Controlling Molecular Orientation of Small Molecular Dopant-Free Hole-Transport Materials: Toward Efficient and Stable Perovskite Solar Cells
Abstract
1. Introduction

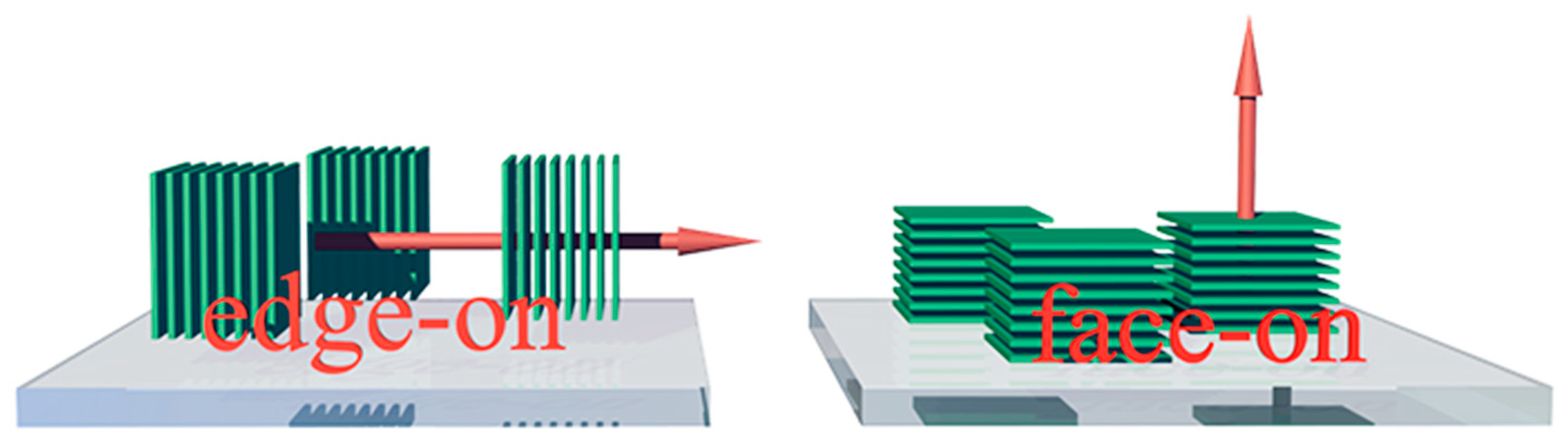
2. Molecular Orientation–Device Performance Relationship
2.1. Charge Carrier Transport and Transfer
2.2. Interfacial Energy Level Alignment
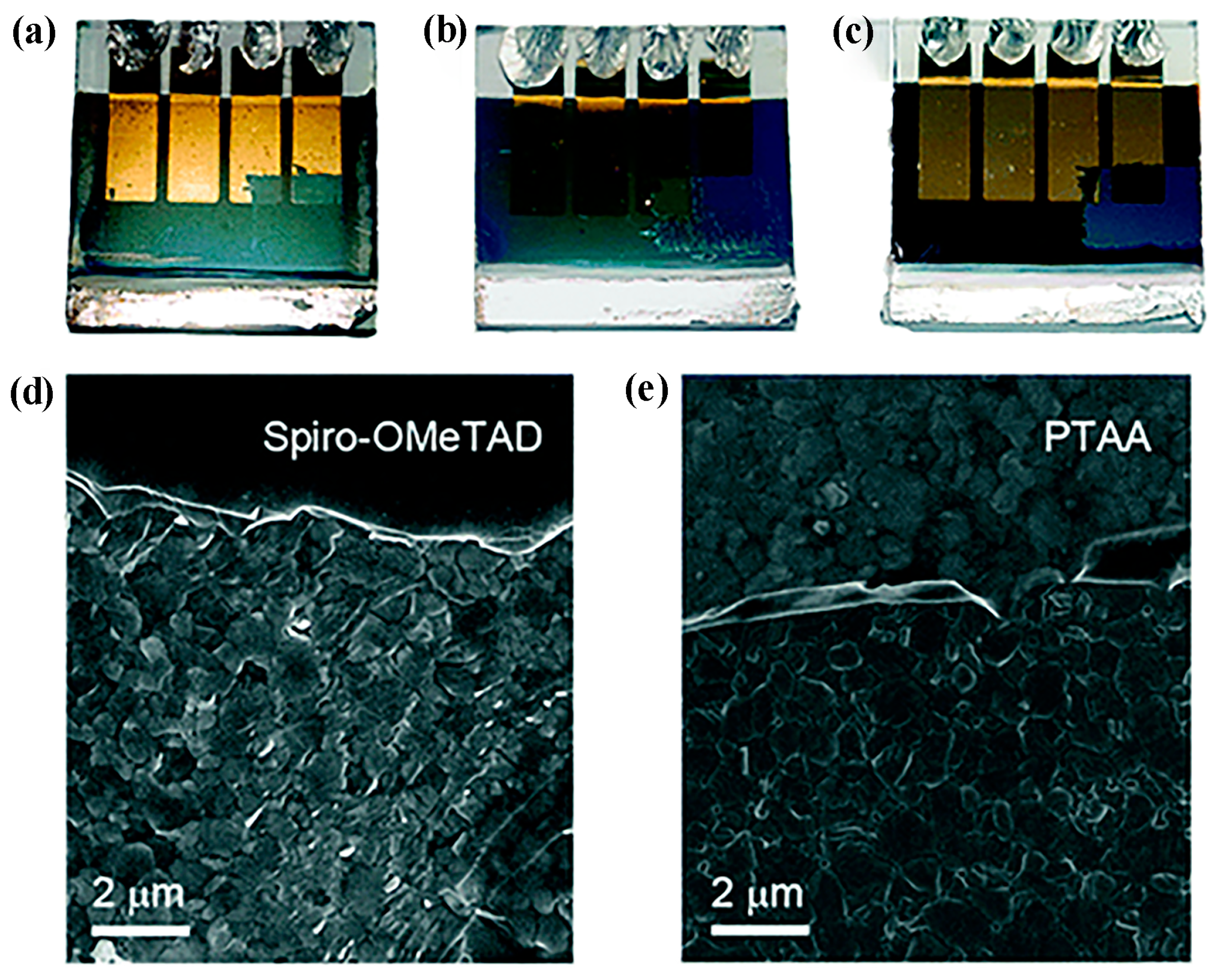
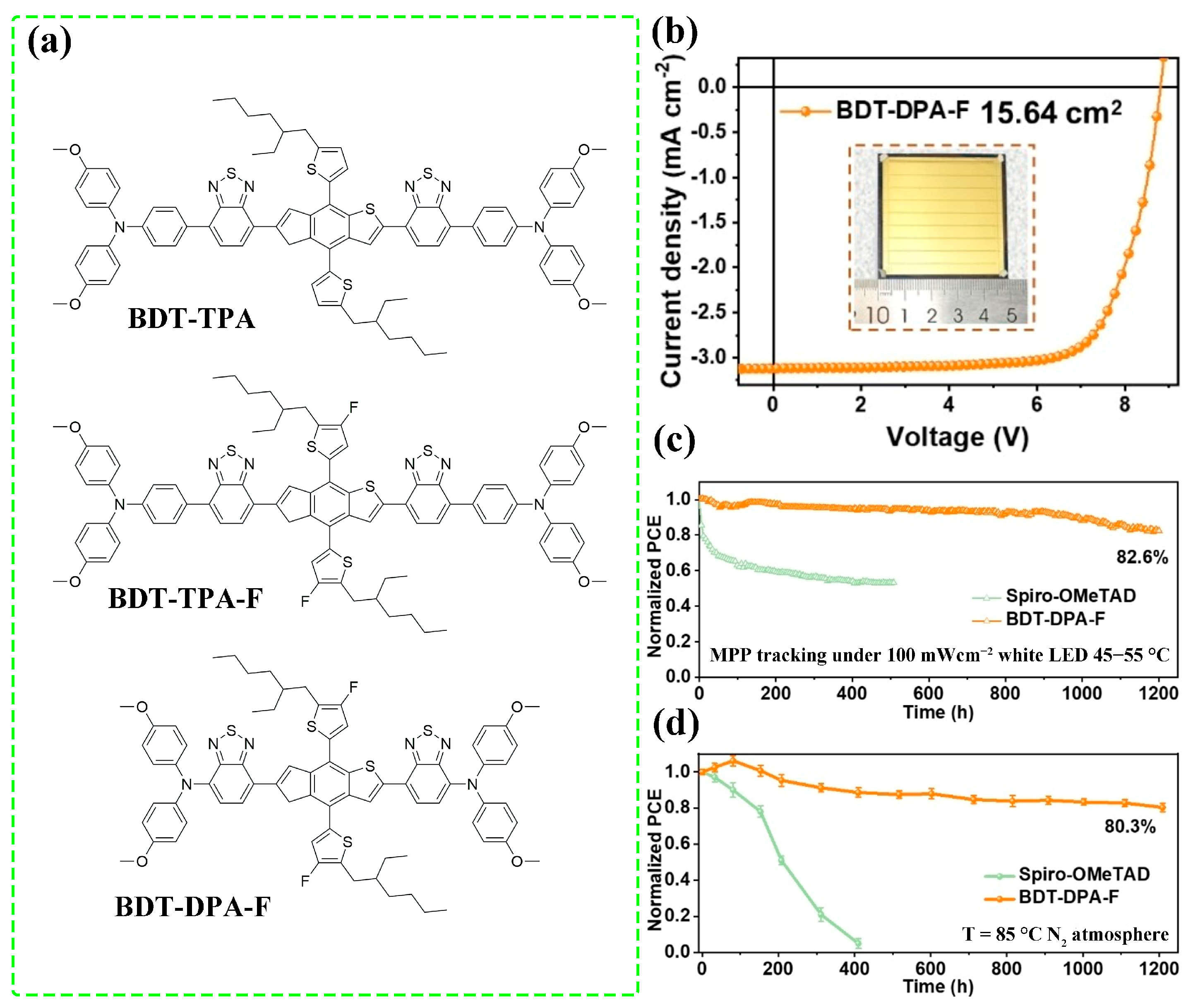
2.3. Device Stability
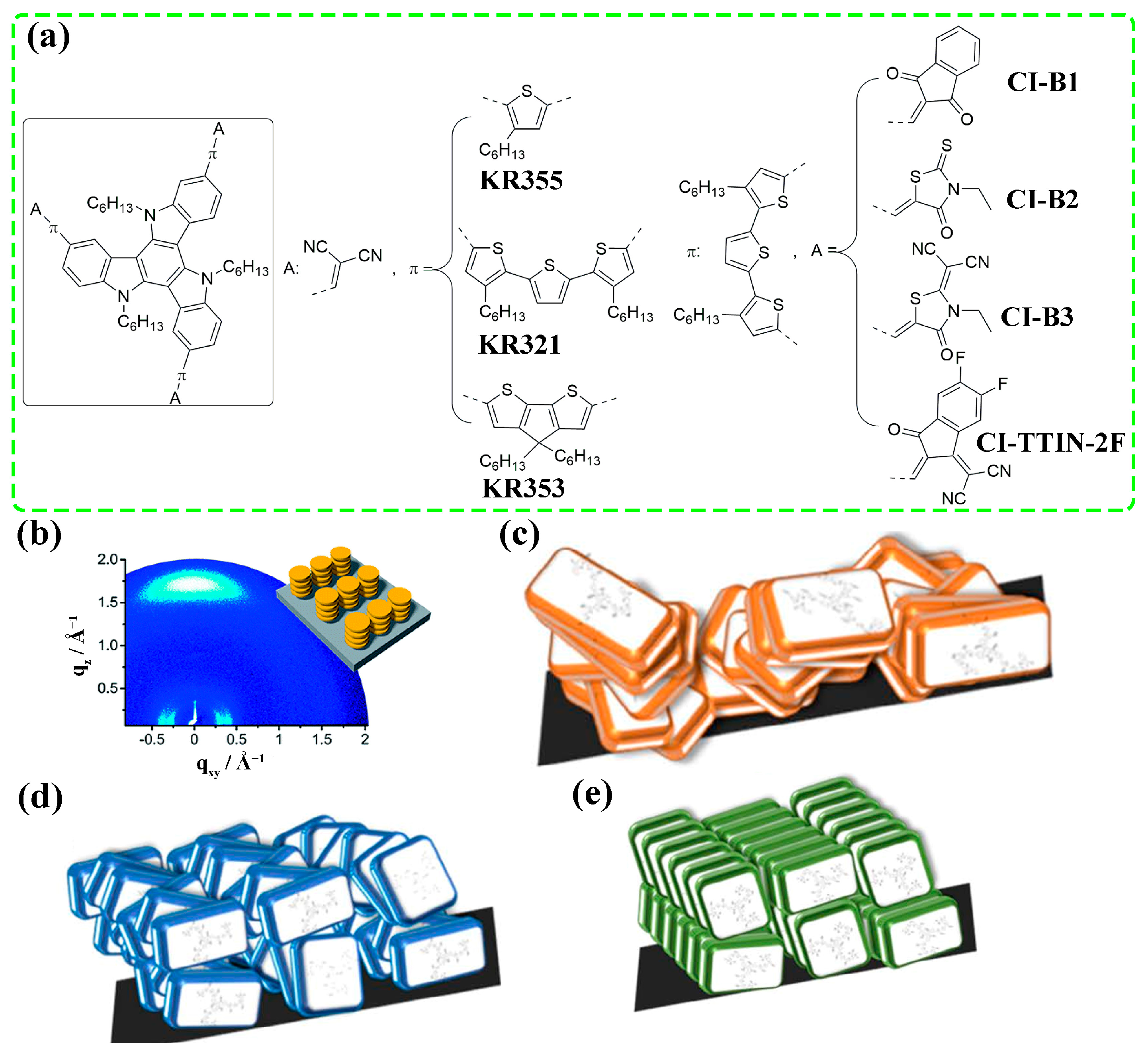
3. Controlling Strategies
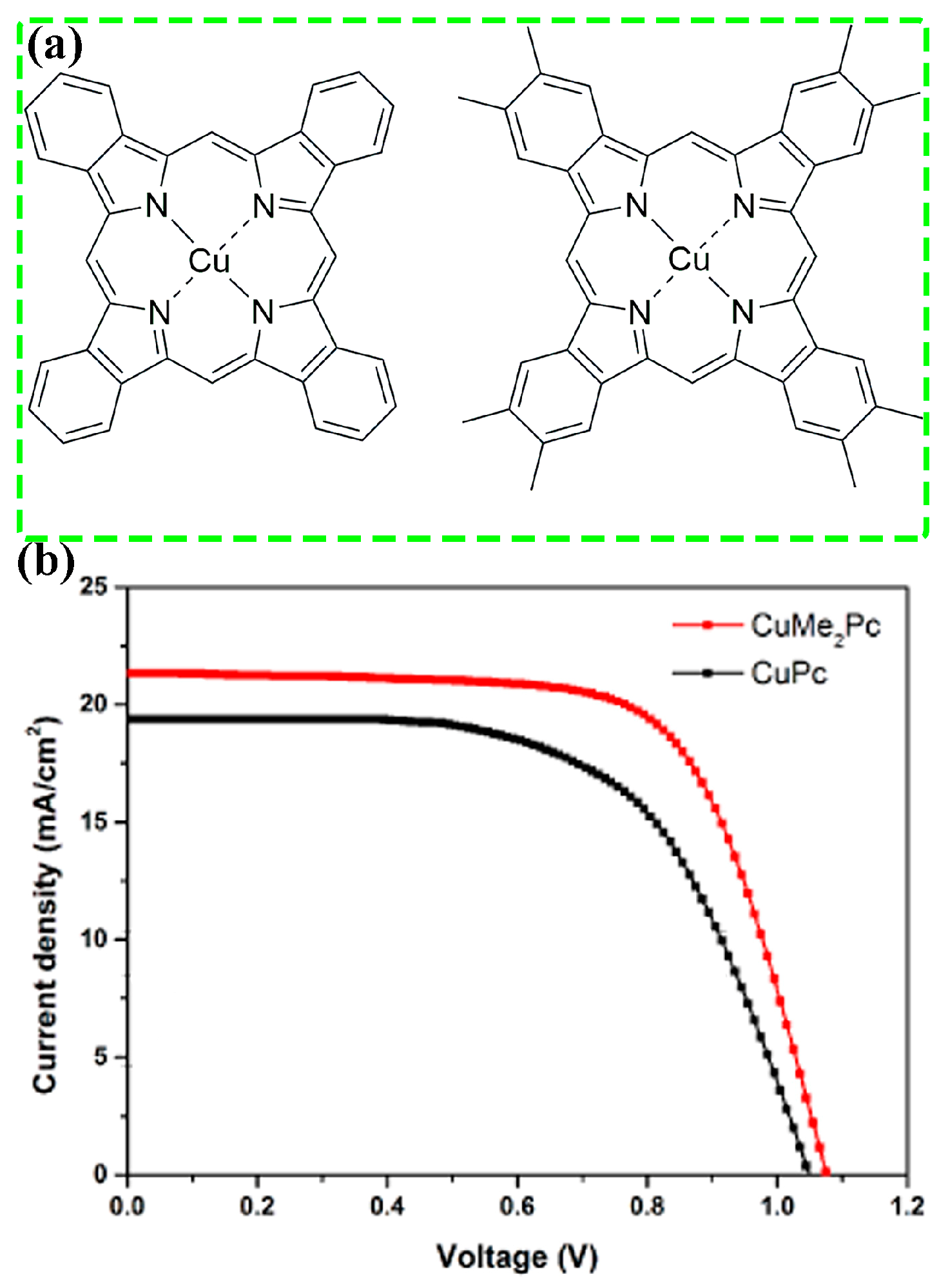
3.1. Molecular Structure
3.2. External Factors
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, B.; Sargent, E.H. What does net zero by 2050 mean to the solar energy materials researcher? Matter 2022, 5, 1322–1325. [Google Scholar] [CrossRef]
