Synthesis, Solution, and Solid State Properties of Homological Dialkylated Naphthalene Diimides—A Systematic Review of Molecules for Next-Generation Organic Electronics
Abstract
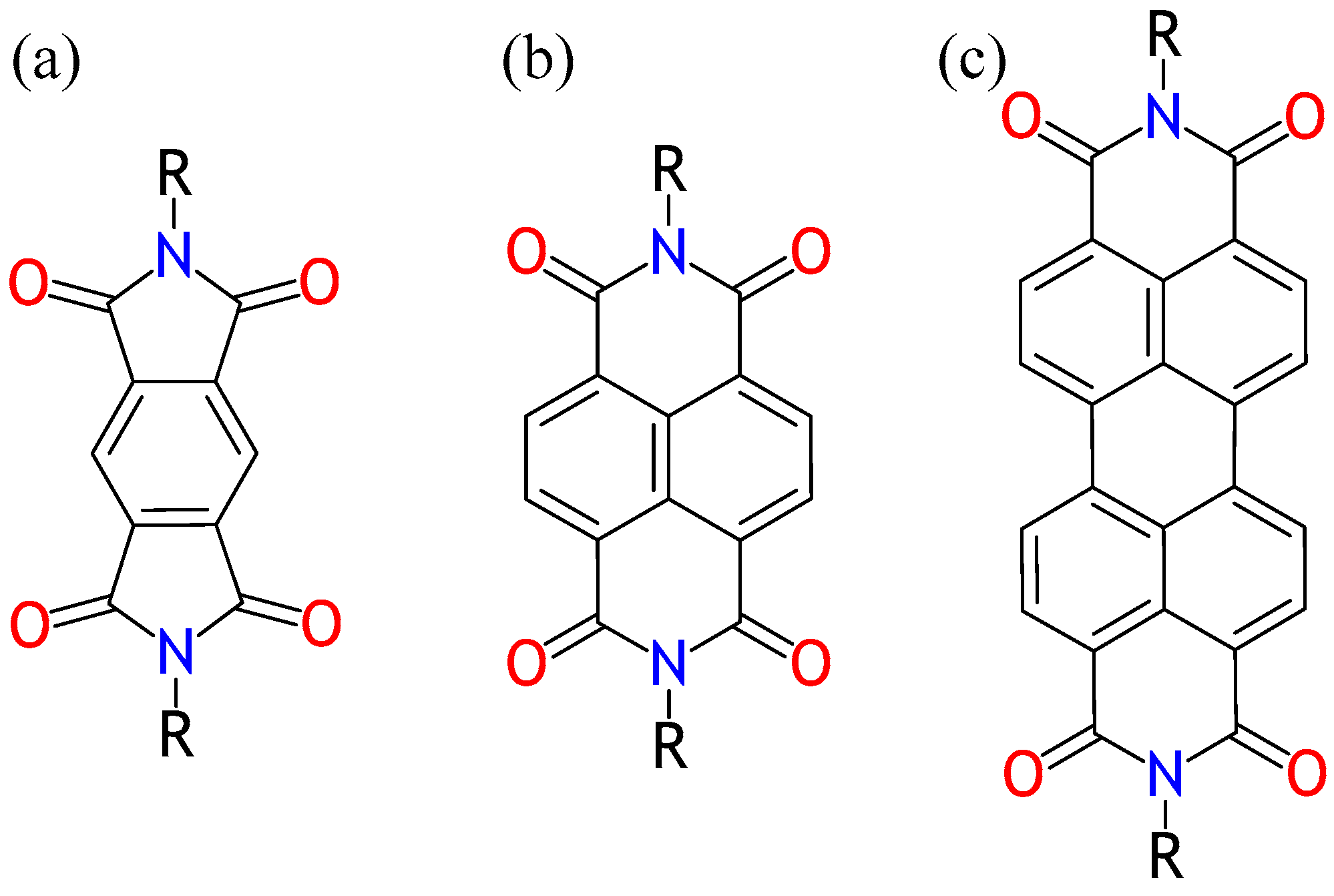
1. Introduction
2. Results and Discussion
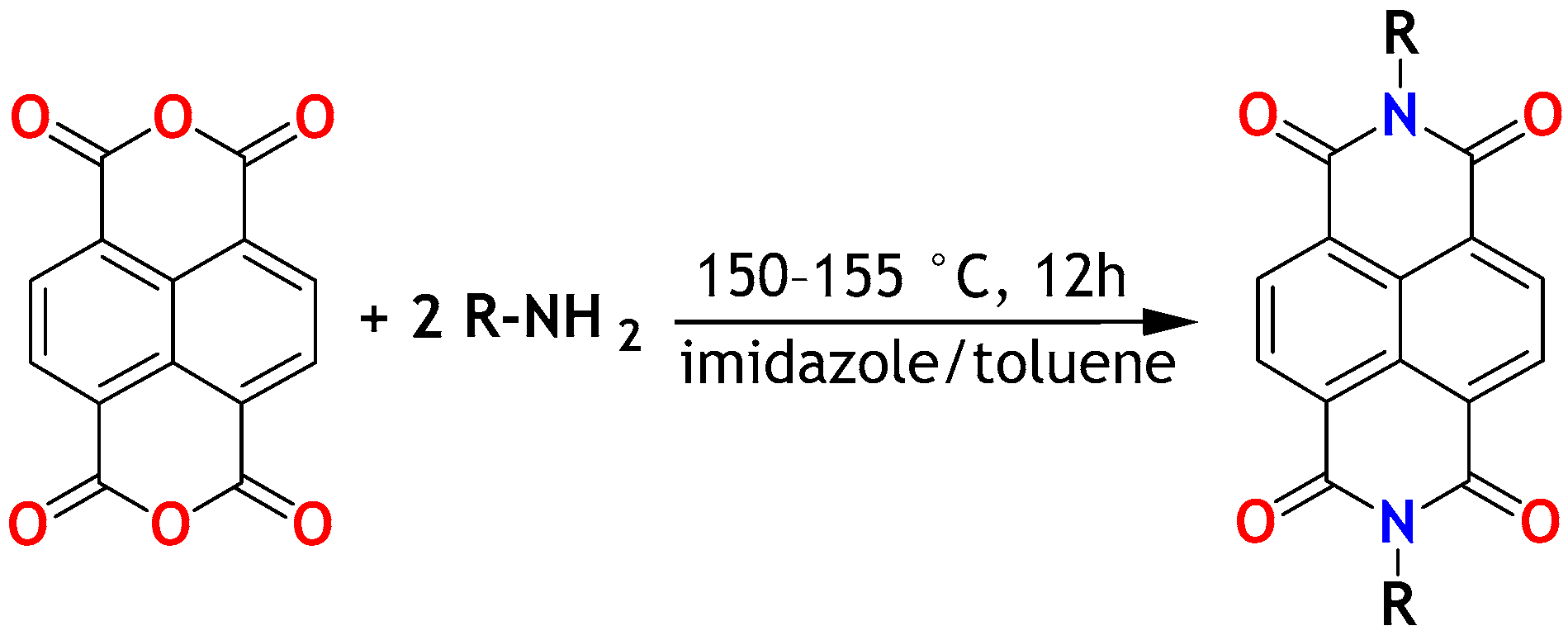
2.1. Remarks on Synthesis: Towards Increasing the Synthesis Scale
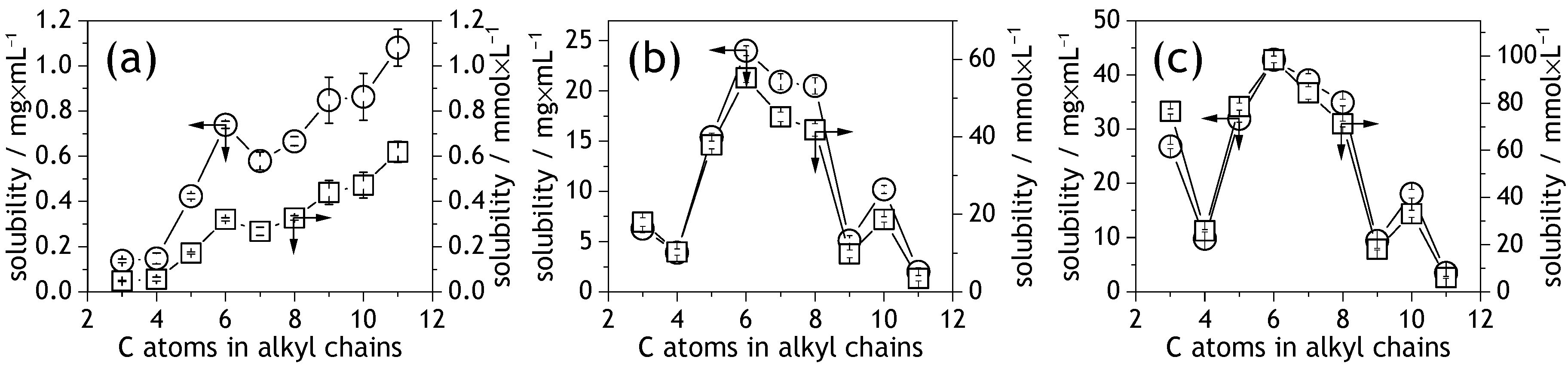
2.2. UV-Vis Spectroscopy: Solubility of the NDIs
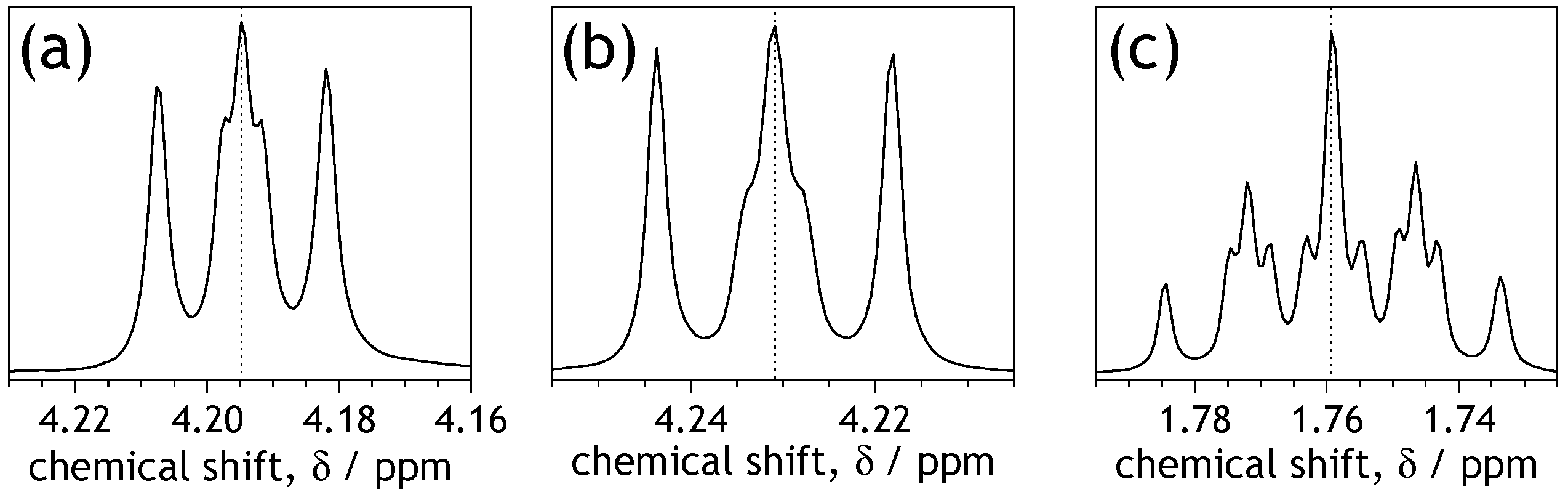
2.3. Characteristic Features of NMR Spectra of the Symmetrically N-Substituted NDIs
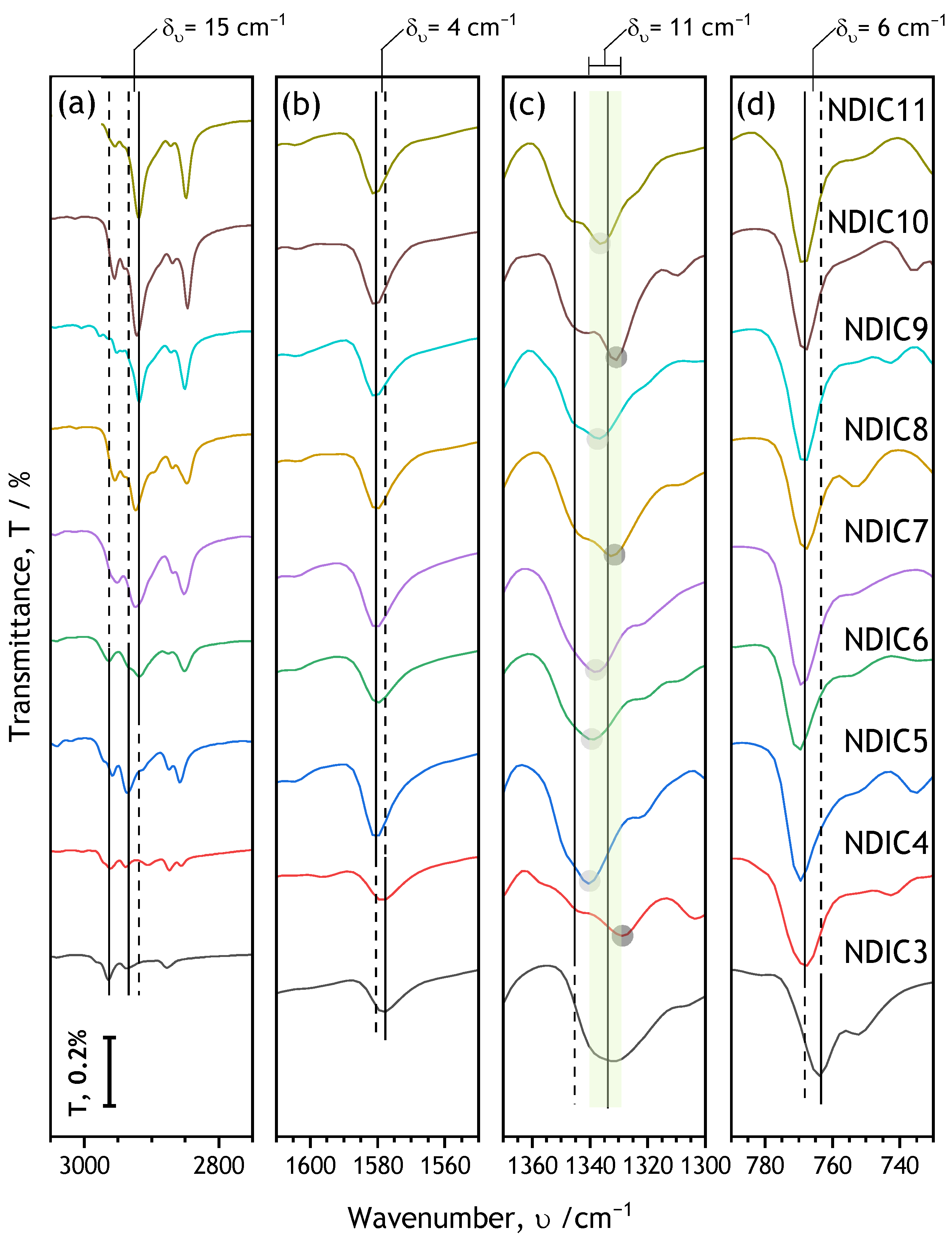
2.4. Effects of Alkyl Chain Length on FTIR Spectra of Solid NDIs
2.5. Crystal Structure of NDIs
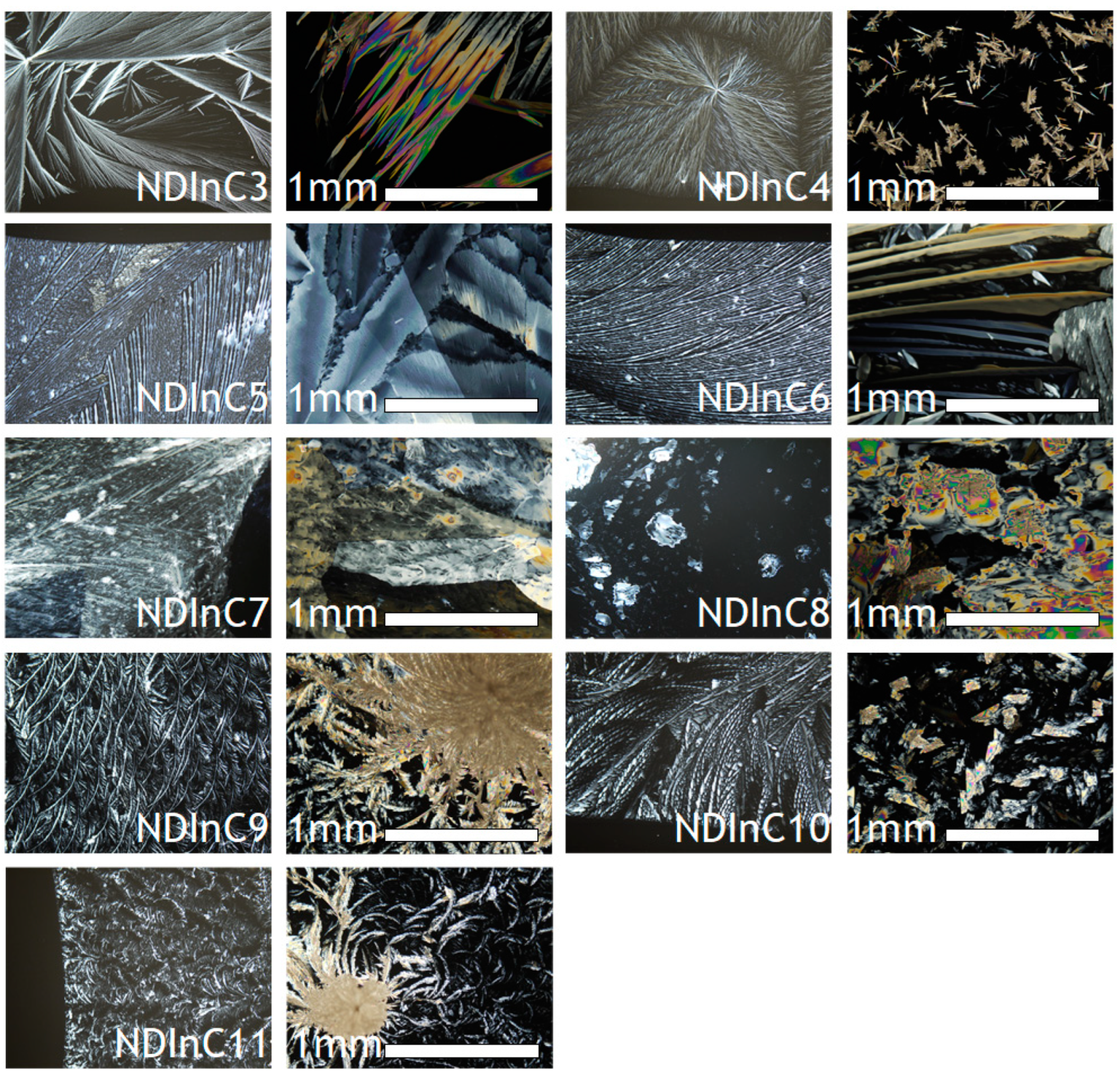
2.6. Crystal Morphologies of NDIs
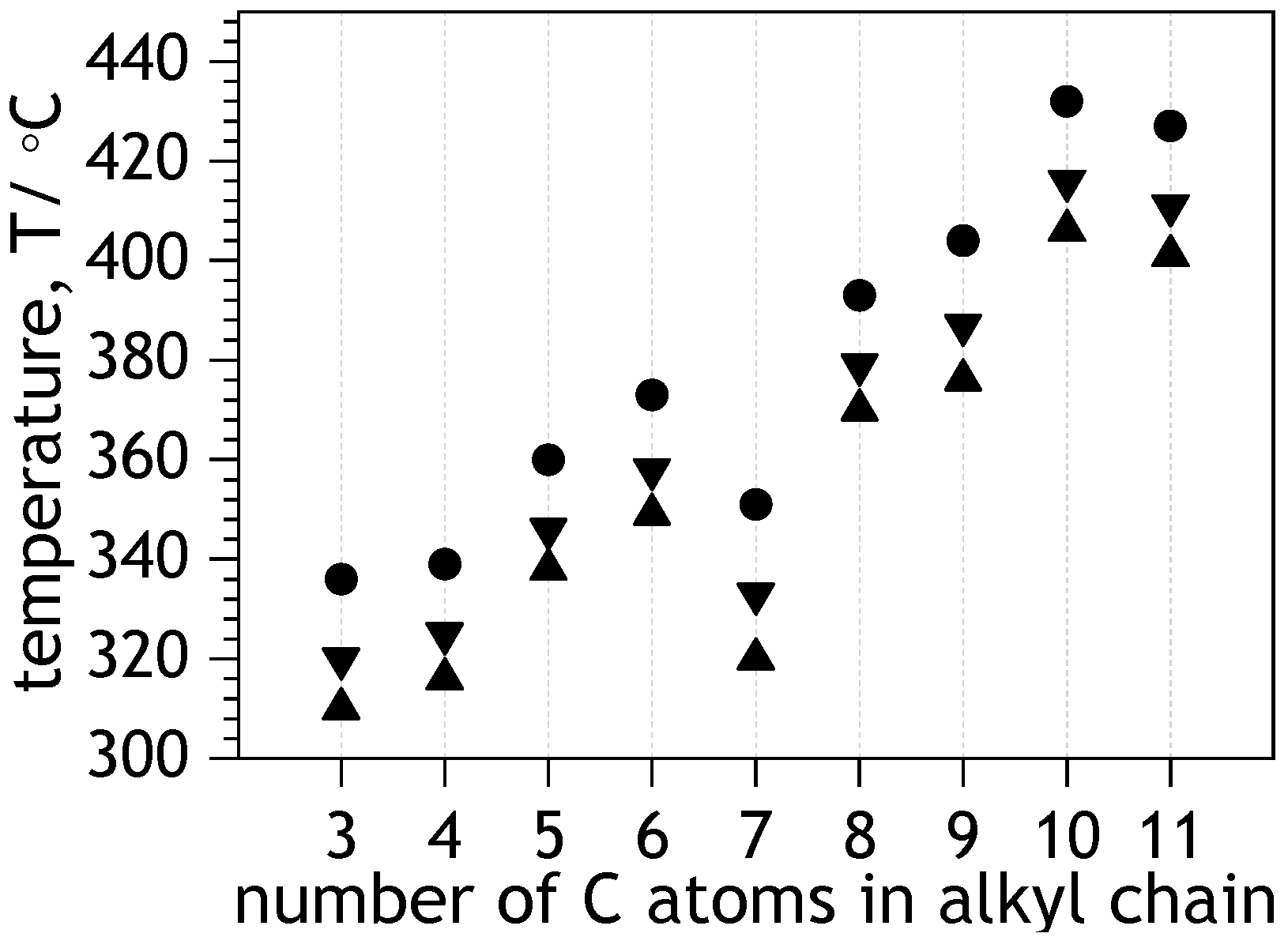
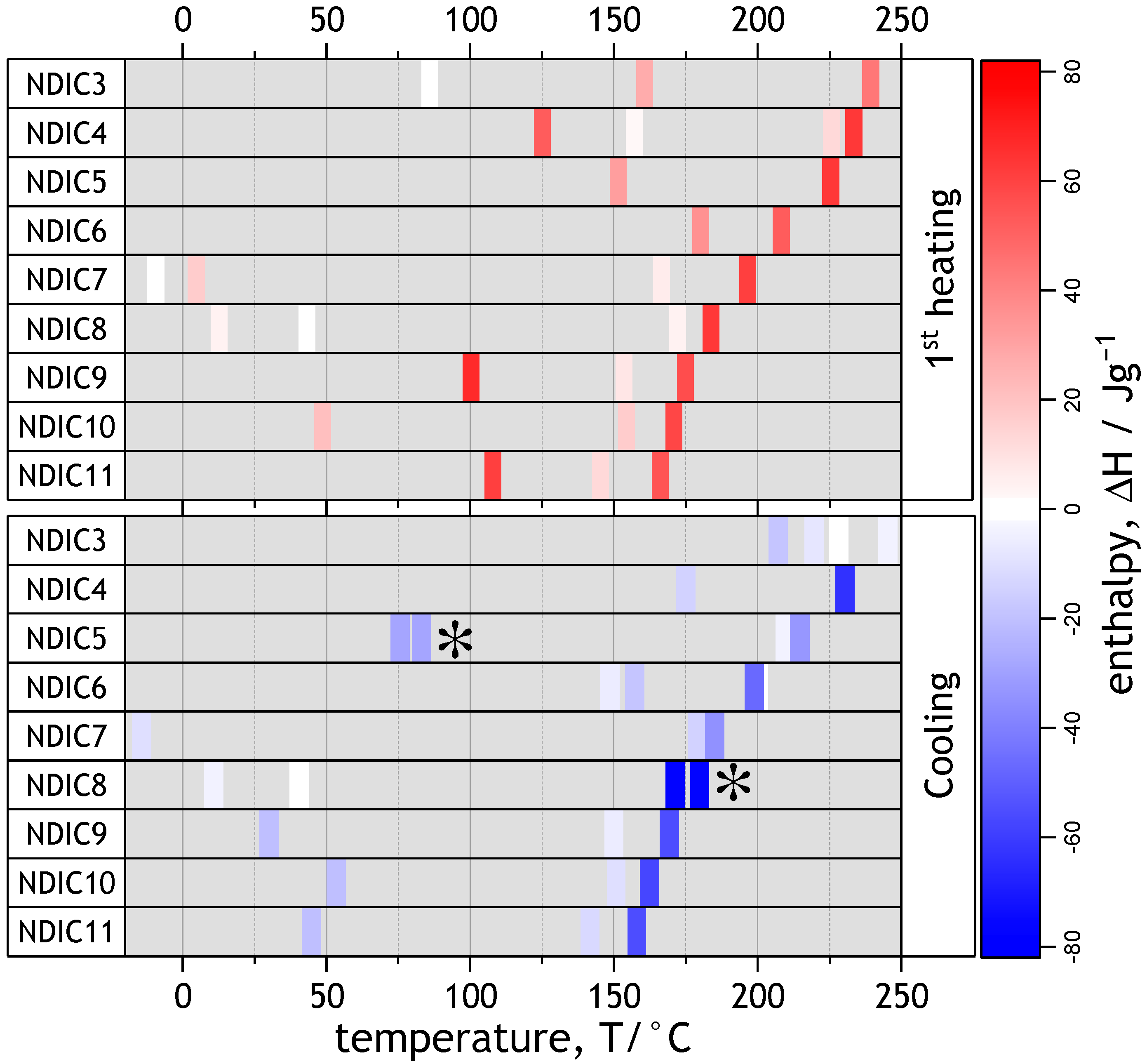
2.7. Thermal Properties of Solid NDIs
2.8. Field-Effect Electron Mobility
3. Materials and Methods
3.1. Materials
3.2. Synthesis
3.3. Preparation of Isotropic Samples
3.4. Infrared Spectroscopy
3.5. UV-Vis Spectroscopy and Solubility Studies
3.6. NMR Spectroscopy
3.7. Thermogravimetric Analysis and Differential Scanning Calorimetry
3.8. Polarized Optical Microscopy
3.9. X-ray Diffraction Measurements
3.10. Determination of Field-Effect Electron Mobility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Medabalmi, V.; Sundararajan, M.; Singh, V.; Baik, M.-H.; Byon, H.R. Naphthalene diimide as a two-electron anolyte for aqueous and neutral pH redox flow batteries. J. Mater. Chem. A 2020, 8, 11218–11223. [Google Scholar] [CrossRef]
- Andric, G.; Boas, J.F.; Bond, A.M.; Fallon, G.D.; Ghiggino, K.P.; Hogan, C.F.; Hutchison, J.A.; Lee, M.A.-P.; Langford, S.J.; Pilbrow, J.R.; et al. Spectroscopy of naphthalene diimides and their anion radicals. Aust. J. Chem. 2004, 57, 1011–1019. [Google Scholar] [CrossRef]
- Pervin, R.; Manian, A.; Chen, Z.; Christofferson, A.J.; Owyong, T.C.; Bradley, S.J.; White, J.M.; Ghiggino, K.P.; Russo, S.P.; Wong, W.W.H. Medium effects on the fluorescence of Imide-substituted naphthalene diimides. J. Photochem. Photobiol. A Chem. 2023, 436, 114364. [Google Scholar] [CrossRef]
- Polander, L.E.; Tiwari, S.P.; Pandey, L.; Seifried, B.M.; Zhang, Q.; Barlow, S.; Risko, C.; Brédas, J.-L.; Kippelen, B.; Marder, S.R. Solution-processed molecular bis(naphthalene diimide) derivatives with high electron mobility. Chem. Mater. 2011, 23, 3408–3410. [Google Scholar] [CrossRef]
- Jung, J.; Selerowicz, A.; Maczugowska, P.; Halagan, K.; Rybakiewicz-Sekita, R.; Zagorska, M.; Stefaniuk-Grams, A. Electron transport in naphthalene diimide derivatives. Materials 2021, 14, 4026. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Huang, J.; Sarjeant, A.; Katz, H.E. Pyromellitic diimides: Minimal cores for high mobility n-channel transistor semiconductors. J. Am. Chem. Soc. 2008, 130, 14410–14411. [Google Scholar] [CrossRef]
