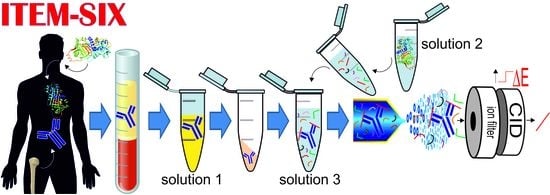
Intact Transition Epitope Mapping—Serological Inspection by Epitope EXtraction (ITEM—SIX) †
Abstract
1. Introduction
2. Results
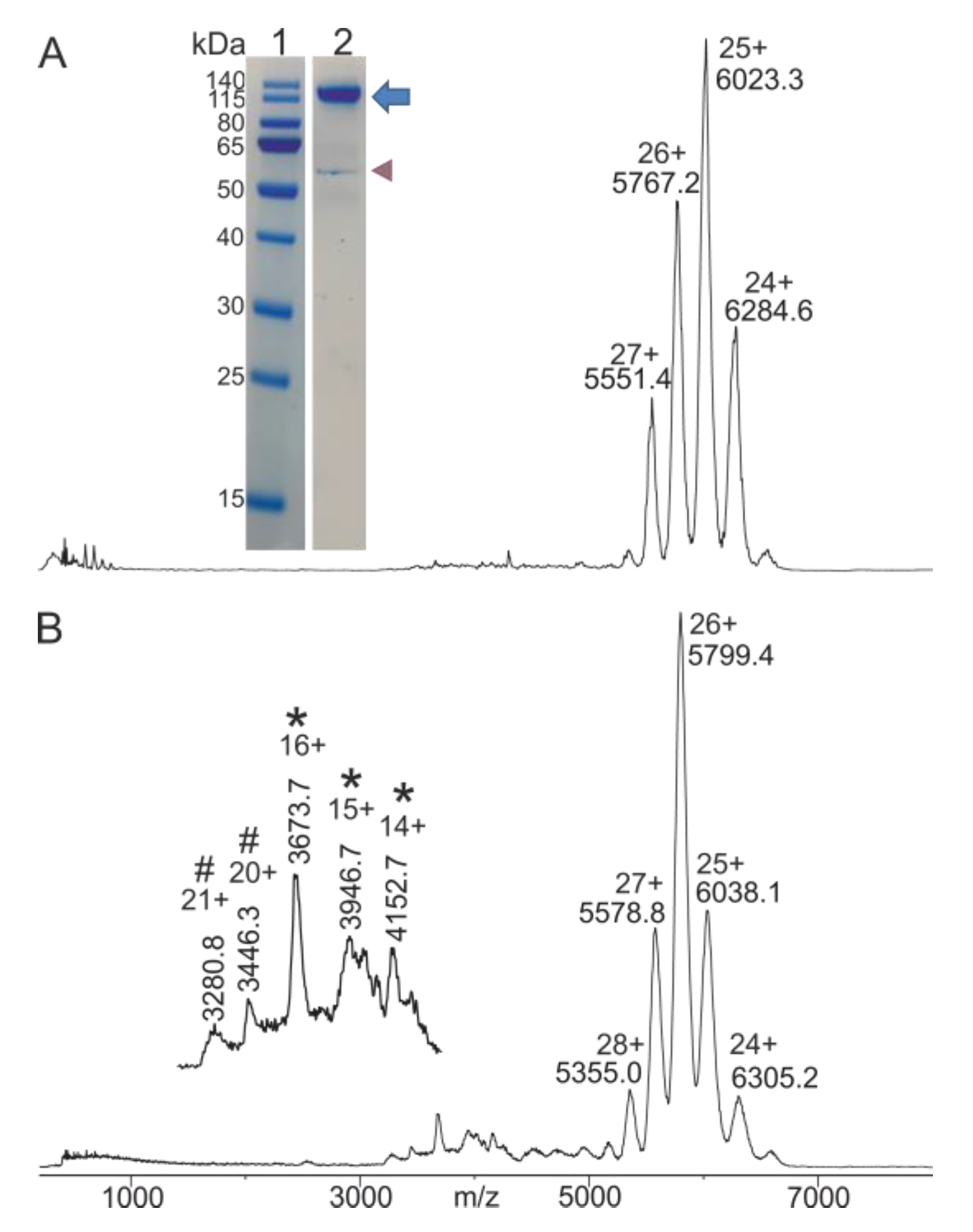
2.1. IgG Preparation from Blood Serum
2.2. Mass Spectrometric Characterization of Starting Materials
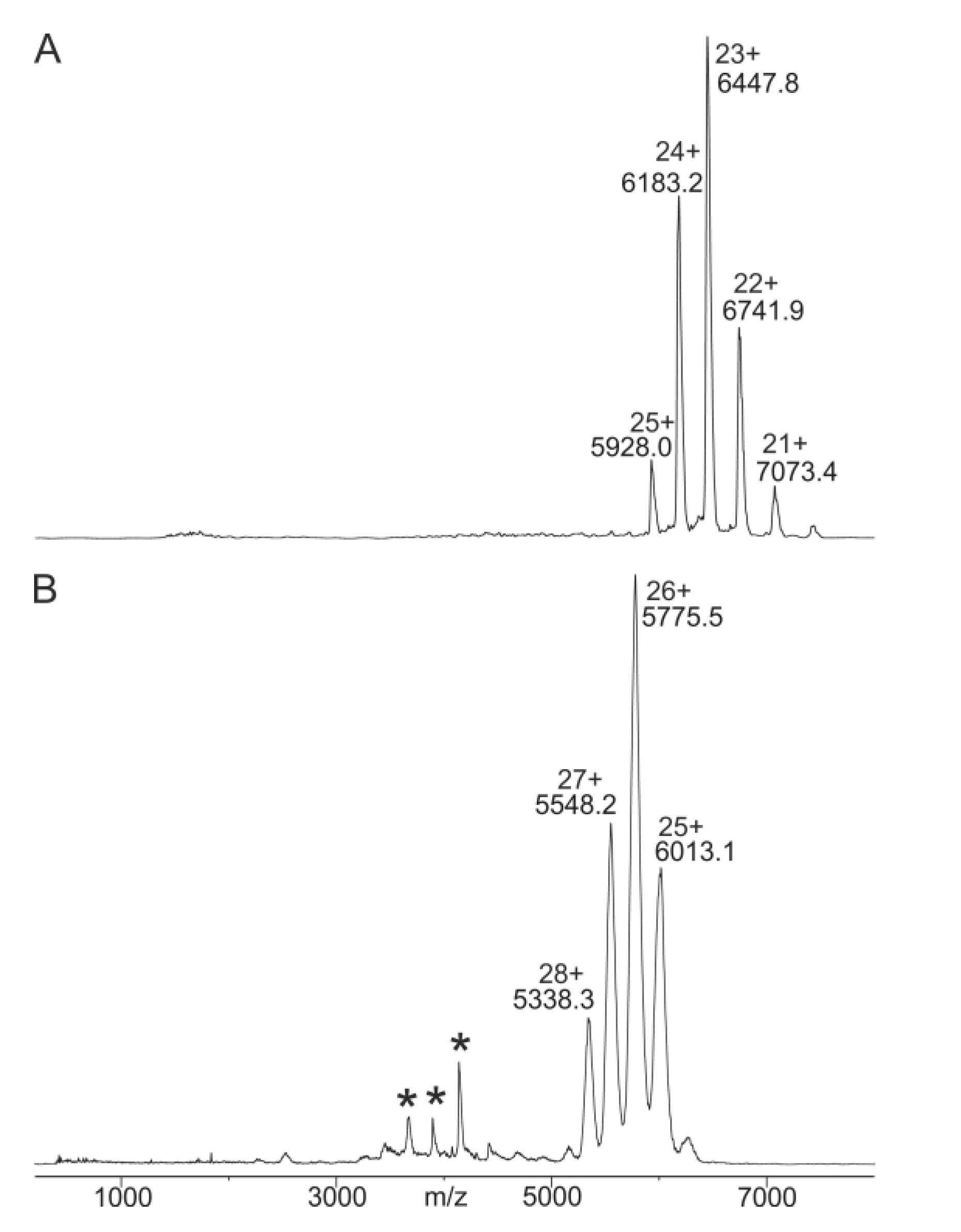
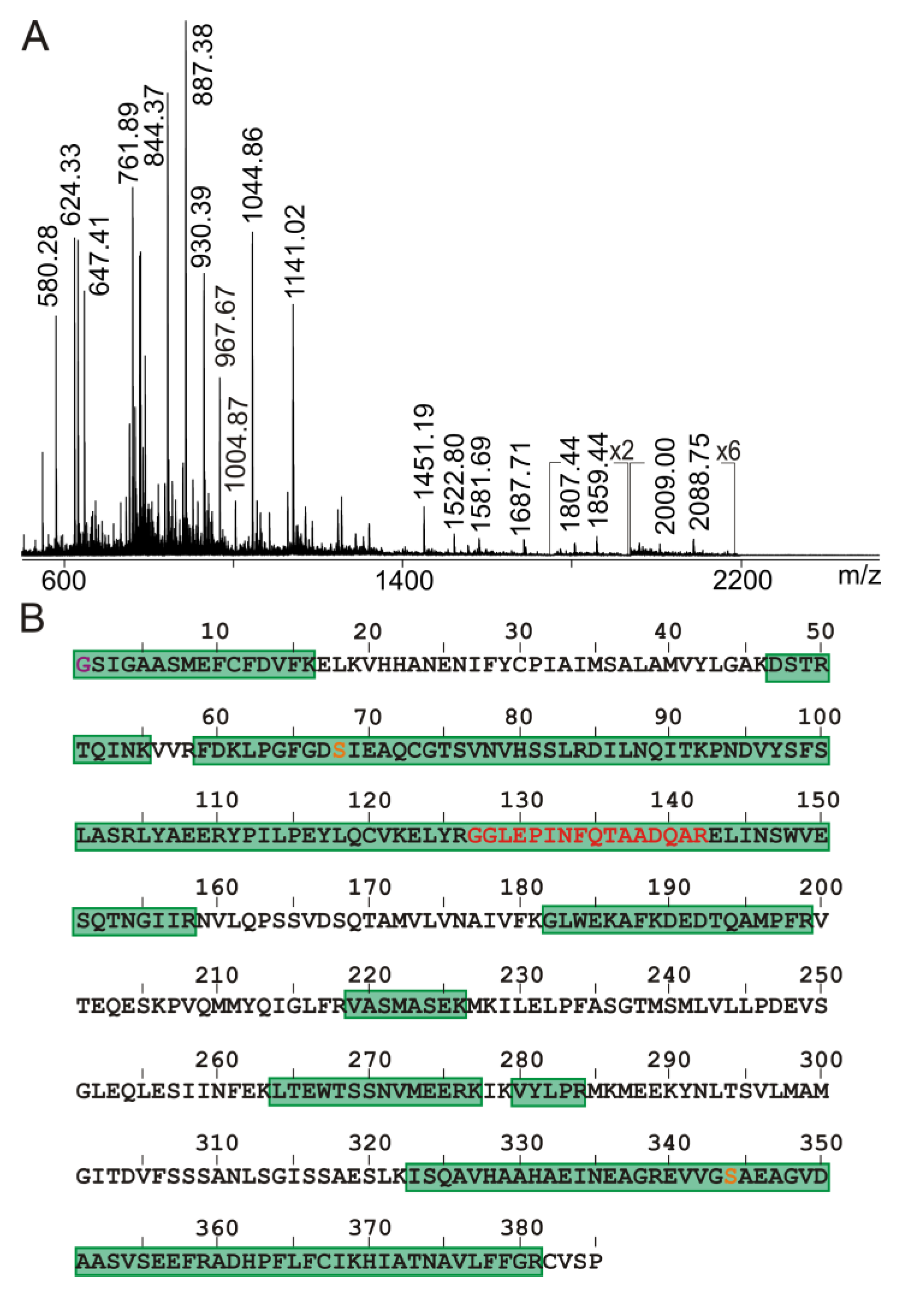
2.3. Anti-Ovalbumin Antibody Epitope Mapping by ITEM—ONE Analysis
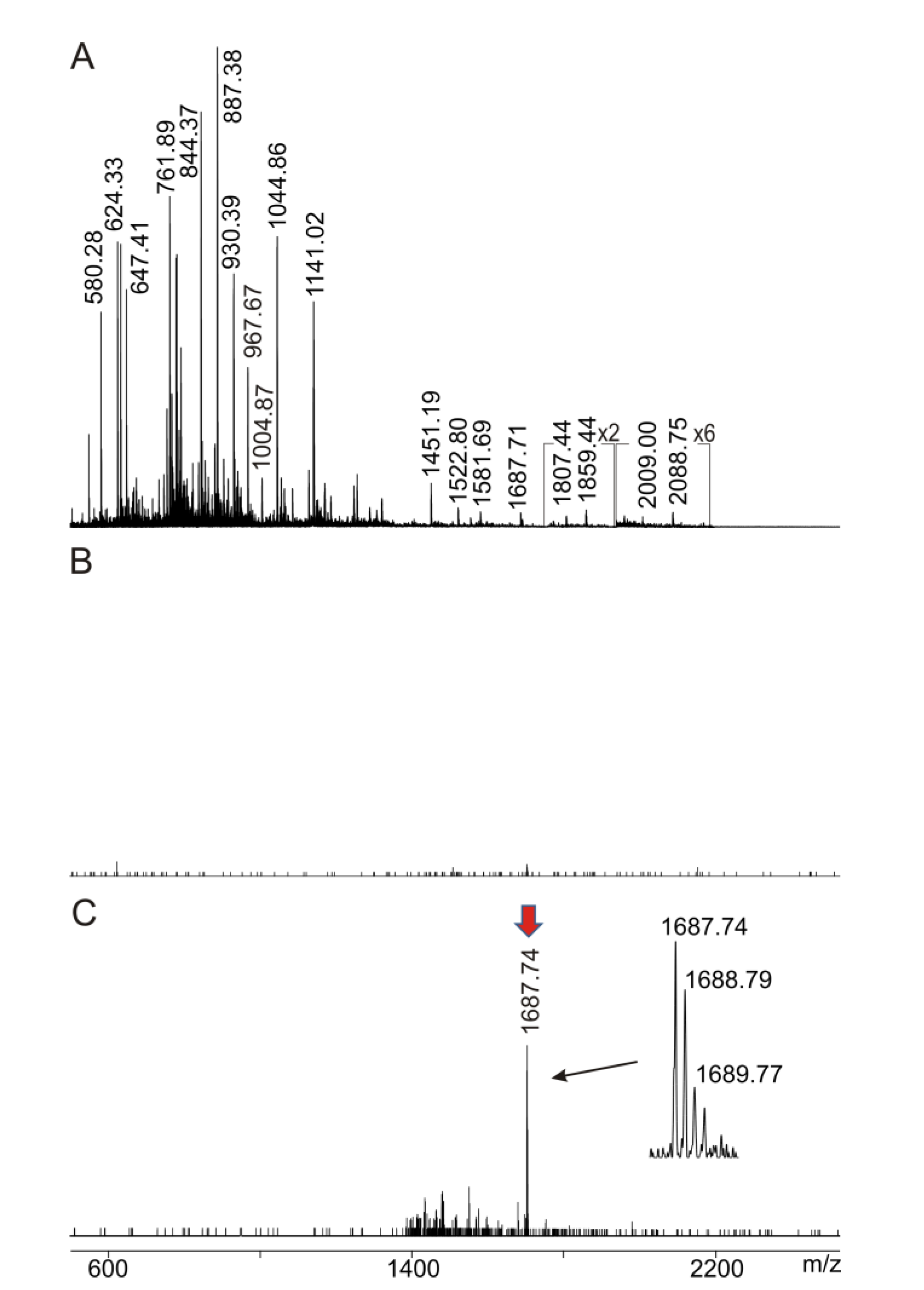
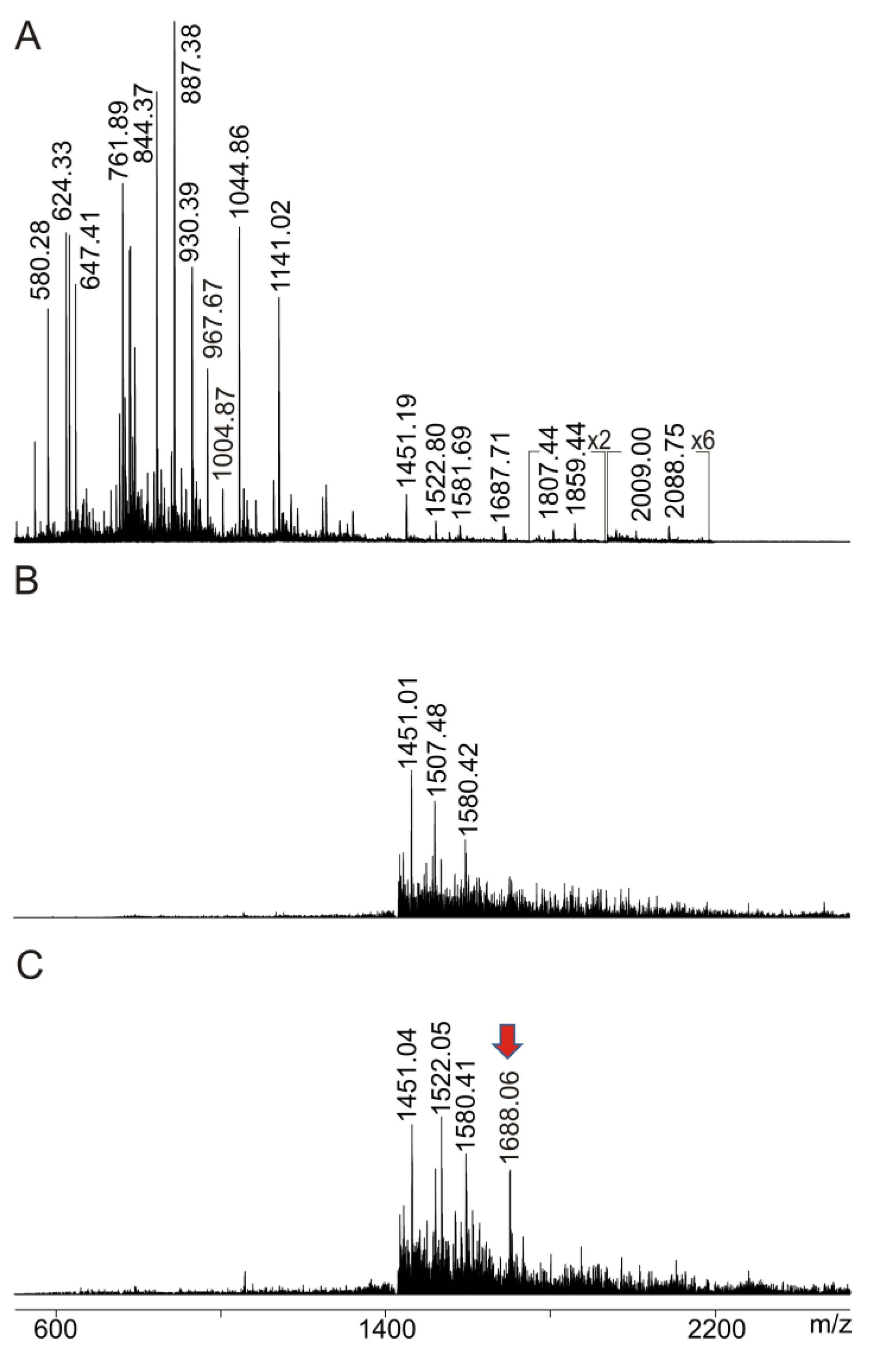
2.4. Intact Transition Epitope Mapping—Serology Inspection by Epitope EXtraction (ITEM—SIX)
3. Discussion
4. Materials and Methods
4.1. IgG from Blood Serum
4.2. Anti-Ovalbumin Antibody
4.3. IgG from mAb-Spiked Serum (Surrogate Seroconversion)
4.4. Ovalbumin Antigen and Tryptic Digestion
4.5. SDS-PAGE Analysis
4.6. Offline nanoESI-MS Analysis of Ovalbumin Peptides
4.7. Offline nanoESI-MS Analysis of Intact IgG
4.8. ITEM—ONE Epitope Mapping and Negative Control
4.9. ITEM—SIX Analysis and Negative Control
4.10. Western Blot Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wine, Y.; Horton, A.P.; Ippolito, G.C.; Georgiou, G. Serology in the 21st century: The molecular-level analysis of the serum antibody repertoire. Curr. Opin. Immunol. 2015, 35, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Fox, T.; Geppert, J.; Dinnes, J.; Scandrett, K.; Bigio, J.; Sulis, G.; Hettiarachchi, D.; Mathangasinghe, Y.; Weeratunga, P.; Wickramasinghe, D.; et al. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst. Rev. 2022, 11, 1–1236. [Google Scholar]
- Haselbeck, A.H.; Im, J.; Prifti, K.; Marks, F.; Holm, M.; Zellweger, R.M. Serology as a Tool to Assess Infectious Disease Landscapes and Guide Public Health Policy. Pathogens 2022, 11, 732. [Google Scholar] [CrossRef] [PubMed]
- Prechl, J. Why current quantitative serology is not quantitative and how systems immunology could provide solutions. Biol. Futur. 2021, 72, 37–44. [Google Scholar] [CrossRef]
