External Catalyst- and Additive-Free Photo-Oxidation of Aromatic Alcohols to Carboxylic Acids or Ketones Using Air/O2
Abstract
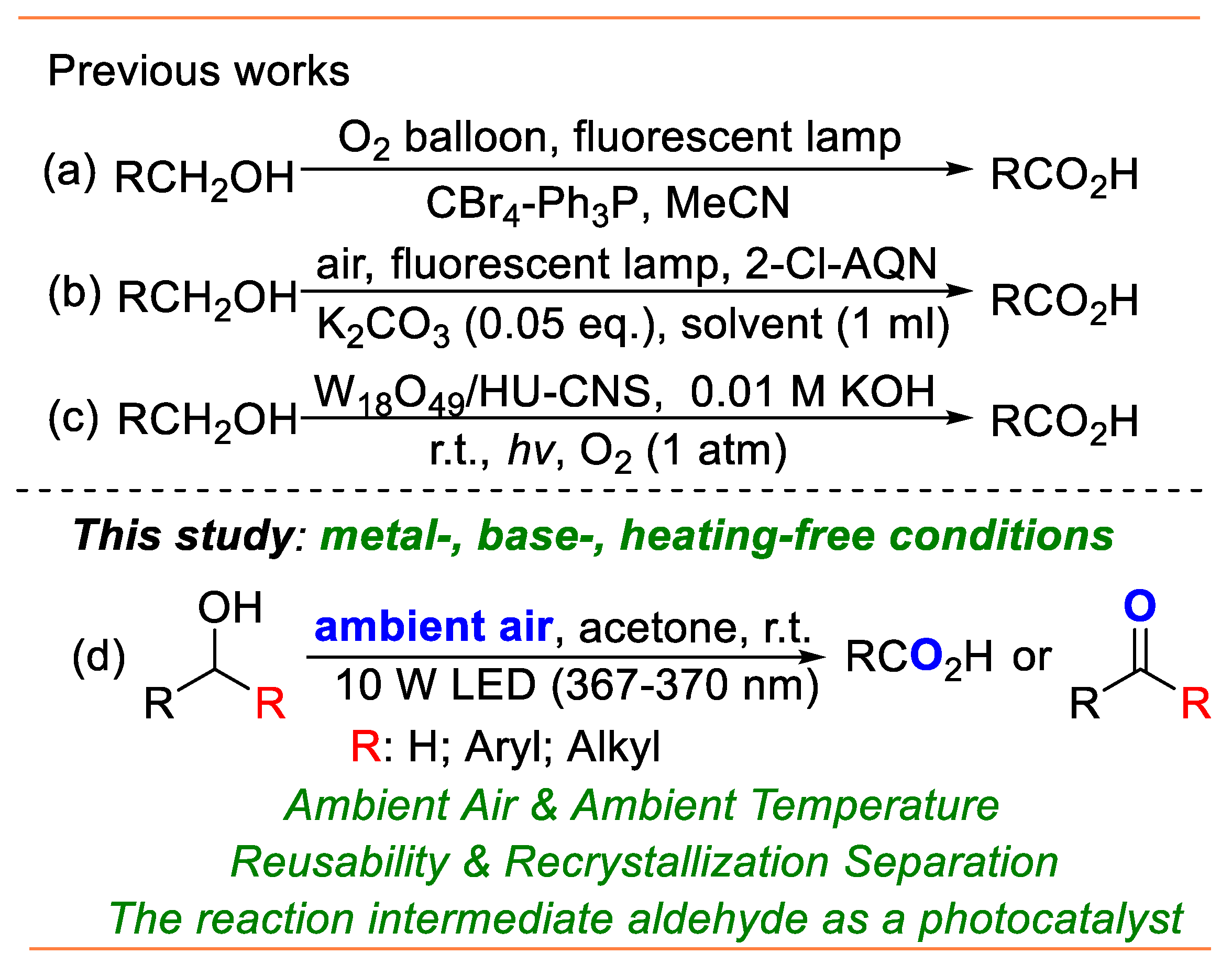
1. Introduction
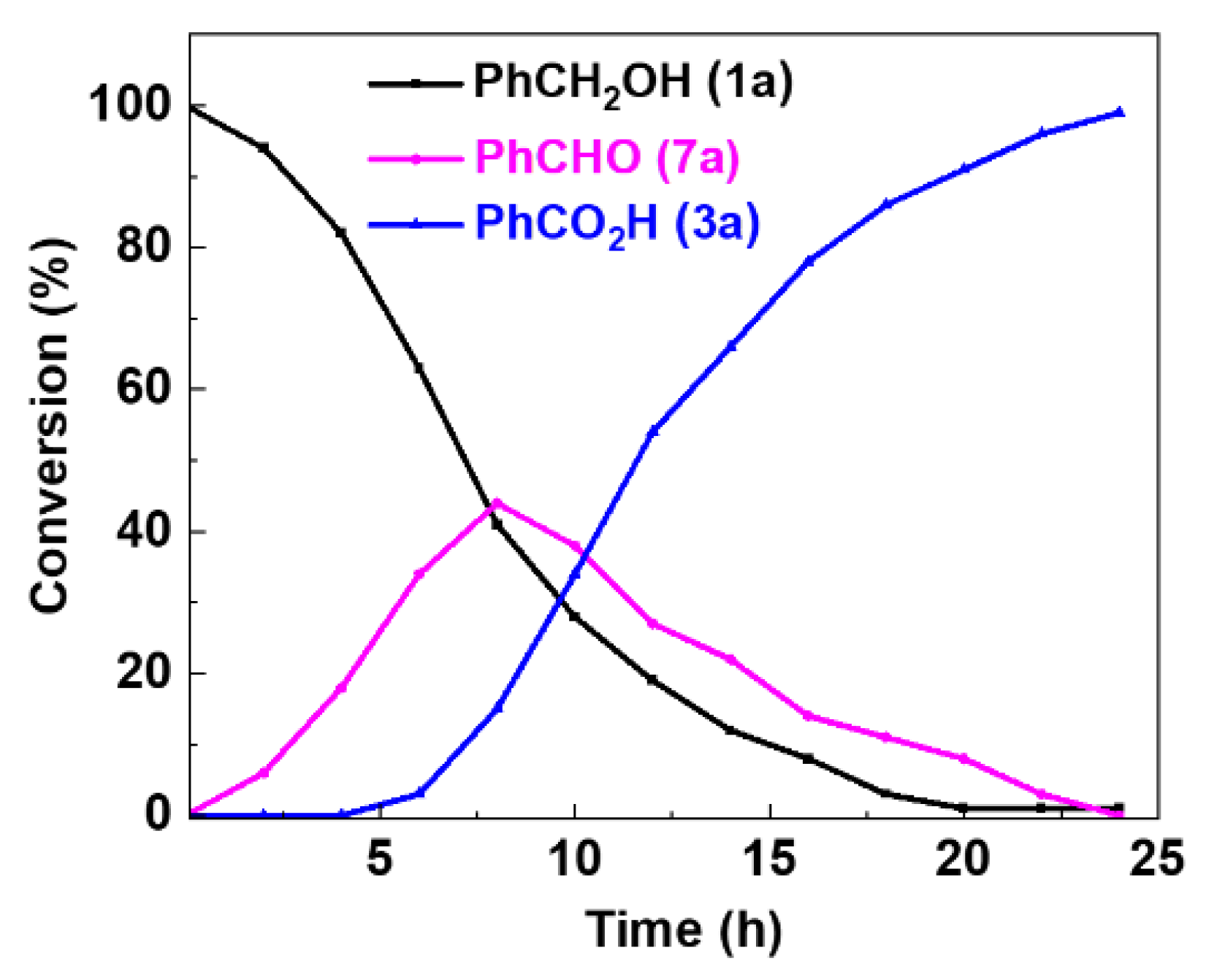
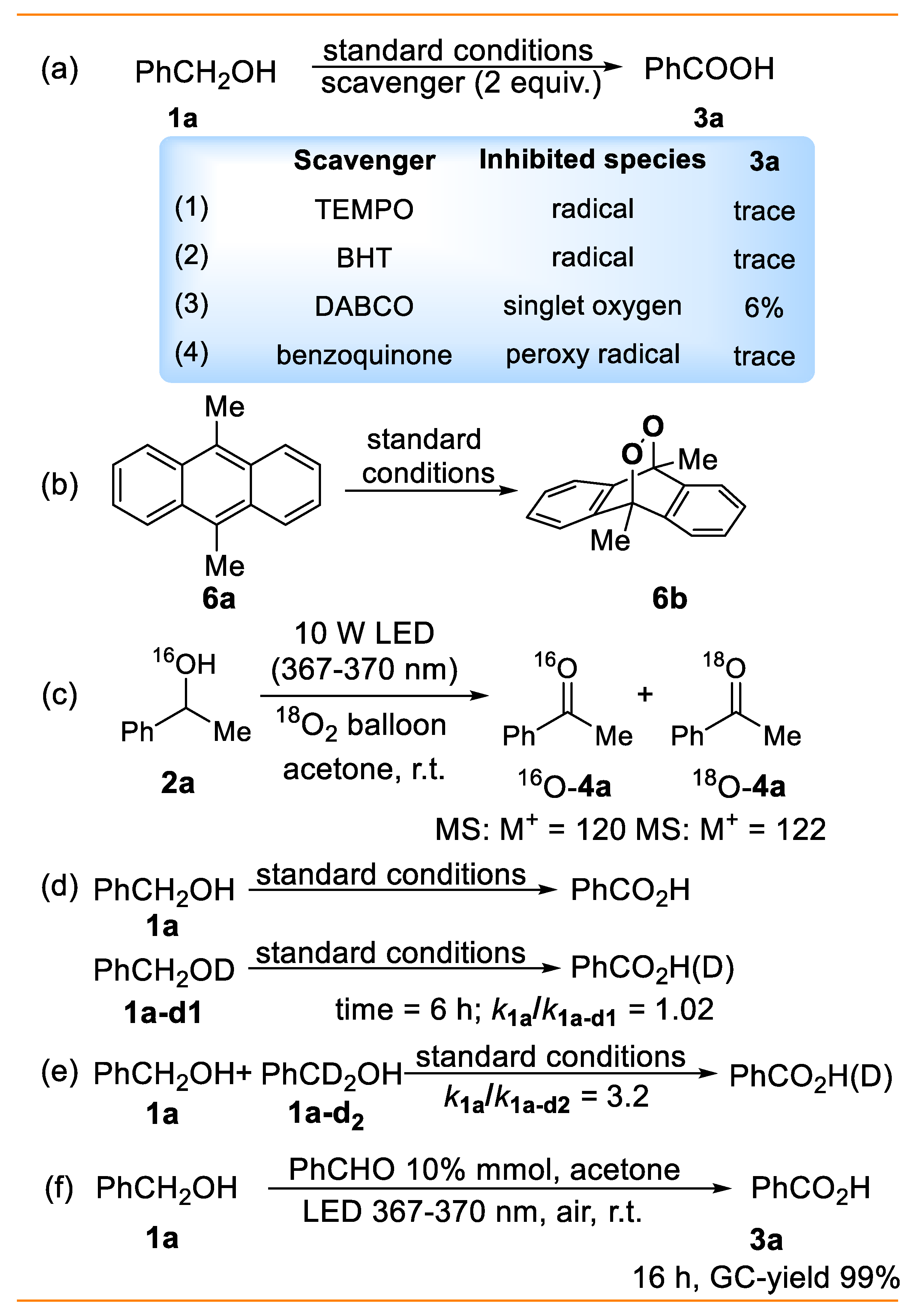
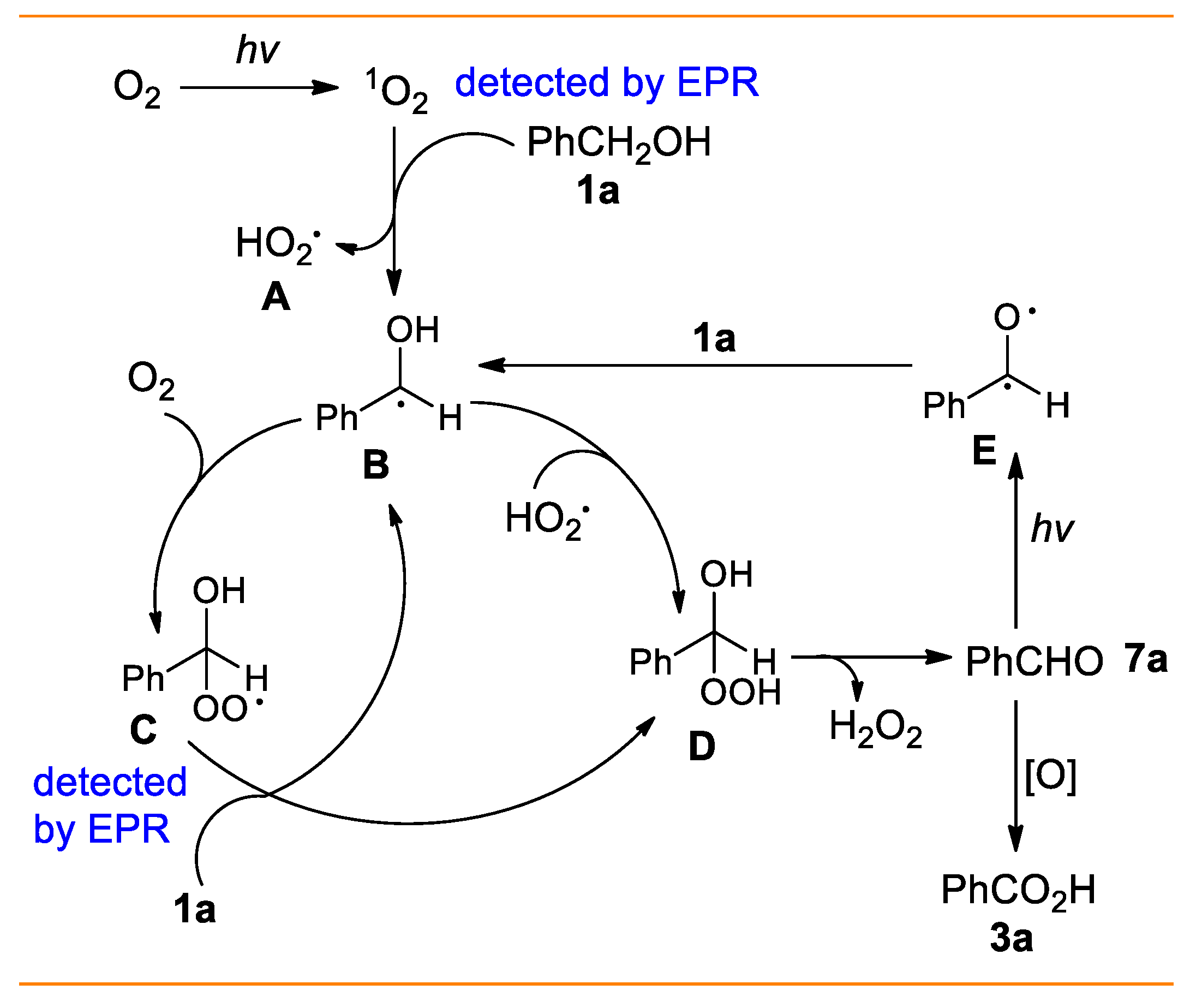
2. Results and Discussion
3. Experimental Section
3.1. General Information
3.2. Typical Procedure for the Synthesis of Benzoic Acid (3a)
3.3. Characterization Data of Products 3a–3y, 4a–4s, and 5a–5d
- Benzoic acid (3a) [57]: White solid (58 mg, 95%); 1H NMR (400 MHz, DMSO-d6) δ 12.96 (s, 1H), 7.95 (d, J = 7.2 Hz, 2H), 7.62 (t, J = 7.2 Hz, 1H), 7.49 (t, J = 7.6 Hz, 2H); 13C NMR (101 MHz, DMSO-d6) δ 167.8, 133.4, 131.3, 129.8, 129.1.
- [1,1′-biphenyl]-4-carboxylic acid (3b) [58]: White solid (94 mg, 95%); 1H NMR (400 MHz, DMSO-d6) δ 13.00 (s, 1H), 8.03 (d, J = 8.4 Hz, 2H), 7.80 (d, J = 8.4 Hz, 2H), 7.73 (d, J = 8.4 Hz, 2H), 7.50 (t, J = 7.6 Hz, 2H), 7.42 (t, J = 7.6 Hz, 1H); 13C NMR (101 MHz, DMSO-d6) δ 167.7, 144.8, 139.5, 130.5, 130.1, 129.6, 128.8, 127.5, 127.3.
