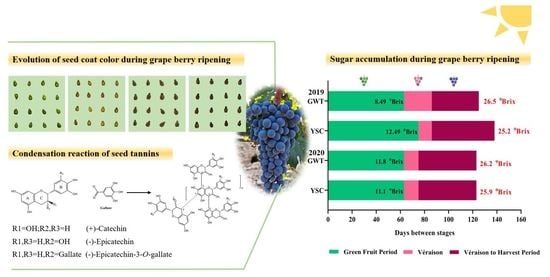
Evolution of Seed-Soluble and Insoluble Tannins during Grape Berry Maturation
Abstract
1. Introduction
2. Results
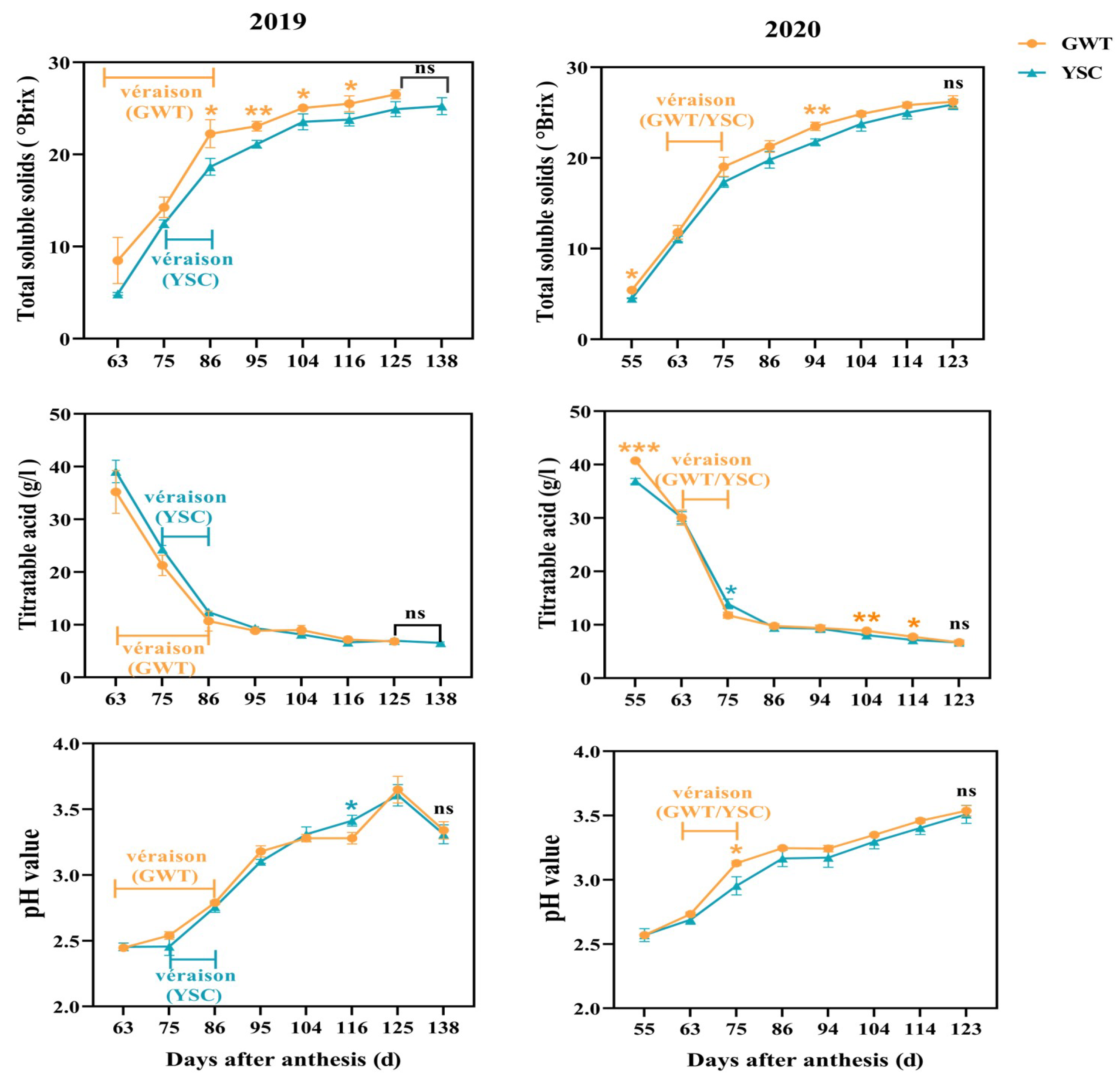
2.1. Comparison of Grape Berry Ripening Process
2.2. Change of Seed Coat Color
2.3. Comparison of Soluble and Insoluble CT Contents
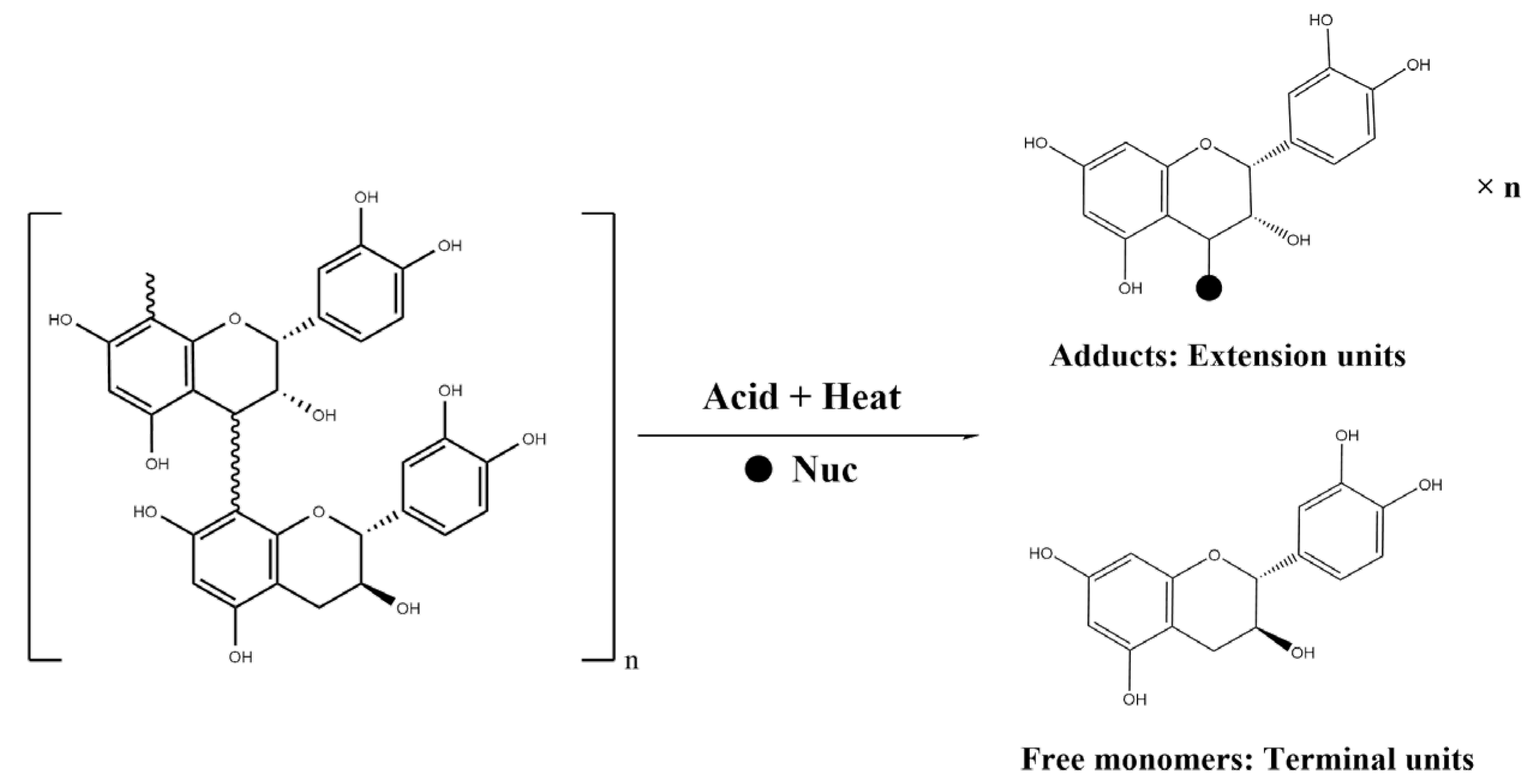
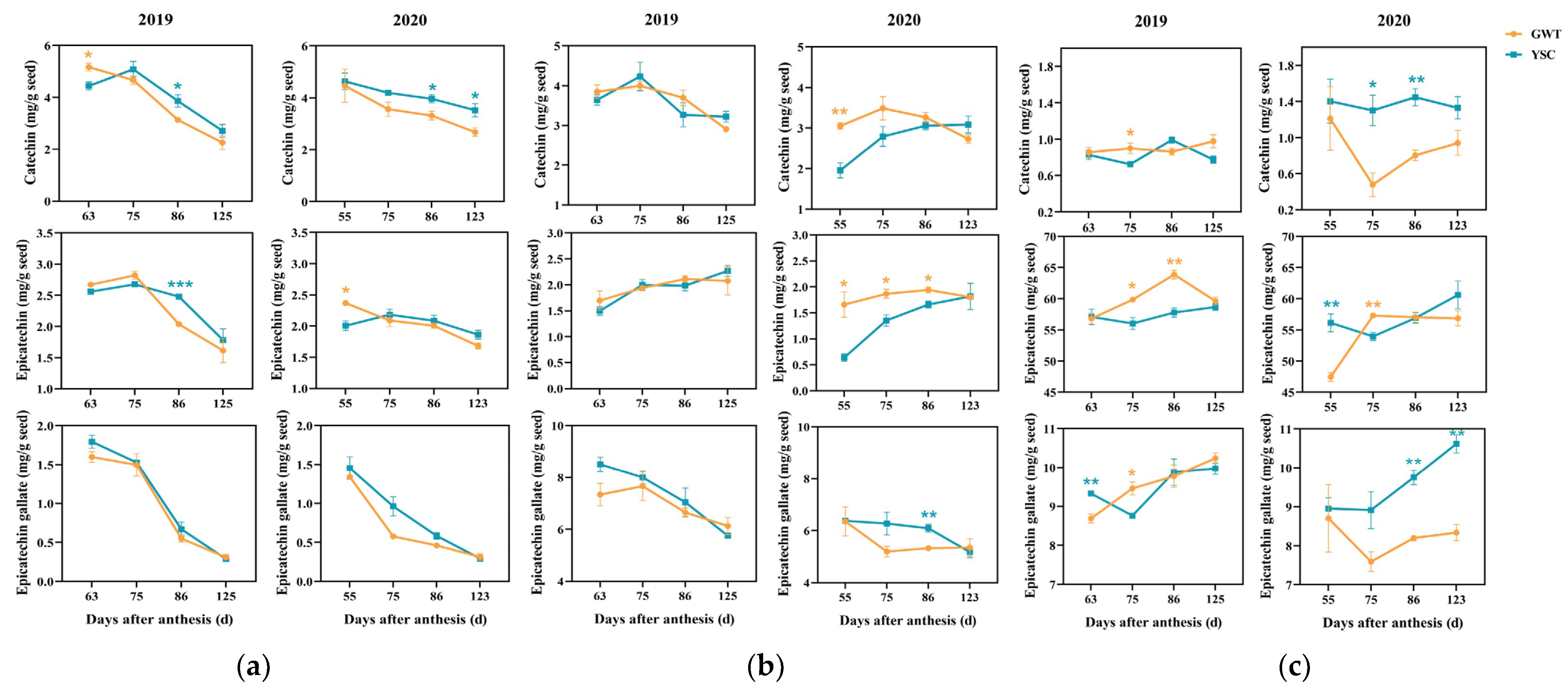
2.4. Comparison of Soluble and Insoluble CT Flavan-3-ol Units
2.4.1. Free Flavan-3-ol Monomers in the Seeds
2.4.2. Flavan-3-ol Composition Units of Seed-Soluble CT
2.4.3. Flavan-3-ol Composition Units of Insoluble CT
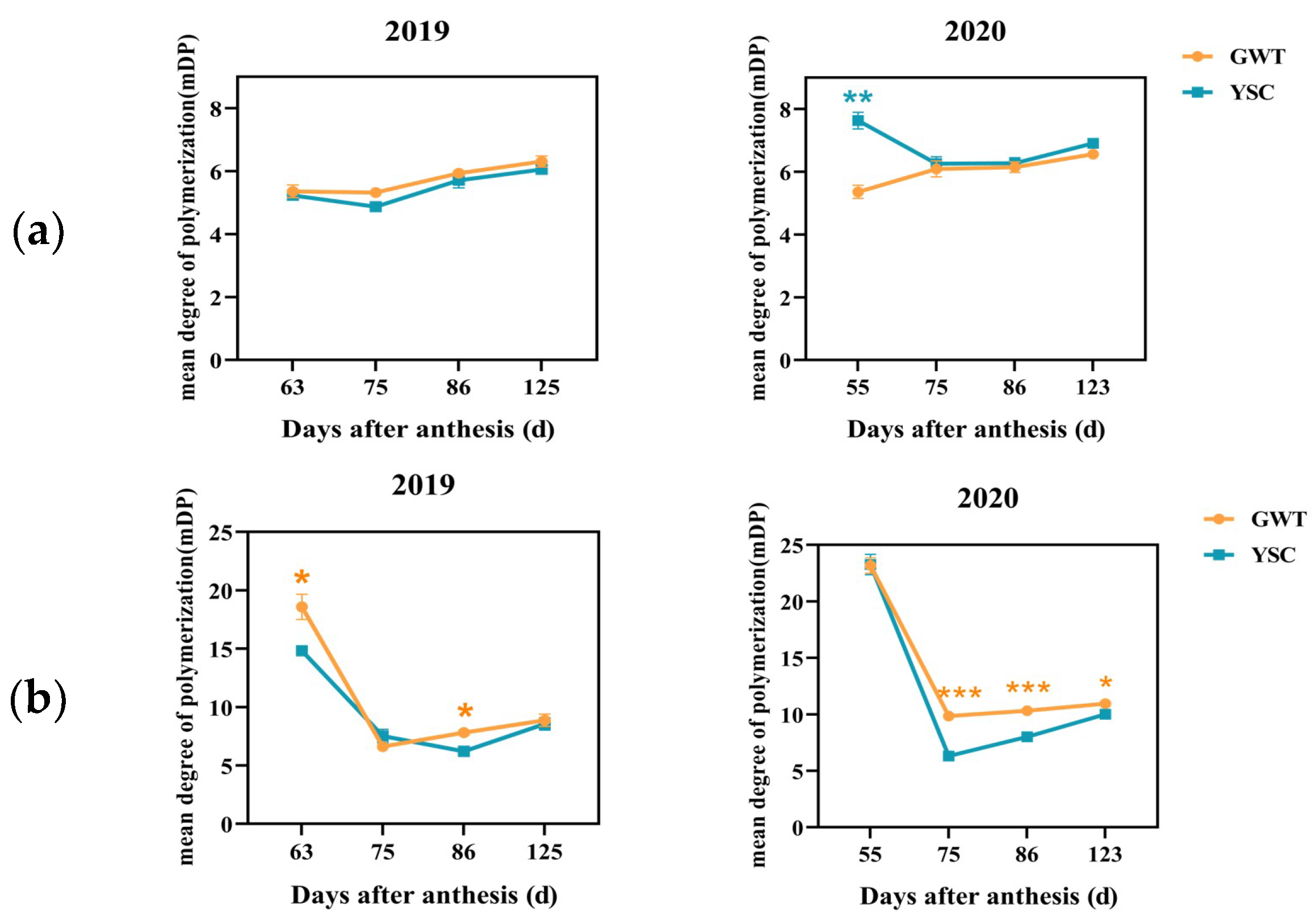
2.4.4. Mean Degree of Polymerization of Soluble and Insoluble CT
2.5. Correlation Analysis between Seed CT and Ripening Parameter
3. Discussion
4. Materials and Methods
4.1. Information of Vineyards and Sampling
4.2. Methods
4.2.1. Determination of Total Soluble Solids, Titratable Acid, and pH in Grape Juice
4.2.2. Seed Coat Color Determination
4.2.3. Extraction and Determination of Soluble and Insoluble CT
4.2.4. Determination of Flavan-3-ol Units
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Egea, M.B.; Pierce, G.; Luo, T.; Becraft, A.; Shay, N. Intake of an enological oak tannin powder alters hepatic gene express patterns indicative of a reduction of inflammation in male mice fed an obesogenic diet. Food Funct. 2022, 13, 9754–9760. [Google Scholar] [CrossRef]
- Wei, X.; Ju, Y.; Ma, T.; Zhang, J.; Fang, Y.; Sun, X. New Perspectives on the Biosynthesis, Transportation, Astringency Perception and Detection Methods of Grape Proanthocyanidins. Crit. Rev. Food Sci. 2021, 61, 2372–2398. [Google Scholar] [CrossRef]
