Design, Synthesis, and Biological Activities of Novel 2-Cyanoacrylate Compounds Containing Substituted Pyrazolyl or 1,2,3-Triazolyl Moiety
Abstract
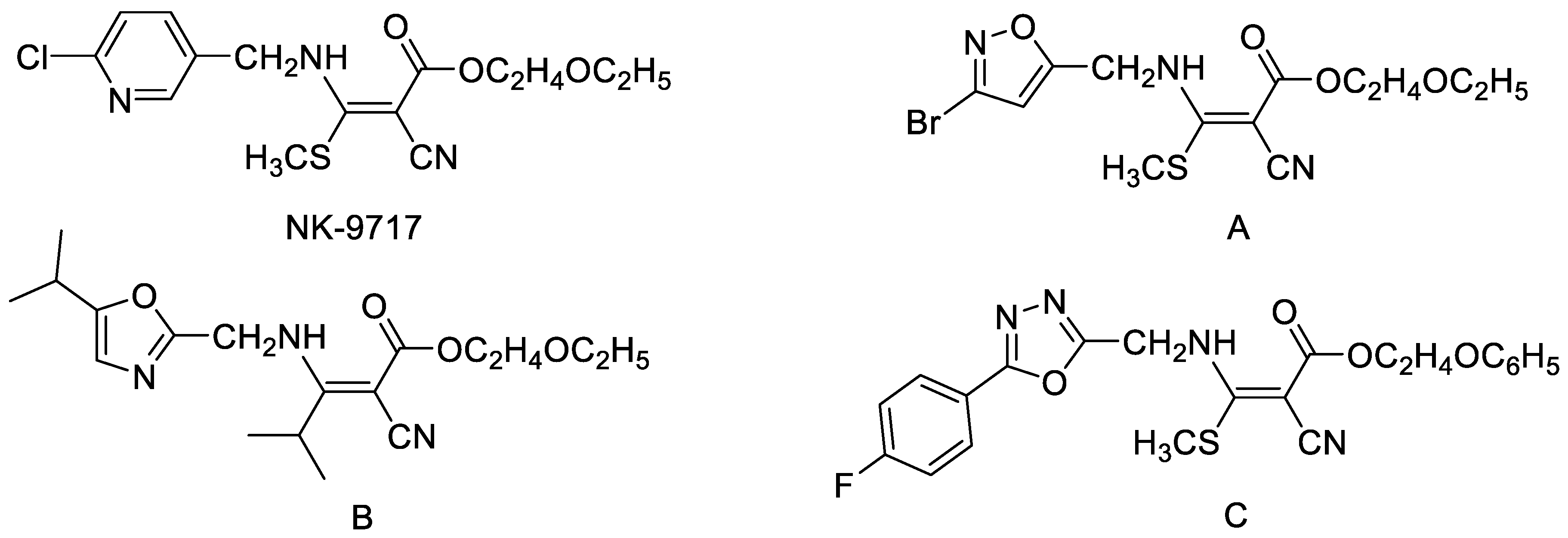
1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biological Activities
3. Materials and Methods
3.1. Chemistry
3.1.1. General Approach to Preparation of 4
3.1.2. General Approach to Preparation of 5
3.1.3. General Approach to Preparation of Title Compounds 9a–9i
3.1.4. General Approach to Preparation of Title Compounds 10a–10o
3.2. Biological Activity Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jia, H.W.; Guo, W.; Li, W.; Li, T.F.; Chen, X.L.; Li, Z.; Xu, X.Y. Design, synthesis and nematicidal activities of novel 1,3-thiazin(thiazol)-4-one derivatives against Meloidogyne incognita. J. Chem. Res. 2019, 43, 161–169. [Google Scholar] [CrossRef]
- Chen, J.X.; Li, Q.X.; Song, B.A. Chemical nematicides: Recent research progress and outlook. J. Agric. Food Chem. 2020, 68, 12175–12188. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.H.; Wang, C.F.; Song, Q.X.; Fan, M.L.; Liu, B.Y.; Wei, D.M.; Liu, J.B. Synthesis and herbicidal activities of 2-ethoxyethyl-2-cyano-3-(substituted)acrylates. Chin. J. Org. Chem. 2014, 34, 2324–2330. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.Q.; Fu, C.R.; Zou, X.M. Synthesis and herbicidal activity of 2-cyano-3-(2-substituted phenyoxypyridin-5-yl) amino acrylates containing substituted phenoxyl group. Chin. J. Org. Chem. 2015, 35, 92–99. [Google Scholar] [CrossRef]
- Wu, S.S.; Miao, W.K.; Wang, T.T.; Fang, J.X. Synthesis and herbicidal activities of 2-cyanocarylates with 4-(6-chloropyridin-3-yl)methoxy benzylamine moieties. Chin. J. Org. Chem. 2015, 35, 1484–1492. [Google Scholar] [CrossRef]
- Wang, L.G.; Wang, F.Y.; Diao, Y.M.; Ni, J.P.; Wei, P. Synthesis and fungicidal activity of 2-cyano-3-substituted amino-3-(2-methylphenyl)propenoate. Chin. J. Org. Chem. 2015, 35, 1254–1258. [Google Scholar]
- Liu, Y.X.; Cai, B.L.; Li, Y.H.; Song, H.B.; Huang, R.Q.; Wang, Q.M. Synthesis, crystal structure, and biological activities of 2-cyanoacrylates containing furan or tetrahydrofuran moieties. J. Agric. Food Chem. 2007, 55, 3011–3017. [Google Scholar] [CrossRef]
