Polysaccharides from Discarded Stems of Trollius chinensis Bunge Elicit Promising Potential in Cosmetic Industry: Characterization, Moisture Retention and Antioxidant Activity
Abstract
1. Introduction
2. Results
2.1. Yields and Main Chemical Compositions of Polysaccharides from TCS and TCP
2.2. Molecular Weights and Monosaccharide Composition of TCSP and TCPP
2.3. Fourier Transform Infrared Spectrometry (FT-IR) Analysis of TCSP and TCPP
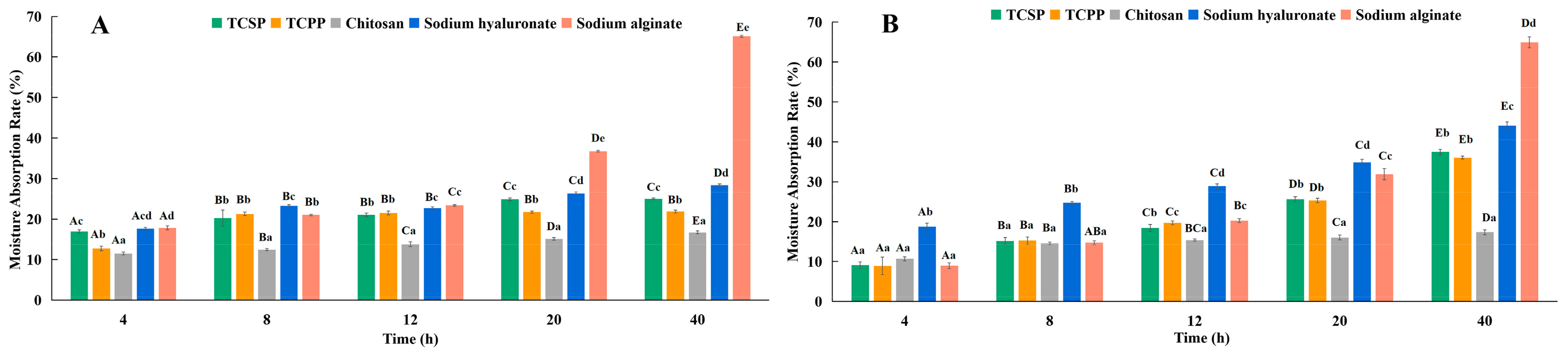
2.4. Moisture Absorption and Retention of TCSP and TCPP
2.5. Antioxidant Activities of TCSP and TCPP
2.5.1. Reducing Capacity
2.5.2. Diphenyl Picryl Hydrazinyl Radical Scavenging Activity
2.5.3. 2,2-Azino-bis (3-Ethylbenzothiazoline–6-sulfonic Acid) Scavenging Activity
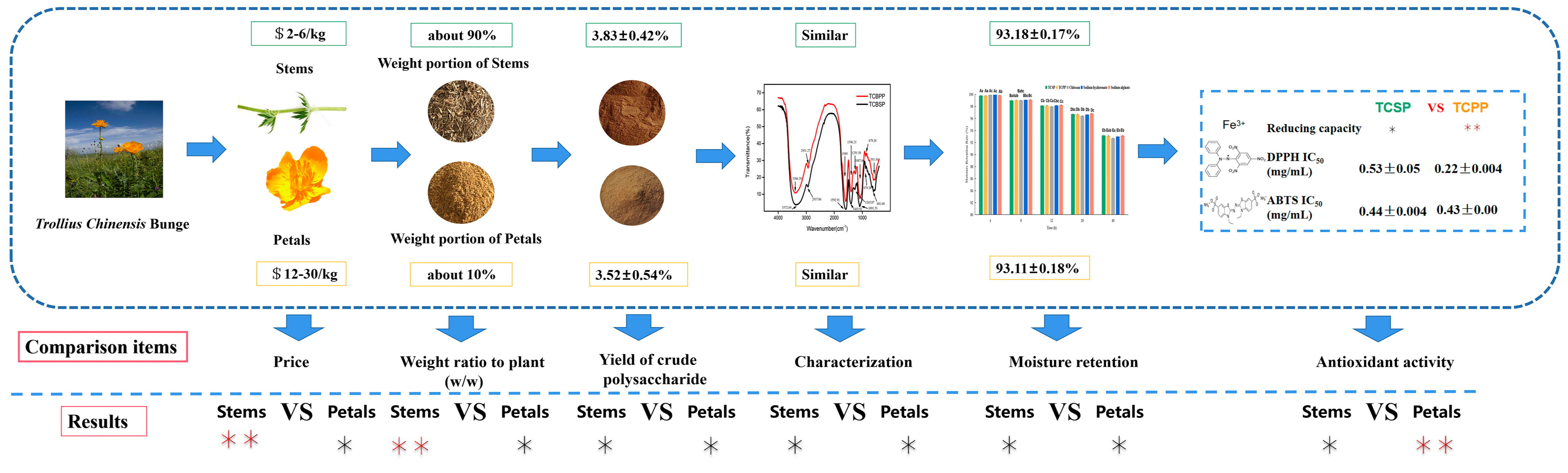
2.6. Comprehensive Comparison of TCSP and TCPP
3. Materials and Methods
3.1. Materials
3.2. Extraction of Crude Polysaccharides from Stems and Petals of TC
3.3. Chemical Composition
3.4. Purification of Crude Polysaccharides
3.5. Molecular Weights and Analysis of Monosaccharide’s Compositions
3.6. FT-IR Ananlysis of TCSP and TCPP
3.7. Measurement of Moisture Absorption and Retention Abilities
3.8. Antioxidant Activity In Vitro
3.8.1. Determination of Reducing Capacity
3.8.2. DPPH Radical Scavenging Activity
3.9. ABTS Radical Scavenging Activity
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, J.Y.; Li, S.Y.; Feng, J.Y.; Sun, Y.; Cai, J.N.; Sun, X.F.; Yang, S.L. Flavone C-glycosides from the flowers of Trollius chinensis and their anti-complementary activity. J. Asian Nat. Prod. Res. 2013, 15, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.M. A Supplement to the Compendium of Materia Medica, 2nd ed.; People’s Health Publishing House: Beijing, China, 1983. [Google Scholar]
- Lu, J.; Qin, P.Z.; Han, X.; Wang, Y.P.; Li, Z.H. Evaluation of antioxidant and antibacterial properties of extracts from Trollius chinensis Bunge. Eur. Food Res. Technol. 2014, 240, 301–310. [Google Scholar] [CrossRef]
- Liang, J.; Wang, M.; Olounfeh, K.M.; Zhao, N.; Wang, S.; Meng, F. Network pharmacology-based identification of potential targets of the flower of Trollius chinensis Bunge acting on anti-inflammatory effectss. Sci. Rep. 2019, 9, 8109. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.-L.; Xu, H.-T.; Yang, J.-J.; Chou, G.-X. Diterpenoid glycosides from the flower of Trollius chinensis Bunge and their nitric oxide inhibitory activities. Bioorg. Chem. 2021, 116, 105312. [Google Scholar] [CrossRef]
- Wei, M.; Yang, L. Determination of orientin in Trollius chinensis using ultrasound-assisted extraction and high performance liquid chromatography: Several often-overlooked sample preparation parameters in an ultrasonic bath. J. Chromatogr. A 2017, 1530, 68–79. [Google Scholar] [CrossRef]
- Lam, K.Y.; Ling, A.P.K.; Koh, R.Y.; Wong, Y.P.; Say, Y.H. A Review on Medicinal Properties of Orientin. Adv. Pharmacol. Sci. 2016, 2016, 4104595. [Google Scholar] [CrossRef]
