Silver Nanoparticles for Waste Water Management
Abstract
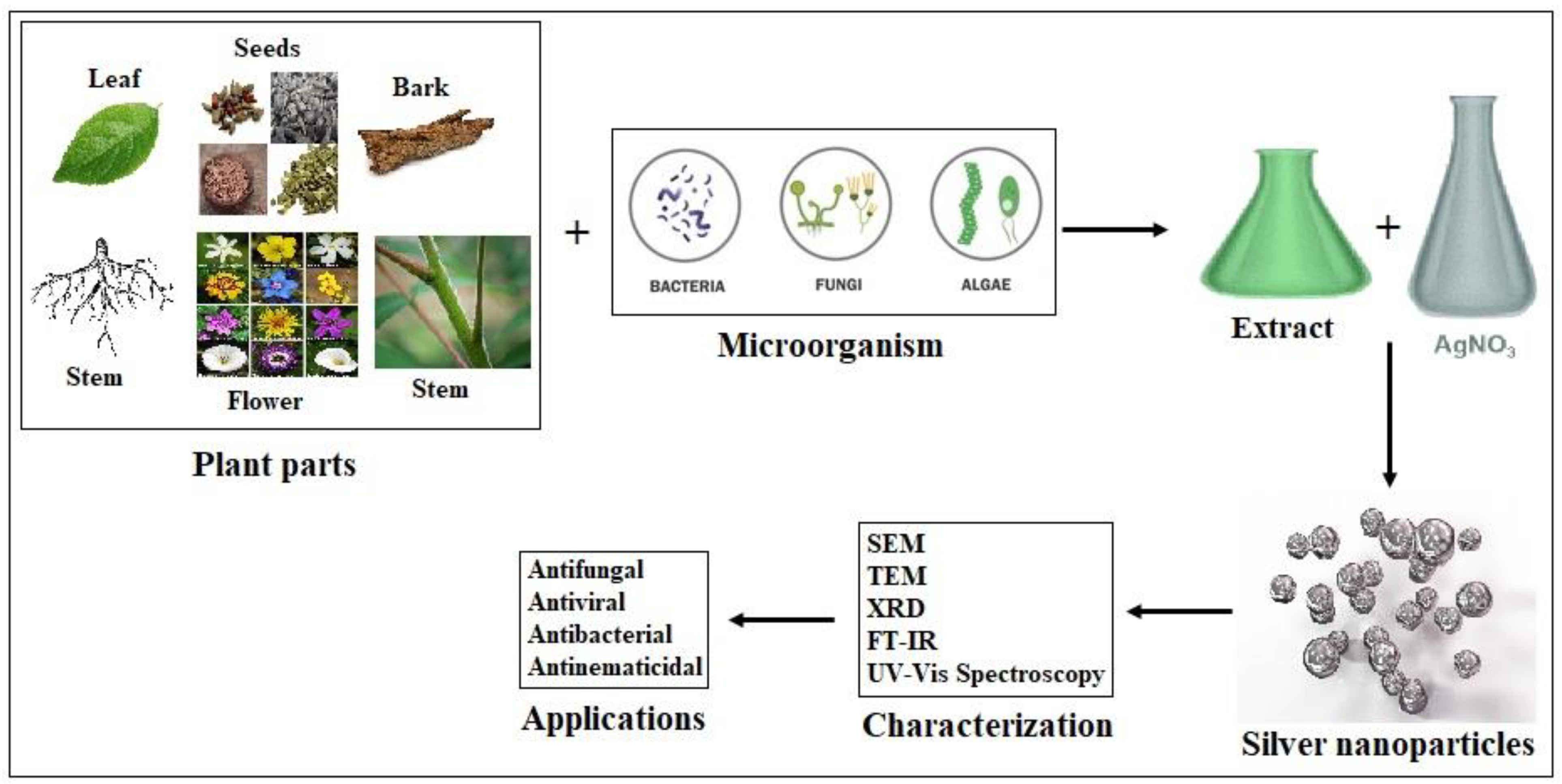
1. Introduction
2. AgNPs in Wastewater Management
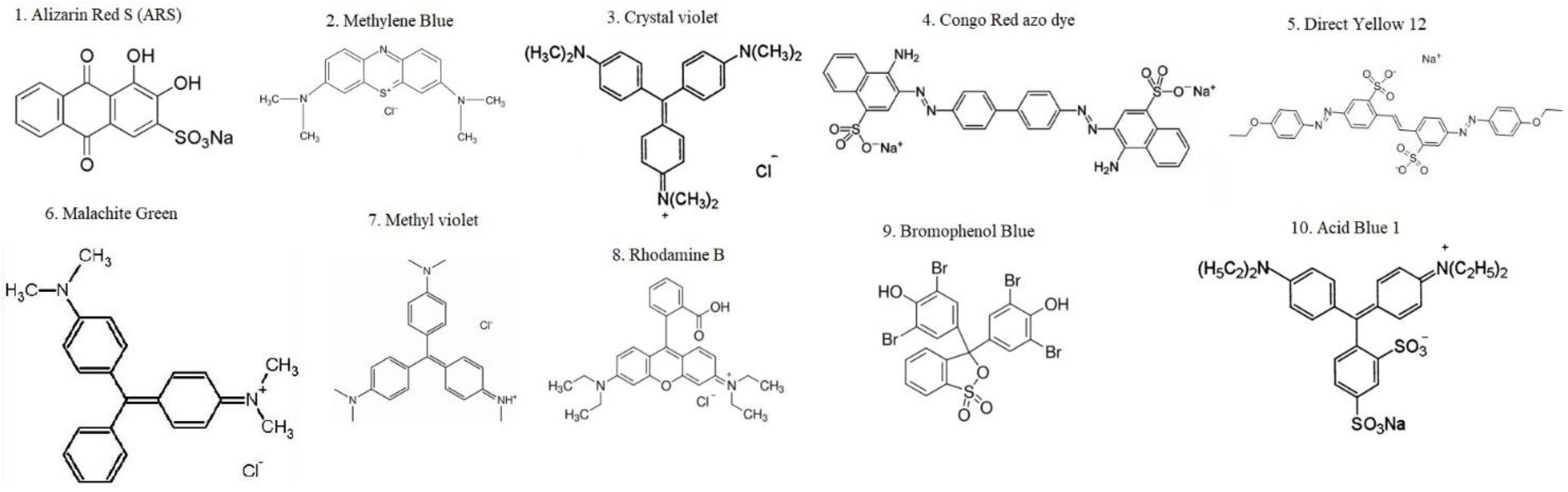
3. Effects of Nanoparticle Composites in Textile Dye Removal
4. Silver Nanoparticles-Composite Activity for Wastewater Treatment
5. Silver Nanoparticles in Agriculture
6. Effect of Textile Dyes on Health and the Environment
7. Challenges in Environmental Safety
8. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Callegari, A.; Tonti, D.; Chergui, M. Photochemically Grown Silver Nanoparticles with Wavelength-Controlled Size and Shape. Nano Lett. 2003, 3, 1565–1568. [Google Scholar] [CrossRef]
- Kordy, M.G.M.; Abdel-Gabbar, M.; Soliman, H.A.; Aljohani, G.; BinSabt, M.; Ahmed, I.A.; Shaban, M. Phyto-Capped Ag Nanoparticles: Green Synthesis, Characterization, and Catalytic and Antioxidant Activities. Nanomaterials 2022, 12, 373. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Feng, M. Water depollution using metal-organic frameworks-catalyzed advanced oxidation processes: A review. J. Hazard. Mater. 2019, 372, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Kędzierska, M.; Potemski, P.; Drabczyk, A.; Kudłacik-Kramarczyk, S.; Głąb, M.; Grabowska, B.; Mierzwiński, D.; Tyliszczak, B. The Synthesis Methodology of PEGylated Fe3O4@Ag Nanoparticles Supported by Their Physicochemical Evaluation. Molecules 2021, 26, 1744. [Google Scholar] [CrossRef] [PubMed]
- Florkiewicz, W.; Pluta, K.; Malina, D.; Rudnicka, K.; Żywicka, A.; Guigou, M.D.; Tyliszczak, B.; Sobczak-Kupiec, A. Investigation on Green Synthesis, Biocompatibility, and Antibacterial Activity of Silver Nanoparticles Prepared Using Cistus incanus. Materials 2021, 14, 5028. [Google Scholar] [CrossRef]
- Jeyaraj, M.; Rajesh, M.; Arun, R.; MubarakAli, D.; Sathishkumar, G.; Sivanandhan, G.; Dev, G.K.; Manickavasagam, M.; Premkumar, K.; Thajuddin, N.; et al. An investigation on the cytotoxicity and caspase-mediated apoptotic effect of biologically synthesized silver nanoparticles using Podophyllum hexandrum on human cervical carcinoma cells. Coll. Surf. B Biointerface 2013, 102, 708–717. [Google Scholar] [CrossRef]
- Maccora, D.; Dini, V.; Battocchio, C.; Fratoddi, I.; Cartoni, A.; Rotili, D.; Castagnola, M.; Faccini, R.; Bruno, I.; Scotognella, T.; et al. Gold Nanoparticles and Nanorods in Nuclear Medicine: A Mini Review. Appl. Sci. 2019, 9, 3232. [Google Scholar] [CrossRef]
- Prosposito, P.; Burratti, L.; Venditti, I. Silver Nanoparticles as Colorimetric Sensors for Water Pollutants. Chemosensors 2020, 8, 26. [Google Scholar] [CrossRef]
- Venditti, I. Engineered Gold-Based Nanomaterials: Morphologies and Functionalities in Biomedical Applications. A Mini Review. Bioengineering 2019, 6, 53. [Google Scholar] [CrossRef]
- Kędzierska, M.; Drabczyk, A.; Jamroży, M.; Kudłacik-Kramarczyk, S.; Głąb, M.; Tyliszczak, B.; Bańkosz, W.; Potemski, P. The Synthesis Methodology and Characterization of Nanogold-Coated Fe3O4 Magnetic Nanoparticles. Materials 2022, 15, 3383. [Google Scholar] [CrossRef]
- Corsi, I.; Winther-Nielsen, M.; Sethi, R.; Punta, C.; Della Torre, C.; Libralato, G.; Lofrano, G.; Sabatini, L.; Aiello, M.; Fiordi, L.; et al. Ecofriendly nanotechnologies and nanomaterials for environmental applications: Key issue and consensus recommendations for sustainable and ecosafe nanoremediation. Ecotoxicol. Environ. Saf. 2018, 154, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Kodoth, A.K.; Badalamoole, V. Silver nanoparticle-embedded pectin-based hydrogel for adsorptive removal of dyes and metal ions. Polym. Bull. 2020, 77, 541–564. [Google Scholar] [CrossRef]
- Corsi, I.; Cherr, G.N.; Lenihan, H.S.; Labille, J.; Hassellov, M.; Canesi, L.; Dondero, F.; Frenzilli, G.; Hristozov, D.; Puntes, V.; et al. Common Strategies and Technologies for the Ecosafety Assessment and Design of Nanomaterials Entering the Marine Environment. ACS Nano 2014, 8, 9694–9709. [Google Scholar] [CrossRef]
- Gottschalk, F.; Sun, T.; Nowack, B. Environmental concentrations of engineered nanomaterials: Review of modeling and analytical studies. Environ. Pollut. 2013, 181, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, I.-L.; Hsieh, Y.-K.; Wang, C.-F.; Chen, I.-C.; Huang, Y.-J. Trojan-Horse Mechanism in the Cellular Uptake of Silver Nanoparticles Verified by Direct Intra- and Extracellular Silver Speciation Analysis. Environ. Sci. Technol. 2015, 49, 3813–3821. [Google Scholar] [CrossRef]
- Ma, R.; Levard, C.; Marinakos, S.M.; Cheng, Y.; Liu, J.; Michel, F.M.; Brown, J.G.E.; Lowry, G.V. Size-Controlled Dissolution of Organic-Coated Silver Nanoparticles. Environ. Sci. Technol. 2012, 46, 752–759. [Google Scholar] [CrossRef]
- Courtois, P.; Rorat, A.; Lemiere, S.; Guyoneaud, R.; Attard, E.; Levard, C.; Vandenbulcke, F. Ecotoxicology of silver nanoparticles and their derivatives introduced in soil with or without sewage sludge: A review of effects on microorganisms, plants and animals. Environ. Pollut. 2019, 253, 578–598. [Google Scholar] [CrossRef]
- McGillicuddy, E.; Murray, I.; Kavanagh, S.; Morrison, L.; Fogarty, A.; Cormican, M.; Dockery, P.; Prendergast, M.; Rowan, N.; Morris, D. Silver nanoparticles in the environment: Sources, detection and ecotoxicology. Sci. Total Environ. 2017, 575, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Minetto, D.; Ghirardini, A.V.; Libralato, G. Saltwater ecotoxicology of Ag, Au, CuO, TiO2, ZnO and C60 engineered nanoparticles: An overview. Environ. Int. 2016, 92, 189–201. [Google Scholar] [CrossRef]