- Liu, Y.X.; Liu, S.H.; Li, Y.H.; Song, H.B.; Wang, Q.M. Synthesis and biological evaluation of arylhydrazino cyanoacrylates and N-aryl pyrazole carboxylates. Bioorg. Med. Chem. Lett. 2009, 19, 2953–2956. [Google Scholar] [CrossRef]
- Wang, Q.M.; Sun, H.K.; Cao, H.Y.; Chen, M.R.; Huang, R.Q. Synthesis and herbicidal activity of 2-cyano-3-substituted pyridine methylamino acrylates. J. Agric. Food Chem. 2003, 51, 5030–5035. [Google Scholar] [CrossRef]
- Liu, Y.X.; Cui, Z.P.; Liu, B.; Cai, B.L.; Li, Y.H.; Wang, Q.M. Design, synthesis, and herbicidal activities of novel 2-cyanoacrylates containing isoxazole moieties. J. Agric. Food Chem. 2010, 58, 2685–2689. [Google Scholar] [CrossRef]
- Zhao, Q.Q.; Liu, S.H.; Li, Y.H.; Wang, Q.M. Design, synthesis, and biological activities of novel 2-cyanoacrylates containing oxazole, oxadiazole, or quinoline moieties. J. Agric. Food Chem. 2009, 57, 2849–2855. [Google Scholar] [CrossRef]
- Shi, Y.J.; Li, Y.; Fang, Y.; Chen, J.; Ye, L.Y.; Ge, S.S.; Dai, H. Synthesis and biological activities of novel cyanoacrylates containing 1,3,4-oxadiazole moiety. Chin. J. Org. Chem. 2016, 36, 2472–2478. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Gao, L.; Li, H.G.; Sun, P.W.; Meng, F.F.; Zhang, Y.; Xie, Y.T.; Sun, B.Q.; Zhou, S.; Ma, Y.; et al. Synthesis, insecticidal activities and structure-activity relationship of phenylpyrazole derivatives containing fluoro-substituted benzene moiety. J. Agric. Food Chem. 2020, 68, 11282–11289. [Google Scholar] [CrossRef]
- Aljohani, F.S.; Rezki, N.; Aouad, M.R.; Hagar, M.; Bakr, B.A.; Shaaban, M.M.; Elwakil, B.H. Novel 1,2,3-triazole-sulphadiazine-ZnO hybrids as potent antimicrobial agents against carbapenem resistant bacteria. Antibiotics 2022, 11, 916. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, P.W.; Zhao, J.H.; Zhang, H.Y.; Wang, X.Y.; Li, L.S.; Xiong, L.X.; Yang, N.; Li, Y.X.; Yuchi, Z.G.; et al. Design, synthesis and biological activity of diamide compounds based on 3-substituent of the pyrazole ring. Pest Manag. Sci. 2022, 78, 2022–2033. [Google Scholar] [CrossRef]