- Guo, L.; Qiao, S.; Hu, J.; Li, D.; Zheng, S.; Shi, D.; Liu, J.; Wang, R. Investigation of the effective components of the flowers of Trollius chinensisfrom the perspectives of intestinal bacterial transformation and intestinal absorption. Pharm. Biol. 2017, 55, 1747–1758. [Google Scholar] [CrossRef]
- Yuan, M.; Wang, R.F.; Wu, X.W.; Aa, Y.N.; Yang, X.W. Investigation on Flos Trollii: Constituents and activities. Chin. J. Nat. Med. 2013, 11, 0449–0455. [Google Scholar] [CrossRef]
- Yan, R.; Cui, Y.; Deng, B.; Bi, J.; Zhang, G. Flavonoid glucosides from the flowers of Trollius chinensis Bunge. J. Nat. Med. 2019, 73, 297–302. [Google Scholar] [CrossRef]
- Kanlayavattanakul, M.; Rodchuea, C.; Lourith, N. Moisturizing effect of alcohol-based hand rub containing okra polysaccharide. Int. J. Cosmet. Sci. 2012, 34, 280–283. [Google Scholar] [CrossRef]
- Egawa, M.; Oguri, M.; Kuwahara, T.; Takahashi, M. Effect of exposure of human skin to a dry environment. Skin Res. Technol. 2010, 8, 212–218. [Google Scholar] [CrossRef]
- Inoue, N.; Sugihara, F.; Wang, X. Ingestion of bioactive collagen hydrolysates enhance facial skin moisture and elasticity and reduce facial aging signs in a randomized double-blind placebo-controlled clinical study. J. Sci. Food Agric. 2016, 96, 4077–4081. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Dermato-Endocrinology 2012, 4, 253–258. [Google Scholar] [CrossRef]
- Chen, B. Research Progress on the Application of Plant Polysaccharide in Cosmetics. Chin. Wild Plant Res. 2020, 39, 44–47. [Google Scholar]
- Li, J.; Chi, Z.; Yu, L.; Jiang, F.; Liu, C. Sulfated modification, characterization, and antioxidant and moisture absorption/retention activities of a soluble neutral polysaccharide from Enteromorpha prolifera. Int. J. Biol. Macromol. 2017, 105, 1544–1553. [Google Scholar] [CrossRef]
- Wang, J.; Jin, W.; Hou, Y.; Niu, X.; Zhang, H.; Zhang, Q. Chemical composition and moisture-absorption/retention ability of polysaccharides extracted from five algae. Int. J. Biol. Macromol. 2013, 57, 26–29. [Google Scholar] [CrossRef]
- Lou, C.W.; Hu, J.J.; Lu, C.T.; Huang, C.C.; Sie, M.Y.; Lin, J.H. Preparation and characterization of low-methoxyl pectin/bletilla striata composite membranes. Adv. Mater. Res. 2011, 287–290, 140–144. [Google Scholar] [CrossRef]
- Gu, F.; Jiang, X.; Chen, Y.; Han, B.; Chen, N.; Wei, C. Study on Moisture-absorption-retention Capacity and Skin Irritation of Polysaccharide from Dendrobium huoshanense. Nat. Prod. Res. Dev. 2018, 30, 1701–1705. [Google Scholar]
