Initial Response of Pentaerythritol Tetranitrate (PETN) under the Coupling Effect of Preheating, Shock and Defect via the Molecular Dynamics Simulations with the Multiscale Shock Technique Method
Abstract
1. Introduction
2. Results and Discussion
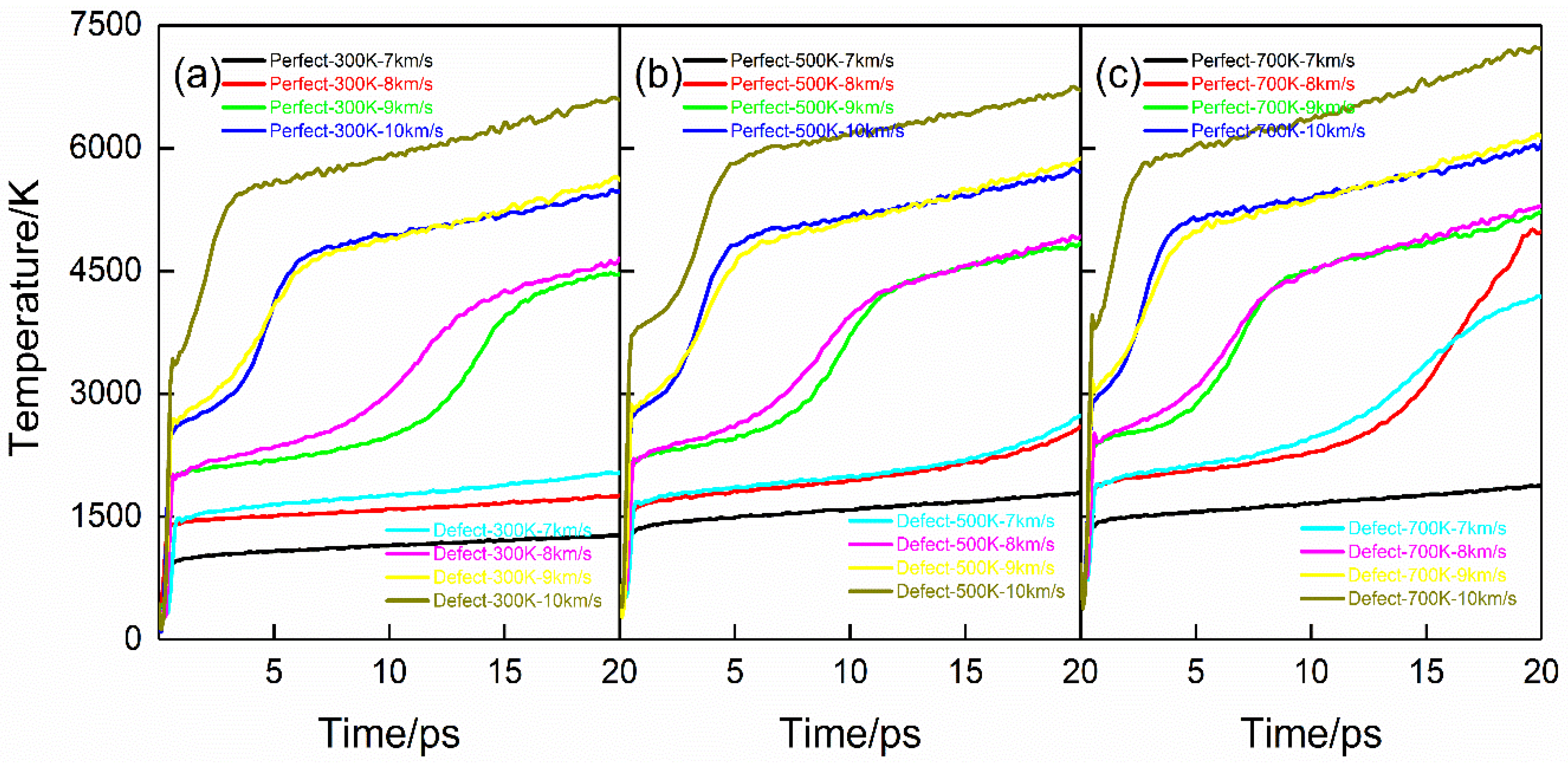
2.1. Thermodynamic Quantities during Shock Compression
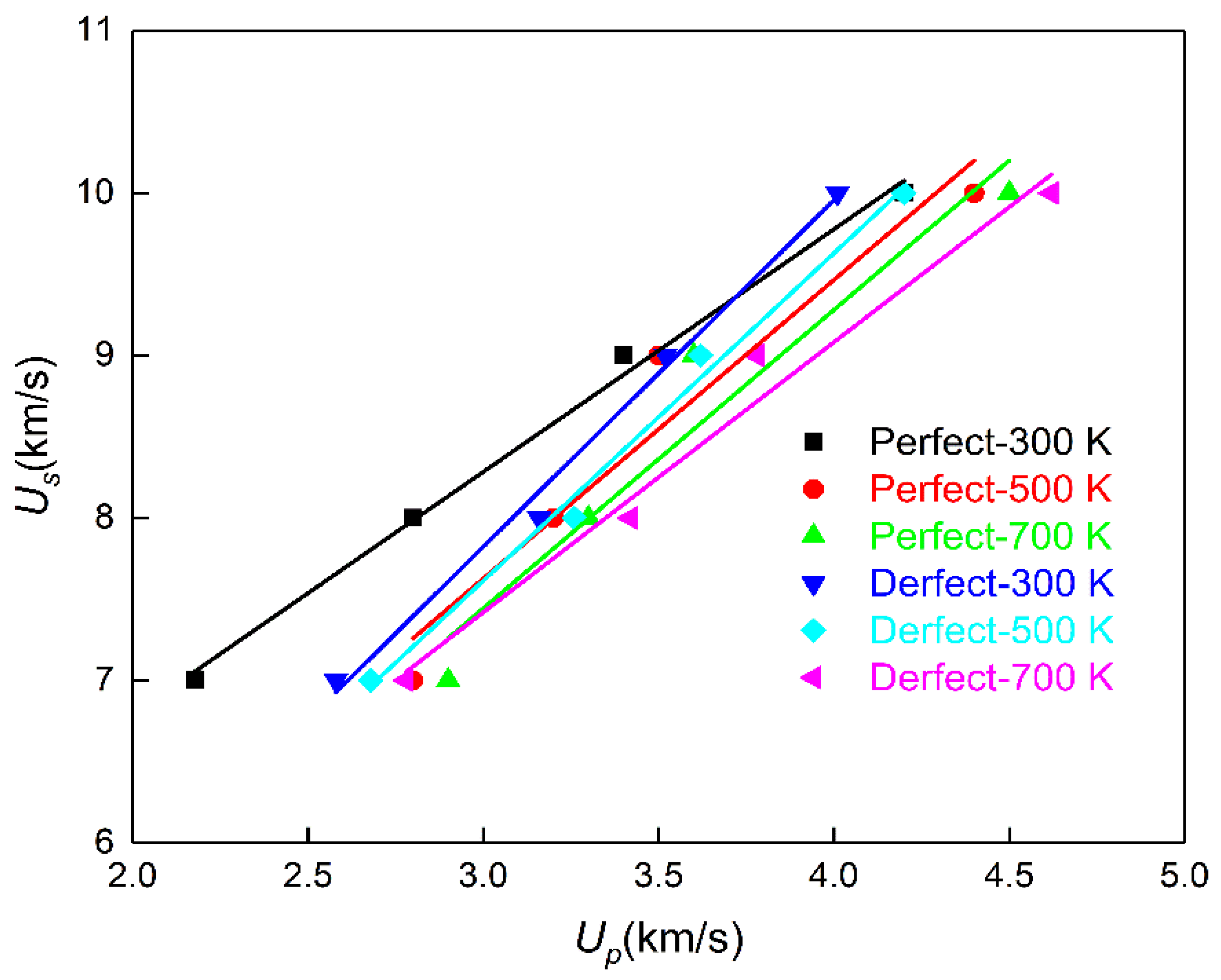
2.2. Relationship between Shock Wave Speed and Particle Speed
2.3. Effects of Initial Preheating Temperature and Defects on PETN Reactivity
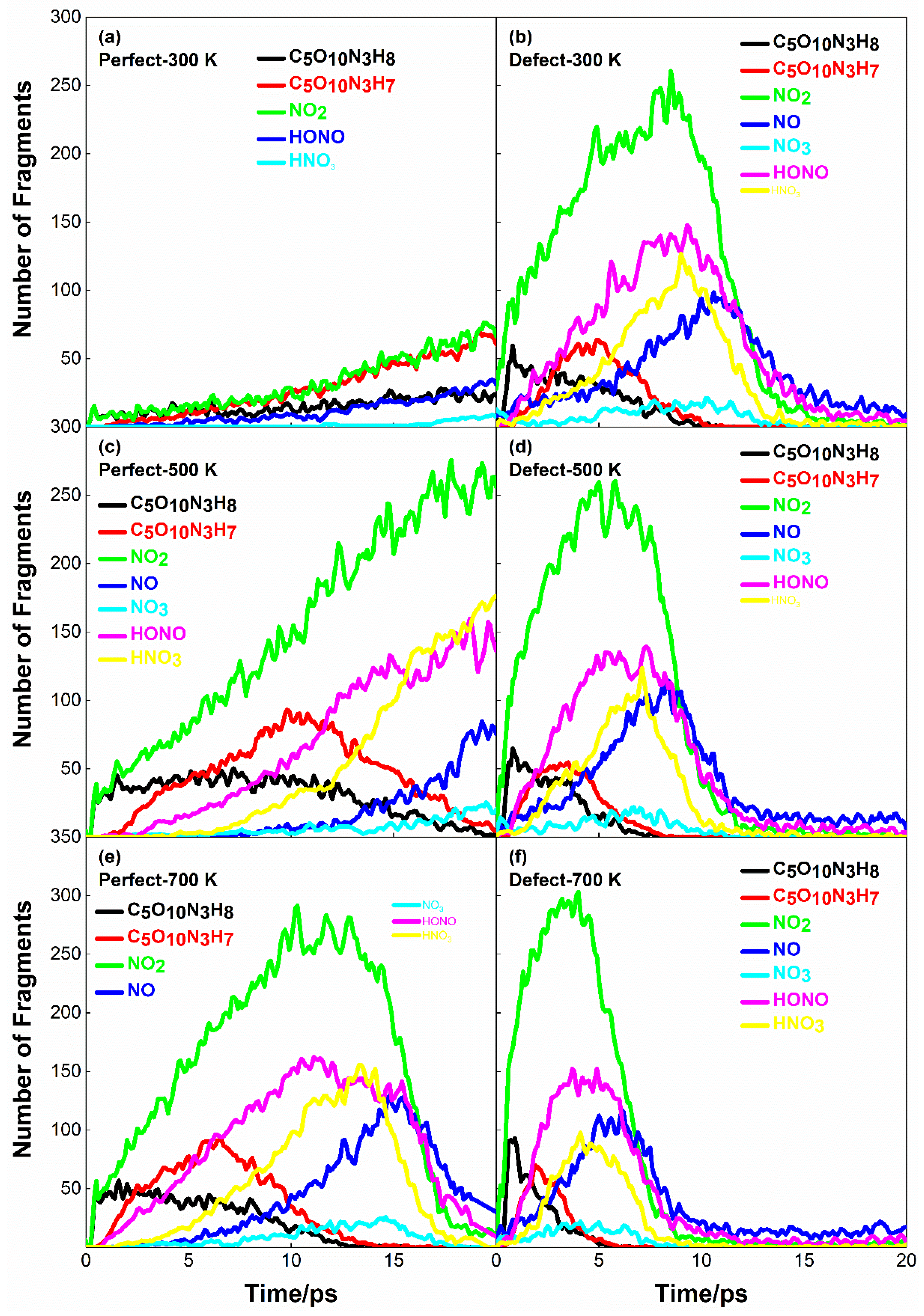
2.4. Initial Decomposition Path and Product Distribution of PETN
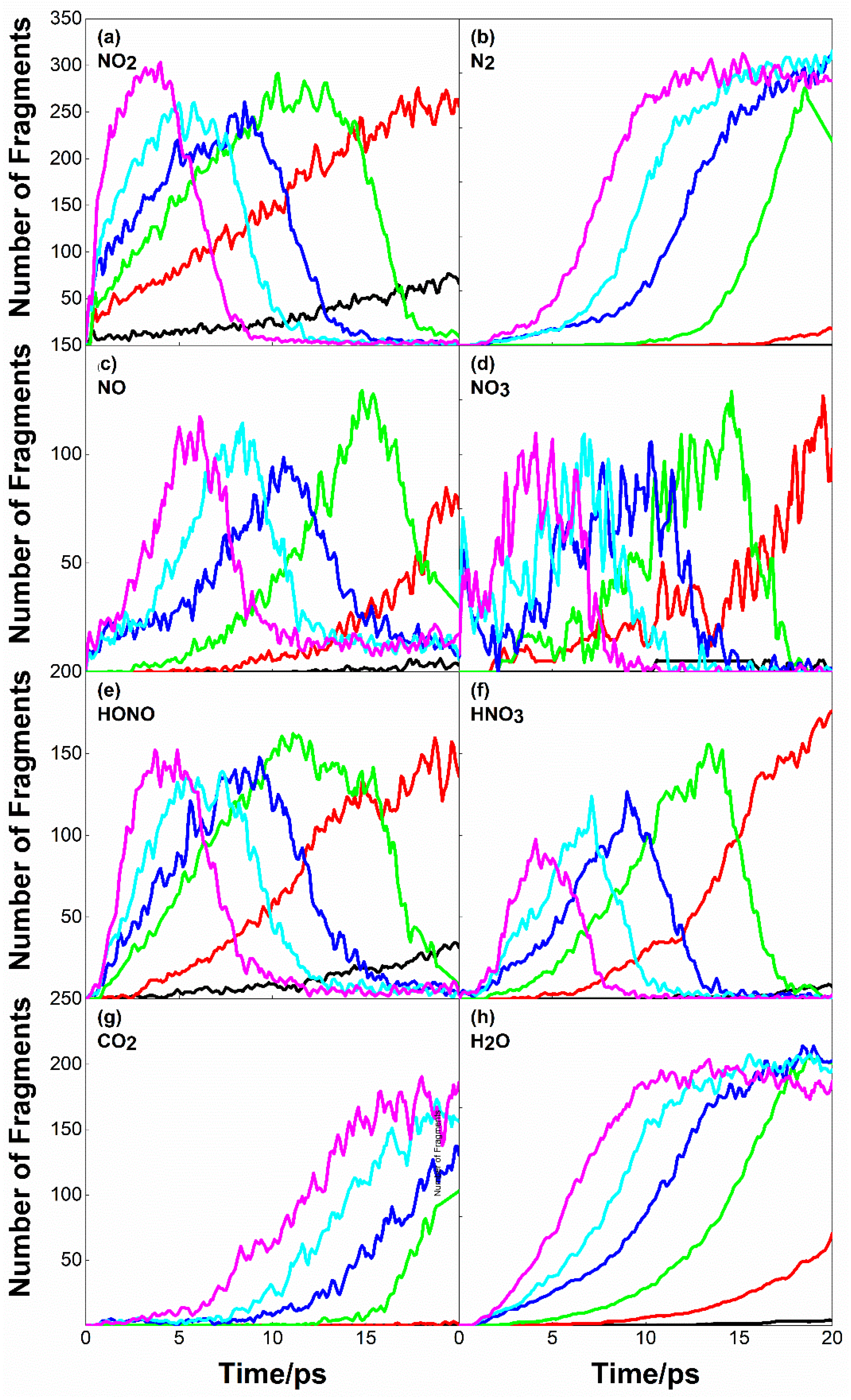
2.5. Initial Response of PETN under Strong Load Coupling
2.6. Chemical Reaction Kinetics
3. Simulation Details
3.1. Details of the Multiscale Shock Technique (MSST) Method
3.2. Details of MD Simulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sakano, M.; Hamilton, B.; Islam, M.M.; Strachan, A. Role of molecular disorder on the reactivity of RDX. J. Phys. Chem. 2018, 122, 27032–27043. [Google Scholar] [CrossRef]
- Munn, R.W.; Tsiaousis, D. Charge-transfer exciton trapping at vacancies in molecular crystals: Photoconductivity quenching, optical damage and detonation. Macromol. Symp. 2004, 212, 73–82. [Google Scholar] [CrossRef]
- Urtiew, P.A.; Tarver, C.M. Shock initiation of energetic materials at different initial temperatures (Review). Combust. Explos. Shock Waves 2005, 41, 766–776. [Google Scholar] [CrossRef]
