Abstract
A method for the technically easy-to-implement synthesis of deuterium-labeled pyrazolo[1,5-a]pyrimidines and 1,2,4-triazolo[1,5-a]pyrimidines have been developed. The regioselectivity of such transformations has been shown. 1H NMR and mass spectrometric methods have proved the quantitative nature of such transformations and the kinetics of deuterium exchange has been studied. Spectrally, at different temperatures (+30 °C, −10 °C and −15 °C), the kinetics of the process was studied both in CD3OD and in deuterated alkali.
1. Introduction
Azolo[1,5-a]pyrimidines derivatives, primarily pyrazolo[1,5-a]pyrimidine and 1,2,4-triazolo[1,5-a]pyrimidine, are known for their high biological activity. Several drugs containing the pyrazolo[1,5-a]pyrimidine backbone are used in medical practice. These include the sleeping pills Zaleplon [1,2], Indiplon and Lorediplon [3,4]; the sedative Ocinaplon [5]; the antifungal drug Pyrazophos [6]; the antiglycemic drug Anagliptin [7,8]; and the antitumor drug Dinaciclib [9,10] (Figure 1). Moreover, an entire series of pyrazolo[1,5-a]pyrimidine derivatives are registered, which have shown an antitumor effect [11,12,13,14,15,16,17,18,19,20], and effects on the central nervous system and serotonin receptors [21,22,23,24,25,26,27]. The same backbone of pyrazolo[1,5-a]pyrimidine is included in a number of derivatives that have shown activity as non-nucleoside inhibitors of HIV-1 reverse transcriptase (NNRTIs) [28] and respiratory syncytial virus (RSV) [29], inhibitors of RNA-polymerase of hepatitis C virus [30], as well as having antibacterial, antifungal [31] and anti-inflammatory properties [32,33,34].
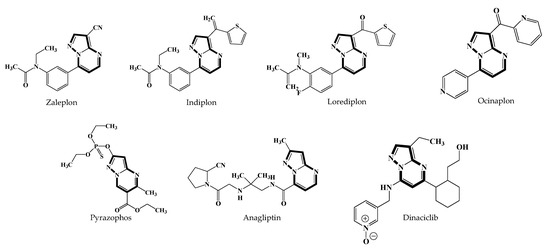
Figure 1.
Examples of drugs containing the pyrazolo[1,5-a]pyrimidine backbone.
The high biological activity of pyrazolo[1,5-a]pyrimidines stimulated interest in the development of methods for introducing various isotopes into its derivatives. In particular, compounds labeled with the [99mTcN]2+ isotope were synthesized and studied for their biodistribution in mice with tumors [35]. A 5-methylpyrazolo[1,5-a]pyrimidine derivative containing the 18F fluorine isotope was used as an imaging marker for positron emission tomography (PET) to detect a tumor [36]. It is known that deuterium-labeled compounds are also used as markers for studying the mechanisms of chemical reactions, as well as in biological research and medicinal chemistry [37,38].
Deuterated derivatives of known drugs differ from the drugs themselves by prolonging the half-life, which leads to an increase in the interval between taking the drug and, consequently, an increase in the effectiveness and safety for the patient [39].
Various methods for introducing a deuterium isotope into molecules of organic compounds are described. Formally, these methods can be divided into 3 groups, namely, proceeding under the conditions of acid or base catalysis and metal catalysis [40]. In the case of metal catalysis, Pd, Ni, Ir, Pt, Ru or their salts were used as metals. These reactions require special conditions. They were carried out under heating [41], under pressure [40,42], in a microwave oven [43], by passing pure deuterium [40], or in deuterated solvents, more often D2O. Deuterium atoms were introduced by H/D exchange into α-aminopyridine derivatives by heating them to 80 °C in a solution of K2CO3 in D2O [44]. Furthermore, by reaction with substituted acetylenes, the resulting deuterium derivatives were cyclized to pyrazolo[1,5-a]pyridine derivatives containing deuterium atoms. The reaction of the same pyridines with acetonitrile in basic D2O solution resulted in 1,2,4-triazolo[1,5-a]pyridine derivatives containing deuterium atoms.
Another method for introducing deuterium into an azine molecule has been described for the preparation of pyrimidine derivatives containing a deuterium atom. This involves a multicomponent cyclization of amidine, benzaldehyde and deuterated triethylamine-D15 in the presence of iodine at 150 °C [45]. An example of the metal catalysis used for introducing deuterium into a pyrimidine molecule is a reaction under microwave-promoted conditions catalyzed by Ruthenium(II)–Carboxylate [46].
It is also important to note that the drug Austedo (deutetrabenazine) (Figure 2), which contains deuterium atoms, is already being used in medical practice [47].
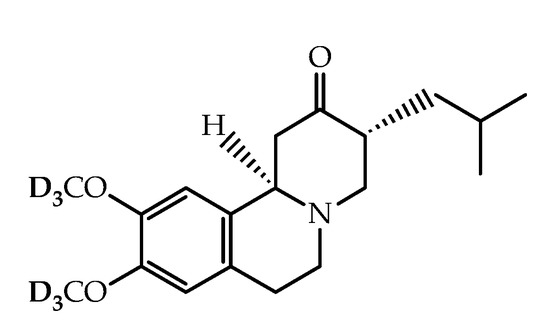
Figure 2.
Drug Austedo (deutetrabenazine).
2. Results and Discussion
From the above, it is clear that the methods used for obtaining deuterated derivatives are not always simple. Therefore, the development of new, easily implemented methods for introducing deuterium atoms into molecules of organic substances is of interest. This is especially important in the synthesis of deuterium-containing bioactive substances. Considering the biological activity and importance of Azolo[1,5-a]pyrimidine derivatives in medicine, interest in developing new methods for introducing deuterium atoms into their molecules is of practical interest and is undoubtedly relevant.
This communication is devoted to a simple, easily and rapidly implemented method for introducing deuterium atoms into the molecules of substituted pyrazolo[1,5-a]- and 1,2,4-triazolo[1,5-a]pyrimidines. It is also essential that the proposed method is regioselective.
When drops of a preliminarily prepared solution of CD3ONa in CD3OD are added to the solution of 6-acetyl-2,7-dimethylpyrazolo[1,5-a]- (1) and 6-acetyl-7-methyl-2-phenylpyrazolo[1,5-a]pyrimidine (2) in CD3OD (Scheme 1), the disappearance of the signals of two methyl groups is almost immediately observed in the 1H NMR spectrum. Comparison of the spectra of substances 1 and 2, as well as the NOESY study of the spectrum of compound 1, indicates the H/D exchange of hydrogen atoms of the methyl (7-CH3) and acetyl (6-COCH3) groups (Figure 3). Thus, in the spectrum of the deuterated product of compound 1, the signal of the methyl groups is retained, for which the NOESY spectrum shows a response between the proton signal of one of the methyl groups (2-CH3) and the aromatic proton (3-H). Note that such an interaction is impossible for other methyl groups, which unambiguously proves that the 7-CH3 and 6-COCH3 protons located in the pyrimidine ring, and not the 2-CH3 protons located in the pyrazole ring, undergo isotopic exchange.
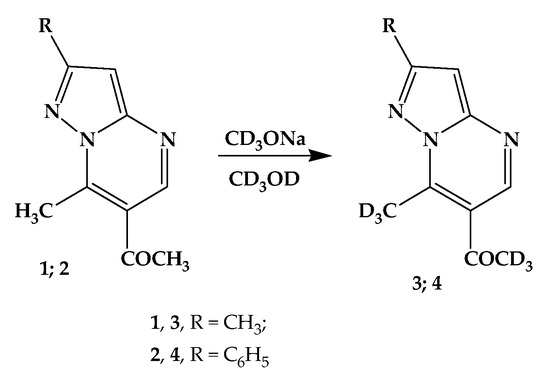
Scheme 1.