- Shukla, D.; Nelson, S.F.; Freeman, D.C.; Rajeswaran, M.; Ahearn, W.G.; Meyer, D.M.; Carey, J.T. Thin-film morphology control in naphthalene-diimide-based semiconductors: High mobility n-type semiconductor for organic thin-film transistors. Chem. Mater. 2008, 20, 7486–7491. [Google Scholar] [CrossRef]
- Mondal, S.; Lin, W.-H.; Chen, Y.-C.; Huang, S.-H.; Yang, R.; Chen, B.-H.; Yang, T.-F.; Mao, S.-W.; Kuo, M.-Y. Solution-processed single-crystal perylene diimide transistors with high electron mobility. Org. Electron. 2015, 23, 64–69. [Google Scholar] [CrossRef]
- Langhals, H.; Demmig, S.; Potrawa, T. The relation between packing effects and solid state fluorescence of dyes. J. Prakt. Chem. 1991, 333, 733–748. [Google Scholar] [CrossRef]
- Vollmann, H. Naphthalene Dyes. DE Patent 661756, 16 April 1933. [Google Scholar]
- Eckert, W. Braunsdorf, Otto Vat Dyes. U.S. Patent 2143830, 23 September 1935. [Google Scholar]
- Vollmann, H. Dyes of the Naphthalene-1,4,5,8-Tetracarboxylic Acid Diimide Series. U.S. Patent 2087133, 18 March 1937. [Google Scholar]
- Vollmann, H.; Becker, H.; Corell, M.; Streeck, H. Beiträge zur kenntnis des pyrens und seiner derivate. Justus Liebigs Ann. Chem. 1937, 531, 1–159. [Google Scholar] [CrossRef]
- Fierz-David, H.E.; Rossi, C. Über die darstellung von naphthoylen-imidazolinen. Helv. Chim. Acta 1938, 21, 1466–1489. [Google Scholar] [CrossRef]
- Yen, S.F.; Gabbay, E.J.; Wilson, W.D. Interaction of aromatic imides with DNA. 1. Spectrophotometric and viscometric studies. Biochemistry 1982, 21, 2070–2076. [Google Scholar] [CrossRef] [PubMed]
- Welford, A.; Maniam, S.; Gann, E.; Jiao, X.; Thomsen, L.; Langford, S.J.; McNeill, C.R. Influence of alkyl side-chain type and length on the thin film microstructure and OFET performance of naphthalene diimide-based organic semiconductors. Org. Electron. 2019, 75, 105378. [Google Scholar] [CrossRef]
- Quinn, J.T.E.; Zhu, J.; Li, X.; Wang, J.; Li, Y. Recent progress in the development of n-type organic semiconductors for organic field effect transistors. J. Mater. Chem. C 2017, 5, 8654–8681. [Google Scholar] [CrossRef]
- Zhan, X.; Facchetti, A.; Barlow, S.; Marks, T.J.; Ratner, M.A.; Wasielewski, M.R.; Marder, S.R. Rylene and related diimides for organic electronics. Adv. Mater. 2011, 23, 268–284. [Google Scholar] [CrossRef]
- Chlebosz, D.; Janus, K.; Danielewicz, K.; Goldeman, W.; Czapik, A.; Glaßer, G.; Mezger, M.; Kiersnowski, A. Recrystallization upon solvent vapor annealing and impact of polymer crystallinity on hole transport in poly(3-hexylthiophene): Small molecule blends. Mol. Syst. Des. Eng. 2020, 5, 1417–1427. [Google Scholar] [CrossRef]