- Deng, C.; Liu, J.; Xue, X.G.; Long, X.P.; Zhang, C.Y. Coupling effect of shock, heat, and defect on the decay of energetic materials: A case of reactive molecular dynamics simulations on 1,3,5-Trinitro-1,3,5-triazinane. J. Phys. Chem. 2018, 122, 27875–27884. [Google Scholar] [CrossRef]
- Dreger, Z.A.; Gupta, Y.M. Decomposition of γ-cyclotrimethylene trinitramine (γ-RDX): Relevance for shock wave initiation. J. Phys. Chem. 2012, 116, 8713–8717. [Google Scholar] [CrossRef] [PubMed]
- Dreger, Z.A.; McCluskey, M.D.; Gupta, Y.M. High pressure-high temperature decomposition of γ-cyclotrimethylene trinitramine. J. Phys. Chem. 2012, 116, 9680–9688. [Google Scholar] [CrossRef] [PubMed]
- Akiki, M.; Menon, S. A model for hot spot formation in shocked energetic materials. Combust. Flame 2015, 162, 1759–1771. [Google Scholar] [CrossRef]
- Eason, R.M.; Sewell, T.D. Molecular dynamics simulations of the collapse of a cylindrical pore in the energetic material α-RDX. J. Dyn. Behav. Mater. 2015, 1, 423–438. [Google Scholar] [CrossRef]
- Tsai, D.H.; Armstrong, R.W. Defect-enhanced structural relaxation mechanism for the evolution of hot spots in rapidly compressed crystals. J. Phys. Chem. 1994, 98, 10997–11000. [Google Scholar] [CrossRef]
- Tsai, D.H. Structural defects and ‘‘hot spot’’ formation in a crystalline solid under rapid compression. I. Vacancy clusters and slip bands. J. Chem. Phys. 1991, 95, 7497–7503. [Google Scholar] [CrossRef]
- Kuklja, M.M.; Tsyshevsky, R.V.; Sharia, O. Effect of polar surfaces on decomposition of molecular materials. J. Am. Chem. Soc. 2014, 136, 13289–13302. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.T.; Huang, F.L. Effects of defects on thermal decomposition of HMX via ReaxFF molecular dynamics simulations. J. Phys. Chem. 2011, 115, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Xue, X.; Zhou, X.; Guo, F.; Long, X.; Zhou, Y.; Li, H.; Zhang, C. Twin induced sensitivity enhancement of HMX versus shock: A molecular reactive force field simulation. J. Phys. Chem. 2013, 117, 24368–24374. [Google Scholar] [CrossRef]
- Wood, M.A.; van Duin, A.C.T.; Strachan, A. Coupled thermal and electromagnetic induced decomposition in the molecular explosive αHMX; A reactive molecular dynamics study. J. Phys. Chem. 2014, 118, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.G.; Wen, Y.S.; Long, X.P.; Li, J.S.; Zhang, C.Y. Influence of dislocations on the shock sensitivity of RDX: Molecular dynamics simulations by reactive force field. J. Phys. Chem. 2015, 119, 13735–13742. [Google Scholar] [CrossRef]
- Wang, N.; Peng, J.; Pang, A.; He, T.; Du, F.; Jaramillo-Botero, A. Thermodynamic simulation of the RDX—Aluminum interface using ReaxFF molecular dynamics. J. Phys. Chem. 2017, 121, 14597–14610. [Google Scholar] [CrossRef]
- Zhong, K.; Liu, J.; Wang, L.Y.; Zhang, C.Y. Influence of Atmospheres on the Initial Thermal Decomposition of 1,3,5-Trinitro-1,3,5-triazinane: Reactive Molecular Dynamics Simulation. J. Phys. Chem. 2019, 123, 1483–1493. [Google Scholar] [CrossRef]
- Sharia, O.; Kuklja, M.M. Rapid materials degradation induced by surfaces and voids: Ab initio modeling of β-octatetramethylene tetranitramine. J. Am. Chem. Soc. 2012, 134, 11815–11820. [Google Scholar] [CrossRef]