H/D exchange in 6-acetyl-2,7-dimethylpyrazolo[1,5-a]- (1) and 6-acetyl-7-methyl-2-phenylpyrazolo[1,5-a]pyrimidine (2).
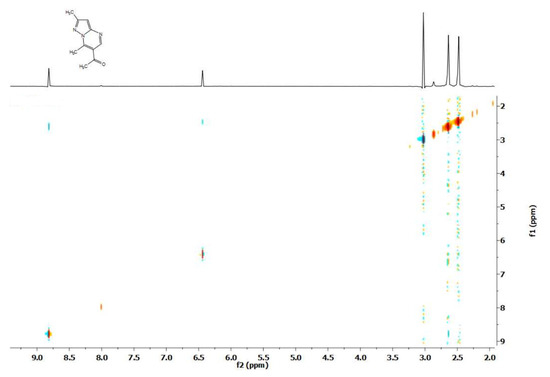
Figure 3.
NOESY spectrum of 6-acetyl-2,7-dimethylpyrazolo[1,5-a]pyrimidine (1).
In order to confirm the deuterium exchange that had taken place (after the addition of CD3ONa), we isolated substance 3 from the NMR ampoule and compared its mass spectrum with the spectrum of compound 1 before deuterium exchange (Figure 4). As a result, the mass of product 3, as expected, was 6 units higher than the mass of the initial substance 1 (respectively, 195 and 189). This confirms the H/D exchange in the two methyl groups.
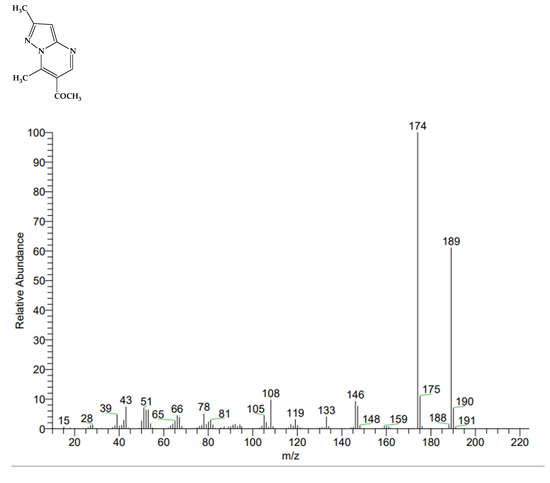
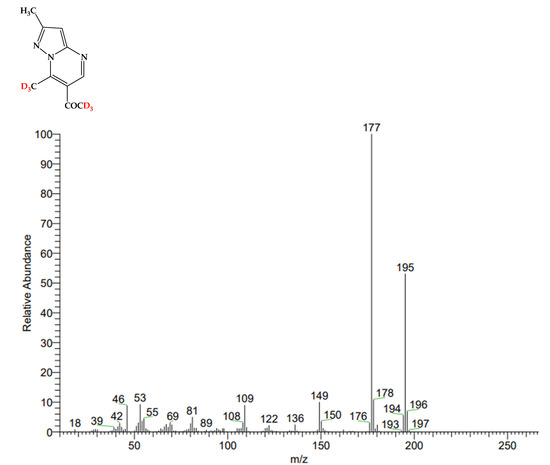
Figure 4.
Mass spectra of substance 1 and deuterated product 3, respectively.
A similar exchange of protons of two methyl groups was also observed in the 1H NMR spectrum of 6-acetyl-7-methyl-1,2,4-triazolo[1,5-a]pyrimidine (CD3ONa solution in CD3OD) (Scheme 2). Almost immediately after the addition of alcoholate-D3 to the NMR ampoule, the signals of both methyl groups (7-CH3 and COCH3) disappeared.
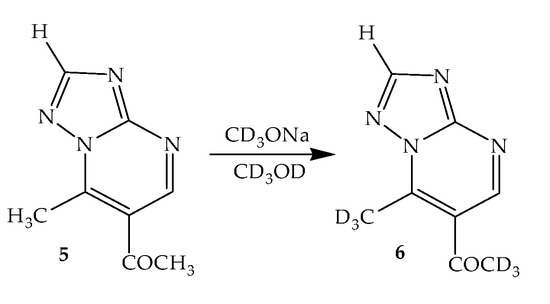
Scheme 2.
H/D exchange in 6-acetyl-7-methyl-1,2,4-triazolo[1,5-a]pyrimidine (5).
Under standard conditions for recording NMR spectra, i.e., at a temperature of +30 °C, it was impossible to study the kinetics of isotope exchange because of the very high transformation rate. Therefore, we tried to study the reaction at low temperatures. According to the results of preliminary studies, the optimal temperature for this was −10 °C (Table 1, Figure 5). The rate of proton exchange in the two methyl groups was different under these conditions. The intensity of the signal of one of the methyl groups (chemical shift 3.2 ppm) (−10 °C) decreases by 6% by 5 min after the addition of deuterated sodium methoxide under these conditions, by 15 min the exchange is 22%, by 20 min the exchange reaches almost 30% and is practically completed after 2 h of measurements. According to the results of the measurements, the rate of exchange of protons of the second methyl group (chemical shift 2.28 ppm) under these conditions turned out to be significantly lower. Thus, we recorded the first results of isotope exchange only 20 min after the start of the measurements, and by 120 min only 20% of the protons in this group had undergone exchange.

Table 1.
The decrease in the concentration of hydrogen atoms in 6-acetyl-7-methyl-1,2,4-triazolo[1,5-a]pyrimidine (5) molecule for 7-CH3 and COCH3 groups in CD3ONa solution in CD3OD at −10 °C in a time period of 0–110 min.
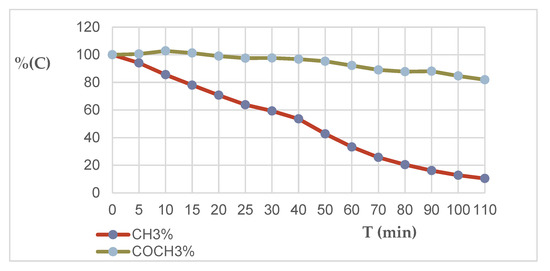
Figure 5.
Diagram of the decrease in the concentration of hydrogen atoms in 6-acetyl-7-methyl-1,2,4-triazolo[1,5-a]pyrimidine (5) molecule for 7-CH3 and COCH3 groups in CD3ONa solution in CD3OD at −10 °C in a time period of 0–110 min.
In the 1H NMR spectrum of triazolopyrimidine 5, recorded by the NOESY method (Figure 6), only one of the methyl groups located in the region of 2.28 ppm has a cross peak with an aromatic proton (9.3 ppm). This methyl group corresponds to the acetyl group occupying position 6. Based on this, we concluded that in the examples described, the protons of the 7-CH3 methyl group (3.17 ppm) are exchanged faster. We obtained similar confirmation when studying the spectrum of pyrazolopyrimidine 2 and, consequently, compound 1.
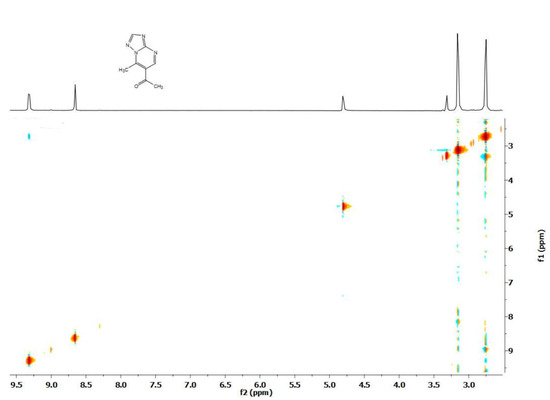
Figure 6.