- Janus, K.; Danielewicz, K.; Chlebosz, D.; Goldeman, W.; Kiersnowski, A. Electron-to hole transport change induced by solvent vapor annealing of naphthalene diimide doped with poly(3-hexylthiophene). Front. Chem. 2021, 9, 641. [Google Scholar] [CrossRef]
- Janasz, L.; Marszalek, T.; Zajaczkowski, W.; Borkowski, M.; Goldeman, W.; Kiersnowski, A.; Chlebosz, D.; Rogowski, J.; Blom, P.; Ulanski, J.; et al. Ultrathin film heterojunctions by combining solution processing and sublimation for ambipolar organic field-effect transistors. J. Mater. Chem. C 2018, 6, 7830–7838. [Google Scholar] [CrossRef]
- Vadehra, G.S.; Maloney, R.P.; Garcia-Garibay, M.A.; Dunn, B. Naphthalene diimide based materials with adjustable redox potentials: Evaluation for organic lithium-ion batteries. Chem. Mater. 2014, 26, 7151–7157. [Google Scholar] [CrossRef]
- Higginbotham, H.F.; Pander, P.; Rybakiewicz, R.; Etherington, M.K.; Maniam, S.; Zagorska, M.; Pron, A.; Monkman, A.P.; Data, P. Triphenylamine disubstituted naphthalene diimide: Elucidation of excited states involved in TADF and application in near-infrared organic light emitting diodes. J. Mater. Chem. C 2018, 6, 8219–8225. [Google Scholar] [CrossRef]
- Rao, P.S.; Gupta, A.; Srivani, D.; Bhosale, S.V.; Bilic, A.; Li, J.; Xiang, W.; Evans, R.A.; Bhosale, S.V. An efficient non-fullerene acceptor based on central and peripheral naphthalene diimides. Chem. Commun. 2018, 54, 5062–5065. [Google Scholar] [CrossRef] [PubMed]
- Pantos, G.D. Naphthalenediimide and Its Congeners: From Molecules to Materials, 1st ed.; The Royal Society of Chemistry: London, UK, 2017. [Google Scholar]
- Al Kobaisi, M.; Bhosale, S.V.; Latham, K.; Raynor, A.M.; Bhosale, S.V. Functional naphthalene diimides: Synthesis, properties, and applications. Chem. Rev. 2016, 116, 11685–11796. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Yu, R.; Gao, C.; Sui, Y.; Deng, Y.; Chen, H.; Guo, T. High-performance n-type thin-film transistor based on bilayer MXene/semiconductor with enhanced electrons transport. Sci. China Mater. 2022, 65, 3087–3095. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, L.; Ma, X.; Jin, R.; Kang, C.; Gao, L. Chiral naphthalenediimides with high-efficiency fluorescence and circularly polarized luminescence in the solid state for the application in organic optoelectronics. Chem. A Eur. J. 2023, 29, e202202476. [Google Scholar] [CrossRef] [PubMed]
- Svirskaite, L.M.; Mandati, S.; Spalatu, N.; Malinauskiene, V.; Karazhanov, S.; Getautis, V.; Malinauskas, T. Asymmetric NDI electron transporting SAM materials for application in photovoltaic devices. Synth. Met. 2022, 291, 117214. [Google Scholar] [CrossRef]
