Menin–MLL1 Interaction Small Molecule Inhibitors: A Potential Therapeutic Strategy for Leukemia and Cancers
Abstract
1. Introduction
2. Type of Hydroxymethyl and Aminomethyl Piperidine Inhibitors
2.1. M-525
2.2. M-808
2.3. M-89
2.4. M-1121
3. Type of Thiophenpyrimidine Inhibitors
3.1. MI-2/MI-3
3.2. MI-136
3.3. MI-538
3.4. MI-463/MI-503
3.5. VTP-50469
3.6. BAY-155
3.7. KO-539
3.8. MI-1481
3.9. MI-3454
4. Type of Macrocyclic Mimics Peptide Inhibitor
MCP-1
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brandi, M.L.; Agarwal, S.K.; Perrier, N.D.; Lines, K.E.; Valk, G.D.; Thakker, R.V. Multiple Endocrine Neoplasia Type 1: Latest Insights. Endocr. Rev. 2021, 42, 133–170. [Google Scholar] [CrossRef]
- Soto-Feliciano, Y.M.; Sánchez-Rivera, F.J.; Perner, F.; Barrows, D.W.; Kastenhuber, E.R.; Ho, Y.J.; Carroll, T.; Xiong, Y.; Anand, D.; Soshnev, A.A.; et al. A Molecular Switch between Mammalian MLL Complexes Dictates Response to Menin-MLL Inhibition. Cancer Discov. 2023, 13, 146–169. [Google Scholar] [CrossRef]
- Dreijerink, K.; Timmers, H.; Brown, M. Twenty years of menin: Emerging opportunities for restoration of transcriptional regulation in MEN1. Endocr. Relat. Cancer 2017, 24, T135–T145. [Google Scholar] [CrossRef]
- Lemmens, I.; Van de Ven, W.J.; Kas, K.; Zhang, C.X.; Giraud, S.; Wautot, V.; Buisson, N.; De Witte, K.; Salandre, J.; Lenoir, G.; et al. Identification of the multiple endocrine neoplasia type 1 (MEN1) gene. The European Consortium on MEN1. Hum. Mol. Genet. 1997, 6, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.; Khan, A.P.; Asangani, I.A.; Cieślik, M.; Prensner, J.R.; Wang, X.; Iyer, M.K.; Jiang, X.; Borkin, D.; Escara-Wilke, J.; et al. Targeting the MLL complex in castration-resistant prostate cancer. Nat. Med. 2015, 21, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Kempinska, K.; Malik, B.; Borkin, D.; Klossowski, S.; Shukla, S.; Miao, H.; Wang, J.; Cierpicki, T.; Grembecka, J. Pharmacologic Inhibition of the Menin-MLL Interaction Leads to Transcriptional Repression of PEG10 and Blocks Hepatocellular Carcinoma. Mol. Cancer Ther. 2018, 17, 26–38. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, E.H.; Hsieh, J.J. MLL fusions: Pathways to leukemia. Cancer Biol. Ther. 2009, 8, 1204–1211. [Google Scholar] [CrossRef]
