Progress and Prospect of Zn Anode Modification in Aqueous Zinc-Ion Batteries: Experimental and Theoretical Aspects
Abstract
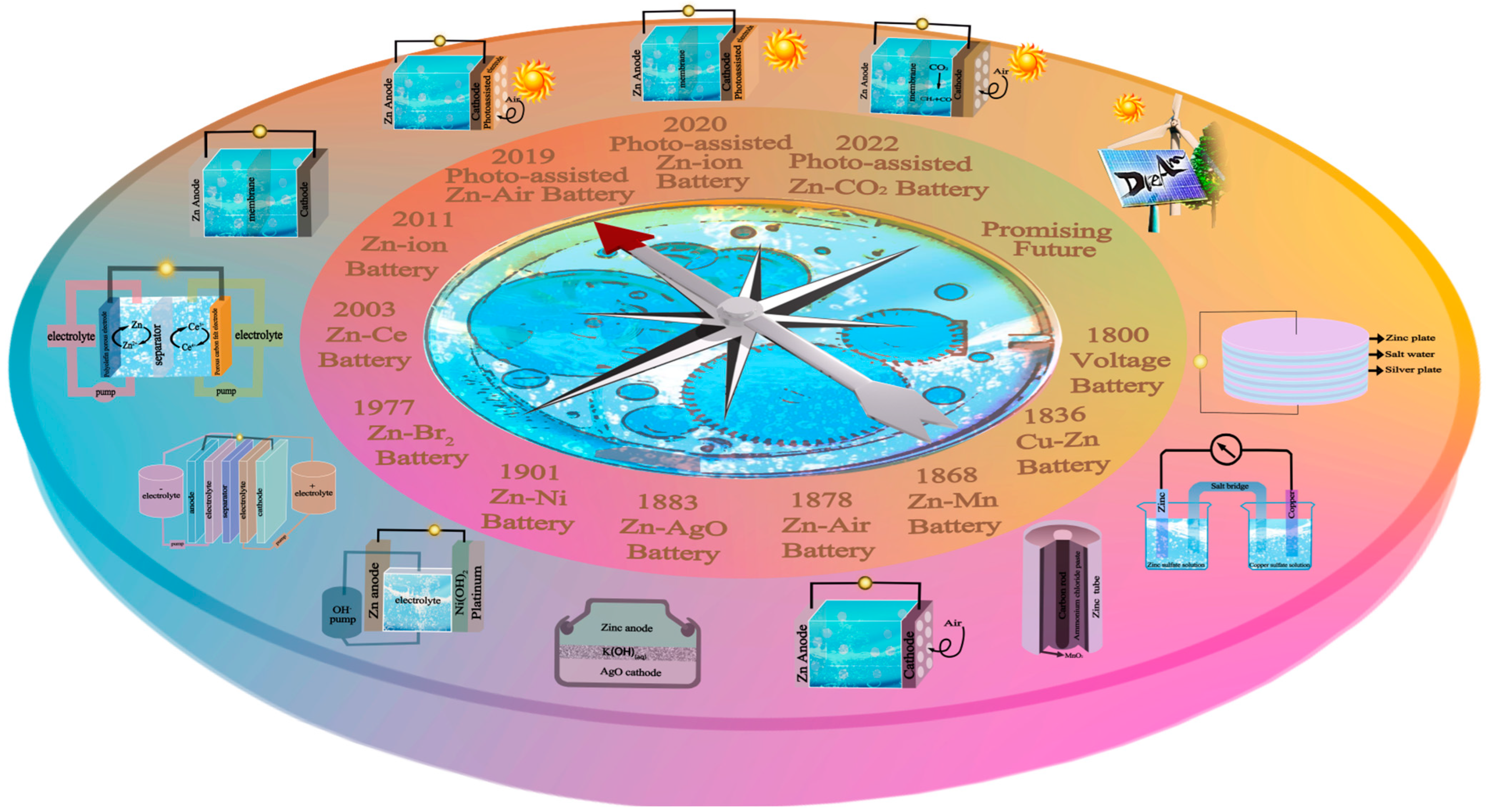
1. Introduction
2. Basic Component and Energy Storage Mechanism
2.1. Basic Components of AZIBs
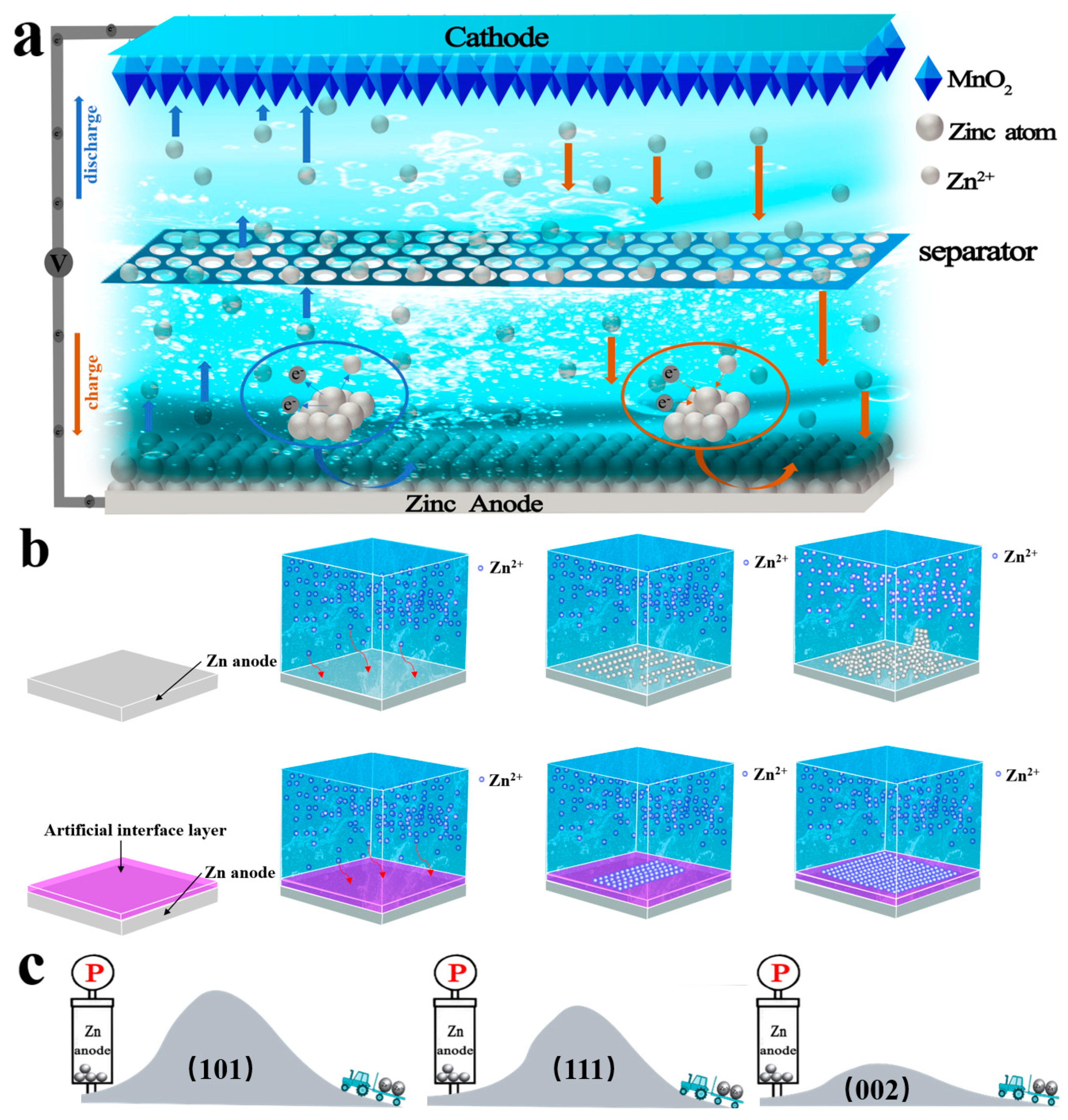
2.2. Energy Storage Mechanism of AZIBs
2.3. Current Challenges of AZIBs
3. Modification Strategy for Zinc Metal Anodes
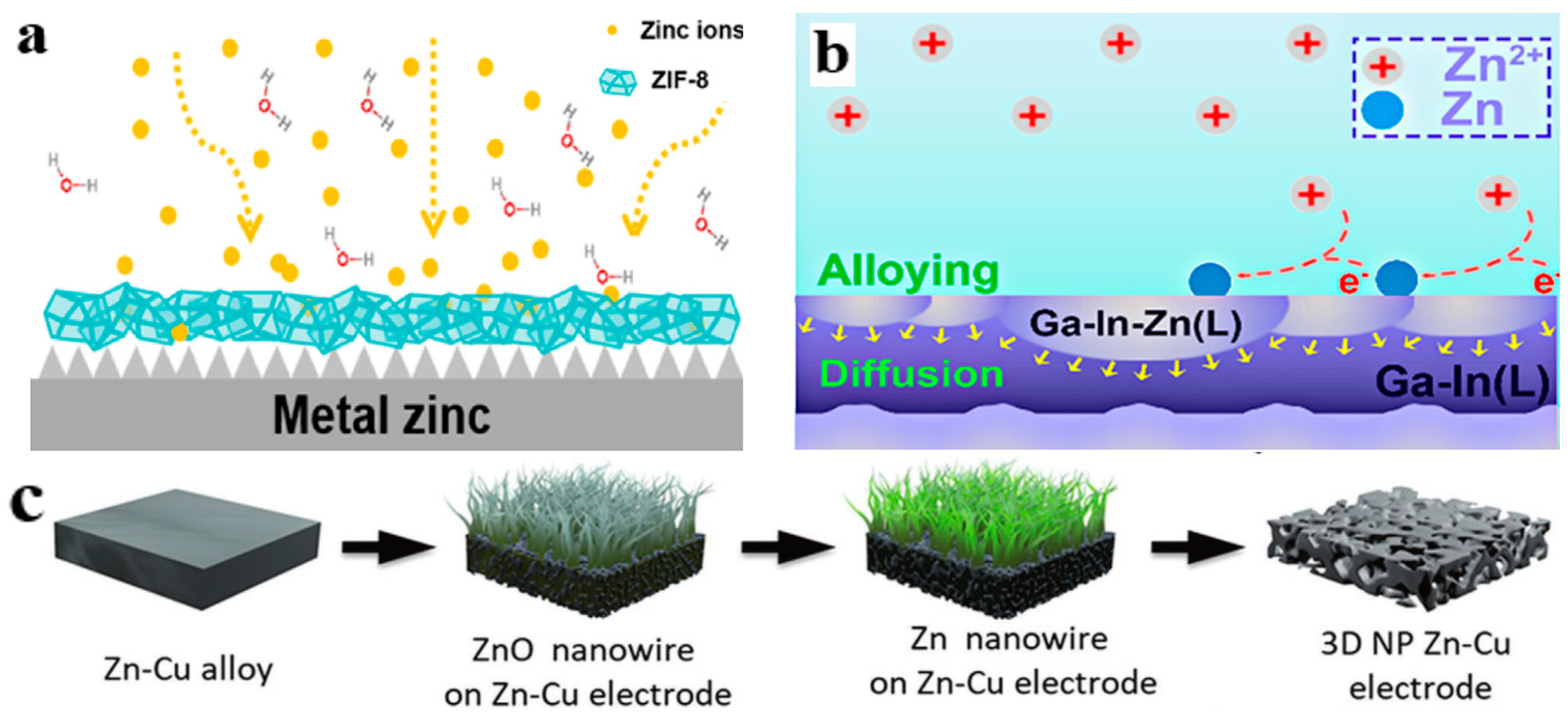
3.1. Dense Artificial Interface Layer
3.2. Porous Framework
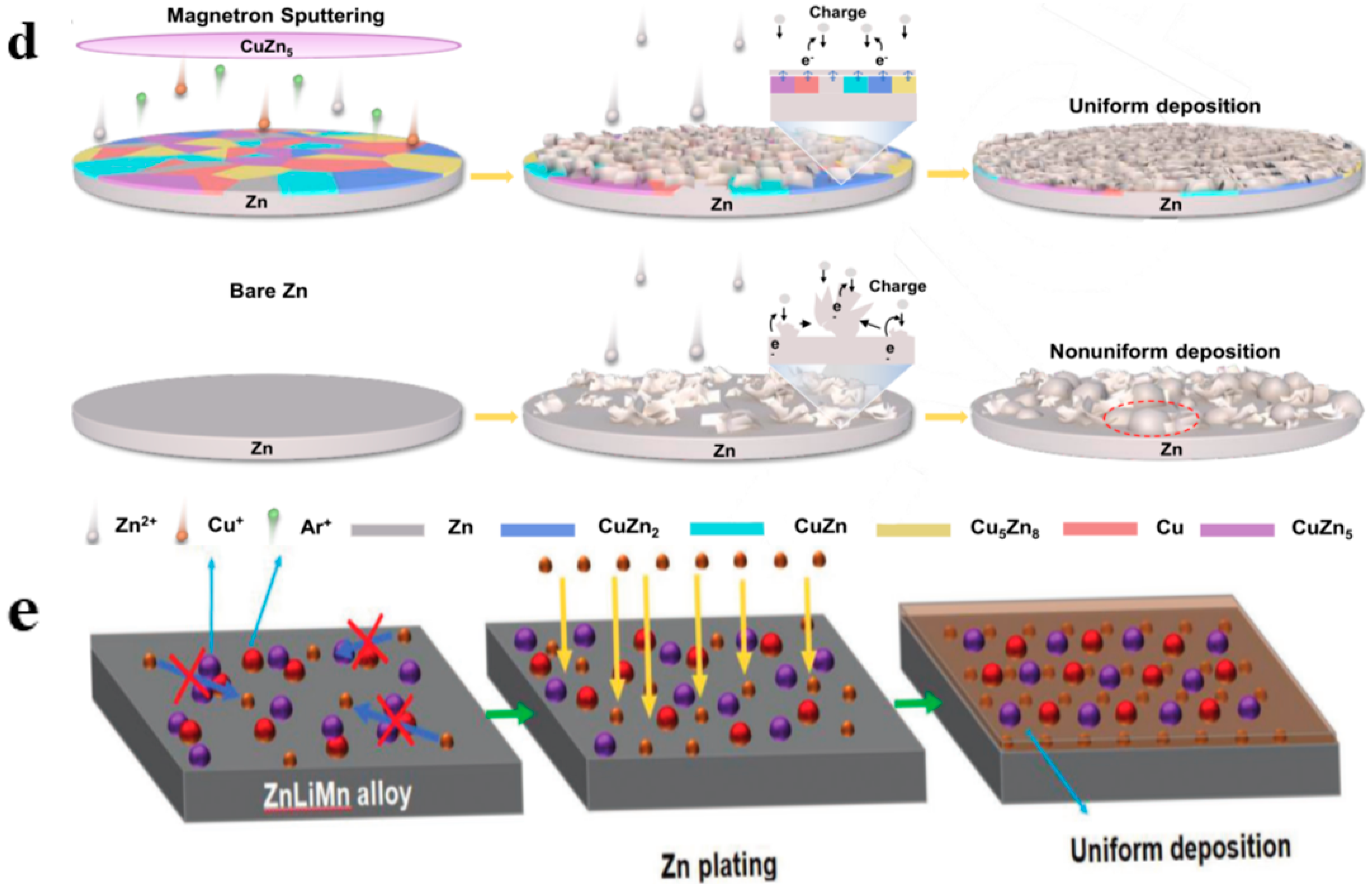
3.3. Construction of Zinc Alloy Anodes
4. Theoretical Study on Modified Zinc Anode
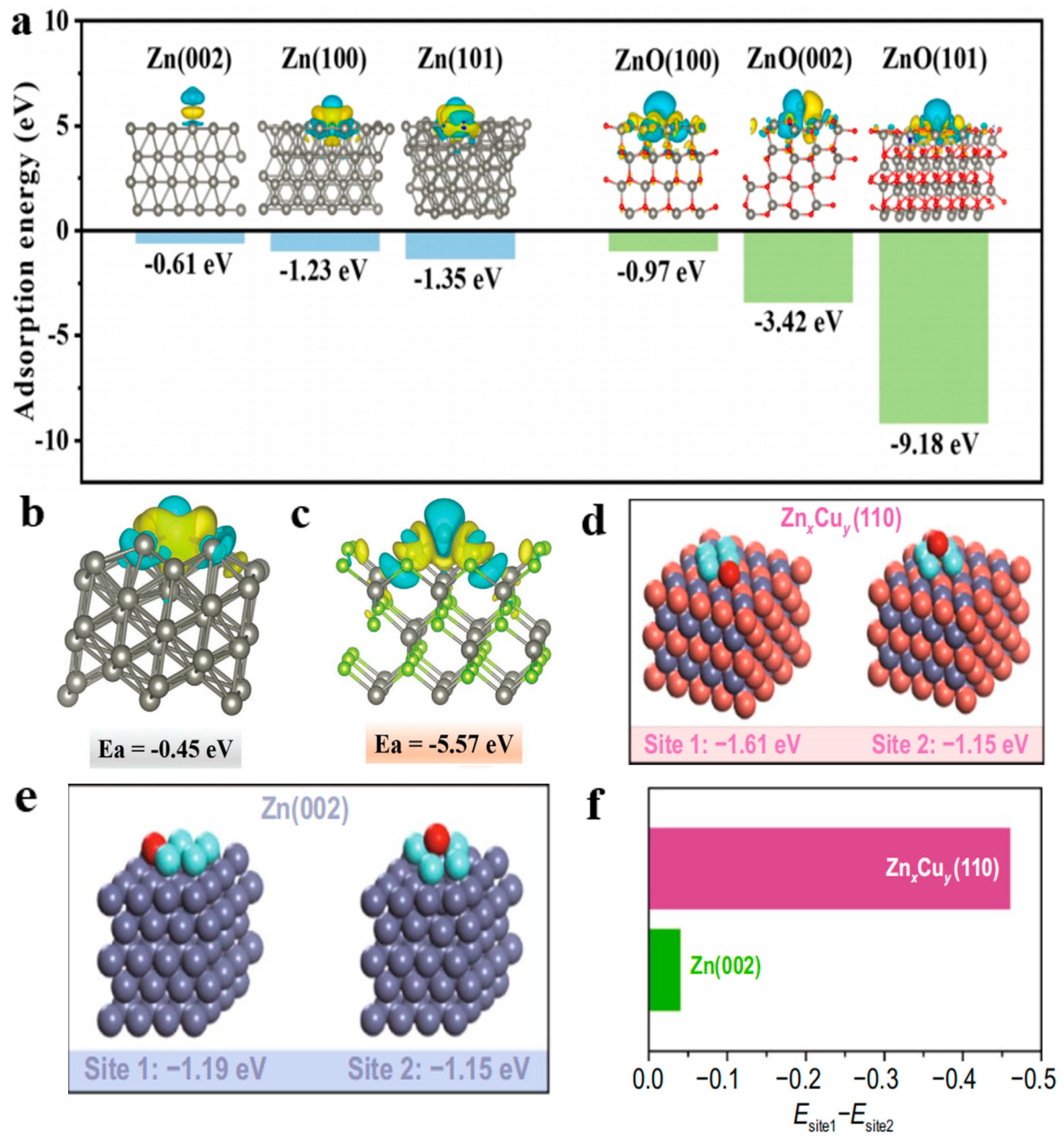
4.1. Theoretical Study of Interfacial Adsorption Energy and Differential Charge Density
4.2. Molecular Dynamics
5. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blanc, L.E.; Kundu, D.; Nazar, L.F. Scientific Challenges for the Implementation of Zn-Ion Batteries. Joule 2020, 4, 771–799. [Google Scholar] [CrossRef]
- Sun, H.; Huyan, Y.; Li, N.; Lei, D.; Liu, H.; Hua, W.; Wei, C.; Kang, F.; Wang, J.G. A Seamless Metal-Organic Framework Interphase with Boosted Zn2+ Flux and Deposition Kinetics for Long-Living Rechargeable Zn Batteries. Nano Lett. 2023, 23, 1726–1734. [Google Scholar] [CrossRef]
- Zhao, R.; Yang, J.; Han, X.; Wang, Y.; Ni, Q.; Hu, Z.; Wu, C.; Bai, Y. Stabilizing Zn Metal Anodes via Cation/Anion Regulation toward High Energy Density Zn-Ion Batteries. Adv. Energy Mater. 2023, 13, 2203542. [Google Scholar] [CrossRef]
- Jia, H.; Liu, K.; Lam, Y.; Tawiah, B.; Xin, J.H.; Nie, W.; Jiang, S.-X. Fiber-Based Materials for Aqueous Zinc Ion Batteries. Adv. Fiber Mater. 2022, 5, 36–58. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Cheng, H.; Ni, Z.; Wang, Y.; Xia, G.; Li, X.; Zeng, X. Research Progress toward Room Temperature Sodium Sulfur Batteries: A Review. Molecules 2021, 26, 1535. [Google Scholar] [CrossRef]
- Salgado, R.M.; Danzi, F.; Oliveira, J.E.; El-Azab, A.; Camanho, P.P.; Braga, M.H. The Latest Trends in Electric Vehicles Batteries. Molecules 2021, 26, 3188. [Google Scholar] [CrossRef]
- Chen, D.; Li, Y.; Zhang, X.; Hu, S.; Yu, Y. Investigation of the discharging behaviors of different doped silicon nanowires in alkaline Si-air batteries. J. Ind. Eng. Chem. 2022, 112, 271–278. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, T.; Wang, D.; Huang, J.; Xiang, Y.; Yu, Y. Ge nanowires on top of a Ge substrate for applications in anodes of Li and Na ion batteries: A first-principles study. RSC Adv. 2022, 12, 9163–9169. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Gao, S.; Hu, S. Si modified by Zn and Fe as anodes in Si-air batteries with ameliorative properties. J. Alloys Compd. 2021, 883, 160902. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, D.; Luo, J.; Xiang, Y. First-principles study of ZIF-8 as anode for Na and K ion batteries. Colloids Surf. A Physicochem. Eng. Asp. 2023, 659, 130802. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, Y.; Wang, D.; Chen, D.; Zhang, X.; Yu, Y. Graphene-coated Ge as anodes in Ge-air batteries with enhanced performance. Carbon 2023, 205, 86–96. [Google Scholar] [CrossRef]
- Yu, Y.; Hu, S. The applications of semiconductor materials in air batteries. Chin. Chem. Lett. 2021, 32, 3277–3287. [Google Scholar] [CrossRef]
- Liu, W.; Lu, B.; Liu, X.; Gan, Y.; Zhang, S.; Shi, S. In Situ Synthesis of the Peapod-Like Cu–SnO2@Copper Foam as Anode with Excellent Cycle Stability and High Area Specific Capacity. Adv. Funct. Mater. 2021, 31, 2101999. [Google Scholar] [CrossRef]
- Liu, W.; Chen, X.; Xiang, P.; Zhang, S.; Yan, J.; Li, N.; Shi, S. Chemically monodisperse tin nanoparticles on monolithic 3D nanoporous copper for lithium-ion battery anodes with ultralong cycle life and stable lithium storage properties. Nanoscale 2019, 11, 4885–4894. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Zhang, Y.; Yu, Y. Conductive Metal–Organic Frameworks for Rechargeable Lithium Batteries. Batteries 2023, 9, 109. [Google Scholar] [CrossRef]