NOESY spectrum of 6-acetyl-7-methyl-1,2,4-triazolo[1,5-a]pyrimidine (5).
A fast H/D exchange of protons of two methyl groups upon addition of CD3ONa to a solution of compound 1 in CD3OD was observed. Due to the rapidity of the reaction, the kinetics of the process at a temperature of −10 °C could not be fixed. However, it was registered at a lower temperature (−15 °C). Therefore, the exchange of both methyl groups approached 50% after 2 min and was practically completed within a few minutes (Table 2, Figure 7).

Table 2.
The decrease in the concentration of hydrogen atoms in 6-acetyl-2,7-dimethylpyrazolo[1,5-a]pyrimidine (1) molecule for 7-CH3 and COCH3 groups in CD3ONa solution in CD3OD at −15 °C in a time period of 0–12 min.
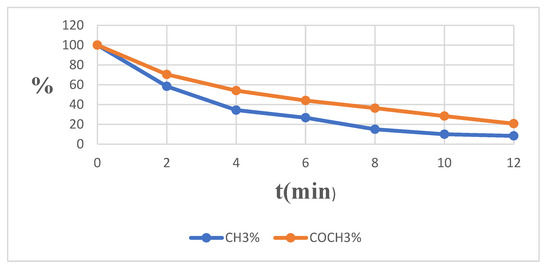
Figure 7.
Diagram of the decrease in the concentration of hydrogen atoms in 6-acetyl-2,7-dimethylpyrazolo[1,5-a]pyrimidine (1) molecule for 7-CH3 and COCH3 groups in CD3ONa solution in CD3OD at −15 °C in a time period of 0–12 min.
When studying the deuteroexchange of the 3-pyrazolyl derivative of pyrazolopyrimidine 7 containing 4 methyl groups, after adding CD3ONa to a solution, protons of two methyl groups in the pyrimidine ring are first exchanged (Scheme 3). In the side pyrazole ring, only the signal of one of the methyl groups disappears. In this case, a cross peak was noted in the NOESY spectrum (Figure 8), indicating the interaction of the methyl group of the acetyl fragment with the aromatic proton of the pyrazole ring, which made it possible to show that the hydrogen atoms of the acetyl group of the pyrazole ring undergo exchange. Interestingly, in this example, the exchange also partially affects the aromatic protons of pyrazolo[1,5-a]pyrimidine.
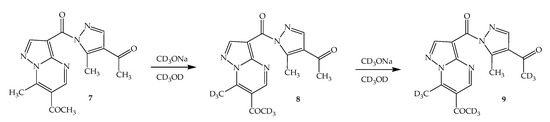
Scheme 3.
H/D exchange in 3-pyrazolyl derivative of pyrazolopyrimidine 7.
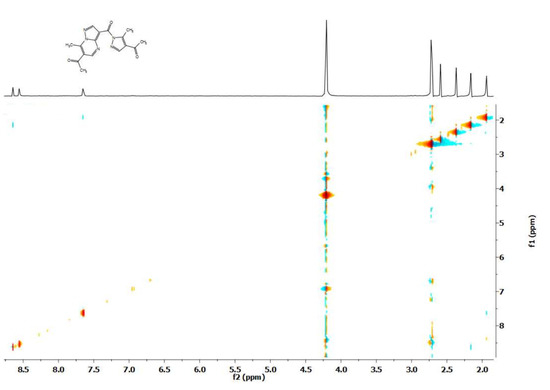
Figure 8.
NOESY spectrum of 6-acetyl-7-methyl-3-[1-(4-acetyl-5-methyl-1H-pyrazole-1-carbonyl)]pyrazolo[1,5-a]pyrimidine (7).
In the study of deuterium exchange in 2-substituted 7-methyl-5-ethoxycarbonylpyrazolo[1,5-a]pyrimidines (10, 11), after adding CD3ONa to the solution, a rapid exchange of hydrogen atoms in the pyrimidine ring for deuterium atoms was immediately noted. However, in these cases, the reaction was accompanied by another transformation, which was also recorded spectrally.
Thus, when comparing the 1H NMR spectra of 2,7-dimethyl-5-ethoxycarbonylpyrazolo[1,5-a]pyrimidine (10) recorded in CD3OD at temperatures of +30 °C and −10 °C, an unusual and at first glance inexplicable difference is noted. At minus temperature (−10 °C), the spectrum of the compound corresponds to the expected one and includes the signals of two methyl (2-CH3 2.55; 7-CH3 2.84 ppm) and one ester (OCH2CH3 4.46, OCH2CH3 1.44 ppm) groups, as well as singlets of two protons 3-H and 6-H (respectively, 6.70 and 7.48 ppm). In the spectrum of the same compound, recorded at +30 °C, the signals of all groups with the corresponding integrals are preserved, but two identical pairs of proton signals of two ethyl groups are observed (two quartets—OCH2CH3 4.46 and DOCH2CH3 3.62 ppm, each of which corresponds to one proton, and two triplets—respectively, 1.44 and 1.19 ppm, 1.5 H each) (Figure 9).
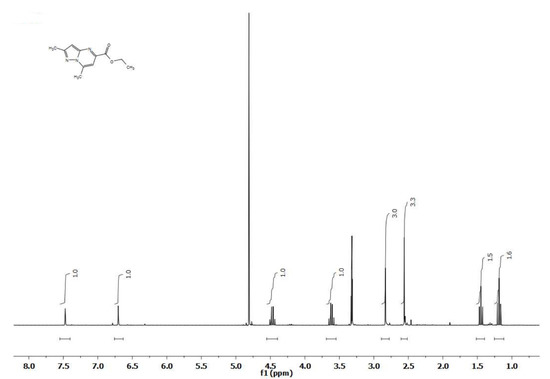
Figure 9.
1H NMR spectra of 2,7-dimethyl-5-ethoxycarbonylpyrazolo[1,5-a]pyrimidine (10) recorded in CD3OD at temperatures of +30 °C.
The study of the spectra recorded in CD3OD at −10 °C, that is, before adding CD3ONa to the ampoule, showed that over time there is a gradual decrease in the signals of the protons of the ethyl group and the proportional appearance of a new pair of signals of another ethyl group. In this case, the signals of all other groups remain unchanged in the spectra (Figure 7). Ultimately, the signals of the ethyl group of the original molecule, namely, those noted in the region of 4.5 ppm (CH2) and 1.48 ppm (CH3) completely disappear and are replaced by ethyl group signals that have chemical shifts in a stronger field, respectively, in the region of 1.2 (CH3) and 3.6 ppm (CH2).
We believe that the observed dynamic change in the NMR spectra is explained by the ongoing transesterification (Scheme 4). In this case, the solvent molecules (CD3OD) interact with the ethoxycarbonyl group displacing the ethoxy group, resulting in the formation of a new 7-methyl-2-methyl-5-d3-metoxycarbonylpyrazolo[1,5-a]pyrimidine. Ethanol (C2H5OD) is formed in the solution, the signals of the groups of which are fixed in the 1H NMR spectrum in the form of a new ethyl group.
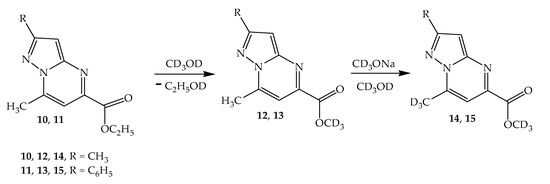
Scheme 4.
