Metal Oxides Nanoparticles: General Structural Description, Chemical, Physical, and Biological Synthesis Methods, Role in Pesticides and Heavy Metal Removal through Wastewater Treatment
Abstract
1. Introduction
1.1. Developmental History of NanoTech
1.2. Production Rates of NMs
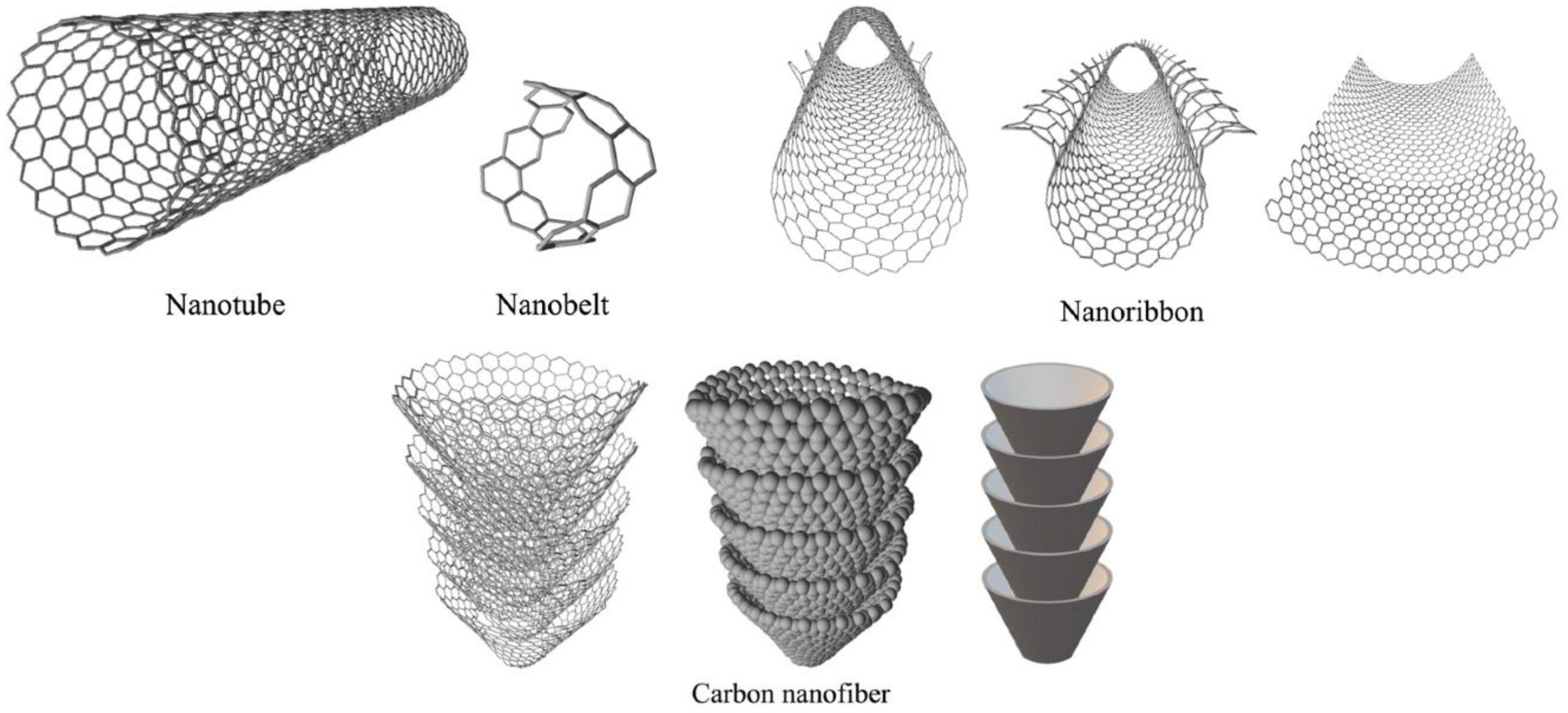
1.3. Classification of NMs
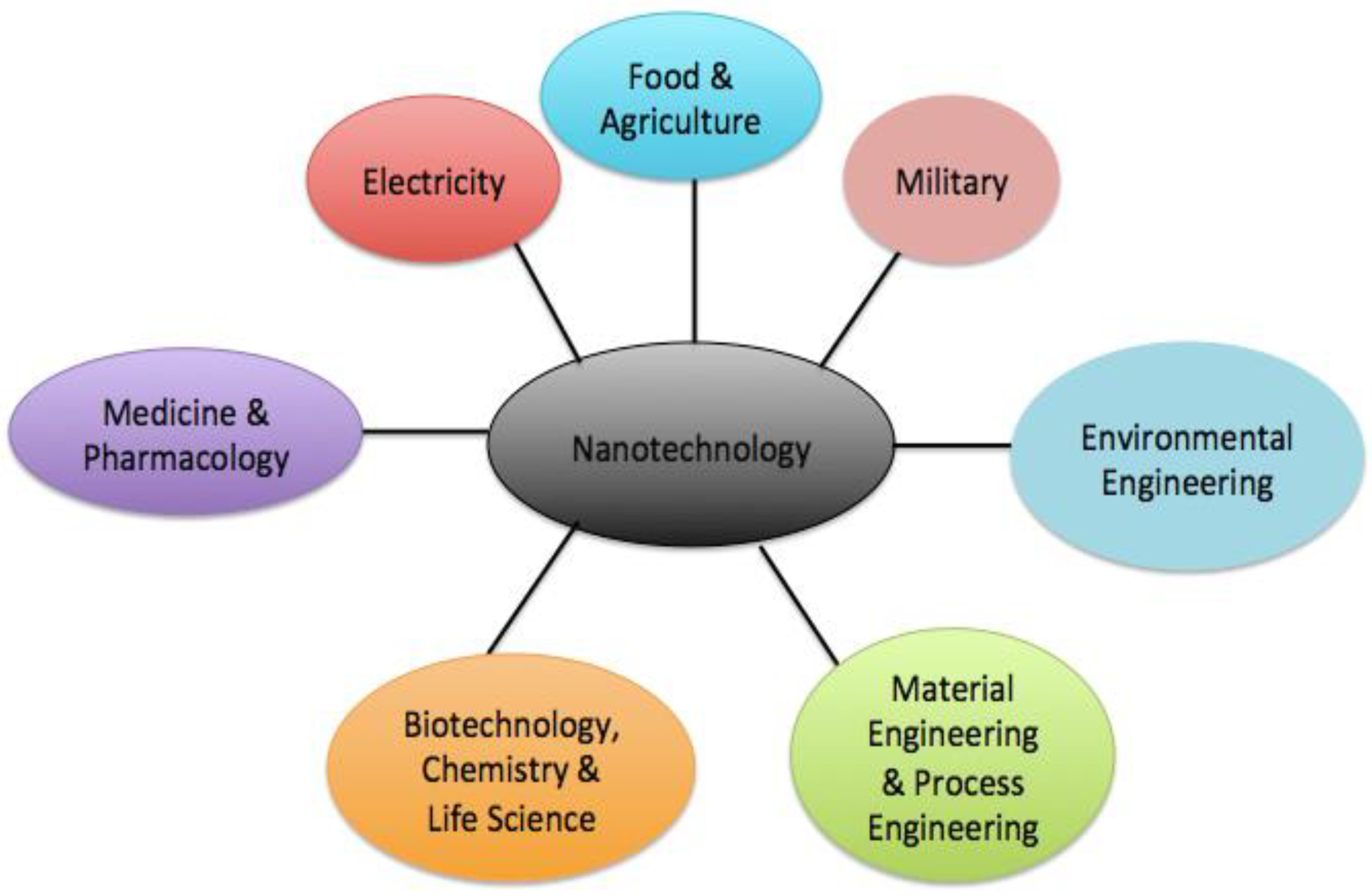
1.4. The Potential Applications of NMs
2. Metal Oxide Nanoparticles
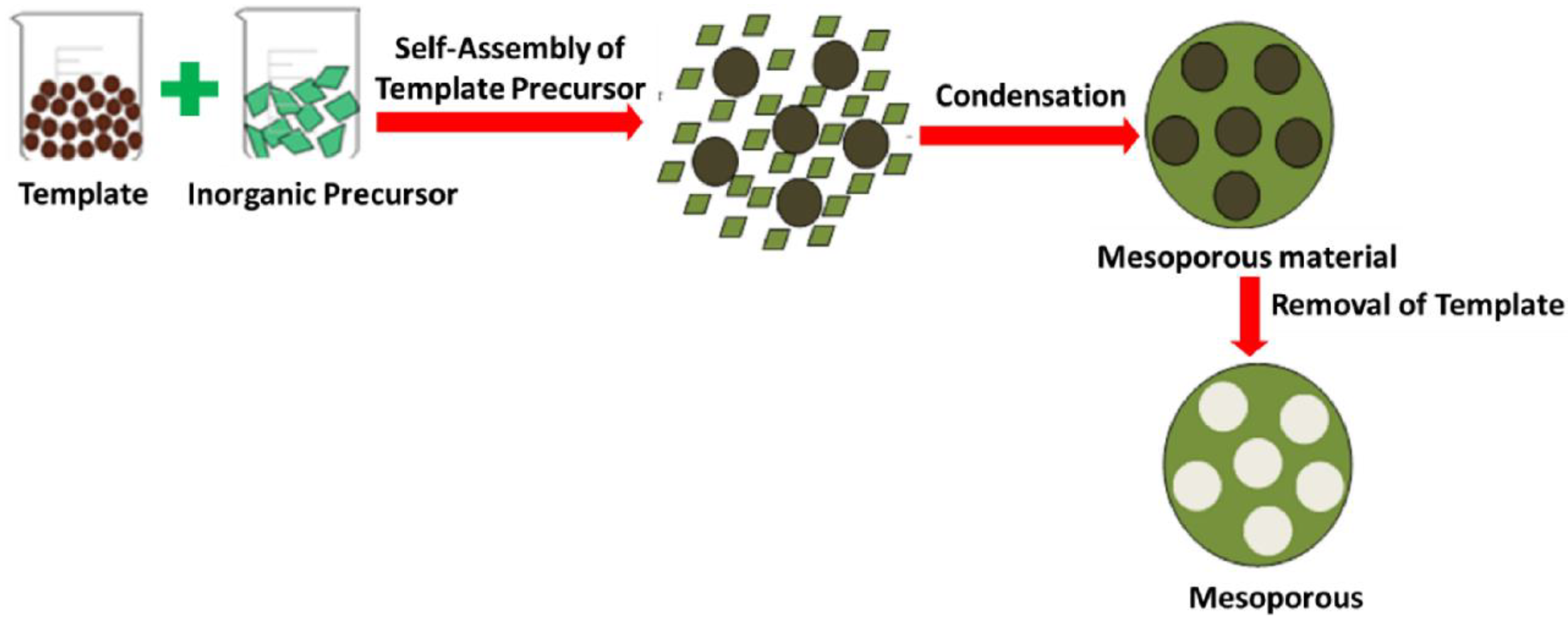
2.1. Preparation of MOx Nanoparticles Using the Template-Assisted Method
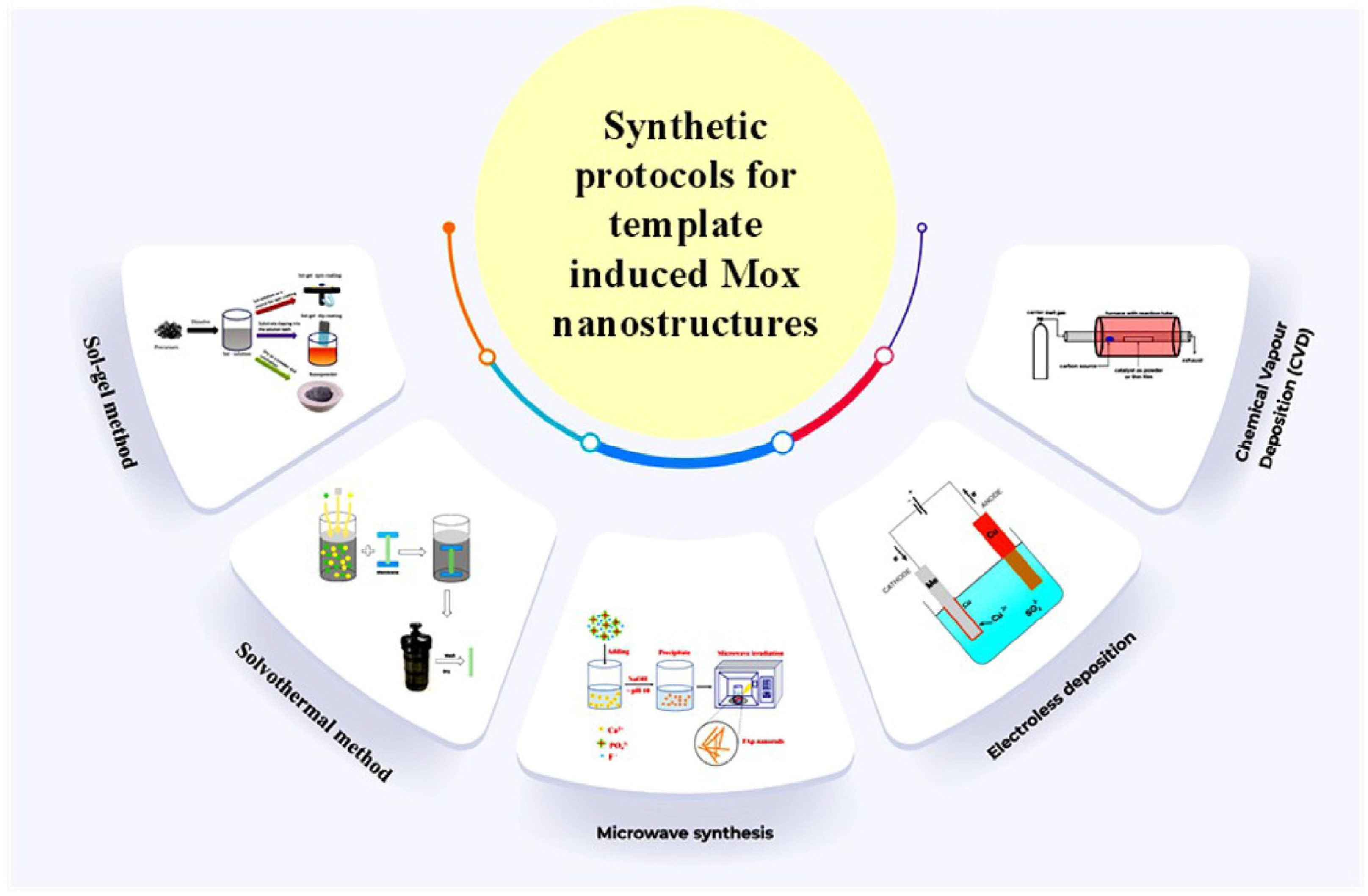
2.2. Non-Biological Synthesis of MOx NPs
2.2.1. Sol-Gel Method
2.2.2. Solvothermal/Hydrothermal Procedure
2.2.3. Deposition by Electroless
2.2.4. Synthesis through Microwave
2.2.5. Chemical Vapor Deposition (CVD)
2.2.6. Heterogeneous Catalysis
2.3. Bio/Green Synthesis of MOx NPs
3. Wastewater Treatment
3.1. Decontamination Methods of Pesticides from Wastewater
3.1.1. Nano Composites
3.1.2. Non-Homogenous Supported Nano Catalysts
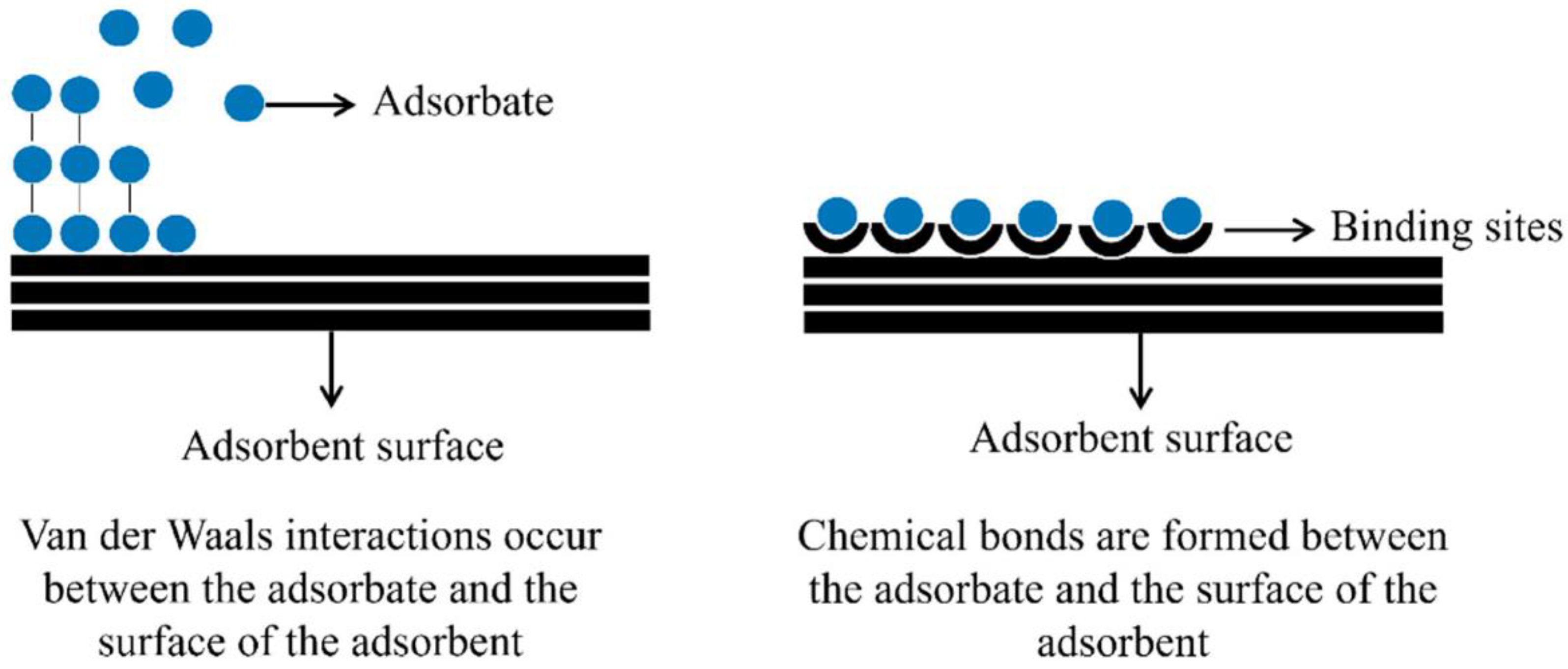
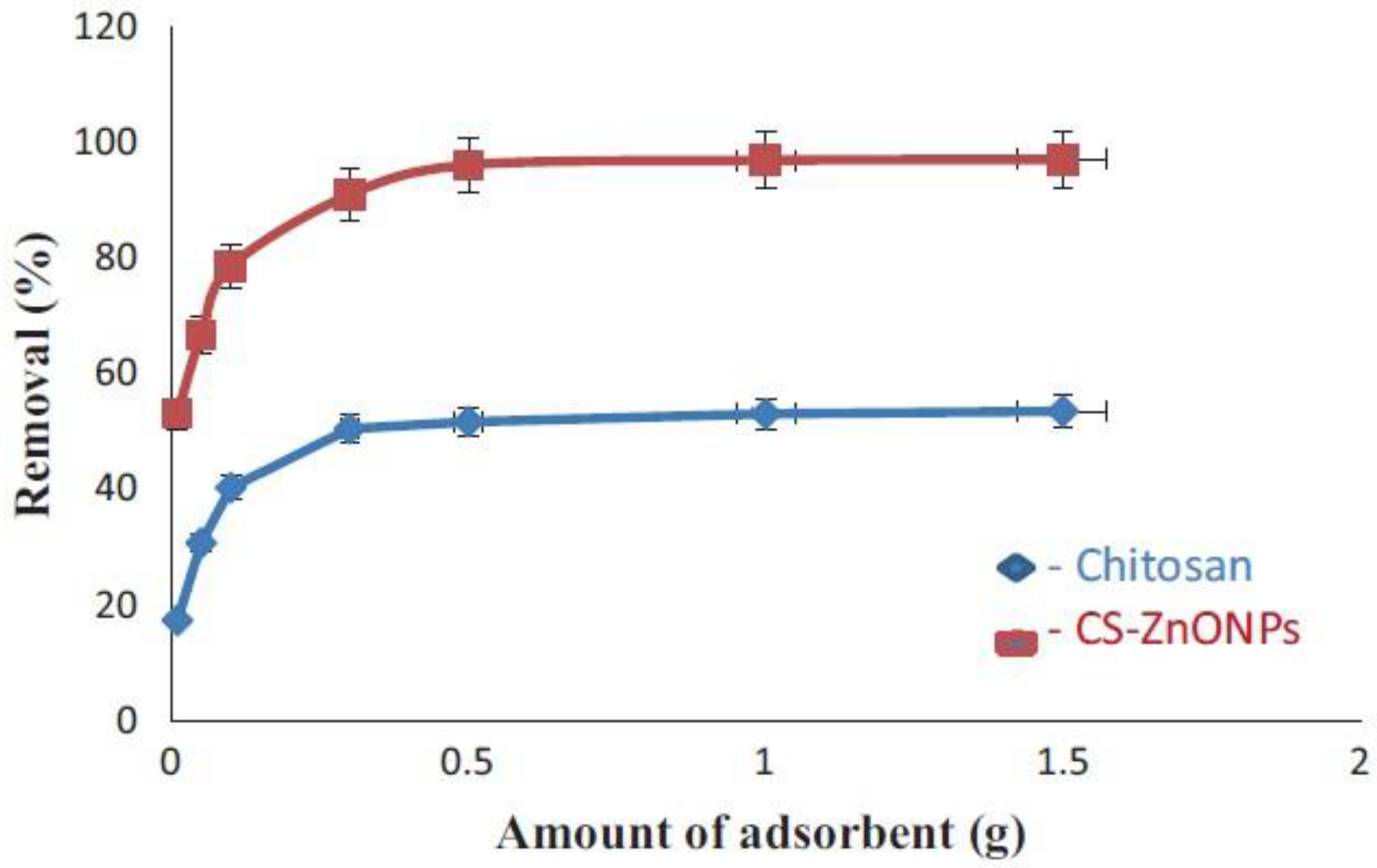
3.1.3. Adsorption
4. Heavy Metals
5. The Potential Applications of Metal Oxides Nanoparticles in Water Treatment
| Nanoparticles | Synthesis Method | Shape | Diameter (nm) | Contaminants | Removal% | Ref. |
|---|---|---|---|---|---|---|
| Iron oxide NPs | Tangerine peel extract | Spherical | 50 nm–1 μm | Cd (II) | 90% | [203] |
| Zinc oxide NPs | Co-precipitation | Spherical | 374.1–730.2 | Phenol | 100% | [204] |
| Fe3O4 @1 | combining Fe3O4 and polyoxometalate. | Mostly spherical | 19.1 | methylene blue (MB), rhodamine B (ChB), safranine T (T), gentian violet (GV), fuchsin basic (FB) | 96.9%, 96.3%, 89.1%, 96.1%, and 94.5%, respectively. | [205] |
| Titanium oxide NPs | Peepal leaf extract | Agglomerated particles | 11–91 | Methylene blue, methylene orange | 64% and 28% | [206] |
| Copper oxide NPs-1 | Mint leaf extract | Mostly spherical | ~150 | Cd (II), Ni (II) and Pb (II). | 18%, 52.5%, and 84% | [207] |
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuhn, R.; Bryant, I.M.; Jensch, R.; Böllmann, J. Applications of Environmental nanotechnologies in remediation, wastewater treatment, drinking water treatment, and agriculture. App. Nano 2022, 3, 54–90. [Google Scholar] [CrossRef]
- Franco, C.A.; Zabala, R.; Cortés, F.B. NanoTech. applied to the enhancement of oil and gas productivity and recovery of Colombian fields. J. Petro. Sci. Eng. 2017, 157, 39–55. [Google Scholar] [CrossRef]
- Saleh, T.A. Nanomaterials: Classification, properties, and Environmental toxicities. Enviro. Tech. Inno. 2020, 20, 101067. [Google Scholar] [CrossRef]
- Gregorczyk, K.; Knez, M. Hybrid nanomaterials through molecular and atomic layer deposition: Top down, bottom up, and in-between approaches to new materials. Prog. Mater. Sci. 2016, 75, 1–37. [Google Scholar] [CrossRef]
- Feynman, R. There’s Plenty of Room at the Bottom. Caltech’s Eng. Sci. 1960, 23, 22–36. [Google Scholar]
- Calipinar, H.; Ulas, D. Development of Nanotechnology in the World and Nanotechnology Standards in Turkey. Proc. Comp. Sci. 2019, 158, 1011–1018. [Google Scholar] [CrossRef]
- Glenn, J.C. Nanotechnology: Future military Environmental health considerations. Techno. Fore. Soc. Cha. 2006, 73, 128–137. [Google Scholar] [CrossRef]
- Bao, Y.J.; Song, K.; Guo, J.; Zhou, X.; Liu, S. Plant-extract-mediated synthesis of metal nanoparticles. J. Chem. 2021, 2021, 6562687. [Google Scholar] [CrossRef]
- Mueller, N.C.; Nowack, B. Exposure modeling of engineered nanoparticles in the environment. Enviro. Sci. Tech. 2008, 42, 4447–4453. [Google Scholar] [CrossRef]
- Naghdi, M.; Metahni, S.; Ouarda, Y.; Brar, S.K.; Das, R.K.; Cledon, M. Instrumental approach toward understanding nano-pollutants. Nanotechnol. Enviro. Eng. 2017, 2, 3. [Google Scholar] [CrossRef]
- Sousa, V.S.; Teixeira, M.R. Metal-based engineered nanoparticles in the drinking water treatment systems: A critical review. Sci. Tot. Env. 2020, 707, 136077. [Google Scholar] [CrossRef]
- Giese, B.F.; Klaessig, B.; Park, R.; Kaegi, M.; Steinfeldt, H.; von Gleich Wigger, A.; Gottschalk, F. Risks, release and concentrations of engineered nanomaterial in the environment. Sci. Rep. 2018, 8, 1565. [Google Scholar] [CrossRef]
- Sheikholeslami, M.; Ganji, D. Nanofluid convective heat transfer using semi analytical and numerical approaches: A review. J. Tai. Inst. Chem. Eng. 2016, 65, 43–77. [Google Scholar] [CrossRef]
- Kargozar, S.; Mozafari, M. Nanotechnology and Nanomedicine: Start small, think big. Mater. Today Proc. 2018, 5, 15492–15500. [Google Scholar] [CrossRef]
- Ghoranneviss, M.; Soni, A.; Talebitaher, A.; Aslan, N. Nanomaterial synthesis, characterization, and application. J. Nanomat. 2015, 2015, 892542. [Google Scholar] [CrossRef]
- Rineesh, N.; Neelakandan, M.; Thomas, S. Applications of silver nanoparticles for medicinal purpose. JSM Nanotechnol. Nanomed. 2018, 6, 1063. [Google Scholar]
- Kolahalam, L.A.; Viswanath, I.K.; Diwakar, B.S.; Govindh, B.; Reddy, V.; Murthy, Y. Review on nanomaterials: Synthesis and applications. Mat. Today Proc. 2019, 18, 2182–2190. [Google Scholar] [CrossRef]
- Bratovcic, A. Different application of nanomaterials and their impact on the environment. SSRG Inte. J. Mat. Sci. Eng. 2019, 5, 1–7. [Google Scholar]
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M.; de Lima, T.M.T.; Delbem, A.C.B.; Monteiro, D.R. Iron Oxide Nanoparticles for Biomedical Applications: A Perspective on Synthesis, Drugs, Antimicrobial Activity, and Toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef]
- Kharisov, B.I.; Kharissova, O.V.; Dias, H.R.; Méndez, U.O.; de la Fuente, I.G.; Peña, Y.; Dimas, Y.P.A.A.V. Iron-Based Nanomaterials in the Catalysis; InTech: Rijeka, Croatia, 2016. [Google Scholar]
- Aragaw, T.A.; Bogale, F.M.; Aragaw, B.A. Iron-based nanoparticles in wastewater treatment: A review on synthesis methods, applications, and removal mechanisms. J. Saudi Chem. Soc. 2021, 25, 101280. [Google Scholar] [CrossRef]
- Ebadi, M.; Buskaran, K.; Bullo, S.; Hussein, M.Z.; Fakurazi, S.; Pastorin, G. Drug delivery system based on magnetic iron oxide nanoparticles coated with (polyvinyl alcohol-zinc/aluminium-layered double hydroxide-sorafenib). Alex. Eng. J. 2021, 60, 733–747. [Google Scholar] [CrossRef]
- Rossi, A.; Zannotti, M.; Cuccioloni, M.; Minicucci, M.; Petetta, L.; Angeletti, M.; Giovannetti, R. Silver Nanoparticle-Based Sensor for the Selective Detection of Nickel Ions. Nanomaterials 2021, 11, 1733. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Nichols, F.; Lu, J.E.; Mercado, R.; Dudschus, R.; Bridges, F.; Chen, S. Platinum Oxide Nanoparticles for Electrochemical Hydrogen Evolution: Influence of Platinum Valence State. Chem. A Eur. J. 2020, 26, 4136–4142. [Google Scholar] [CrossRef]
