Screening out Biomarkers of Tetrastigma hemsleyanum for Anti-Cancer and Anti-Inflammatory Based on Spectrum-Effect Relationship Coupled with UPLC-Q-TOF-MS
Abstract
1. Introduction
2. Results
2.1. Screening for Biomarkers of Anticancer Activity of T. hemsleyanum in HepG2 Hepatocellular Carcinoma Cells
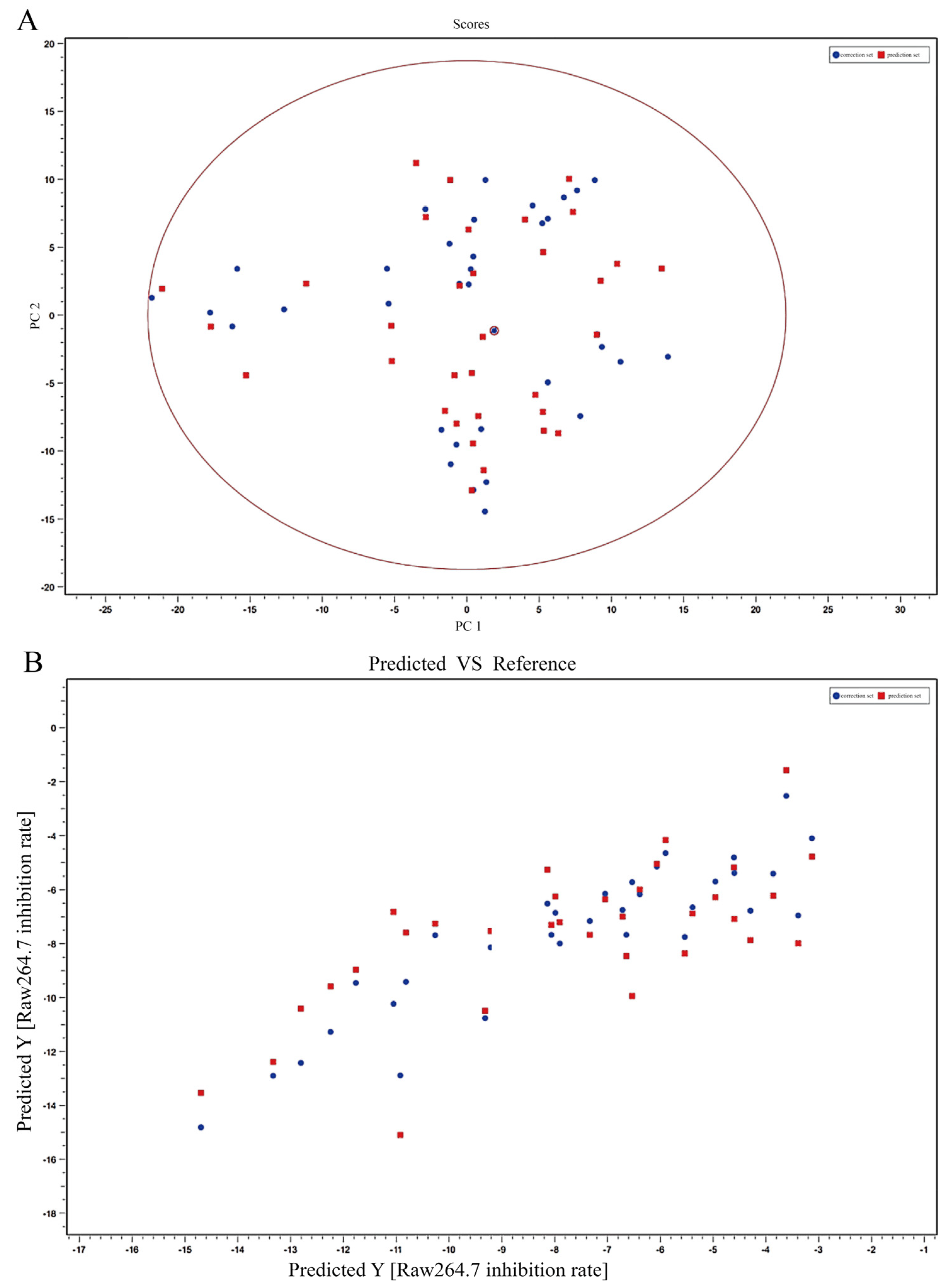
2.1.1. UPLC-Q-TOF-MSE Fingerprint Profiles of T. hemsleyanum Extracts from Different Origins
2.1.2. Anti-Cancer Efficacy Analysis on HepG2 Cells
2.1.3. Spectrum-Effect Relationship of T. hemsleyanum Extracts on HepG2 Anticancer Activity
2.2. Screening for Biomarkers of Anticancer Activity of T. Hemsleyanum in HuH-7 Hepatocellular Carcinoma Cells
2.2.1. Anti-Cancer Efficacy Analysis on HuH-7 Cells
2.2.2. Spectrum-Effect Relationship of T. hemsleyanum for Anti-Cancer with HuH-7
2.3. Screening for Biomarkers of Anti-Inflammatory Activity of T. hemsleyanum on Inflammatory Model Damage in RAW264.7 Cells
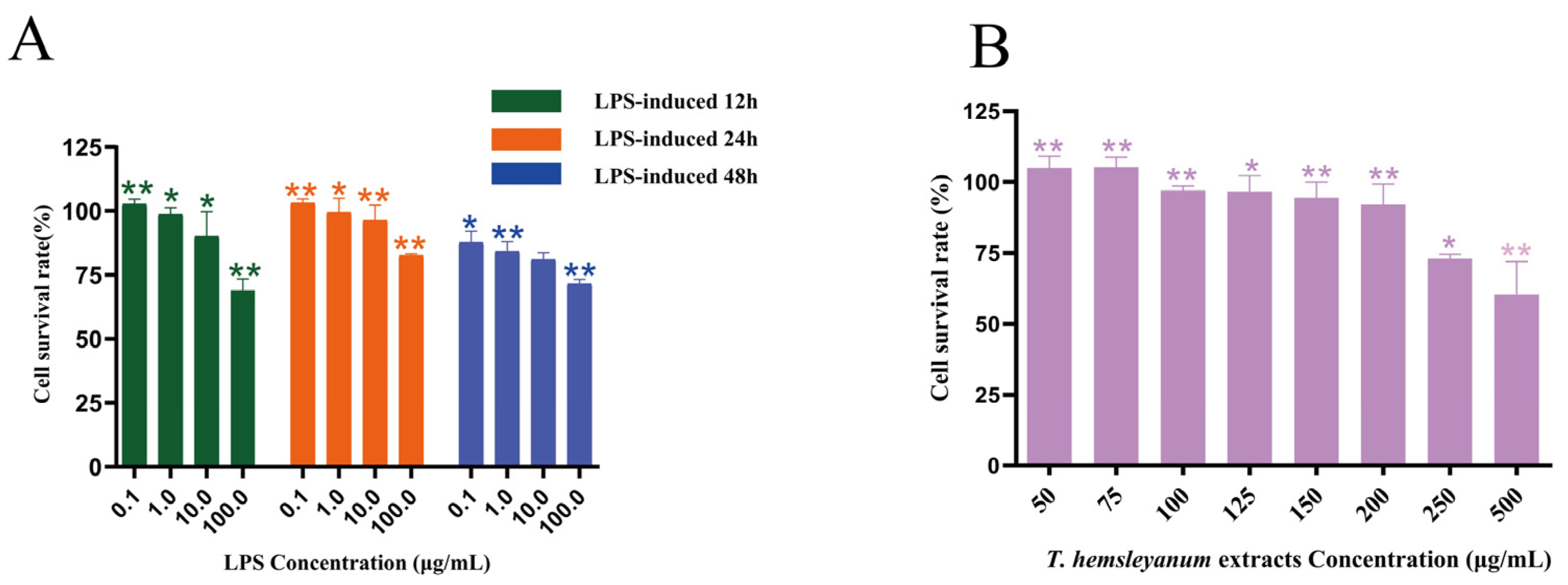
2.3.1. T. hemsleyanum Extracts and LPS Exhibits Cytotoxicity on RAW264.7 Cells
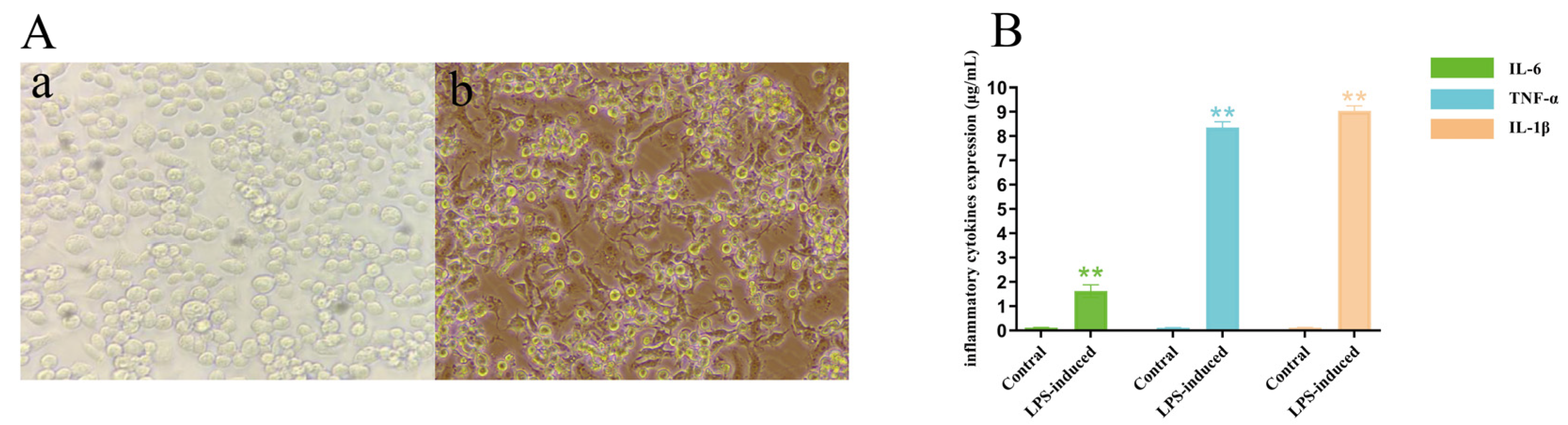
2.3.2. Establishment of a Model of LPS-Induced Inflammation in Macrophage RAW264.7 Cells
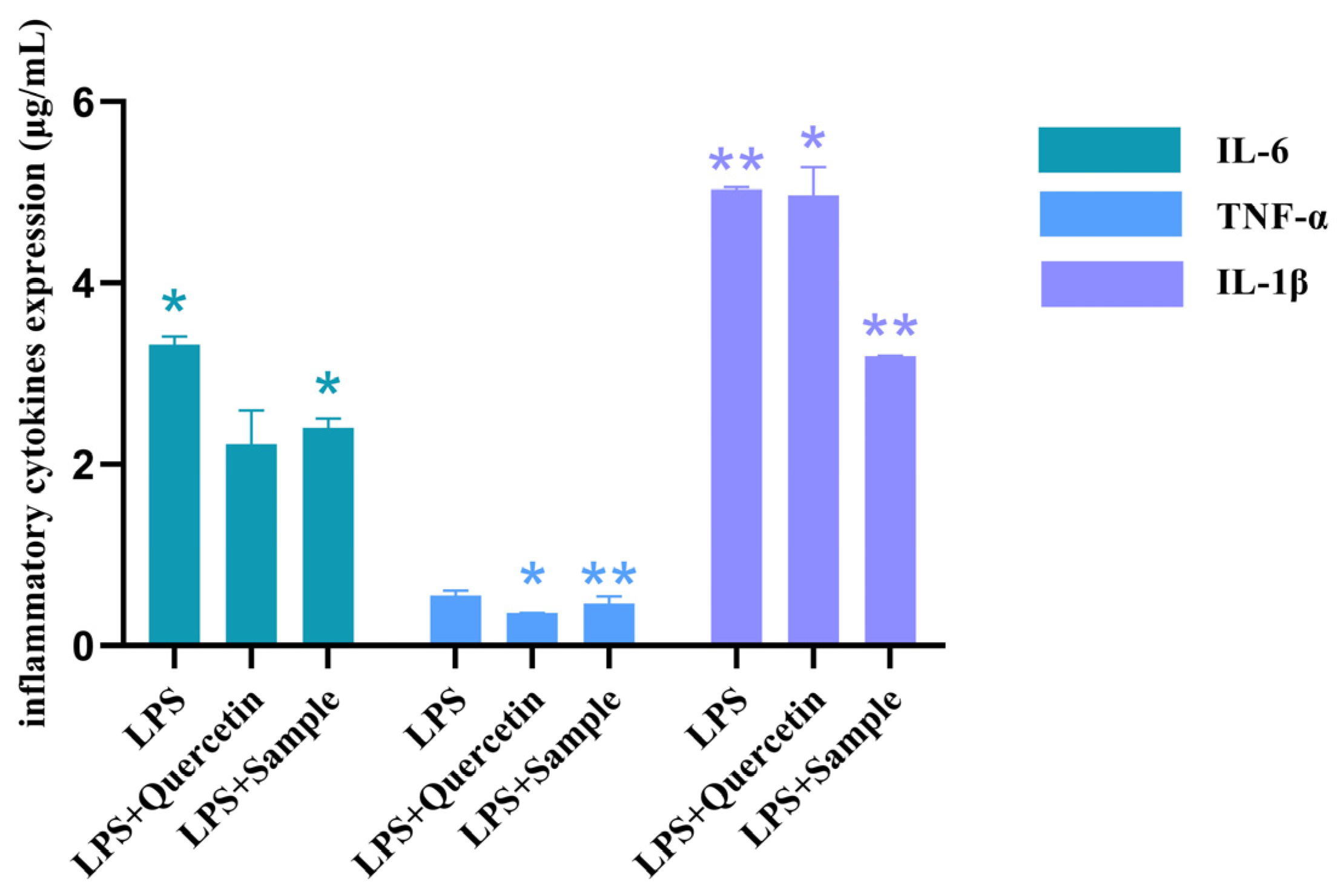
2.3.3. Effect of T. Hemsleyanum Extract on the Secretion Function of Pro-Inflammatory Factors in an Inflammatory Model
2.3.4. Spectrum-Effect Relationship Associated with the Inflammatory Factor IL-1β in the LPS-Induced Inflammation Model
2.3.5. Docking Study
3. Discussion
3.1. Quality-Oriented UPLC-Q-TOF-MS Metabolite Fingerprint
3.2. Variation on Anti-Cancer and Anti-Inflammatory Effects of T. hemsleyanum from Different Origins
3.3. Screening Biomarkers of T. hemsleyanum by the Spectrum-Effect Relationship Method and Chemometric Analysis
4. Materials and Methods
4.1. Reagents and Materials
4.2. Extraction
4.3. Liquid Chromatography Tandem Mass Spectrometry (LC–MS/MS) Analysis for Metabolite Identification
4.4. Assessing the Effect of T. hemsleyanum Extract on Cancer Cell Proliferation
4.4.1. Cell Culture
4.4.2. Cell Viability Detected by CCK-8 Assay
4.5. Assessment of the Effect of T. hemsleyanum Extract on Inflammatory Model Injury
4.5.1. Cytotoxicity Assays
4.5.2. LPS-Induced RAW264.7 Cell Model
4.5.3. Effect of T. hemsleyanum Extract on the Secretion Function of Pro-Inflammatory Cytokines in an Inflammatory Model
4.5.4. Molecular Docking