- Palani, G.; Apsari, R.; Hanafiah, M.M.; Venkateswarlu, K.; Lakkaboyana, S.K.; Kannan, K.; Shivanna, A.T.; Idris, A.M.; Yadav, C.H. Metal-Doped Graphitic Carbon Nitride Nanomaterials for Photocatalytic Environmental Applications—A Review. Nanomaterials 2022, 12, 1754. [Google Scholar] [CrossRef]
- Mansoor, S.; Zahoor, I.; Baba, T.R.; Padder, S.A.; Bhat, Z.A.; Koul, A.M.; Jiang, L. Fabrication of Silver Nanoparticles Against Fungal Pathogens. Front. Nanotechnol. 2021, 3, 679358. [Google Scholar] [CrossRef]
- Lofrano, G.; Libralato, G.; Brown, J. (Eds.) Nanotechnologies for Environmental Remediation: Applications and Implications, 1st ed.; Springer: Cham, Switzerland, 2017; p. 325. [Google Scholar] [CrossRef]
- Mierzwiński, D.; Nosal, P.; Szczepanik, A.; Łach, M.; Guigou, M.D.; Hebda, M.; Korniejenko, K. Concept of Flocks Fragmentation and Averaging Method for the Application of Electrocoagulation in Process for Coke Oven Wastewater Treatment. Materials 2021, 14, 6307. [Google Scholar] [CrossRef] [PubMed]
- Corsi, P.; Venditti, I.; Battocchio, C.; Meneghini, C.; Bruni, F.; Prosposito, P.; Mochi, F.; Capone, B. Designing an Optimal Ion Adsorber at the Nanoscale: The Unusual Nucleation of AgNP/Co2+–Ni2+ Binary Mixtures. J. Phys. Chem. C 2019, 123, 3855–3860. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Bai, J.; Jiang, X.; Fan, G. High sensitivity hydrogen peroxide and hydrazine sensor based on silver nanocubes with rich {100} facets as an enhanced electrochemical sensing platform. Biosens. Bioelectron. 2013, 43, 180–185. [Google Scholar] [CrossRef]
- Krishnappa, S.; Kalikeri, S.; Garampalli, R.K.H.; Kachintaya, C.K. A brief review of the impact of silver nanoparticles on agriculture and certain biological properties: A case study. Int. J. Health Allied Sci. 2022, 11, 62–69. [Google Scholar] [CrossRef]
- Khan, S.A.; Jain, M.; Pandey, A.; Pant, K.K.; Ziora, Z.M.; Blaskovich, M.A.; Shetti, N.P.; Aminabhavi, T.M. Leveraging the potential of silver nanoparticles-based materials towards sustainable water treatment. J. Environ. Manag. 2022, 319, 115675. [Google Scholar] [CrossRef] [PubMed]
- Shetti, N.P.; Malode, S.J.; Nayak, D.S.; Aminabhavi, T.M.; Reddy, K.R. Nanostructured silver doped TiO2/CNTs hybrid as an efficient electrochemical sensor for detection of anti-inflammatory drug, cetirizine. Microchem. J. 2019, 150, 104124. [Google Scholar] [CrossRef]
- Tarannum, N.; Divya; Gautam, Y.K. Facile green synthesis and applications of silver nanoparticles: A state-of-the-art review. RSC Adv. 2019, 9, 34926–34948. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar]
- Puiatti, G.A.; de Carvalho, J.P.; de Matos, A.T.; Lopes, R.P. Green synthesis of Fe0 nanoparticles using Eucalyptus grandis leaf extract: Characterization and application for dye degradation by a (Photo)Fenton-like process. J. Environ. Manag. 2022, 311, 114828. [Google Scholar] [CrossRef]
- Xiao, C.; Li, H.; Zhao, Y.; Zhang, X.; Wang, X. Green synthesis of iron nanoparticle by tea extract (polyphenols) and its selective removal of cationic dyes. J. Environ. Manag. 2020, 275, 111262. [Google Scholar] [CrossRef] [PubMed]
- Pryshchepa, O.; Pomastowski, P.; Buszewski, B. Silver nanoparticles: Synthesis, investigation techniques, and properties. Adv. Colloid Interface Sci. 2020, 284, 102246. [Google Scholar] [CrossRef]
- Marimuthu, S.; Antonisamy, A.J.; Malayandi, S.; Rajendran, K.; Tsai, P.-C.; Pugazhendhi, A.; Ponnusamy, V.K. Silver nanoparticles in dye effluent treatment: A review on synthesis, treatment methods, mechanisms, photocatalytic degradation, toxic effects and mitigation of toxicity. J. Photochem. Photobiol. B Biol. 2020, 205, 111823. [Google Scholar] [CrossRef] [PubMed]
- Sudha, A.; Jeyakanthan, J.; Srinivasan, P. Green synthesis of silver nanoparticles using Lippia nodiflora aerial extract and evaluation of their antioxidant, antibacterial and cytotoxic effects. Resour. Technol. 2017, 3, 506–515. [Google Scholar] [CrossRef]
- Łach, M.; Grela, A.; Pławecka, K.; Guigou, M.D.; Mikuła, J.; Komar, N.; Bajda, T.; Korniejenko, K. Surface Modification of Synthetic Zeolites with Ca and HDTMA Compounds with Determination of Their Phytoavailability and Comparison of CEC and AEC Parameters. Materials 2022, 15, 4083. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.-T.; Song, Y.-H.; Fan, H.-C.; Yu, L. Bioreduction of azo dyes was enhanced by in-situ biogenic palladium nanoparticles. Bioresour. Technol. 2018, 266, 176–180. [Google Scholar] [CrossRef]
- Riaz, U.; Ashraf, S.; Budhiraja, V.; Aleem, S.; Kashyap, J. Comparative studies of the photocatalytic and microwave –assisted degradation of alizarin red using ZnO/poly(1-naphthylamine) nanohybrids. J. Mol. Liq. 2016, 216, 259–267. [Google Scholar] [CrossRef]
- Reghioua, A.; Barkat, D.; Jawad, A.H.; Abdulhameed, A.S.; Al-Kahtani, A.A.; Alothman, Z.A. Parametric optimization by Box–Behnken design for synthesis of magnetic chitosan-benzil/ZnO/Fe3O4 nanocomposite and textile dye removal. J. Environ. Chem. Eng. 2021, 9, 105166. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Wang, Z.; Liu, H. Green synthesis of palladium nanoparticles with carboxymethyl cellulose for degradation of azo-dyes. Mater. Chem. Phys. 2017, 187, 133–140. [Google Scholar] [CrossRef]
