Study on Wastewater Demulsification Technology of Crude Oil in Xinjiang Oilfield
Abstract
:1. Introduction
2. Results
2.1. Basic Parameters
2.2. Viscosity-Temperature Curve
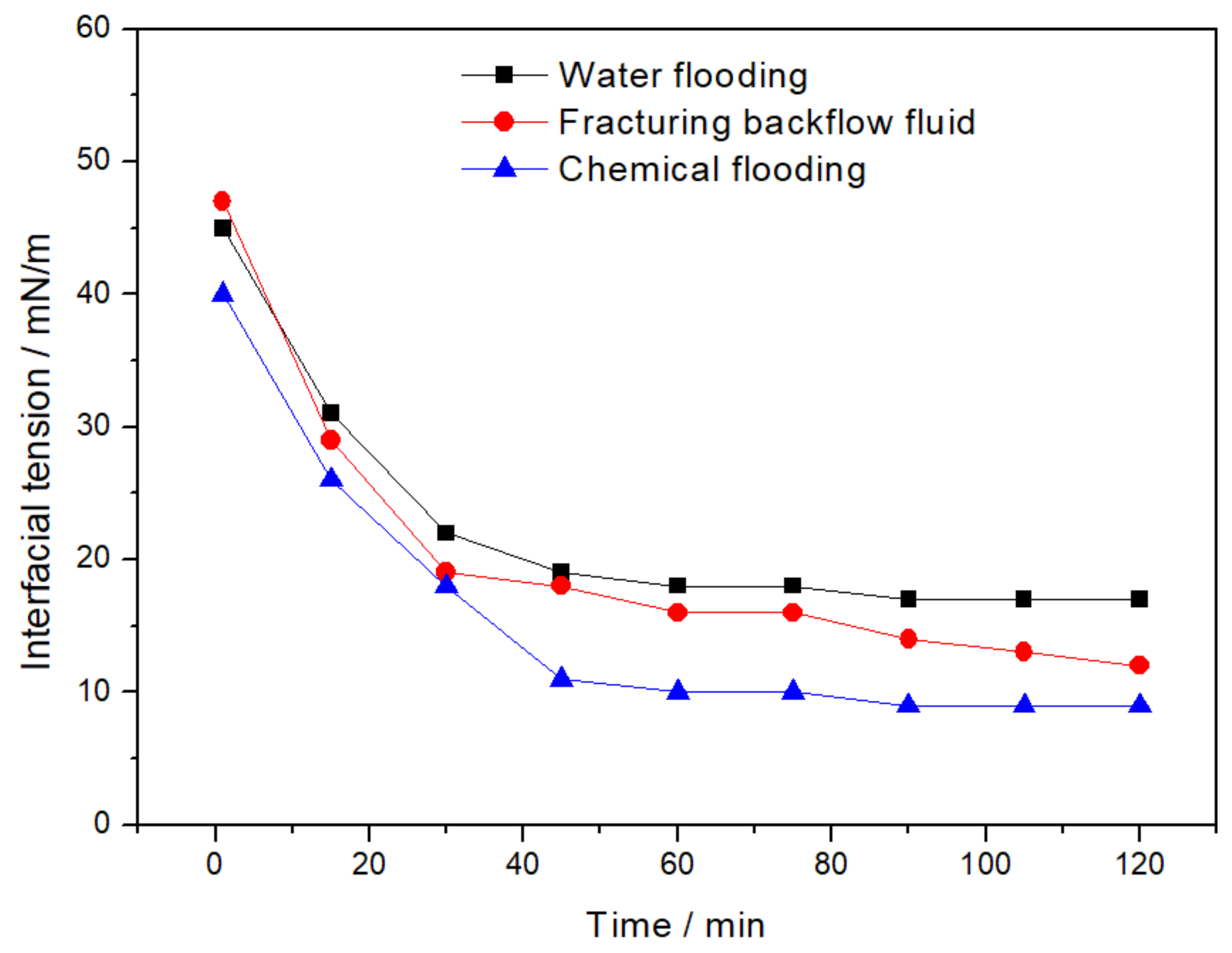
2.3. Interfacial Tension
2.4. Size and Type of Emulsion
2.5. Oil–Water Density Difference
2.6. Zeta Potential
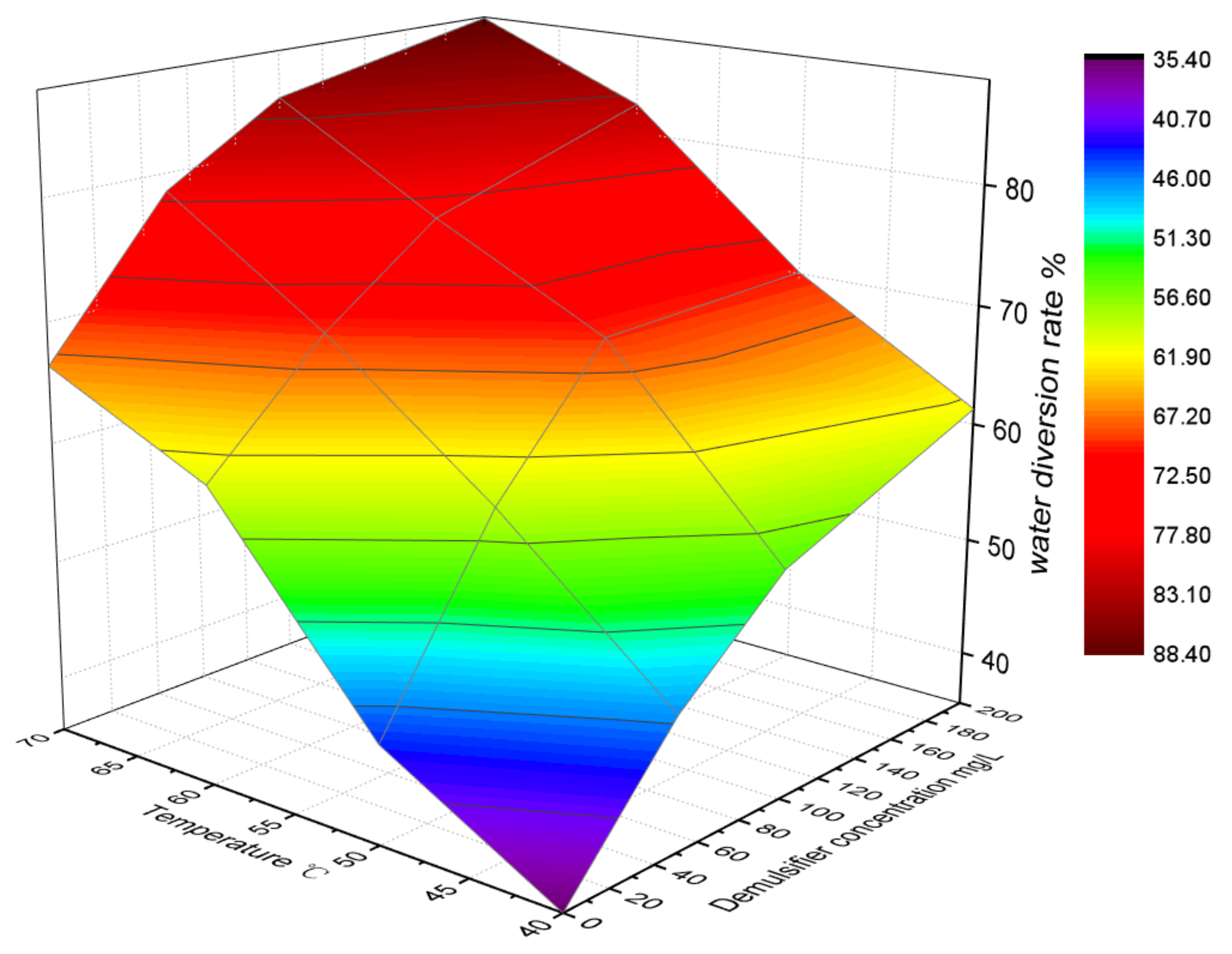
2.7. Water Diversion Rate
3. Discussion
3.1. Reasons for Emulsification
3.2. Comprehensive Demulsification Experiment of WF Emulsion
4. Materials and Methods
4.1. Material and Reagent
4.2. Experimental Method
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ghafoori, S.; Omar, M.; Koutahzadeh, N.; Zendehboudi, S.; Malhas, R.N.; Mohamed, M.; Al-Zubaidi, S.; Redha, K.; Baraki, F.; Mehrvar, M. New advancements, challenges, and future needs on treatment of oilfield produced water: A state-of-the-art review. Sep. Purif. Technol. 2022, 289, 120652. [Google Scholar] [CrossRef]
- Gao, T.; Lin, J.Q.; Zhang, K.; Padervand, M.; Zhang, Y.F.; Zhang, W.; Shi, M.L.; Wang, C.Y. Porous Defective Bi/Bi3NbO7 Nanosheets for Efficient Photocatalytic NO Removal under Visible Light. Processes 2023, 11, 115. [Google Scholar] [CrossRef]
- Padervand, M.; Rhimi, B.; Wang, C.Y. One-pot synthesis of novel ternary Fe3N/Fe2O3/C3N4 photocatalyst for efficient removal of rhodamine B and CO2 reduction. J. Alloy. Compd. 2021, 852, 156955. [Google Scholar] [CrossRef]
- Wang, S.H.; Sun, Y.B.; Zou, J. Analysis of factors affecting apparent viscosity of heavy oil emulsion. Drill. Prod. Technol. 2019, 42, 94–96. [Google Scholar]
- Duy, N.; Nicholas, S.; Christopher, H. Chemical Interactions and Demulsifier Characteristics for Enhanced Oil Recovery Applications. Energy Fuels 2012, 26, 2742–2750. [Google Scholar]
- Zheng, C.; Zhang, L.; Xu, J.; Zen, M.; Fu, G.; Fu, Y. Construction and Performance Evaluation of Nano Microemulsion Oil Displacement System. Xinjiang Oil & Gas. 2023, 19, 89–94. [Google Scholar]
- Zhang, H.; Liao, X.Y.; Hou, J.W. Influence of inorganic salts on emulsion of binary flooding system in Xinjiang oilfield. Inorg. Chem. Ind. 2016, 48, 22–25. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Wang, J.; Zhang, L.; Huang, J.; Li, L.M. Influence of thermochemical demulsification on demulsification of weak base ASP flooding emulsion. Chem. Eng. 2021, 315, 49. [Google Scholar]