- Pokhodylo, N.; Finiuk, N.; Klyuchivska, O.; Tupychak, M.A.; Matiychuk, V.; Goreshnik, E.; Stoika, R. Novel N-(4-thiocyanato-phenyl)-1H-1,2,3-triazole-4-carboxamidesexhibit selective cytotoxic activity at nanomolar doses towards human leukemic T-cells. Eur. J. Med. Chem. 2022, 241, 114633. [Google Scholar] [CrossRef]
- Tang, X.B.; Li, Z.H.; Li, Y.H.; Liu, W.; Yu, P.; Li, L.X.; Guo, Y.; Yang, C. Synthesis and biological evaluation of novel saccharin derivatives containing 1,2,3-triazole moiety. Chem. Res. Chin. Univ. 2015, 31, 71–77. [Google Scholar] [CrossRef]
- Mu, J.X.; Zhai, Z.W.; Tan, C.X.; Weng, J.Q.; Wu, H.K.; Duke, S.O.; Zhang, Y.G.; Liu, X.H. Synthesis and herbicidal activity of 1,2,4-triazole derivatives containing a pyrazole moiety. J. Heterocycl. Chem. 2019, 56, 968–971. [Google Scholar] [CrossRef]
- Fu, Q.; Cai, P.P.; Cheng, L.; Zhong, L.K.; Tan, C.X.; Shen, Z.H.; Han, L.; Xu, T.M.; Liu, X.H. Synthesis and herbicidal activity of novel pyrazole aromatic ketone analogs as HPPD inhibitor. Pest Manag. Sci. 2020, 76, 868–879. [Google Scholar] [CrossRef]
- Wang, B.L.; Zhu, H.W.; Li, Z.M.; Wang, L.Z.; Zhang, X.; Xiong, L.X.; Song, H.B. Synthesis, biological evaluation and SAR analysis of novel poly-heterocyclic compounds containing pyridylpyrazole group. Pest Manag. Sci. 2018, 74, 726–736. [Google Scholar] [CrossRef]
- Xie, F.; Hao, Y.M.; Bao, J.H.; Liu, J.C.; Liu, Y.; Wang, R.N.; Chi, X.C.; Chai, X.Y.; Wang, T.; Yu, S.C.; et al. Design, synthesis, and in vitro evaluation of novel antifungal triazoles containing substituted 1,2,3-triazole-methoxyl side chains. Bioorg. Chem. 2022, 129, 106216. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Y.; Li, H.G.; Sun, P.W.; Gao, L.; Liu, J.B.; Zhou, S.; Xiong, L.X.; Yang, N.; Li, Y.X.; Li, Z.M. Synthesis, biological activities, and SAR studies of novel 1-(2-chloro-4,5-difluorophenyl)-1H-pyrazole derivatives. Bioorg. Med. Chem. Lett. 2020, 30, 127535. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Saini, A.; Gut, J.; Rosenthal, P.J.; Raj, R.; Kumar, V. 4-Aminoquinoline-chalcone/-N-acetylpyrazoline conjugates: Synthesis and antiplasmodial evaluation. Eur. J. Med. Chem. 2017, 138, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Huang, M.L.; Qian, J.Q.; Liu, J.; Meng, C.; Li, Y.Y.; Ming, G.X.; Zhang, T.; Wang, S.L.; Shi, Y.J.; et al. Excellent antitumor and antimetastatic activities based on novel coumarin/pyrazole oxime hybrids. Eur. J. Med. Chem. 2019, 166, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.Y.; Hao, S.Y.; Tian, H.Z.; Bian, H.L.; Hui, L.; Chen, S.W. Synthesis and biological evaluation of 1-(benzofuran-3-yl)-4-(3,4,5-trimethoxyphenyl)-1H-1,2,3-triazole derivatives as tubulin polymerization inhibitors. Bioorg. Chem. 2020, 94, 103392. [Google Scholar] [CrossRef]
- Patel, B.; Zunk, M.; Grant, G.; Rudrawar, S. Design, synthesis and bioactivity evaluation of novel pyrazole linked phenylthiazole derivatives in context of antibacterial activity. Bioorg. Med. Chem. Lett. 2021, 39, 127853. [Google Scholar] [CrossRef]
- Teng, Y.T.; Qin, Y.H.; Song, D.; Liu, X.B.; Ma, Y.G.; Zhang, P.P.; Ma, S.T. A novel series of 11-O-carbamoyl-3-O-descladinosyl clarithromycin derivatives bearing 1,2,3-triazole group: Design, synthesis and antibacterial evaluation. Bioorg. Med. Chem. Lett. 2020, 30, 126850. [Google Scholar] [CrossRef]
- Sabat, N.; Migianu-Griffoni, E.; Tudela, T.; Lecouvey, M.; Kellouche, S.; Carreriras, F.; Gallier, F.; Uziel, J.; Lubin-Germain, N. Synthesis and antitumor activities investigation of a C-nucleoside analogue of ribavirin. Eur. J. Med. Chem. 2020, 188, 112009. [Google Scholar] [CrossRef]
- Ren, B.; Liu, R.C.; Ji, K.G.; Tang, J.J.; Gao, J.M. Design, synthesis and in vitro antitumor evaluation of novel pyrazole-benzimidazole derivatives. Bioorg. Med. Chem. Lett. 2020, 43, 128097. [Google Scholar] [CrossRef]
- Morak-Mlodawska, B.; Jelen, M. Lipophilicity and pharmacokinetic properties of new anticancer dipyridothiazine with 1,2,3-triazole substituents. Molecules 2022, 27, 1253. [Google Scholar] [CrossRef]