- Sorohan, B.M.; Baston, C.; Tacu, D.; Bucșa, C.; Țincu, C.; Vizireanu, P.; Sinescu, I.; Constantinescu, I. Non-HLA Antibodies in Kidney Transplantation: Immunity and Genetic Insights. Biomedicines 2022, 10, 1506. [Google Scholar] [CrossRef]
- Horner, P.J.; Anyalechi, G.E.; Geisler, W.M. What Can Serology Tell Us About the Burden of Infertility in Women Caused by Chlamydia? J. Infect. Dis. 2021, 224, S80–S85. [Google Scholar] [CrossRef]
- Crespo, M.; Llinàs-Mallol, L.; Redondo-Pachón, D.; Butler, C.; Gimeno, J.; Pérez-Sáez, M.J.; Burballa, C.; Buxeda, A.; Arias-Cabrales, C.; Folgueiras, M.; et al. Non-HLA Antibodies and Epitope Mismatches in Kidney Transplant Recipients with Histological Antibody-Mediated Rejection. Front. Immunol. 2021, 12, 703457. [Google Scholar] [CrossRef]
- Loh, J.; Hashimoto, K.; Kwon, C.H.D.; Fujiki, M.; Modaresi Esfeh, J. Positive autoantibodies in living liver donors. World J. Hepatol. 2022, 14, 1757–1766. [Google Scholar] [CrossRef]
- Wickman, M.; Lilja, G.; Söderström, L.; van Hage-Hamsten, M.; Ahlstedt, S. Quantitative analysis of IgE antibodies to food and inhalant allergens in 4-year-old children reflects their likelihood of allergic disease. Allergy 2005, 60, 650–657. [Google Scholar] [CrossRef]
- Ansotegui, I.J.; Melioli, G.; Canonica, G.W.; Caraballo, L.; Villa, E.; Ebisawa, M.; Passalacqua, G.; Savi, E.; Ebo, D.; Gómez, R.M.; et al. IgE allergy diagnostics and other relevant tests in allergy, a World Allergy Organization position paper. World Allergy Organ J. 2020, 13, 100080. [Google Scholar] [CrossRef]
- Jakob, T.; Forstenlechner, P.; Matricardi, P.; Kleine-Tebbe, J. Molecular allergy diagnostics using multiplex assays: Methodological and practical considerations for use in research and clinical routine: Part 21 of the Series Molecular Allergology. Allergo J. Int. 2015, 24, 320–332. [Google Scholar] [CrossRef]
- Van Gool, A.; Corrales, F.; Čolović, M.; Krstić, D.; Oliver-Martos, B.; Martínez-Cáceres, E.; Jakasa, I.; Gajski, G.; Brun, V.; Kyriacou, K.; et al. Analytical techniques for multiplex analysis of protein biomarkers. Expert Rev. Proteom. 2020, 17, 257–273. [Google Scholar] [CrossRef]