- Kimmel, A.V.; Sushko, P.V.; Shluger, A.L.; Kuklja, M.M. Effect of charged and excited states on the decomposition of 1,1-diamino-2,2-dinitroethylene molecules. J. Chem. Phys. 2007, 126, 234711. [Google Scholar] [CrossRef]
- Kuklja, M.M.; Rashkeev, S.N. Shear-strain-induced chemical reactivity of layered molecular crystals. Appl. Phys. Lett. 2007, 90, 151913. [Google Scholar] [CrossRef]
- Kuklja, M.M.; Rashkeev, S.N. Interplay of decomposition mechanisms at shear-strain interface. J. Phys. Chem. 2009, 113, 17–20. [Google Scholar] [CrossRef]
- Kuklja, M.M.; Rashkeev, S.N. Self-accelerated mechanochemistry in nitroarenes. J. Phys. Chem. Lett. 2010, 1, 363–367. [Google Scholar] [CrossRef]
- Shan, T.R.; Wixom, R.R.; Mattsson, A.E.; Thompson, A.P. Atomistic simulation of orientation dependence in shock-induced initiation of pentaerythritol tetranitrate. J. Phys. Chem. 2013, 117, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Dick, J.J. Effect of crystal orientation on shock initiation sensitivity of pentaerythritol tetranitrate explosive. Appl. Phys. Lett. 1984, 44, 859–861. [Google Scholar] [CrossRef]
- Dick, J.J.; Mulford, R.N.; Spencer, W.J.; Pettit, D.R.; Garcia, E.; Shaw, D.C. Shock response of pentaerythritol tetranitrate single crystals. J. Appl. Phys. 1991, 70, 3572–3587. [Google Scholar] [CrossRef]
- Li, Y.; Kalia, R.K.; Nakano, A.; Nomura, K.-I.; Vashishta, P. Multistage reaction pathways in detonating high explosives. Appl. Phys. Lett. 2014, 105, 204103. [Google Scholar] [CrossRef]
- Dlott, D.D.; Fayer, M.D. Shocked molecular solids: Vibrational up pumping, defect hot spot formation, and the onset of chemistry. J. Chem. Phys. 1990, 92, 3798–3812. [Google Scholar] [CrossRef]