- Jaberian, H.; Piri, K.; Nazari, J. Phytochemical composition and in vitro antimicrobial and antioxidant activities of some medicinal plants. Food Chem. 2013, 136, 237–244. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohyd. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Su, L.; Zhang, W.; Zhao, B.; Liu, X.; Huang, Y.; Nan, Y.; Fan, R.; Li, J.; Zhang, S. Dynamic changes of total flavonoids and orientin from stems and leaves of Trollius chinensis at different collecting periods. Chin. Tradit. Herb. Drugs 2012, 43, 2058–2061. [Google Scholar]
- Salwowska, N.M.; Bebenek, K.A.A.; Zazdło, D.; Wcisło-Dziadecka, D.L. Physiochemical properties and application of hyaluronic acid a systematic review. J. Cosmet. Dermatol. 2016, 15, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Rao, N. Purification and Anti-oxidant Activity of the Polysacchatide Isolated from Trollious Chinesis. Master’s Thesis, North University, Zhangjiakou, China, 2012. [Google Scholar]
- Becker, D.J.; Lowe, J.B. Fucose: Biosynthesis and biological function in mammals. Glycobiology 2003, 13, 41R–53R. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, M.; Fawzy, M.A.; Hifney, A.F.; Abdel-Gawad, K.M. Use of the brown seaweed Sargassum latifolium in the design of alginate-fucoidan based films with natural antioxidant properties and kinetic modeling of moisture sorption and polyphenolic release. Food Hydrocoll. 2018, 82, 64–72. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Zhang, T.; Tian, Y.; Jiang, B.; Miao, M.; Mu, W. Purification, preliminary structural characterization and in vitro antioxidant activity of polysaccharides from Acanthus ilicifolius. LWT—Food Sci. Technol. 2014, 56, 9–14. [Google Scholar] [CrossRef]
- Zhu, B.W.; Zhou, D.Y.; Li, T.; Yan, S.; Yang, J.F.; Li, D.M.; Dong, X.P.; Murata, Y. Chemical composition and free radical scavenging activities of a sulphated polysaccharide extracted from abalone gonad (Haliotis Discus Hannai Ino). Food Chem. 2010, 121, 712–718. [Google Scholar] [CrossRef]
- Chen, G.; Fang, C.; Chen, X.; Wang, Z.; Liu, M.; Kan, J. High-pressure ultrasonic-assisted extraction of polysaccharides from Mentha haplocalyx: Structure, functional and biological activities. Ind. Crops Prod. 2019, 130, 273–284. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, F.; Li, R.; Wu, Y.; Liu, S.; Liang, Q. Purification, characterization, antioxidant and moisture-preserving activities of polysaccharides from Rosa rugosa petals. Int. J. Biol. Macromol. 2019, 124, 938–945. [Google Scholar] [CrossRef]
- Galus, S.; Lenart, A. Development and characterization of composite edible films based on sodium alginate and pectin. J. Food Eng. 2013, 115, 459–465. [Google Scholar] [CrossRef]
- Chen, L.; Du, Y.; Wu, H.; Xiao, L. Relationship between molecular structure and moisture-retention ability of carboxymethyl chitin and chitosan. J. Appl. Polym. Sci. 2002, 83, 1233–1241. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, G.; Du, C.; Mou, H.; Cui, J.; Guan, H.; Hwang, H.; Wang, P. Characterization of high yield exopolysaccharide produced by Phyllobacterium sp. 921F exhibiting moisture preserving properties. Int. J. Biol. Macromol. 2017, 101, 562–568. [Google Scholar]