- Garcia, M.; Rodriguez, J. Metal Oxide Nanoparticles Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Maruthupandy, M.; Zuo, Y.; Chen, J.-S.; Song, J.-M.; Niu, H.-L.; Mao, C.-J.; Zhang, S.-Y.; Shen, Y.-H. Synthesis of metal oxide nanoparticles (CuO and ZnO NPs) via biological template and their optical sensor applications. Appl. Surf. Sci. 2017, 397, 167–174. [Google Scholar] [CrossRef]
- Syal, A.; Sud, D. Development of highly selective novel fluorescence quenching probe based on Bi2S3-TiO2 nanoparticles for sensing the Fe(III). Sens. Actuators B Chem. 2018, 266, 1–8. [Google Scholar] [CrossRef]
- Bahal, M.; Kaur, N.; Sharotri, N.; Sud, D. Investigations on Amphoteric Chitosan/TiO2 Bionanocomposites for Application in Visible Light Induced Photocatalytic Degradation. Adv. Polym. Technol. 2019, 2019, 2345631. [Google Scholar] [CrossRef]
- Yu, L.; Xu, L.; Lu, L.; Alhalili, Z.; Zhou, X. Thermal Properties of MXenes and Relevant Applications. ChemPhysChem 2022, 23, e202200203. [Google Scholar] [CrossRef]
- Bykkam, S.; Prasad, D.; Maurya, M.R.; Sadasivuni, K.K.; Cabibihan, J.-J. Comparison study of metal oxides (CeO2, CuO, SnO2, CdO, ZnO and TiO2) decked few layered graphene nanocomposites for dye-sensitized solar cells. Sustainabililty 2021, 13, 7685. [Google Scholar] [CrossRef]
- Kenawy, S.H.; Hassan, M.L. Synthesis and characterization high purity alumina nanorods by a novel and simple method using nanocellulose aerogel template. Heliyon 2019, 5, e01816. [Google Scholar] [CrossRef]
- Ahmad, G.; Nawaz, A.; Nawaz, S.; Shad, N.A.; Sajid, M.M.; Javed, Y. Nanomaterial-based gas sensor for environmental science and technology. In Nanofabrication for Smart Nanosensor Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 229–252. [Google Scholar]
- Abdalla, A.M.; Hossain, S.; Azad, A.; Petra, P.; Begum, F.; Eriksson, S.; Azad, A.K. Nanomaterials for solid oxide fuel cells: A review. Renew. Sust. Ener. Rev. 2018, 82, 353–368. [Google Scholar] [CrossRef]
- Rahman, M.; Hoque, M.A.; Rahman, G.; Gafur, M.; Khan, R.A.; Hossain, M.K. Study on the mechanical, electrical and optical properties of metal-oxide nanoparticles dispersed unsaturated polyester resin nanocomposites. Res. Phys. 2019, 13, 102264. [Google Scholar] [CrossRef]
- Díaz, C.; Valenzuela, M.L.; Laguna-Bercero, M.; Orera, A.; Bobadilla, D.; Abarca, S.; Peña, O. Synthesis and magnetic properties of nanostructured metallic Co, Mn and Ni oxide materials obtained from solid-state metal-macromolecular complex precursors. RSC Adv. 2017, 7, 27729–27736. [Google Scholar] [CrossRef]
- Muthuvinothini, A.; Stella, S. Green synthesis of metal oxide nanoparticles and their catalytic activity for the reduction of aldehydes. Proc. Biochem. 2019, 77, 48–56. [Google Scholar] [CrossRef]
- Jian, W.; Hui, D.; Lau, D. Nanoengineering in biomedicine: Current development and future perspectives. Nanotechnol. Rev. 2020, 9, 700–715. [Google Scholar] [CrossRef]
- Subhan, M.A.; Choudhury, K.P.; Neogi, N. Advances with molecular nanomaterials in industrial manufacturing applications. Nanomanufacturing 2021, 1, 75–97. [Google Scholar] [CrossRef]
- Jun, Y.-W.; Seo, Y.-W.; Cheon, J. Nanoscaling laws of magnetic nanoparticles and their applicabilities in biomedical science. Acc. Chem. Res. 2008, 41, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Rane, A.V.; Kanny, K.; Abitha, V.K.; Thomas, S. Methods for synthesis of nanoparticles and fabrication of nanocomposites. In Synthesis of Inorganic Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 121–139. [Google Scholar]
- Modan, E.M.; Plăiașu, A.G. Advantages and disadvantages of chemical methods in the elaboration of nanomaterials. Ann. Dunarea De Jos Univ. Galati. Fascicle IX Metal. Mat. Sci. 2020, 43, 53–60. [Google Scholar] [CrossRef]
- Poolakkandy, R.R.; Menamparambath, M.M. Soft-template-assisted synthesis: A promising approach for the fabrication of transition metal oxides. Nano Adv. 2020, 2, 5015–5045. [Google Scholar] [CrossRef]
- Paramasivam, G.; Palem, V.V.; Sundaram, T.; Sundaram, V.; Kishore, S.C.; Bellucci, S. Nanomaterials: Synthesis and applications in theranostics. Nanomaterials 2021, 11, 3228. [Google Scholar] [CrossRef]
- Liang, H.W.; Liu, S.; Yu, S.H. Controlled synthesis of one-dimensional inorganic nanostructures using pre-existing one-dimensional nanostructures as templates. Adv. Mater. 2010, 22, 3925–3937. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Angelatos, A.S.; Caruso, F. Template synthesis of nanostructured materials via layer-by-layer assembly. Chem. Mater. 2008, 20, 848–858. [Google Scholar] [CrossRef]
- Pérez-Page, M.; Yu, E.; Li, J.; Rahman, M.; Dryden, D.M.; Vidu, R.; Stroeve, P. Template-based syntheses for shape controlled nanostructures. Adv. Coll. Inter. Sci. 2016, 234, 51–79. [Google Scholar] [CrossRef] [PubMed]
- Yourdkhani, A.; Caruntu, G. Highly ordered transition metal ferrite nanotube arrays synthesized by template-assisted liquid phase deposition. J. Mat. Chem. 2011, 21, 7145–7153. [Google Scholar] [CrossRef]
- Aisu, K.; Suzuki, T.S.; Nakamura, E.; Abe, H.; Suzuki, Y. AAO-template assisted synthesis and size control of one-dimensional TiO2 nanomaterials. J. Ceram. Soci. Jap. 2013, 121, 915–918. [Google Scholar] [CrossRef][Green Version]
- Grote, F.; Kühnel, R.-S.; Balducci, A.; Lei, Y. Template assisted fabrication of free-standing MnO2 nanotube and nanowire arrays and their application in supercapacitors. App. Phys. Let. 2014, 104, 053904. [Google Scholar] [CrossRef]
- Kim, K.W.; Kim, J.; Choi, C.; Yoon, H.K.; Go, M.C.; Lee, J.; Kim, J.K.; Seok, H.; Kim, T.; Wu, K.; et al. Soft Template-Assisted Fabrication of Mesoporous Graphenes for High-Performance Energy Storage Systems. ACS App. Mat. Inter. 2022, 14, 46994–47002. [Google Scholar] [CrossRef]
- Xue, Q.; Zhang, Q. Agar hydrogel template synthesis of Mn3O4 nanoparticles through an ion diffusion method controlled by ion exchange membrane and electrochemical performance. Nanomaterials 2019, 9, 503. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Wang, K.-X.; Chen, J.-S. Template-directed metal oxides for electrochemical energy storage. Ener. Stor. Mater. 2016, 3, 1–17. [Google Scholar] [CrossRef]
- Abdelrahman, E.A.; Tolan, D.A.; Nassar, M.Y. A tunable template-assisted hydrothermal synthesis of hydroxysodalite zeolite nanoparticles using various aliphatic organic acids for the removal of zinc (II) ions from aqueous media. J. Inorg. Organo. Poly. Mat. 2019, 29, 229–247. [Google Scholar] [CrossRef]