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ji, T.; Ji, W.W.; Wang, J.; Chen, H.J.; Peng, X.; Cheng, K.J.; Qiu, D.; Yang, W.J. A comprehensive review on traditional uses, chemical compositions, pharmacology properties and toxicology of Tetrastigma hemsleyanum. J. Ethnopharmacol. 2021, 264, 113247. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zheng, Y.; Xia, P.; Liang, Z. The research progresses and future prospects of Tetrastigma hemsleyanum Diels et Gilg: A valuable Chinese herbal medicine. J. Ethnopharmacol. 2021, 271, 113836. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Qian, C.; Zhou, F.; Guo, J.; Chen, N.; Gao, C.; Jin, B.; Ding, Z. Antipyretic and antitumor effects of a purified polysaccharide from aerial parts of Tetrastigma hemsleyanum. J. Ethnopharmacol. 2020, 253, 112663. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Xia, P.; Liang, Z. Molecular cloning and structural analysis of key enzymes in Tetrastigma hemsleyanum for resveratrol biosynthesis. Int. J. Biol. Macromol. 2021, 190, 19–32. [Google Scholar] [CrossRef]
- Xiong, Y.; Wu, X.; Rao, L. Tetrastigma hemsleyanum (Sanyeqing) root tuber extracts induces apoptosis in human cervical carcinoma HeLa cells. J. Ethnopharmacol. 2015, 165, 46–53. [Google Scholar] [CrossRef]
- Yang, L.; Shi, Y.S.; Ruan, X.; Wu, Q.F.; Qu, A.L.; Yu, M.F.; Qian, X.H.; Li, Z.H.; Ke, Z.J.; He, L.P.; et al. Salt interferences to metabolite accumulation, flavonoid biosynthesis and photosynthetic activity in Tetrastigma hemsleyanum. Environ. Exp. Bot. 2021, 194, 104765. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- Diniz, L.R.L.; Elshabrawy, H.A.; Souza, M.T.S.; Duarte, A.B.S.; Datta, S.; de Sousa, D.P. Catechins: Therapeutic Perspectives in COVID-19-Associated Acute Kidney Injury. Molecules 2021, 26, 5951. [Google Scholar] [CrossRef]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Aslam Gondal, T.; Saeed, F.; Imran, A.; Shahbaz, M.; Tsouh Fokou, P.V.; Umair Arshad, M.; Khan, H.; et al. Kaempferol: A Key Emphasis to Its Anticancer Potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef]
- Colombo, M.; Figueiró, F.; de Fraga Dias, A.; Teixeira, H.F.; Battastini, A.M.O.; Koester, L.S. Kaempferol-loaded mucoadhesive nanoemulsion for intranasal administration reduces glioma growth in vitro. Int. J. Pharm. 2018, 543, 214–223. [Google Scholar] [CrossRef]
- Riahi-Chebbi, I.; Souid, S.; Othman, H.; Haoues, M.; Karoui, H.; Morel, A.; Srairi-Abid, N.; Essafi, M.; Essafi-Benkhadir, K. The Phenolic compound Kaempferol overcomes 5-fluorouracil resistance in human resistant LS174 colon cancer cells. Sci. Rep. 2019, 9, 195. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Cao, L.; Li, R.; Fang, X.; Miao, Y. Protective effect of chlorogenic acid on the focal cerebral ischemia reperfusion rat models. Saudi. Pharm. J. 2017, 25, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Xiang, L. Pharmacological action and potential targets of chlorogenic acid. Adv. Pharmacol. 2020, 87, 71–88. [Google Scholar]