- Al Kindi, M.A.; Colella, A.D.; Chataway, T.K.; Jackson, M.W.; Wang, J.J.; Gordon, T.P. Secreted autoantibody repertoires in Sjögren's syndrome and systemic lupus erythematosus: A proteomic approach. Autoimmun. Rev. 2016, 15, 405–410. [Google Scholar] [CrossRef]
- Lee, A.Y.S.; Chataway, T.; Colella, A.D.; Gordon, T.P.; Wang, J.J. Quantitative Mass Spectrometric Analysis of Autoantibodies as a Paradigm Shift in Autoimmune Serology. Front. Immunol. 2019, 10, 2845. [Google Scholar] [CrossRef]
- de Graaf, S.C.; Hoek, M.; Tamara, S.; Heck, A.J.R. A perspective toward mass spectrometry-based de novo sequencing of endogenous antibodies. MAbs 2022, 14, 2079449. [Google Scholar] [CrossRef] [PubMed]
- Melani, R.D.; Des Soye, B.J.; Kafader, J.O.; Forte, E.; Hollas, M.; Blagojevic, V.; Negrão, F.; McGee, J.P.; Drown, B.; Lloyd-Jones, C.; et al. Next-Generation Serology by Mass Spectrometry: Readout of the SARS-CoV-2 Antibody Repertoire. J. Proteome Res. 2022, 21, 274–288. [Google Scholar] [CrossRef]
- Macht, M.; Fiedler, W.; Kürzinger, K.; Przybylski, M. Mass spectrometric mapping of protein epitope structures of myocardial infarct markers myoglobin and troponin T. Biochemistry 1996, 35, 15633–15639. [Google Scholar] [CrossRef]
- Opuni, K.F.M.; Al-Majdoub, M.; Yefremova, Y.; El-Kased, R.F.; Koy, C.; Glocker, M.O. Mass spectrometric epitope mapping. Mass Spectrom. Rev. 2018, 37, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Suckau, D.; Köhl, J.; Karwath, G.; Schneider, K.; Casaretto, M.; Bitter-Suermann, D.; Przybylski, M. Molecular epitope identification by limited proteolysis of an immobilized antigen-antibody complex and mass spectrometric peptide mapping. Proc. Natl. Acad. Sci. USA 1990, 87, 9848–9852. [Google Scholar] [CrossRef] [PubMed]
- Baschung, Y.; Lupu, L.; Moise, A.; Glocker, M.; Rawer, S.; Lazarev, A.; Przybylski, M. Epitope Ligand Binding Sites of Blood Group Oligosaccharides in Lectins Revealed by Pressure-Assisted Proteolytic Excision Affinity Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2018, 29, 1881–1891. [Google Scholar] [CrossRef]