- Xie, Y.; Kocaefe, D.; Chen, C.; Kocaefe, Y. Review of research on template methods in preparation of nanomaterials. J. Nanomat. 2016, 2016, 2302595. [Google Scholar] [CrossRef]
- Kaur, A.; Bajaj, B.; Kaushik, A.; Saini, A.; Sud, D. A review on template assisted synthesis of multi-functional metal oxide nanostructures: Status and prospects. Mat. Sci. Eng. B 2022, 286, 116005. [Google Scholar] [CrossRef]
- Rawal, I. Facial synthesis of hexagonal metal oxide nanoparticles for low temperature ammonia gas sensing applications. RSC Adv. 2015, 5, 4135–4142. [Google Scholar] [CrossRef]
- Sharma, D.; Rajput, J.; Kaith, B.; Kaur, M.; Sharma, S. Synthesis of ZnO nanoparticles and study of their antibacterial and antifungal properties. Thin Sol. F. 2010, 519, 1224–1229. [Google Scholar] [CrossRef]
- Khan, S.B.; Faisal, M.; Rahman, M.M.; Jamal, A. Exploration of CeO2 nanoparticles as a chemi-sensor and photo-catalyst for Enviromental applications. Sci. Tot. Enviro. 2011, 409, 2987–2992. [Google Scholar] [CrossRef] [PubMed]
- Sifontes, A.; Gonzalez, G.; Ochoa, J.; Tovar, L.; Zoltan, T.; Cañizales, E. Chitosan as template for the synthesis of ceria nanoparticles. Mater. Res. Bull. 2011, 46, 1794–1799. [Google Scholar] [CrossRef]
- Khalil, A.T.; Ovais, M.; Ullah, I.; Ali, M.; Shinwari, Z.K.; Maaza, M. Physical properties, biological applications and biocompatibility studies on biosynthesized single phase cobalt oxide (Co3O4) nanoparticles via Sageretia thea (Osbeck.). Arab. J. Chem 2020, 13, 606–619. [Google Scholar] [CrossRef]
- Khun, K.; Ibupoto, Z.; Liu, X.; Beni, V.; Willander, M. The ethylene glycol template assisted hydrothermal synthesis of Co3O4 nanowires; structural characterization and their application as glucose non-enzymatic sensor. Mater. Sci. Eng. B 2015, 194, 94–100. [Google Scholar] [CrossRef]
- Han, D.; Song, P.; Zhang, H.; Yang, Z.; Wang, Q. Cu2O template-assisted synthesis of porous In2O3 hollow spheres with fast response towards acetone. Mater. Let. 2014, 124, 93–96. [Google Scholar] [CrossRef]
- Xiao, B.; Wang, F.; Zhai, C.; Wang, P.; Xiao, C.; Zhang, M. Facile synthesis of In2O3 nanoparticles for sensing properties at low detection temperature. Sen. Act. B Chem. 2016, 235, 251–257. [Google Scholar] [CrossRef]
- Honarmand, M.; Golmohammadi, M.; Naeimi, A. Biosynthesis of tin oxide (SnO2) nanoparticles using jujube fruit for photocatalytic degradation of organic dyes. Adv. Powder Tech. 2019, 30, 1551–1557. [Google Scholar] [CrossRef]
- Wang, Y.; Lee, J.Y.; Zeng, H.C. Polycrystalline SnO2 nanotubes prepared via infiltration casting of nanocrystallites and their electrochemical application. Chem. Mater. 2005, 17, 3899–3903. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Shaheen, T.I.; Zaghloul, S.; El-Rafie, M.H.; Hebeish, A. Antibacterial activities and UV protection of the in situ synthesized titanium oxide nanoparticles on cotton fabrics. Ind. Eng. Chem. Res. 2016, 55, 2661–2668. [Google Scholar] [CrossRef]
- Koh, J.H.; Park, J.T.; Roh, D.K.; Seo, J.A.; Kim, J.H. Synthesis of TiO2 nanoparticles using amphiphilic POEM-b-PS-b-POEM triblock copolymer template film. Coll. Surf. A Physicochem. Eng. Asp. 2008, 329, 51–57. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Davar, F.; Mazaheri, M. Synthesis of Mn3O4 nanoparticles by thermal decomposition of a [bis (salicylidiminato) manganese (II)] complex. Polyhedrom 2008, 27, 3467–3471. [Google Scholar] [CrossRef]
- Abbasi, B.A.; Iqbal, J.; Mahmood, T.; Ahmad, R.; Kanwal, S.; Afridi, S. Plant-mediated synthesis of nickel oxide nanoparticles (NiO) via Geranium wallichianum: Characterization and different biological applications. Mater. Res. Exp. 2019, 6, 0850a7. [Google Scholar] [CrossRef]
- Cao, Y.; Cao, J.; Zheng, M.; Liu, J.; Ji, G. Synthesis, characterization, and electrochemical properties of ordered mesoporous carbons containing nickel oxide nanoparticles using sucrose and nickel acetate in a silica template. J. Sol. Chem. 2007, 180, 792–798. [Google Scholar] [CrossRef]
- Bokov, D.; Turki Jalil, A.; Chupradit, S.; Suksatan, W.; Javed Ansari, M.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by sol-gel method: Synthesis and application. Adv. Mater. Sci. Eng. 2021, 2021, 5102014. [Google Scholar] [CrossRef]
- Hasnidawani, J.; Azlina, H.; Norita, H.; Bonnia, N.; Ratim, S.; Ali, E. Synthesis of ZnO nanostructures using sol-gel method. Proc. Chem. 2016, 19, 211–216. [Google Scholar] [CrossRef]
- Raja, K.; Jaculine, M.M.; Jose, M.; Verma, S.; Prince, A.; Ilangovan, K.; Sethusankar, K.; Das, S.J. Sol–gel synthesis and characterization of α-Fe2O3 nanoparticles. Superlat. Microstruc. 2015, 86, 306–312. [Google Scholar] [CrossRef]
- Zahera, M.; Khan, S.A.; Khan, I.A.; Sharma, R.K.; Sinha, N.; Al-Shwaiman, H.A.; Al-Zahrani, R.R.; Elgorban, A.M.; Syed, A.; Khan, M.S. Cadmium oxide nanoparticles: An attractive candidate for novel therapeutic approaches. Colloids Surf. A PhysicoChem. Eng. Asp. 2020, 585, 124017. [Google Scholar] [CrossRef]
- Mirtaheri, B.; Shokouhimehr, M.; Beitollahi, A. Synthesis of mesoporous tungsten oxide by template-assisted sol–gel method and its photocatalytic degradation activity. J. Sol. Gel Sci. Tech. 2017, 82, 148–156. [Google Scholar] [CrossRef]
- Sifontes, A.B.; Rosales, M.; Méndez, F.J.; Oviedo, O.; Zoltan, T. Effect of calcination temperature on structural properties and photocatalytic activity of ceria nanoparticles synthesized employing chitosan as template. J. Nanomater. 2013, 2013, 1. [Google Scholar] [CrossRef]
- Williams, L.; Prasad, A.R.; Sowmya, P.; Joseph, A. Characterization and temperature dependent DC conductivity study of bio templated nickel oxide nanoparticles (NiO) and their composites using polyaniline (PANI). Mater. Chem. Phys. 2020, 242, 122469. [Google Scholar] [CrossRef]
- Li, D.Y.; Sun, Y.K.; Gao, P.Z.; Zhang, X.L.; Ge, H.L. Structural and magnetic properties of nickel ferrite nanoparticles synthesized via a template-assisted sol–gel method. Ceram. Int. 2014, 40, 16529–16534. [Google Scholar] [CrossRef]
- Abdel Maksoud, M.I.A.; El-Sayyad, G.S.; Fayad, E.; Alyamani, A.; Abu Ali, O.A.; Elshamy, A.A. Gamma irradiation assisted the sol–gel method for silver modified-nickel molybdate nanoparticles synthesis: Unveiling the antimicrobial, and antibiofilm activities against some pathogenic microbes. J. Inorgan. Organomet. Poly. Mater. 2022, 32, 728–740. [Google Scholar] [CrossRef]
- Singh, M.; Vadher, D.; Dixit, V.; Jariwala, C. Synthesis, optimization and characterization of zinc oxide nanoparticles prepared by sol–gel technique. Mater. Tod. Proc. 2022, 48, 690–692. [Google Scholar] [CrossRef]
