Effect of Electrode Spacing on the Performance of a Membrane-Less Microbial Fuel Cell with Magnetite as an Additive
Abstract
1. Introduction
2. Results and Discussion
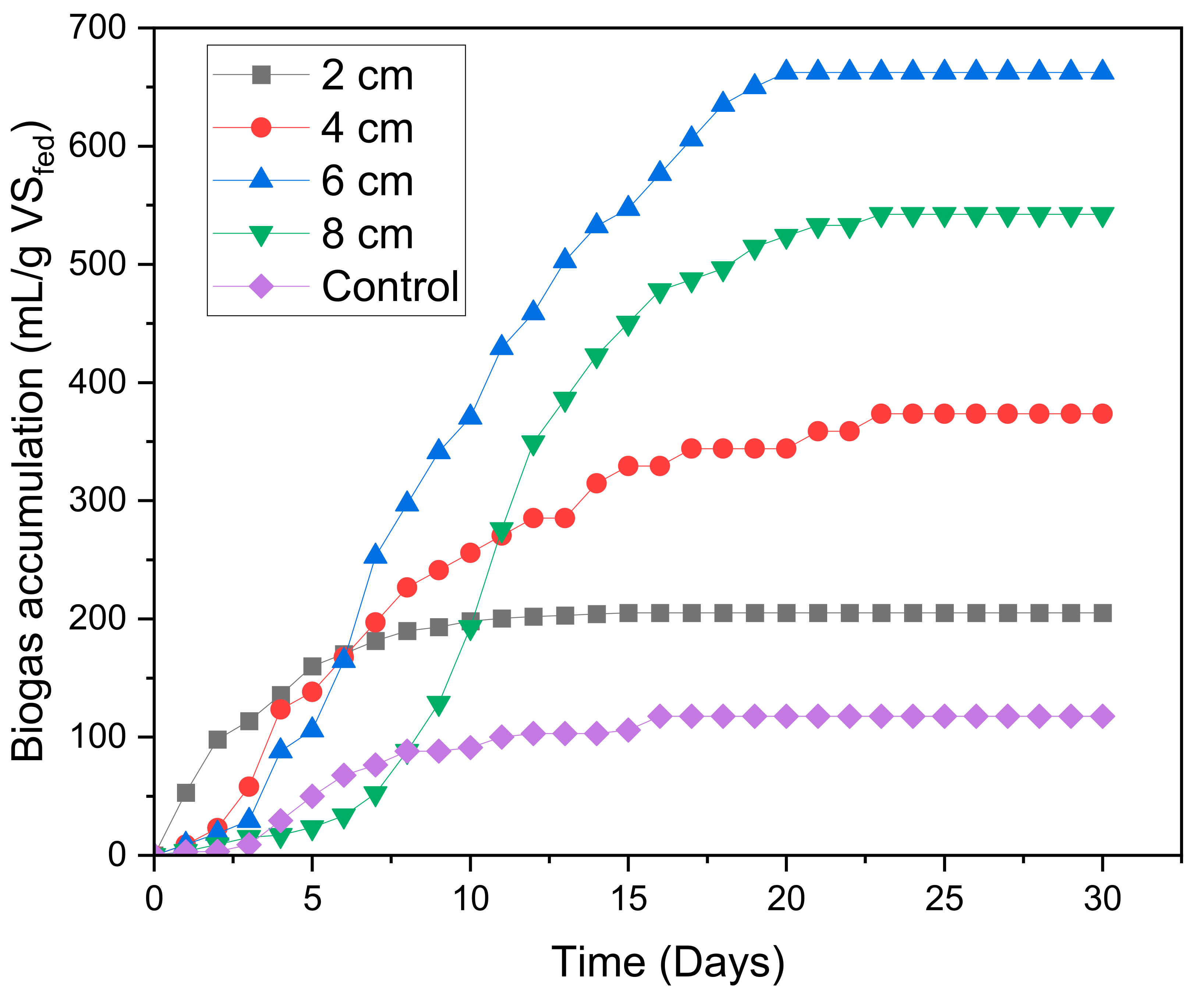