- Amarowicz, R.; Pegg, R.B.; Rahimi-Moghaddam, P.; Barl, B.; Weil, J.A. Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem. 2004, 84, 551–562. [Google Scholar] [CrossRef]
- Yu, G.; Zhang, Q.; Wang, Y.; Yang, Q.; Yu, H.; Li, H.; Fu, L. Sulfated polysaccharides from red seaweed Gelidium amansii: Structural characteristics, anti-oxidant and anti-glycation properties, and development of bioactive films. Food Hydrocoll. 2021, 119, 106820. [Google Scholar] [CrossRef]
- Chen, L.; Huang, G. Antioxidant activities of phosphorylated pumpkin polysaccharide. Int. J. Biol. Macromol. 2019, 125, 256–261. [Google Scholar] [CrossRef]
- Li, F.; Wei, Y.; Liang, L.; Huang, L.; Yu, G.; Li, Q. A novel low-molecular-mass pumpkin polysaccharide: Structural characterization, antioxidant activity, and hypoglycemic potential. Carbohyd. Polym. 2020, 251, 117090. [Google Scholar] [CrossRef]
- Dong, X.; Liu, Y.; Yu, S.; Ji, H.; Feng, Y.; Liu, A.; Yu, J. Extraction, optimization, and biological activities of a low molecular weight polysaccharide from Platycodon grandiflorus. Ind. Crops Prod. 2021, 165, 113427. [Google Scholar] [CrossRef]
- Li, H. Optimization of Enzymatic Extraction Technology of Polysaccharide from Guihna(Osmanthus fragrans) and Investigation on Its Antioxidant Activity. Chin. J. Tradit. Med. Sci. Technol. 2015, 22, 395–397. [Google Scholar]
- Kungel, P.; Correa, V.G.; Correa, R.C.G.; Peralta, R.A.; Sokovic, M.; Calhelha, R.C.; Bracht, A.; Ferreira, I.; Peralta, R.M. Antioxidant and antimicrobial activities of a purified polysaccharide from yerba mate (Ilex paraguariensis). Int. J. Biol. Macromol. 2018, 114, 1161–1167. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, C.F.; Feng, X.; Cheng, L.; Ibrahim, S.A.; Wang, C.T.; Huang, W. Isolation, characterization and antioxidant of polysaccharides from Stropharia rugosoannulata. Int. J. Biol. Macromol. 2020, 155, 883–889. [Google Scholar] [CrossRef]
- Jeddou, K.B.; Chaari, F.; Maktouf, S.; Nouri-Ellouz, O.; Helbert, C.B.; Ghorbel, R.E. Structural, functional, and antioxidant properties of water-soluble polysaccharides from potatoes peels. Food Chem. 2016, 205, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Hamed, M.; Bougatef, H.; Karoud, W.; Krichen, F.; Haddar, A.; Bougatef, A.; Sila, A. Polysaccharides extracted from pistachio external hull: Characterization, antioxidant activity and potential application on meat as preservative. Ind. Crops Prod. 2020, 148, 112315. [Google Scholar] [CrossRef]
- Khaskheli, S.G.; Zheng, W.; Sheikh, S.A.; Khaskheli, A.A.; Liu, Y.; Soomro, A.H.; Feng, X.; Sauer, M.B.; Wang, Y.F.; Huang, W. Characterization of Auricularia auricular polysaccharides and its antioxidant properties in fresh and pickled product. Int. J. Biol. Macromol. 2015, 81, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Wang, P.; Zhou, H.; Li, Y. Extraction, characterization and in vitro antioxidant activity of polysaccharides from Carex meyeriana Kunth using different methods. Int. J. Biol. Macromol. 2018, 120, 2155–2164. [Google Scholar] [CrossRef] [PubMed]