- Singh, V.; Ahn, S.; Byon, H.R. Ammonium-functionalized naphthalene diimides as two-electron-transfer negolyte for aqueous redox flow batteries. Batter. Supercaps. 2022, 5, e202200281. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Gao, C.; Ni, Z.; Zhang, X.; Hu, W.; Dong, H. Recent advances in n-type and ambipolar organic semiconductors and their multi-functional applications. Chem. Soc. Rev. 2023, 52, 1331–1381. [Google Scholar] [CrossRef]
- Liao, Q.; Kang, Q.; Xu, B.; Hou, J. Design and application of an asymmetric naphthalimide-based molecule with improved hydrophobicity for highly stable organic solar cells. J. Am. Chem. Soc. 2022, 2, 1918–1928. [Google Scholar] [CrossRef]
- Rösch, A.T.; Reynaerts, R.; Lamers, B.A.G.; Mali, K.S.; De Feyter, S.; Palmans, A.R.A.; Meijer, E.W. Double lamellar morphologies and odd–even effects in two- and three-dimensional N,N′-bis(n-alkyl)-naphthalenediimide materials. Chem. Mater. 2021, 33, 8800–8811. [Google Scholar] [CrossRef]
- Miyake, Y.; Nagata, T.; Tanaka, H.; Yamazaki, M.; Ohta, M.; Kokawa, R.; Ogawa, T. Entropy-controlled 2D supramolecular structures of N,N′-Bis(n-alkyl)naphthalenediimides on a HOPG surface. ACS Nano 2012, 6, 3876–3887. [Google Scholar] [CrossRef]
- Gryko, D.T.; Rogacki, M.K.; Klajn, J.; Gałęzowski, M.; Stȩpień, D.K.; Cyrański, M.K. Unprecedented 1,3-dipolar cycloaddition: From 1,4,5,8-naphthalene bisimides to a new heterocyclic skeleton. Org. Lett. 2010, 12, 2020–2023. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Shaller, A.D.; Li, A.D.Q. Twisted perylene stereodimers reveal chiral molecular assembly codes. J. Am. Chem. Soc. 2008, 130, 8271–8279. [Google Scholar] [CrossRef] [PubMed]
- Ke, H.; Jiao, C.; Qian, Y.-H.; Lin, M.-J.; Chen, J.-Z. Naphthalene diimide templated synthesis of pillar[6]arenes. Chin. J. Chem. 2015, 33, 339–342. [Google Scholar] [CrossRef]
- Doria, F.; di Antonio, M.; Benotti, M.; Verga, D.; Freccero, M. Substituted heterocyclic naphthalene diimides with unexpected acidity. Synthesis, properties, and reactivity. J. Org. Chem. 2009, 74, 8616–8625. [Google Scholar] [CrossRef]
- Baumgartner, B.; Svirkova, A.; Bintinger, J.; Hametner, C.; Marchetti-Deschmann, M.; Unterlass, M.M. Green and highly efficient synthesis of perylene and naphthalene bisimides in nothing but water. Chem. Commun. 2017, 53, 1229–1232. [Google Scholar] [CrossRef]
- Kakinuma, T.; Kojima, H.; Ashizawa, M.; Matsumoto, H.; Mori, T. Correlation of mobility and molecular packing in organic transistors based on cycloalkyl naphthalene diimides. J. Mater. Chem. C 2013, 1, 5395–5401. [Google Scholar] [CrossRef]
- Shimizu, K.D.; Dewey, T.M.; Rebek, J., Jr. Convergent Functional Groups. 15. Synthetic and structural studies of large and rigid molecular clefts. J. Am. Chem. Soc. 1994, 116, 5145–5149. [Google Scholar] [CrossRef]
- Katz, H.E.; Lovinger, A.J.; Johnson, J.; Kloc, C.; Siegrist, T.; Li, W.; Lin, Y.Y.; Dodabalapur, A. A soluble and air-stable organic semiconductor with high electron mobility. Nature 2000, 404, 478–481. [Google Scholar] [CrossRef]