H/D exchange in 2-substituted 7-methyl-5-ethoxycarbonylpyrazolo[1,5-a]pyrimidine (10, 11).
At +30 °C, after adding one drop of deuterated sodium methoxide (CD3ONa) to the NMR ampoule, both processes rapidly occur—transesterification and H/D exchange, a result of which the signal of one of the methyl groups (7-CH3) disappears in the spectrum, after which 3D-methyl 2-methyl-7-(d3-methyl)-5-ethoxycarbonylpyrazolo[1,5-a]pyrimidine is immediately formed.
At low temperatures (−10 °C), we were able to study the kinetics of the entire deuterium exchange. As the experiment showed, in the beginning, transesterification already begins in CD3OD, which is completed even without the addition of CD3ONa. However, when d3-sodium methoxide is added, transesterification is activated, since there are no signals from the ester group of the starting ester in the spectrum. Under the same conditions (−10 °C), the kinetics of the H/D isotope exchange was studied by NMR spectral (Table 3, Figure 10).

Table 3.
The decrease in the concentration of hydrogen atoms in 2,7-dimethyl-5-ethoxycarbonylpyrazolo[1,5-a]pyrimidine (10) molecule for 7-CH3 group in CD3ONa solution in CD3OD at −10 °C in a time period of 0–75 min.
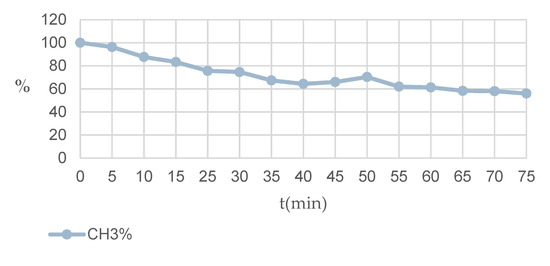
Figure 10.
Diagram of the decrease in the concentration of hydrogen atoms in 2,7-dimethyl-5-ethoxycarbonylpyrazolo[1,5-a]pyrimidine (10) molecule for 7-CH3 group in CD3ONa solution in CD3OD at −10 °C in a time period of 0–75 min.
A similar isotopic exchange, together with transesterification, was also noted for 2-phenyl-7-methyl-5-ethoxycarbonylpyrazolo[1,5-a]pyrimidine 11. Thus, in the spectrum of compound 11, which contains a phenyl group in the pyrazole ring, two processes easily occur not only in a solution of deuterated alkali (CD3ONa in CD3OD), but also in a solution of CD3OD: the deuterium exchange of protons of the methyl group and transesterification with the formation of d1-ethanol (CH3CH2OD). In the 1H NMR spectrum at a temperature of +30 °C after the dissolution of the substance, the signals of the protons of the ethyl group of the ester in the regions of 1.46 (t, CH3) and 4.47 (q, CH2O) almost completely disappear, and the signals of the ethyl group of the formed d1-ethanol CH3CH2OD (1.18—t, CH3 and 3.61—q, CH2O) become the main signals. In this case, the proton signals of all other groups of compound 11 (phenyl and methyl groups, as well as hydrogen atoms directly connected to the pyrimidine and pyrazole rings) are observed in the spectrum without changes. After adding 1–2 drops of CD3ONa solution in CD3OD to the NMR ampoule containing a solution of compound 11 in CD3OD, the 7-CH3 signal almost completely and immediately disappears (the exchange is approximately 80%), and the proton signals in the weak field (C6H5, 3-H and 6-H) remain unchanged. The exchange of protons of the methyl group for deuterium (H/D) according to the 1H NMR spectrum data is practically completed by 20 min after the addition of sodium d3-methoxide.
N-Alkylation of the pyrazolo[1,5-a]pyrimidine skeleton leads to a significant change in the process of deuterium exchange of methyl groups noted by us. This process was studied spectrally using the example of two salts of 6-acetyl-2,7-dimethylpyrazolo[1,5-a]pyrimidine—iodomethylate and iodoethylate. Namely, 6-acetyl-2,4,7-trimethylpyrazolo[1,5-a]pyrimidinium (16) and 6-acetyl-2,7-dimethyl-4-ethylpyrazolo[1,5-a]pyrimidinium (17) iodides. It is important to note that the position of the N-alkyl groups was confirmed by 1H NMR spectral using the NOESY technique. Thus, in the spectrum of 16 iodide, NOE (Nuclear Overhauser Effect) was noted between the protons of the N-methyl group (4.43 ppm) and the protons of the pyrazole (3-H) and pyrimidine (5-H) rings (7.25 and 9.95 ppm, respectively). The cross-peaks interactions of the 5-H proton of the pyrimidine ring with the signals of two adjacent positions of the groups, methyl N-CH3 and acetyl (COCH3), are also clearly visible. Therefore, based on the above, it was unequivocally determined that alkylation occurs at the N-4 nitrogen atom of the pyrimidine ring.
Similar interactions of protons were also noted in the NOESY study of iodoethylate 17. It is noteworthy, that, in this case, only the protons of the N-methylene group participate in the interaction with the neighboring 3-H and 5-H protons.
In the 1H NMR spectrum of iodide 16 recorded in CD3OD without the addition of CD3ONa methylate, an H/D exchange of one of the methyl groups was noted. Instead of the expected signals of four methyl groups, the signals of only three of them were fixed in the spectrum (18) (Scheme 5). Since the signal of the N-methyl group, as a rule, appears in a weaker field (in this case, it is 4.41 ppm), and the signal of the methyl group of the pyrazole ring is usually in the strongest field, the signal in the 2.78 ppm region could correspond to either a methyl group in position 7 or an acetyl group. Based on the NOESY study carried out in DMSO-d6 solution, it was concluded that the methyl group signal was present in the spectrum of the salt 16 in DMSO-d6 in the region of 3.3 ppm and disappeared due to deuterium exchange in the spectrum registered in CD3OD corresponding to 7-CH3.
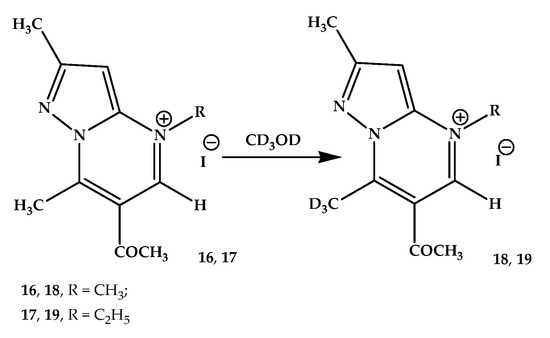
Scheme 5.
H/D exchange in 6-acetyl-2,4,7-trimethylpyrazolo[1,5-a]pyrimidinium (16) and 6-acetyl-2,7-dimethyl-4-ethylpyrazolo[1,5-a]pyrimidinium (17) iodides.
Thus, methylation of the pyrimidine ring, leading to an increase in its electrophilicity, facilitates the nucleophilic isotopic exchange of hydrogen atoms of the 7-methyl group, resulting in a rapid H/D exchange even in CD3OD. It is interesting that, in contrast to the above examples of deuterium exchange of non-alkylated at the nitrogen atom Azolo[1,5-a]pyrimidines (1, 2, 5, 7, 10, 11), with the addition of sodium d3-methoxide, the subsequent exchange of hydrogen atoms of the methyl fragment of the acetyl, or any other group, is not observed for only several minutes, but also during the first two days. Only by the third day does a slight decrease in the signal of the hydrogen atoms of the acetyl group (by about 25%) become noticeable in the spectrum; however, new signals begin to appear, which indicates the occurrence of other processes. The possibility of destruction at this stage should be excluded, since, in the spectrum for several more days of observations, in parallel with the decrease in the integral of the signal of the protons of the acetyl group, the signals of the remaining protons of the initial molecule (N-Me, 2-Me, 3-H and 5-H) are practically unchanged.