- Papachristou, F.; Anninou, N.; Koukoulis, G.; Paraskakis, S.; Sertaridou, E.; Tsalikidis, C.; Pitiakoudis, M.; Simopoulos, C.; Tsaroucha, A. Differential effects of cisplatin combined with the flavonoid apigenin on HepG2, Hep3B, and Huh7 liver cancer cell lines. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2021, 866, 503352. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.M.; Oh, S.J.; Lee, S.Y.; Im, J.H.; Oh, J.M.; Ryu, C.S.; Kwak, H.C.; Lee, J.Y.; Kang, K.W.; Kim, S.K. HepG2 cells as an in vitro model for evaluation of cytochrome P450 induction by xenobiotics. Arch. Pharm. Res. 2015, 38, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.J.; Lin, M.; Zhang, X.; Qin, L.P. Combined Use of Astragalus Polysaccharide and Berberine Attenuates Insulin Resistance in IR-HepG2 Cells via Regulation of the Gluconeogenesis Signaling Pathway. Front. Pharmacol. 2019, 10, 1508. [Google Scholar] [CrossRef]
- Guo, L.; Dial, S.; Shi, L.; Branham, W.; Liu, J.; Fang, J.L.; Green, B.; Deng, H.; Kaput, J.; Ning, B. Similarities and differences in the expression of drug-metabolizing enzymes between human hepatic cell lines and primary human hepatocytes. Drug. Metab. Dispos. 2011, 39, 528–538. [Google Scholar] [CrossRef]
- Villalva-Pérez, J.M.; Ramírez-Vargas, M.A.; Serafín-Fabían, J.I.; Ramírez, M.; Elena Moreno-Godínez, M.; Espinoza-Rojo, M.; Flores-Alfaro, E. Characterization of Huh7 cells after the induction of insulin resistance and post-treatment with metformin. Cytotechnology 2020, 72, 499–511. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Y.; Tao, S.; Sun, S. Geniposide plays anti-tumor effects by down-regulation of microRNA-224 in HepG2 and Huh7 cell lines. Exp. Mol. Pathol. 2020, 112, 104349. [Google Scholar] [CrossRef]
- Su, L.; Zhang, X.; Ma, Y.; Geng, C.; Huang, X.; Hu, J.; Li, T.; Tang, S.; Shen, C.; Gao, Z.; et al. New guaiane-type sesquiterpenoid dimers from Artemisia atrovirens and their antihepatoma activity. Acta Pharm. Sin. B 2021, 11, 1648–1666. [Google Scholar] [CrossRef]
- Majno, G.; Joris, I. Cells, In Tissues and Disease; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Opal, S.M.; DePalo, V.A. Anti-inflammatory cytokines. Chest 2000, 117, 1162–1172. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Feussner, K.; Feussner, I. Comprehensive LC-MS-Based Metabolite Fingerprinting Approach for Plant and Fungal-Derived Samples. Methods Mol. Biol. 2019, 1978, 167–185. [Google Scholar]
- Scholz, M.; Gatzek, S.; Sterling, A.; Fiehn, O.; Selbig, J. Metabolite fingerprinting: Detecting biological features by independent component analysis. Bioinformatics 2004, 20, 2447–2454. [Google Scholar] [CrossRef]
- Xia, P.; Bai, Z.; Liang, T.; Yang, D.; Liang, Z.; Yan, X.; Liu, Y. High-performance liquid chromatography based chemical fingerprint analysis and chemometric approaches for the identification and distinction of three endangered Panax plants in Southeast Asia. J. Sep. Sci. 2016, 39, 3880–3888. [Google Scholar] [CrossRef]