- Li, P.Y. Structural Characterization and Biological Activity of Polysaccharides from Corn Silk. Master’s Thesis, Jilin Institute of Chemical Technology, Jilin City, China, 2019. [Google Scholar]
- Hammami, N.; Ben Gara, A.; Bargougui, K.; Ayedi, H.; Ben Abdalleh, F.; Belghith, K. Improved in vitro antioxidant and antimicrobial capacities of polysaccharides isolated from Salicornia arabica. Int. J. Biol. Macromol. 2018, 120, 2123–2130. [Google Scholar] [CrossRef]
- Xiao, H.; Fu, X.; Cao, C.; Li, C.; Chen, C.; Huang, Q. Sulfated modification, characterization, antioxidant and hypoglycemic activities of polysaccharides from Sargassum pallidum. Int. J. Biol. Macromol. 2019, 121, 407–414. [Google Scholar] [CrossRef]
- Xu, Y.; Niu, X.; Liu, N.; Gao, Y.; Wang, L.; Xu, G.; Yang, Y.; Li, X. Characterization, antioxidant and hypoglycemic activities of degraded polysaccharides from blackcurrant (Ribes nigrum L.) fruits. Food Chem. 2018, 243, 26–35. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, Q.; Xin, Y.; Li, Q.; Liu, Y. Synergistic moisturizing activity and antioxidant activity between crude polysaccharides and flavonoids in discarded stem of Trollius chinensis bunge. Eng. Rep. 2022, 5, e12569. [Google Scholar] [CrossRef]
- Wei, E.; Yang, R.; Zhao, H.; Wang, P.; Zhao, S.; Zhai, W.; Zhang, Y.; Zhou, H. Microwave-assisted extraction releases the antioxidant polysaccharides from seabuckthorn (Hippophae rhamnoides L.) berries. Int. J. Biol. Macromol. 2019, 123, 280–290. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484. [Google Scholar] [CrossRef]
- Witkowska-Banaszczak, E. The GenusTrollius-Review of Pharmacological and Chemical Research. Phytother. Res. 2015, 29, 475–500. [Google Scholar] [CrossRef]
- Wu, D.T.; He, Y.; Fu, M.X.; Gan, R.Y.; Hu, Y.C.; Peng, L.X.; Zou, L. Structural characteristics and biological activities of a pectic-polysaccharide from okra affected by ultrasound assisted metal-free Fenton reaction. Food Hydrocoll. 2022, 122, 107085. [Google Scholar] [CrossRef]
- Barua, S.; Kim, H.; Hong, S.C.; Yoo, S.Y.; Shin, D.; Lee, C.L.; Na, S.J.; Kim, Y.H.; Jo, K.; Yun, G.; et al. Moisturizing effect of serine-loaded solid lipid nanoparticles and polysaccharide-rich extract of root Phragmites communis incorporated in hydrogel bases. Arch. Pharm. Res. 2017, 40, 250–257. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Zhao, M.; Qi, H. Effect of phthaloylation on radical-scavenging and moisture-preserving activities of polysaccharide from Enteromorpha linza. Carbohydr. Polym. 2014, 111, 729–733. [Google Scholar] [CrossRef]