- 4-methylbenzoic acid (3c) [57]: White solid (59 mg, 87%); 1H NMR (400 MHz, DMSO-d6) δ 12.80 (s, 1H), 7.83 (d, J = 8.4 Hz, 2H), 7.29 (d, J = 8.4 Hz, 2H), 2.35 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ 167.9, 143.6, 129.9, 129.7, 128.6, 21.7.
- 4-isopropylbenzoic acid (3d) [57]: White solid (75 mg, 90%); 1H NMR (400 MHz, DMSO-d6) δ 12.81 (s, 1H), 7.87 (d, J = 8.4 Hz, 2H), 7.36 (d, J = 8.4 Hz, 2H), 3.00–2.89 (m, 1H), 1.21 (d, J = 7.2 Hz, 6H); 13C NMR (101 MHz, DMSO-d6) δ 154.1, 130.0, 127.0, 34.0, 24.1.
- 4-(tert-butyl)benzoic acid (3e) [59]: White solid (82 mg, 92%); 1H NMR (400 MHz, DMSO-d6) δ 12.79 (s, 1H), 7.87 (d, J = 8.4 Hz, 2H), 7.50 (d, J = 8.4 Hz, 2H), 1.28 (s, 9H); 13C NMR (101 MHz, DMSO-d6) δ 167.8, 156.3, 129.7, 128.5, 125.9, 35.3, 31.4.
- 4-(trifluoromethoxy)benzoic acid (3f) [60]: Slight yellow solid (94 mg, 91%); 1H NMR (400 MHz, DMSO-d6) δ 13.19 (s, 1H), 8.06 (d, J = 8.4 Hz, 2H), 7.46 (d, J = 8.0 Hz, 2H); 13C NMR (101 MHz, DMSO-d6) δ 166.7, 152.0, 132.2, 130.4, 121.2, 120.5 (q, J = 255.9 Hz). 19F NMR (376 MHz, CDCl3) δ −56.7.