- Gupta, M.; Dey, S.; Marbaniang, D.; Pal, P.; Ray, S.; Mazumder, B. Grape Seed Extract: Having a Potential Health Benefits. J. Food Sci. Technol. 2020, 57, 1205–1215. [Google Scholar] [CrossRef]
- Unusan, N. Proanthocyanidins in Grape Seeds: An Updated Review of Their Health Benefits and Potential Uses in the Food Industry. J. Funct. Foods 2020, 67, 103861. [Google Scholar] [CrossRef]
- Padilla-González, G.F.; Grosskopf, E.; Sadgrove, N.J.; Simmonds, M.S.J. Chemical Diversity of Flavan-3-Ols in Grape Seeds: Modulating Factors and Quality Requirements. Plants 2022, 11, 809. [Google Scholar] [CrossRef] [PubMed]
- Rousserie, P.; Rabot, A.; Geny-Denis, L. From Flavanols Biosynthesis to Wine Tannins: What Place for Grape Seeds? J. Agric. Food. Chem. 2019, 67, 1325–1343. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, M.D.; Smith, P.A. Tannin Quantification in Red Grapes and Wine: Comparison of Polysaccharide- and Protein-Based Tannin Precipitation Techniques and Their Ability to Model Wine Astringency. J. Agric. Food. Chem. 2008, 56, 5528–5537. [Google Scholar] [CrossRef] [PubMed]
- Mcrae, J.M.; Kennedy, J.A. Wine and Grape Tannin Interactions with Salivary Proteins and Their Impact on Astringency: A Review of Current Research. Molecules 2011, 16, 2348–2364. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Peel, G.J.; Wright, E.; Wang, Z.; Dixon, R.A. Early Steps in Proanthocyanidin Biosynthesis in the Model Legume Medicago Truncatula. Plant Physiol. 2007, 145, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J. Flavonoid Transport Mechanisms: How to Go, and with Whom. Trends Plant Sci. 2015, 20, 576–585. [Google Scholar] [CrossRef]
- González-Muñoz, B.; Garrido-Vargas, F.; Pavez, C.; Osorio, F.; Chen, J.; Bordeu, E.; O’brien, J.A.; Brossard, N. Wine Astringency: More Than Just Tannin–Protein Interactions. J. Sci. Food Agric. 2022, 102, 1771–1781. [Google Scholar] [CrossRef] [PubMed]