- Pan, H.; Shao, Y.; Yan, P.; Cheng, Y.; Han, K.; Nie, Z.; Wang, C.; Yang, J.; Li, X.; Mueller, K.T.; et al. Highly Reversible Aqueous Zn/MnO2 Energy Storage System from Chemical Conversion Reactions. ECS Meet. Abstr. 2016, MA2016-03, 749. [Google Scholar] [CrossRef]
- Dai, Q.; Li, L.; Hoang, T.K.A.; Tu, T.; Hu, B.; Jia, Y.; Zhang, M.; Song, L.; Trudeau, M.L. The secondary aqueous zinc-manganese battery. J. Energy Storage 2022, 55, 105397. [Google Scholar] [CrossRef]
- Yu, X.; Liu, G.; Wang, T.; Gong, H.; Qu, H.; Meng, X.; He, J.; Ye, J. Recent Advances in the Research of Photo-Assisted Lithium-Based Rechargeable Batteries. Chem. Asian J. 2022, 28, e202202104. [Google Scholar] [CrossRef]
- Lv, J.; Abbas, S.C.; Huang, Y.; Liu, Q.; Wu, M.; Wang, Y.; Dai, L. A photo-responsive bifunctional electrocatalyst for oxygen reduction and evolution reactions. Nano Energy 2018, 43, 130–137. [Google Scholar] [CrossRef]
- Boruah, B.D.; Mathieson, A.; Wen, B.; Feldmann, S.; Dose, W.M.; De Volder, M. Photo-rechargeable zinc-ion batteries. Energy Environ. Sci. 2020, 13, 2414–2421. [Google Scholar] [CrossRef]
- Li, J.; Zhang, K.; Wang, B.; Peng, H. Light-Assisted Metal–Air Batteries: Progress, Challenges, and Perspectives. Angew. Chem. Int. Ed. 2022, 61, e202213026. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Xu, K.; Liu, N.; Reed, D.; Li, X. Crossroads in the renaissance of rechargeable aqueous zinc batteries. Mater. Today 2021, 45, 191–212. [Google Scholar] [CrossRef]
- Huang, J.; Qiu, X.; Wang, N.; Wang, Y. Aqueous rechargeable zinc batteries: Challenges and opportunities. Curr. Opin. Electrochem. 2021, 30, 100801. [Google Scholar] [CrossRef]
- Xu, X.; Song, M.; Li, M.; Xu, Y.; Sun, L.; Shi, L.; Su, Y.; Lai, C.; Wang, C. A novel bifunctional zinc gluconate electrolyte for a stable Zn anode. Chem. Eng. J. 2023, 454, 140364. [Google Scholar] [CrossRef]
- Tao, F.; Liu, Y.; Ren, X.; Wang, J.; Zhou, Y.; Miao, Y.; Ren, F.; Wei, S.; Ma, J. Different surface modification methods and coating materials of zinc metal anode. J. Energy Chem. 2022, 66, 397–412. [Google Scholar] [CrossRef]
- Jian, Q.; Wan, Y.; Sun, J.; Wu, M.; Zhao, T. A dendrite-free zinc anode for rechargeable aqueous batteries. J. Mater. Chem. A 2020, 8, 20175–20184. [Google Scholar] [CrossRef]
- Gao, X.; Li, Y.; Yin, W.; Lu, X. Recent Advances of Carbon Materials in Anodes for Aqueous Zinc Ion Batteries. Chem. Rec. 2022, 22, e202200092. [Google Scholar] [CrossRef]
- Su, Y.; Yang, X.; Zhang, Q.; Sun, J.; Liu, Z. Carbon nanomaterials for highly stable Zn anode: Recent progress and future outlook. J. Electroanal. Chem. 2022, 904, 115883. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, J.; Luo, J.; Yu, Y.; Liu, X.; Lu, X. Methods for Rational Design of Advanced Zn-Based Batteries. Small Methods 2022, 6, e2200560. [Google Scholar] [CrossRef]
- Li, B.; Zhang, X.; Wang, T.; He, Z.; Lu, B.; Liang, S.; Zhou, J. Interfacial Engineering Strategy for High-Performance Zn Metal Anodes. Nano-Micro Lett. 2021, 14, 6. [Google Scholar] [CrossRef]
- Shang, Y.; Kundu, D. Understanding and Performance of the Zinc Anode Cycling in Aqueous Zinc-Ion Batteries and a Roadmap for the Future. Batter. Supercaps 2022, 5, e202100394. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Wu, X. Toward Long-Life Aqueous Zinc Ion Batteries by Constructing Stable Zinc Anodes. Chem. Rec. 2022, 22, e202200088. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Yi, J.; Liu, X.; Wu, K.; Wang, Z.; Cui, J.; Liu, Y.; Wang, Y.; Xia, Y.; Zhang, J. Highly Reversible Zn Anode Enabled by Controllable Formation of Nucleation Sites for Zn-Based Batteries. Adv. Funct. Mater. 2020, 30, 1908528. [Google Scholar] [CrossRef]
- Tao, H.; Hou, Z.; Zhang, L.; Yang, X.; Fan, L.-Z. Manipulating alloying reaction to achieve the stable and dendrite-free zinc metal anodes. Chem. Eng. J. 2022, 450, 138048. [Google Scholar] [CrossRef]