- Langhals, H. Synthesis of highly pure perylene fluorescent dyes in large-scale amounts—Specific preparation of atropic isomers. Chem. Ber.-Recl. 1985, 118, 4641–4645. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, Y.; Zhao, C.; Li, R.; Xu, W.; Li, X.; Jiang, J. Cyclophanes of perylene tetracarboxylic diimide with different substituents at bay positions. Chem. A Eur. J. 2008, 14, 7000–7010. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Liu, H.; Sun, D.; Li, X. Synthesis and aggregation properties of a series of dumbbell polyhedral oligosilsesquioxane-perylene diimide triads. CrystEngComm 2015, 17, 1453–1463. [Google Scholar] [CrossRef]
- Jones, B.A.; Facchetti, A.; Wasielewski, M.R.; Marks, T.J. Tuning orbital energetics in arylene diimide semiconductors. Materials design for ambient stability of n-type charge transport. J. Am. Chem. Soc. 2007, 129, 15259–15278. [Google Scholar] [CrossRef]
- Shao, H.; Parquette, J.R. A π-conjugated hydrogel based on an Fmoc-dipeptide naphthalene diimide semiconductor. Chem. Commun. 2010, 46, 4285–4287. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Seifert, J.; Romano, N.C.; Gao, M.; Helmus, J.J.; Jaroniec, C.P.; Modarelli, D.A.; Parquette, J.R. Amphiphilic self-assembly of an n-type nanotube. Angew. Chem. Int. Ed. 2010, 49, 7688–7691. [Google Scholar] [CrossRef] [PubMed]
- Erten, S.; Posokhov, Y.; Alp, S.; Içli, S. The study of the solubility of naphthalene diimides with various bulky flanking substituents in different solvents by UV-vis spectroscopy. Dye. Pigment. 2005, 64, 171–178. [Google Scholar] [CrossRef]
- Katz, H.E.; Johnson, J.; Lovinger, A.J.; Li, W. Naphthalenetetracarboxylic diimide-based n-channel transistor semiconductors: Structural variation and thiol-enhanced gold contacts. J. Am. Chem. Soc. 2000, 122, 7787–7792. [Google Scholar] [CrossRef]
- Ichikawa, M.; Yokota, Y.; Jeon, H.-G.; Banoukepa, G.d.R.; Hirata, N.; Oguma, N. Comparative study of soluble naphthalene diimide derivatives bearing long alkyl chains as n-type organic thin-film transistor materials. Org. Electron. 2013, 14, 516–522. [Google Scholar] [CrossRef]
- Bhosale, S.V.; Jani, C.H.; Langford, S.J. Chemistry of naphthalene diimides. Chem. Soc. Rev. 2008, 37, 331–342. [Google Scholar] [CrossRef]
- Ohyama, A.; Miyazawa, J.; Yokota, Y.; Hirata, N.; Oguma, N.; Ichikawa, M. Printing technology based on isotropic liquid phase of naphthalene diimide derivatives for n-type organic transistors. Org. Electron. 2018, 58, 231–237. [Google Scholar] [CrossRef]
- Jameel, M.A.; Yang, C.J.; Wilson, G.J.; Evans, R.A.; Gupta, A.; Langford, S.J. Naphthalene diimide-based electron transport materials for perovskite solar cells. J. Mater. Chem. A 2021, 9, 27170–27192. [Google Scholar] [CrossRef]