- Fan, S.M.; Xu, H.L.; Xie, X.Y.; Cai, B.Y.; Zou, F.X.; Xu, W.; Xie, Z.S.; Huang, X.P. Study on UHPLC fingerprint and determination of eight phenolic components of Tetrastigma hemsleyanum leaves. Chin. J. Med. 2016, 41, 3975–3981. (In Chinese) [Google Scholar]
- Xu, H.L.; Xu, W.; Su, W.C.; Fang, Y.N.; Xu, W.; Wei, Y.C.; Chen, M.; Fan, S.M.; Zhuang, J.X. Study on the correlation between genetic and chemical diversity of Tetrastigma hemsleyanum on the basis of ISSR and UHPLC. Process Biochem. 2019, 84, 220–229. [Google Scholar] [CrossRef]
- Dettmer, K.; Aronov, P.A.; Hammock, B.D. Mass spectrometry-based metabolomics. Mass Spectrom Rev. 2007, 26, 51–78. [Google Scholar] [CrossRef]
- Sen, T.; Samanta, S.K. Medicinal plants, human health and biodiversity: A broad review. Adv. Biochem. Eng. Biotechnol. 2015, 147, 59–110. [Google Scholar]
- Soares-Bezerra, R.J.; Calheiros, A.S.; da Silva Ferreira, N.C.; da Silva Frutuoso, V.; Alves, L.A. Natural Products as a Source for New Anti-Inflammatory and Analgesic Compounds through the Inhibition of Purinergic P2X Receptors. Pharmaceuticals 2013, 6, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Feng, X.; Zhang, Y.; Wang, Y.; Yu, X.; Jia, R.; Yu, T.; Zheng, X.; Chu, Q. Dietary flavone from the Tetrastigma hemsleyanum vine triggers human lung adenocarcinoma apoptosis via autophagy. Food Funct. 2020, 11, 9776–9788. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Peng, X.; Lou, T.; Wang, J.; Qiu, W. Total flavonoids from Tetrastigma hemsleyanum ameliorates inflammatory stress in concanavalin A-induced autoimmune hepatitis mice by regulating Treg/Th17 immune homeostasis. Inflammopharmacology 2019, 27, 1297–1307. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Luo, M.; Ma, L.; Lin, W. Ethylacetate extract from Tetrastigma hemsleyanum inhibits proliferation and induces apoptosis in HepG2 and SMMC-7721 cells. Cancer Manag. Res. 2018, 10, 3793–3799. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhang, Y.Y.; Wang, J.; Ji, Q. Ethylacetate extract from Tetrastigma hemsleyanum induces apoptosis via the mitochondrial caspase-dependent intrinsic pathway in HepG2 cells. Tumour Biol. 2016, 37, 865–876. [Google Scholar] [CrossRef]
- Sun, Y.; Hui, Q.R.; Chen, R.; Li, H.Y.; Peng, H.; Chen, F.; Deng, Z.Y. Apoptosis in human hepatoma HepG2 cells induced by the phenolics of Tetrastigma hemsleyanum leaves and their antitumor effects in H22 tumor-bearing mice. J. Funct. Foods 2018, 40, 349–364. [Google Scholar] [CrossRef]