In case of N-ethylpyrazolo[1,5-a]pyrimidinium iodide 17, as well as N-methyl derivative 16, in the spectrum registered in CD3OD, i.e., without the addition of CD3ONa, H/D exchange occurs immediately (Scheme 5) with the formation of deutero-substituted compound 19. In the same way, i.e., the NOESY study, it was shown that 7-CH3 hydrogen atoms undergo rapid exchange. Further monitoring for two days did not register the H/D exchange of any other protons in the molecule.
The spectra of 6-acetyl-3,7-dimethyl-1,2,4-triazolo[1,5-a]pyrimidinium iodide 20 recorded in DMSO-d6 and CD3OD were identical. This indicates that the isotopic exchange does not proceed in this case in the CD3OD solution. Consequently, the displacement of the N-alkyl group into the triazole ring, that is, its removal from the 7-Me group, led to a decrease in the effect on potential (expected) isotopic exchange. The addition of alcoholate (CD3ONa) to the solution leads to the appearance of many new signals, possibly due to the opening of the pyrimidine ring and subsequent destruction of the molecule (Scheme 6).
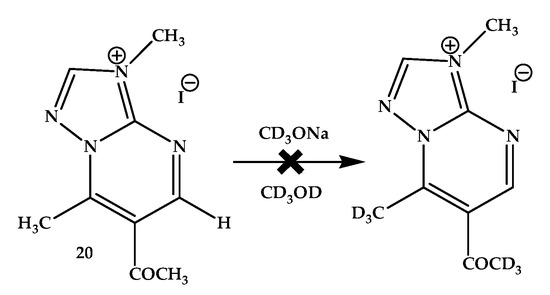
Scheme 6.
Expected H/D exchange in 6-acetyl-3,7-dimethyl-1,2,4-triazolo[1,5-a]pyrimidinium iodide (20).
We studied a similar interactions of two more 1,2,4-triazolo[1,5-a]pyrimidinium iodides with solutions of deuterated sodium methoxide in deuteromethanol. In particular, the NMR spectra of 3,5,7-trimethyl-1,2,4-triazolo[1,5-a]pyrimidinium iodide (21) in CD3OD and CD3ONa/CD3OD were studied (Scheme 7). As in the case of iodide 20 in CD3OD, no selective isotopic exchange of C-alkyl groups was observed without the addition of alcoholate. However, when a small amount of CD3ONa was added to the NMR ampule, an easy, quantitative, and, most importantly, selective basic deuteroexchange of protons of both C-methyl groups of the pyrimidinium salt 22 was noted. The signals of C-methyl groups completely disappeared at room temperature. With an increase in the duration of exposure to the deuterated reagent, the signal also disappeared from one of the aromatic protons (apparently, 2-H, located in the triazole ring—in the neighborhood of the quaternized nitrogen atom).
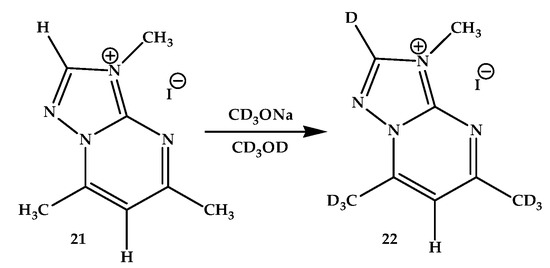
Scheme 7.
H/D exchange in 3,5,7-trimethyl-1,2,4-triazolo[1,5-a]pyrimidinium iodide (21).
It is important to note that, as in the above example, in the spectrum of compound 23 (3,7-dimethyl-6-ethoxycarbonyl-1,2,4-triazolo[1,5-a]pyrimidine) in d4-methanol, no isotopic exchanges were observed. However, when d4-sodium methoxide is added, the proton signal of the 7-methyl group disappeared completely within a few minutes, while the signals of the remaining hydrogen atoms were preserved. Note that, as in the case of compound 21, deuterium exchange of one of the aromatic protons, presumably located in the triazole ring, occurred over time (during 10 days of monitoring) with the formation of salt 24 (Scheme 8).
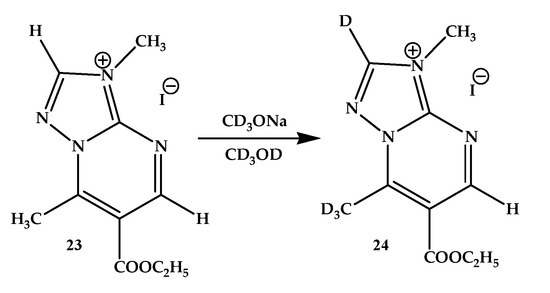
Scheme 8.
H/D exchange in 3,7-dimethyl-6-ethoxycarbonyl-1,2,4-triazolo[1,5-a]pyrimidinium iodide (23).
It should be noted that in the latter case (salt 23), we also observed in the 1H NMR spectrum a partially proceeding (significantly slower than in the case of the ester group in position 5 of compounds 10, 11) transesterification reaction.
Thus, on a number of examples—methyl derivatives of 1,2,4-triazolo[1,5-a]pyrimidinium iodides—it was confirmed that alkylation of the nitrogen atom of the triazole ring does not lead to isotopic exchange of protons of the C-methyl group located in the pyrimidine ring. Such H/D exchange requires the addition of an alcoholate (CD3ONa) to the medium. In the examples of pyrazolo[1,5-a]pyrimidinium iodides, where the nitrogen atom in the pyrimidine part of the molecule is alkylated, the H/D exchange of C-methyl groups in the pyrimidine ring is very easily realized already in a CD3OD solution without the addition of CD3ONa.
The schemes of the deuterium exchange reaction is associated with the attack of the methylate ion at the most electrophilic position in the molecule, which leads to the elimination of a proton. The resulting carbanion is stabilized by the addition of a proton (or, when the reaction is carried out in a solution of deuterated methanol, a deuterium atom).
The H/D isotopic exchange of the protons of methyl groups in all the above examples proceeds according to the mechanism of nucleophilic substitution. However, the nucleophilic substitution in bases (i.e., not salts) and alkyl iodides (i.e., azolopyrimidinium salts) occurs via different pathways and has different driving forces. In the case of the bases of 1, 2, 5, 7, 10 and 11 compounds, in a solution of CD3ONa in CD3OD, under the action of a methoxide ion, the proton of the methyl group of the pyrimidine ring is removed and replaced by a deuterium atom from the solvent molecule at the same position (Scheme 9). As a result, a stepwise exchange of all hydrogen atoms of the methyl group 7-CH3, and then in the acetyl group, by deuterium atoms is realized.
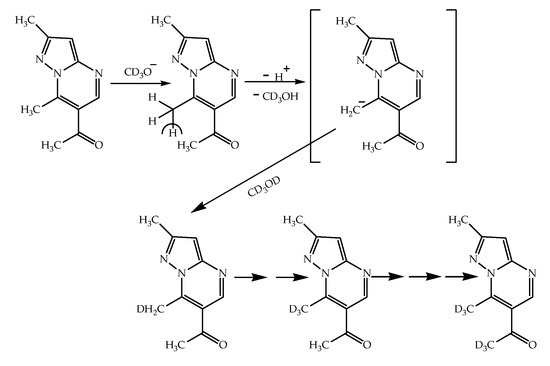
Scheme 9.
