Targeting Carbohydrate Mimetics of Tetrahydrofuran-Containing Acetogenins to Prostate Cancer
Abstract
1. Introduction
2. Results and Discussion
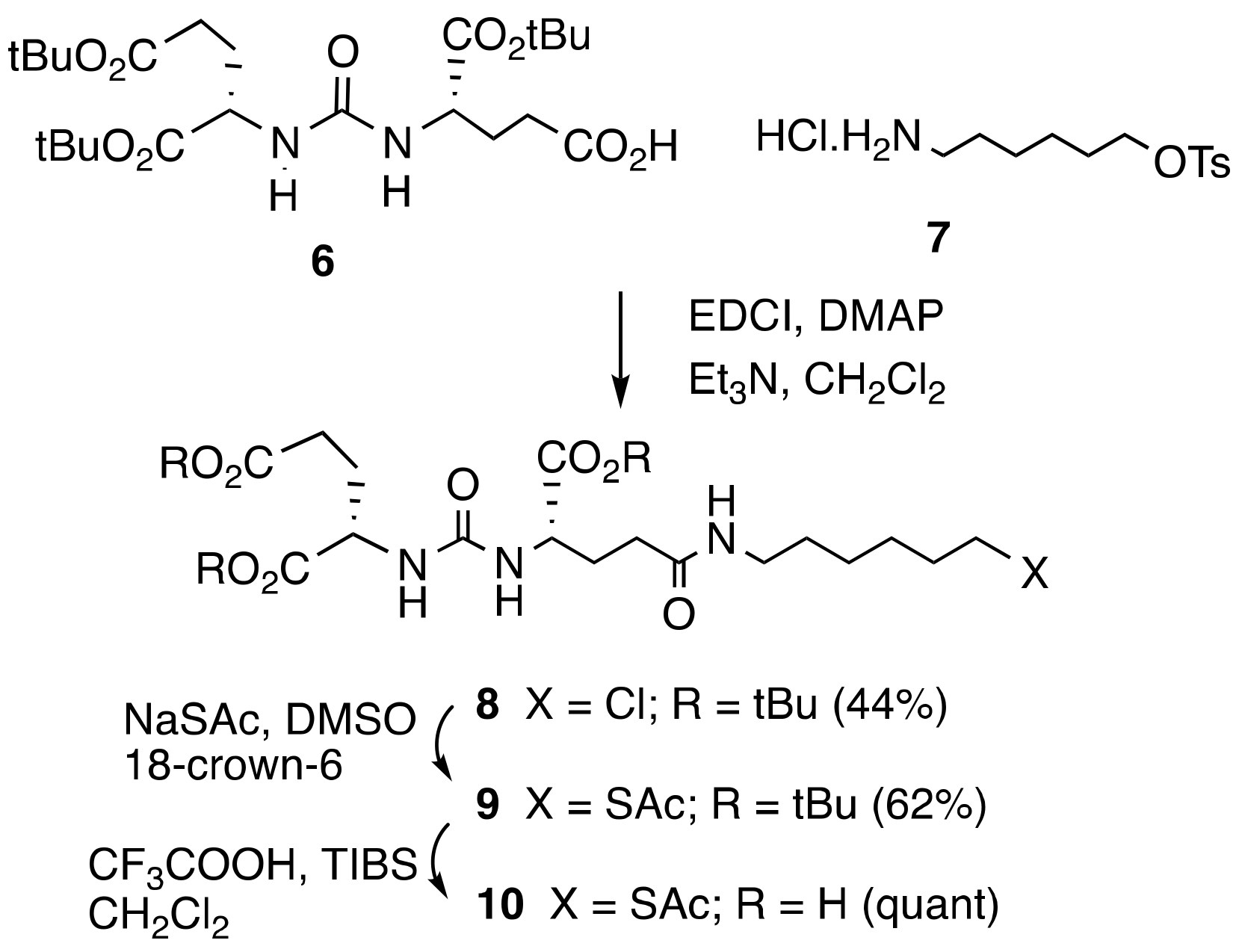
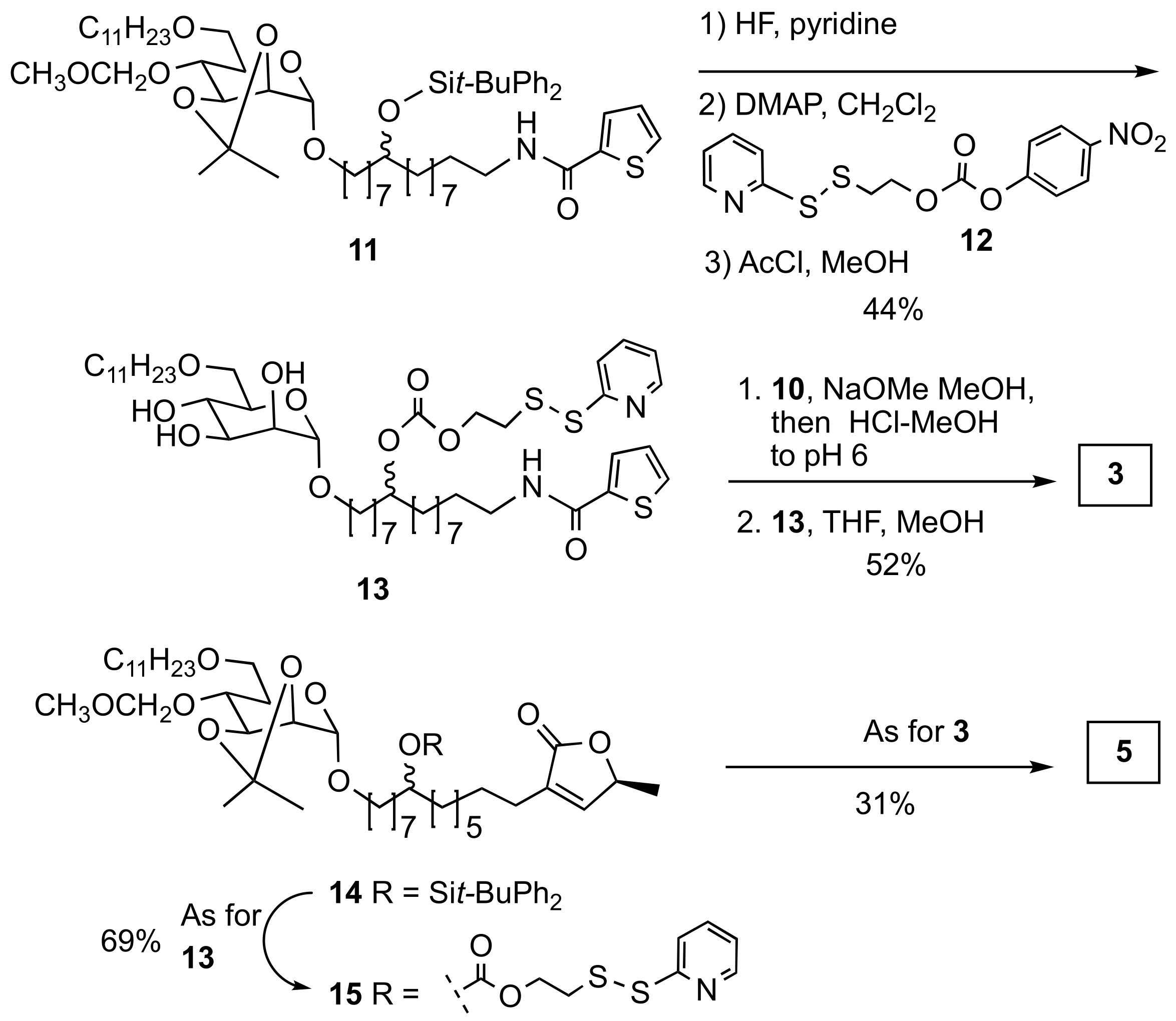
2.1. Synthesis
2.2. Cytotoxicity Data
3. Materials and Methods
3.1. Chemical Synthesis
3.1.1. 6-aminohexyl 4-methylbenzenesulfonate Hydrochloride (7)
3.1.2. Di-tert-butyl(((S)-1-(tert-butoxy)-5-((6-chlorohexyl)amino)-1,5-dioxopentan-2-yl)carbamoyl)-L-glutamate (8)
3.1.3. Tri-tert-butyl (14S,18S)-2,11,16-trioxo-3-thia-10,15,17-triazaicosane-14,18,20-tricarboxylate (9)
3.1.4. (14. S,18S)-2,11,16-trioxo-3-thia-10,15,17-triazaicosane-14,18,20-tricarboxylic Acid (10)
3.1.5. Mannose-thiophene-SS-DUPA (3)
3.1.6. 1-((S)-5-methyl-2-oxo-2,5-dihydro-1H-1λ3-furan-3-yl)-15-(((2S,3S,4S,5S,6R)-3,4,5-trihydroxy-6-((undecyloxy)methyl)tetrahydro-2H-pyran-2-yl)oxy)pentadecan-8-yl (2-(pyridin-2-yldisulfaneyl)ethyl) Carbonate (15)
3.1.7. Mannose-butenolide-SS-DUPA (5)
3.2. Cytotoxicity Measurements
3.2.1. Cell Lines and Cell Number Optimization
3.2.2. IC50 Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alali, F.Q.; Liu, X.X.; McLaughlin, J.L. Annonaceous acetogenins: Recent progress. J. Nat. Prod. 1999, 62, 504–540. [Google Scholar] [CrossRef]
- McLaughlin, J.L. Paw paw and cancer: Annonaceous acetogenins from discovery to commercial products. J. Nat. Prod. 2008, 71, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Kojima, N.; Tanaka, T. Medicinal chemistry of annonaceous acetogenins: Design, synthesis, and biological evaluation of novel analogues. Molecules 2009, 14, 3621–3661. [Google Scholar] [CrossRef] [PubMed]
- Liaw, C.-C.; Wu, T.-Y.; Chang, F.-R.; Wu, Y.-C. Historic perspectives on Annonaceous acetogenins from the chemical bench to preclinical trials. Planta Med. 2010, 76, 1390–1404. [Google Scholar] [CrossRef] [PubMed]