- 4-fluorobenzoic acid (3g) [57]: White solid (66 mg, 94%); 1H NMR (400 MHz, DMSO-d6) δ 13.06 (s, 1H), 8.02–7.98 (m, 2H), 7.34–7.29 (m, 2H); 13C NMR (101 MHz, DMSO-d6) δ 166.9, 165.4 (d, J = 249.0 Hz), 132.7 (d, J = 9.4 Hz), 127.9 (d, J = 2.8 Hz), 116.2 (d, J = 21.9 Hz); 19F NMR (376 MHz, DMSO-d6) δ −106.9.
- 4-chlorobenzoic acid (3h) [57]: White solid (73 mg, 93%); 1H NMR (400 MHz, DMSO-d6) δ 13.20 (s, 1H), 7.94 (d, J = 8.8 Hz, 2H), 7.57 (d, J = 8.8 Hz, 2H); 13C NMR (101 MHz, DMSO-d6) δ 167.0, 138.3, 131.7, 130.1, 129.3.
- 4-bromobenzoic acid (3i) [61]: White solid (95 mg, 95%); 1H NMR (400 MHz, DMSO-d6) δ 13.19 (s, 1H), 7.86 (d, J = 8.4 Hz, 2H), 7.70 (d, J = 8.4 Hz, 2H); 13C NMR (101 MHz, DMSO-d6) δ 167.1, 132.2, 131.8, 130.5, 127.4.
- 4-cyanobenzoic acid (3j) [58]: Gray white solid (55 mg, 75%); 1H NMR (400 MHz, DMSO-d6) δ 13.28 (s, 1H), 8.06 (s, 2H), 8.00 (s, 2H), 13C NMR (101 MHz, DMSO-d6) δ 195.9, 133.6, 118.7, 115.5.
- 4-(methoxycarbonyl)benzoic acid (3k) [60]: White solid (84 mg, 93%); 1H NMR (400 MHz, DMSO-d6) δ 13.36 (s, 1H), 8.05 (s, 4H), 3.87 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ 167.1, 166.1, 135.3, 133.7, 130.1, 129.9, 53.0.
- 2-chlorobenzoic acid (3l) [62]: White solid (73 mg, 94%); 1H NMR (400 MHz, DMSO-d6) δ 13.37 (s, 1H), 7.78 (d, J = 7.6 Hz, 1H), 7.55–7.50 (m, 2H), 7.45–7.39(m, 1H); 13C NMR (101 MHz, DMSO-d6) δ 167.3, 133.1, 132.1, 132.0, 131.3, 131.1, 127.7.
- 2-methylbenzoic acid (3m) [60]: White solid (58 mg, 86%); 1H NMR (400 MHz, DMSO-d6) δ 12.79 (s, 1H), 7.80 (d, J = 8.0 Hz, 1H), 7.43–7.39 (m, 1H), 7.26(t, J = 7.6 Hz, 2H); 13C NMR (101 MHz, DMSO-d6) δ 169.2, 139.5, 132.2, 132.0, 131.0, 130.7, 126.3, 21.8.
- 3-chlorobenzoic acid (3n) [60]: White solid (72 mg, 92%); 1H NMR (400 MHz, DMSO-d6) δ 13.34 (s, 1H), 7.90–7.88 (m, 2H), 7.70–7.67 (m, 1H), 7.53 (t, J = 8.0 Hz, 1H); 13C NMR (101 MHz, DMSO-d6) δ 166.6, 133.9, 133.4, 133.2, 131.2, 129.4, 128.4.
- 3-methylbenzoic acid (3o) [60]: Slight yellow solid (55 mg, 81%); 1H NMR (400 MHz, DMSO-d6) δ 12.89 (s, 1H), 7.76–7.73 (m, 2H), 7.44–7.36 (m, 2H), 2.36 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ 167.9, 138.4, 134.0, 131.2, 130.2, 129.0, 127.0, 21.3.
- 3,4-dichlorobenzoic acid (3p) [62]: White solid (92 mg, 96%); 1H NMR (400 MHz, DMSO-d6) δ 13.50 (s, 1H), 8.04 (d, J = 2.0 Hz, 1H), 7.87 (dd, J = 8.4, 2.0 Hz, 1H), 7.76 (d, J = 8.4 Hz, 1H); 13C NMR (101 MHz, DMSO-d6) δ 165.9, 136.3, 132.0, 131.9, 131.5, 131.5, 129.8.
- terephthalic acid (3q) [57]: White solid (59 mg, 71%); 1H NMR (400 MHz, DMSO-d6) δ 13.29 (s, 2H), 8.03 (s, 4H); 13C NMR (101 MHz, DMSO-d6) δ 167.3, 135.1, 130.1.
- 1-naphthoic acid (3r) [57]: White solid (65 mg, 78%); 1H NMR (400 MHz, DMSO-d6) δ 13.17 (s, 1H), 8.86 (d, J = 8.4 Hz, 1H), 8.17–8.14 (m, 2H), 8.02 (d, J = 7.6 Hz, 1H), 7.66–7.57 (m, 3H); 13C NMR (101 MHz, DMSO-d6) δ 169.2, 134.0, 133.5, 131.2, 130.4, 129.1, 128.2, 128.1, 126.7, 126.0, 125.4.
- anthracene-9-carboxylic acid (3s) [63]: Yellow solid (97 mg, 87%); 1H NMR (400 MHz, DMSO-d6) δ 13.94 (s, 1H), 8.73 (s, 1H), 8.16 (d, J = 8.4 Hz, 2H), 8.05 (d, J = 8.4 Hz, 2H), 7.64–7.57 (m, 4H); 13C NMR (101 MHz, DMSO-d6) δ 170.7, 131.0, 130.2, 129.1, 128.8, 127.6, 127.4, 126.2, 125.4.
- isonicotinic acid (3t) [62]: White solid (45 mg, 74%); 1H NMR (400 MHz, DMSO-d6) δ 13.64 (s, 1H), 8.78 (dd, J = 4.4, 1.6 Hz, 2H), 7.81 (dd, J = 4.4 1.6 Hz, 2H); 13C NMR (101 MHz, DMSO-d6) δ 166.7, 151.1, 138.6, 123.3.
- thiophene-2-carboxylic acid (3u) [57]: Gray white solid (54 mg, 85%); 1H NMR (400 MHz, DMSO-d6) δ 13.06 (s, 1H), 7.88 (dd, J = 4.8, 1.2 Hz, 1H), 7.73 (dd, J = 3.6, 1.2 Hz, 1H), 7.18 (dd, J = 4.8, 3.6 Hz, 1H); 13C NMR (101 MHz, DMSO-d6) δ 163.4, 135.2, 133.8, 133.7, 128.7.
- isobenzofuran-1(3H)-one (3v) [57]: White solid (48 mg, 71%); 1H NMR (400 MHz, CDCl3) δ 7.92 (d, J = 8.0 Hz, 1H), 7.70–7.67 (m, 1H), 7.55–7.49 (m, 2H), 5.32 (s, 2H); 13C NMR (101 MHz, CDCl3) δ 171.1, 146.5, 134.0, 129.0, 125.7, 125.7, 122.1, 69.6.