- Dai, H.; Yao, W.; Sun, S.Y.; Li, L.; Shi, L.; Qian, H.W.; Li, C.J.; Shi, J.; Shi, Y.J. Synthesis and bioactivities of novel pyrazole oxime ethers containing substituted pyrazolyl group. Chin. J. Org. Chem. 2017, 37, 3155–3162. [Google Scholar] [CrossRef]
- Dai, H.; Chen, J.; Hong, Y.; Yuan, B.Y.; Chen, Y.M.; Shi, Y.J.; Ma, R.Y.; Liang, Z.P.; Shi, J. Synthesis and herbicidal activity of novel cyanoacrylates containing substituted pyridyl moiety. Chin. J. Org. Chem. 2017, 37, 739–745. [Google Scholar] [CrossRef]
- Wang, W.; He, H.W.; Zuo, N.; He, H.F.; Peng, H.; Tan, X.S. Synthesis, and herbicidal activity of 2-(substituted phenoxyacetoxy)alkyl-5,5-dimethyl-1,3,2-dioxaphosphinan-2-one. J. Agric. Food Chem. 2012, 60, 7581–7587. [Google Scholar] [CrossRef]

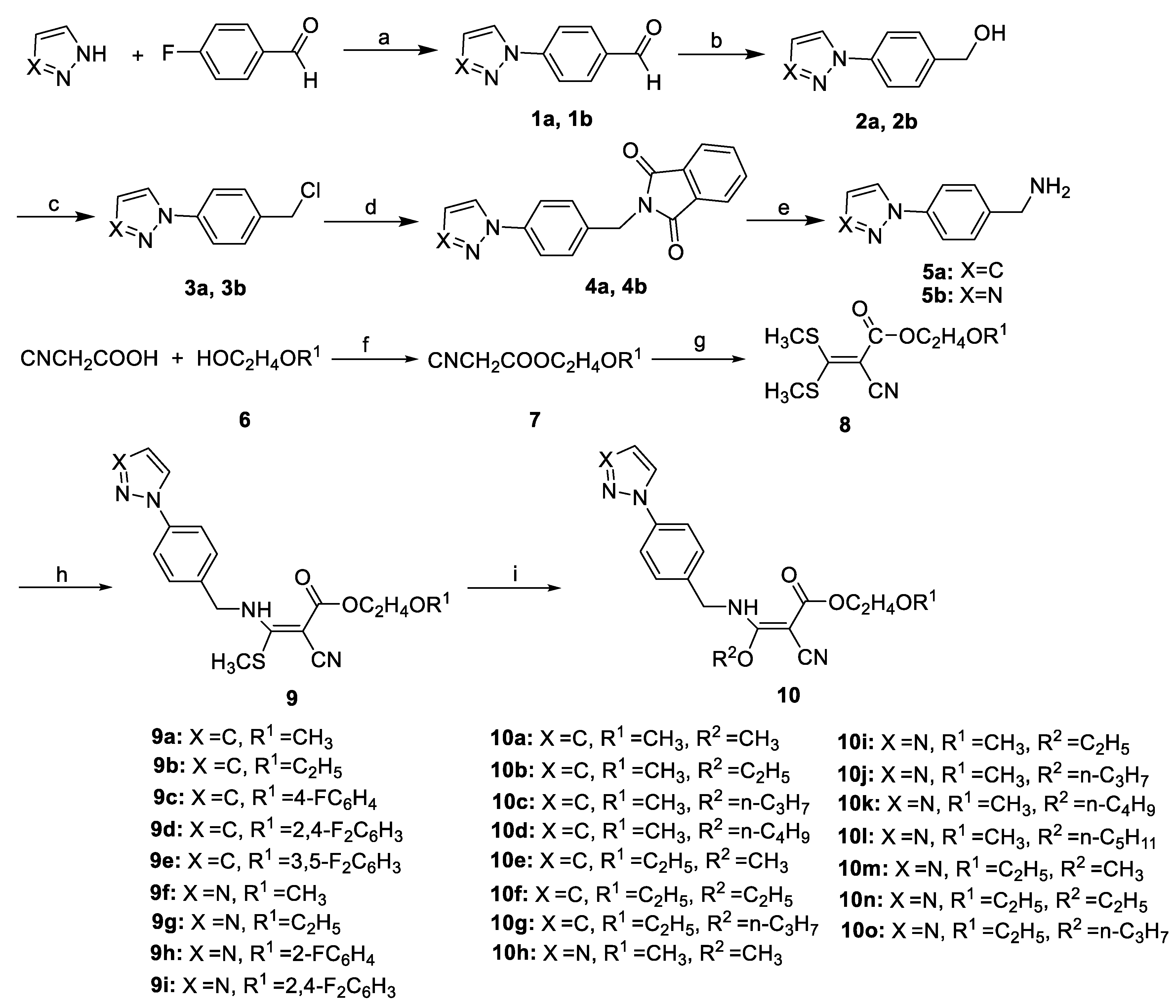
| Compd. | Dose | Brassica juncea | Chenopodium serotinum | Rumex acetosa | Alopecurus aequalis | Polypogon fugax | Poa annua |
|---|---|---|---|---|---|---|---|
| (g/ha) | |||||||
| 9a | 1500 | 100 | 100 | 100 | 45 | 40 | 30 |
| 9b | 1500 | 0 | 0 | 0 | 0 | 0 | 0 |
| 9c | 1500 | 0 | 0 | 0 | 0 | 0 | 0 |
| 9d | 1500 | 100 | 100 | 100 | 50 | 40 | 30 |
| 9e | 1500 | 30 | 0 | 0 | 0 | 0 | 0 |
| 9f | 1500 | 90 | 90 | 30 | 30 | 30 | 20 |
| 9g | 1500 | 100 | 100 | 80 | 70 | 50 | 30 |
| 9h | 1500 | 90 | 90 | 0 | 50 | 30 | 30 |
| 9i | 1500 | 95 | 100 | 20 | 70 | 40 | 30 |
| 10a | 1500 | 100 | 100 | 80 | 80 | 70 | 30 |
| 10b | 1500 | 100 | 80 | 50 | 30 | 30 | 30 |
| 10c | 1500 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10d | 1500 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10e | 1500 | 100 | 100 | 100 | 50 | 45 | 20 |
| 10f | 1500 | 60 | 100 | 100 | 0 | 0 | 0 |
| 10g | 1500 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10h | 1500 | 100 | 100 | 0 | 85 | 80 | 50 |
| 10i | 1500 | 85 | 90 | 60 | 70 | 70 | 30 |
| 10j | 1500 | 50 | 40 | 0 | 30 | 0 | 0 |
| 10k | 1500 | 50 | 80 | 0 | 30 | 0 | 0 |
| 10l | 1500 | 100 | 0 | 0 | 0 | 0 | 0 |
| 10m | 1500 | 90 | 90 | 30 | 70 | 60 | 40 |
| 10n | 1500 | 100 | 100 | 100 | 100 | 100 | 70 |
| 10o | 1500 | 100 | 100 | 60 | 90 | 0 | 0 |
| NK-9717 | 1500 | 100 | 100 | 100 | 30 | 20 | 10 |
| Compd. | Dose | Brassica juncea | Chenopodium serotinum | Rumex acetosa | Alopecurus aequalis | Polypogon fugax | Poa annua |