- El-Kased, R.F.; Koy, C.; Lorenz, P.; Montgomery, H.; Tanaka, K.; Thiesen, H.-J.; Glocker, M.O. A Novel Mass Spectrometric Epitope Mapping Approach without Immobilization of the Antibody. J. Proteom. Bioinform. 2011, 4, 1–9. [Google Scholar]
- Yefremova, Y.; Opuni, K.F.M.; Danquah, B.D.; Thiesen, H.J.; Glocker, M.O. Intact Transition Epitope Mapping (ITEM). J. Am. Soc. Mass. Spectrom. 2017, 28, 1612–1622. [Google Scholar] [CrossRef] [PubMed]
- Loo, J.A. Studying noncovalent protein complexes by electrospray ionization mass spectrometry. Mass Spectrom. Rev. 1997, 16, 1–23. [Google Scholar] [CrossRef]
- Przybylski, M.; Glocker, M.O. Electrospray Mass Spectrometry of Biomacromolecular Complexes with Noncovalent Interactions-New Analytical Perspectives for Supramolecular Chemistry and Molecular Recognition Processes. Angew Chem. Int. Ed. Engl. 1996, 35, 806–826. [Google Scholar] [CrossRef]
- Yefremova, Y.; Danquah, B.D.; Opuni, K.F.; El-Kased, R.; Koy, C.; Glocker, M.O. Mass spectrometric characterization of protein structures and protein complexes in condensed and gas phase. Eur. J. Mass. Spectrom. 2017, 23, 445–459. [Google Scholar] [CrossRef]
- Danquah, B.D.; Opuni, K.F.M.; Roewer, C.; Koy, C.; Glocker, M.O. Mass Spectrometric Analysis of Antibody-Epitope Peptide Complex Dissociation: Theoretical Concept and Practical Procedure of Binding Strength Characterization. Molecules 2020, 25, 4776. [Google Scholar] [CrossRef]
- Danquah, B.D.; Yefremova, Y.; Opuni, K.F.M.; Röwer, C.; Koy, C.; Glocker, M.O. Intact Transition Epitope Mapping—Thermodynamic Weak-force Order (ITEM—TWO). J. Proteom. 2020, 212, 103572. [Google Scholar] [CrossRef]
- Danquah, B.D.; Röwer, C.; Opuni, K.M.; El-Kased, R.; Frommholz, D.; Illges, H.; Koy, C.; Glocker, M.O. Intact Transition Epitope Mapping—Targeted High-Energy Rupture of Extracted Epitopes (ITEM-THREE). Mol. Cell. Proteom. 2019, 18, 1543–1555. [Google Scholar] [CrossRef]
- Röwer, C.; Ortmann, C.; Neamtu, A.; El-Kased, R.F.; Glocker, M.O. Intact Transition Epitope Mapping—Force differences between Original and Unusual Residues (ITEM—FOUR). Biomolecules 2023, 13, 187. [Google Scholar] [CrossRef]