- International Renewable Energy Agency IRENA. Renewable Power Generation Costs in 2021. In eBOOK Partnership; International Renewable Energy Agency: Abu Dhabi, The United Arab Emirates, 2022. [Google Scholar]
- IEA. World Energy Outlook 2022; International Energy Agency: Paris, France, 2022. [Google Scholar]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Kim, H.-S.; Lee, C.-R.; Im, J.-H.; Lee, K.-B.; Moehl, T.; Marchioro, A.; Moon, S.-J.; Humphry-Baker, R.; Yum, J.-H.; Moser, J.E.; et al. Lead Iodide Perovskite Sensitized All-Solid-State Submicron Thin Film Mesoscopic Solar Cell with Efficiency Exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Johnston, M.B.; Snaith, H.J. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 2013, 501, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Chen, Q.; Li, G.; Luo, S.; Song, T.-b.; Duan, H.-S.; Hong, Z.; You, J.; Liu, Y.; Yang, Y. Interface engineering of highly efficient perovskite solar cells. Science 2014, 345, 542–546. [Google Scholar] [CrossRef]
- Saliba, M.; Matsui, T.; Domanski, K.; Seo, J.-Y.; Ummadisingu, A.; Zakeeruddin, S.M.; Correa-Baena, J.-P.; Tress, W.R.; Abate, A.; Hagfeldt, A.; et al. Incorporation of rubidium cations into perovskite solar cells improves photovoltaic performance. Science 2016, 354, 206–209. [Google Scholar] [CrossRef] [PubMed]
- NREL. Best Research-Cell Efficiency Chart. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 1 March 2023).
- Champion Photovoltaic Module Efficiency Chart. Available online: https://www.nrel.gov/pv/module-efficiency.html (accessed on 1 March 2023).