- Ćurko, N.; Kovačević Ganić, K.; Gracin, L.; Đapić, M.; Jourdes, M.; Teissedre, P.L. Characterization of Seed and Skin Polyphenolic Extracts of Two Red Grape Cultivars Grown in Croatia and Their Sensory Perception in a Wine Model Medium. Food Chem. 2014, 145, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Someya, S.; Hu, F.; Tanokura, M. Comparative Study of Catechin Compositions in Five Japanese Persimmons (Diospyros Kaki). Food Chem. 2005, 93, 149–152. [Google Scholar] [CrossRef]
- Vidal, S.; Francis, L.; Guyot, S.; Marnet, N.; Kwiatkowski, M.; Gawel, R.; Cheynier, V.; Waters, E.J. The Mouth-Feel Properties of Grape and Apple Proanthocyanidins in a Wine-Like Medium. J. Sci. Food Agric. 2003, 83, 564–573. [Google Scholar] [CrossRef]
- Fredes, C.; Von Bennewitz, E.; Holzapfel, E.; Saavedra, F. Relation between Seed Appearance and Phenolic Maturity: A Case Study Using Grapes Cv. Carmenere. CHIL J. Agr. Res. 2010, 70, 381–389. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Troup, G.J.; Pilbrow, J.R.; Hutton, D.R.; Hewitt, D.; Hunter, C.R.; Ristic, R.; Iland, P.G.; Jones, G.P. Development of Seed Polyphenols in Berries from Vitis vinifera L. cv. Shiraz. Aust. J. Grape Wine Res. 2000, 6, 244–254. [Google Scholar] [CrossRef]
- Mucalo, A.; Maletic, E.; Goran, Z. Extended Harvest Date Alter Flavonoid Composition and Chromatic Characteristics of Plavac Mali (Vitis vinifera L.) Grape Berries. Foods 2020, 9, 1155. [Google Scholar] [CrossRef] [PubMed]
- Obreque-Slier, E.; López-Solís, R.; Castro-Ulloa, L.; Romero-Díaz, C.; Peña-Neira, Á. Phenolic Composition and Physicochemical Parameters of Carménère, Cabernet Sauvignon, Merlot and Cabernet Franc Grape Seeds (Vitis vinifera L.) During Ripening. LWT-Food Sci. Technol. 2012, 48, 134–141. [Google Scholar] [CrossRef]
