Obesity and Type 2 Diabetes: Adiposopathy as a Triggering Factor and Therapeutic Options
Abstract
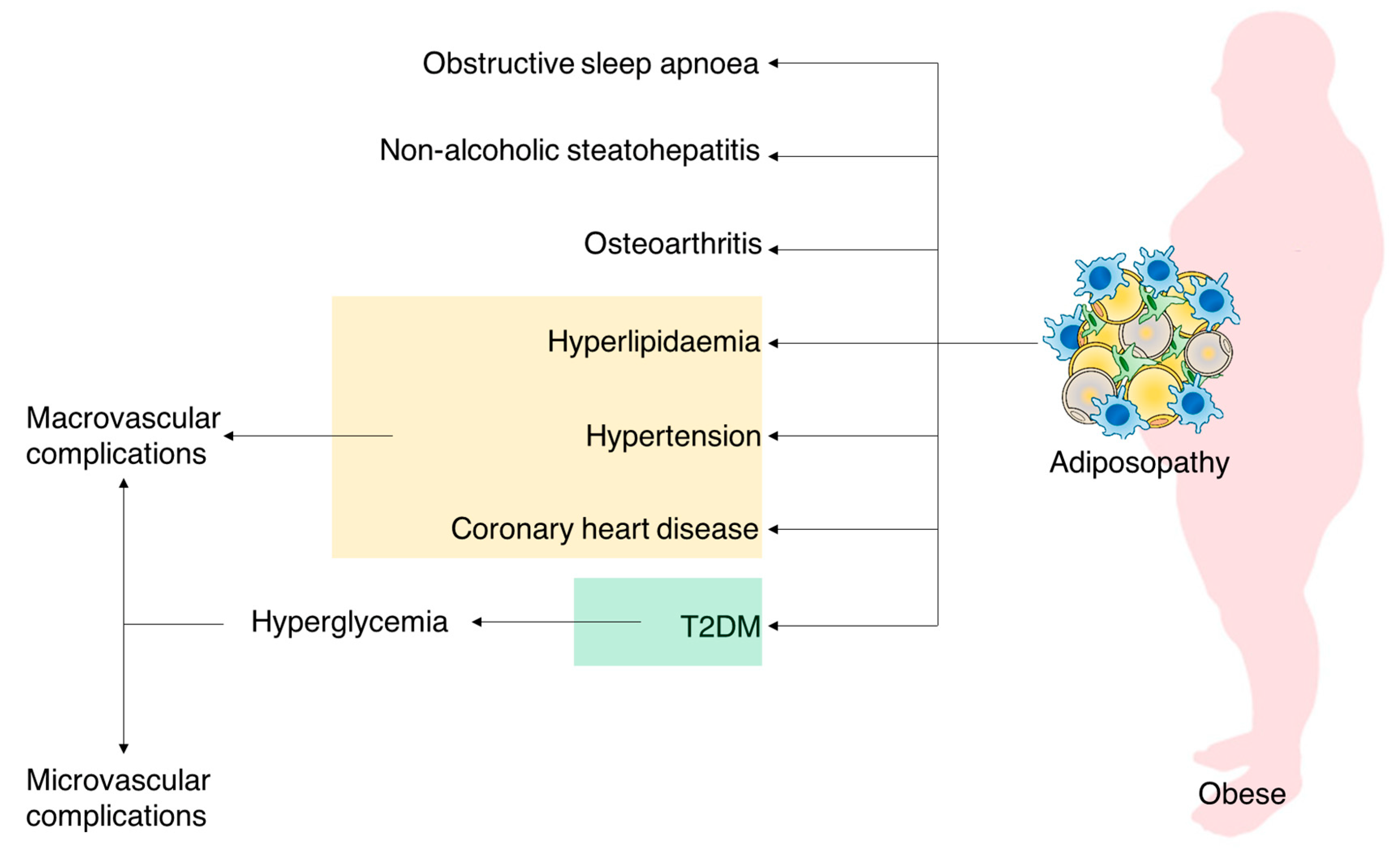
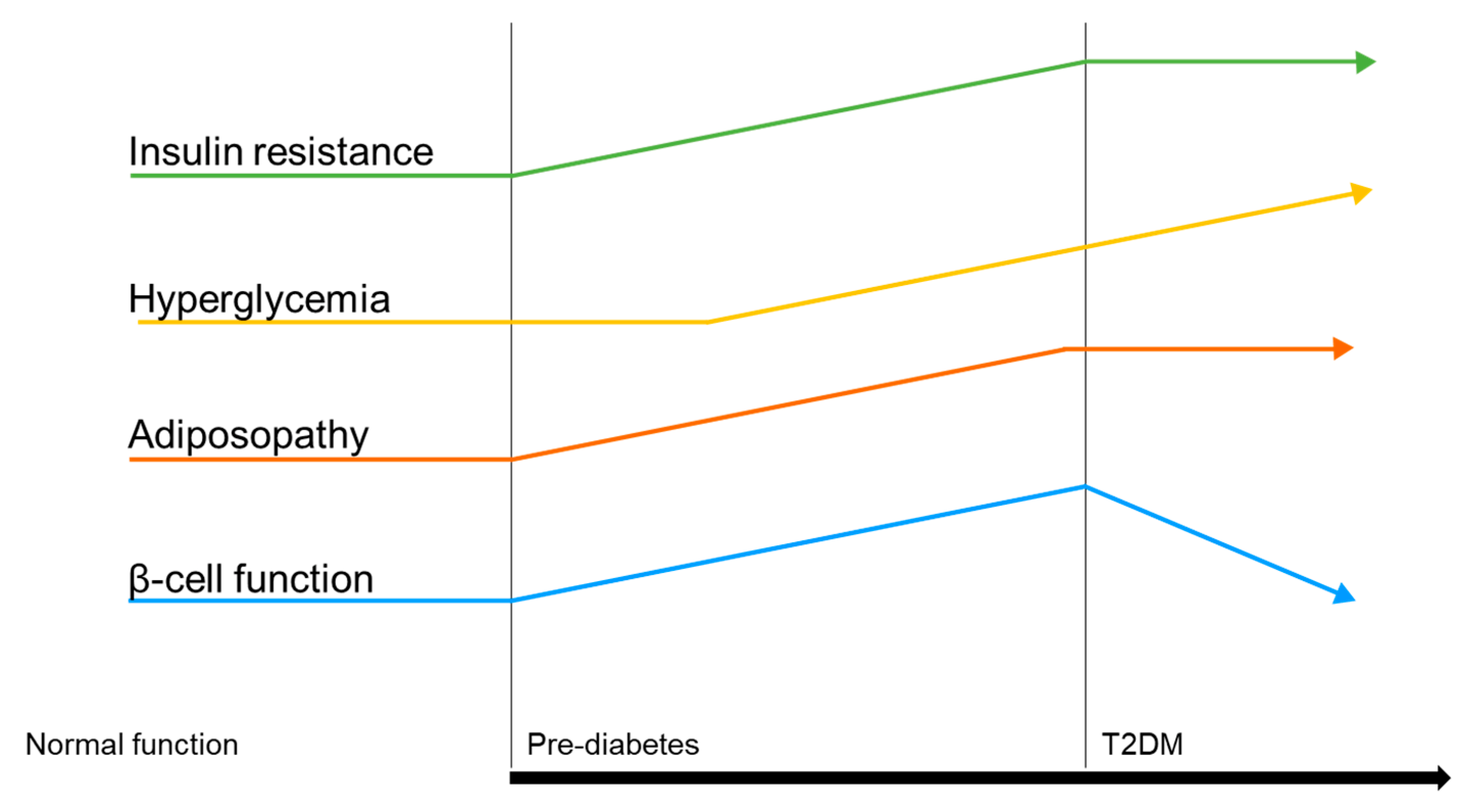
1. Background
Adiposity Impact on T2DM
2. Weight Loss Interventions
2.1. Overview