- De Bastiani, M.; Larini, V.; Montecucco, R.; Grancini, G. The levelized cost of electricity from perovskite photovoltaics. Energy Environ. Sci. 2023, 16, 421–429. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, C.; Lv, P.; Ku, Z.; Lu, J.; Huang, F.; Cheng, Y.-B. Printing strategies for scaling-up perovskite solar cells. Nat. Sci. Rev. 2021, 8, nwab075. [Google Scholar] [CrossRef]
- Zhang, J.; Chang, N.; Fagerholm, C.; Qiu, M.; Shuai, L.; Egan, R.; Yuan, C. Techno-economic and environmental sustainability of industrial-scale productions of perovskite solar cells. Renew. Sustain Energy Rev. 2022, 158, 112146. [Google Scholar] [CrossRef]
- Min, H.; Lee, D.Y.; Kim, J.; Kim, G.; Lee, K.S.; Kim, J.; Paik, M.J.; Kim, Y.K.; Kim, K.S.; Kim, M.G.; et al. Perovskite solar cells with atomically coherent interlayers on SnO2 electrodes. Nature 2021, 598, 444–450. [Google Scholar] [CrossRef]
- Yoo, J.J.; Seo, G.; Chua, M.R.; Park, T.G.; Lu, Y.; Rotermund, F.; Kim, Y.-K.; Moon, C.S.; Jeon, N.J.; Correa-Baena, J.-P.; et al. Efficient perovskite solar cells via improved carrier management. Nature 2021, 590, 587–593. [Google Scholar] [CrossRef]
- Li, Z.; Li, B.; Wu, X.; Sheppard, S.A.; Zhang, S.; Gao, D.; Long, N.J.; Zhu, Z. Organometallic-functionalized interfaces for highly efficient inverted perovskite solar cells. Science 2022, 376, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.L.; Ho-Baillie, A.W.Y.; Basore, P.A.; Young, T.L.; Evans, R.; Egan, R.J. A manufacturing cost estimation method with uncertainty analysis and its application to perovskite on glass photovoltaic modules. Prog. Photovoltaics Res. Appl. 2017, 25, 390–405. [Google Scholar] [CrossRef]
- Rombach, F.M.; Haque, S.A.; Macdonald, T.J. Lessons learned from spiro-OMeTAD and PTAA in perovskite solar cells. Energy Environ. Sci. 2021, 14, 5161–5190. [Google Scholar] [CrossRef]
- Schloemer, T.H.; Christians, J.A.; Luther, J.M.; Sellinger, A. Doping strategies for small molecule organic hole-transport materials: Impacts on perovskite solar cell performance and stability. Chem. Sci. 2019, 10, 1904–1935. [Google Scholar] [CrossRef] [PubMed]
- Kasparavicius, E.; Magomedov, A.; Malinauskas, T.; Getautis, V. Long-Term Stability of the Oxidized Hole-Transporting Materials used in Perovskite Solar Cells. Chem. Eur. J. 2018, 24, 9910–9918. [Google Scholar] [CrossRef]
- Kasparavicius, E.; Franckevičius, M.; Malinauskiene, V.; Genevičius, K.; Getautis, V.; Malinauskas, T. Oxidized Spiro-OMeTAD: Investigation of Stability in Contact with Various Perovskite Compositions. ACS Appl. Energy Mater. 2021, 4, 13696–13705. [Google Scholar] [CrossRef]
- Zhao, Y.; Heumueller, T.; Zhang, J.; Luo, J.; Kasian, O.; Langner, S.; Kupfer, C.; Liu, B.; Zhong, Y.; Elia, J.; et al. A bilayer conducting polymer structure for planar perovskite solar cells with over 1400 h operational stability at elevated temperatures. Nat. Energy 2022, 7, 144–152. [Google Scholar] [CrossRef]
- Song, Z.; McElvany, C.L.; Phillips, A.B.; Celik, I.; Krantz, P.W.; Watthage, S.C.; Liyanage, G.K.; Apul, D.; Heben, M.J. A technoeconomic analysis of perovskite solar module manufacturing with low-cost materials and techniques. Energy Environ. Sci. 2017, 10, 1297–1305. [Google Scholar] [CrossRef]
- Čulík, P.; Brooks, K.; Momblona, C.; Adams, M.; Kinge, S.; Maréchal, F.; Dyson, P.J.; Nazeeruddin, M.K. Design and Cost Analysis of 100 MW Perovskite Solar Panel Manufacturing Process in Different Locations. ACS Energy Lett. 2022, 7, 3039–3044. [Google Scholar] [CrossRef]
- Meng, L.; You, J.; Yang, Y. Addressing the stability issue of perovskite solar cells for commercial applications. Nat. Commun. 2018, 9, 5265. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Song, Z.; Li, Z.; Tang, W. Toward ideal hole transport materials: A review on recent progress in dopant-free hole transport materials for fabricating efficient and stable perovskite solar cells. Energy Environ. Sci. 2020, 13, 4057–4086. [Google Scholar] [CrossRef]
- Mahajan, P.; Padha, B.; Verma, S.; Gupta, V.; Datt, R.; Tsoi, W.C.; Satapathi, S.; Arya, S. Review of current progress in hole-transporting materials for perovskite solar cells. J. Energy Chem. 2022, 68, 330–386. [Google Scholar] [CrossRef]
- Lim, J.; Choi, E.; Kim, M.; Lee, M.; Chen, D.; Green, M.A.; Seidel, J.; Kim, C.; Park, J.; Hao, X.; et al. Revealing the Dynamics of the Thermal Reaction between Copper and Mixed Halide Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2022, 14, 20866–20874. [Google Scholar] [CrossRef]
- Farokhi, A.; Shahroosvand, H.; Monache, G.D.; Pilkington, M.; Nazeeruddin, M.K. The evolution of triphenylamine hole transport materials for efficient perovskite solar cells. Chem. Soc. Rev. 2022, 51, 5974–6064. [Google Scholar] [CrossRef]
- Yu, X.; Gao, D.; Li, Z.; Sun, X.; Li, B.; Zhu, Z.; Li, Z.A. Green-solvent Processable Dopant-free Hole Transporting Materials for Inverted Perovskite Solar Cells. Angew. Chem. Int. Ed. 2023, 62, e202218752. [Google Scholar] [CrossRef]
- Cheng, Q.; Chen, H.; Yang, F.; Chen, Z.; Chen, W.; Yang, H.; Shen, Y.; Ou, X.-M.; Wu, Y.; Li, Y.; et al. Molecular Self-Assembly Regulated Dopant-Free Hole Transport Materials for Efficient and Stable n-i-p Perovskite Solar Cells and Scalable Modules. Angew. Chem. Int. Ed. 2022, 61, e202210613. [Google Scholar] [CrossRef]
- Jeong, M.J.; Yeom, K.M.; Kim, S.J.; Jung, E.H.; Noh, J.H. Spontaneous interface engineering for dopant-free poly(3-hexylthiophene) perovskite solar cells with efficiency over 24%. Energy Environ. Sci. 2021, 14, 2419–2428. [Google Scholar] [CrossRef]
- Qu, G.; Dong, L.; Qiao, Y.; Khan, D.; Chen, Q.; Xie, P.; Yu, X.; Liu, X.; Wang, Y.; Chen, J.; et al. Dopant-Free Phthalocyanine Hole Conductor with Thermal-Induced Holistic Passivation for Stable Perovskite Solar Cells with 23% Efficiency. Adv. Funct. Mater. 2022, 32, 2206585. [Google Scholar] [CrossRef]