The proposed mechanism of H/D nucleophilic exchange in the bases (1, 2, 5, 7, 10 and 11) on the example of compound 1.
In the case of 4-alkyl-substituted pyrazolo[1,5-a]pyrimidinium salts, a different driving force determines the beginning of the isotope exchange. Due to the positive charge on the nitrogen atom, the mobility of hydrogen atoms of the 7-methyl group of the pyrimidine ring increases and, as a result, already in CD3OD—without the addition of alcoholate—H/D exchange becomes possible (Scheme 10). It should be noted that the positive charge on the nitrogen atom of the pyrimidine ring, while promoting an increase in the mobility of hydrogen atoms in the 7-methyl group, does not affect the possibility of detachment of hydrogen atoms in the acetyl group. It remains unaffected by the electronic effects of p-conjugation in the pyrimidine ring. This explains the absence of H/D exchange in the acetyl group in Azolo[1,5-a]pyrimidinium 4-alkyl derivatives in CD3OD solution.
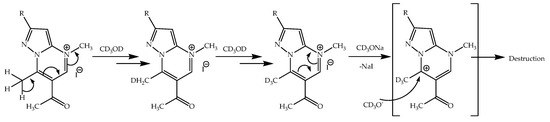
Scheme 10.
The proposed mechanism of H/D nucleophilic exchange in the salts.
When alcoholate is added to the reactor, azolopyrimidine is converted into its neutral form, due to the formation of NaI. The shift in the electron cloud towards the N4 atom makes the 7-position of the pyrimidine ring a nucleophilic attack target. This can lead to the opening of the pyrimidine ring and its destruction (Scheme 10). Note that an ambiguous transformation of the molecule after the addition of an alcoholate was noted earlier.
The process of deuterium exchange of protons of the methyl groups of the pyrimidine ring can be explained by the relatively high CH acidity of these protons. This also correlates the shifts in the signals of these protons in a relatively weak field.
3. Materials and Methods
3.1. General Experimental Details
1H-, 13C-NMR and NOESY spectra were recorded via Varian Mercury-300 VX spectrometer (Varian, Baden, Switzerland) (1H-NMR 300 MHz, 13C-NMR 75 MHz) in a CD3OD at temperatures of 253, 258 and 298 K. Elemental analysis was performed via Eurovector EA 3000 instrument. Melting points were measured on instruments for determining the melting point of organic substances SMP 11 (STUART) and SMP 30 (STUART, Wickford, UK). The purity and identity of the substances were confirmed on a high-performance preparative liquid chromatograph SENMIPREPARATIV HPLC (HPLC Knauer AZURA PREP + Analitical UV Detector),Germany), as well as TLC on Silufol (UV-254). The synthesis of non-deuterated compounds 1, 2, 5, 10, 11, 16 and 17 was carried out according to the previously described procedures [48,49]. 5-Amino-1H-pyrazole-4-carbohydrazide used for the synthesis of 3-pyrazolyl derivative of pyrazolopyrimidine 7 was purchased from Aurora Fine Chemicals LLC, San Diego, CA, USA. CD3OD was purchased from Sigma- Aldrich, St. Louis, MO, USA. All reagents purchased commercially were used without purification.
3.2. Synthetic Procedures
3.2.1. Synthesis of 6-acetyl-7-methyl-3-[1-(4-acetyl-5-methyl-1H-pyrazole-1-carbonyl)]pyrazolo[1,5-a]pyrimidine 7
A mixture of 5-amino-1H-pyrazole-4-carbohydrazide (130 mg, 1.2 mmol) and ethoxymethylideneacetylacetone (400 mg, 2.4 mmol) in 5 mL of absolute ethanol was refluxed for 4 h with a calcium chloride tube. After solvent evaporation, the resulting precipitate was filtered, washed with diethyl ether, recrystallized from hexane and dried to give 6-acetyl-7-methyl-3-[1-(4-acetyl-5-methyl-1H-pyrazole-1-carbonyl)]pyrazolo[1,5-a]pyrimidine 7 as an orange solid in 70% yield. 1H NMR (300 MHz, DMSO/CCl4 1:3, δ, ppm): 2.48 (s, 3 H), 2.76 (s, 3 H), 2.93 (s, 3 H), 3.14 (s, 3 H), 8.12 (s, 1 H), 8.96 (s, 1 H), 9.28 (s, 1 H). 13C NMR (75 MHz, DMSO/CCl4 1:3, δ, ppm): 12.4, 14.52, 28.82, 29.56, 103.68, 120.08, 121.8, 142.27, 146, 149.01, 149.8, 150.07, 152.88, 159.68, 191.86, 195.43. Calculated, %: C 59.07, H 4.65, N 21.53. C16H15N5O3. Found, %: C 59.03, H 4.70, N 21.50; mp: 217–218 ℃.
3.2.2. General Procedure for the Preparation of Deutero-Substituted Azolo[1,5-a]pyrimidines 3, 4, 6, 9, 14, 15
A solution of several mg of compounds 1, 2, 5, 7, 10, 11 in CD3OD was prepared in an NMR ampoule and 1H NMR spectra were recorded. Next, 2 drops of a pre-prepared solution of CD3ONa in CD3OD were added to the ampoule and the dynamics of the proton deuterium exchange in the ampoule was monitored by registering changes in the 1H NMR spectra (Table 4).

Table 4.
Initial and Deutero-Substituted Azolo[1,5-a]pyrimidines, T = 30℃.
4. Conclusions
We have developed an efficient protocol for the synthesis of deuterium-labeled pyrazolo[1,5-a]pyrimidines and 1,2,4-triazolo[1,5-a]pyrimidines. It is important that the method is regioselective and leads to the introduction of deuterium atoms into the methyl group of the pyrimidine fragment of the molecule. The reaction is technically easy to implement and it can be used for labeling for biological research and studying the mechanisms of chemical reactions. It can be assumed that the method will be extended to introduce a tritium label into pharmaceuticals for use in medicine.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28062869/s1, NMR spectra Schemes S1–S3 and Figures S1–S27, Table S1: Kinetic study of the deuterium exchange from compound 5 to 6-d3-acetyl-7-d3-methyl-1,2,4-triazolo[1,5-a]pyrimidine 6 in CD3OD + CD3ONa at T = −10 °C; Table S2: Kinetic study of the deuterium exchange from compound 1 to 6-d3-acetyl-7-d3-methyl-2-methylpyrazolo[1,5-a]pyrimidine 3 in CD3OD + CD3ONa at T = −15 °C; Table S3: Kinetic study of the deuterium exchange from compound 10 to 7-d3-methyl-2-methyl-5-d3-metoxycarbonylpyrazolo[1,5-a]pyrimidine 14 in CD3OD + CD3ONa at T = −10 °C.
Author Contributions
Conceptualization, supervision, project administration, data analysis and writing original draft, G.G.D.; synthesis, methodology, investigation, writing—review and editing, V.K.G. and A.H.H.; formal analysis and validation, H.A.P. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by Russian-Armenian University (RAU).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are included in the article and the Supplementary Materials.
Acknowledgments
The authors wish to thank R. S. Borisov (A.V. Topchiev Institute of Petrochemical Synthesis, RAS, Moscow) for recording the mass spectra.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors upon request.