- Pui, C.H.; Gaynon, P.S.; Boyett, J.M.; Chessells, J.M.; Baruchel, A.; Kamps, W.; Silverman, L.B.; Biondi, A.; Harms, D.O.; Vilmer, E.; et al. Outcome of treatment in childhood acute lymphoblastic leukaemia with rearrangements of the 11q23 chromosomal region. Lancet 2002, 359, 1909–1915. [Google Scholar] [CrossRef]
- Liang, K.; Volk, A.G.; Haug, J.S.; Marshall, S.A.; Woodfin, A.R.; Bartom, E.T.; Gilmore, J.M.; Florens, L.; Washburn, M.P.; Sullivan, K.D.; et al. Therapeutic Targeting of MLL Degradation Pathways in MLL-Rearranged Leukemia. Cell 2017, 168, 59–72.e13. [Google Scholar] [CrossRef]
- Sorensen, P.H.; Chen, C.S.; Smith, F.O.; Arthur, D.C.; Domer, P.H.; Bernstein, I.D.; Korsmeyer, S.J.; Hammond, G.D.; Kersey, J.H. Molecular rearrangements of the MLL gene are present in most cases of infant acute myeloid leukemia and are strongly correlated with monocytic or myelomonocytic phenotypes. J. Clin. Investig. 1994, 93, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Cierpicki, T.; Grembecka, J. Challenges and opportunities in targeting the menin-MLL interaction. Future Med. Chem. 2014, 6, 447–462. [Google Scholar] [CrossRef]
- He, S.; Senter, T.J.; Pollock, J.; Han, C.; Upadhyay, S.K.; Purohit, T.; Gogliotti, R.D.; Lindsley, C.W.; Cierpicki, T.; Stauffer, S.R.; et al. High-affinity small-molecule inhibitors of the menin-mixed lineage leukemia (MLL) interaction closely mimic a natural protein-protein interaction. J. Med. Chem. 2014, 57, 1543–1556. [Google Scholar] [CrossRef] [PubMed]
- Honkanen, E.; Pippuri, A.; Kairisalo, P.; Nore, P.; Karppanen, H.; Paakkari, I. Synthesis and antihypertensive activity of some new quinazoline derivatives. J. Med. Chem. 1983, 26, 1433–1438. [Google Scholar] [CrossRef]
- Xu, S.; Aguilar, A.; Xu, T.; Zheng, K.; Huang, L.; Stuckey, J.; Chinnaswamy, K.; Bernard, D.; Fernández-Salas, E.; Liu, L.; et al. Design of the First-in-Class, Highly Potent Irreversible Inhibitor Targeting the Menin-MLL Protein-Protein Interaction. Angew. Chem. Int. Ed. Engl. 2018, 57, 1601–1605. [Google Scholar] [CrossRef]
- Xu, S.; Aguilar, A.; Huang, L.; Xu, T.; Zheng, K.; McEachern, D.; Przybranowski, S.; Foster, C.; Zawacki, K.; Liu, Z.; et al. Discovery of M-808 as a Highly Potent, Covalent, Small-Molecule Inhibitor of the Menin-MLL Interaction with Strong In Vivo Antitumor Activity. J. Med. Chem. 2020, 63, 4997–5010. [Google Scholar] [CrossRef]