2.2. Bariatric Surgery
2.3. Pharmacotherapy for Chronic Weight Management
3. Management of T2DM and Obesity
3.1. Biguanides
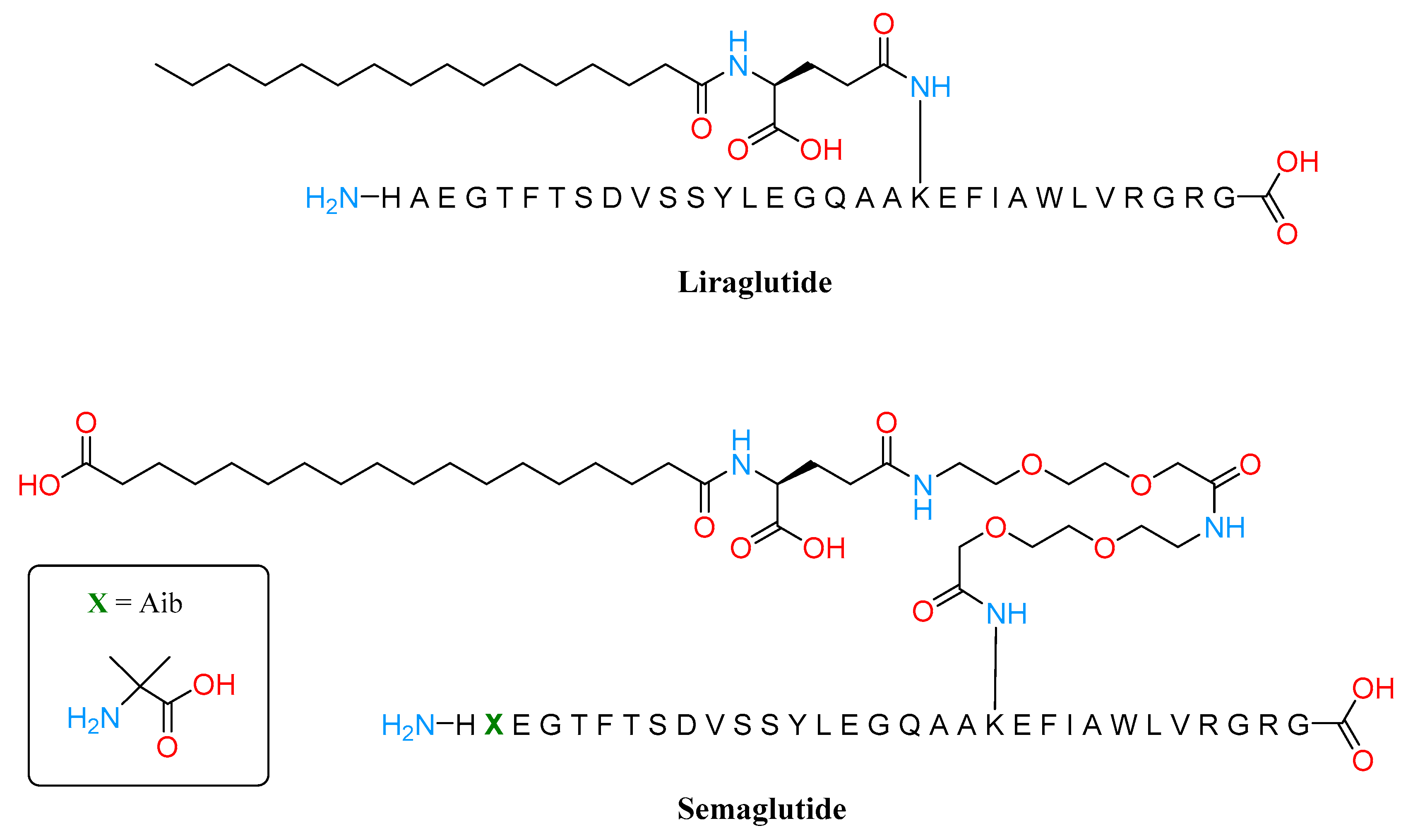
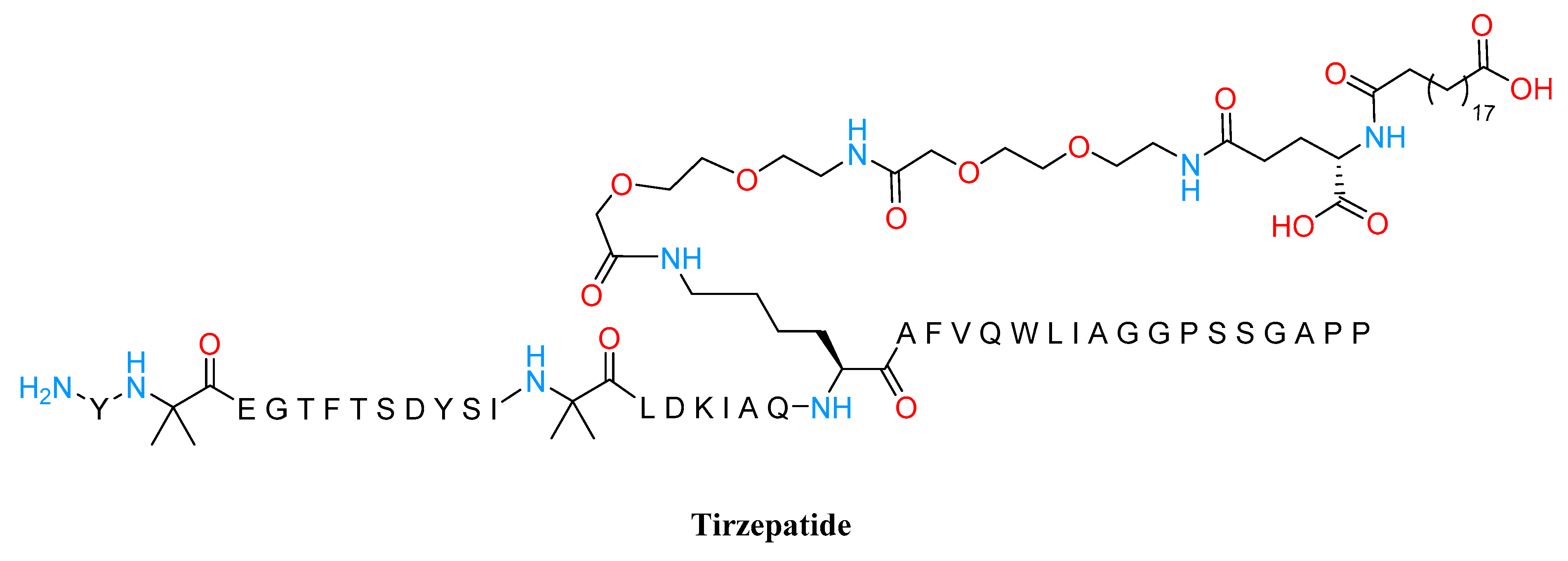
3.2. GLP1 Agonist
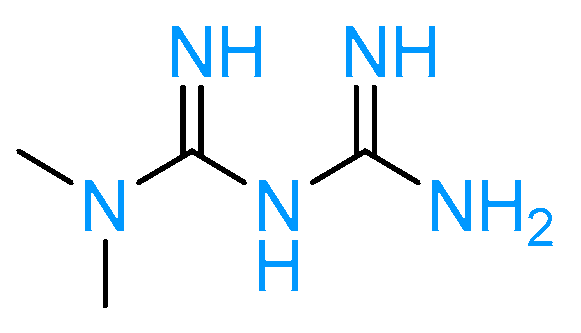
