Abstract
Pesticides are essential for the development of agriculture. It is urgent to develop green, safe and efficient pesticides. Bisindole alkaloids have unique and concise structures and broad biological activities, which make them an important leading skeleton in the creation of new pesticides. In this work, we synthesized bisindole alkaloid barakacin in a simple seven-step process, and simultaneously designed and synthesized a series of its derivatives. Biological activity research indicated that most of these compounds displayed good antiviral activities against tobacco mosaic virus (TMV). Among them, compound 14b exerted a superior inhibitory effect in comparison to commercially available antiviral agent ribavirin, and could be expected to become a novel antiviral candidate. Molecular biology experiments and molecular docking research found that the potential target of compound 14b was TMV coat protein (CP). These compounds also showed broad-spectrum anti-fungal activities against seven kinds of plant fungi.
1. Introduction
Since ancient times, agriculture has been the foundation of the national economy, and crop diseases and insect pests have been the main agricultural disasters perplexing the world. Their characteristics of wide distribution, irregular outbreak and serious harm have brought great difficulties to prevention and control. Pesticides are the key to plant protection and can effectively prevent and control the diseases of various crops [1]. However, the long-term and irregular use of traditional pesticides has caused serious environmental pollution, and also caused some pests to develop drug resistance. The development of novel, efficient and low-risk pesticides has become an important scientific issue that needs to be solved urgently. Natural products have novel structure, rich resources, extensive biological activities and a unique mechanism of action and are valuable resources for new pesticide discovery. Natural products can be made into pesticides directly or in the form of derivatives. They originate from nature and are used in nature, so they have ecological safety [2,3].
Indole ring system exists in a variety of natural products and has a wide range of pharmacological activities. Some of them have been used in various drugs, such as antihistamines [4], antifungals [5], antimicrobials [6], antioxidants [7], plant growth regulators [8], HIV inhibitors [9], anticonvulsants [10], anti-inflammatory analgesics [11], etc. Indole is probably one of the most important components of heterocyclic compounds for drug discovery [12]. Bisindole alkaloids are a class of star molecules in indole compounds. Since the isolation of a bis(indolyl)imidazole topsentin and its analogs from Topsentia genitrix more than a decade ago [13], scientists have successively isolated various novel bisindole alkaloids from marine organisms as well as from microorganisms; they found that these alkaloids had powerful and diverse biological activities, including antiviral, antitumor, antibacterial and anti-inflammatory activities [14]. Their unique and concise structures and broad biological activities make them an important leading skeleton in the creation of new drugs.
Bisindole alkaloid barakacin was first isolated from the ruminal bacterium Pseudomonas aeruginosa strain ZIO in 2012 and found to have weak and non-selective cytotoxic activity against human cancer cell lines LXFA 629L, LXFL 529L (lung), MAXF 401NL (breast), etc. [15]. In 2016, Buzid’ s group accomplished the first synthesis of barakacin with an overall yield of 11% (Scheme S1 in Supplementary Materials) [16]. So far, the research on barakacin is limited to this, and there is no report on the control of crop diseases and insect pests.
Tobacco mosaic virus (TMV) has a history of more than 100 years, and with the development of the first half of the 20th century, TMV has become the preferred model virus for basic research such as disease resistance and breeding [17,18]. After the plant is infected, the virus proliferates rapidly on the leaves, causing extensive damage to the leaves and a large number of necrotic spots on the surface. The severely diseased leaves will shrink, twist or even die. At present, there is no specific drug for TMV. Ribavirin, the widely used antiviral agent, can only give an inhibitory effect of less than 50% at the concentration of 500 µg/mL.
We have been engaged in the discovery of new antiviral agents based on natural products and found a series of alkaloids that had good antiviral activities [19,20,21]. In this work, we chose the alkaloid barakacin as the lead compound, designed and synthesized a series of its derivatives, evaluated their antiviral activities against TMV and investigated their structure-activity relationship and antiviral action mechanism systematically (Figure 1). In addition, the fungicidal activities of these compounds against seven phytopathogenic fungi (Fusarium oxysporium f. sp. cucumeris; Physalospora piricola; Rhizoctonia cerealis; Alternaria solani; Botrytis cinereal; Phytophthora capsici; Sclerotinia sclerotiorum) were also investigated.
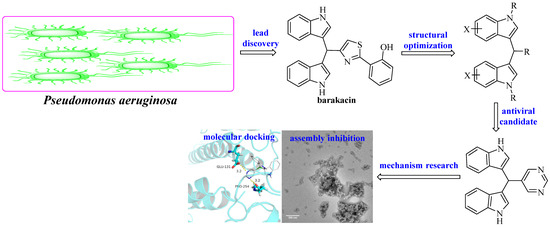
Figure 1.
Molecular design and structural optimization based on barakacin.