|---|---|---|---|---|---|---|---|
| (g/ha) | |||||||
| 9a | 150 | 80 | 20 | 20 | 0 | 0 | 0 |
| 9d | 150 | 90 | 90 | 70 | 0 | 0 | 0 |
| 9f | 150 | 80 | 80 | 20 | 20 | 20 | 0 |
| 9g | 150 | 60 | 80 | 30 | 20 | 20 | 20 |
| 9h | 150 | 70 | 70 | 0 | 20 | 20 | 20 |
| 9i | 150 | 85 | 80 | 0 | 0 | 0 | 0 |
| 10a | 150 | 80 | 60 | 20 | 0 | 0 | 0 |
| 10b | 150 | 80 | 20 | 30 | 0 | 0 | 0 |
| 10e | 150 | 80 | 40 | 20 | 0 | 0 | 0 |
| 10f | 150 | 20 | 50 | 20 | 0 | 0 | 0 |
| 10h | 150 | 75 | 70 | 0 | 0 | 0 | 0 |
| 10i | 150 | 70 | 75 | 20 | 30 | 30 | 20 |
| 10k | 150 | 0 | 30 | 0 | 0 | 0 | 0 |
| 10l | 150 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10m | 150 | 75 | 85 | 20 | 0 | 0 | 0 |
| 10n | 150 | 80 | 80 | 40 | 30 | 30 | 20 |
| 10o | 150 | 60 | 70 | 40 | 20 | 0 | 0 |
| NK-9717 | 150 | 80 | 75 | 40 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Chen, Y.; Qian, Y.; Chen, J.; Du, X.; Shi, Y.; Xu, B.; Hua, S.; Dai, H. Design, Synthesis, and Biological Activities of Novel 2-Cyanoacrylate Compounds Containing Substituted Pyrazolyl or 1,2,3-Triazolyl Moiety. Molecules 2023, 28, 3141. https://doi.org/10.3390/molecules28073141
Wang Y, Chen Y, Qian Y, Chen J, Du X, Shi Y, Xu B, Hua S, Dai H. Design, Synthesis, and Biological Activities of Novel 2-Cyanoacrylate Compounds Containing Substituted Pyrazolyl or 1,2,3-Triazolyl Moiety. Molecules. 2023; 28(7):3141. https://doi.org/10.3390/molecules28073141
Chicago/Turabian StyleWang, Yang, Yudie Chen, Ye Qian, Jia Chen, Xianchao Du, Yujun Shi, Baolin Xu, Sheng Hua, and Hong Dai. 2023. "Design, Synthesis, and Biological Activities of Novel 2-Cyanoacrylate Compounds Containing Substituted Pyrazolyl or 1,2,3-Triazolyl Moiety" Molecules 28, no. 7: 3141. https://doi.org/10.3390/molecules28073141
APA StyleWang, Y., Chen, Y., Qian, Y., Chen, J., Du, X., Shi, Y., Xu, B., Hua, S., & Dai, H. (2023). Design, Synthesis, and Biological Activities of Novel 2-Cyanoacrylate Compounds Containing Substituted Pyrazolyl or 1,2,3-Triazolyl Moiety. Molecules, 28(7), 3141. https://doi.org/10.3390/molecules28073141