- Lu, W.; Xie, C.; Zhang, H.; Li, X. Inhibition of Zinc Dendrite Growth in Zinc-Based Batteries. ChemSusChem 2018, 11, 3996–4006. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Guo, C.; Zhang, T.; Xue, P.; Zhao, R.; Zhou, W.; Li, W.; Elzatahry, A.; Zhao, D.; Chao, D. Hierarchical Confinement Effect with Zincophilic and Spatial Traps Stabilized Zn-Based Aqueous Battery. Nano Lett. 2022, 22, 4223–4231. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Zhou, H.; Zhou, X.; Du, Q.; Tang, J.; Yang, J. Stabilizing Zinc Anodes by a Cotton Towel Separator for Aqueous Zinc-Ion Batteries. ACS Sustain. Chem. Eng. 2022, 10, 8350–8359. [Google Scholar] [CrossRef]
- He, Q.; Yu, B.; Li, Z.; Zhao, Y. Density Functional Theory for Battery Materials. Energy Environ. Mater. 2019, 2, 264–279. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, J.P.; Fu, N.; Zhou, W.B.; Liu, B.; Deng, Q.; Wu, X.W. Comprehensive review on zinc-ion battery anode: Challenges and strategies. InfoMat 2022, 4, e12306. [Google Scholar] [CrossRef]
- Qin, R.; Wang, Y.; Yao, L.; Yang, L.; Zhao, Q.; Ding, S.; Liu, L.; Pan, F. Progress in interface structure and modification of zinc anode for aqueous batteries. Nano Energy 2022, 98, 107333. [Google Scholar] [CrossRef]
- Song, M.; Tan, H.; Chao, D.; Fan, H.J. Recent Advances in Zn-Ion Batteries. Adv. Funct. Mater. 2018, 28, 1802564. [Google Scholar] [CrossRef]
- Xu, C.; Li, B.; Du, H.; Kang, F. Energetic zinc ion chemistry: The rechargeable zinc ion battery. Angew. Chem. Int. Ed. 2012, 51, 933–935. [Google Scholar] [CrossRef]
- Kim, E.; Choi, I.; Nam, K.W. Metal–organic framework for dendrite-free anodes in aqueous rechargeable zinc batteries. Electrochim. Acta 2022, 425, 140648. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Liu, W.; Ni, X.; Qing, P.; Zhao, Q.; Wei, W.; Ji, X.; Ma, J.; Chen, L. Uniform and dendrite-free zinc deposition enabled by in situ formed AgZn3 for the zinc metal anode. J. Mater. Chem. A 2021, 9, 8452–8461. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.-G.; Hua, W.; Ren, L.; Sun, H.; Hou, Z.; Huyan, Y.; Cao, Y.; Wei, C.; Kang, F. Navigating fast and uniform zinc deposition via a versatile metal–organic complex interphase. Energy Environ. Sci. 2022, 15, 1872–1881. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Y.; Zhang, Y.; Li, Y.; Sun, R.; Wang, Z.; Wang, H. Strategies towards the challenges of zinc metal anode in rechargeable aqueous zinc ion batteries. Energy Storage Mater. 2021, 35, 19–46. [Google Scholar] [CrossRef]
- Yuksel, R.; Buyukcakir, O.; Seong, W.K.; Ruoff, R.S. Metal-Organic Framework Integrated Anodes for Aqueous Zinc-Ion Batteries. Adv. Energy Mater. 2020, 10, 1904215. [Google Scholar] [CrossRef]
- Zheng, J.; Zhao, Q.; Tang, T.; Yin, J.; Quilty, C.D.; Renderos, G.D.; Liu, X.; Deng, Y.; Wang, L.; Bock, D.C.; et al. Reversible epitaxial electrodeposition of metals in battery anodes. Science 2019, 366, 645–648. [Google Scholar] [CrossRef]
- Zheng, J.; Huang, Z.; Ming, F.; Zeng, Y.; Wei, B.; Jiang, Q.; Qi, Z.; Wang, Z.; Liang, H. Surface and Interface Engineering of Zn Anodes in Aqueous Rechargeable Zn-Ion Batteries. Small 2022, 18, e2200006. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, R.; Peng, C.; Chen, W.; Wu, T.; Hu, B.; Weng, W.; Yao, Y.; Zeng, J.; Chen, Z.; et al. Horizontally arranged zinc platelet electrodeposits modulated by fluorinated covalent organic framework film for high-rate and durable aqueous zinc ion batteries. Nat. Commun. 2021, 12, 6606. [Google Scholar] [CrossRef]
- Ho, V.C.; Lim, H.; Kim, M.J.; Mun, J. Improving the Performance of Aqueous Zinc-ion Batteries by Inhibiting Zinc Dendrite Growth: Recent Progress. Chem. Asian J. 2022, 17, e202200289. [Google Scholar] [CrossRef]
- Pu, S.D.; Gong, C.; Tang, Y.T.; Ning, Z.; Liu, J.; Zhang, S.; Yuan, Y.; Melvin, D.; Yang, S.; Pi, L.; et al. Achieving Ultrahigh-Rate Planar and Dendrite-Free Zinc Electroplating for Aqueous Zinc Battery Anodes. Adv. Mater. 2022, 34, e2202552. [Google Scholar] [CrossRef]
- Wang, N.; Wu, Z.; Long, Y.; Chen, D.; Geng, C.; Liu, X.; Han, D.; Zhang, J.; Tao, Y.; Yang, Q.-H. MXene-assisted polymer coating from aqueous monomer solution towards dendrite-free zinc anodes. J. Energy Chem. 2022, 73, 277–284. [Google Scholar] [CrossRef]