2. Results and Discussion
2.1. Chemistry
Buzid’s group achieved the synthesis of alkaloid barakacin with an overall yield of 11%, but the yield of the Suzuki coupling step was only 26%, and the raw materials were not easy to obtain, which limited its application in the study of structure-activity relationship (SAR) of these compounds [16]. Therefore, it is necessary to develop a simple and practical route to realize the structural diversity derivation of these compounds. Using the commercially available o-hydroxybenzylnitrile as raw material, compound 1 was obtained by methylation, which reacted with sodium hydrosulfide to produce thioamide 2. Compound 2 reacted with ethyl bromopyruvate to obtain thiazole 3, and then the key intermediate aldehyde 5 was obtained through reduction and oxidation of 3. Aldehyde 5 reacted with indole under the catalysis of I2 to give alkaloid derivative 6. After compound 6 was demethylated, the natural product barakacin (7) was obtained via seven steps in 26% overall yield (Scheme 1). Compared with the methods reported in the literature, the raw materials of our route are easy to obtain, the operation is simple and it is easier to realize the structural diversity derivation of these kinds of compounds. In order to evaluate the effects of hydroxyl on benzene ring and indole NH on biological activity, we designed and synthesized derivatives 8a, 8b and 9a–9e (Scheme 1). To explore the influence of different substituents on the indole benzene ring on the biological activity, compounds 13a–13d were designed and synthesized in a similar way (Scheme 2). Heterocycles are important pharmacophores with a wide range of biological activities. Based on structural simplification and scaffold hopping strategy, we designed and synthesized bisindole derivatives 14a–14g with different ring structures (Scheme 3). To further investigate the substitution effect of the heterocyclic region, we designed and synthesized derivatives 15 and 16a–16c (Scheme 4).
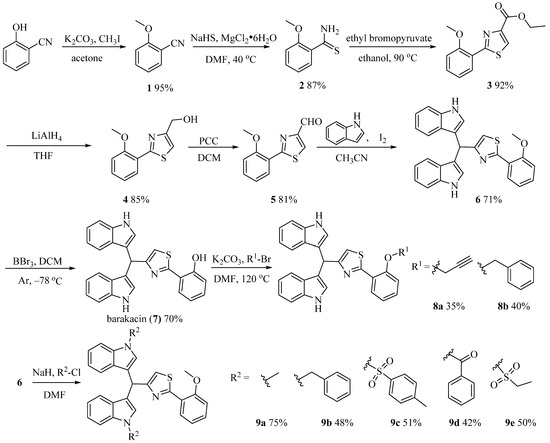
Scheme 1.
Synthesis of barakacin (7) and its derivatives 8a, 8b and 9a–9e.
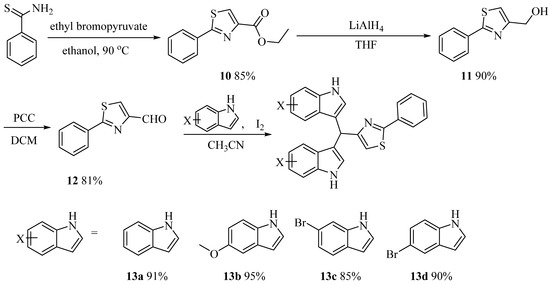
Scheme 2.
Synthesis of 13a–13d.
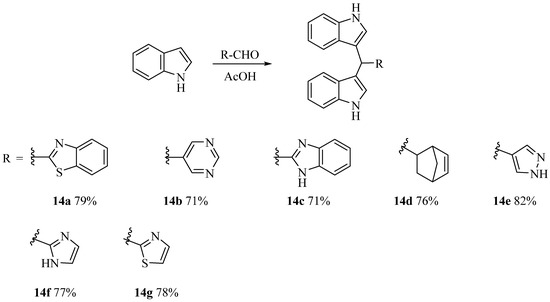
Scheme 3.
Synthesis of 14a–14g.
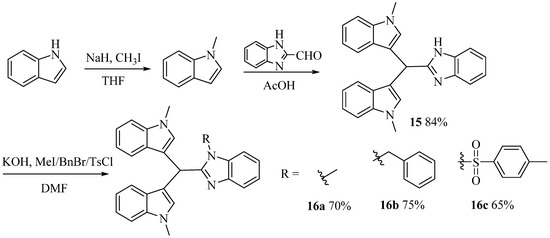
Scheme 4.
Synthesis of 15 and 16a–16c.
2.2. Antiviral Activity Result and Structure-Activity Relationship (SAR)
Alkaloid barakacin (7) and its derivatives 6, 8a, 8b, 9a–9e, 13a–13d, 14a–14g, 15, 16a–16c were screened for their anti-TMV activities with commercial ribavirin as control. Results in Table 1 show that most of these compounds displayed very good in vivo antiviral activities. Among them, compounds 8a, 9d, 13b, 13d, 14a, 14c, 14d and 14g presented similar levels of antiviral activities as ribavirin. The antiviral activities of natural compound barakacin (7) and its derivatives 8b, 9c, 13a, 13c and 14b are better than that of ribavirin. Compound 14b, with advantages of simple structure, convenient synthesis and good biological activity, emerged as a new antiviral candidate.