- Zhan, L.; Pu, J.; Zheng, J.; Hang, S.; Pang, L.; Dai, M.; Ji, C. Tetrastigma hemsleyanum Diels et Gilg ameliorates lipopolysaccharide induced sepsis via repairing the intestinal mucosal barrier. Biomed. Pharmacother. 2022, 148, 112741. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, Y.; Wu, W.; Huang, L.; Guo, D.; Liu, C. Approaches to establish Q-markers for the quality standards of traditional Chinese medicines. Acta Pharm. Sin. B. 2017, 7, 439–446. [Google Scholar] [CrossRef]
- Xiang, Q.; Hu, S.; Ligaba-Osena, A.; Yang, J.; Tong, F.; Guo, W. Seasonal Variation in Transcriptomic Profiling of Tetrastigma hemsleyanum Fully Developed Tuberous Roots Enriches Candidate Genes in Essential Metabolic Pathways and Phytohormone Signaling. Front. Plant Sci. 2021, 12, 659645. [Google Scholar] [CrossRef]
- Xia, P.; Li, Q.; Liang, Z.; Zhang, X.; Yan, K. Spaceflight breeding could improve the volatile constituents of Andrographis paniculata. Ind. Crop. Prod. 2021, 171, 1–9. [Google Scholar] [CrossRef]
- Xu, L.; Cao, M.; Wang, Q.; Xu, J.; Liu, C.; Ullah, N.; Li, J.; Hou, Z.; Liang, Z.; Zhou, W.; et al. Insights into the plateau adaptation of Salvia castanea by comparative genomic and WGCNA analyses. J. Adv. Res. 2022, 42, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.J.; Lin, B.; Song, H.T. Progress in study of spectrum-effect relationship of traditional Chinese medicine and discussions. Chin. J. Med. 2015, 40, 1425–1432. (In Chinese) [Google Scholar]
- Zhang, C.; Liang, J.; Zhou, L.; Yuan, E.; Zeng, J.; Zhu, J.; Zhu, Y.; Zhou, L.; Wang, C.Z.; Yuan, C.S. Components study on antitussive effect and holistic mechanism of Platycodonis Radix based on spectrum-effect relationship and metabonomics analysis. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2021, 1173, 122680. [Google Scholar] [CrossRef]
- Mo, Y.J.; Wang, Y.; Qi, Q.; Yu, X.L.; Luo, J.Y.; Hu, H.Y.; Liu, F.; Wu, J.X.; Lu, Y.; Du, S.Y.; et al. HPLC fingerprint of famous traditional formula Sanpian Decoction and quality value transmitting of Chuanxiong Rhizoma. Chin. J. Med. 2022, 45, 572–578. (In Chinese) [Google Scholar]
- Zhou, X.; Li, Y.; Zhang, M.; Hao, J.; Gu, Q.; Liu, H.; Chen, W.; Shi, Y.; Dong, B.; Zhang, Y.; et al. Spectrum-Effect Relationship between UPLC Fingerprints and Antilung Cancer Effect of Si Jun Zi Tang. Evid.-Based Complement. Altern. Med. 2019, 2019, 7282681. [Google Scholar] [CrossRef]
- Gong, P.Y.; Guo, Y.J.; Tian, Y.S.; Gu, L.F.; Qi, J.; Yu, B.Y. Reverse tracing anti-thrombotic active ingredients from dried Rehmannia Radix based on multidimensional spectrum-effect relationship analysis of steaming and drying for nine cycles. J. Ethnopharmacol. 2021, 276, 114177. [Google Scholar] [CrossRef]
- Liu, C.X.; Chen, S.L.; Xiao, X.H.; Zhang, T.J.; Hou, W.B.; Liao, M.L. A new concept on quality marker of Chinese materia medica: Quality control for Chinese medicinal products. Chin. Tradit. Herbal. Drugs. 2016, 47, 1443–1457. [Google Scholar]