- Nagar, N.; Devra, V. Activation of peroxodisulfate and peroxomonosulfate by green synthesized copper nanoparticles for Methyl Orange degradation: A kinetic study. J. Environ. Chem. Eng. 2017, 5, 5793–5800. [Google Scholar] [CrossRef]
- Tharunya, P.; Subha, V.; Kirubanandan, S.; Sandhaya, S.; Renganathan, S. Green synthesis of superparamagnetic iron oxide nanoparticle from Ficus carica fruit extract, characterization studies and its application on dye degradation studies. Asian J. Pharm. Clin. Res. 2017, 10, 125. [Google Scholar] [CrossRef]
- Gao, Z.; Yi, Y.; Zhao, J.; Xia, Y.; Jiang, M.; Cao, F.; Zhou, H.; Wei, P.; Jia, H.; Yong, X.-Y. Co-immobilization of laccase and TEMPO onto amino-functionalized magnetic Fe3O4 nanoparticles and its application in acid fuchsin decolorization. Bioresour. Bioprocess. 2018, 5, 27. [Google Scholar] [CrossRef]
- Wang, L.; Lu, F.; Liu, Y.; Wu, Y.; Wu, Z. Photocatalytic degradation of organic dyes and antimicrobial activity of silver nanoparticles fast synthesized by flavonoids fraction of Psidium guajava L. leaves. J. Mol. Liq. 2018, 263, 187–192. [Google Scholar] [CrossRef]
- Thi, V.H.T.; Cao, T.H.; Pham, T.N.; Pham, T.T.; Le, M.C. Synergistic Adsorption and Photocatalytic Activity under Visible Irradiation Using Ag-ZnO/GO Nanoparticles Derived at Low Temperature. J. Chem. 2019, 2019, 2979517. [Google Scholar] [CrossRef]
- Cahino, A.; Loureiro, R.G.; Dantas, J.; Madeira, V.S.; Fernandes, P.C.R. Characterization and evaluation of ZnO/CuO catalyst in the degradation of methylene blue using solar radiation. Ceram. Int. 2019, 45, 13628–13636. [Google Scholar] [CrossRef]
- Stan, M.; Lung, I.; Soran, M.-L.; Opris, O.; Leostean, C.; Popa, A.; Copaciu, F.; Lazar, M.D.; Kacso, I.; Silipas, T.-D.; et al. Starch-coated green synthesized magnetite nanoparticles for removal of textile dye Optilan Blue from aqueous media. J. Taiwan Inst. Chem. Eng. 2019, 100, 65–73. [Google Scholar] [CrossRef]
- Banerjee, P.; Mukhopadhyay, A.; Das, P. Graphene oxide–nanobentonite composite sieves for enhanced desalination and dye removal. Desalination 2019, 451, 231–240. [Google Scholar] [CrossRef]
- Jawad, A.H.; Mubarak, N.S.A.; Abdulhameed, A.S. Tunable Schiff’s base-cross-linked chitosan composite for the removal of reactive red 120 dye: Adsorption and mechanism study. Int. J. Biol. Macromol. 2020, 142, 732–741. [Google Scholar] [CrossRef]
- Nga, N.K.; Chau, N.T.T.; Viet, P.H. Preparation and characterization of a chitosan/MgO composite for the effective removal of reactive blue 19 dye from aqueous solution. J. Sci. Adv. Mater. Dev. 2020, 5, 65–72. [Google Scholar] [CrossRef]
- Muinde, V.M.; Onyari, J.M.; Wamalwa, B.; Wabomba, J.N. Adsorption of malachite green dye from aqueous solutions using mesoporous chitosan–zinc oxide composite material. Environ. Chem. Ecotoxicol. 2020, 2, 115–125. [Google Scholar] [CrossRef]
- Reghioua, A.; Barkat, D.; Jawad, A.H.; Abdulhameed, A.S.; Rangabhashiyam, S.; Khan, M.R.; Alothman, Z.A. Magnetic Chitosan-Glutaraldehyde/Zinc Oxide/Fe3O4 Nanocomposite: Optimization and Adsorptive Mechanism of Remazol Brilliant Blue R Dye Removal. J. Polym. Environ. 2021, 29, 3932–3947. [Google Scholar] [CrossRef]
- Mostafa, M.H.; Elsawy, M.A.; Darwish, M.S.; Hussein, L.I.; Abdaleem, A.H. Microwave-Assisted preparation of Chitosan/ZnO nanocomposite and its application in dye removal. Mater. Chem. Phys. 2020, 248, 122914. [Google Scholar] [CrossRef]
- Mydeen, S.S.; Kumar, R.R.; Sambathkumar, S.; Kottaisamy, M.; Vasantha, V. Facile Synthesis of ZnO/AC Nanocomposites using Prosopis Juliflora for Enhanced Photocatalytic Degradation of Methylene Blue and Antibacterial Activity. Optik 2020, 224, 165426. [Google Scholar] [CrossRef]
- Pandey, A.; Shukla, P.; Srivastava, P.K. Remediation of Dyes in Water using Green Synthesized Nanoparticles (NPs). Int. J. Plant Environ. 2020, 6, 68–84. [Google Scholar] [CrossRef]
- Vijayaraghavan, T.; Althaf, R.; Babu, P.; Parida, K.; Vadivel, S.; Ashok, A.M. Visible light active LaFeO3 nano perovskite-RGO-NiO composite for efficient H2 evolution by photocatalytic water splitting and textile dye degradation. J. Environ. Chem. Eng. 2021, 9, 104675. [Google Scholar] [CrossRef]
- Stanley, R.; Jebasingh, J.A.; Manisha Vidyavathy, S.; Stanley, P.K.; Ponmani, P.; Shekinah, M.; Vasanthi, J. Excellent Photocatalytic degradation of Methylene Blue, Rhodamine B and Methyl Orange dyes by Ag-ZnO nanocomposite under natural sunlight irradiation. Optik 2021, 231, 166518. [Google Scholar] [CrossRef]
- Soto-Robles, C.; Nava, O.; Cornejo, L.; Lugo-Medina, E.; Vilchis-Nestor, A.; Castro-Beltrán, A.; Luque, P. Biosynthesis, characterization and photocatalytic activity of ZnO nanoparticles using extracts of Justicia spicigera for the degradation of methylene blue. J. Mol. Struct. 2021, 1225, 129101. [Google Scholar] [CrossRef]
- Shubha, J.P.; Kavalli, K.; Adil, S.F.; Assal, M.E.; Hatshan, M.R.; Dubasi, N. Facile green synthesis of semiconductive ZnO nanoparticles for photocatalytic degradation of dyes from the textile industry: A kinetic approach. J. King Saud Univ. Sci. 2022, 34, 102047. [Google Scholar] [CrossRef]
- Yasin, A.; Fatima, U.; Shahid, S.; Mansoor, S.; Inam, H.; Javed, M.; Iqbal, S.; Alrbyawi, H.; Somaily, H.H.; Pashameah, R.A.; et al. Fabrication of Copper Oxide Nanoparticles Using Passiflora edulis Extract for the Estimation of Antioxidant Potential and Photocatalytic Methylene Blue Dye Degradation. Agronomy 2022, 12, 2315. [Google Scholar] [CrossRef]