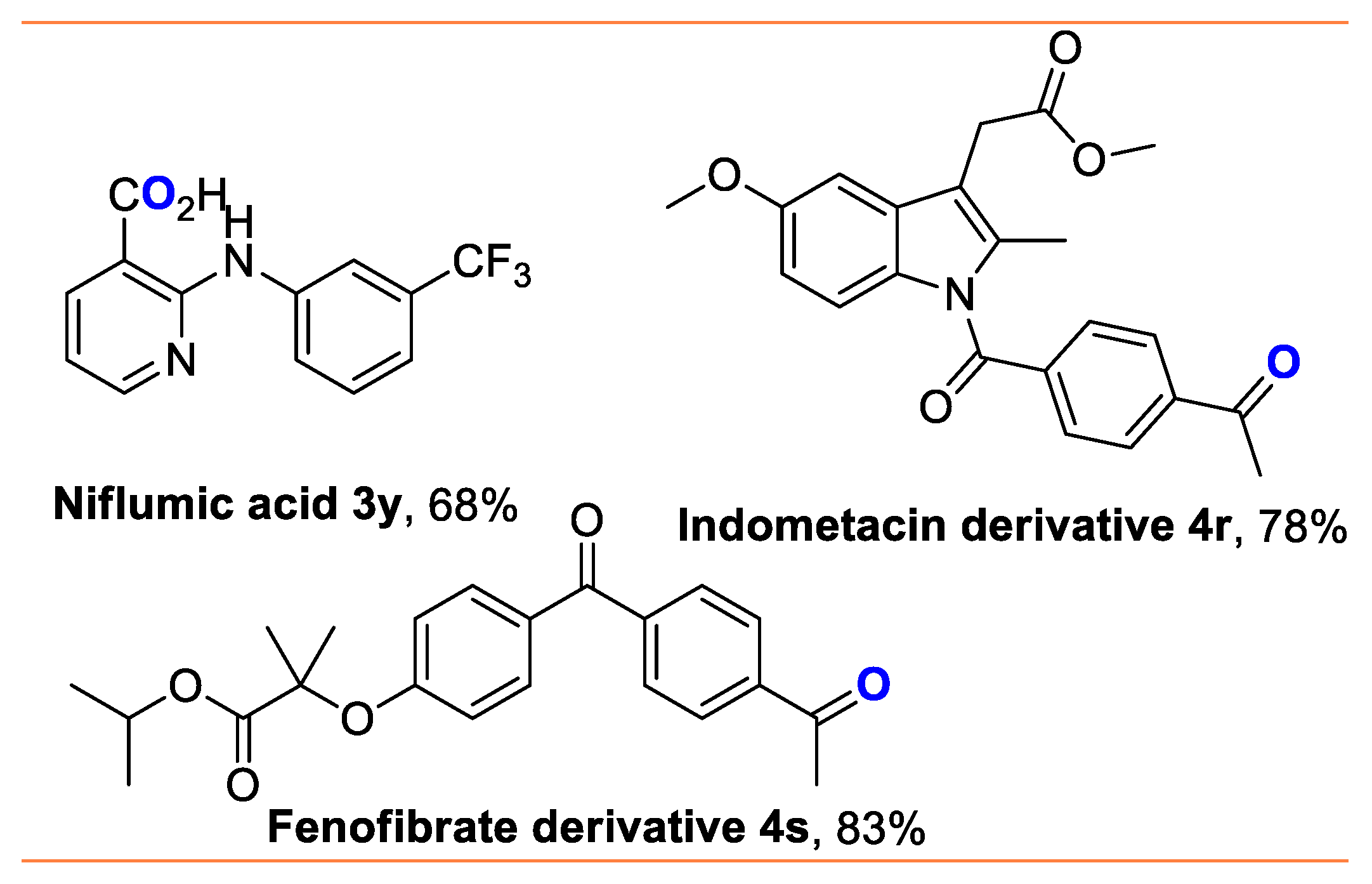
- 2-((3-(trifluoromethyl)phenyl)amino)nicotinic acid (3y) [64]: Gray white solid (96 mg, 68%); 1H NMR (400 MHz, DMSO-d6) δ 10.64 (s, 1H), 8.42 (dd, J = 4.8, 2.0 Hz, 1H), 8.28–8.26 (m, 2H), 7.84 (d, J = 8.0 Hz, 1H), 7.50 (t, J = 8.0 Hz, 1H), 7.30 (d, J = 8.0 Hz, 1H), 6.92 (dd, J = 7.6, 4.8 Hz, 1H); 13C NMR (101 MHz, DMSO-d6) δ 169.5, 155.7, 153.0, 141.2 (d, JC-F = 7.5 Hz), 130.2, 130.1 (d, JC-F = 93.5 Hz), 129.9, 124.8 (q, JC-F = 270.7 Hz), 123.9, 118.7 (JC-F = 3.9 Hz), 116.2 (JC-F = 4.0 Hz), 115.3, 108.9; 19F NMR (376 MHz, CDCl3) δ −61.2.
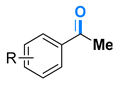
- acetophenone (4a) [65]: Colorless liquid (55 mg, 91%); 1H NMR (400 MHz, DMSO-d6) δ 7.93 (d, J = 8.0 Hz, 2H), 7.53 (t, J = 7.8 Hz, 1H), 7.43 (t, J = 8.0 Hz, 2H), 2.57 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ 198.0, 136.9, 133.0, 128.4, 128.1, 26.4.
- 1-(p-tolyl)ethanone (4b) [65]: Colorless liquid (55 mg, 82%); 1H NMR (400 MHz, DMSO-d6) δ 7.84 (d, J = 8.0 Hz, 2H), 7.24 (d, J = 8.0 Hz, 2H), 2.56 (s, 3H), 2.39 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ 197.8, 143.8, 134.6, 129.1, 128.3, 26.4, 21.5.
- 1-(4-fluorophenyl)ethanone (4c) [65]: Colorless liquid (63 mg, 91%); 1H NMR (400 MHz, DMSO-d6) δ 7.99–7.93 (m, 2H), 7.13–7.07 (m, 2H), 2.57 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ 196.4, 165.7 (d, JC-F = 253.1), 133.5, 130.9 (d, JC-F = 9.3 Hz), 115.6 (d, JC-F = 21.7), 26.4. 19F NMR (376 MHz, CDCl3) δ −105.3.
- 1-(4-chlorophenyl)ethanone (4d) [65]: Colorless liquid (74 mg, 96%);1H NMR (400 MHz, DMSO-d6) δ 7.87 (d, J = 8.4 Hz, 2H), 7.40 (d, J = 8.4 Hz, 2H), 2.56 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ 196.7, 139.5, 135.3, 129.6, 128.8, 26.5.
- 1-(4-bromophenyl)ethanone (4e) [65]: Colorless liquid (97 mg, 97%); 1H NMR (400 MHz, DMSO-d6) δ 7.82 (d, J = 8.4 Hz, 2H), 7.60 (d, J = 8.4 Hz, 2H), 2.58 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ 197.0, 135.8, 131.9, 129.8, 128.3, 26.5.
- benzophenone (4f) [66]: White solid (87 mg, 96%); 1H NMR (400 MHz, DMSO-d6) δ 7.80 (d, J = 7.2 Hz, 4H), 7.57 (t, J = 7.2 Hz, 2H), 7.47 (t, J = 7.6 Hz, 4H); 13C NMR (101 MHz, DMSO-d6) δ 196.6, 137.4, 132.3, 129.9, 128.1.
- phenyl(p-tolyl)methanone (4g) [67]: White solid (88 mg, 90%); 1H NMR (400 MHz, DMSO-d6) δ 7.80–7.77 (m, 2H), 7.73 (d, J = 8.0 Hz, 2H), 7.60–7.56 (m, 1H), 7.47 (t, J = 7.6 Hz, 2H), 7.28 (d, J = 8.0 Hz, 2H), 2.44 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ 196.5, 143.2, 137.9, 134.8, 132.1, 130.3, 129.9, 128.9, 128.2, 21.6.
- (4-fluorophenyl)(phenyl)methanone (4h) [67]: Colorless liquid (93 mg, 93%); 1H NMR (400 MHz, DMSO-d6) δ 7.87–7.82 (m, 2H), 7.78–7.76 (m, 2H), 7.62–7.58 (m, 1H), 7.19–7.13 (m, 2H); 13C NMR (101 MHz, DMSO-d6) δ 195.3, 165.4 (d, JC-F = 252.6 Hz), 137.5, 133.8, 133.7, 132.7, 132.6, 132.5, 129.9, 128.3, 115.5, 115.3. 19F NMR (376 MHz, CDCl3) δ −105.9.
- (4-chlorophenyl)(phenyl)methanone (4i) [68]: Colorless liquid (103 mg, 95%); 1H NMR (400 MHz, DMSO-d6) δ 7.78–7.74 (m, 4H), 7.62–7.58 (m, 1H), 7.51–7.44 (m, 4H); 13C NMR (101 MHz, DMSO-d6) δ 195.5, 138.9, 137.2, 135.8, 132.6, 131.4, 129.9, 128.6, 128.4.
- (4-bromophenyl)(phenyl)methanone (4j) [68]: Colorless liquid (123 mg, 94%); 1H NMR (400 MHz, DMSO-d6) δ 7.78–7.76 (m, 2H), 7.69–7.67 (m, 2H), 7.64–7.61 (m, 3H), 7.49 (t, J = 7.6 Hz, 2H); 13C NMR (101 MHz, DMSO-d6) δ 195.7, 137.1, 136.3, 132.7, 131.6, 131.6, 129.9, 128.4, 127.5.
- 2-chloro-1-phenylethanone (4k) [69]: White solid (70 mg, 90%); 1H NMR (400 MHz, DMSO-d6) δ 7.96 (d, J = 7.6 Hz, 2H), 7.62 (t, J = 7.6 Hz, 2H), 7.50 (t, J = 7.6 Hz, 2H), 4.72 (s, 2H); 13C NMR (101 MHz, DMSO-d6) δ 191.0, 134.2, 134.0, 128.9, 128.5, 46.0.
- 2-bromo-1-phenylethanone (4l) [69]: Grayish white solid (88 mg, 88%); 1H NMR (400 MHz, DMSO-d6) δ 7.98 (d, J = 8.4 Hz, 2H), 7.62 (t, J = 7.6 Hz, 1H), 7.50 (t, J = 7.6 Hz, 2H), 4.46 (s, 2H); 13C NMR (101 MHz, DMSO-d6) δ 191.3, 133.9, 133.9, 128.9, 128.8, 30.9.
- 3-chloro-1-phenylpropan-1-one (4m) [70]: Grayish white solid (70 mg, 83%); 1H NMR (400 MHz, DMSO-d6) δ 7.95 (d, J = 7.6 Hz, 2H), 7.59 (t, J = 7.6 Hz, 1H), 7.47 (t, J = 7.6 Hz, 2H), 3.92 (t, J = 6.8 Hz, 2H), 3.45 (t, J = 6.8 Hz, 2H); 13C NMR (101 MHz, DMSO-d6) δ 196.6, 136.3, 133.5, 128.7, 128.0, 41.2, 38.6.