- Li, B.; Xue, J.; Han, C.; Liu, N.; Ma, K.; Zhang, R.; Wu, X.; Dai, L.; Wang, L.; He, Z. A hafnium oxide-coated dendrite-free zinc anode for rechargeable aqueous zinc-ion batteries. J. Colloid Interface Sci. 2021, 599, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, S.; Xu, L.; Momen, R.; Deng, W.; Hu, J.; Zou, G.; Hou, H.; Ji, X. High-Yield Carbon Dots Interlayer for Ultra-Stable Zinc Batteries. Adv. Energy Mater. 2022, 12, 2200665. [Google Scholar] [CrossRef]
- Zhang, S.; Ye, J.; Ao, H.; Zhang, M.; Li, X.; Xu, Z.; Hou, Z.; Qian, Y. In-situ formation of hierarchical solid-electrolyte interphase for ultra-long cycling of aqueous zinc-ion batteries. Nano Res. 2022, 16, 449–457. [Google Scholar] [CrossRef]
- Shin, J.; Lee, J.; Kim, Y.; Park, Y.; Kim, M.; Choi, J.W. Highly Reversible, Grain-Directed Zinc Deposition in Aqueous Zinc Ion Batteries. Adv. Energy Mater. 2021, 11, 2100676. [Google Scholar] [CrossRef]
- Luo, B.; Wang, Y.; Zheng, S.; Sun, L.; Duan, G.; Lu, J.; Huang, J.; Ye, Z. Ion pumping synergy with atomic anchoring for dendrite-free Zn anodes. Energy Storage Mater. 2022, 51, 610–619. [Google Scholar] [CrossRef]
- Xue, P.; Guo, C.; Li, L.; Li, H.; Luo, D.; Tan, L.; Chen, Z. A MOF-Derivative Decorated Hierarchical Porous Host Enabling Ultrahigh Rates and Superior Long-Term Cycling of Dendrite-Free Zn Metal Anodes. Adv. Mater. 2022, 34, e2110047. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Fu, H.; Wang, Z.-y.; Huang, Y.-d.; He, Z.-j.; Yan, C.; Mao, J.; Dai, K.; Zhang, X.-h.; Zheng, J.-C. A hydrophobic layer of amino acid enabling dendrite-free Zn anodes for aqueous zinc-ion batteries. J. Mater. Chem. A 2022, 10, 17501–17510. [Google Scholar] [CrossRef]
- Zhao, T.; Wu, H.; Wen, X.; Zhang, J.; Tang, H.; Deng, Y.; Liao, S.; Tian, X. Recent advances in MOFs/MOF derived nanomaterials toward high-efficiency aqueous zinc ion batteries. Coord. Chem. Rev. 2022, 468, 214642. [Google Scholar] [CrossRef]
- Zenaidee, M.A.; Loo, J.A. Seeing flying molecular elephants more clearly. Nat. Chem. 2022, 14, 482–483. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ying, Y.; Wang, G.; Hu, K.; Yuan, Y.D.; Ye, H.; Liu, Z.; Lee, J.Y.; Zhao, D. Covalent organic framework film protected zinc anode for highly stable rechargeable aqueous zinc-ion batteries. Energy Storage Mater. 2022, 48, 82–89. [Google Scholar] [CrossRef]
- Xue, R.; Kong, J.; Wu, Y.; Wang, Y.; Kong, X.; Gong, M.; Zhang, L.; Lin, X.; Wang, D. Highly reversible zinc metal anodes enabled by a three-dimensional silver host for aqueous batteries. J. Mater. Chem. A 2022, 10, 10043–10050. [Google Scholar] [CrossRef]
- Zeng, X.; Zhao, J.; Wan, Z.; Jiang, W.; Ling, M.; Yan, L.; Liang, C. Controllably Electrodepositing ZIF-8 Protective Layer for Highly Reversible Zinc Anode with Ultralong Lifespan. J. Phys. Chem. Lett. 2021, 12, 9055–9059. [Google Scholar] [CrossRef] [PubMed]
- So, S.; Ahn, Y.N.; Ko, J.; Kim, I.T.; Hur, J. Uniform and oriented zinc deposition induced by artificial Nb2O5 Layer for highly reversible Zn anode in aqueous zinc ion batteries. Energy Storage Mater. 2022, 52, 40–51. [Google Scholar] [CrossRef]
- Liu, C.; Luo, Z.; Deng, W.; Wei, W.; Chen, L.; Pan, A.; Ma, J.; Wang, C.; Zhu, L.; Xie, L.; et al. Liquid Alloy Interlayer for Aqueous Zinc-Ion Battery. ACS Energy Lett. 2021, 6, 675–683. [Google Scholar] [CrossRef]
- Liu, B.; Wang, S.; Wang, Z.; Lei, H.; Chen, Z.; Mai, W. Novel 3D Nanoporous Zn-Cu Alloy as Long-Life Anode toward High-Voltage Double Electrolyte Aqueous Zinc-Ion Batteries. Small 2020, 16, e2001323. [Google Scholar] [CrossRef]