Table 1.
In vivo antiviral activities of compounds 6, 7, 8a–8b, 9a–9e, 13a–13d, 14a–14g, 15, 16a–16c and ribavirin against TMV at 500 μg/mL a.
Barakacin (7) displayed prominent antiviral activity; the introduction of methyl or propargyl into the hydroxyl of phenyl ring leads to the decrease in activity (inhibition rate: 7 > 8a > 6), while the introduction of benzyl can maintain activity (inhibition rate: 7 ≈ 8b). Indole NH also has a great impact on the antiviral activity: the introduction of benzyl or ethylsulfonyl into indole NH leads to the decrease in activity (inhibition rate: 9b ≈ 9e < 6); the introduction of methyl can maintain the original activity (inhibition rate: 9a ≈ 6); and the introduction of p-toluenesulfonyl or benzoyl can improve the activity (inhibition rate: 9c > 9d > 6), indicating that the introduction of electron-withdrawing aryl in this region is beneficial to the antiviral activity. Compounds 13a–13d have the same level of inhibitory activity, indicating that the introduction of functional groups at position 5 or 6 of indole ring is tolerable. The structure simplification of natural products has always been an important strategy for the creation of new pesticides [22,23]. It can not only save the synthesis costs, but also improve the physical and chemical properties of natural products. Based on structural simplification and scaffold hopping strategy, compounds 14a–14g were synthesized by one-step reaction from commercially available indole and corresponding aldehydes. Except for 14e and 14f, all compounds showed similar or higher level of antiviral activities as ribavirin, especially compound 14b, which exhibited similar biological activity to parent compound 7. Taking 14c as an example, we further investigated the substitution effect on NH. The results showed that the introduction of methyl, benzyl or p-toluenesulfonyl on NH was not conducive to biological activity (inhibition rate: 14c > 15 and 16a–16c).
2.3. Preliminary Mechanism Research
TMV is a baculovirus with a total length of 300 nm, which is composed of CP and RNA; usually, protein monomers first form 20S disk, and then assemble with RNA into complete virus particles; in this process, CP plays a very important role [24]. We selected compound 14b to further evaluate the mode of action of these compounds via our reported method [20] via transmission electron microscopy (FEI Tecnai G2 F20). Results in Figure 2 show that TMV CP and RNA could assemble into virus particles under conventional conditions, but the assembly was blocked when compound 14b was added, indicating that compound 14b can interfere with the assembly process of the virus. Considering the good inactive activity of compound 14b, we speculated that it might act on the CP. We further designed the interaction experiment between 14b and TMV protein (Figure 3). The results show that under normal circumstances, the protein monomer could successfully generate the 20S disk with a regular shape, but when 14b was added, the formation of the 20S disk could hardly be observed, indicating that the compound could act on the viral protein. From the preliminary mechanism research, it can be inferred that these compounds inhibit virus assembly by acting on viral proteins.
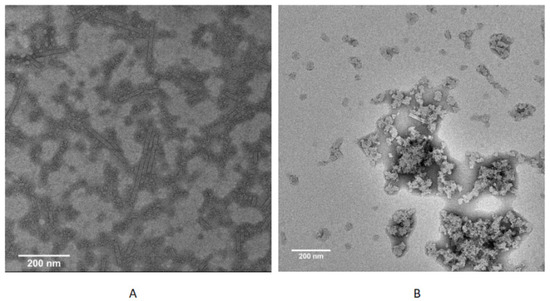
Figure 2.
TMV rod assembly inhibition of compound 14b (200 nm scale bar): (A) 20S CP disk + RNA, (B) 20S CP disk + RNA + 10 μM 14b.
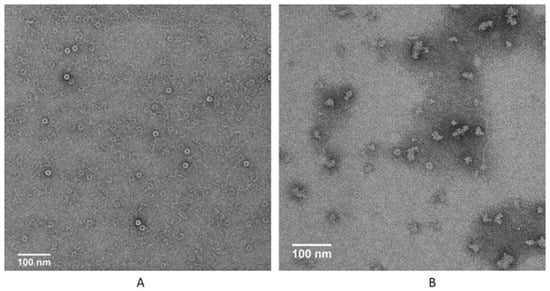
Figure 3.
20S CP disk assembly inhibition of compound 14b (100 nm scale bar): (A) CP, (B) CP + 10 μM 14b.
2.4. Molecular Docking
Molecular docking is an important and advantageous tool to verify the interaction sites and affinities between biological macromolecules and small molecule drugs at the molecular level. It is widely used in new drug creation and target prediction [25,26,27]. In order to evaluate the hypothetical binding mode between compounds 8b, 9a and 14b with TMV CP (PDB code: 1EI7), we carried out molecular docking via AutoDock Vina 1.1.2 [28]. As shown in Figure 4: compound 8b and amino acid residue SER-138 formed a 2.1 Å hydrogen bond (Figure 4A); compound 14b formed a 3.2 Å hydrogen bond with amino acid residues GLU-131 and PRO-254, respectively (Figure 4C); while compound 9a with poor activity did not form a hydrogen bond with the target protein (Figure 4B). The results of molecular docking are consistent with SAR, which further confirms the interaction between these compounds and TMV CP.