- Scherf, M.; Danquah, B.D.; Koy, C.; Lorenz, P.; Steinbeck, F.; Neamtu, A.; Thiesen, H.J.; Glocker, M.O. Epitope Fine Mapping by Mass Spectrometry: Investigations of Immune Complexes Consisting of Monoclonal Anti-HpTGEKP Antibody and Zinc Finger Protein Linker Phospho-Hexapeptides. ChemBioChem 2022, 23, e202200390. [Google Scholar] [CrossRef]
- Kienbaum, M.; Koy, C.; Montgomery, H.V.; Drynda, S.; Lorenz, P.; Illges, H.; Tanaka, K.; Kekow, J.; Guthke, R.; Thiesen, H.J.; et al. MS characterization of apheresis samples from rheumatoid arthritis patients for the improvement of immunoadsorption therapy—A pilot study. Proteom. Clin. Appl. 2009, 3, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Wolter, M.; Rower, C.; Koy, C.; Reimer, T.; Rath, W.; Pecks, U.; Glocker, M.O. A proteome signature for intrauterine growth restriction derived from multifactorial analysis of mass spectrometry-based cord blood serum profiling. Electrophoresis 2012, 33, 1881–1893. [Google Scholar] [CrossRef] [PubMed]
- Honma, K.; Kohno, Y.; Saito, K.; Shimojo, N.; Horiuchi, T.; Hayashi, H.; Suzuki, N.; Hosoya, T.; Tsunoo, H.; Niimi, H. Allergenic epitopes of ovalbumin (OVA) in patients with hen's egg allergy: Inhibition of basophil histamine release by haptenic ovalbumin peptide. Clin. Exp. Immunol. 1996, 103, 446–453. [Google Scholar] [CrossRef]
- Matsuo, H.; Yokooji, T.; Taogoshi, T. Common food allergens and their IgE-binding epitopes. Allergol. Int. 2015, 64, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Holm, B.E.; Bergmann, A.C.; Hansen, P.R.; Koch, C.; Houen, G.; Trier, N.H. Antibodies with specificity for native and denatured forms of ovalbumin differ in reactivity between enzyme-linked immunosorbent assays. APMIS 2015, 123, 136–145. [Google Scholar] [CrossRef]
- Miguel, M.; Manso, M.; Aleixandre, A.; Alonso, M.J.; Salaices, M.; Lopez-Fandino, R. Vascular effects, angiotensin I-converting enzyme (ACE)-inhibitory activity, and antihypertensive properties of peptides derived from egg white. J. Agric. Food Chem. 2007, 55, 10615–10621. [Google Scholar] [CrossRef]