- Li, B.; Yang, K.; Ma, J.; Shi, P.; Chen, L.; Chen, C.; Hong, X.; Cheng, X.; Tang, M.C.; He, Y.B.; et al. Multicomponent Copper-Zinc Alloy Layer Enabling Ultra-Stable Zinc Metal Anode of Aqueous Zn-ion Battery. Angew. Chem. Int. Ed. 2022, 61, e202212587. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Hu, Y.; Hu, K.; Lin, X.; Liu, X.; Reddy, K.M.; Xie, G.; Qiu, H.J. Highly Strengthened and Toughened Zn-Li-Mn Alloys as Long-Cycling Life and Dendrite-Free Zn Anode for Aqueous Zinc-Ion Batteries. Small 2022, 18, e2200787. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, B.; Zhang, T.; Li, T.; Shi, T.; Li, W.; Shen, T.; Huang, X.; Xu, J.; Zhang, X.; et al. Eliminating Dendrites and Side Reactions via a Multifunctional ZnSe Protective Layer toward Advanced Aqueous Zn Metal Batteries. Adv. Funct. Mater. 2021, 31, 2100186. [Google Scholar] [CrossRef]
- Qi, Z.; Xiong, T.; Chen, T.; Yu, C.; Zhang, M.; Yang, Y.; Deng, Z.; Xiao, H.; Lee, W.S.V.; Xue, J. Dendrite-Free Anodes Enabled by a Composite of a ZnAl Alloy with a Copper Mesh for High-Performing Aqueous Zinc-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 28129–28139. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, Q.; Wang, Y.; Liu, W.; Qing, P.; Chen, L. A dendrite-free Zn@CuxZny composite anode for rechargeable aqueous batteries. Electrochim. Acta 2021, 399, 139334. [Google Scholar] [CrossRef]
- Tribbia, M.; Zampardi, G.; La Mantia, F. Towards the commercialization of rechargeable aqueous zinc ion batteries: The challenge of the zinc electrodeposition at the anode. Curr. Opin. Electrochem. 2023, 38, 101230. [Google Scholar] [CrossRef]
- Liu, M.; Yao, L.; Ji, Y.; Zhang, M.; Gan, Y.; Cai, Y.; Li, H.; Zhao, W.; Zhao, Y.; Zou, Z.; et al. Nanoscale Ultrafine Zinc Metal Anodes for High Stability Aqueous Zinc Ion Batteries. Nano Lett. 2023, 23, 541–549. [Google Scholar] [CrossRef]
- Zheng, S.; Zhao, W.; Chen, J.; Zhao, X.; Pan, Z.; Yang, X. 2D Materials Boost Advanced Zn Anodes: Principles, Advances, and Challenges. Nano-Micro Lett. 2023, 15, 46. [Google Scholar] [CrossRef]
- Ge, J.; Zhang, Y.; Xie, Z.; Xie, H.; Chen, W.; Lu, B. Tailored ZnF2/ZnS-rich interphase for reversible aqueous Zn batteries. Nano Res. 2023. [Google Scholar] [CrossRef]
- Huang, Z.; Li, H.; Yang, Z.; Wang, H.; Ding, J.; Xu, L.; Tian, Y.; Mitlin, D.; Ding, J.; Hu, W. Nanosecond laser lithography enables concave-convex zinc metal battery anodes with ultrahigh areal capacity. Energy Storage Mater. 2022, 51, 273–285. [Google Scholar] [CrossRef]
- Li, T.C.; Lim, Y.V.; Xie, X.; Li, X.L.; Li, G.; Fang, D.; Li, Y.; Ang, Y.S.; Ang, L.K.; Yang, H.Y. ZnSe Modified Zinc Metal Anodes: Toward Enhanced Zincophilicity and Ionic Diffusion. Small 2021, 17, e2101728. [Google Scholar] [CrossRef]
- Meng, H.; Ran, Q.; Dai, T.Y.; Shi, H.; Zeng, S.P.; Zhu, Y.F.; Wen, Z.; Zhang, W.; Lang, X.Y.; Zheng, W.T.; et al. Surface-Alloyed Nanoporous Zinc as Reversible and Stable Anodes for High-Performance Aqueous Zinc-Ion Battery. Nano-Micro Lett. 2022, 14, 128. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, W.; Chen, Y.; Chen, L. Ultra-stable Zn metal batteries with dendrite-free Cu-Sn alloy induced high-quality composite Zn mesh. Chem. Eng. J. 2022, 450, 137979. [Google Scholar] [CrossRef]
- Wang, F.; Lu, H.; Li, H.; Li, J.; Wang, L.; Han, D.; Gao, J.; Geng, C.; Cui, C.; Zhang, Z.; et al. Demonstrating U-shaped zinc deposition with 2D metal-organic framework nanoarrays for dendrite-free zinc batteries. Energy Storage Mater. 2022, 50, 641–647. [Google Scholar] [CrossRef]
- Lei, L.; Chen, F.; Wu, Y.; Shen, J.; Wu, X.-J.; Wu, S.; Yuan, S. Surface coatings of two-dimensional metal-organic framework nanosheets enable stable zinc anodes. Sci. China Chem. 2022, 65, 2205–2213. [Google Scholar] [CrossRef]
- He, W.; Gu, T.; Xu, X.; Zuo, S.; Shen, J.; Liu, J.; Zhu, M. Uniform In Situ Grown ZIF-L Layer for Suppressing Hydrogen Evolution and Homogenizing Zn Deposition in Aqueous Zn-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14, 40031–40042. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Zuo, S.W.; Xiao, S.H.; Sun, P.X.; Li, N.W.; Chen, J.S.; Zhang, H.B.; Yu, L. Atomically Dispersed Cu in Zeolitic Imidazolate Framework Nanoflake Array for Dendrite-Free Zn Metal Anode. Small 2022, 18, e2203231. [Google Scholar] [CrossRef]