- Liu, H.Y.; Zhang, X.Y.; Zhang, Z.J. Demulsification of amphiphilic gemini ionic liquids and its demulsification mechanism. Chemosphere 2022, 309, 136650. [Google Scholar]
- Zhang, J.; Ren, H.; Yu, T.; Yin, J.; Zhou, J.; Zhou, H. Research and application progress of fracturing proppants. Xinjiang Oil Gas 2023, 19, 27–34. [Google Scholar]
- Pan, Z.H.; Chen, J.Q.; Li, F.; Wang, C.S.; Xie, R.B. Research on Dehydration of Aging Oil from Liuhua Oilfield by High Frequency and High Voltage Pulse Electric Field. Chin. J. Process Eng. 2015, 12, 969. [Google Scholar]
- Ahmadi, S.; Khormali, A.; Meerovich, K.F. Optimization of the demulsification of water-in-heavy crude oil emulsions using response surface methodology. Fuel 2022, 323, 124270. [Google Scholar] [CrossRef]
- Liu, Y.C.; Wu, D.H.; Tian, F.; Zhang, B. Oil tank bottom sludge treatment based on physicochemical demulsification-destabilization centrifugal. Chin. J. Environ. Eng. 2016, 10, 7188. [Google Scholar]
- Wang, Z.J.; Babadagli, T.F.; Maeda, N. Generation of pickering emulsions by activating natural asphaltenes as nano materials: An experimental analysis for cost-effective heavy-oil recovery. J. Mol. Liq. 2021, 339, 116759. [Google Scholar] [CrossRef]
- Cong, H. Experimental Study on Thermochemical Dehydration and Demulsification of High Viscosity Oil. Liaoning Chem. Ind. 2021, 50, 161–165. [Google Scholar]
- Zou, J.; Patiguli, Y.; Chen, J.; Alimila, A.; Zhao, B.; Hou, J. Study on Demulsification Technology of Heavy Oil Blended in Xinjiang Oilfield. Processes 2023, 11, 409. [Google Scholar] [CrossRef]
- Kong, L.; Sun, Y.; Xie, Y.; Hung, B.; Ma, J.; Hou, J. Development of CO2 capture technology using MOFs. Xinjiang Oil & Gas 2022, 18, 78–83. [Google Scholar]
- He, Y.Q.; Mu, M.; Zhao, Y.; Sun, C.; Ma, J. Study on The Adaptability of High Frequency Electric Pulse Dewatering Technology for Poly Crude Oil in Bohai. Petrochem. Ind. Technol. 2019, 5, 88. [Google Scholar]