- Huynh, A.; Kelton, J.G.; Arnold, D.M.; Daka, M.; Nazy, I. Antibody epitopes in vaccine-induced immune thrombotic thrombocytopaenia. Nature 2021, 596, 565–569. [Google Scholar] [CrossRef]
- Greinacher, A.; Langer, F.; Makris, M.; Pai, M.; Pavord, S.; Tran, H.; Warkentin, T.E. Vaccine-induced immune thrombotic thrombocytopenia (VITT)—Update on diagnosis and management considering different resources: Response to Comment from Yamada et al. J. Thromb. Haemost. 2022, 20, 542–543. [Google Scholar] [CrossRef]
- Muthupalaniappen, L.; Jamil, A. Prick, patch or blood test? A simple guide to allergy testing. Malays. Fam. Physician 2021, 16, 19–26. [Google Scholar] [CrossRef]
- Cohn, E.J. Blood Proteins And Their Therapeutic Value. Science 1945, 101, 51–56. [Google Scholar] [CrossRef]
- Cohn, E.J.; Oncley, J.L.; Strong, L.E.; Hughes, W.L.; Armstrong, S.H. Chemical, Clinical, and Immunological Studies on the Products of Human Plasma Fractionation. I. The Characterization of the Protein Fractions of Human Plasma. J. Clin. Investig. 1944, 23, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Hess, E.L.; Deutsch, H.F. Separation and Properties of the 7-Globulins of the Sera of Normal Cows. J. Am. Chem. Soc. 1948, 70, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Hess, E.L.; Deutsch, H.F. Biophysical studies of blood plasma proteins; separation and properties of the immune globulins of the sera of hyperimmunized cows. J. Am. Chem. Soc. 1949, 71, 1376–1381. [Google Scholar] [CrossRef] [PubMed]
- McKinney, M.M.; Parkinson, A. A simple, non-chromatographic procedure to purify immunoglobulins from serum and ascites fluid. J. Immunol. Methods 1987, 96, 271–278. [Google Scholar] [CrossRef]
- Simpson, D.M.; Beynon, R.J. Acetone precipitation of proteins and the modification of peptides. J. Proteome Res. 2010, 9, 444–450. [Google Scholar] [CrossRef]