- Krishna, G.R.; Devarapalli, R.; Lal, G.; Reddy, C.M. Mechanically flexible organic crystals achieved by introducing weak interactions in structure: Supramolecular shape synthons. J. Am. Chem. Soc. 2016, 138, 13561–13567. [Google Scholar] [CrossRef]
- Alvey, P.M.; Reczek, J.J.; Lynch, V.; Iverson, B.L. A systematic study of thermochromic aromatic donor−acceptor materials. J. Org. Chem. 2010, 75, 7682–7690. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, Y.; Goto, K.; Kubono, K.; Sako, K.; Shinmyozu, T. Photoinduced color change and photomechanical effect of naphthalene diimides bearing alkylamine moieties in the solid state. Chem. A Eur. J. 2014, 20, 7309–7316. [Google Scholar] [CrossRef]
- Reczek, J.J.; Villazor, K.R.; Lynch, V.; Swager, T.M.; Iverson, B.L. Tunable columnar mesophases utilizing C2 symmetric aromatic donor−acceptor complexes. J. Am. Chem. Soc. 2006, 128, 7995–8002. [Google Scholar] [CrossRef]
- Ofir, Y.; Zelichenok, A.; Yitzchaik, S. 1,4; 5,8-naphthalene-tetracarboxylic diimide derivatives as model compounds for molecular layer epitaxy. J. Mater. Chem. 2006, 16, 2142–2149. [Google Scholar] [CrossRef]
- Flamigni, L.; Wyrostek, D.; Voloshchuk, R.; Gryko, D.T. Solvent polarity effect on intramolecular electron transfer in a corrole–naphthalene bisimide dyad. Phys. Chem. Chem. Phys. 2010, 12, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.J.; Lee, K.; Sun, J.; Andreou, A.G.; Katz, H.E. Air-operable, high-mobility organic transistors with semifluorinated side chains and unsubstituted naphthalenetetracarboxylic diimide cores: High mobility and environmental and bias stress stability from the perfluorooctylpropyl side chain. Adv. Funct. Mater. 2010, 20, 2930–2944. [Google Scholar] [CrossRef]
- Stevenson, P.J. Second-order NMR spectra at high field of common organic functional groups. Org. Biomol. Chem. 2011, 9, 2078–2084. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Naqvi, S.; Kumar, R. Naphthalene diimide self-assembled ribbons with high electrical conductivity and mobility without doping. J. Mater. Sci. 2018, 53, 4046–4055. [Google Scholar] [CrossRef]
- Wang, L.; Park, S.-J.; Lee, S.-H.; Kim, Y.-J.; Kook, Y.-B.; Kuo, S.-W.; Van Horn, R.M.; Cheng, S.Z.D.; Lee, M.-H.; Jeong, K.-U. Self-assembled columnar structures of swallow-shaped tetrathiafulvalene-based molecules. Chem. Mater. 2009, 21, 3838–3847. [Google Scholar] [CrossRef]
- Heinz, H.; Castelijns, H.J.; Suter, U.W. Structure and phase transitions of alkyl chains on mica. J. Am. Chem. Soc. 2003, 125, 9500–9510. [Google Scholar] [CrossRef] [PubMed]
- Kiersnowski, A.; Kolman, K.; Lieberwirth, I.; Yordanov, S.; Butt, H.-J.; Hansen, M.R.; Anastasiadis, S.H. New insights into the multilevel structure and phase transitions of synthetic organoclays. Soft Matter 2013, 9, 2291–2301. [Google Scholar] [CrossRef]