- Xu, Q.; Zhou, W.; Xin, T.; Zheng, Z.; Yuan, X.; Liu, J. Practical Zn anodes enabled by a Ti-MOF-derived coating for aqueous batteries. J. Mater. Chem. A 2022, 10, 12247–12257. [Google Scholar] [CrossRef]
- Xin, T.; Wang, Y.; Xu, Q.; Shang, J.; Yuan, X.; Song, W.; Liu, J. Forming an Amorphous ZnO Nanosheet Network by Confined Parasitic Reaction for Stabilizing Zn Anodes and Reducing Water Activity. ACS Appl. Energy Mater. 2022, 5, 2290–2299. [Google Scholar] [CrossRef]
- Long, Y.; Huang, X.; Li, Y.; Yi, M.; Hou, J.; Zhou, X.; Hu, Q.; Zheng, Q.; Lin, D. In-situ regulation of zinc metal surface for Dendrite-Free Zinc-ion hybrid supercapacitors. J. Colloid Interface Sci. 2022, 614, 205–213. [Google Scholar] [CrossRef]
- Deng, C.; Xie, X.; Han, J.; Lu, B.; Liang, S.; Zhou, J. Stabilization of Zn Metal Anode through Surface Reconstruction of a Cerium-Based Conversion Film. Adv. Funct. Mater. 2021, 31, 2103227. [Google Scholar] [CrossRef]
- Liu, C.; Li, Z.; Zhang, X.; Xu, W.; Chen, W.; Zhao, K.; Wang, Y.; Hong, S.; Wu, Q.; Li, M.C.; et al. Synergic Effect of Dendrite-Free and Zinc Gating in Lignin-Containing Cellulose Nanofibers-MXene Layer Enabling Long-Cycle-Life Zinc Metal Batteries. Adv. Sci. 2022, 9, e2202380. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xi, Q.; Li, Y.; Fu, H.; Hua, Y.; Shankar, E.G.; Kakarla, A.K.; Yu, J.S. Regulating Dendrite-Free Zinc Deposition by Red Phosphorous-Derived Artificial Protective Layer for Zinc Metal Batteries. Adv. Sci. 2022, 9, e2200155. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Yang, J.; Zhang, Z.; Wang, C.; Xu, J.; Wang, T. Silk Fibroin Coating Enables Dendrite-free Zinc Anode for Long-Life Aqueous Zinc-Ion Batteries. ChemSusChem 2022, 15, e202200656. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Xiong, T.; Yu, Z.G.; Meng, F.; Chen, B.; Xiao, H.; Xue, J. Suppressing zinc dendrite growth in aqueous battery via Zn–Al alloying with spatially confined zinc reservoirs. J. Power Sources 2023, 558, 232628. [Google Scholar] [CrossRef]
- Wang, Y.; Song, J.; Wong, W.Y.R. Constructing 2D Sandwich-like MOF/MXene Heterostructures for Durable and Fast Aqueous Zinc-Ion Batteries. Angew. Chem. Int. Ed. 2022, 62, e202218343. [Google Scholar] [CrossRef]
- Li, M.; Zhou, X.; He, X.; Lai, C.; Shan, B.; Wang, K.; Jiang, K. Controllable CF4 Plasma in Situ Modification Strategy Enables Durable Zinc Metal Anode. ACS Appl. Mater. Interfaces 2023, 15, 3017–3027. [Google Scholar] [CrossRef]
- Yang, X.; Lv, J.; Cheng, C.; Shi, Z.; Peng, J.; Chen, Z.; Lian, X.; Li, W.; Zou, Y.; Zhao, Y.; et al. Mosaic Nanocrystalline Graphene Skin Empowers Highly Reversible Zn Metal Anodes. Adv. Sci. 2022, 10, 2206077. [Google Scholar] [CrossRef]
- Luan, X.; Qi, L.; Zheng, Z.; Gao, Y.; Xue, Y.; Li, Y. Step by Step Induced Growth of Zinc-Metal Interface on Graphdiyne for Aqueous Zinc-Ion Batteries. Angew. Chem. Int. Ed. 2023, 62, e202215968. [Google Scholar] [CrossRef]
- Xin, W.; Xiao, J.; Li, J.; Zhang, L.; Peng, H.; Yan, Z.; Zhu, Z. Metal-organic frameworks with carboxyl functionalized channels as multifunctional ion-conductive interphase for highly reversible Zn anode. Energy Storage Mater. 2023, 56, 76–86. [Google Scholar] [CrossRef]
- Li, J.; Zheng, Z.; Yu, Z.; She, F.; Lai, L.; Prabowo, J.; Lv, W.; Wei, L.; Chen, Y. Stable Zn electrodes enabled by an ultra-thin Zn phosphate protective layer. J. Mater. Chem. A 2023, 11, 3051–3059. [Google Scholar] [CrossRef]
- Yuan, X.; Yi, J.; Li, C.; Zhao, Z.; Xiong, C. An artificial β-PVDF nanofiber layer for dendrite-free zinc anode in rechargeable aqueous batteries. J. Mater. Sci. 2023, 58, 1708–1720. [Google Scholar] [CrossRef]
- Xu, W.; Liao, X.; Xu, W.; Zhao, K.; Yao, G.; Wu, Q. Ion Selective and water-resistant Cellulose Nanofiber/MXene Membrane Enabled Cycling Zn Anode at High Currents. Adv. Energy Mater. 2023, 2300283. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Zhou, Y.; Qi, H.; Li, X.; Wei, C.; Zou, T.; Wang, W.; Yang, Z. In-situ construction of multifunctional protection interface for ultra-stable zinc anodes. J. Alloys Compd. 2023, 947, 169510. [Google Scholar] [CrossRef]