- Grba, D.N.; Blaza, J.N.; Bridges, H.R.; Agip, A.-N.A.; Yin, Z.; Murai, M.; Miyoshi, H.; Hirst, J. Cryo-electron microscopy reveals how acetogenins inhibit mitochondrial respiratory complex I. J. Biol. Chem. 2022, 298, 101602. [Google Scholar] [CrossRef]
- Xue, D.; Xu, Y.; Kyani, A.; Roy, J.; Dai, L.; Sun, D.; Neamati, N. Multiparameter optimization of oxidative phosphorylation inhibitors for the treatment of pancreatic cancer. J. Med. Chem. 2022, 65, 3404–3419. [Google Scholar] [CrossRef]
- Ahmad, F.; Cherukuri, M.K.; Choyke, P.L. Metabolic reprogramming in prostate cancer. Br. J. Cancer 2021, 125, 1185–1196. [Google Scholar] [CrossRef]
- Naguib, A.; Mathew, G.; Reczek, C.R.; Watrud, K.; Ambrico, A.; Herzka, T.; Salas, I.C.; Lee, M.F.; El-Amine, N.; Zheng, W.; et al. Mitochondrial complex I inhibitors expose a vulnerability for selective killing of Pten-mnull cells. Cell Rep. 2018, 23, 58–67. [Google Scholar] [CrossRef]
- Gonzalez Periche, P.; Ramdular, A.; Bhupathiraju, N.V.S.D.K.; Kalidindi, T.; Johnson, D.S.; Pillarsetty, N.; Mootoo, D.R. Synthesis of carbohydrate analogues of the THF-acetogenin 4-deoxyannomontacin and their cytotoxicity against human prostate cancer cell lines. Carbohydr. Res. 2022, 521, 108671. [Google Scholar] [CrossRef]
- Wang, F.; Kawamura, A.; Mootoo, D.R. Synthesis and antitumor activity of C-9 epimers of the tetrahydrofuran containing acetogenin 4-deoxyannoreticuin. Bioorg. Med. Chem. 2008, 16, 8413–8418. [Google Scholar] [CrossRef]
- Hoffman, R.M. Clinical practice. Screening for prostate cancer. N. Engl. J. Med. 2011, 365, 2013–2019. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Shi, J.F.; Wu, P.; Cheng, X.L.; Wei, X.Y.; Jiang, Z.H. For studies on naturally occurring THF congeners conjugated to tumor vectors: Synthesis and cytotoxic property of annonaceous acetogenin glycoconjugates. Drug Design Dev. Therap. 2020, 14, 4993–5004. [Google Scholar] [CrossRef]
- Shi, J.F.; Wu, P.; Jiang, Z.H.; Wei, X.Y. Synthesis and tumor cell growth inhibitory activity of biotinylated annonaceous acetogenins. Eur. J. Med. Chem. 2014, 71, 219–228. [Google Scholar] [CrossRef]