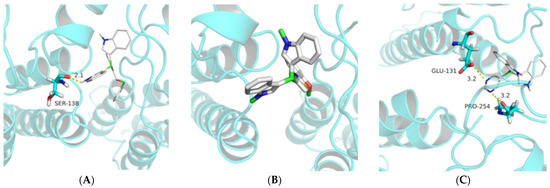
Figure 4.
Molecule Docking Results of Compounds 8b (A), 9a (B) and 14b (C) with TMV CP.
2.5. Fungicidal Activity Result and Structure-Activity Relationship (SAR)
Considering the biodiversity of natural products, we further studied the antifungal activities of these compounds with commercial fungicides carbendazim and chlorothalonil as controls (Table 2). The results showed that the target compounds also exhibited broad-spectrum fungicidal activities, among which compounds 6, 7, 8a, 13a, 14a–14c, 14e and 14g displayed better inhibitory activities against Botrytis cinereal than chlorothalonil. The anti-fungal activities of 8a, 9a–9e, 14b, 16b and 16c against Sclerotinia sclerotiorum are superior to those of commercial fungicides carbendazim and chlorothalonil. The fungicidal activity of 14b against Rhizoctonia cerealis is better than that of carbendazim. In general, these compounds showed good inhibitory activities against Physalospora piricola and Sclerotinia sclerotiorum. For Sclerotinia sclerotiorum: barakacin (7) presented a similar level of fungicidal activity as carbendazim; the introduction of propargyl group into the hydroxyl group of phenyl ring can improve the fungicidal activity; compounds 9a–9e exhibited better activities than 6, indicating that the introduction of substituents into indole NH can improve the fungicidal activity; compound 13a showed higher fungicidal activity than 13b–13d, indicating that the introduction of functional groups at position 5 or 6 of the indole ring is unfavorable to the activity; except for compound 14f, all compounds with simplified structure (14a–14e, 14g, 15, 16a–16c) showed fungicidal activities equivalent to or better than that of natural product 7. Compound 14b also displayed broad-spectrum and efficient fungicidal activity and could be used as novel anti-fungal candidate.

Table 2.
In vitro fungicidal activities of compounds 6, 7, 8a–8b, 9a–9e, 13a–13d, 14a–14g, 15, 16a–16c, chlorothalonil and carbendazim against 7 kinds of fungi a.
3. Materials and Methods
3.1. Synthetic Procedures
3.1.1. Chemicals
The reagents were purchased from commercial sources and were used as received. All anhydrous solvents were dried and purified by standard techniques prior to use. The synthesis steps of derivatives 8a–8b, 9a–9e, 10–12, 13a–13d, 14a–14g, 15, 16a–16c are placed in Supplementary Materials.
3.1.2. Instruments
Melting points were determined on an X-4 melting point apparatus equipped with a binocular microscope (Beijing Tech Instruments Co., Beijing. China). Nuclear magnetic resonance (NMR) spectra were obtained with a 400 MHz (100 MHz for 13C) instrument (Bruker, Billerica, MA, USA) at room temperature in either CDCl3 or DMSO-d6 as the solvent. Chemical shifts were measured relative to residual solvent peaks of DMSO-d6 as internal standards (1H: δ = 2.5 and 3.3 ppm; 13C: δ = 39.9 ppm) or CDCl3 as internal standards (1H: δ = 7.26 ppm; 13C: δ = 77.0 ppm). The following abbreviations are used to designate chemical shift multiplicities: s = singlet, d = doublet, dd = doublet of doublets, t = triplet, m = multiplet and brs = broad singlet. High-resolution mass spectra were obtained with an Ionspec, 7.0 T Fourier transform ion cyclotron resonance mass spectrometer.
3.1.3. Preparation of 2-Methoxybenzonitrile (1)
The 2-Cyanophenol (1.00 g, 8.4 mmol) and K2CO3 (1.16 g, 8.4 mmol) were dissolved in 50 mL of acetone, and CH3I (1.05 mL, 16.8 mmol) was added dropwise to the mixture at 0 °C. The progress of the reaction was monitored by thin layer chromatography (TLC). After the reaction was complete, the solvent was removed in vacuo at 50 °C, water was added, the reaction solution was extracted with ethyl acetate (50 mL × 3), dried with anhydrous Na2SO4, filtered with suction and the solvent was removed in vacuo at 50 °C to obtain 1 as a yellow oil with 95% yield; 1H NMR (400 MHz, CDCl3) δ 7.54 (ddd, J = 7.4, 4.4, 2.6 Hz, 2H), 7.00 (ddd, J = 12.2, 6.5, 2.7 Hz, 2H), 3.93 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 161.2, 134.5, 133.7, 120.8, 116.6, 111.4, 101.6, 56.0.