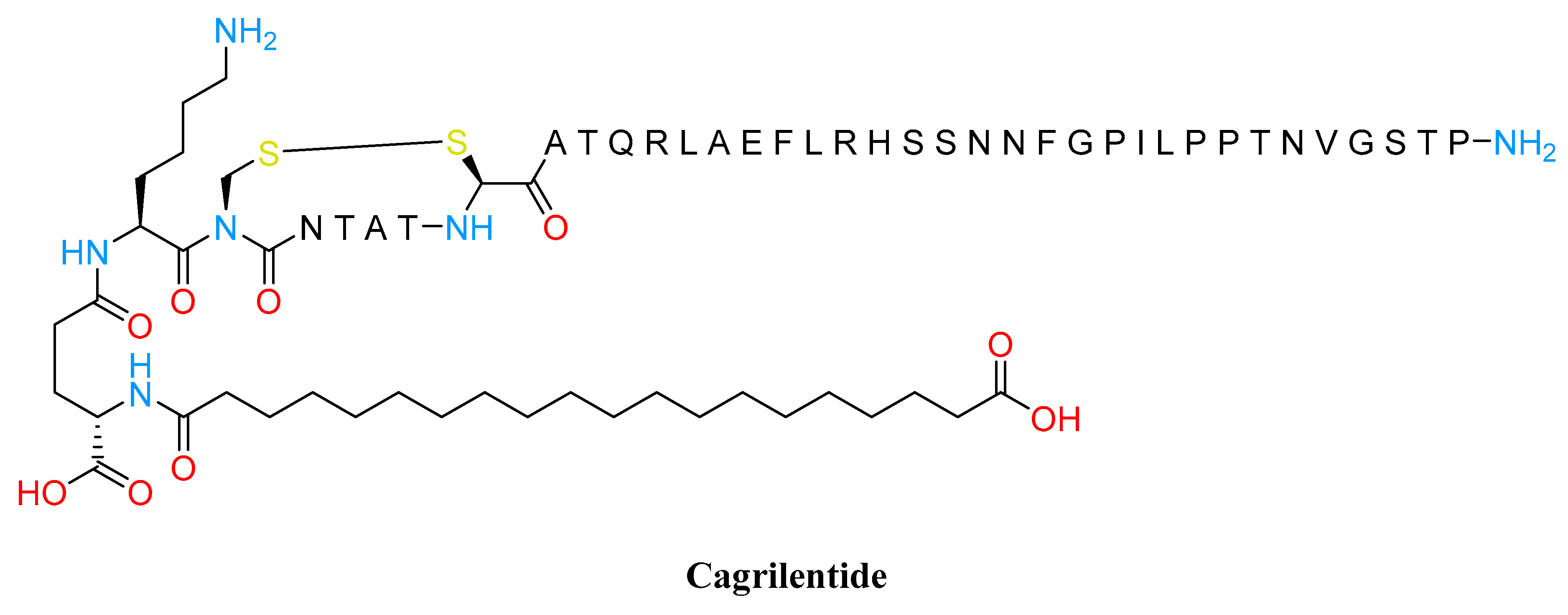
3.3. Amylin Analogs
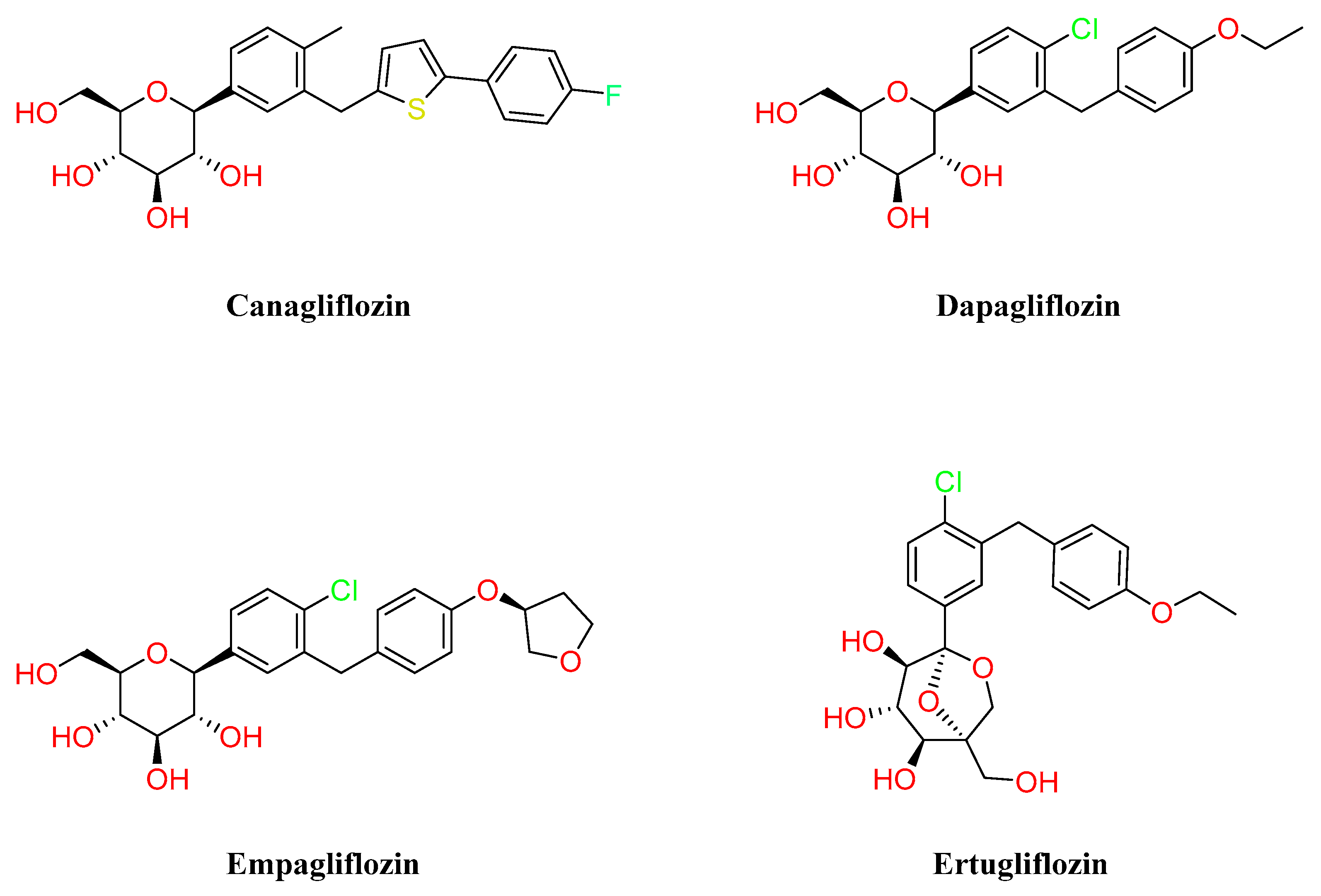
3.4. SGLT-2 Inhibitors
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- IDF Diabetes Atlas|Tenth Edition. Available online: https://diabetesatlas.org/ (accessed on 20 February 2023).