- Queiroz, E.F.; Roblot, F.; Duret, P.; Figadère, B.; Gouyette, A.; Laprévote, O.; Serani, L.; Hocquemiller, R. Synthesis, spectroscopy and cytotoxicity of glycosylated acetogenin derivatives as promising molecules for cancer therapy. J. Med. Chem. 2000, 43, 1604–1610. [Google Scholar] [CrossRef]
- Uspenskaya, A.A.; Machulkin, A.E.; Mazhuga, A.G.; Beloglazkina, A.K. Conjugates of prostate-specific membrane antigen ligands with antitumor drugs. Pharm. Chem. J. 2019, 53, 288–297. [Google Scholar] [CrossRef]
- Afshar-Oromieh, A.; Babich, J.W.; Kratochwil, C.; Giesel, F.L.; Eisenhut, M.; Kopka, K.; Haberkorn, U. The rise of PSMA ligands for diagnosis and therapy of prostate cancer. J. Nucl Med. 2016, 57, 79S–89S. [Google Scholar] [CrossRef]
- Hawkey, N.M.; Sartor, A.O.; Morris, M.J.; Armstrong, A.J. Prostate-specific membrane antigen–targeted theranostics: Past, present, and future approaches. Clin. Adv. Hemat. Oncol. 2022, 20, 227–239. [Google Scholar]
- Pastorino, S.; Riondato, M.; Uccelli, L.; Giampiero, G.; Giovannini, E.; Duce, V.; Ciamiello, A. Toward the discovery and development of PSMA targeted inhibitors for nuclear medicine applications. Cur. Radiopharm. 2020, 13, 63–79. [Google Scholar] [CrossRef]
- Kularatne, S.A.; Wang, K.; Santhapuram, H.-K.R.; Low, P.S. Prostate-specific membrane antigen targeted imaging and therapy of prostate cancer using a PSMA inhibitor as a homing ligand. Mol. Pharm. 2009, 6, 780–789. [Google Scholar] [CrossRef]
- Kularatne, S.A.; Zhou, Z.; Yang, J.; Post, C.B.; Low, P.S. Design, synthesis, and preclinical evaluation of prostate-specific membrane antigen targeted 99mTc-radioimaging agents. Mol. Pharm. 2009, 6, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Kularatne, S.A.; Venkatesh, C.; Santhapuram, H.