- Hu, B.; Zhou, R.; Li, Z.; Ouyang, S.; Li, Z.; Hu, W.; Wang, L.; Jiao, B. Study of the binding mechanism of aptamer to palytoxin by docking and molecular simulation. Sci. Rep. 2019, 9, 15494. [Google Scholar] [CrossRef]

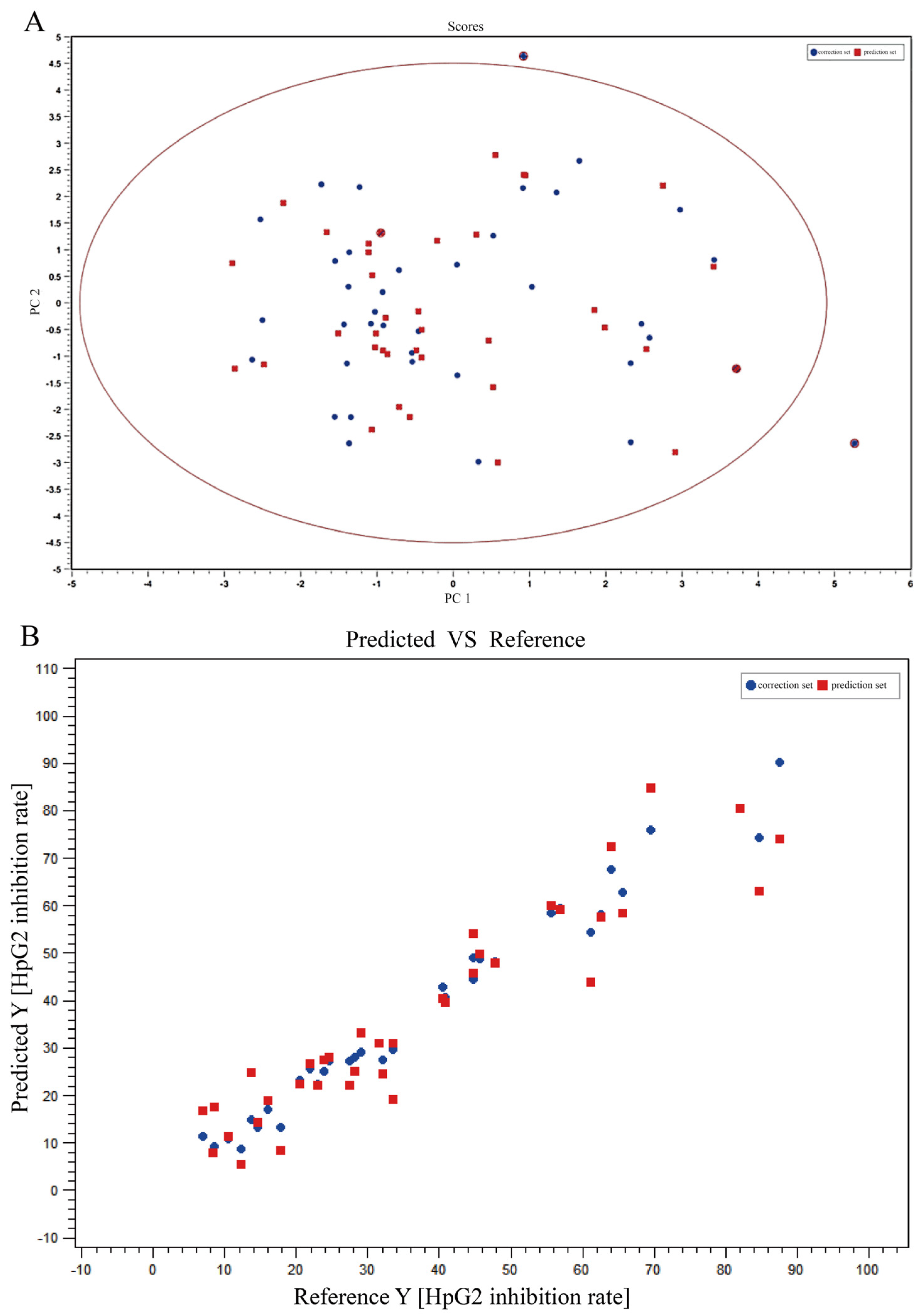
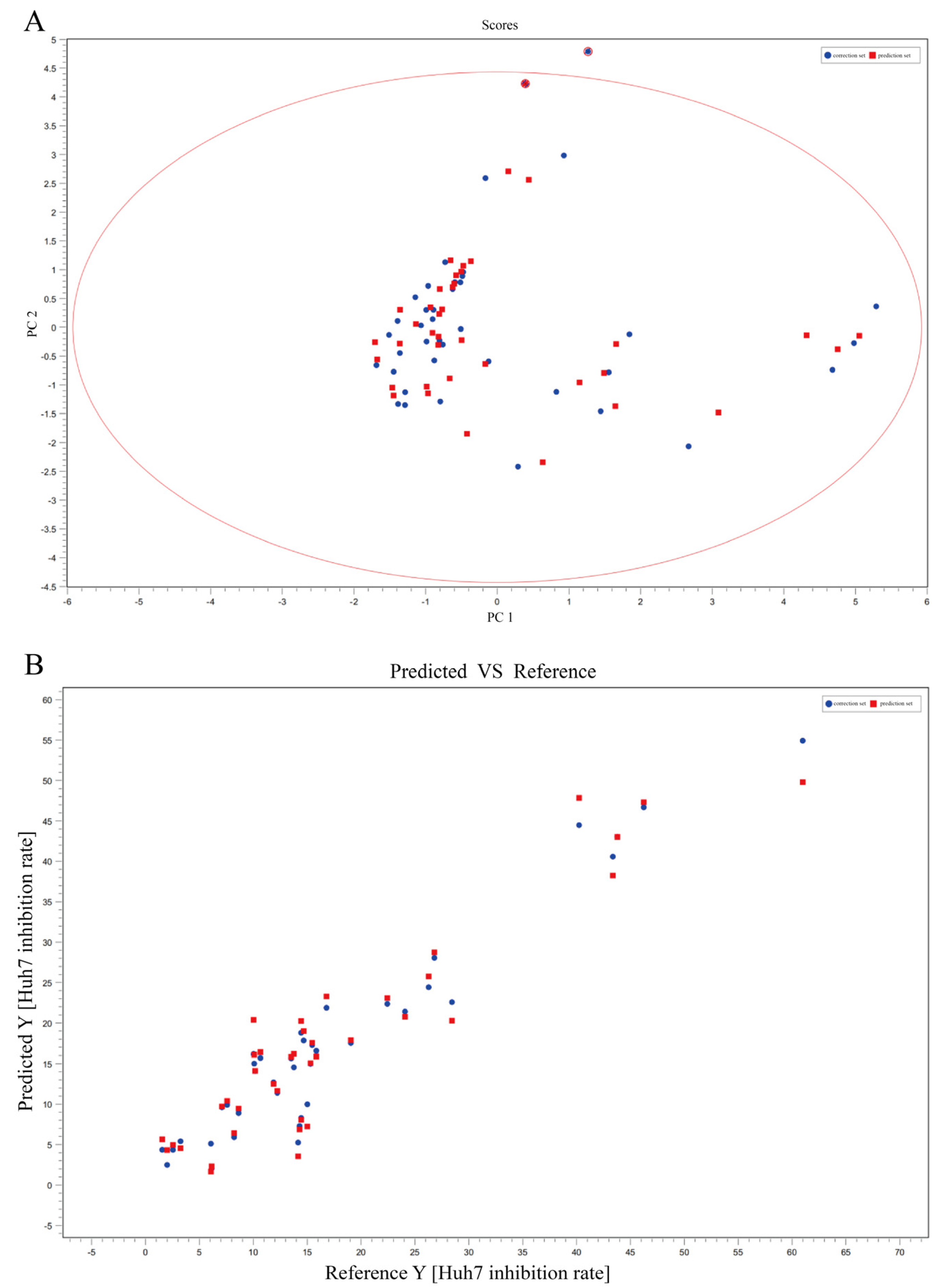

| Sample Name | HepG2 IC50 (μg/mL) | Sample Name | HepG2 IC50 (μg/mL) |
|---|---|---|---|
| AH-HS | 983.8 ± 0.98 ** | ZJ-CA | 301.5 ± 0.86 ** |
| FJ-FZ | 518.0 ± 1.62 ** | ZJ-LQ | 300.4 ± 1.14 ** |
| FJ-SM | 127.3 ± 2.66 | ZJ-FY | 150.7 ± 1.70 |
| GZ-ZY | 292.7 ± 1.05 ** | ZJ-WY | 531.4 ± 0.81 ** |
| GZ-BJ | 659.3 ± 1.64 ** | ZJ-QDH | 178.1 ± 1.17 ** |
| GX-BS | 207.3 ± 1.26 ** | ZJ-LX | 560.3 ± 0.86 ** |
| GX-LS | 619.4 ± 1.93 ** | ZJ-HZ | 335.1 ± 1.51 ** |
| GD-SC | 171.9 ± 1.15 ** | ZJ-JS | 249.1 ± 2.61 |
| JX-JGS | 187.5 ± 2.76 ** | ZJ-SX-SC | 723.8 ± 0.60 ** |
| JX-SR | 283.4 ± 2.43 ** | ZJ-WZ | 301.8 ± 0.87 * |
| SC-CQ | 129.4 ± 2.37 * | ZJ-NB | 466.9 ± 0.83 ** |
| SC-SC | 495.3 ± 1.04 ** | ZJ-PQ-XG | 377.8 ± 1.08 ** |
| ZJ-QY | 404.2 ± 0.79 ** | ZJ-PQ-Y | 315.1 ± 0.87 * |
| ZJ-SX | 897.6 ± 0.76 ** | ZJ-PQ-KG | 98.7 ± 0.48 ** |
| No. | Compound Name | Formula | MW/m/z | Rt/min | R2Y | Q2Y | Fragment Information |
|---|---|---|---|---|---|---|---|
| 1 | chlorogenic acid | C16H18 9 | 354.0951 | 0.80 | 0.88 | 0.65 | 95.9615,165.0341, 191.0492, 195.0430, 262.0531, 341.1071, 353.0871 |
| 2 | quinic acid | C7H12O6 | 192.0634 | 0.83 | 0.93 | 0.72 | 95.9615, 191.0130 |
| 3 | catechin | C15H14 6 | 290.0790 | 5.09 | 0.96 | 0.78 | 146.9597, 166.9872, 174.9493, 178.8363, 222.7850, 245.0751, 289.0650 |
| 4 | kaempferol 3-rutinoside | C27H30 15 | 594.1585 | 6.64 | 0.97 | 0.85 | 178.8363, 248.9543, 316.9427, 403.1532, 425.1367, 429.1726, 475.1776, 593.1465 |
| 5 | Apigenin-8-C-glucoside-arabinoside | C26H28O14 | 564.3201 | 16.51 | 0.97 | 0.88 | 178.8363, 249.1473, 293.2094, 432.2275, 504.3028, 563.3320 |
| 6 | unknown | unknow | 316.1478 | 20.78 | 0.98 | 0.88 | 166.9872, 178.8363, 249.1473, 297.1497, 316.9427, 317.1371 |
| Sample Name | HepG2 IC50 (μg/mL) | Sample Name | HepG2 IC50 (μg/mL) |
|---|---|---|---|
| AH-HS | 1595.99 ± 2.38 ** | ZJ-SX | 486.42 ± 1.17 ** |
| FJ-FZ | 693.00 ± 0.66 ** | ZJ-PQ-KG | 490.92 ± 0.98 ** |
| FJ-SM | 163.96 ± 7.77 ** | ZJ-CA | 646.41 ± 3.78 ** |
| FJ-SC | 851.61 ± 0.16 ** | ZJ-LQ | 380.66 ± 0.06 ** |
| GZ-ZY | 681.19 ± 3.77 ** | ZJ-FY | 650.16 ± 0.16 ** |
| GZ-BJ | 841.75 ± 0.22 ** | ZJ-WL | 610.84 ± 2.45 ** |
| GZ-SC | 630.91 ± 1.05 ** | ZJ-WY | 921.56 ± 0.65 ** |
| GX-HC | 692.04 ± 0.05 ** | ZJ-QDH | 726.21 ± 0.96 * |
| GX-BS | 595.59 ± 3.20 ** | ZJ-LX | 817.72 ± 7.09 ** |