- Tokamkoff, A.; Fayer, M.D.; Dlott, D.D. Chemical reaction initiation and hot-spot formation in shocked energetic molecular materials. J. Phys. Chem. 1993, 97, 1901–1913. [Google Scholar] [CrossRef]
- Wu, C.J.; Ree, F.H.; Yoo, C.S. A quantum mechanical molecular dynamics study of binary collisions of pentaerythritol tetranitrate (PETN): Its correlation to shock sensitivity. Propellants Explos. Pyrotech. 2004, 29, 296–303. [Google Scholar] [CrossRef]
- Tsyshevsky, R.V.; Sharia, O.; Kuklja, M.M. Thermal decomposition mechanisms of nitroesters: Ab initio modeling of pentaerythritol tetranitrate. J. Phys. Chem. 2013, 117, 18144–18153. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Liu, H.; Yang, Z.; Li, Q.K.; He, Y.H. Shock-induced hot spot formation and spalling in 1,3,5-trinitroperhydro-1,3,5-triazine containing a cube Void. ACS Omega 2019, 4, 8031–8038. [Google Scholar] [CrossRef] [PubMed]
- Strachan, A.; van Duin, A.C.T.; Chakraborty, D.; Dasgupta, S.; Goddard, W.A., III. Shock Waves in High-Energy Materials: The Initial Chemical Events in Nitramine RDX. Phys. Rev. Lett. 2003, 91, 098301. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.T.; Lou, J.F.; Zhang, Y.P.; Song, H.J.; Huang, F.L. Hot spot formation and chemical reaction initiation in shocked HMX crystals with nanovoids: A large-scale reactive molecular dynamics study. Phys. Chem. Chem. Phys. 2016, 18, 17627–17645. [Google Scholar] [CrossRef] [PubMed]
- Reed, E.J.; Fried, L.E.; Joannopoulos, J.D. A method for tractable dynamical studies of single and double shock compression. Phys. Rev. Lett. 2003, 90, 235503. [Google Scholar] [CrossRef]