- Chennaiah, A.; Bhowmick, S.; Vankar, Y.D. Conversion of Glycals into Vicinal-1,2-Diazides and 1,2-(or 2,1)-Azidoacetates Using Hypervalent Iodine Reagents and Me 3 SiN 3. Application in the Synthesis of N-Glycopeptides, Pseudo-Trisaccharides and an Iminosugar. RSC Adv. 2017, 7, 41755–41762. [Google Scholar] [CrossRef]
- Rajasekaran, P.; Ande, C.; Vankar, Y.D. Synthesis of (5,6 & 6,6)-Oxa-Oxa Annulated Sugars as Glycosidase Inhibitors from 2-Formyl Galactal Using Iodocyclization as a Key Step. Arkivoc 2022, 2022, 5–23. [Google Scholar] [CrossRef]
- Tseng, P.; Ande, C.; Moremen, K.W.; Crich, D. Influence of Side Chain Conformation on the Activity of Glycosidase Inhibitors. Angew. Chem. Int. Ed. 2023, 62. [Google Scholar] [CrossRef] [PubMed]
- Artasensi, A.; Angeli, A.; Lammi, C.; Bollati, C.; Gervasoni, S.; Baron, G.; Matucci, R.; Supuran, C.T.; Vistoli, G.; Fumagalli, L. Discovery of a Potent and Highly Selective Dipeptidyl Peptidase IV and Carbonic Anhydrase Inhibitor as “Antidiabesity” Agents Based on Repurposing and Morphing of WB-4101. J. Med. Chem 2023, 2022, 13946–13966. [Google Scholar] [CrossRef]
- Artasensi, A.; Pedretti, A.; Vistoli, G.; Fumagalli, L. Type 2 Diabetes Mellitus: A Review of Multi-Target Drugs. Molecules 2020, 25, 1987. [Google Scholar] [CrossRef]
- Gregg, E.W.; Cheng, Y.J.; Srinivasan, M.; Lin, J.; Geiss, L.S.; Albright, A.L.; Imperatore, G. Trends in Cause-Specific Mortality among Adults with and without Diagnosed Diabetes in the USA: An Epidemiological Analysis of Linked National Survey and Vital Statistics Data. Lancet 2018, 391, 2430–2440. [Google Scholar] [CrossRef]
- Yang, M.; Liu, S.; Zhang, C. The Related Metabolic Diseases and Treatments of Obesity. Healthcare 2022, 10, 1616. [Google Scholar] [CrossRef]
- Kyrou, I.; Randeva, H.S.; Tsigos, C.; Kaltsas, G.; Weickert, M.O. Clinical Problems Caused by Obesity; National Institutes of Health: Bethesda, MD, USA, 2000. [Google Scholar]
- García-Molina, L.; Lewis-Mikhael, A.-M.; Riquelme-Gallego, B.; Cano-Ibáñez, N.; Oliveras-López, M.-J.; Bueno-Cavanillas, A. Improving Type 2 Diabetes Mellitus Glycaemic Control through Lifestyle Modification Implementing Diet Intervention: A Systematic Review and Meta-Analysis. Eur. J. Nutr. 2020, 59, 1313–1328. [Google Scholar] [CrossRef]
- Lingvay, I.; Sumithran, P.; Cohen, R.V.; le Roux, C.W. Obesity Management as a Primary Treatment Goal for Type 2 Diabetes: Time to Reframe the Conversation. Lancet 2022, 399, 394–405. [Google Scholar] [CrossRef]
- Bays, H.E. Current and Investigational Antiobesity Agents and Obesity Therapeutic Treatment Targets. Obes. Res. 2004, 12, 1197–1211. [Google Scholar] [CrossRef]
- Iacobini, C.; Pugliese, G.; Blasetti Fantauzzi, C.; Federici, M.; Menini, S. Metabolically Healthy versus Metabolically Unhealthy Obesity. Metabolism 2019, 92, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ballantyne, C.M. Metabolic Inflammation and Insulin Resistance in Obesity. Circ. Res. 2020, 126, 1549–1564. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, C.N.; Deyoung, S.M.; Saltiel, A.R. Macrophages Block Insulin Action in Adipocytes by Altering Expression of Signaling and Glucose Transport Proteins. Am. J. Physiol.-Endocrinol. Metab. 2007, 292, E166–E174. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Grijalva, A.; Skowronski, A.; van Eijk, M.; Serlie, M.J.; Ferrante, A.W. Obesity Activates a Program of Lysosomal-Dependent Lipid Metabolism in Adipose Tissue Macrophages Independently of Classic Activation. Cell Metab. 2013, 18, 816. [Google Scholar] [CrossRef]
- Kusminski, C.M.; Bickel, P.E.; Scherer, P.E. Targeting Adipose Tissue in the Treatment of Obesity-Associated Diabetes. Nat. Rev. Drug Discov. 2016, 15, 639–660. [Google Scholar] [CrossRef]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms Linking Obesity to Insulin Resistance and Type 2 Diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef]
- Caleyachetty, R.; Barber, T.M.; Mohammed, N.I.; Cappuccio, F.P.; Hardy, R.; Mathur, R.; Banerjee, A.; Gill, P. Ethnicity-Specific BMI Cutoffs for Obesity Based on Type 2 Diabetes Risk in England: A Population-Based Cohort Study. Lancet Diabetes Endocrinol. 2021, 9, 419–426. [Google Scholar] [CrossRef]
- Aronne, L.J.; Hall, K.D.; Jakicic, J.M.; Leibel, R.L.; Lowe, M.R.; Rosenbaum, M.; Klein, S. Describing the Weight-Reduced State: Physiology, Behavior, and Interventions. Obesity 2021, 29, S9–S24. [Google Scholar] [CrossRef]
- Batterham, R.L.; Cummings, D.E. Mechanisms of Diabetes Improvement Following Bariatric/Metabolic Surgery. Diabetes Care 2016, 39, 893–901. [Google Scholar] [CrossRef]
- Dixon, J.B.; Zimmet, P.; Alberti, K.G.; Rubino, F. Bariatric Surgery: An IDF Statement for Obese Type 2 Diabetes. Diabet. Med. 2011, 28, 628. [Google Scholar] [CrossRef]
- Mingrone, G.; Panunzi, S.; de Gaetano, A.; Guidone, C.; Iaconelli, A.; Capristo, E.; Chamseddine, G.; Bornstein, S.R.; Rubino, F. Metabolic Surgery versus Conventional Medical Therapy in Patients with Type 2 Diabetes: 10-Year Follow-up of an Open-Label, Single-Centre, Randomised Controlled Trial. Lancet 2021, 397, 293–304. [Google Scholar] [CrossRef]
- Sandoval, D.A.; Patti, M.E. Glucose Metabolism after Bariatric Surgery: Implications for T2DM Remission and Hypoglycaemia. Nat. Rev. Endocrinol. 2023, 19, 164–176. [Google Scholar] [CrossRef]