References
- Hassan, A.S.; Hafez, T.S.; Osman, S.A. Synthesis, characterization, and cytotoxicity of some new 5-aminopyrazole and pyrazolo[1,5-a]pyrimidine derivatives. Sci. Pharm. 2015, 83, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Stallman, H.M.; Kohler, M.; White, J. Medication induced sleepwalking: A systematic review. Sleep Med. Rev. 2018, 37, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Lemon, M.D.; Strain, J.D.; Hegg, A.M.; Farver, D.K. Indiplon in the management of insomnia. Drug Des. Dev. Ther. 2009, 3, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Horoszok, L.; Baleeiro, T.; D’Aniello, F.; Gropper, S.; Santos, B.; Guglietta, A.; Roth, T.A. single-dose, randomized, double-blind, double dummy, placebo and positive-controlled, five-way cross-over study to assess the pharmacodynamic effects of lorediplon in a phase advance model of insomnia in healthy Caucasian adult male subjects. Hum. Psychopharmacol. Clin. Exp. 2014, 29, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Lippa, A.; Czobor, P.; Stark, J.; Beer, B.; Kostakis, E.; Gravielle, M.; Skolnick, P. Selective anxiolysis produced by ocinaplon, a GABAA receptor modulator. Proc. Natl. Acad. Sci. USA 2005, 102, 7380–7385. [Google Scholar] [CrossRef]
- Talekar, N.S.; Huang, C.C. Efficacy of pyrazophos in controlling agromyzid flies on legumes in Taiwan. Int. J. Pest Manag. 1993, 39, 188–192. [Google Scholar] [CrossRef]
- Hamasaki, H.; Hamasaki, Y. Efficacy of anagliptin as compared to linagliptin on metabolic parameters over 2 years of drug consumption: A retrospective cohort study. World J. Diabetes 2018, 9, 165–171. [Google Scholar] [CrossRef]
- Chihara, A.; Tanaka, A.; Morimoto, T.; Sakuma, M.; Shimabukuro, M.; Nomiyama, T.; Node, K. Differences in lipid metabolism between anagliptin and sitagliptin in patients with type 2 diabetes on statin therapy: A secondary analysis of the REASON trial. Cardiovasc. Diabetol. 2019, 18, 158. [Google Scholar] [CrossRef]
- Criscitiello, C.; Viale, G.; Esposito, A.; Curigliano, G. Dinaciclib for the treatment of breast cancer. Expert Opin. Investig. Drugs 2014, 23, 1305–1312. [Google Scholar] [CrossRef]
- Stephenson, J.J.; Nemunaitis, J.; Joy, A.A.; Martin, J.C.; Jou, Y.M.; Zhang, D.; Edelman, M.J. Randomized phase 2 study of the cyclin-dependent kinase inhibitor dinaciclib (MK-7965) versus erlotinib in patients with non-small cell lung cancer. Lung Cancer 2014, 83, 219–223. [Google Scholar] [CrossRef]
- Paulovich, A.G.; Toczyski, D.P.; Hartwell, L.H. When checkpoints fail. Cell 1997, 88, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Gopalsamy, A.; Yang, H.; Ellingboe, J.W.; Tsou, H.R.; Zhang, N.; Honores, E.; Rabindran, S.K. Pyrazolo[1,5-a]pyrimidin-7-yl phenyl amides as novel anti-proliferative agents: Parallel synthesis for lead optimization of amide region. Bioorganic Med. Chem. Lett. 2005, 15, 1591–1594. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, Y.F.; Zhao, X.L.; Yuan, X.Y.; Gong, P. Synthesis and anti-tumor activities of novel pyrazolo[1,5-a]pyrimidines. Arch. Pharm. Int. J. Pharm. Med. Chem. 2006, 339, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.D.; Honores, E.; Wu, B.; Johnson, S.; Powell, D.; Miranda, M.; Krishnamurthy, G. Synthesis, SAR study and biological evaluation of novel pyrazolo[1,5-a]pyrimidin-7-yl phenyl amides as anti-proliferative agents. Bioorganic Med. Chem. 2009, 17, 2091–2100. [Google Scholar] [CrossRef]
- Heathcote, D.A.; Patel, H.; Kroll, S.H.; Hazel, P.; Periyasamy, M.; Alikian, M.; Ali, S. A novel pyrazolo[1,5-a]pyrimidine is a potent inhibitor of cyclin-dependent protein kinases 1, 2, and 9, which demonstrates antitumor effects in human tumor xenografts following oral administration. J. Med. Chem. 2010, 53, 8508–8522. [Google Scholar] [CrossRef]
- Malumbres, M.; Barbacid, M. Cell cycle, CDKs and cancer: A changing paradigm. Nat. Rev. Cancer 2009, 9, 153–166. [Google Scholar] [CrossRef]
- Williamson, D.S.; Parratt, M.J.; Bower, J.F.; Moore, J.D.; Richardson, C.M.; Dokurno, P.; Torrance, C.J. Structure-guided design of pyrazolo[1,5-a]pyrimidines as inhibitors of human cyclin-dependent kinase 2. Bioorganic Med. Chem. Lett. 2005, 15, 863–867. [Google Scholar] [CrossRef]
- Paruch, K.; Dwyer, M.P.; Alvarez, C.; Brown, C.; Chan, T.Y.; Doll, R.J.; Guzi, T.J. Pyrazolo[1,5-a]pyrimidines as orally available inhibitors of cyclin-dependent kinase 2. Bioorganic Med. Chem. Lett. 2007, 17, 6220–6223. [Google Scholar] [CrossRef]
- Boyer, S.J. Small molecule inhibitors of KDR (VEGFR-2) kinase: An overview of structure activity relationships. Curr. Top. Med. Chem. 2002, 2, 973–1000. [Google Scholar] [CrossRef]
- Fraley, M.E.; Rubino, R.S.; Hoffman, W.F.; Hambaugh, S.R.; Arrington, K.L.; Hungate, R.W.; Thomas, K.A. Optimization of a pyrazolo[1,5-a]pyrimidine class of KDR kinase inhibitors: Improvements in physical properties enhance cellular activity and pharmacokinetics. Bioorganic Med. Chem. Lett. 2002, 12, 3537–3541. [Google Scholar] [CrossRef]
- Woods, M.J.; Williams, D.C. Multiple forms and locations for the peripheral-type benzodiazepine receptor. Biochem. Pharmacol. 1996, 52, 1805–1814. [Google Scholar] [CrossRef] [PubMed]
- Selleri, S.; Bruni, F.; Costagli, C.; Costanzo, A.; Guerrini, G.; Ciciani, G.; Martini, C. Synthesis and BZR affinity of pyrazolo[1,5-a]pyrimidine derivatives. Part 1: Study of the structural features for BZR recognition. Bioorganic Med. Chem. 1999, 7, 2705–2711. [Google Scholar] [CrossRef] [PubMed]
- Selleri, S.; Bruni, F.; Costagli, C.; Costanzo, A.; Guerrini, G.; Ciciani, G.; Martini, C. 2-Arylpyrazolo[1,5-a]pyrimidin-3-yl acetamides. New potent and selective peripheral benzodiazepine receptor ligands. Bioorganic Med. Chem. 2001, 9, 2661–2671. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.; Hanani, R.; Hibbs, D.; Damont, A.; Da Pozzo, E.; Selleri, S.; Kassiou, M. Pyrazolo[1,5-a]pyrimidine acetamides: 4-Phenyl alkyl ether derivatives as potent ligands for the 18 kDa translocator protein (TSPO). Bioorganic Med. Chem. Lett. 2010, 20, 5799–5802. [Google Scholar] [CrossRef] [PubMed]