- Alcantara-Cobos, A.; Gutiérrez-Segura, E.; Solache-Ríos, M.; Amaya-Chávez, A.; Solís-Casados, D. Tartrazine removal by ZnO nanoparticles and a zeolite-ZnO nanoparticles composite and the phytotoxicity of ZnO nanoparticles. Microporous Mesoporous Mater. 2020, 302, 110212. [Google Scholar] [CrossRef]
- Rajagopal, S.; Paramasivam, B.; Muniyasamy, K. Photocatalytic removal of cationic and anionic dyes in the textile wastewater by H2O2 assisted TiO2 and micro-cellulose composites. Sep. Purif. Technol. 2020, 252, 117444. [Google Scholar] [CrossRef]
- Nsom, M.V.; Etape, E.P.; Tendo, J.F.; Namond, B.V.; Chongwain, P.T.; Yufanyi, M.D.; William, N. A Green and Facile Approach for Synthesis of Starch-Pectin Magnetite Nanoparticles and Application by Removal of Methylene Blue from Textile Effluent. J. Nanomater. 2019, 2019, 4576135. [Google Scholar] [CrossRef]
- Rashid, T.; Iqbal, D.; Hazafa, A.; Hussain, S.; Sher, F.; Sher, F. Formulation of zeolite supported nano-metallic catalyst and applications in textile effluent treatment. J. Environ. Chem. Eng. 2020, 8, 104023. [Google Scholar] [CrossRef]
- Zeng, Q.; Liu, Y.; Shen, L.; Lin, H.; Yu, W.; Xu, Y.; Li, R.; Huang, L. Facile preparation of recyclable magnetic Ni@filter paper composite materials for efficient photocatalytic degradation of methyl orange. J. Colloid Interface Sci. 2021, 582, 291–300. [Google Scholar] [CrossRef]
- Dubey, S.P.; Dwivedi, A.D.; Kim, I.-C.; Sillanpaa, M.; Kwon, Y.-N.; Lee, C. Synthesis of graphene–carbon sphere hybrid aerogel with silver nanoparticles and its catalytic and adsorption applications. Chem. Eng. J. 2014, 244, 160–167. [Google Scholar] [CrossRef]
- Jiao, T.; Guo, H.; Zhang, Q.; Peng, Q.; Tang, Y.; Yan, X.; Li, B. Reduced Graphene Oxide-Based Silver Nanoparticle-Containing Composite Hydrogel as Highly Efficient Dye Catalysts for Wastewater Treatment. Sci. Rep. 2015, 5, 11873. [Google Scholar] [CrossRef]
- Al-Rawashdeh, N.A.F.; Allabadi, O.; Aljarrah, M.T. Photocatalytic Activity of Graphene Oxide/Zinc Oxide Nanocomposites with Embedded Metal Nanoparticles for the Degradation of Organic Dyes. ACS Omega 2020, 5, 28046–28055. [Google Scholar] [CrossRef]
- Naseem, K.; Rehman, M.Z.U.; Ahmad, A.; Dubal, D.; AlGarni, T.S. Plant Extract Induced Biogenic Preparation of Silver Nanoparticles and Their Potential as Catalyst for Degradation of Toxic Dyes. Coatings 2020, 10, 1235. [Google Scholar] [CrossRef]
- Yang, W.; Hu, W.; Zhang, J.; Wang, W.; Cai, R.; Pan, M.; Huang, C.; Chen, X.; Yan, B.; Zeng, H. Tannic acid/Fe3+ functionalized magnetic graphene oxide nanocomposite with high loading of silver nanoparticles as ultra-efficient catalyst and disinfectant for wastewater treatment. Chem. Eng. J. 2021, 405, 126629. [Google Scholar] [CrossRef]
- Nguyen, T.-D.; Dang, C.-H.; Mai, D.-T. Biosynthesized AgNP capped on novel nanocomposite 2-hydroxypropyl-β-cyclodextrin/alginate as a catalyst for degradation of pollutants. Carbohydr. Polym. 2018, 197, 29–37. [Google Scholar] [CrossRef]
- Veisi, H.; Azizi, S.; Mohammadi, P. Green synthesis of the silver nanoparticles mediated by Thymbra spicata extract and its application as a heterogeneous and recyclable nanocatalyst for catalytic reduction of a variety of dyes in water. J. Clean. Prod. 2018, 170, 1536–1543. [Google Scholar] [CrossRef]
- Kaur, P.; Thakur, R.; Malwal, H.; Manuja, A.; Chaudhury, A. Biosynthesis of biocompatible and recyclable silver/iron and gold/iron core-shell nanoparticles for water purification technology. Biocatal. Agric. Biotechnol. 2018, 14, 189–197. [Google Scholar] [CrossRef]
- Das, R.; Sypu, V.S.; Paumo, H.K.; Bhaumik, M.; Maharaj, V.; Maity, A. Silver decorated magnetic nanocomposite (Fe3O4@PPy-MAA/Ag) as highly active catalyst towards reduction of 4-nitrophenol and toxic organic dyes. Appl. Catal. B Environ. 2019, 244, 546–558. [Google Scholar] [CrossRef]
- Alkayal, N.S.; Hussein, M.A. Photocatalytic Degradation of Atrazine under Visible Light Using Novel Ag@Mg4Ta2O9 Nanocomposites. Sci. Rep. 2019, 9, 7470. [Google Scholar] [CrossRef] [PubMed]
- Albukhari, S.M.; Ismail, M.; Akhtar, K.; Danish, E.Y. Catalytic reduction of nitrophenols and dyes using silver nanoparticles @ cellulose polymer paper for the resolution of waste water treatment challenges. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 577, 548–561. [Google Scholar] [CrossRef]
- Azzam, E.M.; Fathy, N.A.; El-Khouly, S.M.; Sami, R.M. Enhancement the photocatalytic degradation of methylene blue dye using fabricated CNTs/TiO2/AgNPs/Surfactant nanocomposites. J. Water Process Eng. 2019, 28, 311–321. [Google Scholar] [CrossRef]
- Elbakry, S.; Ali, M.E.; Abouelfadl, M.; Badway, N.A.; Salam, K.M. Photocatalytic degradation of organic compounds by TFC membranes functionalized with Ag/rGO nanocomposites. J. Photochem. Photobiol. A Chem. 2022, 430, 113957. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Zhang, Y.; van Bochove, B.; Mäkilä, E.; Seppälä, J.; Xu, W.; Willför, S.; Xu, C. Robust shape-retaining nanocellulose-based aerogels decorated with silver nanoparticles for fast continuous catalytic discoloration of organic dyes. Sep. Purif. Technol. 2020, 242, 116523. [Google Scholar] [CrossRef]