- Zhou, S.; Su, Y.; Li, G.; Wang, X.; Liu, D.; Zhu, G. Zincophilic polyurethane-based porous film enables dendrite-free zinc anode for reversible aqueous zinc-based batteries. Colloids Surf. A Physicochem. Eng. Asp. 2023, 661, 130960. [Google Scholar] [CrossRef]
- Zhang, L.; Hou, Y. Comprehensive Analyses of Aqueous Zn Metal Batteries: Characterization Methods, Simulations, and Theoretical Calculations. Adv. Energy Mater. 2021, 11, 2003823. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, S.; Zhang, Q.; Song, Y.; Chang, N.; Pan, Y.; Zhang, H.; Li, X. Dendrite-Free Zinc Deposition Induced by Tin-Modified Multifunctional 3D Host for Stable Zinc-Based Flow Battery. Adv. Mater. 2020, 32, e1906803. [Google Scholar] [CrossRef]
- Nie, X.; Miao, L.; Yuan, W.; Ma, G.; Di, S.; Wang, Y.; Shen, S.; Zhang, N. Cholinium Cations Enable Highly Compact and Dendrite-Free Zn Metal Anodes in Aqueous Electrolytes. Adv. Funct. Mater. 2022, 32, 2203905. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, D.; Ouyang, K.; Yang, M.; Shen, S.; Wang, Y.; Mi, H.; Sun, L.; He, C.; Zhang, P. A Multifunctional Anti-Proton Electrolyte for High-Rate and Super-Stable Aqueous Zn-Vanadium Oxide Battery. Nano-Micro Lett. 2022, 14, 154. [Google Scholar] [CrossRef]
- Yan, M.; Dong, N.; Zhao, X.; Sun, Y.; Pan, H. Tailoring the Stability and Kinetics of Zn Anodes through Trace Organic Polymer Additives in Dilute Aqueous Electrolyte. ACS Energy Lett. 2021, 6, 3236–3243. [Google Scholar] [CrossRef]
- Qiu, M.; Sun, P.; Wang, Y.; Ma, L.; Zhi, C.; Mai, W. Anion-Trap Engineering toward Remarkable Crystallographic Reorientation and Efficient Cation Migration of Zn Ion Batteries. Angew. Chem. Int. Ed. 2022, 61, e202210979. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, F.; Yang, F.; Liu, X.; Yu, Y.; Zheng, D.; Lu, X. Unshared Pair Electrons of Zincophilic Lewis Base Enable Long-life Zn Anodes under “Three High” Conditions. Angew. Chem. Int. Ed. 2022, 61, e202208051. [Google Scholar] [CrossRef]
- Zhao, M.; Rong, J.; Huo, F.; Lv, Y.; Yue, B.; Xiao, Y.; Chen, Y.; Hou, G.; Qiu, J.; Chen, S. Semi-Immobilized Ionic Liquid Regulator with Fast Kinetics toward Highly Stable Zinc Anode under −35 to 60 °C. Adv. Mater. 2022, 34, e2203153. [Google Scholar] [CrossRef] [PubMed]
- Yao, N.; Chen, X.; Fu, Z.H.; Zhang, Q. Applying Classical, Ab Initio, and Machine-Learning Molecular Dynamics Simulations to the Liquid Electrolyte for Rechargeable Batteries. Chem. Rev. 2022, 122, 10970–11021. [Google Scholar] [CrossRef] [PubMed]





| Modification Strategy | Performance | Refs. | ||||||
|---|---|---|---|---|---|---|---|---|
| Full Battery | Half Battery | |||||||
| Electrolyte | Current Density | Capacity Retention Rate | Electrolyte | Area Specific Current | Area Specific Capacity | Time | ||
| Ti3C2Tx MXene | ZnSO4 + Na2SO4 | 16 A g−1 | 85.0% after 2600 cycles | ZnSO4 | 0.20 mA cm−2 | 0.20 mAh cm−2 | 2000 h | [53] |
| Zn@CDs | ZnSO4 | 5 A g−1 | 81.6% after 500 cycles | ZnSO4 | 1.00 mA cm−2 | 1.00 mAh cm−2 | 3000 h | [55] |
| Zn@InF3 | ZnSO4 + MnSO4 | 3C | 80.0% after 1000 cycles | ZnSO4 | 0.50 mA cm−2 | 0.50 mAh cm−2 | 6000 h | [56] |
| TCNQ@Zn | \ | \ | \ | ZnSO4 | 1.00 mA cm−2 | 1.00 mAh cm−2 | 2000 h | [58] |
| 3D-ZGC@Zn | ZnSO4 | 2 A g−1 | 80.8% after 6000 cycles | ZnSO4 | 10.0 mA cm−2 | 1.00 mAh cm−2 | 400 h | [59] |
| Cys-Zn@Zn | ZnSO4 | 1 A g−1 | 78.7% after 1000 cycles | ZnSO4 | 2.00 mA cm−2 | 2.00 mAh cm−2 | 2000 h | [60] |
| TpPa-SO3H@Zn | ZnSO4 | 5 mA cm−2 | 94.7% after 1000 cycles | ZnSO4 | 1.00 mA cm−2 | 5.00 mAh cm−2 | 1000 h | [63] |
| Zn@ZIF-8 | ZnSO4 + MnSO4 | 0.5 A g−1 | 76.0% after 250 cycles | ZnSO4 | 10.0 mA cm−2 | 1.00 mAh cm−2 | 5000 h | [65] |
| Nb2O5@Zn | Zn(OTf)2 | 2 A g−1 | 78.6% after 500 cycles | ZnSO4 | 1.00 mA cm−2 | 0.50 mAh cm−2 | 1000 h | [66] |
| GaIn@Zn | ZnSO4 | \ | \ | ZnSO4 | 0.25 mA cm−2 | 0.05 mAh cm−2 | 2100 h | [67] |
| Cu-Zn@Zn | Zn(OTf)2 | 2 A g−1 | 88.2% after 600 cycles | Zn(OTf)2 | 1.00 mA cm−2 | 1.00 mAh cm−2 | 5496 h | [69] |