3.1.4. Preparation of 2-Methoxybenzothioamide (2)
An amount of 70% NaHS (3.00 g, 52.57 mmol) and MgCl2·6H2O (5.34 g, 26.29 mmol) were added to 20 mL of DMF, and compound 1 dissolved in 10 mL of DMF was added dropwise to the system under vigorous stirring. The reaction was stirred at 40 °C for 6 h. After the reaction was completed by TLC monitoring, part of the solvent was removed, 100 mL of 1 M hydrochloric acid was added, and the mixture was stirred for 0.5 h. The reaction solution was extracted with ethyl acetate (30 mL × 3), the organic layer was washed with water, dried over anhydrous Na2SO4, and concentrated to obtain compound 2 as a reddish-brown solid with 87% yield, mp. 139–141 °C. 1H NMR (400 MHz, CDCl3) δ 9.05 (s, 1H), 8.63 (dd, J = 8.0, 1.8 Hz, 1H), 8.18 (s, 1H), 7.47 (ddd, J = 8.4, 7.3, 1.8 Hz, 1H), 7.20–6.87 (m, 2H), 3.97 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 199.2, 156.3, 136.4, 133.7, 124.9, 121.3, 111.5, 56.1.
3.1.5. Preparation of Ethyl 2-(2-Methoxyphenyl)thiazole-4-carboxylate (3)
Compound 2 (2.00 g, 12 mmol) was dissolved in 200 mL of ethanol, and under stirring at 0 °C, ethyl 3-bromo-2-oxopropionate (3.50 g, 18 mmol) was gradually added dropwise to the system. Then, the ice bath was removed, and the reaction system was heated to 90 °C. After the completion of the reaction by TLC monitoring, the solvent was evaporated under reduced pressure to obtain a crude product, which was purified by flash chromatography on silica gel using petroleum ether and ethyl acetate (4:1, v/v) as eluent to give title compound 3 as a pale yellow solid with 92% yield, mp. 137–139 °C; 1H NMR (400 MHz, CDCl3) δ 8.45 (dd, J = 7.9, 1.7 Hz, 1H), 8.13 (s, 1H), 7.50–7.27 (m, 1H), 7.09–6.86 (m, 2H), 4.37 (q, J = 7.1 Hz, 2H), 3.96 (s, 3H), 1.36 (t, J = 7.1 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 163.0, 162.0, 156.5, 146.0, 131.4, 129.1, 127.9, 121.5, 121.1, 111.2, 61.3, 55.6, 14.4.
3.1.6. Preparation of (2-(2-Methoxyphenyl)thiazol-4-yl)methanol (4)
LiAlH4 (0.14 g, 3.8 mmol) was dissolved in 20 mL of THF, and under stirring at 0 °C, it was slowly dropped into a solution of compound 3 (1.00 g, 3.8 mmol) in THF. The mixture was stirred at room temperature and monitored by TLC. After the reaction was completed, water was added to quench it, the result solution was extracted with ethyl acetate, dried over anhydrous Na2SO4, and concentrated to obtain compound 4 as a yellow solid with 85% yield, mp. 116–118 °C; 1H NMR (400 MHz, CDCl3) δ 8.40 (dd, J = 7.8, 1.7 Hz, 1H), 7.54–7.35 (m, 1H), 7.39 (s, 1H), 7.19–6.99 (m, 2H), 4.86 (s, 2H), 4.04 (s, 3H), 2.95 (s, 1H). 13C NMR (100 MHz, CDCl3) δ 162.9, 156.4, 154.9, 130.9, 128.4, 121.9, 121.1, 115.5, 111.4, 61.1, 55.6.
3.1.7. Preparation of 2-(2-Methoxyphenyl)thiazole-4-carbaldehyde (5)
Under stirring at room temperature, PCC (1.46 g, 6.8 mmol) was dissolved in 45 mL of dichloromethane, and a solution of compound 4 (1.00 g, 4.5 mmol) in dichloromethane (40 mL) was added dropwise to it. After 4 h of reaction, the mixture was washed with 10% hydrochloric acid (30 mL × 2) and extracted with ethyl acetate (50 mL × 3). The organic layer was washed with brine (50 mL), water (50 mL × 2), then dried over anhydrous Na2SO4, filtered by suction, and concentrated in vacuo. The residue was purified by flash chromatography on silica gel using petroleum ether and ethyl acetate (4:1, v/v) as eluent to obtain the target compound 5 as a white solid with 81% yield, mp. 81–83 °C; 1H NMR (400 MHz, CDCl3) δ 10.14 (s, 1H), 8.49 (dd, J = 7.9, 1.7 Hz, 1H), 8.22 (s, 1H), 7.63–7.44 (m, 1H), 7.11 (ddd, J = 22.3, 14.6, 4.7 Hz, 2H), 4.06 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 185.4, 163.7, 156.6, 153.7, 131.7, 128.8, 128.0, 121.3, 111.4, 55.6.