- Crowell, A.M.J.; Wall, M.J.; Doucette, A.A. Maximizing recovery of water-soluble proteins through acetone precipitation. Anal. Chim. Acta 2013, 796, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Opuni, K.F.M.; Koy, C.; Russ, M.; Reepmeyer, M.; Danquah, B.D.; Weresow, M.; Alef, A.; Lorenz, P.; Thiesen, H.J.; Glocker, M.O. ITEM-THREE analysis of a monoclonal anti-malaria antibody reveals its assembled epitope on the pfMSP1(19) antigen. J. Biol. Chem. 2020, 295, 14987–14997. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, J.; Peng, Y.; Xie, Y.; Xiao, Y. A Promising Tool in Serological Diagnosis: Current Research Progress of Antigenic Epitopes in Infectious Diseases. Pathogens 2022, 11, 1095. [Google Scholar] [CrossRef]
- Beans, J.A.; Trinidad, S.B.; Blacksher, E.; Hiratsuka, V.Y.; Spicer, P.; Woodahl, E.L.; Boyer, B.B.; Lewis, C.M., Jr.; Gaffney, P.M.; Garrison, N.A.; et al. Communicating Precision Medicine Research: Multidisciplinary Teams and Diverse Communities. Public Health Genom. 2022, 25, 1–9. [Google Scholar] [CrossRef]
- Betancourt, L.H.; Sanchez, A.; Pla, I.; Kuras, M.; Zhou, Q.; Andersson, R.; Marko-Varga, G. Quantitative Assessment of Urea In-Solution Lys-C/Trypsin Digestions Reveals Superior Performance at Room Temperature over Traditional Proteolysis at 37 °C. J. Proteome Res. 2018, 17, 2556–2561. [Google Scholar] [CrossRef]
- Happersberger, H.P.; Przybylski, M.; Glocker, M.O. Selective bridging of bis-cysteinyl residues by arsonous acid derivatives as an approach to the characterization of protein tertiary structures and folding pathways by mass spectrometry. Anal. Biochem. 1998, 264, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Kilshaw, P.J.; McEwan, F.J.; Baker, K.C.; Cant, A.J. Studies on the specificity of antibodies to ovalbumin in normal human serum: Technical considerations in the use of ELISA methods. Clin. Exp. Immunol. 1986, 66, 481–489. [Google Scholar]
- Wang, H.; Tu, Z.C.; Liu, G.X.; Liu, C.M.; Huang, X.Q.; Xiao, H. Comparison of glycation in conventionally and microwave-heated ovalbumin by high resolution mass spectrometry. Food Chem. 2013, 141, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Barendregt, A.; Kamerling, J.P.; Heck, A.J. Analyzing protein micro-heterogeneity in chicken ovalbumin by high-resolution native mass spectrometry exposes qualitatively and semi-quantitatively 59 proteoforms. Anal. Chem. 2013, 85, 12037–12045. [Google Scholar] [CrossRef] [PubMed]