- Bordiga, M.; Travaglia, F.; Locatelli, M.; Coïsson, J.D.; Arlorio, M. Characterisation of Polymeric Skin and Seed Proanthocyanidins During Ripening in Six Vitis vinifera L. cv. Food Chem. 2011, 127, 180–187. [Google Scholar] [CrossRef]
- Hanlin, R.L.; Downey, M.O. Condensed Tannin Accumulation and Composition in Skin of Shiraz and Cabernet Sauvignon Grapes During Berry Development. Am. J. Enol. Vitic. 2009, 60, 13–23. [Google Scholar] [CrossRef]
- Conde, C.; Silva, P.; Fontes, N.; Dias, A.C.P.; Tavares, R.M.; Sousa, M.J.; Agasse, A.; Delrot, S.; Gerós, H. Biochemical changes throughout grape berry development and fruit and wine quality. Food (Glob. Sci. Books) 2007, 1, 1–22. [Google Scholar]
- Schelezki, O.J.; Smith, P.A.; Hranilovic, A.; Bindon, K.A.; Jeffery, D.W. Comparison of consecutive harvests versus blending treatments to produce lower alcohol wines from Cabernet Sauvignon grapes: Impact on polysaccharide and tannin content and composition. Food Chem. 2018, 244, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Arias Pérez, I.; Ferrero, S.; Sáenz-Navajas, M.-P.; Fernández, P.; Blanca, L.; Astraín, J.; Barón, C.; Ferreira, V.; Escudero, A. Some clues about the changes in wine aroma composition associated to the maturation of “neutral” grapes. Food Chem. 2020, 320, 126610. [Google Scholar] [CrossRef] [PubMed]
- Symma, N.; Hensel, A. Advanced Analysis of Oligomeric Proanthocyanidins: Latest Approaches in Liquid Chromatography and Mass Spectrometry Based Analysis. Phytochem. Rev. 2021, 201, 12557. [Google Scholar] [CrossRef]
- Ristic, R.; Iland, P.G. Relationships between Seed and Berry Development of Vitis vinifera L. cv Shiraz: Developmental Changes in Seed Morphology and Phenolic Composition. Aust. J. Grape Wine Res. 2005, 11, 43–58. [Google Scholar]