-K.; Wang, K.; Vaitilingam, B.; Henne, W.A.; Low, P.S. Synthesis and biological analysis of prostate-specific membrane antigen-targeted anticancer prodrugs. J. Med. Chem. 2010, 53, 7767–7777. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Yang, J.; Zhang, R.; Yang, Z.; Yang, Z.; Wang, Y.; Xu, Y.; He, Z. Prostate-specific membrane antigen targeted therapy of prostate cancer using a DUPA−paclitaxel conjugate. Mol. Pharm. 2018, 15, 1842–1852. [Google Scholar] [CrossRef] [PubMed]
- Srinivasarao, M.; Low, P.S. Ligand targeted drug delivery. Chem. Rev. 2017, 117, 12133–12164. [Google Scholar] [CrossRef]
- Hansen, J.B.; Buchardt, O. A novel synthesis of tri-, di-, and mono-9-acridinyl derivatives of tetra-, tri-, and di-amines. J. Chem. Soc. Chem. Commun. 1983, 4, 162–164. [Google Scholar] [CrossRef]
- Leamon, C.P.; Parker, M.A.; Vlahov, I.R.; Xu, L.-C.; Reddy, J.A.; Vetzel, M.; Douglas, N. Synthesis and biological evaluation of EC20: A new folate-derived, 99mTc-based radiopharmaceutical. Bioconj. Chem. 2002, 13, 1200–1210. [Google Scholar] [CrossRef]
- Satyam, A. Design and synthesis of releasable folate–drug conjugates using a novel heterobifunctional disulfide-containing linker. Bioorg. Med. Chem. Lett. 2008, 18, 3196–3199. [Google Scholar] [CrossRef]
- Dubikovskaya, E.A.; Thorne, S.H.; Pillow, T.H.; Contag, C.H.; Wender, P.A. Overcoming multidrug resistance of small-molecule therapeutics through conjugation with releasable octaarginine transporters. Proc. Natl. Acad. Sci. USA 2008, 105, 12128. [Google Scholar] [CrossRef]
- Xu, B.; Zhou, F.; Yan, M.-M.; Cai, D.-S.; Guo, W.-B.; Yang, Y.-Q.; Jia, X.-H.; Zhang, W.-X.; Li, T.; Ma, T.; et al. PSMA-oriented target delivery of novel anticancer prodrugs: Design, synthesis, and biological evaluations of oligopeptide-camptothecin conjugates. Int. J. Mol. Sci. 2018, 19, 3251. [Google Scholar] [CrossRef]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef]