- Postu, P.A.; Ion, L.; Drochioiu, G.; Petre, B.A.; Glocker, M.O. Mass spectrometric characterization of the zein protein composition in maize flour extracts upon protein separation by SDS-PAGE and 2D gel electrophoresis. Electrophoresis 2019, 40, 2747–2758. [Google Scholar] [CrossRef]
- Röwer, C.; Koy, C.; Hecker, M.; Reimer, T.; Gerber, B.; Thiesen, H.J.; Glocker, M.O. Mass spectrometric characterization of protein structure details refines the proteome signature for invasive ductal breast carcinoma. J. Am. Soc. Mass Spectrom. 2011, 22, 440–456. [Google Scholar] [CrossRef]
- Yang, J.; Rower, C.; Koy, C.; Russ, M.; Ruger, C.P.; Zimmermann, R.; von Fritschen, U.; Bredell, M.; Finke, J.C.; Glocker, M.O. Mass spectrometric characterization of limited proteolysis activity in human plasma samples under mild acidic conditions. Methods 2015, 89, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; Garcia-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE database resources in 2022: A hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022, 50, D543–D552. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zammataro, A.; Koy, C.; Ruß, M.; Röwer, C.; Glocker, M.O. Intact Transition Epitope Mapping—Serological Inspection by Epitope EXtraction (ITEM—SIX). Molecules 2023, 28, 3092. https://doi.org/10.3390/molecules28073092
Zammataro A, Koy C, Ruß M, Röwer C, Glocker MO. Intact Transition Epitope Mapping—Serological Inspection by Epitope EXtraction (ITEM—SIX). Molecules. 2023; 28(7):3092. https://doi.org/10.3390/molecules28073092
Chicago/Turabian StyleZammataro, Agatino, Cornelia Koy, Manuela Ruß, Claudia Röwer, and Michael O. Glocker. 2023. "Intact Transition Epitope Mapping—Serological Inspection by Epitope EXtraction (ITEM—SIX)" Molecules 28, no. 7: 3092. https://doi.org/10.3390/molecules28073092
APA StyleZammataro, A., Koy, C., Ruß, M., Röwer, C., & Glocker, M. O. (2023). Intact Transition Epitope Mapping—Serological Inspection by Epitope EXtraction (ITEM—SIX). Molecules, 28(7), 3092. https://doi.org/10.3390/molecules28073092