- Choudhary, N.; Yadav, V.K.; Yadav, K.K.; Almohana, A.I.; Almojil, S.F.; Gnanamoorthy, G.; Kim, D.-H.; Islam, S.; Kumar, P.; Jeon, B.-H. Application of Green Synthesized MMT/Ag Nanocomposite for Removal of Methylene Blue from Aqueous Solution. Water 2021, 13, 3206. [Google Scholar] [CrossRef]
- Bandi, R.; Alle, M.; Park, C.-W.; Han, S.-Y.; Kwon, G.-J.; Kim, J.-C.; Lee, S.-H. Rapid synchronous synthesis of Ag nanoparticles and Ag nanoparticles/holocellulose nanofibrils: Hg(II) detection and dye discoloration. Carbohydr. Polym. 2020, 240, 116356. [Google Scholar] [CrossRef]
- Chandra, R.; Nath, M. Controlled synthesis of AgNPs@ZIF-8 composite: Efficient heterogeneous photocatalyst for degradation of methylene blue and congo red. J. Water Process Eng. 2020, 36, 101266. [Google Scholar] [CrossRef]
- Rabbi, M.A.; Rahman, M.M.; Minami, H.; Habib, M.R.; Ahmad, H. Ag impregnated sub-micrometer crystalline jute cellulose particles: Catalytic and antibacterial properties. Carbohydr. Polym. 2020, 233, 115842. [Google Scholar] [CrossRef] [PubMed]
- Saratale, G.D.; Saratale, R.G.; Cho, S.-K.; Ghodake, G.; Bharagava, R.N.; Park, Y.; Mulla, S.I.; Kim, D.-S.; Kadam, A.; Nair, S.; et al. Investigation of photocatalytic degradation of reactive textile dyes by Portulaca oleracea-functionalized silver nanocomposites and exploration of their antibacterial and antidiabetic potentials. J. Alloys Compd. 2020, 833, 155083. [Google Scholar] [CrossRef]
- Lai, Y.-R.; Lai, J.-T.; Wang, S.S.-S.; Kuo, Y.-C.; Lin, T.-H. Silver nanoparticle-deposited whey protein isolate amyloid fibrils as catalysts for the reduction of methylene blue. Int. J. Biol. Macromol. 2022, 213, 1098–1114. [Google Scholar] [CrossRef]
- Gola, D.; Kriti, A.; Bhatt, N.; Bajpai, M.; Singh, A.; Arya, A.; Chauhan, N.; Srivastava, S.K.; Tyagi, P.K.; Agrawal, Y. Silver nanoparticles for enhanced dye degradation. Curr. Res. Green Sustain. Chem. 2021, 4, 100132. [Google Scholar] [CrossRef]
- Liao, G.; Li, Q.; Zhao, W.; Pang, Q.; Gao, H.; Xu, Z. In-situ construction of novel silver nanoparticle decorated polymeric spheres as highly active and stable catalysts for reduction of methylene blue dye. Appl. Catal. A Gen. 2018, 549, 102–111. [Google Scholar] [CrossRef]
- Heidari, H.; Karbalaee, M. Silver-nanoparticle Supported on Nanocrystalline Cellulose Using Cetyltrimethylammonium Bromide: Synthesis and Catalytic Performance for Decolorization of Dyes. J. Nanostruct. 2021, 11, 48–56. [Google Scholar] [CrossRef]
- Vatanpour, V.; Keskin, B.; Mehrabani, S.A.N.; Karimi, H.; Arabi, N.; Behroozi, A.H.; Shokrollahi-Far, A.; Gul, B.Y.; Koyuncu, I. Investigation of boron nitride/silver/graphene oxide nanocomposite on separation and antibacterial improvement of polyethersulfone membranes in wastewater treatment. J. Environ. Chem. Eng. 2021, 10, 107035. [Google Scholar] [CrossRef]
- Masekela, D.; Hintsho-Mbita, N.C.; Ntsendwana, B.; Mabuba, N. Thin Films (FTO/BaTiO3/AgNPs) for Enhanced Piezo-Photocatalytic Degradation of Methylene Blue and Ciprofloxacin in Wastewater. ACS Omega 2022, 7, 24329–24343. [Google Scholar] [CrossRef]
- Naveas, N.; Manso-Silván, M.; Carmona, E.; Garrido, K.; Hernández-Montelongo, J.; Recio-Sánchez, G. Green synthesized silver nanoparticles decorated on nanostructured porous silicon as an efficient platform for the removal of organic dye methylene blue. Green Chem. Lett. Rev. 2022, 15, 108–115. [Google Scholar] [CrossRef]
- Jiang, Z.-J.; Liu, C.-Y.; Sun, L.-W. Catalytic Properties of Silver Nanoparticles Supported on Silica Spheres. J. Phys. Chem. B 2005, 109, 1730–1735. [Google Scholar] [CrossRef] [PubMed]
- Ghaedi, M.; Heidarpour, S.; Kokhdan, S.N.; Sahraie, R.; Daneshfar, A.; Brazesh, B. Comparison of silver and palladium nanoparticles loaded on activated carbon for efficient removal of Methylene blue: Kinetic and isotherm study of removal process. Powder Technol. 2012, 228, 18–25. [Google Scholar] [CrossRef]
- Das, S.K.; Khan, M.R.; Parandhaman, T.; Laffir, F.; Guha, A.K.; Sekaran, G.; Mandal, A.B. Nano-silica fabricated with silver nanoparticles: Antifouling adsorbent for efficient dye removal, effective water disinfection and biofouling control. Nanoscale 2013, 5, 5549–5560. [Google Scholar] [CrossRef] [PubMed]
- Karthik, C.; Swathi, N.; Pandi Prabha, S. Green synthesized rGO-AgNP hybrid nanocomposite—An effective antibacterial adsorbent for photocatalytic removal of DB-14 dye from aqueous solution. J. Environ. Chem. Eng. 2020, 8, 103577. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Babitha, N.; Christy, S.R.; Palani, G.; Gurumoorthy, M.; Kannan, K.; Chithambaram, V. Enhanced photocatalytic and Antibacterial Activity of Copper oxide Nanoparticles Synthesized by Facile Combustion methods from Mussaendafrondosa Plant Extract. Phys. Chem. Solid State 2020, 23, 443–449. [Google Scholar] [CrossRef]
- Rajendrachari, S.; Taslimi, P.; Karaoglanli, A.C.; Uzun, O.; Alp, E.; Jayaprakash, G.K. Photocatalytic degradation of Rhodamine B (RhB) dye in waste water and enzymatic inhibition study using cauliflower shaped ZnO nanoparticles synthesized by a novel One-pot green synthesis method. Arab. J. Chem. 2021, 14, 103180. [Google Scholar] [CrossRef]
- Shashanka, R.; Jayaprakash, G.K.; Prakashaiah, B.G.; Kumar, M.; Swamy, B.K. Electrocatalytic determination of ascorbic acid using a green synthesised magnetite nano-flake modified carbon paste electrode by cyclic voltammetric method. Mater. Res. Innov. 2022, 26, 229–239. [Google Scholar] [CrossRef]
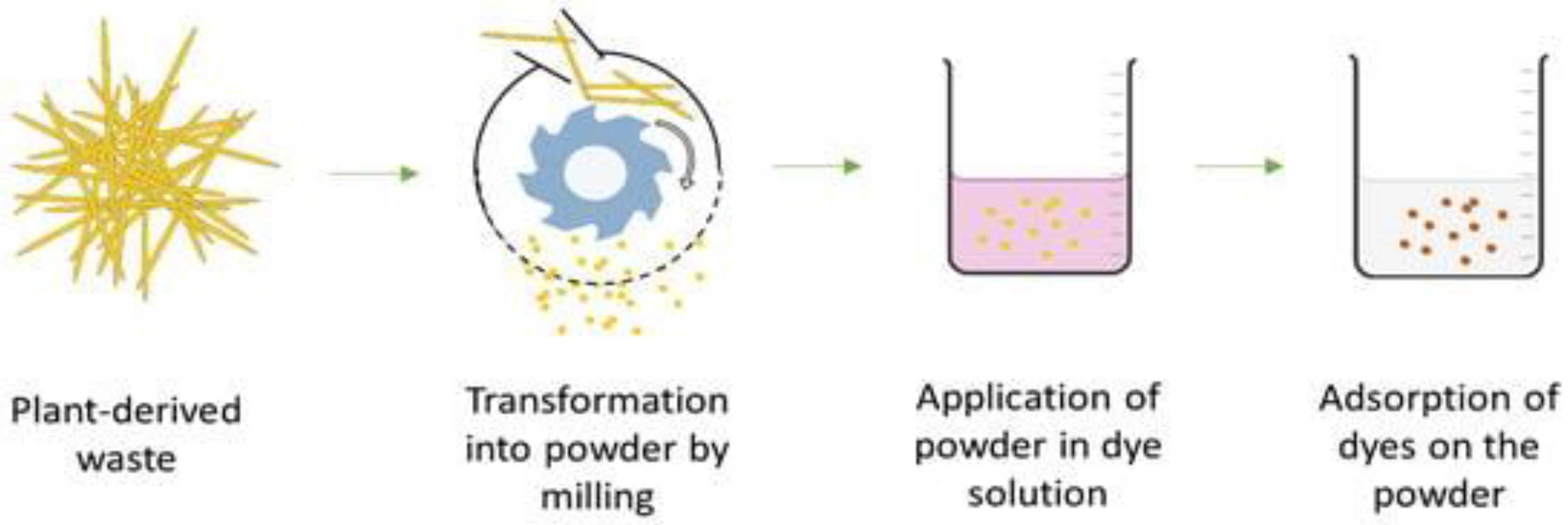