- Zhang, H.; Liu, G.Q.; Zhang, X.F.; Luo, Z. Stability analysis and demulsification technology of electric desalting wastewater. Chem. Ind. Eng. Prog. 2022, 41, 5047–5054. [Google Scholar]
- Yu, H.J.; Liu, H.L.; Liu, D.J.; Zhang, J.; Han, B. Research on the Application of Ultrasonic Demulsification Technology of Produced Fluid. Oil-Gas Field Surf. Eng. 2022, 41, 12. [Google Scholar]
- Li, J.J.; Tang, X.D. Application Progress of Ultrasonic Technique in Petrochemical Industry. Technol. Dev. Chem. Ind. 2006, 22, 93–97. [Google Scholar]
- Liu, H.L.; Hou, J.W.; Liu, Y.S.; Fan, H. Study on the emulsion type in the ASP flooding Process. Fresenius Environ. Bull. 2018, 27, 5752–5758. [Google Scholar]
- Hou, J.W.; Chen, S.P.; Zeng, X.F.; Li, Z.H.; Nie, X.B.; Chen, Q.S. Emulsion Performance Research of Surfactant-polymer Flooding System of Qizhong District. Sci. Technol. Eng. 2015, 15, 1671. [Google Scholar]
- Hou, J.W.; Lu, Z.W.; Jiao, Q.J.; Guo, W.J.; Nie, X.B.; Han, Z.J.; Li, Z.H. Type Transformation of Emulsion during Surfactant/Polymer Flooding in Xinjiang Oilfield. Oilfield Chem. 2016, 33, 112. [Google Scholar]
- Fu, W.N.; Zhang, X. Study on ultrasonic demulsification and centrifugal separation technology for oil sludge. Inn. Mong. Petrochem. Ind. 2021, 6, 13. [Google Scholar]
- Yi, M.H.; Fan, W.; Li, M.S.; Ma, H. Experimental study on demulsification and dehydration of aged heavy oil. J. Chongqing Univ. Sci. Technol. (Nat. Sci. Ed.) 2010, 12, 76–78. [Google Scholar]
- Wan, T.; Qin, J.; Zhang, J. Practices of zipper fracturing for tight reservoir development with small well spacing. Xinjiang Oil Gas 2022, 18, 26–32. [Google Scholar]






| Demulsification Technology | Region of Use | Characteristic |
|---|---|---|
| Blended oil | Xinjiang Oilfield, Shengli Field, and Liaohe Oilfield | Narrow application range and high control difficulty |
| Thermochemistry | Most oilfields | Excessive energy consumption |
| Ultrasonic | Saudi Aramco Oilfield and Bohai Oilfield | Narrow application range and high energy consumption |
| Evaporation technology | Liaohe Oilfield | Low energy consumption but high time cost |
| Biotechnology | Daqing Oilfield and Huabei Oilfield | Narrow application range and biological resistance |