- Barthold, D.; Brouwer, E.; Barton, L.J.; Arterburn, D.E.; Basu, A.; Courcoulas, A.; Crawford, C.L.; Fedorka, P.N.; Fischer, H.; Kim, B.B.; et al. Minimum Threshold of Bariatric Surgical Weight Loss for Initial Diabetes Remission. Diabetes Care 2022, 45, 92–99. [Google Scholar] [CrossRef]
- Carlsson, L.M.S.; Peltonen, M.; Ahlin, S.; Anveden, Å.; Bouchard, C.; Carlsson, B.; Jacobson, P.; Lönroth, H.; Maglio, C.; Näslund, I.; et al. Bariatric Surgery and Prevention of Type 2 Diabetes in Swedish Obese Subjects. N. Engl. J. Med. 2012, 367, 695–704. [Google Scholar] [CrossRef]
- Arterburn, D.E.; Telem, D.A.; Kushner, R.F.; Courcoulas, A.P. Benefits and Risks of Bariatric Surgery in Adults. JAMA 2020, 324, 879. [Google Scholar] [CrossRef]
- Kelley, D.E.; Bray, G.A.; Pi-Sunyer, F.X.; Klein, S.; Hill, J.; Miles, J.; Hollander, P. Clinical Efficacy of Orlistat Therapy in Overweight and Obese Patients With Insulin-Treated Type 2 Diabetes. Diabetes Care 2002, 25, 1033–1041. [Google Scholar] [CrossRef]
- Davies, M.J.; Bergenstal, R.; Bode, B.; Kushner, R.F.; Lewin, A.; Skjøth, T.V.; Andreasen, A.H.; Jensen, C.B.; DeFronzo, R.A. Efficacy of Liraglutide for Weight Loss Among Patients With Type 2 Diabetes. JAMA 2015, 314, 687. [Google Scholar] [CrossRef]
- Gadde, K.M.; Allison, D.B.; Ryan, D.H.; Peterson, C.A.; Troupin, B.; Schwiers, M.L.; Day, W.W. Effects of Low-Dose, Controlled-Release, Phentermine plus Topiramate Combination on Weight and Associated Comorbidities in Overweight and Obese Adults (CONQUER): A Randomised, Placebo-Controlled, Phase 3 Trial. Lancet 2011, 377, 1341–1352. [Google Scholar] [CrossRef] [PubMed]
- Hollander, P.; Gupta, A.K.; Plodkowski, R.; Greenway, F.; Bays, H.; Burns, C.; Klassen, P.; Fujioka, K. Effects of Naltrexone Sustained- Release/Bupropion Sustained-Release Combination Therapy on Body Weight and Glycemic Parameters in Overweight and Obese Patients With Type 2 Diabetes. Diabetes Care 2013, 36, 4022–4029. [Google Scholar] [CrossRef]
- Sjöström, L.; Rissanen, A.; Andersen, T.; Boldrin, M.; Golay, A.; Koppeschaar, H.P.; Krempf, M. Randomised Placebo-Controlled Trial of Orlistat for Weight Loss and Prevention of Weight Regain in Obese Patients. Lancet 1998, 352, 167–172. [Google Scholar] [CrossRef]
- Pi-Sunyer, X.; Astrup, A.; Fujioka, K.; Greenway, F.; Halpern, A.; Krempf, M.; Lau, D.C.W.; le Roux, C.W.; Violante Ortiz, R.; Jensen, C.B.; et al. A Randomized, Controlled Trial of 3.0 Mg of Liraglutide in Weight Management. N. Engl. J. Med. 2015, 373, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Garvey, W.T.; Ryan, D.H.; Look, M.; Gadde, K.M.; Allison, D.B.; Peterson, C.A.; Schwiers, M.; Day, W.W.; Bowden, C.H. Two-Year Sustained Weight Loss and Metabolic Benefits with Controlled-Release Phentermine/Topiramate in Obese and Overweight Adults (SEQUEL): A Randomized, Placebo-Controlled, Phase 3 Extension Study. Am. J. Clin. Nutr. 2012, 95, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Greenway, F.L.; Fujioka, K.; Plodkowski, R.A.; Mudaliar, S.; Guttadauria, M.; Erickson, J.; Kim, D.D.; Dunayevich, E.; COR-I Study Group. Effect of Naltrexone plus Bupropion on Weight Loss in Overweight and Obese Adults (COR-I): A Multicentre, Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet 2010, 376, 595–605. [Google Scholar] [CrossRef]
- Goswami, G.; Shinkazh, N.; Davis, N. Optimal Pharmacologic Treatment Strategies in Obesity and Type 2 Diabetes. J. Clin. Med. 2014, 3, 595–613. [Google Scholar] [CrossRef]
- Toft-Nielsen, M.B.; Damholt, M.B.; Madsbad, S.; Hilsted, L.M.; Hughes, T.E.; Michelsen, B.K.; Holst, J.J. Determinants of the Impaired Secretion of Glucagon-like Peptide-1 in Type 2 Diabetic Patients. J. Clin. Endocrinol. Metab. 2001, 86, 3717–3723. [Google Scholar] [CrossRef]
- Xu, G.; Kaneto, H.; Laybutt, D.R.; Duvivier-Kali, V.F.; Trivedi, N.; Suzuma, K.; King, G.L.; Weir, G.C.; Bonner-Weir, S. Downregulation of GLP-1 and GIP Receptor Expression by Hyperglycemia: Possible Contribution to Impaired Incretin Effects in Diabetes. Diabetes 2007, 56, 1551–1558. [Google Scholar] [CrossRef]
- Pathak, V.; Vasu, S.; Flatt, P.R.; Irwin, N. Effects of Chronic Exposure of Clonal β-Cells to Elevated Glucose and Free Fatty Acids on Incretin Receptor Gene Expression and Secretory Responses to GIP and GLP-1. Diabetes Obes. Metab. 2014, 16, 357–365. [Google Scholar] [CrossRef]
- Højberg, P.V.; Vilsbøll, T.; Rabøl, R.; Knop, F.K.; Bache, M.; Krarup, T.; Holst, J.J.; Madsbad, S. Four Weeks of Near-Normalisation of Blood Glucose Improves the Insulin Response to Glucagon-like Peptide-1 and Glucose-Dependent Insulinotropic Polypeptide in Patients with Type 2 Diabetes. Diabetologia 2009, 52, 199–207. [Google Scholar] [CrossRef]
- Piteau, S.; Olver, A.; Kim, S.-J.; Winter, K.; Pospisilik, J.A.; Lynn, F.; Manhart, S.; Demuth, H.-U.; Speck, M.; Pederson, R.A.; et al. Reversal of Islet GIP Receptor Down-Regulation and Resistance to GIP by Reducing Hyperglycemia in the Zucker Rat. Biochem. Biophys. Res. Commun. 2007, 362, 1007–1012. [Google Scholar] [CrossRef]
- Asmar, M.; Tangaa, W.; Madsbad, S.; Hare, K.; Astrup, A.; Flint, A.; Bülow, J.; Holst, J.J. On the Role of Glucose-Dependent Insulintropic Polypeptide in Postprandial Metabolism in Humans. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E614–E621. [Google Scholar] [CrossRef]
- Rowlands, J.; Heng, J.; Newsholme, P.; Carlessi, R. Pleiotropic Effects of GLP-1 and Analogs on Cell Signaling, Metabolism, and Function. Front. Endocrinol. 2018, 9, 672. [Google Scholar] [CrossRef]