- Haque, A.N.M.A.; Sultana, N.; Sayem, A.S.M.; Smriti, S.A. Sustainable Adsorbents from Plant-Derived Agricultural Wastes for Anionic Dye Removal: A Review. Sustainability 2022, 14, 11098. [Google Scholar] [CrossRef]
- Pallavi, N.; Mehta, C.M.; Srivastava, R.; Arora, S.; Sharma, A.K. Impact assessment of silver nanoparticles on plant growth and soil bacterial diversity. 3 Biotech 2016, 6, 254. [Google Scholar] [CrossRef]
- Kale, S.K.; Parishwad, G.V.; Husainy, A.S.N.; Patil, A.S. Emerging Agriculture Applications of Silver Nanoparticles. ES Food Agrofor. 2021, 3, 17–22. [Google Scholar] [CrossRef]
- Palani, G.; Kannan, K.; Radhika, D.; Vijayakumar, P.; Pakiyaraj, K. Bioengineered metal and metal oxide nanoparticles for photocatalytic and biological applications: A review. Phys. Chem. Solid State 2020, 21, 571–583. [Google Scholar] [CrossRef]
- Nadaf, N.Y.; Kanase, S.S. Antibacterial activity of silver nanoparticles singly and in combination with third generation antibiotics against bacteria causing hospital acquired infections biosynthesised by isolated Bacillus marisflavi YCIS MN 5. Dig. J. Nanomater. Biostruct. 2015, 10, 1189–1199. [Google Scholar]
- Darweesh, M.A.; Elgendy, M.Y.; Ayad, M.I.; Ahmed, A.M.M.; Elsayed, N.K.; Hammad, W. A unique, inexpensive, and abundantly available adsorbent: Composite of synthesized silver nanoparticles (AgNPs) and banana leaves powder (BLP). Heliyon 2022, 8, 9279. [Google Scholar] [CrossRef] [PubMed]
- Graily-Moradi, F.; Mallak, A.M.; Ghorbanpour, M. Biogenic Synthesis of Gold Nanoparticles and Their Potential Application in Agriculture. In Biogenic Nano-Particles and Their Use in Agro-Ecosystems; National Institutes of Health: Washington, DC, USA, 2020; pp. 187–204. [Google Scholar] [CrossRef]
- Castillo-Henriquez, L.; Alfaro-Aguilar, K.; Ugalde-Alvarez, J.; Vega-Fernandez, L.; Montes de Oca-Vasquez, G.; Vega-Baudrit, J.R. Green Synthesis of Gold and Silver Nanoparticles from Plant Extracts and Their Possible Applications as Antimicrobial Agents in the Agricultural Area. Nanomaterials 2020, 10, 1763. [Google Scholar] [CrossRef]
- Tariq, M.; Mohammad, K.N.; Ahmed, B.; Siddiqui, M.A.; Lee, J. Biological Synthesis of Silver Nanoparticles and Prospects in Plant Disease Management. Molecules 2022, 27, 4754. [Google Scholar] [CrossRef]
- Mitra, C.; Gummadidala, P.M.; Afshinnia, K.; Merrifield, R.C.; Baalousha, M.; Lead, J.R.; Chanda, A. Citrate-Coated Silver Nanoparticles Growth-Independently Inhibit Aflatoxin Synthesis in Aspergillus parasiticus. Environ. Sci. Technol. 2017, 51, 8085–8093. [Google Scholar] [CrossRef]
- Ibrahim, E.; Fouad, H.; Zhang, M.; Zhang, Y.; Qiu, W.; Yan, C.; Li, B.; Mo, J.; Chen, J. Biosynthesis of silver nanoparticles using endophytic bacteria and their role in inhibition of rice pathogenic bacteria and plant growth promotion. RSC Adv. 2019, 9, 29293–29299. [Google Scholar] [CrossRef]
- Alonso-Díaz, A.; Floriach-Clark, J.; Fuentes, J.; Capellades, M.; Sanchez-Coll, N.; Laromaine, A. Enhancing Localized Pesticide Action through Plant Foliage by Silver-Cellulose Hybrid Patches. ACS Biomater. Sci. Eng. 2019, 5, 413–419. [Google Scholar] [CrossRef]
- Terra, A.L.M.; Kosinski, R.D.C.; Moreira, J.B.; Costa, J.A.V.; De Morais, M.G. Microalgae biosynthesis of silver nanoparticles for application in the control of agricultural pathogens. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2019, 54, 709–716. [Google Scholar] [CrossRef]
- Beena, V.; Ajitha, S.; Rayar, S.L.; Parvathiraja, C.; Kannan, K.; Palani, G. Enhanced Photocatalytic and Antibacterial Activities of ZnSe Nanoparticles. J. Inorg. Organomet. Polym. Mater. 2021, 31, 4390–4401. [Google Scholar] [CrossRef]
- Chen, J.; Sun, L.; Cheng, Y.; Lu, Z.; Shao, K.; Li, T.; Hu, C.; Han, H. Graphene Oxide-Silver Nanocomposite: Novel Agricultural Antifungal Agent against Fusarium graminearum for Crop Disease Prevention. ACS Appl. Mater. Interfaces 2016, 8, 24057–24070. [Google Scholar] [CrossRef] [PubMed]
- El-Mohamady, R.S.; Ghattas, T.; Zawrah, M.; El-Hafeiz, Y.A. Inhibitory effect of silver nanoparticles on bovine herpesvirus-1. Int. J. Veter- Sci. Med. 2018, 6, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Pandey, S.; Bhattacharya, A.; Mishra, A.; Nautiyal, C. Protective role of biosynthesized silver nanoparticles against early blight disease in Solanum lycopersicum. Plant Physiol. Biochem. 2017, 121, 216–225. [Google Scholar] [CrossRef]
- Deshmukh, M.A.; Kang, B.-C.; Ha, T.-J. Non-enzymatic electrochemical glucose sensors based on polyaniline/reduced-graphene-oxide nanocomposites functionalized with silver nanoparticles. J. Mater. Chem. C 2020, 8, 5112–5123. [Google Scholar] [CrossRef]
- Sher, F.; Hanif, K.; Iqbal, S.Z.; Imran, M. Implications of advanced wastewater treatment: Electrocoagulation and electroflocculation of effluent discharged from a wastewater treatment plant. J. Water Process Eng. 2020, 33, 101101. [Google Scholar] [CrossRef]
- Burkinshaw, S.M.; Salihu, G. The role of auxiliaries in the immersion dyeing of textile fibres: Part 1 an overview. Dye Pigment. 2019, 161, 519–530. [Google Scholar] [CrossRef]
- Ben Fradj, A.; Boubakri, A.; Hafiane, A.; Ben Hamouda, S. Removal of azoic dyes from aqueous solutions by chitosan enhanced ultrafiltration. Results Chem. 2020, 2, 100017. [Google Scholar] [CrossRef]