- Avila, F.; Mora, M.; Fredes, C. A Method to Estimate Grape Phenolic Maturity Based on Seed Images. Comput. Electron. Agr. 2014, 101, 76–83. [Google Scholar] [CrossRef]
- Bastante, M.; Rodríguez-Pulido, F.; Heredia, F.J.; González-Miret, M. Assessment of Sensory and Texture Profiles of Grape Seeds at Real Maturity Stages Using Image Analysis. Foods 2021, 10, 1098. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.A.; Melgosa, M.; Pérez, M.M.; Hita, E.; Negueruela, A.I. Note. Visual and Instrumental Color Evaluation in Red Wines. Food Sci. Technol. Int. 2001, 7, 439–444. [Google Scholar] [CrossRef]
- Downey, M.O.; Harvey, J.S.; Robinson, S.P. Analysis of Tannins in Seeds and Skins of Shiraz Grapes Throughout Berry Development. Aust. J. Grape Wine Res. 2003, 9, 15–27. [Google Scholar] [CrossRef]
- Liu, A.; Wang, J.; Yao, X.; Xia, N.; Sun, Q.; Duan, C.; Pan, Q. Dissecting Seed Proanthocyandin Composition and Accumulation under Different Berry Ripening Process in Wine Grapes. Horticulturae 2023, 9, 61. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Jones, G.P. Analysis of Proanthocyanidin Cleavage Products Following Acid-Catalysis in the Presence of Excess Phloroglucinol. J. Agric. Food. Chem. 2001, 49, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Harbertson, J.F.; Kennedy, J.A.; Adams, D.O. Tannin in Skins and Seeds of Cabernet Sauvignon, Syrah, and Pinot Noir Berries During Ripening. Am. J. Enol. Vitic. 2002, 53, 54–59. [Google Scholar] [CrossRef]
- Pastor Del Rio, J.L.; Kennedy, J.A. Development of Proanthocyanidins in Vitis Vinifera L. cv. Pinot Noir Grapes and Extraction into Wine. Am. J. Enol. Vitic. 2006, 57, 125–132. [Google Scholar] [CrossRef]