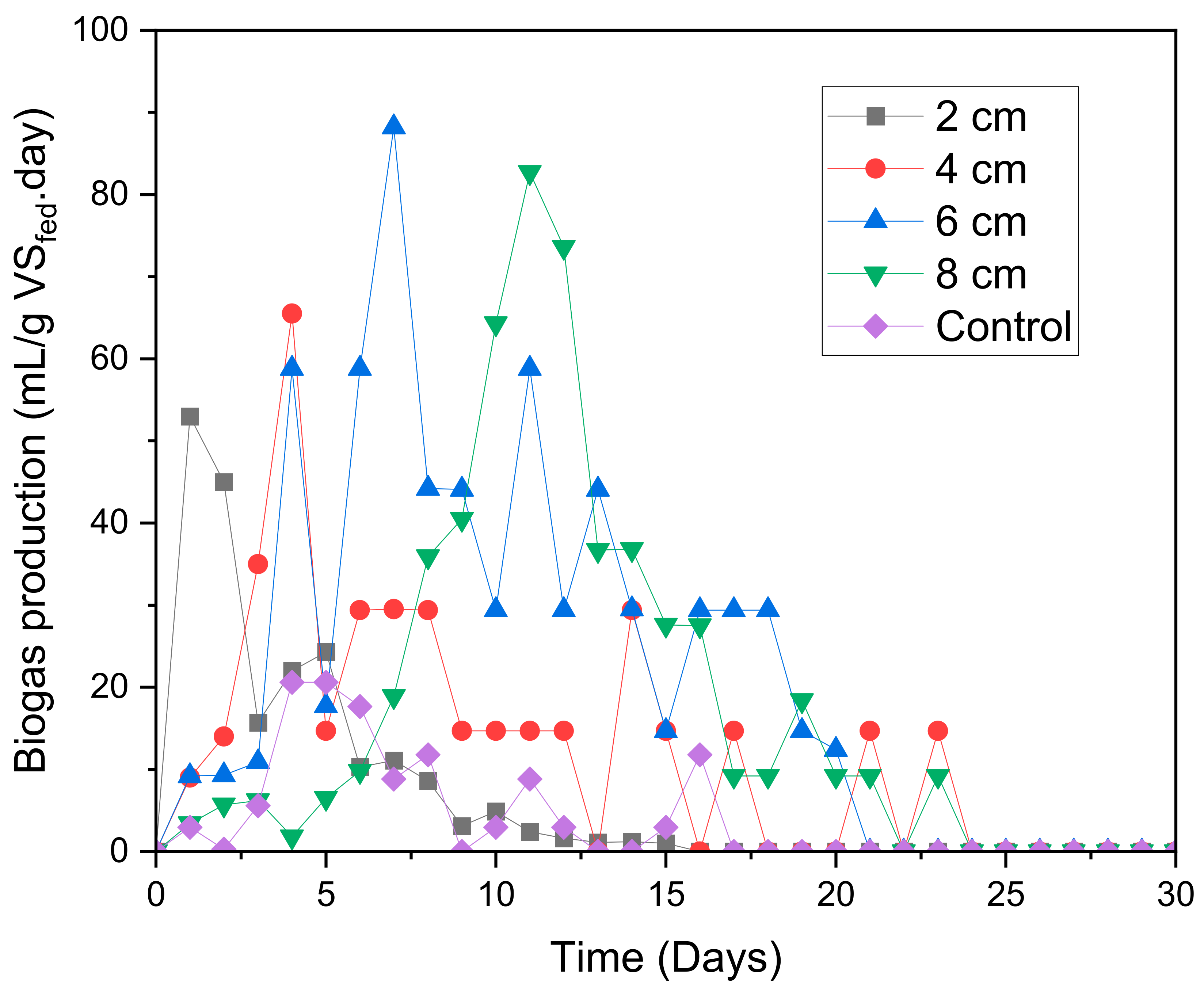
2.1. Biogas Generation
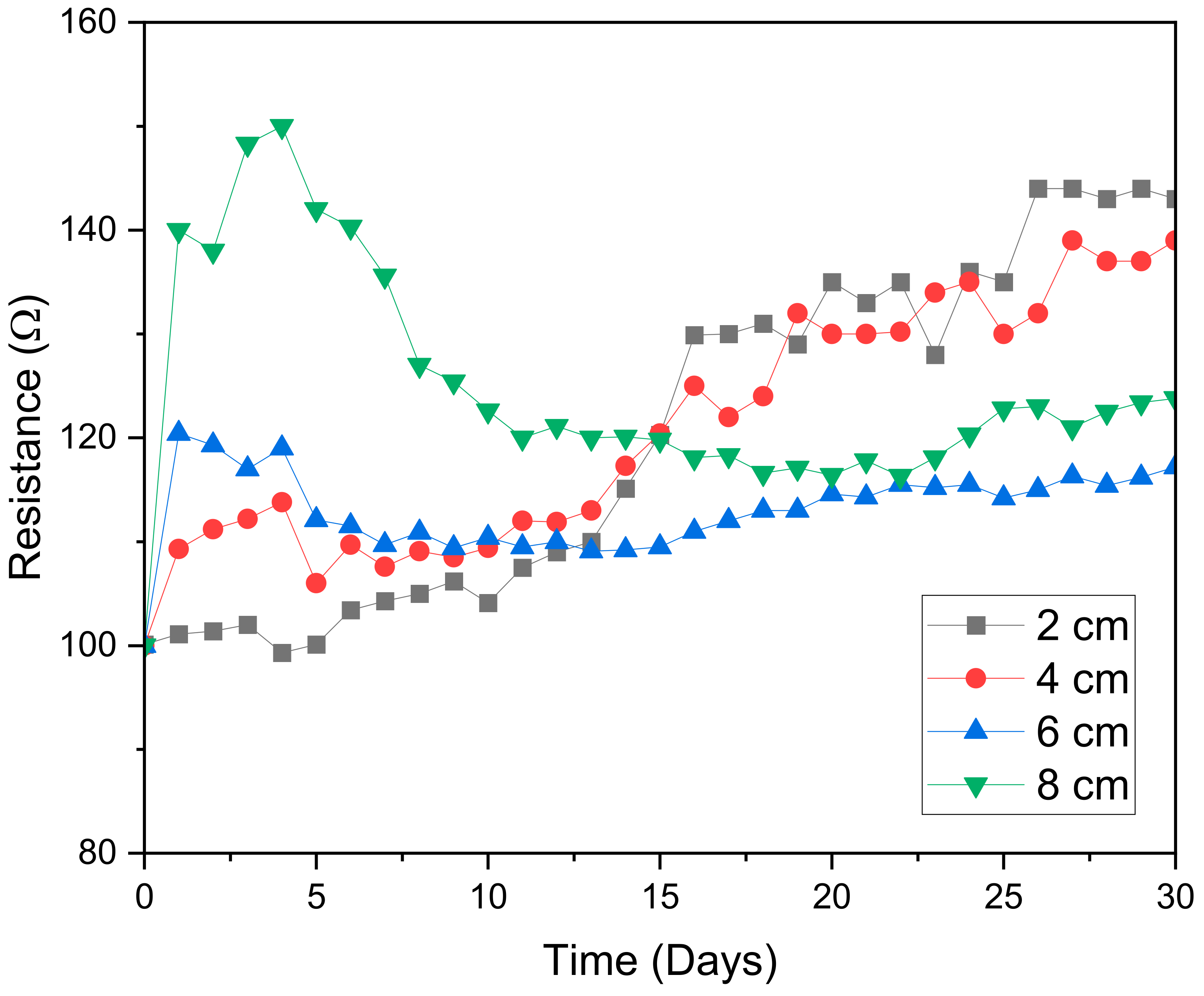