- Aguilar, A.; Zheng, K.; Xu, T.; Xu, S.; Huang, L.; Fernandez-Salas, E.; Liu, L.; Bernard, D.; Harvey, K.P.; Foster, C.; et al. Structure-Based Discovery of M-89 as a Highly Potent Inhibitor of the Menin-Mixed Lineage Leukemia (Menin-MLL) Protein-Protein Interaction. J. Med. Chem. 2019, 62, 6015–6034. [Google Scholar] [CrossRef] [PubMed]
- Borkin, D.; He, S.; Miao, H.; Kempinska, K.; Pollock, J.; Chase, J.; Purohit, T.; Malik, B.; Zhao, T.; Wang, J.; et al. Pharmacologic inhibition of the Menin-MLL interaction blocks progression of MLL leukemia in vivo. Cancer Cell 2015, 27, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Aguilar, A.; Xu, S.; Huang, L.; Chinnaswamy, K.; Sleger, T.; Wang, B.; Gross, S.; Nicolay, B.N.; Ronseaux, S.; et al. Discovery of M-1121 as an Orally Active Covalent Inhibitor of Menin-MLL Interaction Capable of Achieving Complete and Long-Lasting Tumor Regression. J. Med. Chem. 2021, 64, 10333–10349. [Google Scholar] [CrossRef]
- Pollock, J.; Borkin, D.; Lund, G.; Purohit, T.; Dyguda-Kazimierowicz, E.; Grembecka, J.; Cierpicki, T. Rational Design of Orthogonal Multipolar Interactions with Fluorine in Protein-Ligand Complexes. J. Med. Chem. 2015, 58, 7465–7474. [Google Scholar] [CrossRef] [PubMed]
- Grembecka, J.; He, S.; Shi, A.; Purohit, T.; Muntean, A.G.; Sorenson, R.J.; Showalter, H.D.; Murai, M.J.; Belcher, A.M.; Hartley, T.; et al. Menin-MLL inhibitors reverse oncogenic activity of MLL fusion proteins in leukemia. Nat. Chem. Biol. 2012, 8, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Zha, J.; Shi, Y.; Li, Y.; Yuan, D.; Chen, Q.; Lin, F.; Fang, Z.; Yu, Y.; Dai, Y.; et al. Co-inhibition of HDAC and MLL-menin interaction targets MLL-rearranged acute myeloid leukemia cells via disruption of DNA damage checkpoint and DNA repair. Clin. Epigenetics 2019, 11, 137. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Fang, L.; Heuberger, J.; Kranz, A.; Schipper, J.; Scheckenbach, K.; Vidal, R.O.; Sunaga-Franze, D.Y.; Müller, M.; Wulf-Goldenberg, A.; et al. The Wnt-Driven Mll1 Epigenome Regulates Salivary Gland and Head and Neck Cancer. Cell Rep. 2019, 26, 415–428.e5. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.Y.; Yuan, J.B.; Zhang, L.; He, C.X.; Lin, X.; Xu, B.; Jin, G.H. Loss of MLL Induces Epigenetic Dysregulation of Rasgrf1 to Attenuate Kras-Driven Lung Tumorigenesis. Cancer Res. 2022, 82, 4153–4163. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, L.; Peng, H.; Dai, S.; Quan, Y.; Wang, M.; Wang, J.; Bi, Z.; Zheng, Y.; Zhou, S.; et al. An organoid-based drug screening identified a menin-MLL inhibitor for endometrial cancer through regulating the HIF pathway. Cancer Gene Ther. 2021, 28, 112–125. [Google Scholar] [CrossRef]
- Borkin, D.; Pollock, J.; Kempinska, K.; Purohit, T.; Li, X.; Wen, B.; Zhao, T.; Miao, H.; Shukla, S.; He, M.; et al. Property Focused Structure-Based Optimization of Small Molecule Inhibitors of the Protein-Protein Interaction between Menin and Mixed Lineage Leukemia (MLL). J. Med. Chem. 2016, 59, 892–913. [Google Scholar] [CrossRef]
- Zhang, D.; An, X.; Li, Q.; Man, X.; Chu, M.; Li, H.; Zhang, N.; Dai, X.; Yu, H.; Li, Z. Thioguanine Induces Apoptosis in Triple-Negative Breast Cancer by Regulating PI3K-AKT Pathway. Front. Oncol. 2020, 10, 524922. [Google Scholar] [CrossRef]
- Chou, C.W.; Tan, X.; Hung, C.N.; Lieberman, B.; Chen, M.; Kusi, M.; Mitsuya, K.; Lin, C.L.; Morita, M.; Liu, Z.; et al. Menin and Menin-Associated Proteins Coregulate Cancer Energy Metabolism. Cancers 2020, 12, 2715. [Google Scholar] [CrossRef]
- Teinturier, R.; Luo, Y.; Decaussin-Petrucci, M.; Vlaeminck-Guillem, V.; Vacherot, F.; Firlej, V.; Bonnavion, R.; Abou Ziki, R.; Gherardi, S.; Goddard, I.; et al. Men1 disruption in Nkx3.1-deficient mice results in AR(low)/CD44(+) microinvasive carcinoma development with the dysregulated AR pathway. Oncogene 2021, 40, 1118–1127. [Google Scholar] [CrossRef]
- Luo, Y.; Vlaeminck-Guillem, V.; Baron, S.; Dallel, S.; Zhang, C.X.; Le Romancer, M. MEN1 silencing aggravates tumorigenic potential of AR-independent prostate cancer cells through nuclear translocation and activation of JunD and β-catenin. J. Exp. Clin. Cancer Res. 2021, 40, 270. [Google Scholar] [CrossRef]
- Xu, B.; Li, S.H.; Zheng, R.; Gao, S.B.; Ding, L.H.; Yin, Z.Y.; Lin, X.; Feng, Z.J.; Zhang, S.; Wang, X.M.; et al. Menin promotes hepatocellular carcinogenesis and epigenetically up-regulates Yap1 transcription. Proc. Natl. Acad. Sci. USA 2013, 110, 17480–17485. [Google Scholar] [CrossRef]