- Güleç, F.; Sher, F.; Karaduman, A. Catalytic performance of Cu- and Zr-modified beta zeolite catalysts in the methylation of 2-methylnaphthalene. Pet. Sci. 2018, 16, 161–172. [Google Scholar] [CrossRef]
- Kausar, A.; Naeem, K.; Hussain, T.; Nazli, Z.-I.; Bhatti, H.N.; Jubeen, F.; Nazir, A.; Iqbal, M. Preparation and characterization of chitosan/clay composite for direct Rose FRN dye removal from aqueous media: Comparison of linear and non-linear regression methods. J. Mater. Res. Technol. 2019, 8, 1161–1174. [Google Scholar] [CrossRef]
- Shindhal, T.; Rakholiya, P.; Varjani, S.; Pandey, A.; Ngo, H.H.; Guo, W.; Ng, H.Y.; Taherzadeh, M.J. A critical review on advances in the practices and perspectives for the treatment of dye industry wastewater. Bioengineered 2020, 12, 70–87. [Google Scholar] [CrossRef]
- Kanchana, R.; Fernandes, A.; Bhat, B.; Budkule, S.; Dessai, S.; Mohan, R. Dyeing of textiles with natural dyes-an eco-friendly approach. Int. J. Chem. Tech. Res. 2013, 5, 2102–2109. [Google Scholar]
- Tony, M.A. An industrial ecology approach: Green cellulose-based bio-adsorbent from sugar industry residue for treating textile industry wastewater effluent. Int. J. Environ. Anal. Chem. 2021, 101, 167–183. [Google Scholar] [CrossRef]
- Roy, A.; Bulut, O.; Some, S.; Mandal, A.K.; Yilmaz, M.D. Green synthesis of silver nanoparticles: Biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019, 9, 2673–2702. [Google Scholar] [CrossRef] [PubMed]
- Jain, M. Ecological approach to reduce carbon footprint of textile industry. Int. J. Appl. Home Sci. 2017, 4, 623–633. Available online: http://scientificresearchjournal.com/wp-content/uploads/2017/06/Home-Science-Vol-4_A-623-633-Full-Paper.pdf (accessed on 26 July 2017).
- Lara, L.; Cabral, I.; Cunha, J. Ecological Approaches to Textile Dyeing: A Review. Sustainability 2022, 14, 8353. [Google Scholar] [CrossRef]
- Gong, K.; Rather, L.J.; Zhou, Q.; Wang, W.; Li, Q. Natural dyeing of merino wool fibers with Cinnamomum camphora leaves extract with mordants of biological origin: A greener approach of textile coloration. J. Text. Inst. 2020, 111, 1038–1046. [Google Scholar] [CrossRef]
- Sharma, P.; Pant, S.; Rai, S.; Yadav, R.B.; Dave, V. Green Synthesis of Silver Nanoparticle Capped with Allium cepa and Their Catalytic Reduction of Textile Dyes: An Ecofriendly Approach. J. Polym. Environ. 2018, 26, 1795–1803. [Google Scholar] [CrossRef]
- Karn, B.; Kuiken, T.; Otto, M. Nanotechnology and in Situ Remediation: A Review of the Benefits and Potential Risks. Environ. Health Perspect. 2009, 117, 1813–1831. [Google Scholar] [CrossRef]
- Kumar, S.S.; Shantkriti, S.; Muruganandham, T.; Murugesh, E.; Rane, N.; Govindwar, S. Bioinformatics aided microbial approach for bioremediation of wastewater containing textile dyes. Ecol. Inform. 2016, 31, 112–121. [Google Scholar] [CrossRef]
- Kummara, S.; Patil, M.B.; Uriah, T. Synthesis, characterization, biocompatible and anticancer activity of green and chemically synthesized silver nanoparticles—A comparative study. Biomed. Pharmacother. 2016, 84, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Azanaw, A.; Birlie, B.; Teshome, B.; Jemberie, M. Textile effluent treatment methods and eco-friendly resolution of textile wastewater. Case Stud. Chem. Environ. Eng. 2022, 6, 100230. [Google Scholar] [CrossRef]
- Venil, C.K.; Velmurugan, P.; Dufossé, L.; Devi, P.R.; Ravi, A.V. Fungal Pigments: Potential Coloring Compounds for Wide Ranging Applications in Textile Dyeing. J. Fungi 2020, 6, 68. [Google Scholar] [CrossRef] [PubMed]
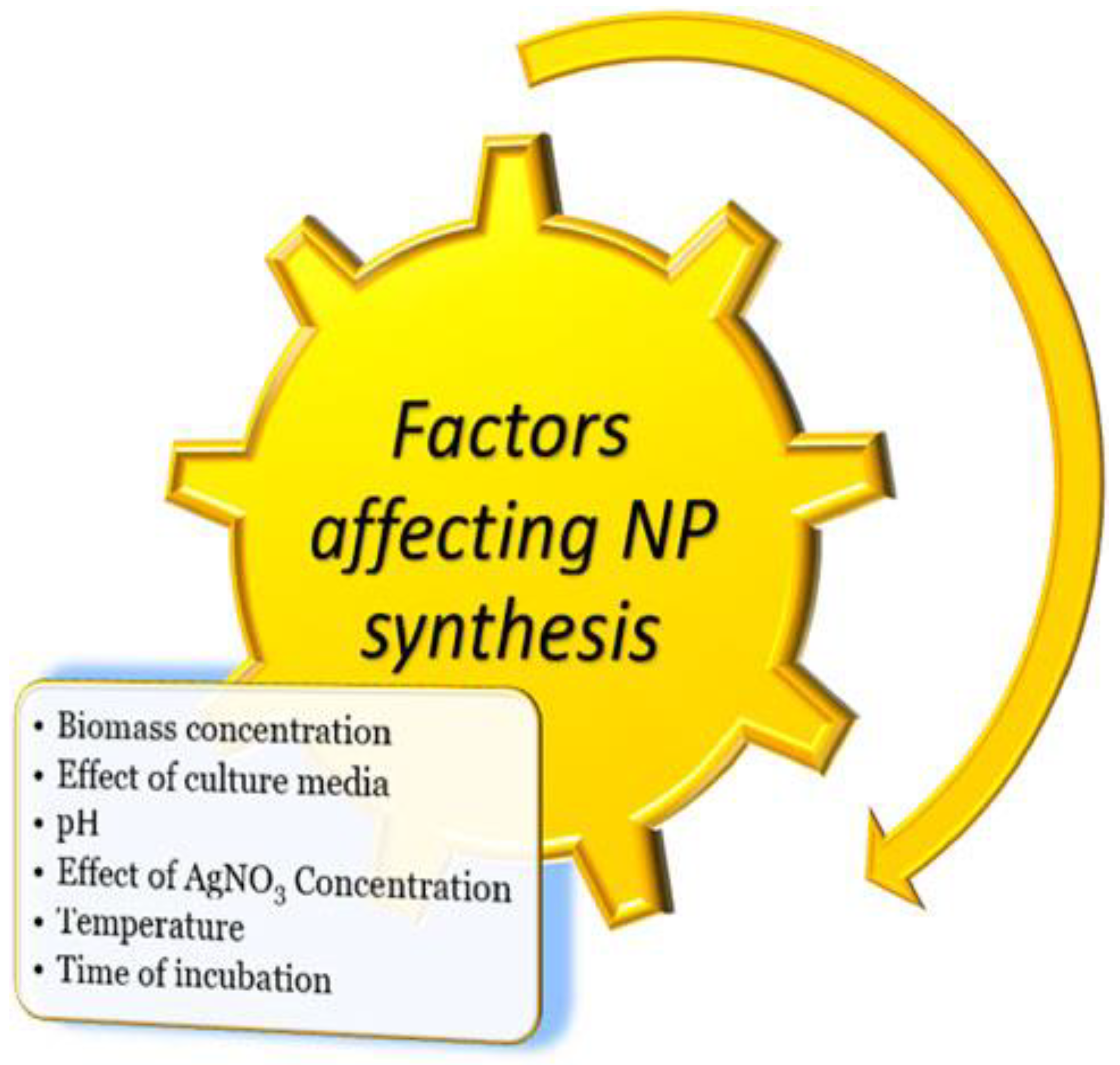
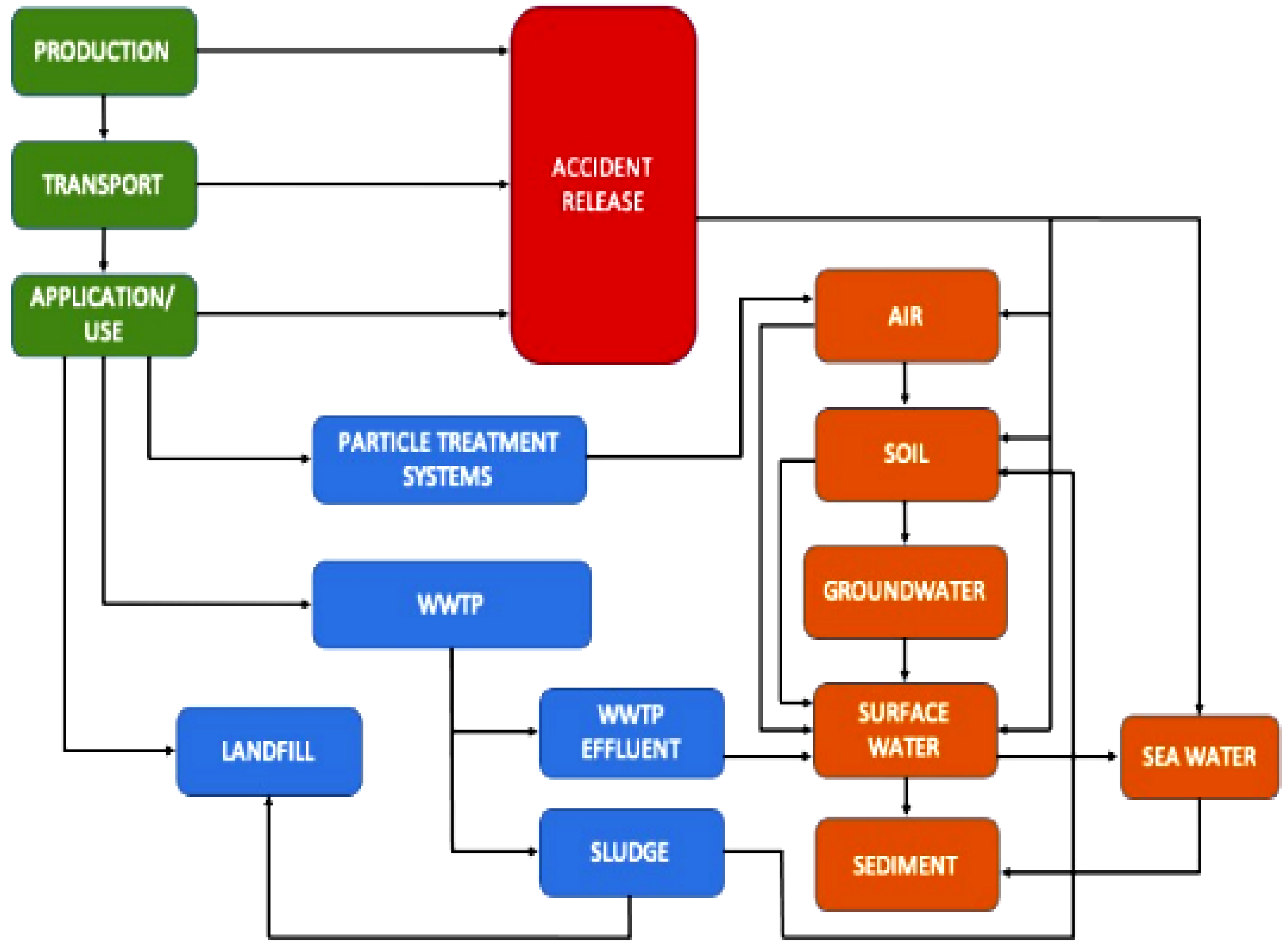
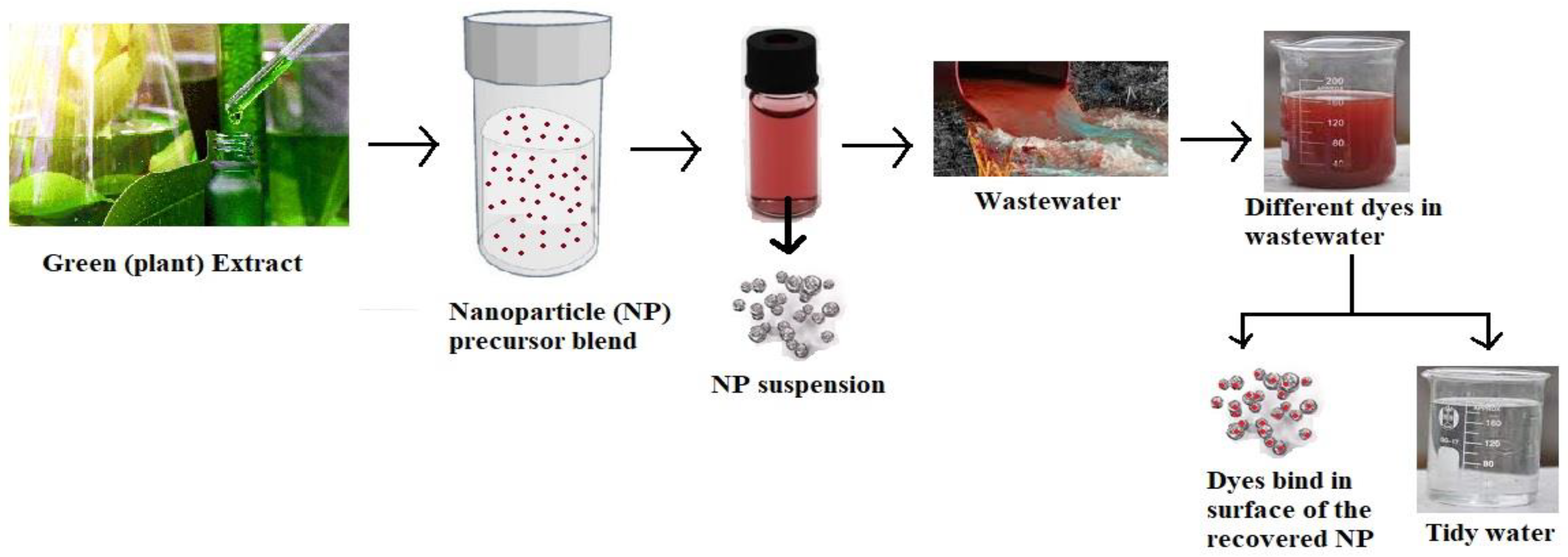
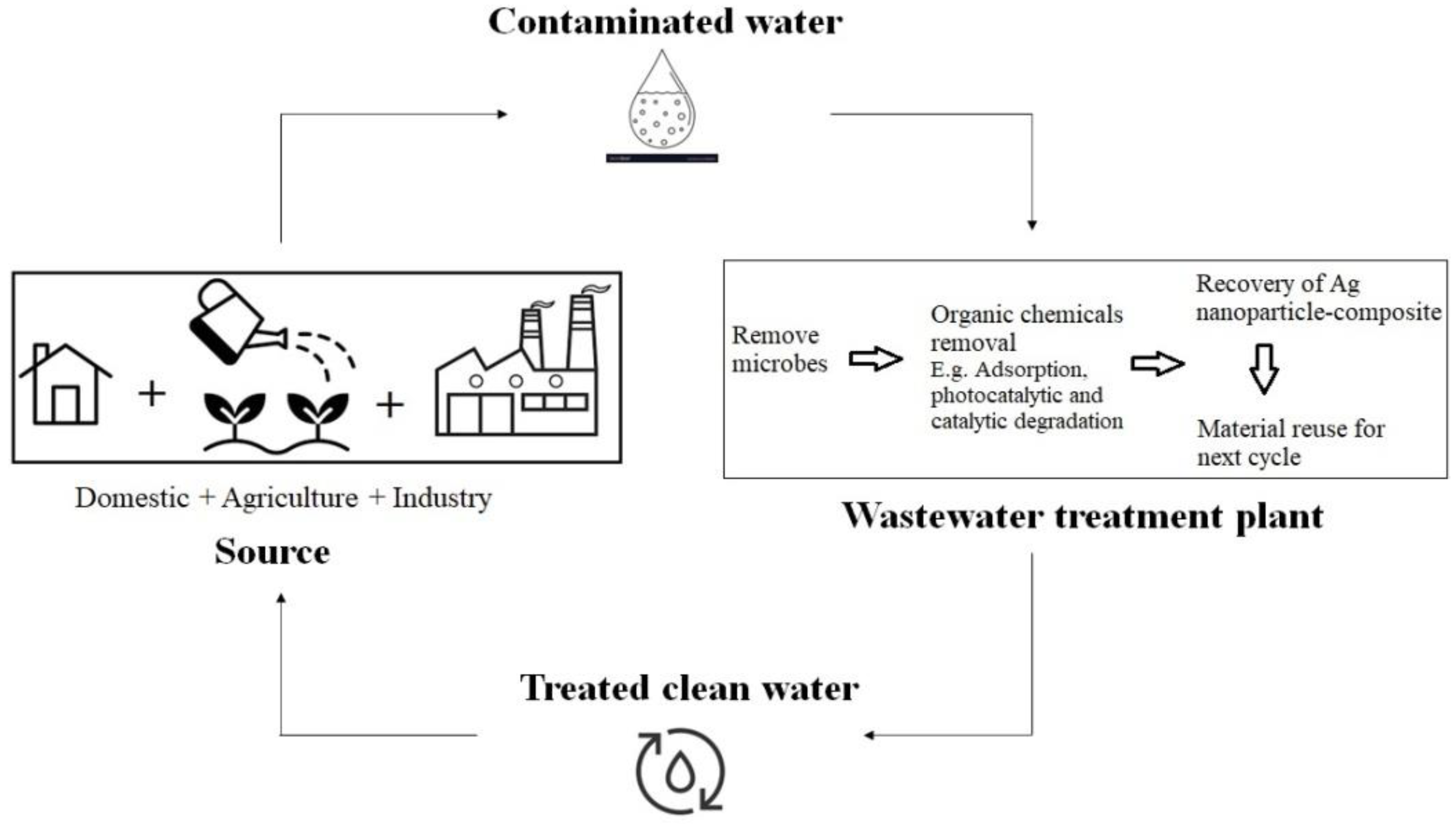

| No | Nanomaterial Type | Type of Process | Nanoparticle Material | Textile Dyes | Removal Efficiency | References |
|---|---|---|---|---|---|---|
| 1 | Powder | Photocatalytic and microwave-assisted degradation method | ZnO/poly (1-naphthylamine) nanohybrids | Alizarin red | 85% | [38] |
| 2 | Powder | Catalytic degradation method | Pd | Azo dyes | 93 and 91% | [40] |
| 3 | Powder | Catalytic degradation method | Cu | Methyl orange | Less than 80% | [41] |
| 4 | Powder | Photocatalytic degradation method | Fe2O3 | Acid blue | 87% | [42] |
| 5 | Decorated | Enzymatic reaction | Fe3O4 | Acid fuchsin | Up to 80% | [43] |
| 6 | Powder | Photocatalytic degradation method | Ag | Methyl orange and Coomassie brilliant blue | 60%; 70% | [44] |
| 7 | Powder | Biogenic method | Biogenic Pd | Acid blue 1 and red, methyl orange and reactive black 5 | Less than 95% | [37] |
| 8 | Powder | Photocatalytic degradation method | Ag–ZnO/GO | Methylene blue | 85% | [45] |
| 9 | Powder | Photocatalytic degradation method | ZnO/CuO | Methylene blue | 93% | [46] |
| 10 | Powder | Adsorption | Fe3O4 | Optilan blue | 50 mg/L with 0.6 g/L | [47] |
| 11 | Powder | Desalination | GO-PEG-NB | Ternary dyes | 99% | [48] |
| 12 | Powder | Adsorption–photocatalysis | Ze-nanZnO; nanZnO | Tartrazine | 87 and 81% | [49] |
| 13 | Film | Adsorption | CS/MgO | Reactive blue (RB) 19 | 77.62% | [50] |
| 14 | Powder | Adsorption | CS–ZnO | Malachite green (MG) | 98.5% | [51] |
| 15 | Powder | Photocatalytic degradation method | TiO2 + MC (micro cellulose) | Methylene blue, methyl violet and acid violet | 99% | [52] |
| 16 | Powder | Photo degradation method | CS/ZnO | Methylene blue | CS: 86.7%; MB: 81% | [53] |
| 17 | Powder | Photocatalytic degradation method | ZnO/AC | Methylene blue | 92.2% | [54] |