- Milita, S.; Liscio, F.; Cowen, L.; Cavallini, M.; Drain, B.A.; Degousée, T.; Luong, S.; Fenwick, O.; Guagliardi, A.; Schroeder, B.C.; et al. Polymorphism in N,N′-dialkyl-naphthalene diimides. J. Mater. Chem. C 2020, 8, 3097–3112. [Google Scholar] [CrossRef]
- He, T.; Stolte, M.; Burschka, C.; Hansen, N.H.; Musiol, T.; Kälblein, D.; Pflaum, J.; Tao, X.; Brill, J.; Würthner, F. Single-crystal field-effect transistors of new Cl 2-NDI polymorph processed by sublimation in air. Nat. Commun. 2015, 6, 5954. [Google Scholar] [CrossRef] [PubMed]
- Dharmarwardana, M.; Arimilli, B.S.; Luzuriaga, M.A.; Kwon, S.; Lee, H.; Appuhamillage, G.A.; McCandless, G.T.; Smaldone, R.A.; Gassensmith, J.J. The thermo-responsive behavior in molecular crystals of naphthalene diimides and their 3D printed thermochromic composites. CrystEngComm 2018, 20, 6054–6060. [Google Scholar] [CrossRef]
- Ichikawa, M.; Iwasaki, K.; Ohyama, A.; Miyazawa, J.; Yokota, Y.; Hirata, N.; Oguma, N. Comparative study of long alkyl chain substituted naphthalene diimide derivatives as n-type organic thin-film transistor materials. Jpn. J. Appl. Phys. 2017, 56, 111601. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, Y.; Liu, Y. 25th anniversary article: Recent advances in n-type and ambipolar organic field-effect transistors. Adv. Mater. 2013, 25, 5372–5391. [Google Scholar] [CrossRef]
- Bao, Z.; Locklin, J. Organic Field-Effect Transistors, 1st ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Fidyk, J.; Waliszewski, W.; Sleczkowski, P.; Kiersnowski, A.; Pisula, W.; Marszalek, T. Switching from electron to hole transport in solution-processed organic blend field-effect transistors. Polymers 2020, 12, 2662. [Google Scholar] [CrossRef]
- Ray, S.; Bousmina, M. Biodegradable polymers and their layered silicate nanocomposites: In greening the 21st century materials world. Prog. Mater. Sci. 2005, 50, 962–1079. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C—Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Leight, K.R.; Esarey, B.E.; Murray, A.E.; Reczek, J.J. Predictable Tuning of Absorption Properties in Modular Aromatic Donor–Acceptor Liquid Crystals. Chem. Mater. 2012, 24, 3318–3328. [Google Scholar] [CrossRef]
| Yield [%] (15 mmol Scale) | Amine Boiling Point [°C] | |
|---|---|---|
| NDIC3 | 35 | 47.8 |
| NDIC4 | 51 | 78.0 |
| NDIC5 | 88 | 104 |
| NDIC6 | 82 | 131 |
| NDIC7 | 75 | 155 |
| NDIC8 | 85 | 176 |
| NDIC9 | 78 | 200 |
| NDIC10 | 79 | 217 |
| NDIC11 | 68 | 242 |
| Crystal System | Space Group | a | B | C | α | β | γ | dπ-π [Å] | |
|---|---|---|---|---|---|---|---|---|---|
| NDIC3 | orthorhombic | Pbca | 6.96 | 17.24 | 27.58 | 90.0 | 90.0 | 90.0 | n/a |
| (7.15) | (17.49) | (27.80) | (90.0) | (90.0) | (90.0) | ||||