- Bullinger, L.; Döhner, K.; Döhner, H. Genomics of Acute Myeloid Leukemia Diagnosis and Pathways. J. Clin. Oncol. 2017, 35, 934–946. [Google Scholar] [CrossRef] [PubMed]
- Dzama, M.M.; Steiner, M.; Rausch, J.; Sasca, D.; Schönfeld, J.; Kunz, K.; Taubert, M.C.; McGeehan, G.M.; Chen, C.W.; Mupo, A.; et al. Synergistic targeting of FLT3 mutations in AML via combined menin-MLL and FLT3 inhibition. Blood 2020, 136, 2442–2456. [Google Scholar] [CrossRef] [PubMed]
- Brzezinka, K.; Nevedomskaya, E.; Lesche, R.; Haegebarth, A.; Ter Laak, A.; Fernández-Montalván, A.E.; Eberspaecher, U.; Werbeck, N.D.; Moenning, U.; Siegel, S.; et al. Characterization of the Menin-MLL Interaction as Therapeutic Cancer Target. Cancers 2020, 12, 201. [Google Scholar] [CrossRef] [PubMed]
- Fiskus, W.; Daver, N.; Boettcher, S.; Mill, C.P.; Sasaki, K.; Birdwell, C.E.; Davis, J.A.; Das, K.; Takahashi, K.; Kadia, T.M.; et al. Activity of menin inhibitor ziftomenib (KO-539) as monotherapy or in combinations against AML cells with MLL1 rearrangement or mutant NPM1. Leukemia 2022, 36, 2729–2733. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, K.W.; LeBlanc, T.W.; Chen, H. Novel agents and regimens in acute myeloid leukemia: Latest updates from 2022 ASH Annual Meeting. J. Hematol. Oncol. 2023, 16, 17. [Google Scholar] [CrossRef]
- Shi, A.; Murai, M.J.; He, S.; Lund, G.; Hartley, T.; Purohit, T.; Reddy, G.; Chruszcz, M.; Grembecka, J.; Cierpicki, T. Structural insights into inhibition of the bivalent menin-MLL interaction by small molecules in leukemia. Blood 2012, 120, 4461–4469. [Google Scholar] [CrossRef] [PubMed]
- Rozovskaia, T.; Feinstein, E.; Mor, O.; Foa, R.; Blechman, J.; Nakamura, T.; Croce, C.M.; Cimino, G.; Canaani, E. Upregulation of Meis1 and HoxA9 in acute lymphocytic leukemias with the t(4:11) abnormality. Oncogene 2001, 20, 874–878. [Google Scholar] [CrossRef]
- Armstrong, S.A.; Staunton, J.E.; Silverman, L.B.; Pieters, R.; den Boer, M.L.; Minden, M.D.; Sallan, S.E.; Lander, E.S.; Golub, T.R.; Korsmeyer, S.J. MLL translocations specify a distinct gene expression profile that distinguishes a unique leukemia. Nat. Genet. 2002, 30, 41–47. [Google Scholar] [CrossRef]
- Ayton, P.M.; Cleary, M.L. Transformation of myeloid progenitors by MLL oncoproteins is dependent on Hoxa7 and Hoxa9. Genes Dev. 2003, 17, 2298–2307. [Google Scholar] [CrossRef]