| ZnLiMn | ZnSO4 + MnSO4 | 1C | 96.0% after 400 cycles | ZnSO4 | 1.00 mA cm−2 | 1.00 mAh cm−2 | 1000 h | [70] |
| Zn@ZnSe | \ | \ | \ | ZnSO4 | 1.00 mA cm−2 | 1.00 mAh cm−2 | 1500 h | [71] |
| ZnAl@Cu-mesh | Zn(OTf)2 | 2 A g−1 | 95.0% after 2000 cycles | Zn(OTf)2 | 0.50 mA cm−2 | 0.25 mAh cm−2 | 240 h | [72] |
| Zn@ZnxCuy | \ | \ | \ | ZnSO4 | 0.25 mA cm−2 | 0.25 mAh cm−2 | 3800 h | [73] |
| LLP@ Zn-foil | ZnSO4 + MnSO4 | 1 A g−1 | 75.0% after 500 cycles | ZnSO4 | 0.50 mA cm−2 | 0.50 mAh cm−2 | 1100 h | [78] |
| ZnSe@Zn | \ | \ | \ | ZnSO4 | 1.00 mA cm−2 | 0.50 mAh cm−2 | 1700 h | [79] |
| ZnxCuy@Zn | Zn(OTf)2 + SDS + Mn(OTf)2 | 1 A g−1 | 84.0% after 800 cycles | Zn(OTf)2 | 0.50 mA cm−2 | 0.50 mAh cm−2 | 1900 h | [80] |
| Zn@Cu-Sn@SSM | Zn(OTf)2 | 2 A g−1 | 84.0% after 1000 cycles | ZnSO4 | 10.0 mA cm−2 | 3.00 mAh cm−2 | 1050 h | [81] |
| Zn-TCPP@Zn | Zn(OTf)2 | 4 A g−1 | 82.5% after 1000 cycles | ZnSO4 | 0.20 mA cm−2 | 0.20 mAh cm−2 | 2600 h | [82] |
| UiO-67-2D | ZnSO4 | 2 A g−1 | 81.0% after 1500 cycles | ZnSO4 | 3.00 mA cm−2 | 0.50 mAh cm−2 | 800 h | [83] |
| Zn@ZIF-L | Zn(OTf)2 | 0.5C | 84.9% after 250 cycles | Zn(OTf)2 | 0.25 mA cm−2 | 0.25 mAh cm−2 | 800 h | [84] |
| CuZIF-L@TM/Zn | \ | \ | \ | ZnSO4 | 1.00 mA cm−2 | 1.00 mAh cm−2 | 1100 h | [85] |
| Zn@TiO2/NC | ZnSO4 | 0.5 A g−1 | 75.0% after 1000 cycles | ZnSO4 | 5.00 mA cm−2 | 1.00 mAh cm−2 | 1100 h | [86] |
| Zn@ZnS/NC | \ | \ | \ | ZnSO4 | 0.20 mA cm−2 | 0.50 mAh cm−2 | 2000 h | [87] |
| ZIF-8@Zn | ZnSO4 | 5.0 A g−1 | 96% after 13,000 cycles | ZnSO4 | 2.00 mA cm−2 | 2.00 mAh cm−2 | 800 h | [88] |
| Zn@CCF | \ | \ | \ | ZnSO4 | 4.40 mA cm−2 | 4.40 mAh cm−2 | 1200 h | [89] |
| Zn@LM | ZnSO4 + MnSO4 | 1 A g−1 | 90.0% after 1000 cycles | ZnSO4 | 0.50 mA cm−2 | 0.50 mAh cm−2 | 800 h | [90] |
| Zn@ZnP-NC | \ | \ | \ | ZnSO4 | 2.00 mA cm−2 | 1.00 mAh cm−2 | 1100 h | [91] |
| Silk II-SF@Zn | \ | \ | \ | ZnSO4 | 10.0 mA cm−2 | 10.0 mAh cm−2 | 3300 h | [92] |
| ZnAl | Zn(OTf)2 | 5 A g−1 | 95.0% after 1000 cycles | Zn(OTf)2 | 0.50 mA cm−2 | 0.25 mAh cm−2 | 300 h | [93] |
| Zn@Cu-HHTP@MX | \ | 4 A g−1 | 92.5% after 1000 cycles | \ | \ | \ | \ | [94] |
| Zn@ZnF2 | \ | \ | \ | ZnSO4 | 1.00 mA cm−2 | 1.00 mAh cm−2 | 2500 h | [95] |
| NOC@Zn | \ | \ | \ | ZnSO4 | 1.00 mA cm−2 | 1.00 mAh cm−2 | 3040 h | [96] |
| Zn@GDY | \ | \ | \ | ZnSO4 | 10.0 mA cm−2 | 1.00 mAh cm−2 | 16,000 h | [97] |
| Zn@UiO-66-(COOH)2 | ZnSO4 | 1 A g−1 | 91.0% after 2400 cycles | ZnSO4 | 2.00 mA cm−2 | 2.00 mAh cm−2 | 2800 h | [98] |
| PPA-Zn | ZnSO4 + MnSO4 | 1 A g−1 | 78.0% after 500 cycles | ZnSO4 | 2.00 mA cm−2 | 1.00 mAh cm−2 | 6500 h | [99] |
| β-PVDF/BMI@Zn | \ | \ | \ | ZnSO4 | 2.00 mA cm−2 | 0.25 mAh cm−2 | 1000 h | [100] |
| CNF/MXene@Zn | Zn(OTf)2 | 2 A g−1 | 93.2% after 500 cycles | Zn(OTf)2 | 1.00 mA cm−2 | \ | 2800 h | [101] |
| 20F-Zn | ZnSO4 + MnSO4 | 2 A g−1 | 68.4% after 500 cycles | ZnSO4 | 2.00 mA cm−2 | 1.00 mAh cm−2 | 1275 h | [102] |
| PUZ-1@Zn | Zn(OTf)2 | 1 A g−1 | 70.3% after 3400 cycles | Zn(OTf)2 | 0.50 mA cm−2 | 0.50 mAh cm−2 | 1800 h | [103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, K.; Wang, D.; Yu, Y. Progress and Prospect of Zn Anode Modification in Aqueous Zinc-Ion Batteries: Experimental and Theoretical Aspects. Molecules 2023, 28, 2721. https://doi.org/10.3390/molecules28062721
Feng K, Wang D, Yu Y. Progress and Prospect of Zn Anode Modification in Aqueous Zinc-Ion Batteries: Experimental and Theoretical Aspects. Molecules. 2023; 28(6):2721. https://doi.org/10.3390/molecules28062721
Chicago/Turabian StyleFeng, Kaiyong, Dongxu Wang, and Yingjian Yu. 2023. "Progress and Prospect of Zn Anode Modification in Aqueous Zinc-Ion Batteries: Experimental and Theoretical Aspects" Molecules 28, no. 6: 2721. https://doi.org/10.3390/molecules28062721
APA StyleFeng, K., Wang, D., & Yu, Y. (2023). Progress and Prospect of Zn Anode Modification in Aqueous Zinc-Ion Batteries: Experimental and Theoretical Aspects. Molecules, 28(6), 2721. https://doi.org/10.3390/molecules28062721