| 18 | Powder | Adsorption | CHT-GLA/ZnO/Fe3O4 | Brilliant Blue R | 176.6 mg/g at 60 °C | [39] |
| 19 | Ni@FP | Coated on Cellulose filter paper | Dyeing wastewater | Methylene orange | 93.4% | [55] |
| 20 | Dry powdered gel | Photocatalytic degradation | LaFeO3- RGO–NiO | Congo red | 96.5% | [56] |
| 21 | Powder | Photocatalytic degradation method | Ag–ZnO | Methylene blue, methyl orange and rhodamine B dyes | 98.5% | [57] |
| 22 | Powder | Photocatalytic degradation method | ZnO | Methylene blue | 90% | [58] |
| 23 | Powder | Photocatalytic method | ZnO | Alizarin red S (AZ) and methylene blue (MB) dyes | 99.9 and 96.8% | [59] |
| 24 | Powder | Photocatalytic degradation method | CuO | Methylene blue (MB) | 93% | [60] |
| No | AgNPs-Composites | AgNPs-Composites Synthesis Method | Type of Pollutant | Name of the Pollutant | Treatment Efficiency | References |
|---|---|---|---|---|---|---|
| 1 | AgNPs capped 2-hydroxypropyl β-cyclodextrin/alginate nanocomposite | Leave extract from Jasminum subtriplinerve | Organic pollutant and dyes | 4-NP, MO, rhodamineB | Kinetic (pseudo-first order) rate 1.51 × 10−3 s−1 to 2.23 × 10−3 s−1 | [71] |
| 812 | Silver nanoparticles (AgNPs) | Leave extract from Thymbra spicata | Organic pollutant and dyes | 4-NP, MO andrhodamine B | Catalytic activity loss | [72] |
| 3 | FeO/AgNPs (Fe–Ag core-shell nanoparticles) | Pomegranate fruit peel extract | Dyes | Aniline blue dye | 90%; 0.25 mg mL−1 | [73] |
| 4 | Fe3O4/PPy-MAA/Ag | Polymer matrix | Organic pollutant and dyes | 4-NP and MB, MO | 42.5 wt% (20 min) | [74] |
| 5 | Silver-doped Mg4Ta2O9 nanoparticles | Irradiation of UV lamp | Dyes herbicide | rhodamine B, methyl orange, atrazine | 2.0 wt% | [75] |
| 6 | Cellulose polymer paper in silver nanoparticles | Leave extract from Durantaerecta | Organic pollutant | 4-NP, 2-NP (2-nitrophenol), (2-Nitroaniline) 2-NA, TNP | 6–12 min, Stable catalyst for five cycles. 95–99% | [76] |
| 7 | TiO2/CNTs/AgNPs/Surfactant (C10) nanocomposite | Trisodium citrate solution | Dye | Methylene blue (MB) | Degraded in 180 min; 0.5 gL−1, 100% | [77] |
| 8 | CAg-NPs | Citrus paradisi | Dye | Congo red (CR), MB, malachite green (MG), rhodamine B (RhB) and 4-NP | MB: 93.29; MG: 83.73; 4-NP: 88.9; RhB: 60.53 | [78] |
| 9 | CNF/PEI/Ag NPs composite | Bleached birch kraft pulp | Organic dye | MB | 96% after 4 min | [79] |
| 10 | rGO-AgNP (graphene oxide silver nanoparticle hybrid nanocomposite) | Brassica nigra aqueous extract | Dye | Direct blue-14 (DB-14) | 95.41% | [80] |
| 11 | GO−ZnO−Ag | Simple one-pot method | Organic dye | MB | 100%, 40 min | [69] |
| 12 | AgNPs/holocellulosenanofibrils (AgNPs/HCNF) | Microwave-assisted | Dye | MB | 94–98%, catalytic activity with five cycles | [81] |
| 13 | AgNPs/ZIF-8 composite | NaBH4 and trisodium citrate solution | Dyes | MB and CR | MB: 97.25%; CR: 100% pH ≥ 7 | [82] |
| 14 | AgNPs impregnated sub-micrometercrystalline jute cellulose (SCJC) particles | Extract of leaves of M. erythrophylla | Dyes | CR and MB | 100%, 14 min with 0.005 mg/mL | [83] |
| 15 | AgNPs | Extract of leaves from Portulacaoleracea (PNL) | Textile dyes | Reactive green 19A, R blue 59, R red 120, R red 141, and R red 2 | 180 min, 50; 35% fourth and fifth cycles | [84] |
| 16 | Ag@MGO-TA/Fe3+ nanocomposite | Graphite flakes | Organic pollutants | Methylene blue | 0.05 mg/mL | [85] |
| 17 | CH-AgNPs | Trisodium citrate solution | Dye | Orange and blue dyes | 97.4 and 100% | [86] |
| 18 | MMT/Ag nanocomposite | Montmorillonite (MMT) clay and AgNPs | Dye | Methylene blue | 99.90% for 25 ppm; 96.50% for 50 ppm; 89% for 100 ppm and 81.14% for 200 ppm | [87] |
| 19 | Ag/CTAB/NCCnanohybride | Microcrystalline cellulose | Dye | Methyl orange, 4-nitrophenol | 14.2 × 10−3 (s−1); 5.4 × 10−3 (s−1) | [88] |
| 20 | Ag/rGO nanocomposite and Ag/rGO/CA/TFC membranes | - | Organic compounds | Methylene blue | 98%; 92% | [78] |
| 21 | FBN-GO-Ag | - | Wastewater | Reactive black 5 and reactive red 120 | 88.9 and 77.7% | [89] |
| 22 | BaTiO3/AgNPs | BaTiO3 | Dye | Methylene blue and ciprofloxacin | 72 and 98% | [90] |
| 23 | AgNP/WPI-AF | Whey protein isolate | Dye | Methylene blue | - | [85] |
| 24 | AgNPs decorated on nanostructured porous silicon | Peumo extract | Organic dyes | Methylene blue | Degradation rate 8.6/min | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palani, G.; Trilaksana, H.; Sujatha, R.M.; Kannan, K.; Rajendran, S.; Korniejenko, K.; Nykiel, M.; Uthayakumar, M. Silver Nanoparticles for Waste Water Management. Molecules 2023, 28, 3520. https://doi.org/10.3390/molecules28083520
Palani G, Trilaksana H, Sujatha RM, Kannan K, Rajendran S, Korniejenko K, Nykiel M, Uthayakumar M. Silver Nanoparticles for Waste Water Management. Molecules. 2023; 28(8):3520. https://doi.org/10.3390/molecules28083520
Chicago/Turabian StylePalani, Geetha, Herri Trilaksana, R. Merlyn Sujatha, Karthik Kannan, Sundarakannan Rajendran, Kinga Korniejenko, Marek Nykiel, and Marimuthu Uthayakumar. 2023. "Silver Nanoparticles for Waste Water Management" Molecules 28, no. 8: 3520. https://doi.org/10.3390/molecules28083520
APA StylePalani, G., Trilaksana, H., Sujatha, R. M., Kannan, K., Rajendran, S., Korniejenko, K., Nykiel, M., & Uthayakumar, M. (2023). Silver Nanoparticles for Waste Water Management. Molecules, 28(8), 3520. https://doi.org/10.3390/molecules28083520