3.1.8. Preparation of 4-(Di(1H-indol-3-yl)methyl)-2-(2-methoxyphenyl)thiazole (6)
Indole (0.468 g, 4 mmol) and compound 5 (0.219 g, 1 mmol) were dissolved in acetonitrile (45 mL), and I2 (0.127 g, 0.5 mmol) was added with stirring. After reacting for 30 min at room temperature, the reaction was quenched by adding 5% Na2S2O3 solution (20 mL). The organic solvent was removed in vacuo at 55 °C, and the resulting solution was extracted with ethyl acetate (50 mL × 3). Then, the organic phase was washed with brine (100 mL), dried over anhydrous Na2SO4, filtered with suction and concentrated. The residue was purified by column chromatography with petroleum ether and ethyl acetate (5:1, v/v) as eluent to obtain compound 6 as a pale yellow solid with 71% yield, mp. 210–212 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.82 (d, J = 1.8 Hz, 2H), 8.24 (dd, J = 7.9, 1.7 Hz, 1H), 7.45 (d, J = 8.2 Hz, 2H), 7.44–7.40 (m, 1H), 7.33 (d, J = 8.1 Hz, 2H), 7.29 (s, 1H), 7.22 (d, J = 8.0 Hz, 1H), 7.09–7.00 (m, 5H), 6.88 (dd, J = 11.0, 3.9 Hz, 2H), 6.07 (s, 1H), 3.99 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 160.7, 159.2, 156.4, 136.9, 131.2, 128.1, 127.0, 124.0, 122.1, 121.3, 119.6, 118.5, 117.5, 116.5, 112.7, 111.9, 56.3, 36.9. HRMS (ESI) calcd for C27H22N3OS [M+H]+ 436.1478, found 436.1472.
3.1.9. Preparation of Barakacin (7)
Compound 6 (1.00 g, 2.3 mmol) was dissolved in dichloromethane, and a solution of 1 mol/L BBr3 in CH2Cl2 (28 mL, 27.6 mmol) was added dropwise at −78 °C under an inert atmosphere. The reaction solution was stirred at room temperature overnight, and then H2O (28 mL, 27.6 mmol) was added. The reaction solution was partitioned and extracted with ethyl acetate (30 mL × 3), the organic phase was washed with brine (100 mL), dried over anhydrous Na2SO4, filtered with suction and concentrated. The residue was subjected to column chromatography with petroleum ether and ethyl acetate (2:1, v/v) as eluent to obtain compound 7 as a red solid with 70% yield, mp. 118–120 °C; 1H NMR (400 MHz, DMSO-d6) δ 11.71 (s, 1H), 10.87 (s, 2H), 8.00–7.79 (m, 1H), 7.42 (dd, J = 24.7, 5.9 Hz, 2H), 7.43–7.22 (m, 4H), 7.15–6.98 (m, 4H), 6.99–6.84 (m, 4H), 6.09 (d, J = 11.8 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δ 165.0, 158.3, 155.4, 136.4, 131.1, 127.2, 126.5, 123.4, 120.9, 119.5, 119.0, 118.3, 116.8, 116.5, 114.6, 111.5, 36.1. HRMS (ESI) calcd for C26H18N3OS [M-H]− 420.1176, found 420.1179.
3.2. Biological Assay
Each test was repeated three times at 25 ± 1 °C. The active effect is expressed in percentage scale of 0–100 (0: no activity; 100: total inhibited).
Specific steps for the anti-TMV activity and fungicidal activity tests and mode of action research were carried out using the literature method [19,20], which also can be seen in Supplementary Materials.
4. Conclusions
In summary, using cheap o-hydroxybenzonitrile as raw material, the natural product barakacin was synthesized in seven steps with a total yield of 26%, which provided a practical method for obtaining the structural diversity derivatives of these compounds. A series of barakacin derivatives were designed based on structural simplification and scaffold hopping strategy, synthesized, and evaluated for their antiviral activities. These compounds were found to have good anti-TMV activities for the first time. Through systematic SAR research, compound 14b was found to be a new antiviral candidate with advantages of simple structure, convenient synthesis and good biological activity. Mode of action research revealed that these compounds might inhibit virus assembly by acting on viral proteins. Further fungicidal activity research indicated that these compounds also exerted broad-spectrum anti-fungal activities, especially for Physalospora piricola and Sclerotinia sclerotiorum. Compound 14b with broad-spectrum and efficient fungicidal activity emerged as a novel anti-fungal candidate. This work provides a case for the discovery of new pesticides based on the structural simplification of natural products.