- Gelmetti, I.; Montcada, N.F.; Pérez-Rodríguez, A.; Barrena, E.; Ocal, C.; García-Benito, I.; Molina-Ontoria, A.; Martín, N.; Vidal-Ferran, A.; Palomares, E. Energy alignment and recombination in perovskite solar cells: Weighted influence on the open circuit voltage. Energy Environ. Sci. 2019, 12, 1309–1316. [Google Scholar] [CrossRef]
- Yan, P.; Yang, D.; Wang, H.; Yang, S.; Ge, Z. Recent advances in dopant-free organic hole-transporting materials for efficient, stable and low-cost perovskite solar cells. Energy Environ. Sci. 2022, 15, 3630–3669. [Google Scholar] [CrossRef]
- Yuan, L.; Zhu, W.; Zhang, Y.; Li, Y.; Chan, C.C.S.; Qin, M.; Qiu, J.; Zhang, K.; Huang, J.; Wang, J.; et al. Conformally bonded molecular interface retarded iodine migration for durable perovskite solar cells. Energy Environ. Sci. 2023. [Google Scholar] [CrossRef]
- Li, Y.; Scheel, K.R.; Clevenger, R.G.; Shou, W.; Pan, H.; Kilway, K.V.; Peng, Z. Highly Efficient and Stable Perovskite Solar Cells Using a Dopant-Free Inexpensive Small Molecule as the Hole-Transporting Material. Adv. Energy Mater. 2018, 8, 1801248. [Google Scholar] [CrossRef]
- Gao, W.-J.; Xia, J.; Xiao, J.; Yu, H.-J.; Wang, D.; Shinohara, A.; Jia, C.; Kuang, D.-B.; Shao, G. Cooperative effects of Dopant-Free Hole-Transporting materials and polycarbonate film for sustainable perovskite solar cells. Chem. Eng. J. 2022, 437, 135197. [Google Scholar] [CrossRef]
- Stolterfoht, M.; Caprioglio, P.; Wolff, C.M.; Márquez, J.A.; Nordmann, J.; Zhang, S.; Rothhardt, D.; Hörmann, U.; Amir, Y.; Redinger, A.; et al. The impact of energy alignment and interfacial recombination on the internal and external open-circuit voltage of perovskite solar cells. Energy Environ. Sci. 2019, 12, 2778–2788. [Google Scholar] [CrossRef]
- Kim, H.; Lee, K.S.; Paik, M.J.; Lee, D.Y.; Lee, S.-U.; Choi, E.; Yun, J.S.; Seok, S.I. Polymethyl Methacrylate as an Interlayer Between the Halide Perovskite and Copper Phthalocyanine Layers for Stable and Efficient Perovskite Solar Cells. Adv. Funct. Mater. 2022, 32, 2110473. [Google Scholar] [CrossRef]
- Yin, X.; Zhou, J.; Song, Z.; Dong, Z.; Bao, Q.; Shrestha, N.; Bista, S.S.; Ellingson, R.J.; Yan, Y.; Tang, W. Dithieno[3,2-b:2′,3′-d]pyrrol-Cored Hole Transport Material Enabling over 21% Efficiency Dopant-Free Perovskite Solar Cells. Adv. Funct. Mater. 2019, 29, 1904300. [Google Scholar] [CrossRef]
- Chen, W.; Qi, D.-C.; Huang, H.; Gao, X.; Wee, A.T.S. Organic–Organic Heterojunction Interfaces: Effect of Molecular Orientation. Adv. Funct. Mater. 2011, 21, 410–424. [Google Scholar] [CrossRef]
- Pandey, M.; Kumari, N.; Nagamatsu, S.; Pandey, S.S. Recent advances in the orientation of conjugated polymers for organic field-effect transistors. J. Mater. Chem. C 2019, 7, 13323–13351. [Google Scholar] [CrossRef]
- Liu, R.; Yang, W.; Xu, W.; Deng, J.; Ding, C.; Guo, Y.; Zheng, L.; Sun, J.; Li, M. Impact of Chemical Design on the Molecular Orientation of Conjugated Donor–Acceptor Polymers for Field-Effect Transistors. ACS Appl. Polym. Mater. 2022, 4, 2233–2250. [Google Scholar] [CrossRef]
- Khan, D.; Liu, X.; Qu, G.; Nath, A.R.; Xie, P.; Xu, Z.-X. Nexuses Between the Chemical Design and Performance of Small Molecule Dopant-Free Hole Transporting Materials in Perovskite Solar Cells. Small 2022, 19, 2205926. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Guo, S.; Zhou, K.; Ma, W. Control of the molecular orientation in small molecule-based organic photovoltaics. Sustain. Energy Fuels 2020, 4, 4934–4955. [Google Scholar] [CrossRef]
- Wang, C.; Dong, H.; Jiang, L.; Hu, W. Organic semiconductor crystals. Chem. Soc. Rev. 2018, 47, 422–500. [Google Scholar] [CrossRef] [PubMed]
- Hartnett, P.E.; Timalsina, A.; Matte, H.S.S.R.; Zhou, N.; Guo, X.; Zhao, W.; Facchetti, A.; Chang, R.P.H.; Hersam, M.C.; Wasielewski, M.R.; et al. Slip-Stacked Perylenediimides as an Alternative Strategy for High Efficiency Nonfullerene Acceptors in Organic Photovoltaics. J. Am. Chem. Soc. 2014, 136, 16345–16356. [Google Scholar] [CrossRef]
- Hecht, M.; Würthner, F. Supramolecularly Engineered J-Aggregates Based on Perylene Bisimide Dyes. Acc. Chem. Res. 2021, 54, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tee, C.K.; Shtein, M.; Martin, D.C.; Anthony, J. Controlled solution deposition and systematic study of charge-transport anisotropy in single crystal and single-crystal textured TIPS pentacene thin films. Org. Electron. 2009, 10, 696–703. [Google Scholar] [CrossRef]
- Hourani, W.; Rahimi, K.; Botiz, I.; Koch, F.P.V.; Reiter, G.; Lienerth, P.; Heiser, T.; Bubendorff, J.-L.; Simon, L. Anisotropic charge transport in large single crystals of π-conjugated organic molecules. Nanoscale 2014, 6, 4774–4780. [Google Scholar] [CrossRef]
- Hofmann, A.; Schmid, M.; Brütting, W. The Many Facets of Molecular Orientation in Organic Optoelectronics. Adv. Opt. Mater. 2021, 9, 2101004. [Google Scholar] [CrossRef]
- Noh, Y.-Y.; Kim, J.-J.; Yoshida, Y.; Yase, K. Effect of Molecular Orientation of Epitaxially Grown Platinum(II) Octaethyl Porphyrin Films on the Performance of Field-Effect Transistors. Adv. Mater. 2003, 15, 699–702. [Google Scholar] [CrossRef]
- Zhou, Z.; Wu, Q.; Cheng, R.; Zhang, H.; Wang, S.; Chen, M.; Xie, M.; Chan, P.K.L.; Grätzel, M.; Feng, S.-P. Orientation-Engineered Small-Molecule Semiconductors as Dopant-Free Hole Transporting Materials for Efficient and Stable Perovskite Solar Cells. Adv. Funct. Mater. 2021, 31, 2011270. [Google Scholar] [CrossRef]
- Bissesar, S.; van Raamsdonk, D.M.E.; Gibbons, D.J.; Williams, R.M. Spin Orbit Coupling in Orthogonal Charge Transfer States: (TD-)DFT of Pyrene-Dimethylaniline. Molecules 2022, 27, 891. [Google Scholar] [CrossRef] [PubMed]
- Rand, B.P.; Cheyns, D.; Vasseur, K.; Giebink, N.C.; Mothy, S.; Yi, Y.; Coropceanu, V.; Beljonne, D.; Cornil, J.; Brédas, J.-L.; et al. The Impact of Molecular Orientation on the Photovoltaic Properties of a Phthalocyanine/Fullerene Heterojunction. Adv. Funct. Mater. 2012, 22, 2987–2995. [Google Scholar] [CrossRef]