- benzoyl cyanide (4n) [71]: Colorless liquid (60 mg, 92%); 1H NMR (400 MHz, DMSO-d6) δ 8.14 (d, J = 7.6 Hz, 2H), 7.79 (t, J = 7.6 Hz, 1H), 7.61 (t, J = 7.6 Hz, 2H); 13C NMR (101 MHz, DMSO-d6) δ 167.8, 136.8, 133.3, 130.4, 129.5, 112.7.
- 9H-fluoren-9-one (4o) [66]: White solid (82 mg, 91%); 1H NMR (400 MHz, DMSO-d6) δ 7.65 (d, J = 7.6 Hz, 2H), 7.52–7.46 (m, 4H), 7.31–7.27 (m, 2H); 13C NMR (101 MHz, DMSO-d6) δ 193.9, 144.4, 134.7, 134.1, 129.0, 124.3, 120.3.
- 9H-xanthen-9-one (4p) [72]: Grayish white solid (88 mg, 90%); 1H NMR (400 MHz, DMSO-d6) δ 8.35 (dd, J = 8.0, 1.6 Hz, 2H), 7.73 (td, J = 7.6, 1.6 Hz, 2H), 7.49 (d, J = 8.0 Hz, 2H), 7.40–7.36 (m, 2H); 13C NMR (101 MHz, DMSO-d6) δ 177.2, 156.1, 134.8, 126.7, 123.9, 121.8, 118.0.
- 9H-thioxanthen-9-one (4q) [73]: Light yellow solid (93 mg, 88%); 1H NMR (400 MHz, DMSO-d6) δ 8.62 (dd, J = 8.0, 1.2 Hz, 2H), 7.65–7.57 (m, 4H), 7.51–7.47 (m, 2H); 13C NMR (101 MHz, DMSO-d6) δ 180.0, 137.3, 132.3, 129.9, 129.2, 126.3, 126.0.
- methyl 2-(1-(4-acetylbenzoyl)-5-methoxy-2-methyl-1H-indol-3-yl)acetate (4r) [44]: Grayish white solid (148 mg, 78%); 1H NMR (400 MHz, DMSO-d6) δ 8.06 (d, J = 8.4 Hz, 2H), 7.79 (d, J = 8.4 Hz, 2H), 6.96 (d, J = 2.4 Hz, 1H), 6.85 (d, J = 9.2 Hz, 1H), 6.65 (dd, J = 9.2, 2.4 Hz, 1H), 3.83 (s, 3H), 3.71 (s, 3H), 3.67 (s, 2H), 2.68 (s, 3H), 2.36 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ 197.3, 171.3, 168.5, 156.2, 139.8, 139.6, 135.9, 130.8, 130.7, 129.7, 128.6, 115.1, 112.9, 111.7, 101.4, 55.7, 52.2, 30.1, 26.9, 13.5.
- isopropyl 2-(4-(4-acetylbenzoyl)phenoxy)-2-methylpropanoate (4s) [44]: White solid (153 mg, 83%); 1H NMR (400 MHz, DMSO-d6) δ 8.04 (d, J = 8.0 Hz, 2H), 7.80 (d, J = 8.0 Hz, 2H), 7.75 (d, J = 8.0 Hz, 2H), 6.86 (d, J = 8.4 Hz, 2H), 5.11–5.05 (m, 1H), 2.66 (s, 3H), 1.66 (s, 6H), 1.20 (d, J = 6.0 Hz, 6H); 13C NMR (101 MHz, DMSO-d6) δ 197.6, 194.7, 173.0, 160.0, 142.0, 139.2, 132.1, 129.9, 129.7, 128.1, 117.2, 79.4, 69.4, 26.8, 25.3, 21.5, 1.0.
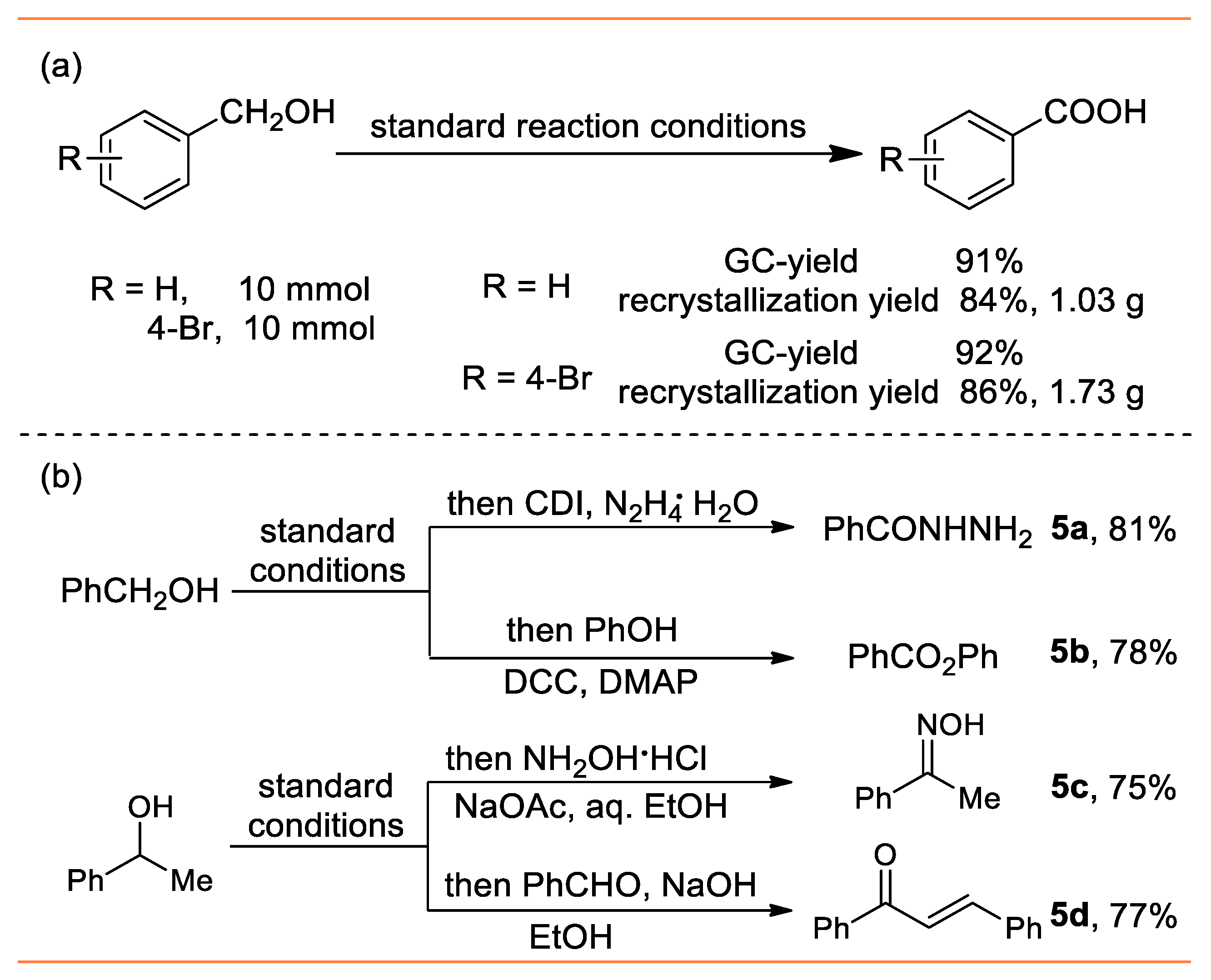
- benzohydrazide (5a) [49]: Grayish white solid (55 mg, 81%); 1H NMR (400 MHz, DMSO-d6) δ 9.79 (s, 1H), 7.83 (d, J = 7.2 Hz, 2H), 7.52–7.42 (m, 3H), 4.51 (s, 2H); 13C NMR (101 MHz, DMSO-d6) δ 166.5, 133.8, 131.6, 128.8, 127.5.
- phenyl benzoate (5b) [50]: Colorless liquid (77 mg, 78%); 1H NMR (400 MHz, DMSO-d6) δ 8.23 (d, J = 7.2 Hz, 2H), 7.65 (t, J = 7.2 Hz, 1H), 7.53 (t, J = 7.6 Hz, 2H), 7.45 (t, J = 8.0 Hz, 2H), 7.31–7.23 (m, 3H); 13C NMR (101 MHz, DMSO-d6) δ 165.2, 150.9, 133.5, 130.1, 129.5, 129.5, 128.5, 125.9, 121.7.
- acetophenone oxime (5c) [51]: White solid (51 mg, 75%); 1H NMR (400 MHz, DMSO-d6) δ 10.30 (s, 1H), 6.74–6.72 (m, 2H), 6.48–6.43 (m, 3H), 1.24 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ 153.4, 137.5, 129.1, 128.9, 126.1, 12.1.