- del Prato, S.; Gallwitz, B.; Holst, J.J.; Meier, J.J. The Incretin/Glucagon System as a Target for Pharmacotherapy of Obesity. Obes. Rev. 2022, 23, e13372. [Google Scholar] [CrossRef]
- Michałowska, J.; Miller-Kasprzak, E.; Bogdański, P. Incretin Hormones in Obesity and Related Cardiometabolic Disorders: The Clinical Perspective. Nutrients 2021, 13, 351. [Google Scholar] [CrossRef]
- Nauck, M.A.; Meier, J.J. Incretin Hormones: Their Role in Health and Disease. Diabetes Obes. Metab. 2018, 20 (Suppl. 1), 5–21. [Google Scholar] [CrossRef]
- Mroz, P.A.; Finan, B.; Gelfanov, V.; Yang, B.; Tschöp, M.H.; DiMarchi, R.D.; Perez-Tilve, D. Optimized GIP Analogs Promote Body Weight Lowering in Mice through GIPR Agonism Not Antagonism. Mol. Metab. 2019, 20, 51–62. [Google Scholar] [CrossRef]
- NamKoong, C.; Kim, M.S.; Jang, B.-T.; Lee, Y.H.; Cho, Y.-M.; Choi, H.J. Central Administration of GLP-1 and GIP Decreases Feeding in Mice. Biochem. Biophys. Res. Commun. 2017, 490, 247–252. [Google Scholar] [CrossRef]
- Rudovich, N.; Kaiser, S.; Engeli, S.; Osterhoff, M.; Gögebakan, O.; Bluher, M.; Pfeiffer, A.F.H. GIP Receptor MRNA Expression in Different Fat Tissue Depots in Postmenopausal Non-Diabetic Women. Regul. Pept. 2007, 142, 138–145. [Google Scholar] [CrossRef]
- Samms, R.J.; Coghlan, M.P.; Sloop, K.W. How May GIP Enhance the Therapeutic Efficacy of GLP-1? Trends Endocrinol. Metab. 2020, 31, 410–421. [Google Scholar] [CrossRef]
- DeFronzo, R.A. Insulin Resistance, Lipotoxicity, Type 2 Diabetes and Atherosclerosis: The Missing Links. The Claude Bernard Lecture 2009. Diabetologia 2010, 53, 1270–1287. [Google Scholar] [CrossRef]
- Mohammad, S.; Ramos, L.S.; Buck, J.; Levin, L.R.; Rubino, F.; McGraw, T.E. Gastric Inhibitory Peptide Controls Adipose Insulin Sensitivity via Activation of CAMP-Response Element-Binding Protein and P110β Isoform of Phosphatidylinositol 3-Kinase. J. Biol. Chem. 2011, 286, 43062–43070. [Google Scholar] [CrossRef]
- Alruwaili, H.; Dehestani, B.; le Roux, C.W. Clinical Impact of Liraglutide as a Treatment of Obesity. Clin. Pharmacol. 2021, 13, 53–60. [Google Scholar] [CrossRef]
- Pratley, R.E.; Nauck, M.; Bailey, T.; Montanya, E.; Cuddihy, R.; Filetti, S.; Thomsen, A.B.; Søndergaard, R.E.; Davies, M. Liraglutide versus Sitagliptin for Patients with Type 2 Diabetes Who Did Not Have Adequate Glycaemic Control with Metformin: A 26-Week, Randomised, Parallel-Group, Open-Label Trial. Lancet 2010, 375, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Marso, S.P.; Neeland, I.J. Liraglutide for Weight Management: A Critical Review of the Evidence. Obes. Sci. Pract. 2017, 3, 3–14. [Google Scholar] [CrossRef]
- le Roux, C.; Aroda, V.; Hemmingsson, J.; Cancino, A.P.; Christensen, R.; Pi-Sunyer, X. Comparison of Efficacy and Safety of Liraglutide 3.0 Mg in Individuals with BMI above and below 35 Kg/M2: A Post-Hoc Analysis. Obes. Facts 2017, 10, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Wadden, T.A.; Hollander, P.; Klein, S.; Niswender, K.; Woo, V.; Hale, P.M.; Aronne, L. Weight Maintenance and Additional Weight Loss with Liraglutide after Low-Calorie-Diet-Induced Weight Loss: The SCALE Maintenance Randomized Study. Int. J. Obes. 2013, 37, 1443–1451. [Google Scholar] [CrossRef]
- Ahmann, A.J.; Capehorn, M.; Charpentier, G.; Dotta, F.; Henkel, E.; Lingvay, I.; Holst, A.G.; Annett, M.P.; Aroda, V.R. Efficacy and Safety of Once-Weekly Semaglutide Versus Exenatide ER in Subjects With Type 2 Diabetes (SUSTAIN 3): A 56-Week, Open-Label, Randomized Clinical Trial. Diabetes Care 2018, 41, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Pratley, R.E.; Aroda, V.R.; Lingvay, I.; Lüdemann, J.; Andreassen, C.; Navarria, A.; Viljoen, A. Semaglutide versus Dulaglutide Once Weekly in Patients with Type 2 Diabetes (SUSTAIN 7): A Randomised, Open-Label, Phase 3b Trial. Lancet Diabetes Endocrinol. 2018, 6, 275–286. [Google Scholar] [CrossRef]
- O’Neil, P.M.; Birkenfeld, A.L.; McGowan, B.; Mosenzon, O.; Pedersen, S.D.; Wharton, S.; Carson, C.G.; Jepsen, C.H.; Kabisch, M.; Wilding, J.P.H. Efficacy and Safety of Semaglutide Compared with Liraglutide and Placebo for Weight Loss in Patients with Obesity: A Randomised, Double-Blind, Placebo and Active Controlled, Dose-Ranging, Phase 2 Trial. Lancet 2018, 392, 637–649. [Google Scholar] [CrossRef]
- Knudsen, L.B.; Lau, J. The Discovery and Development of Liraglutide and Semaglutide. Front. Endocrinol. 2019, 10, 155. [Google Scholar] [CrossRef]
- Coskun, T.; Sloop, K.W.; Loghin, C.; Alsina-Fernandez, J.; Urva, S.; Bokvist, K.B.; Cui, X.; Briere, D.A.; Cabrera, O.; Roell, W.C.; et al. LY3298176, a Novel Dual GIP and GLP-1 Receptor Agonist for the Treatment of Type 2 Diabetes Mellitus: From Discovery to Clinical Proof of Concept. Mol. Metab. 2018, 18, 3–14. [Google Scholar] [CrossRef]
- Nauck, M.A.; D‘Alessio, D.A. Tirzepatide, a Dual GIP/GLP-1 Receptor Co-Agonist for the Treatment of Type 2 Diabetes with Unmatched Effectiveness Regrading Glycaemic Control and Body Weight Reduction. Cardiovasc. Diabetol. 2022, 21, 169. [Google Scholar] [CrossRef]
- Min, T.; Bain, S.C. The Role of Tirzepatide, Dual GIP and GLP-1 Receptor Agonist, in the Management of Type 2 Diabetes: The SURPASS Clinical Trials. Diabetes Ther. 2021, 12, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Study Record | Beta ClinicalTrials.Gov. Available online: https://beta.clinicaltrials.gov/study/NCT05364931?distance=50&term=PROXYMO-ADV&rank=1 (accessed on 21 March 2023).