- Manaa, M.R.; Reed, E.J.; Fried, L.E.; Goldman, N. Nitrogen-rich heterocycles as reactivity retardants in shocked insensitive explosives. J. Am. Chem. Soc. 2009, 131, 5483–5487. [Google Scholar] [CrossRef]
- He, Z.H.; Chen, J.; Wu, Q. Initial decomposition of condensed-phase 1,3,5-Triamino-2,4,6-trinitrobenzene under shock loading. J. Phys. Chem. 2017, 121, 8227–8235. [Google Scholar] [CrossRef]
- Trotter, J. Bond lengths and angles in pentaerythritol tetranitrate. Acta Cryst. 1963, 16, 698. [Google Scholar] [CrossRef]
- Kuklja, M.M.; Kunz, A.B. Ab initio simulation of defects in energetic materials: Hydrostatic compression of cyclotrimethylene trinitramine. J. Appl. Phys. 1999, 86, 4428–4434. [Google Scholar] [CrossRef]
- Plimpton, S.J. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Aktulga, H.M.; Fogarty, J.C.; Pandit, S.A.; Grama, A.Y. Parallel reactive molecular dynamics: Numerical methods and algorithmic techniques. Parallel Comput. 2012, 38, 245–259. [Google Scholar] [CrossRef]
- Liu, L.C.; Liu, Y.; Zybin, S.V.; Sun, H.; Goddard, W.A., III. ReaxFF-lg: Correction of the ReaxFF Reactive Force Field for London Dispersion, with Applications to the Equations of State for Energetic Materials. J. Phys. Chem. 2011, 115, 11016–11022. [Google Scholar] [CrossRef] [PubMed]





| Us (km·s−1) | Preheating Temperature (K) | Kp | Kd | × 100% |
|---|---|---|---|---|
| 7 | 300 | 0.00688 | 0.09167 | 92.5% |
| 500 | 0.05543 | 0.15448 | 64.1% | |
| 700 | 0.07523 | 0.28369 | 73.5% | |