- Huang, J.; Gurung, B.; Wan, B.; Matkar, S.; Veniaminova, N.A.; Wan, K.; Merchant, J.L.; Hua, X.; Lei, M. The same pocket in menin binds both MLL and JUND but has opposite effects on transcription. Nature 2012, 482, 542–546. [Google Scholar] [CrossRef]
- Sarkar, D.; Wang, X.Y.; Fisher, P.B. Targeting JunD: A potential strategy to counteract hormone-refractory prostate cancer. Cell Cycle 2011, 10, 3433. [Google Scholar] [CrossRef] [PubMed]
- Millena, A.C.; Vo, B.T.; Khan, S.A. JunD Is Required for Proliferation of Prostate Cancer Cells and Plays a Role in Transforming Growth Factor-β (TGF-β)-induced Inhibition of Cell Proliferation. J. Biol. Chem. 2016, 291, 17964–17976. [Google Scholar] [CrossRef] [PubMed]
- Elliott, B.; Millena, A.C.; Matyunina, L.; Zhang, M.; Zou, J.; Wang, G.; Zhang, Q.; Bowen, N.; Eaton, V.; Webb, G.; et al. Essential role of JunD in cell proliferation is mediated via MYC signaling in prostate cancer cells. Cancer Lett. 2019, 448, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, L.K.; Teh, S.; Sud, S.; Kerk, S.; Zebolsky, A.; Treichel, S.; Thomas, D.; Halbrook, C.J.; Lee, H.J.; Kremer, D.; et al. Menin regulates the serine biosynthetic pathway in Ewing sarcoma. J. Pathol. 2018, 245, 324–336. [Google Scholar] [CrossRef]
- Gale, R.E.; Green, C.; Allen, C.; Mead, A.J.; Burnett, A.K.; Hills, R.K.; Linch, D.C. The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood 2008, 111, 2776–2784. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, W.; Deng, Q.; Tang, S.; Wang, P.; Xu, P.; Wang, J.; Yu, M. The prognostic and diagnostic value of circulating tumor cells in bladder cancer and upper tract urothelial carcinoma: A meta-analysis of 30 published studies. Oncotarget 2017, 8, 59527–59538. [Google Scholar] [CrossRef]
- Borkin, D.; Klossowski, S.; Pollock, J.; Miao, H.; Linhares, B.M.; Kempinska, K.; Jin, Z.; Purohit, T.; Wen, B.; He, M.; et al. Complexity of Blocking Bivalent Protein-Protein Interactions: Development of a Highly Potent Inhibitor of the Menin-Mixed-Lineage Leukemia Interaction. J. Med. Chem. 2018, 61, 4832–4850. [Google Scholar] [CrossRef]