- Pundir, A.; Chopra, L. Comprehensive study of synthetic tool for ZnO based nanoparticles. Mater. Tod. Proc. 2022, 52, 339–344. [Google Scholar] [CrossRef]
- Liu, Y.; Akula, K.C.; Dandamudi, K.P.R.; Liu, Y.; Xu, M.; Sanchez, A.; Zhu, D.; Deng, S. Effective depolymerization of polyethylene plastic wastes under hydrothermal and solvothermal liquefaction conditions. Chem. Eng. J. 2022, 446, 137238. [Google Scholar] [CrossRef]
- Nandihalli, N.; Gregory, D.H.; Mori, T. Energy-Saving Pathways for Thermoelectric Nanomaterial Synthesis: Hydrothermal/Solvothermal, Microwave-Assisted, Solution-Based, and Powder Processing. Adv. Sci. 2022, 9, 2106052. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Y.; Liao, Y.; Zhang, Z.; Luo, S.; Li, L.; Wu, Y.; Qing, Y. Tuning carbonized wood fiber via sacrificial template-assisted hydrothermal synthesis for high-performance lithium/sodium-ion batteries. J. Power Sour. 2022, 546, 231993. [Google Scholar] [CrossRef]
- Alfaro, A.; León, A.; Guajardo-Correa, E.; Reuquen, E.; Torres, F.; Mery, M.; Segura, R.; Zapata, P.A.; Orihuela, P.A. MgO nanoparticles coated with polyethylene glycol as carrier for 2-Methoxyestradiol anticancer drug. PLoS ONE 2019, 14, e0214900. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Peng, L.; Han, T.; Liu, B.; Zhu, D.; Zhao, C.; Xu, J.; Tang, Y.; Wang, J.; He, S. Hydrothermal synthesis of nanoparticles-assembled NiO microspheres and their sensing properties. Phys. E Low Dimen. Sys. Nanostruc. 2020, 118, 113655. [Google Scholar] [CrossRef]
- Yáñez-Vilar, S.; Sánchez-Andújar, M.; Gómez-Aguirre, C.; Mira, J.; Señarís-Rodríguez, M.A.; Castro-García, S. A simple solvothermal synthesis of MFe2O4 (M = Mn, Co and Ni) nanoparticles. J. Sol. Chem. 2009, 182, 2685–2690. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Zhang, H.; Liu, P.; Li, G.; An, T.; Zhao, H. Soft-template assisted synthesis of mesoporous CuO/Cu2O composite hollow microspheres as efficient visible-light photocatalyst. Mater. Let. 2016, 182, 47–51. [Google Scholar] [CrossRef]
- Tang, J.; Ou, Q.; Zhou, H.; Qi, L.; Man, S. Seed-mediated electroless deposition of gold nanoparticles for highly uniform and efficient SERS enhancement. Nanomaterials 2019, 9, 185. [Google Scholar] [CrossRef]
- Balela, M.D.L.; Amores, K.L.S. Electroless deposition of copper nanoparticle for antimicrobial coating. Mater. Chem. Phys. 2019, 225, 393–398. [Google Scholar] [CrossRef]
- Reddy, P.R.; Ajith, K.; Udayashankar, N. Structural and optical analysis of silver nanoparticles grown on porous anodic alumina membranes by electro-less deposition. Mater. Tod. Proc. 2019, 19, 2633–2638. [Google Scholar] [CrossRef]
- Ren, W.F.; Li, J.T.; Zhang, S.J.; Lin, A.L.; Chen, Y.H.; Gao, Z.G.; Zhou, Y.; Deng, L.; Huang, L.; Sun, S.G. Fabrication of multi-shell coated silicon nanoparticles via in-situ electroless deposition as high performance anodes for lithium ion batteries. J. Ener. Chem. 2020, 48, 160–168. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.; Gu, A.; You, C.; Oh, K.; Lee, S.; Im, Y. Synthesis of vertically conformal ZnO/CuO core–shell nanowire arrays by electrophoresis-assisted electroless deposition. J. Phys. Chem. C 2014, 118, 7377–7385. [Google Scholar] [CrossRef]
- Preda, N.; Enculescu, M.; Enculescu, I. Polymer sphere array assisted ZnO electroless deposition. Soft Mater. 2013, 11, 457–464. [Google Scholar] [CrossRef]
- Sassin, M.B.; Mansour, A.N.; Pettigrew, K.A.; Rolison, D.R.; Long, J.W. Electroless deposition of conformal nanoscale iron oxide on carbon nanoarchitectures for electrochemical charge storage. ACS Nano 2010, 4, 4505–4514. [Google Scholar] [CrossRef] [PubMed]
- McBean, C.L.; Liu, H.; Scofield, M.E.; Li, L.; Wang, L.; Bernstein, A.; Wong, S.S. Generalizable, electroless, template-assisted synthesis and electrocatalytic mechanistic understanding of perovskite LaNiO3 nanorods as viable, supportless oxygen evolution reaction catalysts in alkaline media. ACS Appl. Mater. Interf. 2017, 9, 24634–24648. [Google Scholar] [CrossRef]
- Ali, T.T.; Narasimharao, K.; Basahel, S.N.; Mokhtar, M.; Alsharaeh, E.H.; Mahmoud, H.A. Template assisted microwave synthesis of rGO-ZrO2 composites: Efficient photocatalysts under visible light. J. Nanosci. Nanotech. 2019, 19, 5177–5188. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, A.; Wojciechowska, W.; Kurc, B.; Pigłowska, M.; Synoradzki, K.; Gabała, E.; Moszyński, D.; Szybowicz, M.; Siwińska-Ciesielczyk, K.; Jesionowski, T. Highly crystalline TiO2-MoO3 composite materials synthesized via a template-assisted microwave method for electrochemical application. Crystals 2020, 10, 493. [Google Scholar] [CrossRef]
- Hu, X.; Gong, J.; Zhang, L.; Yu, J.C. Continuous size tuning of monodisperse ZnO colloidal nanocrystal clusters by a microwave-polyol process and their application for humidity sensing. Adv. Mater. 2008, 20, 4845–4850. [Google Scholar] [CrossRef]
- Soren, S.; Bessoi, M.; Parhi, P. A rapid microwave initiated polyol synthesis of cerium oxide nanoparticle using different cerium precursors. Ceram. Int. 2015, 41, 8114–8118. [Google Scholar] [CrossRef]
- Wang, X.-D.; Vinodgopal, K.; Dai, G.-P. Synthesis of carbon nanotubes by catalytic chemical vapor deposition. In Perspective of Carbon Nanotubes; IntechOpen: London, UK, 2019; pp. 1–19. [Google Scholar]
- Arzaee, N.A.; Noh, M.F.M.; Ab Halim, A.; Rahim, M.A.F.A.; Mohamed, N.A.; Safaei, J.; Aadenan, A.; Nasir, S.N.S.; Ismail, A.F.; Teridi, M.A.M. Aerosol-assisted chemical vapour deposition of α-Fe2O3 nanoflowers for photoelectrochemical water splitting. Ceram. Int. 2019, 45, 16797–16802. [Google Scholar] [CrossRef]
- Singh, P.; Kumar, A.; Kaur, D. ZnO nanocrystalline powder synthesized by ultrasonic mist-chemical vapour deposition. Opt. Mater. 2008, 30, 1316–1322. [Google Scholar] [CrossRef]
- Du, J.; Qi, W.; Zuo, J.; Li, X.; Gu, X.; Li, K.; Zhang, K.; Gong, C.; Zou, J. Hydrophilic TiO2 nanowires prepared on Ti5Si3 layer by chemical vapour deposition. J. Chem. Res. 2017, 41, 304–308. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; RanjithKumar, D.; Lee, Y.R. Direct growth of iron oxide nanoparticles filled multi-walled carbon nanotube via chemical vapour deposition method as high-performance supercapacitors. Int. Hydro. Ener. 2019, 44, 2349–2360. [Google Scholar] [CrossRef]
- Smazna, D.; Shree, S.; Polonskyi, O.; Lamaka, S.; Baum, M.; Zheludkevich, M.; Faupel, F.; Adelung, R.; Mishra, Y.K. Mutual interplay of ZnO micro-and nanowires and methylene blue during cyclic photocatalysis process. J. Enviro. Chem. Eng. 2019, 7, 103016. [Google Scholar] [CrossRef]
- Pan, Y.; Shen, X.; Yao, L.; Bentalib, A.; Peng, Z. Active sites in heterogeneous catalytic reaction on metal and metal oxide: Theory and practice. Catalyst 2018, 8, 478. [Google Scholar] [CrossRef]