- Baxter, G.S.; Murphy, O.E.; Blackburn, T.P. Further characterization of 5-hydroxytryptamine receptors (putative 5-HT2B) in rat stomach fundus longitudinal muscle. Br. J. Pharmacol. 1994, 112, 323–331. [Google Scholar] [CrossRef]
- Wesolowska, A. In the search for selective ligands of 5-HT5, 5-HT6 and 5-HT7 serotonin receptors. Pol. J. Pharmacol. 2002, 54, 327–341. [Google Scholar]
- Ivachtchenko, A.V.; Dmitriev, D.E.; Golovina, E.S.; Kadieva, M.G.; Koryakova, A.G.; Kysil, V.M.; Vorobiev, A.A. (3-Phenylsulfonylcycloalkano[e and d]pyrazolo[1,5-a]pyrimidin-2-yl)amines: Potent and selective antagonists of the serotonin 5-HT6 receptor. J. Med. Chem. 2010, 53, 5186–5196. [Google Scholar] [CrossRef]
- Tian, Y.; Du, D.; Rai, D.; Wang, L.; Liu, H.; Zhan, P.; Liu, X. Fused heterocyclic compounds bearing bridgehead nitrogen as potent HIV-1 NNRTIs. Part 1: Design, synthesis and biological evaluation of novel 5,7-disubstituted pyrazolo[1,5-a]pyrimidine derivatives. Bioorganic Med. Chem. 2014, 22, 2052–2059. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Popovici-Muller, J.; Shipps, G.W., Jr.; Rosner, K.E.; Deng, Y.; Wang, T.; Curran, P.J.; Girijavallabhan, V. Pyrazolo[1,5-a]pyrimidine-based inhibitors of HCV polymerase. Bioorganic Med. Chem. Lett. 2009, 19, 6331–6336. [Google Scholar] [CrossRef]
- Aggarwal, R.; Sumran, G.; Garg, N.; Aggarwal, A. A regioselective synthesis of some new pyrazol-1′-ylpyrazolo[1,5-a]pyrimidines in aqueous medium and their evaluation as antimicrobial agents. Eur. J. Med. Chem. 2011, 46, 3038–3046. [Google Scholar] [CrossRef] [PubMed]
- Kosugi, T.; Mitchell, D.R.; Fujino, A.; Imai, M.; Kambe, M.; Kobayashi, S.; Kataoka, K.I. Mitogen-activated protein kinase-activated protein kinase 2 (MAPKAP-K2) as an antiinflammatory target: Discovery and in vivo activity of selective pyrazolo[1,5-a]pyrimidine inhibitors using a focused library and structure-based optimization approach. J. Med. Chem. 2012, 55, 6700–6715. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Altman, M.D.; Baker, J.; Brubaker, J.D.; Chen, H.; Chen, Y.; Yang, R. Discovery of 5-amino-N-(1H-pyrazol-4-yl)pyrazolo[1,5-a]pyrimidine-3-carboxamide inhibitors of IRAK4. ACS Med. Chem. Lett. 2015, 6, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Altman, M.D.; Baker, J.; Brubaker, J.D.; Chen, H.; Chen, Y.; Yang, R. Preparation and optimization of pyrazolo[1,5-a]pyrimidines as new potent PDE4 inhibitors. Bioorganic Med. Chem. Lett. 2016, 26, 454–459. [Google Scholar] [CrossRef]
- Ding, R.; He, Y.; Xu, J.; Liu, H.; Wang, X.; Feng, M.; Zhang, J. Synthesis and biological evaluation of pyrazolo[1,5-a]pyrimidine-containing 99mTc nitrido radiopharmaceuticals as imaging agents for tumors. Molecules 2010, 15, 8723–8733. [Google Scholar] [CrossRef]
- Tigreros, A.; Aranzazu, S.L.; Bravo, N.F.; Zapata-Rivera, J.; Portilla, J. Pyrazolo[1,5-a]pyrimidines-based fluorophores: A comprehensive theoretical-experimental study. RSC Adv. 2020, 10, 39542–39552. [Google Scholar] [CrossRef]
- Ding, S.; Yan, Y.; Jiao, N. Copper-catalyzed direct oxidative annulation of N-iminopyridinium ylides with terminal alkynes using O2 as oxidant. Chem. Commun. 2013, 49, 4250–4252. [Google Scholar] [CrossRef]
- Ling, L.; Chen, J.; Song, J.; Zhang, Y.; Li, X.; Song, L.; Shi, F.; Li, Y.; Wu, C. From N-benzoylpyridinium imides to pyrazolo[1,5-a]pyridines: A mechanistic discussion on a stoichiometric Cu protocol. Org. Biomol. Chem. 2013, 11, 3894–3902. [Google Scholar] [CrossRef]
- Gant, T.G. Using deuterium in drug discovery: Leaving the label in the drug. J. Med. Chem. 2014, 57, 3595–3611. [Google Scholar] [CrossRef]
- Grocholska, P.; Bąchor, R. Trends in the hydrogen−deuterium exchange at the carbon centers. Preparation of internal standards for quantitative analysis by LC-MS. Molecules 2021, 26, 2989. [Google Scholar] [CrossRef]
- Mutsumi, T.; Iwata, H.; Maruhashi, K.; Monguchi, Y.; Sajiki, H. Halogen–deuterium exchange reaction mediated by tributyltin hydride using THF-d8 as the deuterium source. Tetrahedron 2011, 67, 1158–1165. [Google Scholar] [CrossRef]
- Rubio Moreno, M.; Campos, J.; Carmona, E. Rhodium-catalyzed, efficient deutero-and tritiosilylation of carbonyl compounds from hydrosilanes and deuterium or tritium. Org. Lett. 2011, 13, 5236–5239. [Google Scholar] [CrossRef] [PubMed]
- Donald, C.S.; Moss, T.A.; Noonan, G.M.; Roberts, B.; Durham, E.C. Deuterodehalogenation—A mild method for synthesising deuterated heterocycles. Tetrahedron Lett. 2014, 55, 3305–3307. [Google Scholar] [CrossRef]
- Vorob’ev, A.Y.; Supranovich, V.I.; Borodkin, G.I.; Shubin, V.G. New approach toward the synthesis of deuterated pyrazolo[1,5-a]pyridines and 1,2,4-triazolo[1,5-a]pyridines. Beilstein J. Org. Chem. 2017, 13, 800–805. [Google Scholar] [CrossRef]
- Gao, Q.; Wu, M.; Zhang, K.; Yang, N.; Liu, M.; Li, J.; Xu, Y. I2-catalyzed aerobic α, β-dehydrogenation and deamination of tertiary alkylamines: Highly selective synthesis of polysubstituted pyrimidines via hidden acyclic enamines. Org. Lett. 2020, 22, 5645–5649. [Google Scholar] [CrossRef] [PubMed]
- Drev, M.; Grošelj, U.; Ledinek, B.; Perdih, F.; Svete, J.; Štefane, B.; Požgan, F. Microwave-promoted ortho-C–H bond (hetero)arylation of arylpyrimidines in water catalyzed by ruthenium(II)–carboxylate. ChemCatChem 2018, 10, 3824–3832. [Google Scholar] [CrossRef]
- Schmidt, C. First deuterated drug approved. Nat. Biotechnol. 2017, 35, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Danagulyan, G.G.; Ostrovskii, V.A.; Gharibyan, V.K. Regioselectivity of Alkylation of Azolo[1,5-a]pyrimidines. Russ. J. Org. Chem. 2022, 58, 1648–1651. [Google Scholar] [CrossRef]
- Danagulyan, G.G.; Ostrovskii, V.A.; Panosyan, H.A.; Gharibyan, V.K.; Arakelyan, M.R.; Boyakhchyan, A.P. Synthesis and regioselectivity of alkylation of substituted 4-(1H-pyrazol-1-yl)pyrimidines, pyrazolo[1,5-a]- and 1,2,4-triazolo[1,5-a]pyrimidines. Chem. J. Armen. 2022, 75, 80–91. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).