- Klossowski, S.; Miao, H.; Kempinska, K.; Wu, T.; Purohit, T.; Kim, E.; Linhares, B.M.; Chen, D.; Jih, G.; Perkey, E.; et al. Menin inhibitor MI-3454 induces remission in MLL1-rearranged and NPM1-mutated models of leukemia. J. Clin. Investig. 2020, 130, 981–997. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, L.; Huang, J.; Bernard, D.; Karatas, H.; Navarro, A.; Lei, M.; Wang, S. Structure-based design of high-affinity macrocyclic peptidomimetics to block the menin-mixed lineage leukemia 1 (MLL1) protein-protein interaction. J. Med. Chem. 2013, 56, 1113–1123. [Google Scholar] [CrossRef]
- Kurmasheva, R.T.; Bandyopadhyay, A.; Favours, E.; Pozo, V.D.; Ghilu, S.; Phelps, D.A.; McGeehan, G.M.; Erickson, S.W.; Smith, M.A.; Houghton, P.J. Evaluation of VTP-50469, a menin-MLL1 inhibitor, against Ewing sarcoma xenograft models by the pediatric preclinical testing consortium. Pediatr. Blood Cancer 2020, 67, e28284. [Google Scholar] [CrossRef]
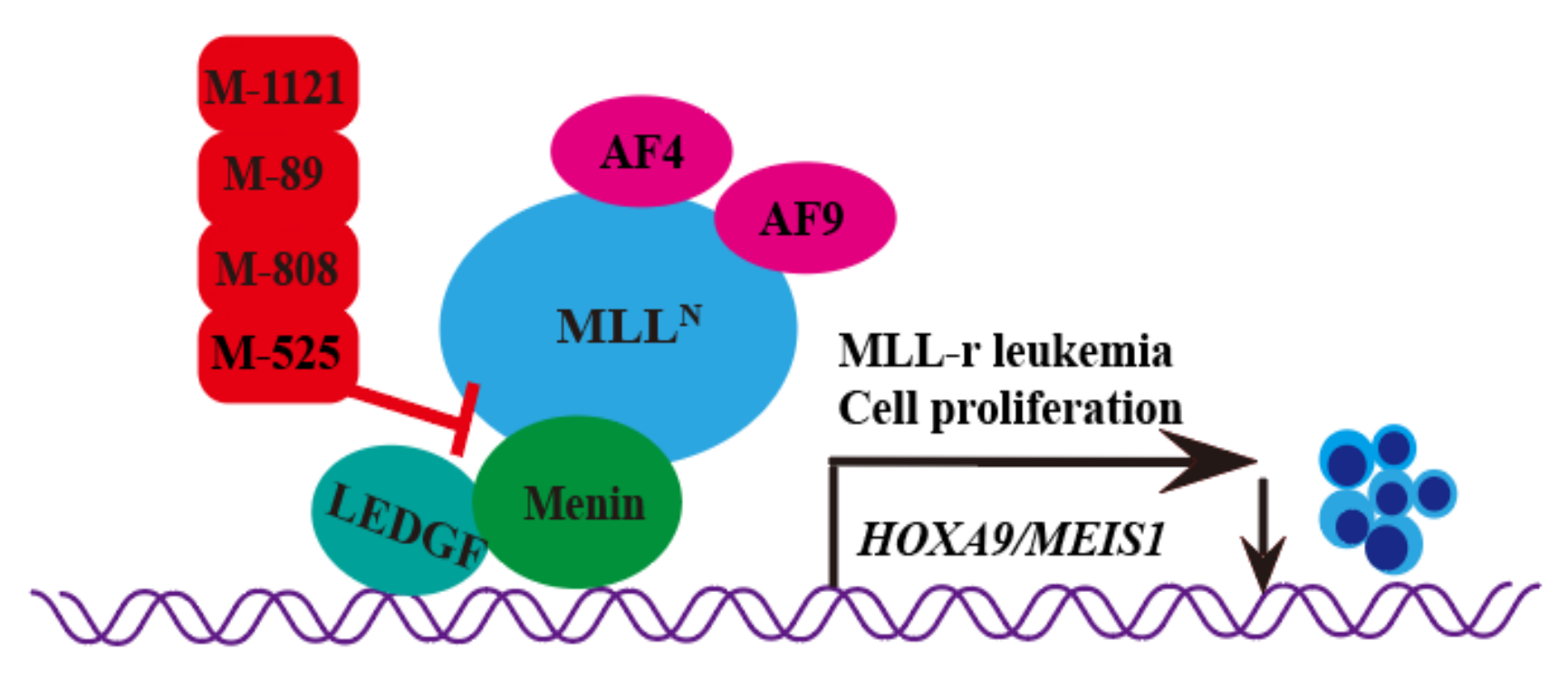
| Classification | Structure | Name | Diseases | Test Model | IC50 | Targeting | Reference |
|---|---|---|---|---|---|---|---|
| Hydroxymethyl and aminomethyl piperidine | 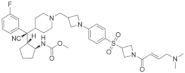 | MI-525 | Leukemia | In vitro | 3 nM | HOXA9 and MEIS1 | [14] |
 | M-808 | Leukemia | In vivo In vitro | 4 nM | HOXA9 and MEIS1 | [15] | |
 | M-89 | Leukemia | In vitro | 25 nM | HOXA9 and MEIS1 | [16,17] | |
 | M-1121 | Leukemia | In vitro | 10.3 nM | HOXA9 and MEIS1 | [18] |
| Classification | Structure | Name | Diseases | Test Model | IC50 | Targeting | Reference |
|---|---|---|---|---|---|---|---|
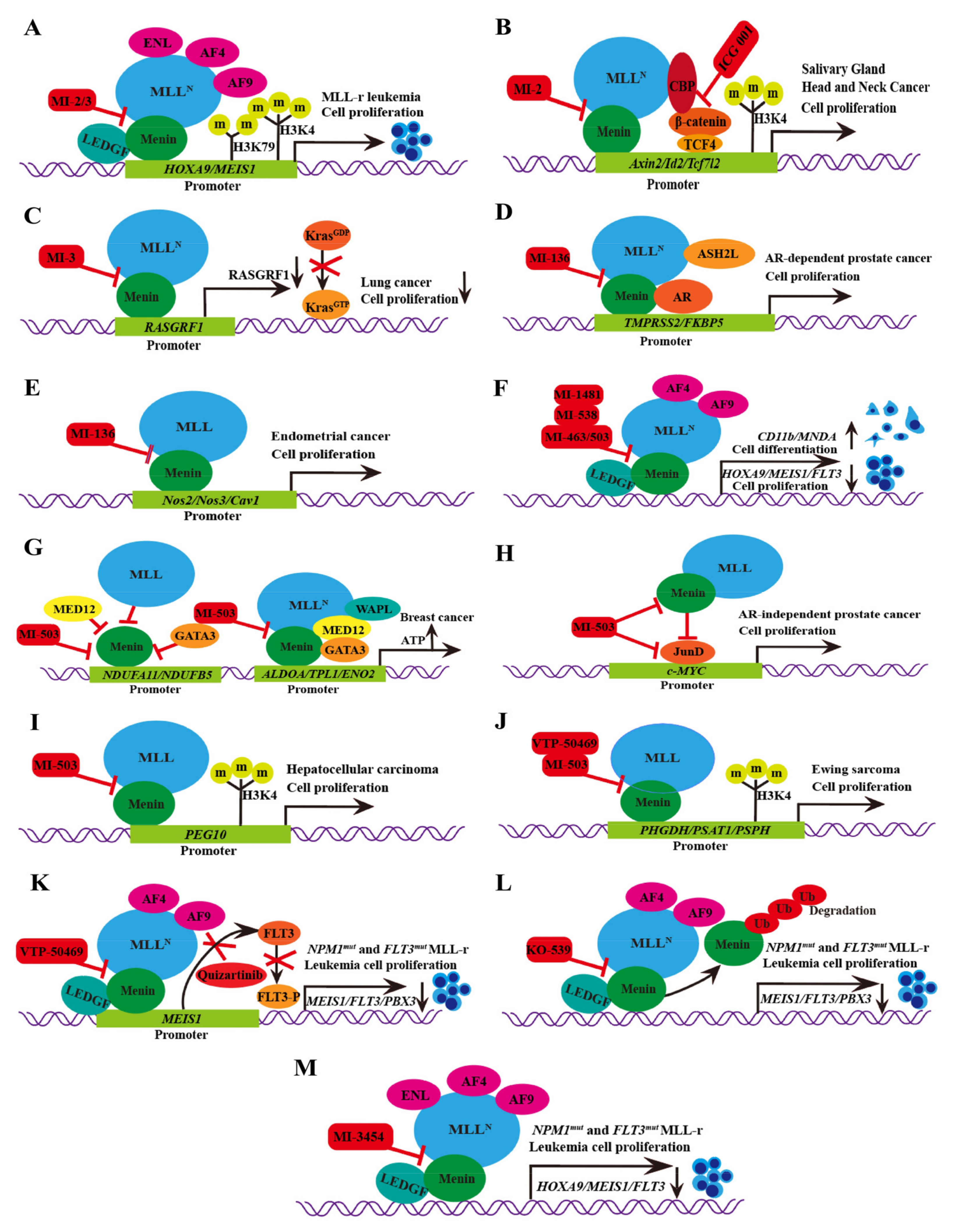
| Thiophenpyrimidine |  | MI-2 | Leukemia | In vitro | 446 nM | HOXA9 and MEIS1 | [20,21] |
| Head and neck tumors | In vivo | Non determined | Wnt signaling pathway | [22] | |||
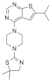 | MI-3 | Leukemia | In vitro | 648 nM | HOXA9 and MEIS1 | [20,21] | |
| Lung cancer | In vivo In vitro | Non determined | Rasgrf 1 | [23] | |||
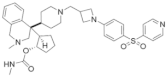
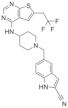 | MI-136 | Prostate cancer | In vivo In vitro | 5.59 nM | AR signaling pathway | [5] | |
| Endometrial cancer | In vivo In vitro | 4.5 μM | HIF signaling pathway | [24] | |||
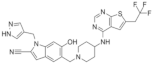 | MI-538 | Leukemia | In vivo In vitro | 21 nM | HOXA9 and MEIS1 | [25] | |