- Hu, X.; Yip, A.C. Heterogeneous Catalysis: Enabling a Sustainable Future; Frontiers Media SA: Lausanne, Switzerland, 2021; p. 667675. [Google Scholar]
- Nur, H. The design and synthesis of heterogeneous catalyst systems for synthesis of useful organic compounds. Akta Kim. 2007, 3, 1–10. [Google Scholar]
- Valden, M.; Lai, X.; Goodman, D. Onset of catalytic activity of gold clusters on titania with the appearance of nonmetallic properties. Science 1998, 281, 1647–1650. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Bell, A.T.; Iglesia, E. The relationship between the electronic and redox properties of dispersed metal oxides and their turnover rates in oxidative dehydrogenation reactions. J. Catal. 2002, 209, 35–42. [Google Scholar] [CrossRef]
- Singha, R.K.; Shukla, A.; Yadav, A.; Konathala, L.S.; Bal, R. Effect of metal-support interaction on activity and stability of Ni-CeO2 catalyst for partial oxidation of methane. App. Catalys. B Enviro. 2017, 202, 473–488. [Google Scholar] [CrossRef]
- Liu, J.; Qiao, S.Z.; Hu, Q.H.; Lu, G.Q. Magnetic nanocomposites with mesoporous structures: Synthesis and applications. Small 2011, 7, 425–443. [Google Scholar] [CrossRef]
- Nassar, N.N. Rapid removal and recovery of Pb (II) from wastewater by magnetic nanoadsorbents. J. Hazard. Mater. 2010, 184, 538–546. [Google Scholar] [CrossRef]
- Gupta, V.; Nayak, A. Cadmium removal and recovery from aqueous solutions by novel adsorbents prepared from orange peel and Fe2O3 nanoparticles. Chem. Eng. J. 2012, 180, 81–90. [Google Scholar] [CrossRef]
- Pérez-Beltrán, C.H.; García-Guzmán, J.J.; Ferreira, B.; Estévez-Hernández, O.; López-Iglesias, D.; Cubillana-Aguilera, L.; Link, W.; Stănică, N.; da Costa, A.M.R.; Palacios-Santander, J.M. One-minute and green synthesis of magnetic iron oxide nanoparticles assisted by design of experiments and high energy ultrasound: Application to biosensing and immunoprecipitation. Mater. Sci. Eng. C 2021, 123, 112023. [Google Scholar] [CrossRef] [PubMed]
- Goudarzi, M.; Salavati-Niasari, M.; Yazdian, F.; Amiri, M. Sonochemical assisted thermal decomposition method for green synthesis of CuCo2O4/CuO ceramic nanocomposite using Dactylopius Coccus for anti-tumor investigations. J. Alloy. Comp. 2019, 788, 944–953. [Google Scholar] [CrossRef]
- Samuel, M.S.; Ravikumar, M.; John J, A.; Selvarajan, E.; Patel, H.; Chander, P.S.; Soundarya, J.; Vuppala, S.; Balaji, R.; Chandrasekar, N. A review on green synthesis of nanoparticles and their diverse biomedical and enviromental applications. Catalyst 2022, 12, 459. [Google Scholar] [CrossRef]
- Chouke, P.B.; Shrirame, T.; Potbhare, A.K.; Mondal, A.; Chaudhary, A.R.; Mondal, S.; Thakare, S.R.; Nepovimova, E.; Valis, M.; Kuca, K.; et al. Bioinspired metal/metal oxide nanoparticles: A road map to potential applications. Mater. Tod. Adv. 2022, 16, 100314. [Google Scholar] [CrossRef]
- Gade, A.; Bonde, P.; Ingle, A.; Marcato, P.; Duran, N.; Rai, M. Exploitation of Aspergillus niger for synthesis of silver nanoparticles. J. Bio. Mater. Bioenerg. 2008, 2, 243–247. [Google Scholar] [CrossRef]
- Lakshminarayanan, S.; Shereen, M.F.; Niraimathi, K.; Brindha, P.; Arumugam, A. One-pot green synthesis of iron oxide nanoparticles from Bauhinia tomentosa: Characterization and application towards synthesis of 1, 3 diolein. Sci. Rep. 2021, 11, 8643. [Google Scholar] [CrossRef] [PubMed]
- Alhalili, Z. Green synthesis of copper oxide nanoparticles CuO NPs from Eucalyptus Globoulus leaf extract: Adsorption and design of experiments. Arab. J. Chem. 2022, 15, 103739. [Google Scholar] [CrossRef]
- Üstün, E.; Önbaş, S.C.; Çelik, S.K.; Ayvaz, M.Ç.; Şahin, N. Green synthesis of iron oxide nanoparticles by using Ficus carica leaf extract and its antioxidant activity. Biointer. Res. Appl. Chem. 2022, 12, 2108–2116. [Google Scholar]
- Patil, S.P.; Chaudhari, R.Y.; Nemade, M.S. Azadirachta indica leaves mediated green synthesis of metal oxide nanoparticles: A review. Talanta Open 2022, 5, 100083. [Google Scholar] [CrossRef]
- Shah, Y.; Maharana, M.; Sen, S. Peltophorum pterocarpum leaf extract mediated green synthesis of novel iron oxide particles for application in photocatalytic and catalytic removal of organic pollutants. Bio. Conver. Biorefin. 2022, 1–14. [Google Scholar] [CrossRef]
- Abdelmigid, H.M.; Hussien, N.A.; Alyamani, A.A.; Morsi, M.M.; AlSufyani, N.M.; Kadi, H.A. Green synthesis of zinc oxide nanoparticles using pomegranate fruit peel and solid coffee grounds vs. chemical method of synthesis, with their biocompatibility and antibacterial properties investigation. Molecules 2022, 27, 1236. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; Warsi, M.F.; Zulfiqar, S.; Sami, A.; Ullah, S.; Rasheed, A.; Alsafari, I.A.; Agboola, P.O.; Shakir, I.; Baig, M.M. Green nickel/nickel oxide nanoparticles for prospective antibacterial and Enviromental remediation applications. Ceram. Int. 2022, 48, 8331–8340. [Google Scholar] [CrossRef]
- Samuel, M.S.; Selvarajan, E.; Mathimani, T.; Santhanam, N.; Phuong, T.N.; Brindhadevi, K.; Pugazhendhi, A. Green synthesis of cobalt-oxide nanoparticle using jumbo Muscadine (Vitis rotundifolia): Characterization and photo-catalytic activity of acid Blue-74. J. Photochem. Photobio. B Bio. 2020, 211, 112011. [Google Scholar] [CrossRef]
- Ahmed, M.; Messih, M.A.; El-Sherbeny, E.; El-Hafez, S.F.; Khalifa, A.M. Synthesis of metallic silver nanoparticles decorated mesoporous SnO2 for removal of methylene blue dye by coupling adsorption and photocatalytic processes. J. Photochem. Photobio. A Chem. 2017, 346, 77–88. [Google Scholar] [CrossRef]
- Butnariu, I.C.; Stoian, O.; Voicu, Ş.; Iovu, H.; Paraschiv, G. Nanomaterials Used in Treatment of Wastewater: A Review. Ann. Fac. Eng. Hunedoara 2019, 17, 175–179. [Google Scholar]
- Abigail, M.; Samuel, S.M.; Ramalingam, C. Addressing the Enviromental impacts of butachlor and the available remediation strategies: A systematic review. Int. J. Enviro. Sci. Tech. 2015, 12, 4025–4036. [Google Scholar] [CrossRef]
- Chidambaram, R. Application of rice husk nanosorbents containing 2, 4-dichlorophenoxyacetic acid herbicide to control weeds and reduce leaching from soil. J. Tai. Inst. Chem. Eng. 2016, 63, 318–326. [Google Scholar]
- Samuel, M.S.; Bhattacharya, J.; Parthiban, C.; Viswanathan, G.; Singh, N.P. Ultrasound-assisted synthesis of metal organic framework for the photocatalytic reduction of 4-nitrophenol under direct sunlight. Ultrason. Sonochem. 2018, 49, 215–221. [Google Scholar] [CrossRef]
- Needhidasan, S.; Ramalingam, C. Stratagems employed for 2, 4-dichlorophenoxyacetic acid removal from polluted water sources. Clean Techno. Enviro. Pol. 2017, 19, 1607–1620. [Google Scholar]