Supplementary Materials
The following supplementary materials can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28073032/s1, Section S1: Scheme S1, synthesis of barakacin; Section S2: synthetic procedures of derivatives 8a–8b, 9a–9e, 10–12, 13a–13d, 14a–14g, 15, 16a–16c; Section S3: detailed bio-assay procedures for the fungicidal activity, anti-TMV activity, and mode of action research; Section S4: References in Supplementary Materials; Section S5: copies of 1H & 13C NMR spectra (Figures S1–S64). References [29,30,31,32,33,34,35] were cited in Supplementary Materials.
Author Contributions
Conceptualization, Z.W., A.L. and Q.W.; methodology, Y.G.; software, X.H. and L.Y.; validation, H.Z. and S.L.; formal analysis, Q.M. and P.Z.; investigation, Y.Z. and Z.Z.; resources, Z.W.; data curation, Y.G.; writing—original draft preparation, Z.W.; writing—review and editing, Z.W.; visualization, Y.G.; supervision, Z.W., A.L. and Q.W.; project administration, Z.W.; funding acquisition, A.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the S&T Program of Hebei, grant number 21326504D; and Hebei Natural Science Foundation, grant number B2020202028.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data used to support the findings of this study are included within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Grigalunas, M.; Brakmann, S.; Waldmann, H. Chemical evolution of natural product structure. J. Am. Chem. Soc. 2022, 144, 3314–3329. [Google Scholar] [CrossRef] [PubMed]
- Begg, G.S.; Cook, S.M.; Dye, R.; Ferrante, M.; Franck, P.; Lavigne, C.; Lövei, G.L.; Mansion-Vaquie, A.; Pell, J.K.; Petit, S.; et al. A functional overview of conservation biological control. Crop Prot. 2017, 97, 145–158. [Google Scholar] [CrossRef]
- Battaglia, S.; Boldrini, E.; Settimo, F.D.; Dondio, G.; Motta, C.L.; Marini, A.M.; Primofiore, G. Indole amide derivatives: Synthesis, structure-activity relationships and molecular modelling studies of a new series of histamine H1-receptor antagonists. Eur. J. Med. Chem. 1999, 34, 93–105. [Google Scholar] [CrossRef]
- Przhevalskii, N.M.; Magedov, I.V.; Drozd, V.N. New derivatives of indole. Synthesis of s-(indolyl-3) diethyl dithiocarbamates. Chem. Heterocycl. Comp. 1997, 33, 1475–1476. [Google Scholar] [CrossRef]
- Hiari, Y.M.A.; Qaisi, A.M.; Abadelah, M.W.; Voelter, W. Synthesis and antibacterial activity of some substituted 3-(aryl)- and 3- (heteroaryl)indoles. Monatsh. Chem. 2006, 137, 243–248. [Google Scholar] [CrossRef]
- Creager, A.N.H. The life of a virus: Tobacco mosaic virus as an experimental model, 1930–1965. Endeavour 2002, 26, 76–427. [Google Scholar]
- Creager, A.N.H.; Scholthof, K.B.G.; Citovsky, V.; Scholthof, H.B. Tobacco mosaic virus. Pioneering research for a century. Plant cell 1999, 11, 301–308. [Google Scholar]
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef]
- Tan, D.X.; Chen, L.D.; Poeggeler, B.; Manchester, L.C.; Reiter, R.J. Melatonin: A potent, endogenous hydroxyl radical scavenger. Endocr. J. 1993, 1, 57–60. [Google Scholar]
- Abele, E.; Abele, R.; Dzenitis, O.; Lukevics, E. Indole and isatin oximes: Synthesis, reactions, and biological activity (review). Chem. Heterocycl. Comp. 2003, 39, 3–35. [Google Scholar] [CrossRef]
- Suzen, S.; Buyukbingol, E. Evaluation of anti-HIV activity of 5-(2-phenyl-3’-indolyl)-2-thiohydantoin. IL Farmaco 1998, 53, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Bartik, K.; Braekman, J.C.; Daloze, D.; Stoller, C.; Huysecom, J.; Vandevyver, G.; Ottinger, R. Topsentins, new toxic bis-indole alkaloids from the marine sponge Topsentia genitrix. Can. J. Chem. 1987, 65, 2118–2121. [Google Scholar] [CrossRef]
- Pandey, K.P.; Rahman, M.T.; Cook, J.M. Bisindole alkaloids from the Alstonia species: Recent isolation, bioactivity, biosynthesis, and synthesis. Molecules 2021, 26, 3459. [Google Scholar] [CrossRef]
- Zendah, I.; Shaaban, K.A.; Helmke, E.; Maier, A.; Fiebig, H.H.; Laatsch, H. Barakacin: A thiazolyl-indole alkaloid isolated from a ruminal Pseudomonas sp. Z. Naturforsch. 2012, 67, 417–420. [Google Scholar] [CrossRef]