| 8 | 300 | 0.03000 | 0.33122 | 90.9% |
| 500 | 0.13643 | 0.46627 | 70.7% | |
| 700 | 0.24234 | 0.74792 | 67.6% | |
| 9 | 300 | 0.26535 | 0.93081 | 71.5% |
| 500 | 0.45057 | 1.26102 | 64.3% | |
| 700 | 0.77840 | 1.55408 | 50.1% | |
| 10 | 300 | 1.26635 | 1.90714 | 33.6% |
| 500 | 1.71408 | 2.25261 | 24.1% | |
| 700 | 2.04698 | 2.29648 | 10.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, T.; He, Y. Initial Response of Pentaerythritol Tetranitrate (PETN) under the Coupling Effect of Preheating, Shock and Defect via the Molecular Dynamics Simulations with the Multiscale Shock Technique Method. Molecules 2023, 28, 2911. https://doi.org/10.3390/molecules28072911
Zhang Y, Wang T, He Y. Initial Response of Pentaerythritol Tetranitrate (PETN) under the Coupling Effect of Preheating, Shock and Defect via the Molecular Dynamics Simulations with the Multiscale Shock Technique Method. Molecules. 2023; 28(7):2911. https://doi.org/10.3390/molecules28072911
Chicago/Turabian StyleZhang, Yaping, Tao Wang, and Yuanhang He. 2023. "Initial Response of Pentaerythritol Tetranitrate (PETN) under the Coupling Effect of Preheating, Shock and Defect via the Molecular Dynamics Simulations with the Multiscale Shock Technique Method" Molecules 28, no. 7: 2911. https://doi.org/10.3390/molecules28072911
APA StyleZhang, Y., Wang, T., & He, Y. (2023). Initial Response of Pentaerythritol Tetranitrate (PETN) under the Coupling Effect of Preheating, Shock and Defect via the Molecular Dynamics Simulations with the Multiscale Shock Technique Method. Molecules, 28(7), 2911. https://doi.org/10.3390/molecules28072911