- Needhidasan, S.; Samuel, M.; Chidambaram, R. Electronic waste–an emerging threat to the environment of urban India. J. Enviro. Health Sci. Eng. 2014, 12, 36. [Google Scholar] [CrossRef]
- Samuel, M.S.; Bhattacharya, J.; Raj, S.; Santhanam, N.; Singh, H.; Singh, N.P. Efficient removal of Chromium (VI) from aqueous solution using chitosan grafted graphene oxide (CS-GO) nanocomposite. Int. J. Bio. Macromol. 2019, 121, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Samuel, M.S.; Subramaniyan, V.; Bhattacharya, J.; Parthiban, C.; Chand, S.; Singh, N.P. A GO-CS@ MOF [Zn (BDC)(DMF)] material for the adsorption of chromium (VI) ions from aqueous solution. Compo. B Eng. 2018, 152, 116–125. [Google Scholar] [CrossRef]
- Saleh, I.A.; Zouari, N.; Al-Ghouti, M.A. Removal of pesticides from water and wastewater: Chemical, physical and biological treatment approaches. Enviro. Tech. Inno. 2020, 19, 101026. [Google Scholar] [CrossRef]
- da Silva Bruckmann, F.; Ledur, C.M.; da Silva, I.Z.; Dotto, G.L.; Rhoden, C.R.B. A DFT theoretical and experimental study about tetracycline adsorption onto magnetic graphene oxide. J. Mol. Liq. 2022, 353, 118837. [Google Scholar] [CrossRef]
- Nunes, F.B.; da Silva Bruckmann, F.; da Rosa Salles, T.; Rhoden, C.R.B. Study of phenobarbital removal from the aqueous solutions employing magnetite-functionalized chitosan. Enviro. Sci. Poll. Res. 2022, 30, 12658–12671. [Google Scholar] [CrossRef]
- Oviedo, L.R.; Muraro, P.C.L.; Pavoski, G.; Espinosa, D.C.R.; Ruiz, Y.P.M.; Galembeck, A.; Rhoden, C.R.B.; da Silva, W.L. Synthesis and characterization of nanozeolite from (agro) industrial waste for application in heterogeneous photocatalysis. Enviro. Sci. Poll. Res. 2022, 29, 3794–3807. [Google Scholar] [CrossRef] [PubMed]
- Hussein-Al-Ali, S.H.; El Zowalaty, M.E.; Hussein, M.Z.; Geilich, B.M.; Webster, T.J. Synthesis, characterization, and antimicrobial activity of an ampicillin-conjugated magnetic nanoantibiotic for medical applications. Int. J. Nanomed. 2014, 9, 3801. [Google Scholar] [CrossRef]
- da Rosa Salles, T.; da Silva Bruckamann, F.; Viana, A.R.; Krause, L.M.F.; Mortari, S.R.; Rhoden, C.R.B. Magnetic nanocrystalline cellulose: Azithromycin adsorption and in vitro biological activity against melanoma cells. J. Poly. Env. 2022, 30, 2695–2713. [Google Scholar] [CrossRef]
- Fu, S.; Sun, Z.; Huang, P.; Li, Y.; Hu, N. Some basic aspects of polymer nanocomposites: A critical review. Nanomater. Sci. 2019, 1, 2–30. [Google Scholar] [CrossRef]
- Ayekoe, C.Y.P.; Robert, D.; Lanciné, D.G. Combination of coagulation-flocculation and heterogeneous photocatalysis for improving the removal of humic substances in real treated water from Agbô River (Ivory-Coast). Catalys. Tod. 2017, 281, 2–13. [Google Scholar] [CrossRef]
- Cross, A.; Miller, J.; Danghyan, V.; Mukasyan, A.; Wolf, E. Highly active and stable Ni-Cu supported catalysts prepared by combustion synthesis for hydrogen production from ethanol. Appl. Catal. A Gen. 2019, 572, 124–133. [Google Scholar] [CrossRef]
- Omanović-Mikličanin, E.; Badnjević, A.; Kazlagić, A.; Hajlovac, M. Nanocomposites: A brief review. Health Tech. 2020, 10, 51–59. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Yoldi, M.; Fuentes-Ordoñez, E.; Korili, S.; Gil, A. Zeolite synthesis from industrial wastes. Micro. Meso. Mater. 2019, 287, 183–191. [Google Scholar] [CrossRef]
- Mintova, S.; Jaber, M.; Valtchev, V. Nanosized microporous crystals: Emerging applications. Chem. Soci. Rev. 2015, 44, 7207–7233. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.H.; Tran, H.N.; Fu, C.-C.; Lu, Y.-T.; Juang, R.-S. Roles of adsorption and photocatalysis in removing organic pollutants from water by activated carbon–supported titania composites: Kinetic aspects. J. Tai. Inst. Chem. Eng. 2020, 109, 51–61. [Google Scholar] [CrossRef]
- Feng, J.; Ran, X.; Wang, L.; Xiao, B.; Lei, L.; Zhu, J.; Liu, Z.; Xi, X.; Feng, G.; Dai, Z.; et al. The synergistic effect of adsorption-photocatalysis for removal of organic pollutants on mesoporous Cu2V2O7/Cu3V2O8/g-C3N4 heterojunction. Int. J. Mol. Sci. 2022, 23, 14264. [Google Scholar] [CrossRef]
- Bruckmann, F.D.S.; Rossato Viana, A.; Tonel, M.Z.; Fagan, S.B.; Garcia, W.J.D.S.; Oliveira, A.H.D.; Dorneles, L.S.; Roberto Mortari, S.; Silva, W.L.D.; Silva, I.Z.D.; et al. Influence of magnetite incorporation into chitosan on the adsorption of the methotrexate and in vitro cytotoxicity. Enviro. Sci. Poll. Res. 2022, 29, 70413–70434. [Google Scholar] [CrossRef]
- Rahimi, B.; Jafari, N.; Abdolahnejad, A.; Farrokhzadeh, H.; Ebrahimi, A. Application of efficient photocatalytic process using a novel BiVO/TiO2-NaY zeolite composite for removal of acid orange 10 dye in aqueous solutions: Modeling by response surface methodology (RSM). J. Enviro. Chem. Eng. 2019, 7, 103253. [Google Scholar] [CrossRef]
- Falyouna, O.; Eljamal, O.; Maamoun, I.; Tahara, A.; Sugihara, Y. Magnetic zeolite synthesis for efficient removal of cesium in a lab-scale continuous treatment system. J. Colloid Interface Sci. 2020, 571, 66–79. [Google Scholar] [CrossRef]
- Feijoo, S.; González-Rodríguez, J.; Fernández, L.; Vázquez-Vázquez, C.; Feijoo, G.; Moreira, M.T. Fenton and photo-fenton nanocatalysts revisited from the perspective of life cycle assessment. Catalyst 2019, 10, 23. [Google Scholar] [CrossRef]
- Rhoden, C.R.B.; da Silva Bruckmann, F.; da Rosa Salles, T.; Junior, C.G.K.; Mortari, S.R. Study from the influence of magnetite onto removal of hydrochlorothiazide from aqueous solutions applying magnetic graphene oxide. J. Water Pro. Eng. 2021, 43, 102262. [Google Scholar] [CrossRef]
- Ma, T.; Sheng, Y.; Meng, Y.; Sun, J. Multistage remediation of heavy metal contaminated river sediments in a mining region based on particle size. Chemosphere 2019, 225, 83–92. [Google Scholar] [CrossRef]
- Park, C.M.; Kim, Y.M.; Kim, K.H.; Wang, D.; Su, C.; Yoon, Y. Potential utility of graphene-based nano spinel ferrites as adsorbent and photocatalyst for removing organic/inorganic contaminants from aqueous solutions: A mini review. Chemosphere 2019, 221, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Eskandarimakvand, M.; Sabzalipour, S.; Cheraghi, M.; Orak, N. Evaluation of Efficiency of Iron Oxide Nanoparticles (Fe3O4@ CNT) in Removal of Malathion in Aqueous Medium Using Response Surface Methodology (RSM). Pollution 2022, 8, 281–293. [Google Scholar]
- Ziyu, L.; Zhigang, J.; Wenwen, L.; Jianhong, L.; Shan, J.; Shengbiao, L.; Rongsun, Z. Synthesis of Ag/AgCl nanoparticles immobilized on CoFe2O4 fibers and their photocatalytic degradation for methyl orange. Rare Metal Mater. Eng. 2017, 46, 3669–3674. [Google Scholar] [CrossRef]