- Xiang, B.; Li, Y.; Pham, C.H.; Paesani, F.; Xiong, W. Ultrafast direct electron transfer at organic semiconductor and metal interfaces. Sci. Adv. 2017, 3, e1701508. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Giordano, A.J.; Shallcross, R.C.; Fleming, S.R.; Barlow, S.; Armstrong, N.R.; Marder, S.R.; Saavedra, S.S. Surface Modification of Indium–Tin Oxide with Functionalized Perylene Diimides: Characterization of Orientation, Electron-Transfer Kinetics and Electronic Structure. J. Phys. Chem. C 2016, 120, 20040–20048. [Google Scholar] [CrossRef]
- Ayzner, A.L.; Nordlund, D.; Kim, D.-H.; Bao, Z.; Toney, M.F. Ultrafast Electron Transfer at Organic Semiconductor Interfaces: Importance of Molecular Orientation. J. Phys. Chem. Lett. 2015, 6, 6–12. [Google Scholar] [CrossRef]
- Padgaonkar, S.; Amsterdam, S.H.; Bergeron, H.; Su, K.; Marks, T.J.; Hersam, M.C.; Weiss, E.A. Molecular-Orientation-Dependent Interfacial Charge Transfer in Phthalocyanine/MoS2 Mixed-Dimensional Heterojunctions. J. Phys. Chem. C 2019, 123, 13337–13343. [Google Scholar] [CrossRef]
- Igci, C.; Paek, S.; Rakstys, K.; Kanda, H.; Shibayama, N.; Jankauskas, V.; Roldán-Carmona, C.; Kim, H.; Asiri, A.M.; Nazeeruddin, M.K. D–π–A-Type Triazatruxene-Based Dopant-Free Hole Transporting Materials for Efficient and Stable Perovskite Solar Cells. Sol. RRL 2020, 4, 2000173. [Google Scholar] [CrossRef]
- Wu, W.-Q.; Wang, Q.; Fang, Y.; Shao, Y.; Tang, S.; Deng, Y.; Lu, H.; Liu, Y.; Li, T.; Yang, Z.; et al. Molecular doping enabled scalable blading of efficient hole-transport-layer-free perovskite solar cells. Nat. Commun. 2018, 9, 1625. [Google Scholar] [CrossRef]
- Wang, S.; Sakurai, T.; Wen, W.; Qi, Y. Energy Level Alignment at Interfaces in Metal Halide Perovskite Solar Cells. Adv. Mater. Interfaces 2018, 5, 1800260. [Google Scholar] [CrossRef]
- Westbrook, R.J.E.; Sanchez-Molina, D.I.; Marin-Beloqui, J.M.; Bronstein, D.H.; Haque, D.S.A. Effect of Interfacial Energetics on Charge Transfer from Lead Halide Perovskite to Organic Hole Conductors. J. Phys. Chem. C 2018, 122, 1326–1332. [Google Scholar] [CrossRef]
- Yan, W.; Li, Y.; Li, Y.; Ye, S.; Liu, Z.; Wang, S.; Bian, Z.; Huang, C. High-performance hybrid perovskite solar cells with open circuit voltage dependence on hole-transporting materials. Nano Energy 2015, 16, 428–437. [Google Scholar] [CrossRef]
- Ryu, S.; Noh, J.H.; Jeon, N.J.; Kim, Y.C.; Yang, W.S.; Seo, J.; Seok, S.I. Voltage output of efficient perovskite solar cells with high open-circuit voltage and fill factor. Energy Environ. Sci. 2014, 7, 2614–2618. [Google Scholar] [CrossRef]
- Duhm, S.; Heimel, G.; Salzmann, I.; Glowatzki, H.; Johnson, R.L.; Vollmer, A.; Rabe, J.P.; Koch, N. Orientation-dependent ionization energies and interface dipoles in ordered molecular assemblies. Nat. Mater. 2008, 7, 326–332. [Google Scholar] [CrossRef]
- Kahn, A. Fermi level, work function and vacuum level. Mater. Horiz. 2016, 3, 7–10. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, F.; Guo, Y.; Wu, H.; He, L.; Liu, Z.; Cai, B.; Guo, Y.; Brett, C.J.; Li, Y.; et al. Conformational and Compositional Tuning of Phenanthrocarbazole-Based Dopant-Free Hole-Transport Polymers Boosting the Performance of Perovskite Solar Cells. J. Am. Chem. Soc. 2020, 142, 17681–17692. [Google Scholar] [CrossRef]
- Hai, J.; Wu, H.; Yin, X.; Song, J.; Hu, L.; Jin, Y.; Li, L.; Su, Z.; Xu, Z.; Wang, H.; et al. Dopant-Free Hole Transport Materials Based on a Large Conjugated Electron-Deficient Core for Efficient Perovskite Solar Cells. Adv. Funct. Mater. 2021, 31, 2105458. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Y.; Qin, C.; Yang, X.; Yasuda, T.; Islam, A.; Zhang, K.; Peng, W.; Chen, W.; Han, L. A dopant-free hole-transporting material for efficient and stable perovskite solar cells. Energy Environ. Sci. 2014, 7, 2963–2967. [Google Scholar] [CrossRef]
- Wan, Z.; Yang, J.; Xia, J.; Shu, H.; Yao, X.; Luo, J.; Jia, C. A new strategy for constructing a dispiro-based dopant-free hole-transporting material: Spatial configuration of spiro-bifluorene changes from a perpendicular to parallel arrangement. Chem. Sci. 2021, 12, 8548–8555. [Google Scholar] [CrossRef]
- Chen, W.; Pham, N.D.; Wang, H.; Jia, B.; Wen, X. Spectroscopic Insight into Efficient and Stable Hole Transfer at the Perovskite/Spiro-OMeTAD Interface with Alternative Additives. ACS Appl. Mater. Interfaces 2021, 13, 5752–5761. [Google Scholar] [CrossRef]
- Daboczi, M.; Hamilton, I.; Xu, S.; Luke, J.; Limbu, S.; Lee, J.; McLachlan, M.A.; Lee, K.; Durrant, J.R.; Baikie, I.D.; et al. Origin of Open-Circuit Voltage Losses in Perovskite Solar Cells Investigated by Surface Photovoltage Measurement. ACS Appl. Mater. Interfaces 2019, 11, 46808–46817. [Google Scholar] [CrossRef]
- Wei, J.; Wang, Q.; Huo, J.; Gao, F.; Gan, Z.; Zhao, Q.; Li, H. Mechanisms and Suppression of Photoinduced Degradation in Perovskite Solar Cells. Adv. Energy Mater. 2021, 11, 2002326. [Google Scholar] [CrossRef]
- Byeon, J.; Kim, J.; Kim, J.-Y.; Lee, G.; Bang, K.; Ahn, N.; Choi, M. Charge Transport Layer-Dependent Electronic Band Bending in Perovskite Solar Cells and Its Correlation to Light-Induced Device Degradation. ACS Energy Lett. 2020, 5, 2580–2589. [Google Scholar] [CrossRef]
- DuBose, J.T.; Kamat, P.V. TiO2-Assisted Halide Ion Segregation in Mixed Halide Perovskite Films. J. Am. Chem. Soc. 2020, 142, 5362–5370. [Google Scholar] [CrossRef]
- Abudulimu, A.; Sandoval-Torrientes, R.; Zimmermann, I.; Santos, J.; Nazeeruddin, M.K.; Martín, N. Hole transporting materials for perovskite solar cells and a simple approach for determining the performance limiting factors. J. Mater. Chem. A 2020, 8, 1386–1393. [Google Scholar] [CrossRef]
- Tong, C.-J.; Cai, X.; Zhu, A.-Y.; Liu, L.-M.; Prezhdo, O.V. How Hole Injection Accelerates Both Ion Migration and Nonradiative Recombination in Metal Halide Perovskites. J. Am. Chem. Soc. 2022, 144, 6604–6612. [Google Scholar] [CrossRef]