- Buzid, A.; Muimhneacháin, E.Ó.; Reen, F.J.; Hayes, P.E.; Pardo, L.M.; Shang, F.J.; O’Gara, F.; Sperry, J.; Luong, J.H.T.; Glennon, J.D.; et al. Synthesis and electrochemical detection of a thiazolyl-indole natural product isolated from the nosocomial pathogen Pseudomonas aeruginosa. Anal. Bioanal. Electrochem. 2016, 408, 6361–6367. [Google Scholar] [CrossRef]
- El-Gendy Adel, A.; Abdou Naida, A.; El-Taber, Z.S.; El-Banna Hosny, A. Synthesis and biological activity of functionalized indole-2-carboxyates, triazino and pyridazino indoles. Alex. J. Pharm. Sci. 1997, 7, 99–103. [Google Scholar]
- Kumar, A.; Archana; Sharma, S.; Malik, N.; Sharma, P.; Kushik, K.; Saxena, K.K.; Srivastava, V.K. Synthesis of anti-inflammatory, analgesic and COX-II inhibitory activities of indolylpyrazolines. Indian J. Chem. B. 2004, 43, 1532–1536. [Google Scholar]
- Li, G.; Guo, J.C.; Wang, Z.W.; Liu, Y.X.; Song, H.B.; Wang, Q.M. Marine natural products for drug discovery: First discovery of kealiinines A–C and their derivatives as novel antiviral and antiphytopathogenic fungus agents. J. Agric. Food Chem. 2018, 66, 7310–7318. [Google Scholar] [CrossRef]
- Guo, J.C.; Hao, Y.N.; Ji, X.F.; Wang, Z.W.; Liu, Y.X.; Ma, D.J.; Li, Y.Q.; Pang, H.L.; Ni, J.P.; Wang, Q.M. Optimization, structure-activity relationship and mode of action of nortopsentin analogues containing thiazole and oxazole moieties. J. Agric. Food Chem. 2019, 67, 10018–10031. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.F.; Wang, Z.W.; Dong, J.; Liu, Y.X.; Lu, A.D.; Wang, Q.M. Discovery of topsentin alkaloids and their derivatives as novel antiviral and anti-phytopathogenic fungus agents. J. Agric. Food Chem. 2016, 64, 9143–9151. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Sparks, T.C.; Duke, S.O. Structure simplification of natural products as a lead generation approach in agrochemical discovery. J. Agric. Food chem. 2021, 69, 8324–8346. [Google Scholar] [CrossRef] [PubMed]
- Butler, P.J.G. The current picture of the structure and assembly of tobacco mosaic virus. J. Gen. Virol. 1984, 65, 253–279. [Google Scholar] [CrossRef]
- Hao, G.F.; Jiang, W.; Ye, Y.N.; Wu, F.X.; Zhu, X.L.; Guo, F.B.; Yang, G.F. ACFIS: A web server for fragment-based drug discovery. Nucleic Acids Res. 2016, 44, 550–556. [Google Scholar] [CrossRef]
- Dong, A.Y.; Wang, Z.; Huang, J.J.; Song, B.A.; Hao, G.F. Bioinformatic tools support decision-making in plant disease management. Trends Plant Sci. 2021, 26, 953–967. [Google Scholar] [CrossRef]
- Vidal-Limon, A.; Aguilar-Toalá, J.E.; Liceaga, A.M. Integration of molecular docking analysis and molecular dynamics simulations for studying food proteins and bioactive peptides. J. Agric. Food Chem. 2022, 70, 934–943. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. Autodock vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Wang, Z.W.; Wei, P.; Wang, L.Z.; Wang, Q.M. Design, synthesis, and anti-tobacco mosaic virus (TMV) activity of phenanthroindolizidines and their analogues. J. Agric. Food Chem. 2012, 60, 10212–10219. [Google Scholar] [CrossRef]
- Gooding, G.V., Jr.; Hebert, T.T. A simple technique for purification of tobacco mosaic virus in large quantities. Phytopathology 1967, 57, 1285–1290. [Google Scholar]
- Li, S.Z.; Wang, D.M.; Jiao, S.M. Pesticide Experiment Methods-Fungicide Sector; Li, S.Z., Ed.; Agriculture Press of China: Beijing, China, 1991; pp. 93–94. [Google Scholar]
- Zhao, H.P.; Liu, Y.X.; Cui, Z.P.; Beattie, D.; Gu, Y.C.; Wang, Q.M. Design, synthesis, and biological activities of arylmethylamine substituted chlorotriazine and methylthiotriazine compounds. J. Agric. Food Chem. 2011, 59, 11711–11717. [Google Scholar] [CrossRef] [PubMed]
- Leberman, R. Isolation of plant viruses by means of simple coacervates. Virol 1966, 30, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Fraenkel Conrat, H.; Williams, R.C. Reconstitution of active tobacco mosaic virus fromits inactive protein and nucleic acid components. Proc. Natl. Acad. Sci. USA 1955, 41, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Seyedi, S.S.; Shukri, M.; Hassandarvish, P.; Oo, A.; Muthu, S.E.; Abubakar, S.; Zandi, K. Computational approach towards exploring potential anti-chikungunya activity of selected flavonoids. Sci. Rep. 2016, 6, 24027. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).