- Kunduru, K.R.; Nazarkovsky, M.; Farah, S.; Pawar, R.P.; Basu, A.; Domb, A.J. Nanotechnology for water purification: Applications of Nanotechnol. methods in wastewater treatment. Water Purif. 2017, 1, 33–74. [Google Scholar]
- Sadiq, H.; Sher, F.; Sehar, S.; Lima, E.C.; Zhang, S.; Iqbal, H.M.; Zafar, F.; Nuhanović, M. Green synthesis of ZnO nanoparticles from Syzygium Cumini leaves extract with robust photocatalysis applications. J. Mol. Liq. 2021, 335, 116567. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, V.; Jolly, S.S.; Kim, K.H.; Rawat, M.; Kukkar, D.; Tsang, Y.F. Biogenic synthesis of silver nanoparticles and its photocatalytic applications for removal of organic pollutants in water. J. Ind. Eng. Chem. 2019, 80, 247–257. [Google Scholar] [CrossRef]
- Geankoplis, C.; Hersel, A.; Lepek, D. Introduction to Eng. principles and units. In Process Principles; Prentice Hall: Hoboken, NJ, USA, 2018; p. 13. [Google Scholar]
- Alhalili, Z.; Smiri, M. The Influence of the Calcination Time on Synthesis of Nanomaterials with Small Size, High Crystalline Nature and Photocatalytic Activity in the TiO2 Nanoparticles Calcined at 500 °C. Crystals 2022, 12, 1629. [Google Scholar] [CrossRef]
- IIqbal, A.; Haq, A.U.; Cerrón-Calle, G.A.; Naqvi, S.A.R.; Westerhoff, P.; Garcia-Segura, S. Green synthesis of flower-shaped copper oxide and nickel oxide nanoparticles via Capparis decidua leaf extract for synergic adsorption-photocatalytic degradation of pesticides. Catalyst 2021, 11, 806. [Google Scholar] [CrossRef]
- Rahmanifar, B.; Moradi Dehaghi, S. Removal of organochlorine pesticides by chitosan loaded with silver oxide nanoparticles from water. Clean Techno. Enviro. Policy 2014, 16, 1781–1786. [Google Scholar] [CrossRef]
- Bruckmann, F.S.; Schnorr, C.; Oviedo, L.R.; Knani, S.; Silva, L.F.; Silva, W.L.; Dotto, G.L.; Bohn Rhoden, C.R. Adsorption and Photocatalytic Degradation of Pesticides into Nanocomposites: A Review. Molecules 2022, 27, 6261. [Google Scholar] [CrossRef] [PubMed]
- da Silva Bruckmann, F.; Zuchetto, T.; Ledur, C.M.; dos Santos, C.L.; da Silva, W.L.; Fagan, S.B.; da Silva, I.Z.; Rhoden, C.R.B. Methylphenidate adsorption onto graphene derivatives: Theory and experiment. New J. Chem. 2022, 46, 4283–4291. [Google Scholar] [CrossRef]
- Hu, H.; Xu, K. Physicochemical technologies for HRPs and risk control. In High-Risk Pollutants in Wastewater; Elsevier: Amsterdam, The Netherlands, 2020; pp. 169–207. [Google Scholar]
- Abegunde, S.M.; Idowu, K.S.; Adejuwon, O.M.; Adeyemi-Adejolu, T. A review on the influence of chemical modification on the performance of adsorbents. Res Enviro. Sustain. 2020, 1, 100001. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, G.; Guo, X.; Xu, Y. Designing a novel N-doped adsorbent with ultrahigh selectivity for CO2: Waste biomass pyrolysis and two-step activation. Bio. Conver. Biorefin. 2021, 11, 2843–2854. [Google Scholar] [CrossRef]
- Dehaghi, S.M.; Rahmanifar, B.; Moradi, A.M.; Azar, P.A. Removal of permethrin pesticide from water by chitosan–zinc oxide nanoparticles composite as an adsorbent. J. Saudi Chem. Soci. 2014, 18, 348–355. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Sant, S.; Wang, B.; Laurent, S.; Sen, T. Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy. Adv. Drug Deliv. Rev. 2011, 63, 24–46. [Google Scholar] [CrossRef]
- Chakraborty, R.; Asthana, A.; Singh, A.K.; Jain, B.; Susan, A.B.H. Adsorption of heavy metal ions by various low-cost adsorbents: A review. Int. J. Enviro. Analyt. Chem. 2022, 102, 342–379. [Google Scholar] [CrossRef]
- Jaishankar, M.; Mathew, B.B.; Shah, S.; Gowda, K. Biosorption of few heavy metal ions using agricultural wastes. J. Envir. Poll Hum. Health. 2014, 2, 1–6. [Google Scholar]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Sankhla, M.S.; Kumari, M.; Nandan, M.; Kumar, R.; Agrawal, P. Heavy metals contamination in water and their hazardous effect on human health-a review. Int. J. Curr. Microbiol. App. Sci. 2016, 5, 759–766. [Google Scholar] [CrossRef]
- Jan, A.T.; Azam, M.; Siddiqui, K.; Ali, A.; Choi, I.; Haq, Q.M.R. Heavy metals and human health: Mechanistic insight into toxicity and counter defense system of antioxidants. Int. J. Mol. Sci. 2015, 16, 29592–29630. [Google Scholar] [CrossRef]
- Su, L.; Zhang, X.; Yuan, X.; Zhao, Y.; Zhang, D.; Qin, W. Evaluation of joint toxicity of nitroaromatic compounds and copper to Photobacterium phosphoreum and QSAR analysis. J. Hazard. Mater. 2012, 241, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Engwa, G.A.; Ferdinand, P.U.; Nwalo, F.N.; Unachukwu, M.N. Mechanism and health effects of heavy metal toxicity in humans. In Poisoning in the Modern World-New Tricks for an Old Dog; IntechOpen: London, UK, 2019; Volume 10, pp. 70–90. [Google Scholar]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Inter. Toxic. 2014, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Chaemiso, T.D.; Nefo, T. Removal methods of heavy metals from laboratory wastewater. J. Nat. Sci. Res. 2019, 9, 36–42. [Google Scholar]
- Demiral, H.; Güngör, C. Adsorption of copper (II) from aqueous solutions on activated carbon prepared from grape bagasse. J. Clean. Prod. 2016, 124, 103–113. [Google Scholar] [CrossRef]
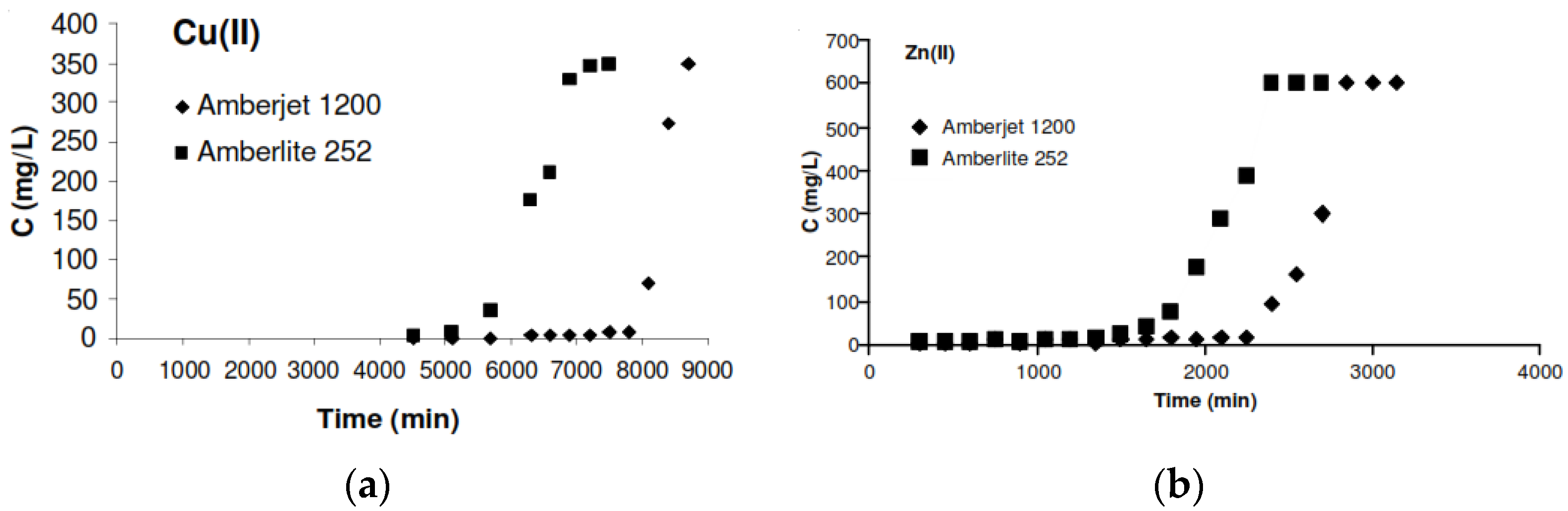
- Budak, T. Removal of heavy metals from wastewater using synthetic ion exchange resin. Asia. J. Chem. 2013, 25, 4207–4210. [Google Scholar] [CrossRef]
- Divyapriya, G.; Singh, S.; Martínez-Huitle, C.; Scaria, J.; Karim, A.; Nidheesh, P. Treatment of real wastewater by photoelectrochemical methods: An overview. Chemosphere 2021, 276, 130188. [Google Scholar] [CrossRef]
- Tortajada, C. Contributions of recycled wastewater to clean water and sanitation Sustainable Development Goals. NPJ Clean Water 2020, 3, 22. [Google Scholar] [CrossRef]
- Epelle, E.I.; Okoye, P.U.; Roddy, S.; Gunes, B.; Okolie, J.A. Okolie, Advances in the Applications of Nanomaterials for Wastewater Treatment. Environments 2022, 9, 141. [Google Scholar] [CrossRef]
- Ahmad, I.Z.; Ahmad, A.; Tabassum, H.; Kuddus, M. Applications of Nanoparticles in the Treatment of Wastewater.; Springer: Cham, Switzerland, 2017; pp. 1–25. [Google Scholar]
- Naseem, T.; Durrani, T. The role of some important metal oxide nanoparticles for wastewater and antibacterial applications: A review. Enviro. Chem. Ecotoxico. 2021, 3, 59–75. [Google Scholar] [CrossRef]