- Kim, Y.C.; Yang, T.-Y.; Jeon, N.J.; Im, J.; Jang, S.; Shin, T.J.; Shin, H.-W.; Kim, S.; Lee, E.; Kim, S.; et al. Engineering interface structures between lead halide perovskite and copper phthalocyanine for efficient and stable perovskite solar cells. Energy Environ. Sci. 2017, 10, 2109–2116. [Google Scholar] [CrossRef]
- Yuan, G.; Xie, W.; Song, Q.; Ma, S.; Ma, Y.; Shi, C.; Xiao, M.; Pei, F.; Niu, X.; Zhang, Y.; et al. Inhibited cracks development by compressive strain in perovskite solar cells with improved mechanical stability. Adv. Mater. 2023, 2211257. [Google Scholar] [CrossRef]
- Yang, D.; Yang, R.; Priya, S.; Liu, F. Recent Advances in Flexible Perovskite Solar Cells: Fabrication and Applications. Angew. Chem. Int. Ed. 2019, 58, 4466–4483. [Google Scholar] [CrossRef]
- Lyu, M.; Park, S.; Lee, H.; Ma, B.S.; Park, S.H.; Hong, K.-H.; Kim, H.; Kim, T.-S.; Noh, J.H.; Son, H.J.; et al. Simultaneous Enhanced Efficiency and Stability of Perovskite Solar Cells Using Adhesive Fluorinated Polymer Interfacial Material. ACS Appl. Mater. Interfaces 2021, 13, 35595–35605. [Google Scholar] [CrossRef]
- Dou, J.; Song, Q.; Ma, Y.; Wang, H.; Yuan, G.; Wei, X.; Niu, X.; Ma, S.; Yang, X.; Dou, J.; et al. Improved interfacial adhesion for stable flexible inverted perovskite solar cells. J. Energy Chem. 2023, 76, 288–294. [Google Scholar] [CrossRef]
- Qin, H.; Xu, L.; Zhong, D. First-Principles Study of Zinc Phthalocyanine Molecules Adsorbed on Methylammonium Lead Iodide Surfaces. J. Phys. Chem. C 2020, 124, 5167–5173. [Google Scholar] [CrossRef]
- Javaid, S.; Lee, G. The impact of molecular orientation on carrier transfer characteristics at a phthalocyanine and halide perovskite interface. RSC Adv. 2021, 11, 31776–31782. [Google Scholar] [CrossRef]
- Wang, J.; Wu, X.; Liu, Y.; Qin, T.; Zhang, K.; Li, N.; Zhao, J.; Ye, R.; Fan, Z.; Chi, Z.; et al. Dopant-Free Hole-Transporting Material with Enhanced Intermolecular Interaction for Efficient and Stable n-i-p Perovskite Solar Cells. Adv. Energy Mater. 2021, 11, 2100967. [Google Scholar] [CrossRef]
- Kaya, I.C.; Ozdemir, R.; Usta, H.; Sonmezoglu, S. A dopant-free 2,7-dioctyl[1]benzothieno[3,2-b][1]benzothiophene (C8-BTBT)-based hole transporting layer for highly stable perovskite solar cells with efficiency over 22%. J. Mater. Chem. A 2022, 10, 12464–12472. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; Zhang, J.; Li, X.; Cheng, C.; Tian, Y.; Jen, A.K.-Y.; Xu, B. Intensive Exposure of Functional Rings of a Polymeric Hole-Transporting Material Enables Efficient Perovskite Solar Cells. Adv. Mater. 2018, 30, e1804028. [Google Scholar] [CrossRef]
- Niu, T.; Zhu, W.; Zhang, Y.; Xue, Q.; Jiao, X.; Wang, Z.; Xie, Y.-M.; Li, P.; Chen, R.; Huang, F.; et al. D-A-π-A-D-type Dopant-free Hole Transport Material for Low-Cost, Efficient, and Stable Perovskite Solar Cells. Joule 2021, 5, 249–269. [Google Scholar] [CrossRef]
- Heilmeier, G.H.; Harrison, S.E. Charge Transport in Copper Phthalocyanine Single Crystals. Phys. Rev. 1963, 132, 2010–2016. [Google Scholar] [CrossRef]
- Yang, G.; Wang, Y.-L.; Xu, J.-J.; Lei, H.-W.; Chen, C.; Shan, H.-Q.; Liu, X.-Y.; Xu, Z.-X.; Fang, G.-J. A facile molecularly engineered copper (II) phthalocyanine as hole transport material for planar perovskite solar cells with enhanced performance and stability. Nano Energy 2017, 31, 322–330. [Google Scholar] [CrossRef]
- Azmi, R.; Nam, S.Y.; Sinaga, S.; Akbar, Z.A.; Lee, C.-L.; Yoon, S.C.; Jung, I.H.; Jang, S.-Y. High-performance dopant-free conjugated small molecule-based hole-transport materials for perovskite solar cells. Nano Energy 2018, 44, 191–198. [Google Scholar] [CrossRef]
- Melville, O.A.; Lessard, B.H.; Bender, T.P. Phthalocyanine-Based Organic Thin-Film Transistors: A Review of Recent Advances. ACS Appl. Mater. Interfaces 2015, 7, 13105–13118. [Google Scholar] [CrossRef]
- Lawton, E.A. The Thermal Stability of Copper Phthalocyanine. J. Phys. Chem. 1958, 62, 384. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, Y.; Hu, J.; Yang, G.; Guo, Z.; Xia, J.; Xu, Z.; Fang, G. Octamethyl-substituted Pd(ii) phthalocyanine with long carrier lifetime as a dopant-free hole selective material for performance enhancement of perovskite solar cells. J. Mater. Chem. A 2017, 5, 24416–24424. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, X.; Liu, X.; Feng, Y.; Shan, H.; Dong, L.; Fang, G.; Xu, Z.-X. A study of different central metals in octamethyl-substituted phthalocyanines as dopant-free hole-transport layers for planar perovskite solar cells. Org. Electron. 2018, 56, 276–283. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Shan, H.; Chen, Q.; Liu, T.; Sun, X.; Ma, D.; Zhang, Z.; Xu, J.; Xu, Z.-X. Tetra-alkyl-substituted copper (II) phthalocyanines as dopant-free hole-transport layers for planar perovskite solar cells with enhanced open circuit voltage and stability. Dye. Pigment 2017, 139, 619–626. [Google Scholar] [CrossRef]
- Duong, T.; Peng, J.; Walter, D.; Xiang, J.; Shen, H.; Chugh, D.; Lockrey, M.; Zhong, D.; Li, J.; Weber, K.; et al. Perovskite Solar Cells Employing Copper Phthalocyanine Hole-Transport Material with an Efficiency over 20% and Excellent Thermal Stability. ACS Energy Lett. 2018, 3, 2441–2448. [Google Scholar] [CrossRef]
- Hu, Q.; Rezaee, E.; Dong, L.; Dong, Q.; Shan, H.; Chen, Q.; Li, M.; Cai, S.; Wang, L.; Xu, Z.-X. Molecularly Designed Zinc (II) Phthalocyanine Derivative as Dopant-Free Hole-Transporting Material of Planar Perovskite Solar Cell with Preferential Face-on Orientation. Sol. RRL 2019, 3, 1900182. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, Q.; Dong, L.; Zhang, Z.; Li, C.; Yang, S.; Cai, S.; Xu, Z.-X. Carbon-chain length substituent effects on Cu(II) phthalocyanines as dopant-free hole-transport materials for perovskite solar cells. Sol. Energy 2019, 184, 649–656. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Rezaee, E.; Chen, Q.; Feng, Y.; Sun, X.; Dong, L.; Hu, Q.; Li, C.; Xu, Z.-X. Tetra-Propyl-Substituted Copper (II) Phthalocyanine as Dopant-Free Hole Transporting Material for Planar Perovskite Solar Cells. Sol. RRL 2018, 2, 1800050. [Google Scholar] [CrossRef]
