treatment of Colon Cancer. Molecules 2023, 28, 3147. https://doi.org/10.3390/molecules28073147
Chang R, Chu X, Zhang J, Fu R, Feng C, Jia D, Wang R, Yan H, Li G, Li J. Liposome-Based Co-Immunotherapy with TLR Agonist and CD47-SIRPα Checkpoint Blockade for Efficient Treatment of Colon Cancer. Molecules. 2023; 28(7):3147. https://doi.org/10.3390/molecules28073147
Chicago/Turabian StyleChang, Rui, Xiaohong Chu, Jibing Zhang, Rongrong Fu, Changshun Feng, Dianlong Jia, Rui Wang, Hui Yan, Guangyong Li, and Jun Li. 2023. "Liposome-Based Co-Immunotherapy with TLR Agonist and CD47-SIRPα Checkpoint Blockade for Efficient Treatment of Colon Cancer" Molecules 28, no. 7: 3147. https://doi.org/10.3390/molecules28073147
APA StyleChang, R., Chu, X., Zhang, J., Fu, R., Feng, C., Jia, D., Wang, R., Yan, H., Li, G., & Li, J. (2023). Liposome-Based Co-Immunotherapy with TLR Agonist and CD47-SIRPα Checkpoint Blockade for Efficient Treatment of Colon Cancer. Molecules, 28(7), 3147. https://doi.org/10.3390/molecules28073147