- Nahra, R.; Wang, T.; Gadde, K.M.; Oscarsson, J.; Stumvoll, M.; Jermutus, L.; Hirshberg, B.; Ambery, P. Effects of Cotadutide on Metabolic and Hepatic Parameters in Adults With Overweight or Obesity and Type 2 Diabetes: A 54-Week Randomized Phase 2b Study. Diabetes Care 2021, 44, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, T.; Thomas, L.; Baader-Pagler, T.; Haebel, P.; Simon, E.; Reindl, W.; Bajrami, B.; Rist, W.; Uphues, I.; Drucker, D.J.; et al. BI 456906: Discovery and Preclinical Pharmacology of a Novel GCGR/GLP-1R Dual Agonist with Robust Anti-Obesity Efficacy. Mol. Metab. 2022, 66, 101633. [Google Scholar] [CrossRef]
- Jiang, H.; Pang, S.; Zhang, Y.; Yu, T.; Liu, M.; Deng, H.; Li, L.; Feng, L.; Song, B.; Han-Zhang, H.; et al. A Phase 1b Randomised Controlled Trial of a Glucagon-like Peptide-1 and Glucagon Receptor Dual Agonist IBI362 (LY3305677) in Chinese Patients with Type 2 Diabetes. Nat. Commun. 2022, 13, 3613. [Google Scholar] [CrossRef]
- Urva, S.; Coskun, T.; Loh, M.T.; Du, Y.; Thomas, M.K.; Gurbuz, S.; Haupt, A.; Benson, C.T.; Hernandez-Illas, M.; D’Alessio, D.A.; et al. LY3437943, a Novel Triple GIP, GLP-1, and Glucagon Receptor Agonist in People with Type 2 Diabetes: A Phase 1b, Multicentre, Double-Blind, Placebo-Controlled, Randomised, Multiple-Ascending Dose Trial. Lancet 2022, 400, 1869–1881. [Google Scholar] [CrossRef] [PubMed]
- Hope, D.C.D.; Vincent, M.L.; Tan, T.M.M. Striking the Balance: GLP-1/Glucagon Co-Agonism as a Treatment Strategy for Obesity. Front. Endocrinol. 2021, 12, 735019. [Google Scholar] [CrossRef]
- Targher, G.; Mantovani, A.; Byrne, C.D. Mechanisms and Possible Hepatoprotective Effects of Glucagon-like Peptide-1 Receptor Agonists and Other Incretin Receptor Agonists in Non-Alcoholic Fatty Liver Disease. Lancet Gastroenterol. Hepatol. 2023, 8, 179–191. [Google Scholar] [CrossRef]
- Tan, T.M.-M. Co-Agonist Therapeutics Come of Age for Obesity. Nat. Rev. Endocrinol. 2023, 19, 66–67. [Google Scholar] [CrossRef]
- Nestor, J.J.; Zhang, X.; Jaw-Tsai, S.; Parkes, D.G.; Becker, C.K. Design and characterization of a surfactant-conjugated, long-acting, balanced GLP-1/glucagon receptor dual agonist. Pept. Sci. 2021, 113, e24221. [Google Scholar] [CrossRef]
- Martins, F.F.; Santos-Reis, T.; Marinho, T.S.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Hypothalamic Anorexigenic Signaling Pathways (Leptin, Amylin, and Proopiomelanocortin) Are Semaglutide (GLP-1 Analog) Targets in Obesity Control in Mice. Life Sci. 2023, 313, 121268. [Google Scholar] [CrossRef]
- Boyle, C.N.; Lutz, T.A.; le Foll, C. Amylin—Its Role in the Homeostatic and Hedonic Control of Eating and Recent Developments of Amylin Analogs to Treat Obesity. Mol. Metab. 2018, 8, 203. [Google Scholar] [CrossRef]
- Kruse, T.; Hansen, J.L.; Dahl, K.; Schäffer, L.; Sensfuss, U.; Poulsen, C.; Schlein, M.; Hansen, A.M.K.; Jeppesen, C.B.; Dornonville de la Cour, C.; et al. Development of Cagrilintide, a Long-Acting Amylin Analogue. J. Med. Chem. 2021, 64, 11183–11194. [Google Scholar] [CrossRef]
- Li, C.; Xu, J.J.; Hu, H.T.; Shi, C.Y.; Yu, C.J.; Sheng, J.Z.; Wu, Y.T.; Huang, H.F. Amylin Receptor Insensitivity Impairs Hypothalamic POMC Neuron Differentiation in the Male Offspring of Maternal High-Fat Diet-Fed Mice. Mol. Metab. 2021, 44, 101135. [Google Scholar] [CrossRef]
- Dunican, K.C.; Adams, N.M.; Desilets, A.R. The Role of Pramlintide for Weight Loss. Ann. Pharmacother. 2010, 44, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Lau, D.C.W.; Erichsen, L.; Francisco, A.M.; Satylganova, A.; le Roux, C.W.; McGowan, B.; Pedersen, S.D.; Pietiläinen, K.H.; Rubino, D.; Batterham, R.L. Once-Weekly Cagrilintide for Weight Management in People with Overweight and Obesity: A Multicentre, Randomised, Double-Blind, Placebo-Controlled and Active-Controlled, Dose-Finding Phase 2 Trial. Lancet 2021, 398, 2160–2172. [Google Scholar] [CrossRef]
- Enebo, L.B.; Berthelsen, K.K.; Kankam, M.; Lund, M.T.; Rubino, D.M.; Satylganova, A.; Lau, D.C.W. Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of Concomitant Administration of Multiple Doses of Cagrilintide with Semaglutide 2·4 Mg for Weight Management: A Randomised, Controlled, Phase 1b Trial. Lancet 2021, 397, 1736–1748. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.A.; Defronzo, R.A. Lowering Plasma Glucose Concentration by Inhibiting Renal Sodium-Glucose Cotransport. J. Intern. Med. 2014, 276, 352–363. [Google Scholar] [CrossRef]
- Ribola, F.A.; Cançado, F.B.; Schoueri, J.H.M.; de Toni, V.F.; Medeiros, V.H.R.; Feder, D. Effects of SGLT2 Inhibitors on Weight Loss in Patients with Type 2 Diabetes Mellitus. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 199–211. [Google Scholar] [PubMed]
- Lee, P.C.; Ganguly, S.; Goh, S.-Y. Weight Loss Associated with Sodium-Glucose Cotransporter-2 Inhibition: A Review of Evidence and Underlying Mechanisms. Obes. Rev. 2018, 19, 1630–1641. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Kaku, K.; Seino, Y.; Inagaki, N.; Haneda, M.; Sasaki, T.; Fukatsu, A.; Kakiuchi, H.; Samukawa, Y. Efficacy and Safety of the SGLT2 Inhibitor Luseogliflozin in Japanese Patients With Type 2 Diabetes Mellitus Stratified According to Baseline Body Mass Index: Pooled Analysis of Data From 52-Week Phase III Trials. Clin. Ther. 2016, 38, 843–862.e9. [Google Scholar] [CrossRef]
- Lavalle-González, F.J.; Januszewicz, A.; Davidson, J.; Tong, C.; Qiu, R.; Canovatchel, W.; Meininger, G. Efficacy and Safety of Canagliflozin Compared with Placebo and Sitagliptin in Patients with Type 2 Diabetes on Background Metformin Monotherapy: A Randomised Trial. Diabetologia 2013, 56, 2582–2592. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; DeFronzo, R.A.; Norton, L. Novel Hypothesis to Explain Why SGLT2 Inhibitors Inhibit Only 30-50% of Filtered Glucose Load in Humans. Diabetes 2013, 62, 3324–3328. [Google Scholar] [CrossRef]