| NDIC4 | triclinic | P | 5.22 | 7.84 | 11.13 | 103.7 | 94.3 | 93.9 | 3.34 |
| (5.32) | (8.17) | (11.04) | (105.1) | (93.4) | (92.9) | ||||
| NDIC5 | monoclinic | P21/c | 5.03 | 8.11 | 24.21 | 90.0 | 90.8 | 90.0 | 3.22 |
| (5.12) | (7.94) | (26.24) | (90.0) | (91.6) | (90.0) | ||||
| NDIC6 | triclinic | P | 4.90 | 8.28 | 14.52 | 96.3 | 98.1 | 93.6 | 3.32 |
| (4.91) | (8.38) | (14.55) | (96.0) | (99.1) | (93.1) | ||||
| NDIC7 | monoclinic | P21/c | 7.87 | 4.84 | 33.02 | 90.0 | 92.0 | 90.0 | 3.27 |
| (-) | (-) | (-) | (-) | (-) | (-) | ||||
| NDIC8 | triclinic | P | 4.77 | 6.53 | 22.71 | 87.9 | 88.9 | 75.8 | 3.36 |
| (4.86) | (6.63) | (22.87) | (88.7) | (89.9) | (75.3) | ||||
| NDIC9 | monoclinic | P21/c | 7.85 | 4.84 | 37.74 | 90.0 | 95.0 | 90.0 | 3.17 |
| (7.95) | (4.91) | (38.06) | (90.0) | (94.9) | (90.0) | ||||
| NDIC10 | triclinic | P | 4.73 | 6.55 | 25.58 | 94.2 | 95.3 | 104.3 | 3.31 |
| (4.81) | (6.70) | (26.09) | (93.2) | (95.8) | (105.2) | ||||
| NDIC11 | monoclinic | P21/c | 7.85 | 4.87 | 42.51 | 90.0 | 92.1 | 90.0 | 3.14 |
| (-) | (-) | (-) | (-) | (-) | (-) |
| Electron Mobility—μe/10−4 × cm2V−1s−1 | Threshold Voltage—Vth/V | |
|---|---|---|
| NDIC3 | 0.16 ± 0.03 | 41 ± 3 |
| NDIC4 | 0.55 ± 0.13 | 34 ± 2 |
| NDIC5 | 0.12 ± 0.01 | 46 ± 1 |
| NDIC6 | 1.55 ± 0.31 | 37 ± 1 |
| NDIC7 | 2.49 ± 0.79 | 47 ± 6 |
| NDIC8 | 1.24 ± 0.46 | 47 ± 3 |
| NDIC9 | 0.15 ± 0.03 | 46 ± 3 |
| NDIC10 | 0.08 ± 0.01 | 54 ± 3 |
| NDIC11 | 0.56 ± 0.05 | 46 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chlebosz, D.; Goldeman, W.; Janus, K.; Szuster, M.; Kiersnowski, A. Synthesis, Solution, and Solid State Properties of Homological Dialkylated Naphthalene Diimides—A Systematic Review of Molecules for Next-Generation Organic Electronics. Molecules 2023, 28, 2940. https://doi.org/10.3390/molecules28072940
Chlebosz D, Goldeman W, Janus K, Szuster M, Kiersnowski A. Synthesis, Solution, and Solid State Properties of Homological Dialkylated Naphthalene Diimides—A Systematic Review of Molecules for Next-Generation Organic Electronics. Molecules. 2023; 28(7):2940. https://doi.org/10.3390/molecules28072940
Chicago/Turabian StyleChlebosz, Dorota, Waldemar Goldeman, Krzysztof Janus, Michał Szuster, and Adam Kiersnowski. 2023. "Synthesis, Solution, and Solid State Properties of Homological Dialkylated Naphthalene Diimides—A Systematic Review of Molecules for Next-Generation Organic Electronics" Molecules 28, no. 7: 2940. https://doi.org/10.3390/molecules28072940
APA StyleChlebosz, D., Goldeman, W., Janus, K., Szuster, M., & Kiersnowski, A. (2023). Synthesis, Solution, and Solid State Properties of Homological Dialkylated Naphthalene Diimides—A Systematic Review of Molecules for Next-Generation Organic Electronics. Molecules, 28(7), 2940. https://doi.org/10.3390/molecules28072940