- Rustioni, L.; Failla, O. Grape Seed Ripening Evaluation by Ortho-Diphenol Quantification. Ital. J. Food Sci. 2016, 28, 510–516. [Google Scholar]
- Vanderweide, J.; Forte, A.; Peterlunger, E.; Sivilotti, P.; Medina-Meza, I.G.; Falchi, R.; Rustioni, L.; Sabbatini, P. Increase in Seed Tannin Extractability and Oxidation Using a Freeze-Thaw Treatment in Cool-Climate Grown Red (Vitis Vinifera L.) Cultivars. Food Chem. 2020, 308, 125571. [Google Scholar] [CrossRef]
- Gombau, J.; Pons-Mercadé, P.; Conde, M.; Asbiro, L.; Pascual, O.; Gómez-Alonso, S.; García-Romero, E.; Miquel Canals, J.; Hermosín-Gutiérrez, I.; Zamora, F. Influence of Grape Seeds on Wine Composition and Astringency of Tempranillo, Garnacha, Merlot and Cabernet Sauvignon Wines. Food Sci. Nutr. 2020, 8, 3442–3455. [Google Scholar] [CrossRef]
- Peleg, H.; Gacon, K.; Schlich, P.; Noble, A.C. Bitterness and Astringency of Flavan-3-Ol Monomers, Dimers and Trimers. J. Sci. Food Agric. 1999, 79, 1123–1128. [Google Scholar] [CrossRef]
- Cheynier, V.; Duenas-Paton, M.; Salas, E.; Maury, C.; Souquet, J.-M.; Sarni-Manchado, P.; Fulcrand, H. Structure and Properties of Wine Pigments and Tannins. Am. J. Enol. Vitic. 2006, 57, 298–305. [Google Scholar] [CrossRef]
- Gawel, R.; Francis, L.; Waters, E.J. Statistical Correlations between the in-Mouth Textural Characteristics and the Chemical Composition of Shiraz Wines. J. Agric. Food Chem. 2007, 55, 2683–2687. [Google Scholar] [CrossRef]
- Poncet-Legrand, C.; Edelmann, A.; Putaux, J.L.; Cartalade, D.; Sarni-Manchado, P.; Vernhet, A. Poly(L-Proline) Interactions with Flavan-3-Ols Units: Influence of the Molecular Structure and the Polyphenol/Protein Ratio. Food Hydrocoll. 2006, 20, 687–697. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Guyot, S.; Renard, C.M.G.C. Non-Covalent Interaction between Procyanidins and Apple Cell Wall Material: Part I. Effect of Some Environmental Parameters. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2004, 1672, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Gallego, R.; García-Marino, M.; Miguel Hernández-Hierro, J.; Rivas-Gonzalo, J.C.; Teresa Escribano-Bailón, M. Statistical Correlation between Flavanolic Composition, Colour and Sensorial Parameters in Grape Seed During Ripening. Anal. Chim. Acta 2010, 660, 22–28. [Google Scholar] [CrossRef]
- Charlton, A.J.; Baxter, N.J.; Khan, M.L.; Moir, A.J.G.; Haslam, E.; Davies, A.P.; Williamson, M.P. Polyphenol/Peptide Binding and Precipitation. J. Agric. Food. Chem. 2002, 50, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Gawel, R.; Oberholster, A.; Francis, I.L. A ‘Mouth-Feel Wheel’: Terminology for Communicating the Mouth-Feel Characteristics of Red Wine. Aust. J. Grape Wine Res. 2000, 6, 203–207. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Zheng, W.; Martínez De Toda, F. Strategies in Vineyard Establishment to Face Global Warming in Viticulture: A Mini Review. J. Sci. Food Agric. 2021, 101, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Torregrosa, L.; Bigard, A.; Doligez, A.; Lecourieux, D.; Rienth, M.; Luchaire, N.; Pieri, P.; Chatbanyong, R.; Shahood, R.; Farnos, M.; et al. Developmental, Molecular and Genetic Studies on Grapevine Response to Temperature Open Breeding Strategies for Adaptation to Warming. OENO One 2017, 51, 155–165. [Google Scholar] [CrossRef]
- Drappier, J.; Thibon, C.; Rabot, A.; Geny-Denis, L. Relationship between Wine Composition and Temperature: Impact on Bordeaux Wine Typicity in the Context of Global Warming. Crit. Rev. Food Sci. Nutr. 2019, 59, 14–30. [Google Scholar] [CrossRef]