- Oh, S.; Khan, N.; Jin, S.-M.; Tran, H.; Yoon, N.; Song, C.E.; Lee, H.K.; Shin, W.S.; Lee, J.-C.; Moon, S.-J.; et al. Alkyl side-chain dependent self-organization of small molecule and its application in high-performance organic and perovskite solar cells. Nano Energy 2020, 72, 104708. [Google Scholar] [CrossRef]
- Molina, D.; Follana-Berná, J.; Sastre-Santos, Á. Phthalocyanines, porphyrins and other porphyrinoids as components of perovskite solar cells. J. Mater. Chem. C 2023. [Google Scholar] [CrossRef]
- Lee, U.-H.; Azmi, R.; Sinaga, S.; Hwang, S.; Eom, S.H.; Kim, T.-W.; Yoon, S.C.; Jang, S.-Y.; Jung, I.H. Diphenyl-2-pyridylamine-Substituted Porphyrins as Hole-Transporting Materials for Perovskite Solar Cells. ChemSusChem 2017, 10, 3780–3787. [Google Scholar] [CrossRef]
- Azmi, R.; Lee, U.-H.; Wibowo, F.T.A.; Eom, S.H.; Yoon, S.C.; Jang, S.-Y.; Jung, I.H. Performance Improvement in Low-Temperature-Processed Perovskite Solar Cells by Molecular Engineering of Porphyrin-Based Hole Transport Materials. ACS Appl. Mater. Interfaces 2018, 10, 35404–35410. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.H.; Park, S.; Heo, J.H.; Lee, H.-S.; Yoon, S.; Kang, J.; Im, S.H.; Kim, H.; Lee, W.; Kim, B.; et al. Enhancement of charge transport properties of small molecule semiconductors by controlling fluorine substitution and effects on photovoltaic properties of organic solar cells and perovskite solar cells. Chem. Sci. 2016, 7, 6649–6661. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Liao, Q.; Huang, J.; Zhang, Z.; Su, M.; Chen, Z.; Wu, Z.; Wang, D.; Lai, Z.; Woo, H.Y.; et al. Intramolecular Noncovalent Interaction-Enabled Dopant-Free Hole-Transporting Materials for High-Performance Inverted Perovskite Solar Cells. Angew. Chem. Int. Ed. 2022, 61, e202113749. [Google Scholar] [CrossRef]
- Rakstys, K.; Paek, S.; Gao, P.; Gratia, P.; Marszalek, T.; Grancini, G.; Cho, K.T.; Genevicius, K.; Jankauskas, V.; Pisula, W.; et al. Molecular engineering of face-on oriented dopant-free hole transporting material for perovskite solar cells with 19% PCE. J. Mater. Chem. A 2017, 5, 7811–7815. [Google Scholar] [CrossRef]
- Liu, C.; Igci, C.; Yang, Y.; Syzgantseva, O.A.; Syzgantseva, M.A.; Rakstys, K.; Kanda, H.; Shibayama, N.; Ding, B.; Zhang, X.; et al. Dopant-Free Hole Transport Materials Afford Efficient and Stable Inorganic Perovskite Solar Cells and Modules. Angew. Chem. Int. Ed. 2021, 60, 20489–20497. [Google Scholar] [CrossRef]
- Shibayama, N.; Maekawa, H.; Nakamura, Y.; Haruyama, Y.; Niibe, M.; Ito, S. Control of Molecular Orientation of Spiro-OMeTAD on Substrates. ACS Appl. Mater. Interfaces 2020, 12, 50187–50191. [Google Scholar] [CrossRef]
- Basova, T.; Hassan, A.; Durmuſ, M.; Gürek, A.G.; Ahsen, V. Liquid crystalline metal phthalocyanines: Structural organization on the substrate surface. Coord. Chem. Rev. 2016, 310, 131–153. [Google Scholar] [CrossRef]
- Stecker, C.; Liu, Z.; Hieulle, J.; Zhang, S.; Ono, L.K.; Wang, G.; Qi, Y. Atomic Scale Investigation of the CuPc–MAPbX3 Interface and the Effect of Non-Stoichiometric Perovskite Films on Interfacial Structures. ACS Nano 2021, 15, 14813–14821. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Rudd, P.N.; Yang, S.; Yuan, Y.; Huang, J. Imperfections and their passivation in halide perovskite solar cells. Chem. Soc. Rev. 2019, 48, 3842–3867. [Google Scholar] [CrossRef]
- Yang, Z.; Babu, B.H.; Wu, S.; Liu, T.; Fang, S.; Xiong, Z.; Han, L.; Chen, W. Review on Practical Interface Engineering of Perovskite Solar Cells: From Efficiency to Stability. Sol. RRL 2020, 4, 1900257. [Google Scholar] [CrossRef]
- Ren, G.; Zhang, Z.; Deng, Y.; Li, Z.; Liu, C.; Wang, M.; Guo, W. Organic iodides in efficient and stable perovskite solar cells: Strong surface passivation and interaction. Energy Environ. Sci. 2023, 16, 565–573. [Google Scholar] [CrossRef]
- Yamada, K.; Suzuki, M.; Suenobu, T.; Nakayama, K.-i. High Vertical Carrier Mobilities of Organic Semiconductors Due to a Deposited Laid-Down Herringbone Structure Induced by a Reduced Graphene Oxide Template. ACS Appl. Mater. Interfaces 2020, 12, 9489–9497. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.B.; Kim, H.H.; Lee, H.; Kang, B.; Lee, S.; Sim, M.; Kim, M.; Lee, W.H.; Cho, K. Boosting Photon Harvesting in Organic Solar Cells with Highly Oriented Molecular Crystals via Graphene–Organic Heterointerface. ACS Nano 2015, 9, 8206–8219. [Google Scholar] [CrossRef] [PubMed]
- Mativetsky, J.M.; Wang, H.; Lee, S.S.; Whittaker-Brooks, L.; Loo, Y.-L. Face-on stacking and enhanced out-of-plane hole mobility in graphene-templated copper phthalocyanine. Chem. Commun. 2014, 50, 5319–5321. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Wu, T.; Cui, D.; Lin, X.; Luo, X.; Wang, Y.; Han, L. The Application of Graphene Derivatives in Perovskite Solar Cells. Small Methods 2020, 4, 2000507. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Wu, C.; Han, X. Controlling Molecular Orientation of Small Molecular Dopant-Free Hole-Transport Materials: Toward Efficient and Stable Perovskite Solar Cells. Molecules 2023, 28, 3076. https://doi.org/10.3390/molecules28073076
Li W, Wu C, Han X. Controlling Molecular Orientation of Small Molecular Dopant-Free Hole-Transport Materials: Toward Efficient and Stable Perovskite Solar Cells. Molecules. 2023; 28(7):3076. https://doi.org/10.3390/molecules28073076
Chicago/Turabian StyleLi, Wenhui, Chuanli Wu, and Xiuxun Han. 2023. "Controlling Molecular Orientation of Small Molecular Dopant-Free Hole-Transport Materials: Toward Efficient and Stable Perovskite Solar Cells" Molecules 28, no. 7: 3076. https://doi.org/10.3390/molecules28073076
APA StyleLi, W., Wu, C., & Han, X. (2023). Controlling Molecular Orientation of Small Molecular Dopant-Free Hole-Transport Materials: Toward Efficient and Stable Perovskite Solar Cells. Molecules, 28(7), 3076. https://doi.org/10.3390/molecules28073076