| GX-SC | 1216.54 ± 4.45 ** | ZJ-HZ | 631.32 ± 0.63 ** |
| GX-LS | 938.08 ± 9.26 ** | ZJ-JS | 372.99 ± 0.11 ** |
| GD-SC | 445.63 ± 4.04 ** | ZJ-SX-SC | 757.40 ± 0.02 ** |
| JX-JGS | 216.26 ± 1.29 | ZJ-WZ | 997.00 ± 2.14 ** |
| JX-SR | 706.21 ± 37.26 ** | ZJ-NB | 351.74 ± 0.46 ** |
| SC-CQ | 248.57 ± 0.10 * | ZJ-PQ-XG | 230.62 ± 0.08 ** |
| SC-SC | 653.59 ± 0.40 ** | ZJ-PQ-Y | 415.28 ± 1.44 ** |
| ZJ-QY | 524.93 ± 2.94 ** |
| No. | Compound Name | Formula | MW/m/z | Rt/min | R2Y | Q2Y | Fragment Information |
|---|---|---|---|---|---|---|---|
| 1 | chlorogenic acid | C16H18O9 | 354.0951 | 0.81 | 0.89 | 0.82 | 191.0492, 195.0430, 353.0871 |
| 2 | quinic acid | C7H12O6 | 192.0634 | 1.20 | 0.92 | 0.85 | 87.0020, 111.0035, 128.0281, 191.0130 |
| 3 | kaempferol 3-rutinoside | C27H30O15 | 594.1585 | 8.41 | 0.92 | 0.86 | 112.9822, 146.9597,174.9493, 178.8363, 222.7850, 248.9543, 316.9427, 433.1093, 593.1465 |
| 4 | linolenic acid | C18H30O2 | 278.2246 | 19.27 | 0.93 | 0.85 | 128.2259, 166.9872, 178.8363, 197.9579, 249.1473, 265.1455, 271.2263, 277.2089 |
| 5 | unknown | 340.1877 | 20.15 | 0.93 | 0.85 | 146.9597, 166.9872, 178.8363, 249.1473, 265.1455, 334.9948, 339.1977 | |
| 6 | unknown | 310.1698 | 20.78 | 0.93 | 0.85 | 166.9872, 178.8363, 249.1473, 265.1455, 309.1671 |
| Sample Name | Inflammatory Cytokine IL-1β Expression (μg/mL) | Sample Name | Inflammatory Cytokine IL-1β Expression (μg/mL) |
|---|---|---|---|
| AH-HS | 6.53 ± 0.37 ** | ZJ-FY-1 | 6.64 ± 0.25 ** |
| CQ-JFS | 5.39 ± 0.16 ** | ZJ-FY-2 | 10.26 ± 0.33 ** |
| FJ-FZ | 6.39 ± 0.40 ** | ZJ-FY-3 | 4.29 ± 0.04 * |
| FJ-SC | 12.81 ± 0.56 ** | ZJ-FY-4 | 11.77 ± 0.13 ** |
| FJ-SM | 5.84 ± 0.93 ** | ZJ-FY-5 | 3.39 ± 0.16 ** |
| GD-SC | 9.32 ± 0.28 * | ZJ-HZ | 3.13 ± 0.11 * |
| GX-BS | 4.61 ± 0.79 ** | ZJ-LQ | 7.90 ± 0.07 ** |
| GX-HC | 14.70 ± 0.76 * | ZJ-LX | 6.05 ± 0.04 ** |
| GX-LS | 4.60 ± 0.12 ** | ZJ-NB | 4.71 ± 0.14 ** |
| GX-SC | 9.21 ± 0.09 | ZJ-PQ-KG | 8.19 ± 0.12 ** |
| GZ-BJ | 16.16 ± 0.79 ** | ZJ-PQ-XG | 4.96 ± 0.05 ** |
| GZ-SC | 7.04 ± 0.14 ** | ZJ-PQ-Y | 4.71 ± 0.17 * |
| GZ-ZY | 13.33 ± 0.37 ** | ZJ-QDH | 10.92 ± 0.11 ** |
| JX-JGS | 9.99 ± 0.06 | ZJ-QY | 10.82 ± 0.12 ** |
| JX-SC | 6.72 ± 0.15 ** | ZJ-SX | 3.61 ± 0.36 ** |
| JX-SR | 5.54 ± 0.06 ** | ZJ-SX-SC | 3.86 ± 0.06 ** |
| SC-CQ | 5.90 ± 0.09 ** | ZJ-WL | 10.42 ± 0.37 ** |
| ZJ-CA | 13.25 ± 0.55 * | ZJ-WY | 7.33 ± 0.39 ** |
| ZJ-DQH | 7.99 ± 0.22 ** | ZJ-WZ | 8.14 ± 0.28 ** |
| ZJ-FY | 11.05 ± 0.96 ** |
| No. | Compound Name | Formula | MW/m/z | Rt/min | R2Y | Q2Y | Fragment Information |
|---|---|---|---|---|---|---|---|
| 1 | chlorogenic acid | C16H18O9 | 354.0951 | 0.80 | 0.64 | 0.15 | 129.0136, 165.0341, 195.0430, 262.0531, 327.0864, 341.1071, 357.0960, 377.0816, 379.0777 |