- Abe, M.; Murai, M.; Ichimaru, N.; Kenmochi, A.; Yoshida, T.; Kubo, A.; Kimura, Y.; Moroda, A.; Akabe, H.; Nishioka, T.; et al. Dynamic function of the alkyl spacer of acetogenins in their inhibitory action with mitochondrial complex I (NADH-ubiquinone oxidoreductase). Biochemistry 2005, 44, 14898–14906. [Google Scholar] [CrossRef]
- Ohta, K.; Fushimi, T.; Okamura, M.; Akatsuka, A.; Dan, S.; Iwasaki, H.; Yamashita, M.; Kojima, N. Structure–antitumor activity relationship of hybrid acetogenins focusing on connecting groups between heterocycles and the linker moiety. RSC Adv. 2022, 12, 15728–15739. [Google Scholar] [CrossRef]

| Test Compound | LNCaP | PC3 | Selectivity LNCaP/PC-3 |
|---|---|---|---|
| 2 | 0.48 | 0.06 | 0.125 |
| 3 | 0.81 | 2.1 | 2.6 |
| 4 | 3.4 | 4.5 | 1.3 |
| 5 | 4.8 | 58 | 12 |
| Doxorubicin | 0.71 | 2.3 | 3.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Periche, P.G.; Lin, J.; Bhupathiraju, N.V.S.D.K.; Kalidindi, T.; Johnson, D.S.; Pillarsetty, N.; Mootoo, D.R. Targeting Carbohydrate Mimetics of Tetrahydrofuran-Containing Acetogenins to Prostate Cancer. Molecules 2023, 28, 2884. https://doi.org/10.3390/molecules28072884
Periche PG, Lin J, Bhupathiraju NVSDK, Kalidindi T, Johnson DS, Pillarsetty N, Mootoo DR. Targeting Carbohydrate Mimetics of Tetrahydrofuran-Containing Acetogenins to Prostate Cancer. Molecules. 2023; 28(7):2884. https://doi.org/10.3390/molecules28072884
Chicago/Turabian StylePeriche, Patricia Gonzalez, Jacky Lin, Naga V. S. D. K. Bhupathiraju, Teja Kalidindi, Delissa S. Johnson, Nagavarakishore Pillarsetty, and David R. Mootoo. 2023. "Targeting Carbohydrate Mimetics of Tetrahydrofuran-Containing Acetogenins to Prostate Cancer" Molecules 28, no. 7: 2884. https://doi.org/10.3390/molecules28072884
APA StylePeriche, P. G., Lin, J., Bhupathiraju, N. V. S. D. K., Kalidindi, T., Johnson, D. S., Pillarsetty, N., & Mootoo, D. R. (2023). Targeting Carbohydrate Mimetics of Tetrahydrofuran-Containing Acetogenins to Prostate Cancer. Molecules, 28(7), 2884. https://doi.org/10.3390/molecules28072884