- Nakayama, H.; Ohtsuka, Y.; Kawahara, M.; Nakamura, Y.; Iwata, S.; Yoshinobu, S.; Soga, R.; Oshige, T.; Kawano, S.; Kakino, S.; et al. Changes in Body Composition during SGLT2 Inhibitor Treatment and Their Relevance to the Improvement of Insulin Sensitivity. Diabetes Res. Clin. Pract. 2016, 120, S50–S51. [Google Scholar] [CrossRef]
- Bolinder, J.; Ljunggren, Ö.; Johansson, L.; Wilding, J.; Langkilde, A.M.; Sjöström, C.D.; Sugg, J.; Parikh, S. Dapagliflozin Maintains Glycaemic Control While Reducing Weight and Body Fat Mass over 2 Years in Patients with Type 2 Diabetes Mellitus Inadequately Controlled on Metformin. Diabetes Obes. Metab. 2014, 16, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Wen, W.; Li, J.; Xu, J.; Zhao, M.; Chen, H.; Sun, J. Systematic Review and Meta-Analysis of Randomized Controlled Trials on the Effect of SGLT2 Inhibitor on Blood Leptin and Adiponectin Level in Patients with Type 2 Diabetes. Horm. Metab. Res. 2019, 51, 487–494. [Google Scholar] [CrossRef]
- Ito, D.; Shimizu, S.; Inoue, K.; Saito, D.; Yanagisawa, M.; Inukai, K.; Akiyama, Y.; Morimoto, Y.; Noda, M.; Shimada, A. Comparison of Ipragliflozin and Pioglitazone Effects on Nonalcoholic Fatty Liver Disease in Patients With Type 2 Diabetes: A Randomized, 24-Week, Open-Label, Active-Controlled Trial. Diabetes Care 2017, 40, 1364–1372. [Google Scholar] [CrossRef] [PubMed]
- Jesudason, D.R.; Pedersen, E.; Clifton, P.M. Weight-Loss Diets in People with Type 2 Diabetes and Renal Disease: A Randomized Controlled Trial of the Effect of Different Dietary Protein Amounts. Am. J. Clin. Nutr. 2013, 98, 494–501. [Google Scholar] [CrossRef]
- Chesterman, T.; Thynne, T.R. Harms and Benefits of Sodium-Glucose Co-Transporter 2 Inhibitors. Aust. Prescr. 2020, 43, 168–171. [Google Scholar] [CrossRef]
| Drug | Company | Dose, Administration | Approval | Mode of Action | Weight Loss (Placebo/Drug) |
|---|---|---|---|---|---|
| Orlistat (Xenical®) | Roche Pharmaceuticals | 120 mg, TTD | 1999–present (EU, USA) | Inhibitor of gastrointestinal lipase | −6.1% to −10.2% [32] |
| Liraglutide (Saxenda®) | Novo Nordisk | with titration 3.0 mg OD | 2014–present (EU, USA) | GLP1R agonist | −2.6% to −8% [33] |
| Semaglutide (Wegovy®) | Novo Nordisk | 2.4 mg, OW | 2021–present (EU, USA) | GLP1R agonist | −2.4% to −14.9% [34] |
| Phentermine/ Topiramate (Qsymia®) | Vivus | with titration 15 mg/92 mg, OD | 2012–present (USA) | Central norepinephrine release | −1.2% to −9.3% (dose-dependent) [30,35] |
| Bupropion/ Naltrexone (Mysimba®) | Orexigen Therapeutics | 360 mg/32 mg, TD | 2014–present (EU, USA) | Increased central norepinephrine and dopamine and opioid receptor antagonist | −1.3% to −6.1% (dose-dependent) [36] |
| Metformin | DPP-IVi | GLP1RA | SGLT-2i | TZD | SU and GLN | Pramlintide | |
|---|---|---|---|---|---|---|---|
| Hypoglycemia | Neutral | Neutral | Neutral | Neutral | Neutral | Mild/Moderate | Neutral |
| Weight | Slight Loss | Neutral | Loss | Loss | Gain | Gain | Loss |
| Renal/GU | Contraindicated if eGFR <30 mL/min/1.73 m2 | Dose adjustment is necessary (except linagliptin) Effective in Reducing albuminuria | Exenatide not indicated CrCl <30 | Not indicated for eGFR <45 mL/min/1.73 m2 | Neutral | More Hypo Risk | Neutral |
| Genital Mycotic infection | |||||||
| Possible benefit of Liraglutide | Possible benefit of Empagliflozin | ||||||
| GI | Moderate | Neutral | Moderate | Neutral | Neutral | Neutral | Moderate |
| Cardiac | Neutral | Possible increase in hospitalization with alogliptin and saxagliptin | Liraglutide Prevents MACE events | Empagliflozin reduce CV mortality Canagliflozin reduce MACE events | Moderate risk for CHF | Possible ASCVD risk | Neutral |
| May reduce stroke risk | |||||||
| Bone | Neutral | Neutral | Neutral | Mild Fracture Risk | Neutral | Neutral | Neutral |
| Ketoacidosis | Neutral | Neutral | Neutral | DKA can occur in various Stress settings | Neutral | Neutral | Neutral |
 cardiovascular
cardiovascular  disease.
disease.  Possible benefit→Use with caution→Possible adverse effects.
Possible benefit→Use with caution→Possible adverse effects.| Drug | Company | Targets | Sequence Modified | Phase | Ref |
|---|---|---|---|---|---|
| Cotadutide | Altimmune | GLP1/GCGR | Glucagon | Phase II | [67] |
| BI 456906 | Boehringer Ingelheim | GLP1/GCGR | Glucagon | Phase II | [68] |
| LY3305677 | Eli Lilly | GLP1/GCGR | OXM | Phase I | [69] |
| LY3437943 | Eli Lilly | GLP1/GIP/GCGR | - | Phase I | [70] |
| JNJ-54729518 | J&J | GLP1/GCGR | OXM | Phase II | [71] |
| HM15211 | Hanmi | GLP1/GIP/GCGR | Glucagon | Phase II | [72] |
| NNC9204-1706 | Novo | GLP1/GIP/GCGR | - | Phase I | [73] |
| Alt-801 | Altimmune | GLP1/GCGR | GLP1 andglucagon | Phase I | [74] |
| G3215 | Imperial College/Zihipp Ltd. | GLP1/GCGR | OXM | Phase I | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Artasensi, A.; Mazzolari, A.; Pedretti, A.; Vistoli, G.; Fumagalli, L. Obesity and Type 2 Diabetes: Adiposopathy as a Triggering Factor and Therapeutic Options. Molecules 2023, 28, 3094. https://doi.org/10.3390/molecules28073094
Artasensi A, Mazzolari A, Pedretti A, Vistoli G, Fumagalli L. Obesity and Type 2 Diabetes: Adiposopathy as a Triggering Factor and Therapeutic Options. Molecules. 2023; 28(7):3094. https://doi.org/10.3390/molecules28073094
Chicago/Turabian StyleArtasensi, Angelica, Angelica Mazzolari, Alessandro Pedretti, Giulio Vistoli, and Laura Fumagalli. 2023. "Obesity and Type 2 Diabetes: Adiposopathy as a Triggering Factor and Therapeutic Options" Molecules 28, no. 7: 3094. https://doi.org/10.3390/molecules28073094
APA StyleArtasensi, A., Mazzolari, A., Pedretti, A., Vistoli, G., & Fumagalli, L. (2023). Obesity and Type 2 Diabetes: Adiposopathy as a Triggering Factor and Therapeutic Options. Molecules, 28(7), 3094. https://doi.org/10.3390/molecules28073094