| 2 | quercetin | C15H10O7 | 302.0427 | 1.44 | 0.75 | 0.36 | 96.9529, 117.0156, 138.9945, 166.9872, 178.8363, 206.9831, 272.9558, 280.9307, 300.9876 |
| 3 | quinic acid | C7H12O6 | 192.0634 | 2.14 | 0.81 | 0.44 | 87.0020, 111.0035, 128.0281, 191.0130 |
| 4 | kaempferol 3-rutinoside | C27H30O15 | 594.1585 | 7.01 | 0.82 | 0.44 | 112.9822, 178.8363, 222.7850, 248.9543, 316.9427, 403.1532, 425.1367, 429.1726, 475.1776, 593.1465 |
| 5 | rutinum | C27H30O16 | 610.1534 | 7.35 | 0.82 | 0.43 | 174.9493, 248.9543, 265.9428, 300.0258, 301.0255, 310.9286, 316.9427, 384.9264, 609.1483 |
| 6 | apigenin8-C-glucoside-arabinoside | C26H28O14 | 564.3201 | 15.63 | 0.83 | 0.37 | 130.9611, 178.8363, 249.1473, 275.1978, 294.2126, 361.1941,384.9350, 504.3028, 563.3320 |
| 7 | linolenic acid | C18H30O2 | 278.2246 | 20.88 | 0.83 | 0.16 | 129.9681, 146.9597, 166.9872, 178.8363, 248.8543, 249.1473, 250.1482, 277.2089 |
| Compound Name | Docking Score | Interacting Residues |
|---|---|---|
| Rutin | −5.918359555 | Gln38, Val41, Glu64, Lys65 |
| Quinic acid | −5.571761051 | Glu37, Gln38, Gln39, Lys65 |
| Vitexin | −5.211264998 | Glu37, Gln38, Gln39, Lys63, Lys65 |
| Quercetin | −5.203226381 | Met20, Glu37, Lys65 |
| Chlorogenic acid | −5.105666547 | Glu37, Gln38, Val41, Lys65 |
| Kaempfeorl | −4.263310555 | Glu37, Lys65 |
| Linolenic acid (Ala) | 0.607761907 | Arg11, Gln39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, J.; Li, X.; Lin, M.; Yu, J.; Zeng, Z.; Ye, F.; Hu, G.; Miu, Q.; He, Q.; Zhang, X.; et al. Screening out Biomarkers of Tetrastigma hemsleyanum for Anti-Cancer and Anti-Inflammatory Based on Spectrum-Effect Relationship Coupled with UPLC-Q-TOF-MS. Molecules 2023, 28, 3021. https://doi.org/10.3390/molecules28073021
Xia J, Li X, Lin M, Yu J, Zeng Z, Ye F, Hu G, Miu Q, He Q, Zhang X, et al. Screening out Biomarkers of Tetrastigma hemsleyanum for Anti-Cancer and Anti-Inflammatory Based on Spectrum-Effect Relationship Coupled with UPLC-Q-TOF-MS. Molecules. 2023; 28(7):3021. https://doi.org/10.3390/molecules28073021
Chicago/Turabian StyleXia, Jie, Xiuyue Li, Min Lin, Jiani Yu, Zhongda Zeng, Fei Ye, Guanjun Hu, Qiang Miu, Qiuling He, Xiaodan Zhang, and et al. 2023. "Screening out Biomarkers of Tetrastigma hemsleyanum for Anti-Cancer and Anti-Inflammatory Based on Spectrum-Effect Relationship Coupled with UPLC-Q-TOF-MS" Molecules 28, no. 7: 3021. https://doi.org/10.3390/molecules28073021
APA StyleXia, J., Li, X., Lin, M., Yu, J., Zeng, Z., Ye, F., Hu, G., Miu, Q., He, Q., Zhang, X., & Liang, Z. (2023). Screening out Biomarkers of Tetrastigma hemsleyanum for Anti-Cancer and Anti-Inflammatory Based on Spectrum-Effect Relationship Coupled with UPLC-Q-TOF-MS. Molecules, 28(7), 3021. https://doi.org/10.3390/molecules28073021