 | MI-463 | Leukemia | In vitro | 15.3 nM | HOXA9 and MEIS1 | [17] | |
| Breast cancer | In vitro | 13.99 μM | Apoptosis | [26] | |||
 | MI-503 | Leukemia | In vivo In vitro | 14.7 nM | HOXA9 and MEIS1 | [17] | |
| Breast cancer | In vitro | Non determined | Glycolytic and Oxidative phosphorylation | [27] | |||
| Prostate cancer | In vivo In vitro | 3.1 μM | AR signaling pathway;menin and JunD | [28,29,30] | |||
| Hepatocellular carcinoma | In vivo In vitro | 14 nM | PEG10 | [6] | |||
| Ewing’s sarcoma | In vitro | 3 μM | Serine biosynthesis pathway | [31] | |||
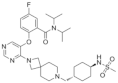 | VTP-50469 | Leukemia | In vitro | 3 nM | MEIS1 and FLT3 | [32] | |
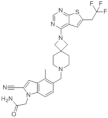 | BAY-155 | Leukemia | In vitro | 8 nM | MEIS1 | [33] | |
 | KO-539 | Leukemia | In vivo In vitro | Non determined | MEIS1, FLT3 and PBX3 | [34,35] | |
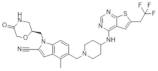 | MI-1481 | Leukemia | In vitro | 3.6 nM | HOXA9 and MEIS1 | [36] | |
 | MI-3454 | Leukemia | In vivo In vitro | 0.51 nM | HOXA9, MEIS1 and FLT3 | [37] |
| Classification | Structure | Name | Diseases | Test Model | IC50 | Targeting | Reference |
|---|---|---|---|---|---|---|---|
| Macrocyclic mimics perptide |  | MCP-1 | Leukemia | In vitro | 18 nM | HOXA9 and MEIS1 | [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Q.; Xu, M.; Kang, Z.; Zhang, M.; Luo, Y. Menin–MLL1 Interaction Small Molecule Inhibitors: A Potential Therapeutic Strategy for Leukemia and Cancers. Molecules 2023, 28, 3026. https://doi.org/10.3390/molecules28073026
Shi Q, Xu M, Kang Z, Zhang M, Luo Y. Menin–MLL1 Interaction Small Molecule Inhibitors: A Potential Therapeutic Strategy for Leukemia and Cancers. Molecules. 2023; 28(7):3026. https://doi.org/10.3390/molecules28073026
Chicago/Turabian StyleShi, Qing, Meiqi Xu, Zhijian Kang, Manjie Zhang, and Yakun Luo. 2023. "Menin–MLL1 Interaction Small Molecule Inhibitors: A Potential Therapeutic Strategy for Leukemia and Cancers" Molecules 28, no. 7: 3026. https://doi.org/10.3390/molecules28073026
APA StyleShi, Q., Xu, M., Kang, Z., Zhang, M., & Luo, Y. (2023). Menin–MLL1 Interaction Small Molecule Inhibitors: A Potential Therapeutic Strategy for Leukemia and Cancers. Molecules, 28(7), 3026. https://doi.org/10.3390/molecules28073026