- (E)-chalcone (5d) [52]: Light yellow solid (80 mg, 77%); 1H NMR (400 MHz, DMSO-d6) δ 8.03 (d, J = 7.2 Hz, 2H), 7.82 (d, J = 16.0 Hz, 1H), 7.66–7.49 (m, 6H), 7.46–7.41 (m, 3H); 13C NMR (101 MHz, DMSO-d6) δ 190.5, 144.8, 138.2, 134.8, 132.8, 130.5, 128.9, 128.6, 128.5, 128.4, 122.1.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cherepakhin, V.; Williams, T.J. Direct Oxidation of Primary Alcohols to Carboxylic Acids. Synthesis 2021, 53, 1023–1034. [Google Scholar]
- Rafiee, M.; Konz, Z.M.; Graaf, M.D.; Koolman, H.F.; Stahl, S.S. Electrochemical oxidation of alcohols and aldehydes to carboxylic acids catalyzed by 4-acetamido-TEMPO: An alternative to “Anelli” and “Pinnick” oxidations. ACS Catal. 2018, 8, 6738–6744. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, W.; Lin, Q.; Ye, M.; Xue, L.; Liu, J.; Wang, Y.; Cheng, H. Direct microdroplet synthesis of carboxylic acids from alcohols by preparative paper spray ionization without phase transfer catalysts. ACS Sustain. Chem. Eng. 2019, 7, 6486–6491. [Google Scholar] [CrossRef]
- Rafiee, M.; Alherech, M.; Karlen, S.D.; Stahl, S.S. Electrochemical aminoxyl-mediated oxidation of primary alcohols in lignin to carboxylic acids: Polymer modification and depolymerization. J. Am. Chem. Soc. 2019, 141, 15266–15276. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Zheng, Q.; Chen, J.; Tu, T. Acceptorless dehydrogenation of primary alcohols to carboxylic acids by self-supported NHC-Ru single-site catalysts. J. Catal. 2022, 408, 165–172. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Qian, Q.; Li, Y.; Bediako, B.B.A.; Zhang, J.; Yang, J.; Li, Z.; Han, B. Synthesis of carboxylic acids via the hydrocarboxylation of alcohols with CO2 and H2. Green Chem. 2022, 24, 1973–1977. [Google Scholar] [CrossRef]
- Thottathil, J.K.; Moniot, J.L.; Mueller, R.H.; Wong, M.K.; Kissick, T.P. Conversion of L-pyroglutamic acid to 4-alkyl-substituted L-prolines. The synthesis of trans-4-cyclohexyl-L-proline. J. Org. Chem. 1986, 51, 3140–3143. [Google Scholar] [CrossRef]
- Mahmood, A.; Robinson, G.E.; Powell, L. An improved oxidation of an alcohol using aqueous permanganate and phase-transfer catalyst. Org. Process Res. Dev. 1999, 3, 363–364. [Google Scholar] [CrossRef]
- Alduhaish, O.; Adil, S.F.; Assal, M.E.; Shaik, M.R.; Kuniyil, M.; Manqari, K.M.; Sekou, D.; Khan, M.; Khan, A.; Dewidar, A.Z.J.P. Synthesis and characterization of CoxOy–MnCO3 and CoxOy–Mn2O3 catalysts: A comparative catalytic assessment towards the aerial oxidation of various kinds of alcohols. Processes 2020, 8, 910. [Google Scholar] [CrossRef]
- Mazitschek, R.; Mülbaier, M.; Giannis, A. IBX-Mediated Oxidation of Primary Alcohols and Aldehydes To Form Carboxylic Acids. Angew. Chem. Int. Ed. 2002, 41, 4059–4061. [Google Scholar] [CrossRef]
- Gioia, M.L.J.M. Synthesis and preliminary evaluation of the anti-cancer activity on A549 lung cancer cells of a series of unsaturated disulfides. MedChemComm 2019, 10, 116–119. [Google Scholar]
- Lee, T.V. Oxidation Adjacent to Oxygen of Alcohols by Activated DMSO Methods. Compr. Org. Synth. 1991, 7, 291–303. [Google Scholar]
- Zhao, M.; Zhang, X.-W.; Wu, C.-D. Structural transformation of porous polyoxometalate frameworks and highly efficient biomimetic aerobic oxidation of aliphatic alcohols. ACS Catal. 2017, 7, 6573–6580. [Google Scholar] [CrossRef]
- Anderson, R.; Gri Ff In, K.; Johnston, P.; Alsters, P. Selective Oxidation of Alcohols to Carbonyl Compounds and Carboxylic Acids with Platinum Group Metal Catalysts. Adv. Syn. Catal. 2003, 345, 517–523. [Google Scholar] [CrossRef]
- Donze, C.; Korovchenko, P.; Gallezot, P.; Besson, M. Aerobic selective oxidation of (hetero) aromatic primary alcohols to aldehydes or carboxylic acids over carbon supported platinum. Appl. Catal. B-Environ. 2007, 70, 621–629. [Google Scholar] [CrossRef]
- Dai, Z.; Qin, L.; Jiang, H.; Qi, L.; Hua, L.; Jing, Z.; Peng, T. Ni(II)–N′NN′ pincer complexes catalyzed dehydrogenation of primary alcohols to carboxylic acids and H2 accompanied by alcohol etherification. Catal. Sci. Technol. 2017, 7, 2506–2511. [Google Scholar] [CrossRef]
- Könning, D.; Olbrisch, T.; Sypaseuth, F.D.; Tzschucke, C.C.; Christmann, M. Oxidation of allylic and benzylic alcohols to aldehydes and carboxylic acids. Chem. Commun. 2014, 50, 5014–5016. [Google Scholar] [CrossRef] [PubMed]
- Fraga-Dubreuil, J.; Garcia-Verdugo, E.; Hamley, P.A.; Vaquero, E.M.; Dudd, L.M.; Pearson, I.; Housley, D.; Partenheimer, W.; Thomas, W.B.; Whiston, K. Catalytic selective partial oxidations using O2 in supercritical water: The continuous synthesis of carboxylic acids. Green Chem. 2007, 9, 1238–1245. [Google Scholar] [CrossRef]
- Han, L.; Xing, P.; Jiang, B. Selective aerobic oxidation of alcohols to aldehydes, carboxylic acids, and imines catalyzed by a Ag-NHC complex. Org. Lett. 2014, 16, 3428–3431. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.S.; Mannel, D.S.; Root, T.W.; Stahl, S.S. Aerobic oxidation of diverse primary alcohols to carboxylic acids with a heterogeneous Pd–Bi–Te/C (PBT/C) catalyst. Org. Process Res. Dev. 2017, 21, 1388–1393. [Google Scholar] [CrossRef]
- Higashimoto, S.; Kitao, N.; Yoshida, N.; Sakura, T.; Azuma, M.; Ohue, H.; Sakata, Y. Selective photocatalytic oxidation of benzyl alcohol and its derivatives into corresponding aldehydes by molecular oxygen on titanium dioxide under visible light irradiation. J. Catal. 2009, 266, 279–285. [Google Scholar] [CrossRef]
- Ye, X.; Li, Y.; Luo, P.; He, B.; Cao, X.; Lu, T. Iron sites on defective BiOBr nanosheets: Tailoring the molecular oxygen activation for enhanced photocatalytic organic synthesis. Nano Res. 2022, 15, 1509–1516. [Google Scholar] [CrossRef]
- Xiao, X.; Jiang, J.; Zhang, L. Selective oxidation of benzyl alcohol into benzaldehyde over semiconductors under visible light: The case of Bi12O17Cl2 nanobelts. Appl. Catal. B-Environ. 2013, 142, 487–493. [Google Scholar] [CrossRef]
- Su, F.; Mathew, S.C.; Lipner, G.; Fu, X.; Antonietti, M.; Blechert, S.; Wang, X. mpg-C3N4-catalyzed selective oxidation of alcohols using O2 and visible light. J. Am. Chem. Soc. 2010, 132, 16299–16301. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Yuan, J.; Lei, A. Transition-metal-free aerobic oxidation of primary alcohols to carboxylic acids. New J. Chem. 2013, 37, 1700–1703. [Google Scholar] [CrossRef]
- Kim, S.; Lee, H.E.; Suh, J.M.; Mi, H.L.; Min, K. Sequential Connection of Mutually Exclusive Catalytic Reactions by a Method Controlling the Presence of an MOF Catalyst: One-Pot Oxidation of Alcohols to Carboxylic Acids. Inorg. Chem. 2020, 59, 17573–17582. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhang, J.; Ma, S. Iron Catalysis for Room-Temperature Aerobic Oxidation of Alcohols to Carboxylic Acids. J. Am. Chem. Soc. 2016, 138, 8344–8347. [Google Scholar] [CrossRef]