| Microwave | Laboratory stage | Narrow application range and high energy consumption |
| Centrifugal technology | Ansai Oilfield, Caofeidian Oilfield, and Dagang Oilfield | Narrow application range and high energy consumption |
| Electrochemistry | Daqing Oilfield, Xinjiang Oilfield, and Nanhai Oilfield | Narrow application range and high energy consumption |
| Name | Cl− mg/L | KPS/LPS mg/L | Ca2+ mg/L | pH | HCO3− mg/L | Mineralization Degree mg/L | Suspended Solids Content mg/L |
|---|---|---|---|---|---|---|---|
| WF | 9827.42 | 0 | 146.6 | 7.45 | 983 | 17,637.2 | 900 |
| CF | 8062.4 | 304 | 678.16 | 8.78 | 4303.44 | 19,209.63 | 544 |
| FB | 12,529.42 | 0 | 1128 | 7.33 | 1098.67 | 21,911 | 276 |
| WF Emulsion | CF Emulsion | FB Emulsion | |
|---|---|---|---|
| Mineralization degree, mg/L | 17,637.2 | 19,209.63 | 21,911 |
| Viscosity at 40 °C, mPa·s | 45 | 16 | 22 |
| Interfacial tension, mN/m | 18 | 10 | 13 |
| Size and type of emulsion, μm | 40 | 120 | ∞ |
| pH | 8.21 | 8.73 | 7.33 |
| Zeta, mV | −62 | −38 | −20 |
| Suspended solids content, mg/L | 900 | 544 | 276 |
| Density difference, g/cm3 | 0.1296 | 0.1423 | 0.1485 |
| Water diversion rate, % | 61% | 86% | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Ma, L.; Gao, Y.; Qin, Y.; Jiao, Z.; Guo, R.; Hou, J. Study on Wastewater Demulsification Technology of Crude Oil in Xinjiang Oilfield. Molecules 2023, 28, 2873. https://doi.org/10.3390/molecules28062873
Ma J, Ma L, Gao Y, Qin Y, Jiao Z, Guo R, Hou J. Study on Wastewater Demulsification Technology of Crude Oil in Xinjiang Oilfield. Molecules. 2023; 28(6):2873. https://doi.org/10.3390/molecules28062873
Chicago/Turabian StyleMa, Jingui, Liqiang Ma, Yongdi Gao, Yue Qin, Zhihao Jiao, Ruibo Guo, and Junwei Hou. 2023. "Study on Wastewater Demulsification Technology of Crude Oil in Xinjiang Oilfield" Molecules 28, no. 6: 2873. https://doi.org/10.3390/molecules28062873
APA StyleMa, J., Ma, L., Gao, Y., Qin, Y., Jiao, Z., Guo, R., & Hou, J. (2023). Study on Wastewater Demulsification Technology of Crude Oil in Xinjiang Oilfield. Molecules, 28(6), 2873. https://doi.org/10.3390/molecules28062873