- Li, H.; Xu, J.; Liu, Y.; Ai, S.; Qin, F.; Li, Z.; Zhang, H.; Huang, Z. Antioxidant and moisture-retention activities of the polysaccharide from Nostoc commune. Carbohydr. Polym. 2011, 83, 1821–1827. [Google Scholar] [CrossRef]
- Hu, Y.C.; Li, J.; Wang, J.; Zhang, X.Y.; Wu, X.Y.; Li, X.; Guo, Z.B.; Zou, L.; Wu, D.T. Physicochemical characteristics and biological activities of soluble dietary fibers isolated from the leaves of different quinoa cultivars. Food Res. Int. 2023, 163, 122166. [Google Scholar] [CrossRef]



| Sample | Yield (%) | Total Sugar Content (%) | Uronic Acid (%) | Flavonoids (%) | Protein (%) |
|---|---|---|---|---|---|
| TCSP | 3.83 ± 0.42 a | 56.99 ± 8.12 a | 7.56 ± 0.45 a | 8.06 ± 1.82 a | 0.92 ± 0.24 a |
| TCPP | 3.52 ± 0.54 a | 46.09 ± 1.68 a | 4.76 ± 0.81 b | 9.52 ± 3.42 a | 0.85 ± 0.21 a |
| Sample | Mn 1 × 105 Da | Mw 1 × 105 Da | PDI (Mw/Mn) | Monosaccharide’s Composition (% mol) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Man | Rha | GlcA | GalA | Glu | Gal | Xyl | Ara | Fuc | ||||
| TCSP | 1.71 | 6.07 | 3.55 | 10.10 ± 0.23 b | 3.11 ± 0.04 a | 3.09 ± 0.07 | 30.29 ± 3.72 b | 13.36 ± 1.2 a | 11.16 ± 0.86 a | 6.33 ± 0.59 | 14.78 ± 2.33 a | 7.79 ± 0.73 a |
| TCPP | 7.33 | 9.72 | 1.33 | 7.81 ± 0.16 a | 5.04 ± 0.12 b | ND | 5.13 ± 2.38 a | 28.15 ± 2.36 b | 14.98 ± 1.03 b | ND | 16.60 ± 2.09 b | 13.29 ± 1.15 b |
| Sample | DPPH-IC50 (mg/mL) | ABTS-IC50 (mg/mL) | Sample |
|---|---|---|---|
| TCSP | 0.5320 ± 0.0496 a | 0.4390 ± 0.0040 a | TCSP |
| TCPP | 0.2193 ± 0.0061 b | 0.4273 ± 0.0035 a | TCPP |
| Vc | 0.0034 ± 0.0002 c | 0.0026 ± 0.0002 b | Vc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Guo, Q.; Zhang, S.; Bao, Y.; Chen, M.; Gao, L.; Zhang, Y.; Zhou, H. Polysaccharides from Discarded Stems of Trollius chinensis Bunge Elicit Promising Potential in Cosmetic Industry: Characterization, Moisture Retention and Antioxidant Activity. Molecules 2023, 28, 3114. https://doi.org/10.3390/molecules28073114
Liu Y, Guo Q, Zhang S, Bao Y, Chen M, Gao L, Zhang Y, Zhou H. Polysaccharides from Discarded Stems of Trollius chinensis Bunge Elicit Promising Potential in Cosmetic Industry: Characterization, Moisture Retention and Antioxidant Activity. Molecules. 2023; 28(7):3114. https://doi.org/10.3390/molecules28073114
Chicago/Turabian StyleLiu, Yang, Qiwei Guo, Saimin Zhang, Yilin Bao, Mengling Chen, Lin Gao, Yang Zhang, and Hongli Zhou. 2023. "Polysaccharides from Discarded Stems of Trollius chinensis Bunge Elicit Promising Potential in Cosmetic Industry: Characterization, Moisture Retention and Antioxidant Activity" Molecules 28, no. 7: 3114. https://doi.org/10.3390/molecules28073114
APA StyleLiu, Y., Guo, Q., Zhang, S., Bao, Y., Chen, M., Gao, L., Zhang, Y., & Zhou, H. (2023). Polysaccharides from Discarded Stems of Trollius chinensis Bunge Elicit Promising Potential in Cosmetic Industry: Characterization, Moisture Retention and Antioxidant Activity. Molecules, 28(7), 3114. https://doi.org/10.3390/molecules28073114