- Kobayashi, M.S. Remarkable Effect of Bimetallic Nanocluster Catalysts for Aerobic Oxidation of Alcohols: Combining Metals Changes the Activities and the Reaction Pathways to Aldehydes/Carboxylic Acids or Esters. J. Am. Chem. Soc. 2010, 132, 15096–15098. [Google Scholar]
- Liu, K.-J.; Jiang, S.; Lu, L.-H.; Tang, L.-L.; Tang, S.-S.; Tang, H.-S.; Tang, Z.; He, W.-M.; Xu, X. Bis (methoxypropyl) ether-promoted oxidation of aromatic alcohols into aromatic carboxylic acids and aromatic ketones with O2 under metal-and base-free conditions. Green Chem. 2018, 20, 3038–3043. [Google Scholar] [CrossRef]
- Hirashima, S.-I.; Itoh, A. Aerobic oxidation of alcohols under visible light irradiation of fluorescent lamp. Green Chem. 2007, 9, 318–320. [Google Scholar] [CrossRef]
- Shimada, Y.; Hattori, K.; Tada, N.; Miura, T.; Itoh, A. Facile aerobic photooxidation of alcohols using 2-chloroanthraquinone under visible light irradiation. Synthesis 2013, 45, 2684–2688. [Google Scholar] [CrossRef]
- Hirashima, S.-I.; Hashimoto, S.; Masaki, Y.; Itoh, A. Aerobic photo-oxidation of alcohols in the presence of a catalytic inorganic bromo source. Tetrahedron 2006, 62, 7887–7891. [Google Scholar] [CrossRef]
- Sugai, T.; Itoh, A. Aerobic oxidation under visible light irradiation of a fluorescent lamp with a combination of carbon tetrabromide and triphenyl phosphine. Tetrahedron Lett. 2007, 48, 9096–9099. [Google Scholar] [CrossRef]
- Xiao, C.; Zhang, L.; Hao, H.; Wang, W. High selective oxidation of benzyl alcohol to benzylaldehyde and benzoic acid with surface oxygen vacancies on W18O49/holey ultrathin g-C3N4 nanosheets. ACS Sustain. Chem. Eng. 2019, 7, 7268–7276. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, M.; Wang, Y.; Su, Y.; Yang, X.; Chen, C.; Xu, J. Au/mesoporous-TiO2 as catalyst for the oxidation of alcohols to carboxylic acids with molecular oxygen in water. Appl. Catal. A-Gen. 2014, 475, 347–354. [Google Scholar] [CrossRef]
- Schilling, W.; Riemer, D.; Zhang, Y.; Hatami, N.; Das, S. Metal-free catalyst for visible-light-induced oxidation of unactivated alcohols using air/oxygen as an oxidant. ACS Catal. 2018, 8, 5425–5430. [Google Scholar] [CrossRef]
- Meng, C.; Yang, K.; Fu, X.; Yuan, R. Photocatalytic oxidation of benzyl alcohol by homogeneous CuCl2/solvent: A model system to explore the role of molecular oxygen. ACS Catal. 2015, 5, 3760–3766. [Google Scholar] [CrossRef]
- Qiu, C.; Wang, S.; Zuo, J.; Zhang, B.J.C. Photocatalytic CO2 reduction coupled with alcohol oxidation over porous carbon nitride. Catalysts 2022, 12, 672. [Google Scholar] [CrossRef]
- Chandra, S.; Jagdale, P.; Medha, I.; Tiwari, A.K.; Bartoli, M.; Nino, A.D.; Olivito, F.J.T. Biochar-Supported TiO2-Based Nanocomposites for the Photocatalytic Degradation of Sulfamethoxazole in Water—A Review. Toxics 2021, 9, 313. [Google Scholar] [CrossRef]
- Xu, J.; Yue, X.; He, L.; Shen, J.; Ouyang, Y.; Liang, C.; Li, W. Photoinduced protocol for aerobic oxidation of aldehydes to carboxylic acids under mild conditions. ACS Sustain. Chem. Eng. 2022, 10, 14119–14125. [Google Scholar] [CrossRef]
- Shi, H.; Li, J.; Wang, T.; Rudolph, M.; Hashmi, A.S.K. Catalyst-and additive-free sunlight-induced autoxidation of aldehydes to carboxylic acids. Green Chem. 2022, 24, 5835–5841. [Google Scholar] [CrossRef]
- Liu, K.-J.; Deng, J.-H.; Yang, J.; Gong, S.-F.; Lin, Y.-W.; He, J.-Y.; Cao, Z.; He, W.-M. Selective oxidation of (hetero) sulfides with molecular oxygen under clean conditions. Green Chem. 2020, 22, 433–438. [Google Scholar] [CrossRef]
- Liu, K.-J.; Wang, Z.; Lu, L.-H.; Chen, J.-Y.; Zeng, F.; Lin, Y.-W.; Cao, Z.; Yu, X.; He, W.-M. Synergistic cooperative effect of CF3SO2Na and bis (2-butoxyethyl) ether towards selective oxygenation of sulfides with molecular oxygen under visible-light irradiation. Green Chem. 2021, 23, 496–500. [Google Scholar] [CrossRef]
- Liu, K.-J.; Duan, Z.-H.; Zeng, X.-L.; Sun, M.; Tang, Z.; Jiang, S.; Cao, Z.; He, W.-M. Clean Oxidation of (Hetero) benzylic Csp3–H Bonds with Molecular Oxygen. ACS Sustain. Chem. Eng. 2019, 7, 10293–10298. [Google Scholar] [CrossRef]
- Liu, K.-J.; Zeng, T.-Y.; Zeng, J.-L.; Gong, S.-F.; He, J.-Y.; Lin, Y.-W.; Tan, J.-X.; Cao, Z.; He, W.-M. Solvent-dependent selective oxidation of 5-hydroxymethylfurfural to 2, 5-furandicarboxylic acid under neat conditions. Chin. Chem. Lett. 2019, 30, 2304–2308. [Google Scholar] [CrossRef]
- Ou, J.; He, S.; Wang, W.; Tan, H.; Liu, K. Highly efficient oxidative cleavage of olefins with O2 under catalyst-, initiator-and additive-free conditions. Org. Chem. Front. 2021, 8, 3102–3109. [Google Scholar] [CrossRef]
- Liu, K.-J.; Fu, Y.-L.; Xie, L.-Y.; Wu, C.; He, W.-B.; Peng, S.; Wang, Z.; Bao, W.-H.; Cao, Z.; Xu, X. Green and efficient: Oxidation of aldehydes to carboxylic acids and acid anhydrides with air. ACS Sustain. Chem. Eng. 2018, 6, 4916–4921. [Google Scholar] [CrossRef]
- Ou, J.; Tan, H.; He, S.; Wang, W.; Hu, B.; Yu, G.; Liu, K. 1, 2-Dibutoxyethane-Promoted Oxidative Cleavage of Olefins into Carboxylic Acids Using O2 Under Clean Conditions. J. Org. Chem. 2021, 86, 14974–14982. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Bai, X.; Deng, Q.; Wang, J.; Zhang, G.; Xiao, C.; Mei, Y.; Wang, Y. SAR studies on 1, 2, 4-triazolo [3, 4-b][1,3,4] thiadiazoles as inhibitors of Mtb shikimate dehydrogenase for the development of novel antitubercular agents. RSC Adv. 2015, 5, 97089–97101. [Google Scholar] [CrossRef]
- Kwon, E.-M.; Kim, C.G.; Bak, J.S.; Jun, J.-G. Preparation of Benzoyloxy Benzophenone Derivatives and Their Inhibitory Effects of ICAM-1 Expression. Bull. Korean Chem. Soc. 2012, 33, 1939–1944. [Google Scholar] [CrossRef]
- Senadi, G.C.; Mutra, M.R.; Lu, T.-Y.; Wang, J.-J. Oximes as reusable templates for the synthesis of ureas and carbamates by an in situ generation of carbamoyl oximes. Green Chem. 2017, 19, 4272–4277. [Google Scholar] [CrossRef]
- Bashary, R.; Khatik, G.L. Design, and facile synthesis of 1, 3 diaryl-3-(arylamino) propan-1-one derivatives as the potential alpha-amylase inhibitors and antioxidants. Bioorg. Chem. 2019, 82, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Nojima, K.; Isogami, C. Photolysis of aldrin in the presence of benzaldehyde in a solid-vapor-air system. Chem. Pharm. Bull. 1996, 44, 1580–1584. [Google Scholar] [CrossRef]
- Li, G.; Yan, Q.; Gong, X.; Dou, X.; Yang, D. Photocatalyst-Free Regioselective C–H Thiocyanation of 4-Anilinocoumarins under Visible Light. ACS Sustain. Chem. Eng. 2019, 7, 14009–14015. [Google Scholar] [CrossRef]
- Zhang, Y.; Teuscher, K.B.; Ji, H. Direct α-heteroarylation of amides (α to nitrogen) and ethers through a benzaldehyde-mediated photoredox reaction. Chem. Sci. 2016, 7, 2111–2118. [Google Scholar] [CrossRef]