- Jiang, M.; Qi, Y.; Liu, H.; Chen, Y. The role of nanomaterials and nanotechnologies in wastewater treatment: A bibliometric analysis. Nanoscale Res. Let. 2018, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Le, T.T.N.; Le, V.T.; Dao, M.U.; Nguyen, Q.V.; Vu, T.T.; Nguyen, M.H.; Tran, D.L.; Le, H.S. Preparation of magnetic graphene oxide/chitosan composite beads for effective removal of heavy metals and dyes from aqueous solutions. Chem. Eng. Communic. 2019, 206, 1337–1352. [Google Scholar] [CrossRef]
- Qing, Y.; Hang, Y.; Wanjaul, R.; Jiang, Z.; Hu, B. Adsorption behavior of noble metal ions (Au, Ag, Pd) on nanometer-size titanium dioxide with ICP-AES. Analy. Sci. 2003, 19, 1417–1420. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tsai, C.H.; Chang, W.C.; Saikia, C.E.; Kao, H.M. Functionalization of cubic mesoporous silica SBA-16 with carboxylic acid via one-pot synthesis route for effective removal of cationic dyes. J. Hazard. Mater. 2016, 309, 236–248. [Google Scholar] [CrossRef]
- Sachan, D.; Ramesh, A.; Das, G. Green synthesis of silica nanoparticles from leaf biomass and its application to remove heavy metals from synthetic wastewater: A comparative analysis. Enviro. Nanotech. Monit. Manag. 2021, 16, 100467. [Google Scholar] [CrossRef]
- Garg, R.; Garg, R.; Eddy, N.O.; Almohana, A.I.; Almojil, S.F.; Khan, M.A.; Hong, S.H. Biosynthesized silica-based zinc oxide nanocomposites for the sequestration of heavy metal ions from aqueous solutions. J. KSU Sci. 2022, 34, 101996. [Google Scholar] [CrossRef]
- Arshad, F.; Selvaraj, M.; Banat, F.; Haija, M.A. Removal of metal ions and organics from real refinery wastewater using double-functionalized graphene oxide in alginate beads. J. Wat. Proc. Eng. 2020, 38, 101635. [Google Scholar] [CrossRef]
- Saien, J.; Shahrezaei, F. Organic pollutants removal from Petro. refinery wastewater with nanotitania photocatalyst and UV light emission. Int. J. Photoenerg. 2012, 2012, 703074. [Google Scholar] [CrossRef]
- Bernabeu, A.; Vercher, R.F.; Santos-Juanes, L.; Simón, P.J.; Lardín, C.; Martínez, M.A.; Vicente, J.A.; González, R.; Llosá, C.; Arques, A.; et al. Solar photocatalysis as a tertiary treatment to remove emerging pollutants from wastewater treatment plant effluents. Catalys. Tod. 2011, 161, 235–240. [Google Scholar] [CrossRef]
- Besha, A.T.; Liu, Y.; Fang, C.; Bekele, D.N.; Naidu, R. Assessing the interactions between micropollutants and nanoparticles in engineered and natural aquatic environments. Criti. Revi. Enviro. Sci. Tech. 2020, 50, 135–215. [Google Scholar] [CrossRef]
- Ehrampoush, M.H.; Miria, M.; Salmani, M.H.; Mahvi, A.H. Cadmium removal from aqueous solution by green synthesis iron oxide nanoparticles with tangerine peel extract. J. Enviro. Healt. Sci. Eng. 2015, 13, 84. [Google Scholar] [CrossRef] [PubMed]
- Hassan, I.; Mustafa, M.A.; Elhassan, B. Use of zinc oxide nanoparticle for the removal of phenol contaminated water. SSRG-IJMSE 2017, 3, 1–5. [Google Scholar] [CrossRef]
- Ji, Y.; Ma, C.; Li, J.; Zhao, H.; Chen, Q.; Li, M.; Liu, H. A magnetic adsorbent for the removal of cationic dyes from wastewater. Nanomaterials 2018, 8, 710. [Google Scholar] [CrossRef]
- Jaybhaye, S.; Shinde, N.; Jaybhaye, S.; Narayan, H. Photocatalytic Degradation of Organic Dyes Using Titanium Dioxide (TiO2) and Mg-TiO2 Nanoparticles. J. Nanotechnol. Nanomater. 2022, 3, 67–76. [Google Scholar]
- Mahmoud, A.E.D.; Al-Qahtani, K.M.; Alflaij, S.O.; Al-Qahtani, S.F.; Alsamhan, F.A. Green copper oxide nanoparticles for lead, nickel, and cadmium removal from contaminated water. Sci. Rep. 2021, 11, 12547. [Google Scholar] [CrossRef]
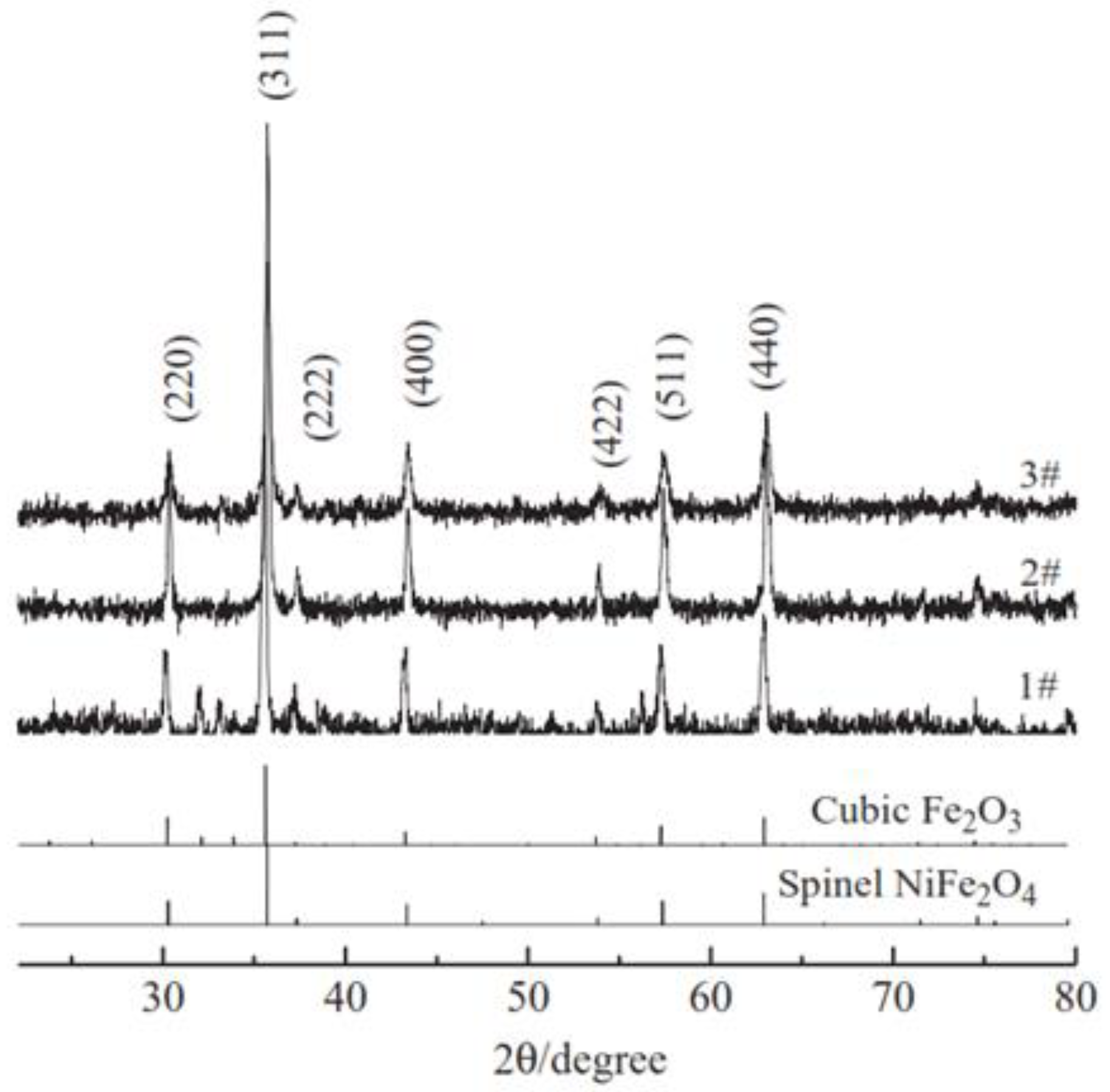
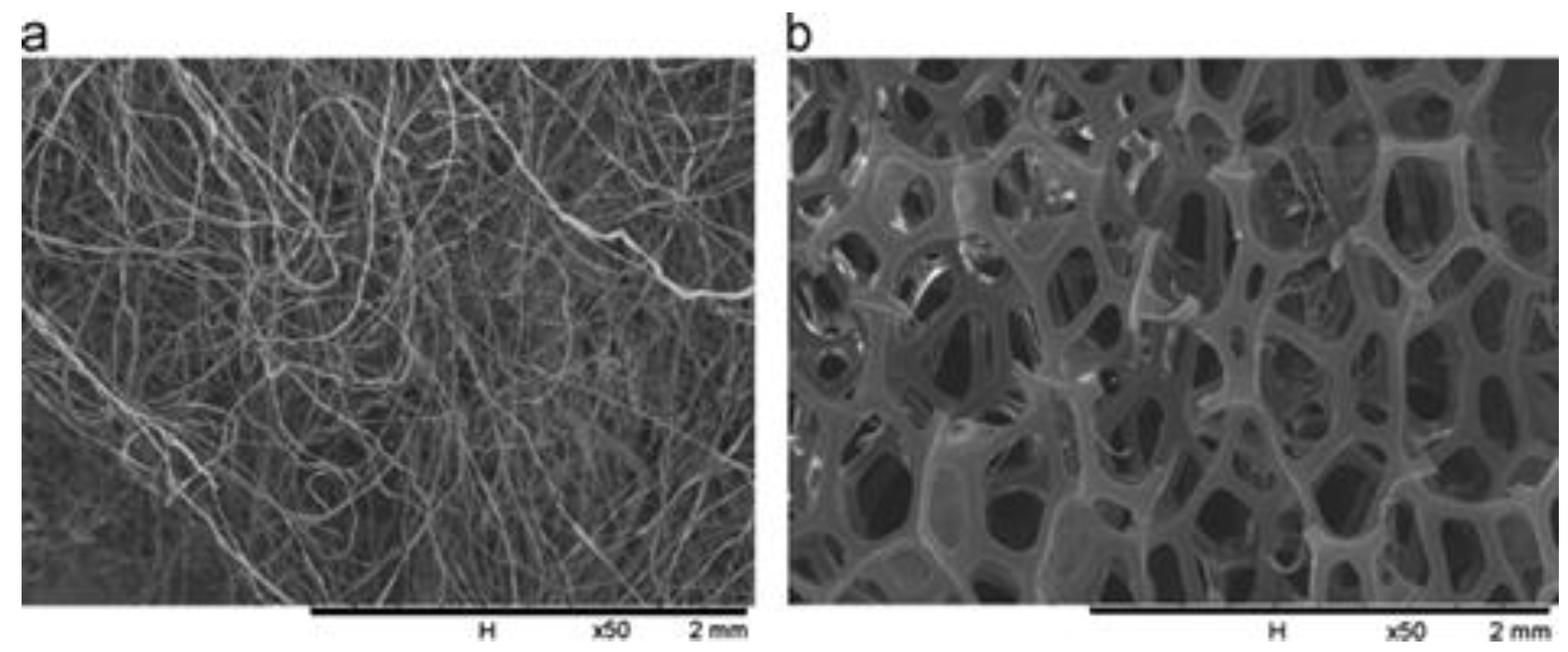

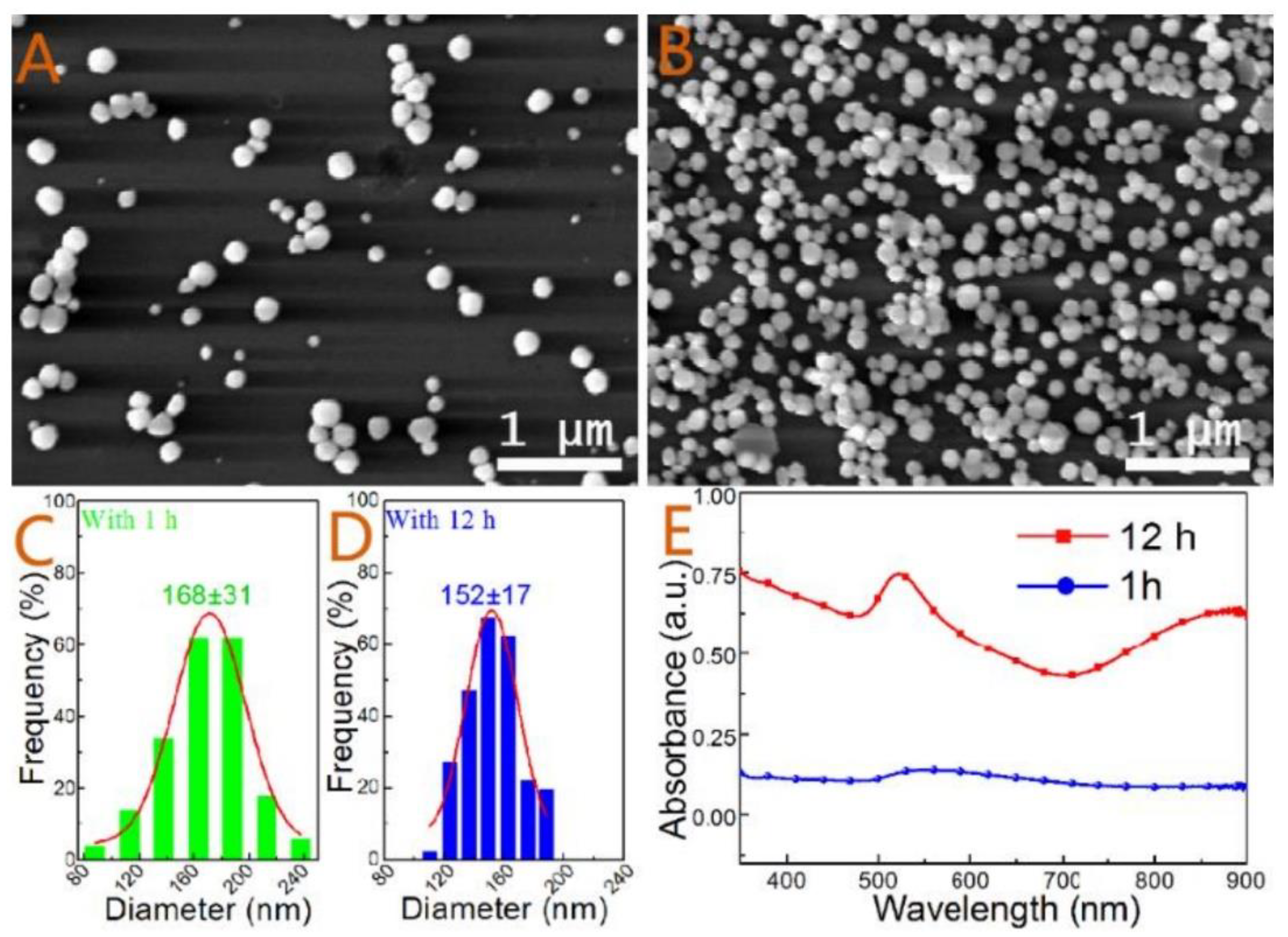

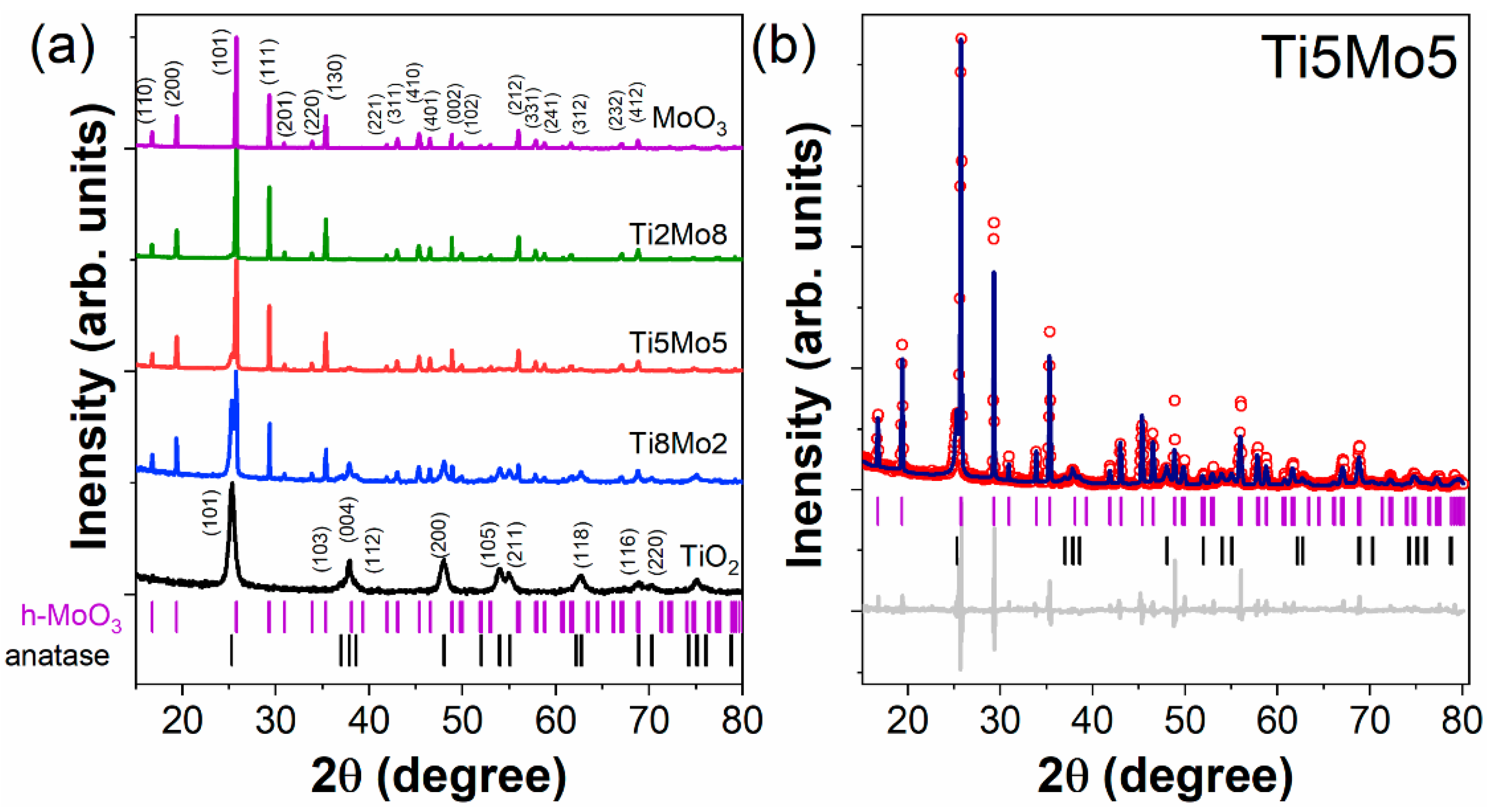
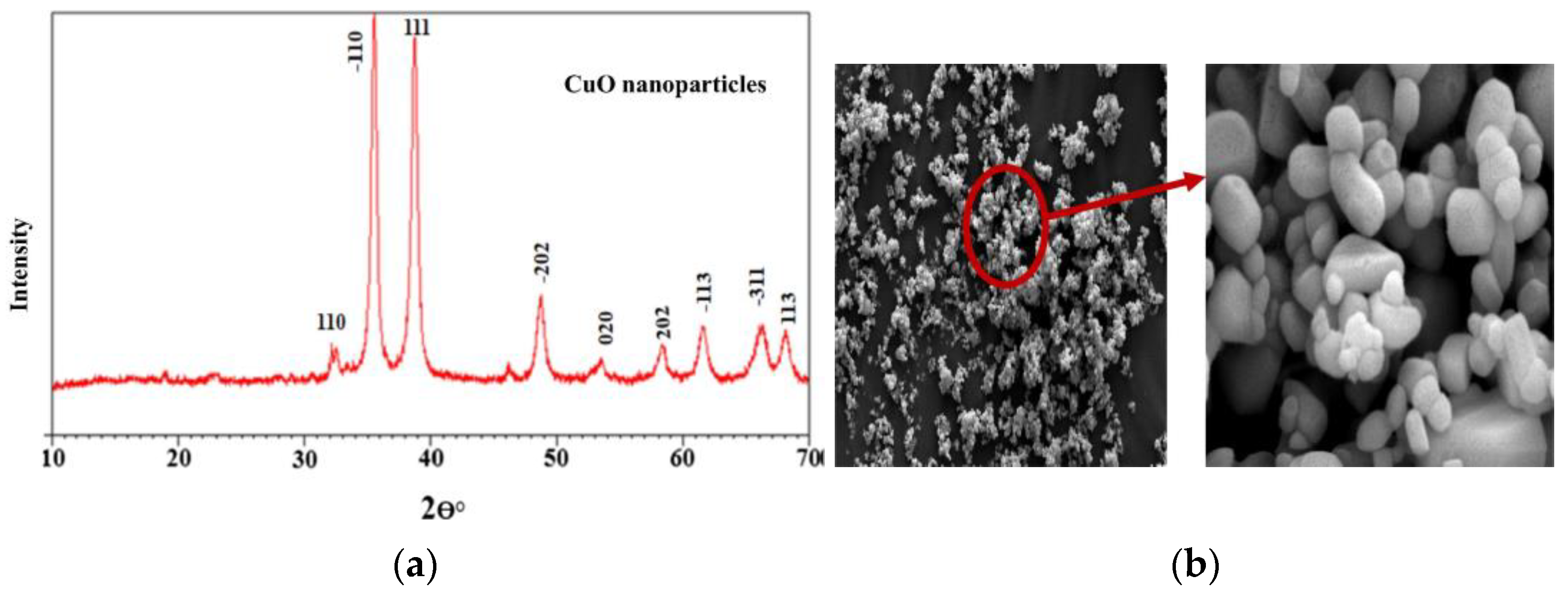
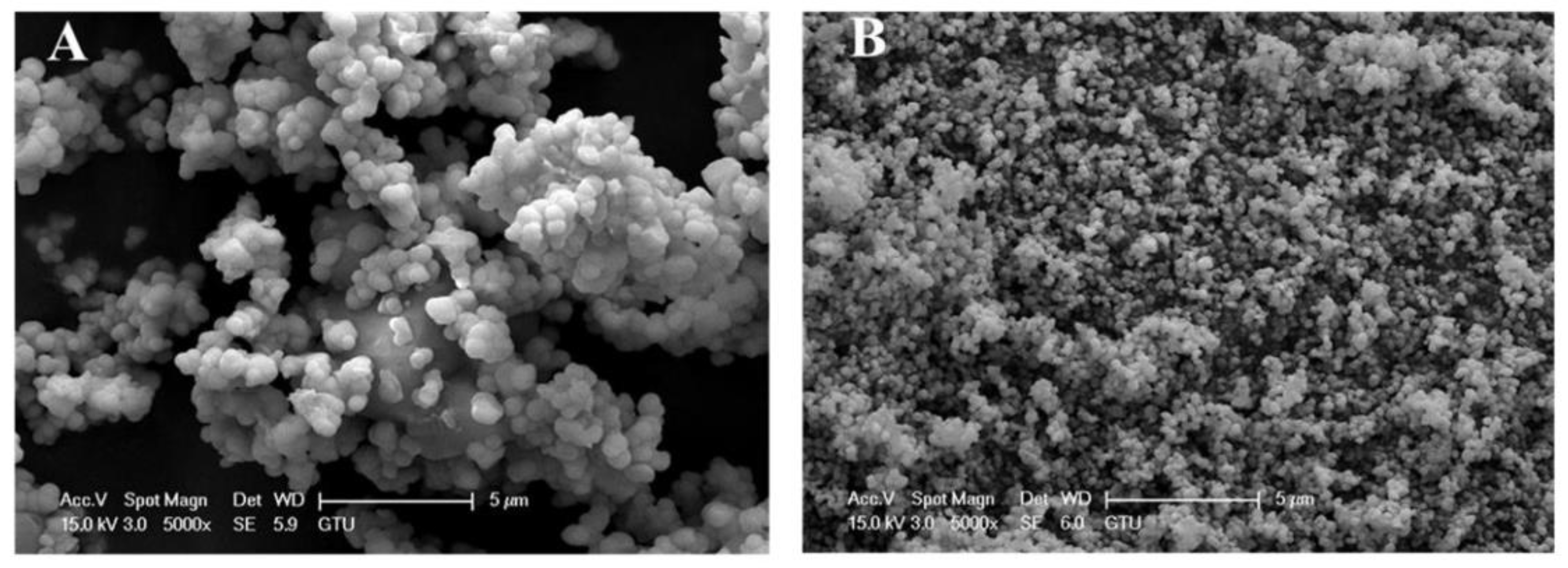

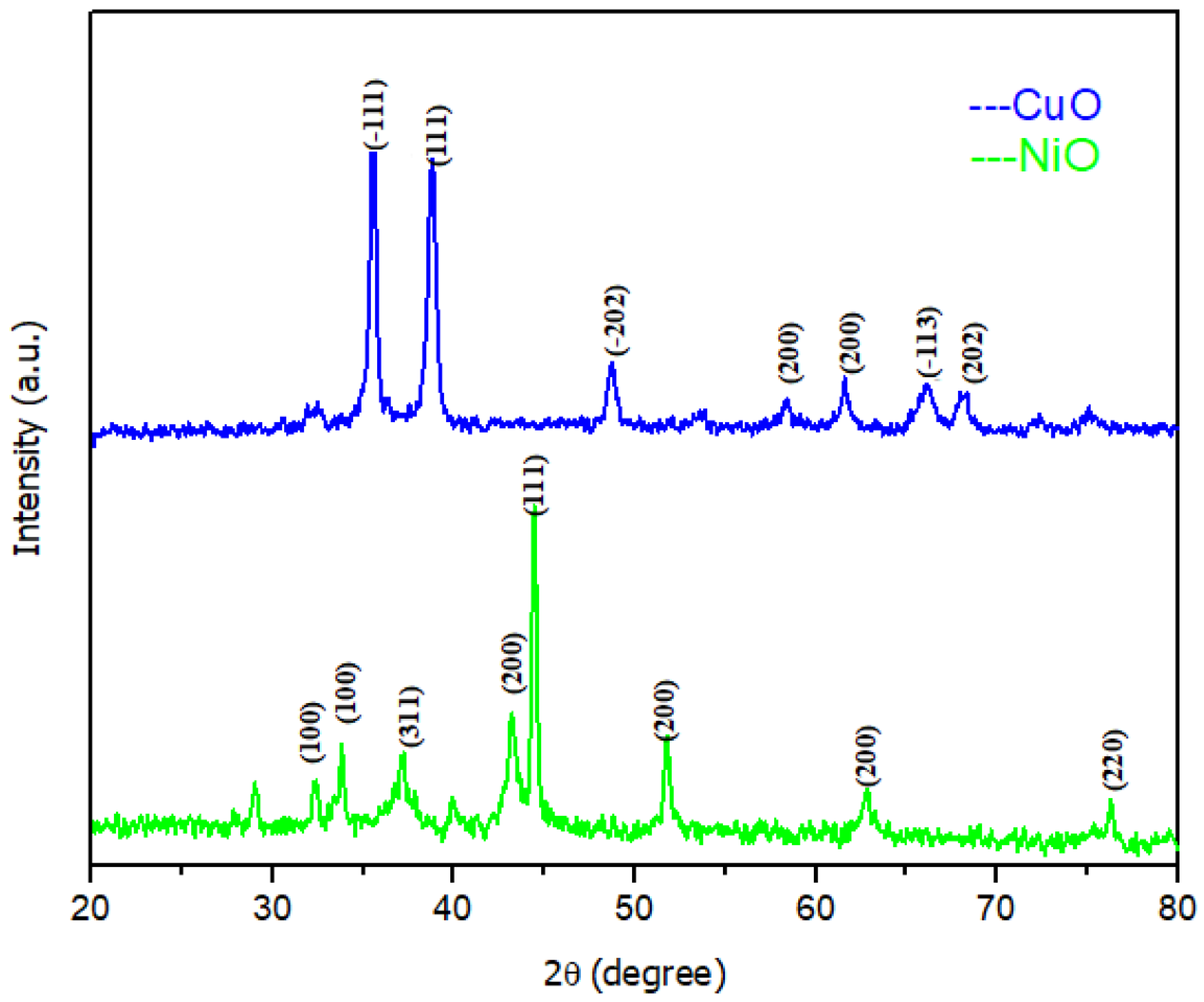

| Formed MOx NPs | Particles Size without Template | Particles Size with Template | Phase/Crystal Structure | Ref | |
|---|---|---|---|---|---|
| With Template | Template-Free | ||||
| ZnO | 20 nm | 28 nm | Wurtzite | Hexagonal | [57,58] |
| CeO2 | 4 nm (105) | 15–36 nm | cubic fluorite | face centred cubic | [59,60] |
| Co3O4 | 10 nm | 20–37 nm | Cubic | face centred cubic | [61,62] |
| In2O3 | 15 nm | 20–30 nm | Cubic | rhombohedral | [63,64] |
| SnO2 | 6–15 nm | 18 nm | tetragonal rutile | tetragonal rutile | [65,66] |
| TiO2 | 30–40 nm | 100 nm | anatase | Rutile | [67,68] |
| Mn3O4 | 5 nm | 25 nm | tetragonal | hausmannite tetragonal | [52,69] |
| NiO | 8 and 26 nm | 31 nm | hexagonal | face-centred cubic | [70,71] |
| Plant | Source of NPS | Metal Oxide | Size | Application | Ref. |
|---|---|---|---|---|---|
| Ficus carica | Leaf | Fe3O4 | 43–57 nm | Antioxidant | [124] |
| Azadirachta indica | Leaf | CuO | NA | Anticancer | [125] |
| Peltophorum pterocarpum | Leaf | Fe3O4 | 85 nm | Rhodamine degradation | [126] |
| Terminalia chebula | Seed | Fe3O4 | NA | Methylene blue degradation | [81] |
| Punica granatum | Peel | ZnO | 118.6 nm | Antibacterial property | [127] |
| Lactuca serriols | Seed | NiO | NA | Degradation of dye | [128] |
| Vitis rotundifolia | Fruit | CoO | NA | Degradation of acid blue dye | [129] |
| Malathion Removal% | Contact Time (min) | pH | Malathion Concentration (mg/L) | Nanoparticle Value (g/L) | Experiment Run |
|---|---|---|---|---|---|
| 21.7 | 50 | 9 | 35 | 1.05 | 1 |
| 23 | 100 | 9 | 35 | 0.55 | 2 |
| 64.5 | 100 | 5 | 85 | 1.05 | 3 |
| 71.33 | 125 | 3 | 35 | 1.05 | 4 |
| 74 | 100 | 7 | 60 | 0.3 | 5 |
| 17 | 100 | 9 | 85 | 0.55 | 6 |
| 82 | 60 | 5 | 35 | 0.4 | 7 |
| 57.7 | 25 | 5 | 110 | 1.3 | 8 |
| 3.4 | 75 | 11 | 60 | 0.80 | 9 |
| 72 | 50 | 7 | 100 | 0.80 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhalili, Z. Metal Oxides Nanoparticles: General Structural Description, Chemical, Physical, and Biological Synthesis Methods, Role in Pesticides and Heavy Metal Removal through Wastewater Treatment. Molecules 2023, 28, 3086. https://doi.org/10.3390/molecules28073086
Alhalili Z. Metal Oxides Nanoparticles: General Structural Description, Chemical, Physical, and Biological Synthesis Methods, Role in Pesticides and Heavy Metal Removal through Wastewater Treatment. Molecules. 2023; 28(7):3086. https://doi.org/10.3390/molecules28073086
Chicago/Turabian StyleAlhalili, Zahrah. 2023. "Metal Oxides Nanoparticles: General Structural Description, Chemical, Physical, and Biological Synthesis Methods, Role in Pesticides and Heavy Metal Removal through Wastewater Treatment" Molecules 28, no. 7: 3086. https://doi.org/10.3390/molecules28073086
APA StyleAlhalili, Z. (2023). Metal Oxides Nanoparticles: General Structural Description, Chemical, Physical, and Biological Synthesis Methods, Role in Pesticides and Heavy Metal Removal through Wastewater Treatment. Molecules, 28(7), 3086. https://doi.org/10.3390/molecules28073086