- Rodríguez-Pulido, F.; González-Miret, M.L.; Heredia, F.J. Application of Imaging Techniques for the Evaluation of Phenolic Maturity of Grape Seeds. Opt. Pura Apl. 2017, 50, 1–11. [Google Scholar] [CrossRef]
- Brill, M.H. Acquisition and Reproduction of Color Images: Colorimetric and Multispectral Approaches. Color Res. Appl. 2002, 27, 304–305. [Google Scholar] [CrossRef]

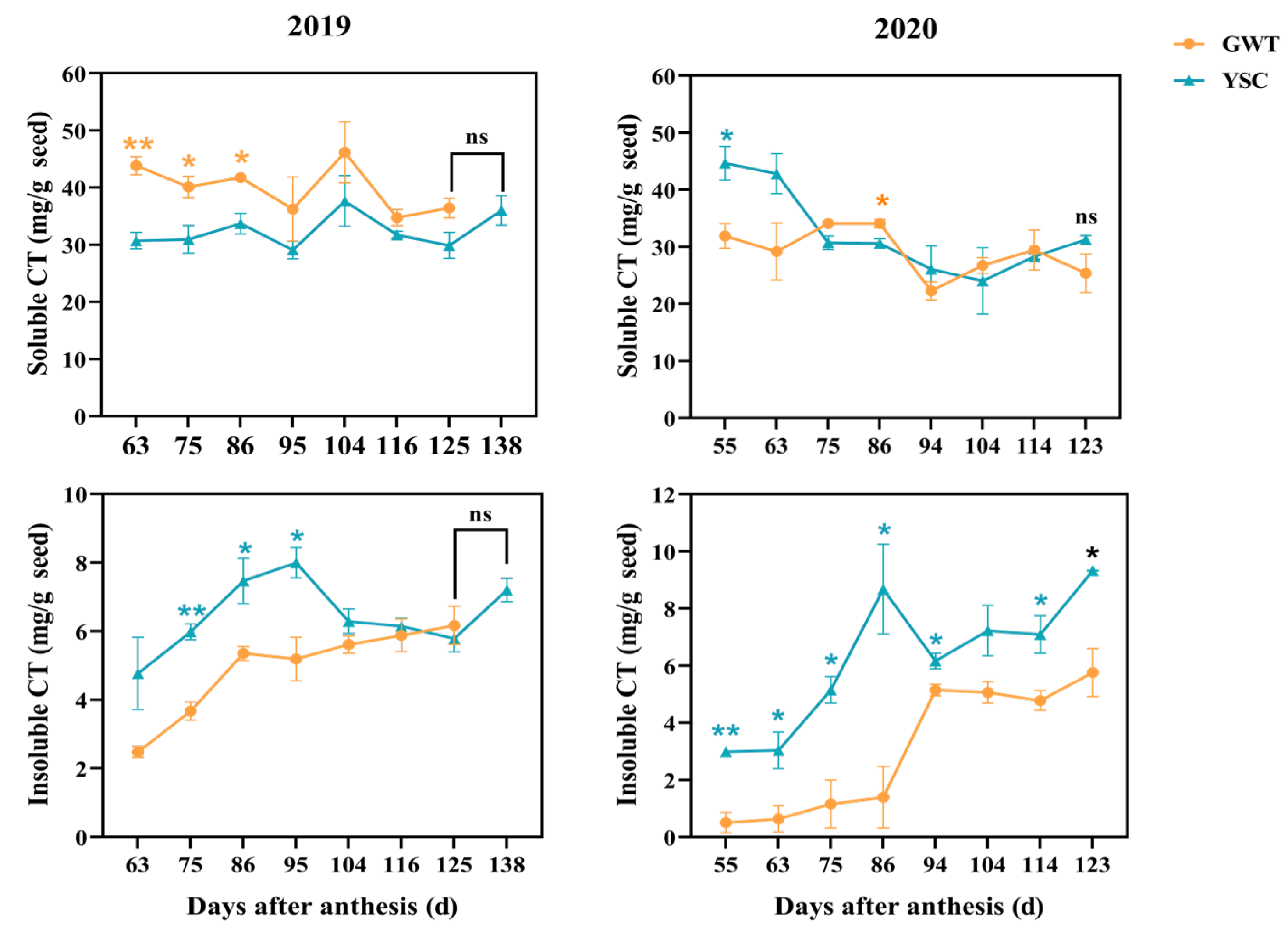


| CT Type | Year | Plot | Year × Plot |
|---|---|---|---|
| Soluble CT | *** | ns | *** |
| Insoluble CT | * | *** | ns |
| Year | Date of Grape Sampling and Days after Full Flowering | |||||||
|---|---|---|---|---|---|---|---|---|
| 2019 | 25 July | 6 August | 17 August | 26 August | 5 September | 16 September | 25 September | 8 October |
| 63 | 75 | 86 | 95 | 104 | 116 | 125 | 138 | |
| 2020 | 19 July | 27 July | 8 August | 19 August | 28 August | 7 September | 17 September | 26 September |
| 55 | 63 | 75 | 86 | 94 | 104 | 114 | 123 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Yao, X.; Xia, N.; Sun, Q.; Duan, C.; Pan, Q. Evolution of Seed-Soluble and Insoluble Tannins during Grape Berry Maturation. Molecules 2023, 28, 3050. https://doi.org/10.3390/molecules28073050
Wang J, Yao X, Xia N, Sun Q, Duan C, Pan Q. Evolution of Seed-Soluble and Insoluble Tannins during Grape Berry Maturation. Molecules. 2023; 28(7):3050. https://doi.org/10.3390/molecules28073050
Chicago/Turabian StyleWang, Jingjing, Xuechen Yao, Nongyu Xia, Qi Sun, Changqing Duan, and Qiuhong Pan. 2023. "Evolution of Seed-Soluble and Insoluble Tannins during Grape Berry Maturation" Molecules 28, no. 7: 3050. https://doi.org/10.3390/molecules28073050
APA StyleWang, J., Yao, X., Xia, N., Sun, Q., Duan, C., & Pan, Q. (2023). Evolution of Seed-Soluble and Insoluble Tannins during Grape Berry Maturation. Molecules, 28(7), 3050. https://doi.org/10.3390/molecules28073050