- Xia, J.-B.; Zhu, C.; Chen, C. Visible light-promoted metal-free C–H activation: Diarylketone-catalyzed selective benzylic mono-and difluorination. J. Am. Chem. Soc. 2013, 135, 17494–17500. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Ru, S.; Dai, G.; Zhai, Y.; Lin, H.; Han, S.; Wei, Y. An Efficient Iron(III)-Catalyzed Aerobic Oxidation of Aldehydes in Water for the Green Preparation of Carboxylic Acids. Angew. Chem. Int. Ed. 2017, 56, 3867–3871. [Google Scholar] [CrossRef] [PubMed]
- Villano, R.; Acocella, M.R.; Scettri, A. Fe3O4 nanoparticles/ethyl acetoacetate system for the efficient catalytic oxidation of aldehydes to carboxylic acids. Tetrahedron Lett. 2014, 55, 2442–2445. [Google Scholar] [CrossRef]
- Ma, C.; Zhao, C.-Q.; Xu, X.-T.; Li, Z.-M.; Wang, X.-Y.; Zhang, K.; Mei, T.-S. Nickel-Catalyzed Carboxylation of Aryl and Heteroaryl Fluorosulfates Using Carbon Dioxide. Org. Lett. 2019, 21, 2464–2467. [Google Scholar] [CrossRef]
- Marcé, P.; Lynch, J.; Blacker, A.J.; Williams, J.M.J. Conversion of nitroalkanes into carboxylic acids via iodide catalysis in water. Chem. Commun. 2016, 52, 1013–1016. [Google Scholar] [CrossRef]
- Mishra, A.K.; Moorthy, J.N. Mechanochemical catalytic oxidations in the solid state with in situ-generated modified IBX from 3,5-di-tert-butyl-2-iodobenzoic acid (DTB-IA)/Oxone. Org. Chem. Front. 2017, 4, 343–349. [Google Scholar] [CrossRef]
- Yang, X.; Tang, S.; Lu, T.; Chen, C.; Zhou, L.; Su, Y.; Xu, J. Sulfonic Acid Resin–Catalyzed Oxidation of Aldehydes to Carboxylic Acids by Hydrogen Peroxide. Synth. Commun. 2013, 43, 979–985. [Google Scholar] [CrossRef]
- Hajimohammadi, M.; Azizi, N.; Tollabimazraeno, S.; Tuna, A.; Duchoslav, J.; Knör, G. Cobalt (II) Phthalocyanine Sulfonate Supported on Reduced Graphene Oxide (RGO) as a Recyclable Photocatalyst for the Oxidation of Aldehydes to Carboxylic Acids. Catal. Lett. 2021, 151, 36–44. [Google Scholar] [CrossRef]
- Yarhosseini, M.; Javanshir, S.; Dolatkhah, Z.; Dekamin, M.G. An improved solvent-free synthesis of flunixin and 2-(arylamino) nicotinic acid derivatives using boric acid as catalyst. Chem. Cent. J. 2017, 11, 124. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, N.; Lu, L.; Zhu, G. Regioselective Hydration of Terminal Alkynes Catalyzed by a Neutral Gold(I) Complex [(IPr)AuCl] and One-Pot Synthesis of Optically Active Secondary Alcohols from Terminal Alkynes by the Combination of [(IPr)AuCl] and Cp*RhCl[(R,R)-TsDPEN]. J. Org. Chem. 2015, 80, 3538–3546. [Google Scholar] [CrossRef] [PubMed]
- Seth, S.; Jhulki, S.; Moorthy, J.N. Catalytic and Chemoselective Oxidation of Activated Alcohols and Direct Conversion of Diols to Lactones with In Situ-Generated Bis-IBX Catalyst. Eur. J. Org. Chem. 2013, 2013, 2445–2452. [Google Scholar] [CrossRef]
- Mondal, M.; Bora, U. Eco-friendly Suzuki–Miyaura coupling of arylboronic acids to aromatic ketones catalyzed by the oxime-palladacycle in biosolvent 2-MeTHF. New J. Chem. 2016, 40, 3119–3123. [Google Scholar] [CrossRef]
- Burange, A.S.; Kale, S.R.; Zboril, R.; Gawande, M.B.; Jayaram, R.V. Magnetically retrievable MFe2O4 spinel (M = Mn, Co, Cu, Ni, Zn) catalysts for oxidation of benzylic alcohols to carbonyls. RSC Adv. 2014, 4, 6597–6601. [Google Scholar] [CrossRef]
- Ye, M.; Wen, Y.; Li, H.; Fu, Y.; Wang, Q. Metal-free hydration of aromatic haloalkynes to α-halomethyl ketones. Tetrahedron Lett. 2016, 57, 4983–4986. [Google Scholar] [CrossRef]
- Dragutan, I.; Dragutan, V.; Demonceau, A. Targeted drugs by olefin metathesis: Piperidine-based iminosugars. RSC Adv. 2012, 2, 719–736. [Google Scholar] [CrossRef]
- Bergonzini, G.; Cassani, C.; Lorimer-Olsson, H.; Hörberg, J.; Wallentin, C.-J. Visible-Light-Mediated Photocatalytic Difunctionalization of Olefins by Radical Acylarylation and Tandem Acylation/Semipinacol Rearrangement. Chem. Eur. J. 2016, 22, 3292–3295. [Google Scholar] [CrossRef] [PubMed]
- Burange, A.S.; Kale, S.R.; Jayaram, R.V. Oxidation of alkyl aromatics to ketones by tert-butyl hydroperoxide on manganese dioxide catalyst. Tetrahedron Lett. 2012, 53, 2989–2992. [Google Scholar] [CrossRef]
- Yu, T.; Guo, M.; Wen, S.; Zhao, R.; Wang, J.; Sun, Y.; Liu, Q.; Zhou, H. Poly(ethylene glycol) dimethyl ether mediated oxidative scission of aromatic olefins to carbonyl compounds by molecular oxygen. RSC Adv. 2021, 11, 13848–13852. [Google Scholar] [CrossRef] [PubMed]

 | ||||
|---|---|---|---|---|
| Entry | Light Source (nm) | Solvent b | Atmosphere | Yield c (%) |
| 1 | 390–395 | MeCN | Oxygen | 63 |
| 2 | 390–395 | EtOH | Oxygen | 54 |
| 3 | 390–395 | 1,4-dioxane | Oxygen | 21 |
| 4 | 390–395 | DCE | Oxygen | 56 |
| 5 | 390–395 | DMF | Oxygen | 62 |
| 6 | 390–395 | DMSO | Oxygen | 63 |
| 7 | 390–395 | Acetone | Oxygen | 74 |
| 8 | 385–390 | Acetone | Oxygen | 75 |
| 9 | 380–385 | Acetone | Oxygen | 76 |
| 10 | 375–380 | Acetone | Oxygen | 80 |
| 11 | 370–375 | Acetone | Oxygen | 78 |
| 12 | 367–370 | Acetone | Oxygen | 98 |
| 13 | 365–367 | Acetone | Oxygen | 71 |
| 14 | 395–400 | Acetone | Oxygen | 64 |
| 15 | 400–405 | Acetone | Oxygen | 57 |
| 16 | 367–370 | Acetone | Air | 98 |
| 17 | 367–370 | Acetone | N2 | 0 |
| 18 | Acetone | air | 0 | |
 | ||||
| R | ||||
 | 4-H | 3a | 95% | 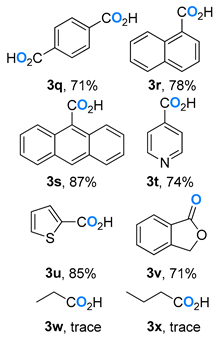 |
| 4-Ph | 3b | 95% | ||
| 4-Me | 3c | 87% | ||
| 4-iPr | 3d | 90% | ||
| 4-tBu | 3e | 92% | ||
| 4-OCF3 | 3f | 91% | ||
| 4-F | 3g | 94% | ||
| 4-Cl | 3h | 93% | ||
| 4-Br | 3i | 95% | ||
| 4-CN | 3j | 75% | ||
| 4-CO2Me | 3k | 93% | ||
| 2-Cl | 3l | 94% | ||
| 2-Me | 3m | 86% | ||
| 3-Cl | 3n | 92% | ||
| 3-Me | 3o | 81% | ||
| 3,4-dl-Cl | 3p | 96% | ||
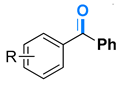
 | 4-H | 4a | 91% | 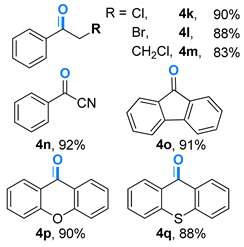 |
| 4-Me | 4b | 82% | ||
| 4-F | 4c | 91% | ||
| 4-Cl | 4d | 96% | ||
| 4-Br | 4e | 97% | ||
 | 4-H | 4f | 96% | |
| 4-Me | 4g | 90% | ||
| 4-F | 4h | 93% | ||
| 4-Cl | 4i | 95% | ||
| 4-Br | 4j | 94% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Ou, J.; Luo, K.; Liang, R.; Liu, J.; Li, N.; Hu, B.; Liu, K. External Catalyst- and Additive-Free Photo-Oxidation of Aromatic Alcohols to Carboxylic Acids or Ketones Using Air/O2. Molecules 2023, 28, 3031. https://doi.org/10.3390/molecules28073031
Xu M, Ou J, Luo K, Liang R, Liu J, Li N, Hu B, Liu K. External Catalyst- and Additive-Free Photo-Oxidation of Aromatic Alcohols to Carboxylic Acids or Ketones Using Air/O2. Molecules. 2023; 28(7):3031. https://doi.org/10.3390/molecules28073031
Chicago/Turabian StyleXu, Meng, Jinhua Ou, Kejun Luo, Rongtao Liang, Jian Liu, Ni Li, Bonian Hu, and Kaijian Liu. 2023. "External Catalyst- and Additive-Free Photo-Oxidation of Aromatic Alcohols to Carboxylic Acids or Ketones Using Air/O2" Molecules 28, no. 7: 3031. https://doi.org/10.3390/molecules28073031
APA StyleXu, M., Ou, J., Luo, K., Liang, R., Liu, J., Li, N., Hu, B., & Liu, K. (2023). External Catalyst- and Additive-Free Photo-Oxidation of Aromatic Alcohols to Carboxylic Acids or Ketones Using Air/O2. Molecules, 28(7), 3031. https://doi.org/10.3390/molecules28073031