2.2. Current, Resistance and Voltage
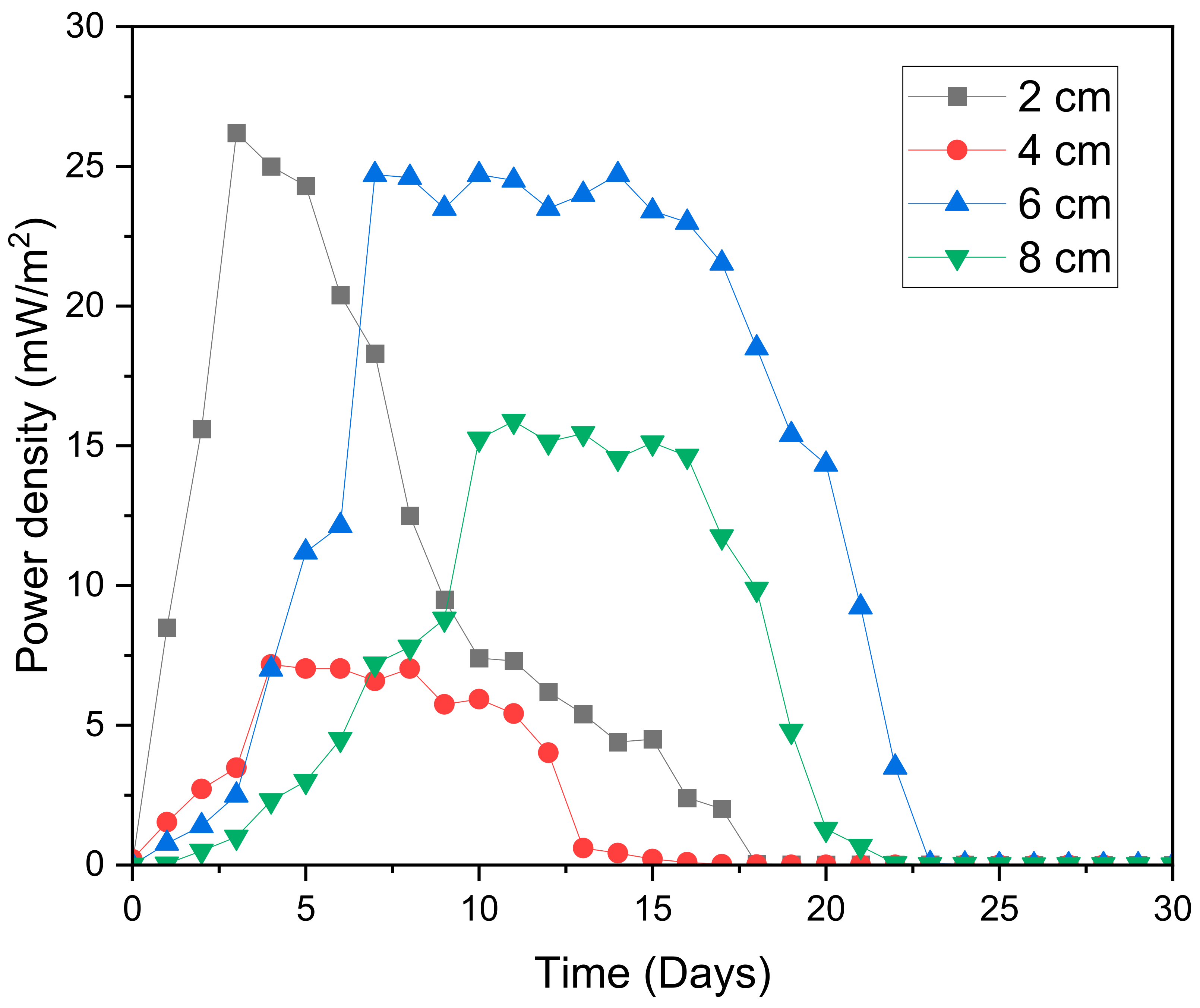
2.3. Electrochemical Efficiencies
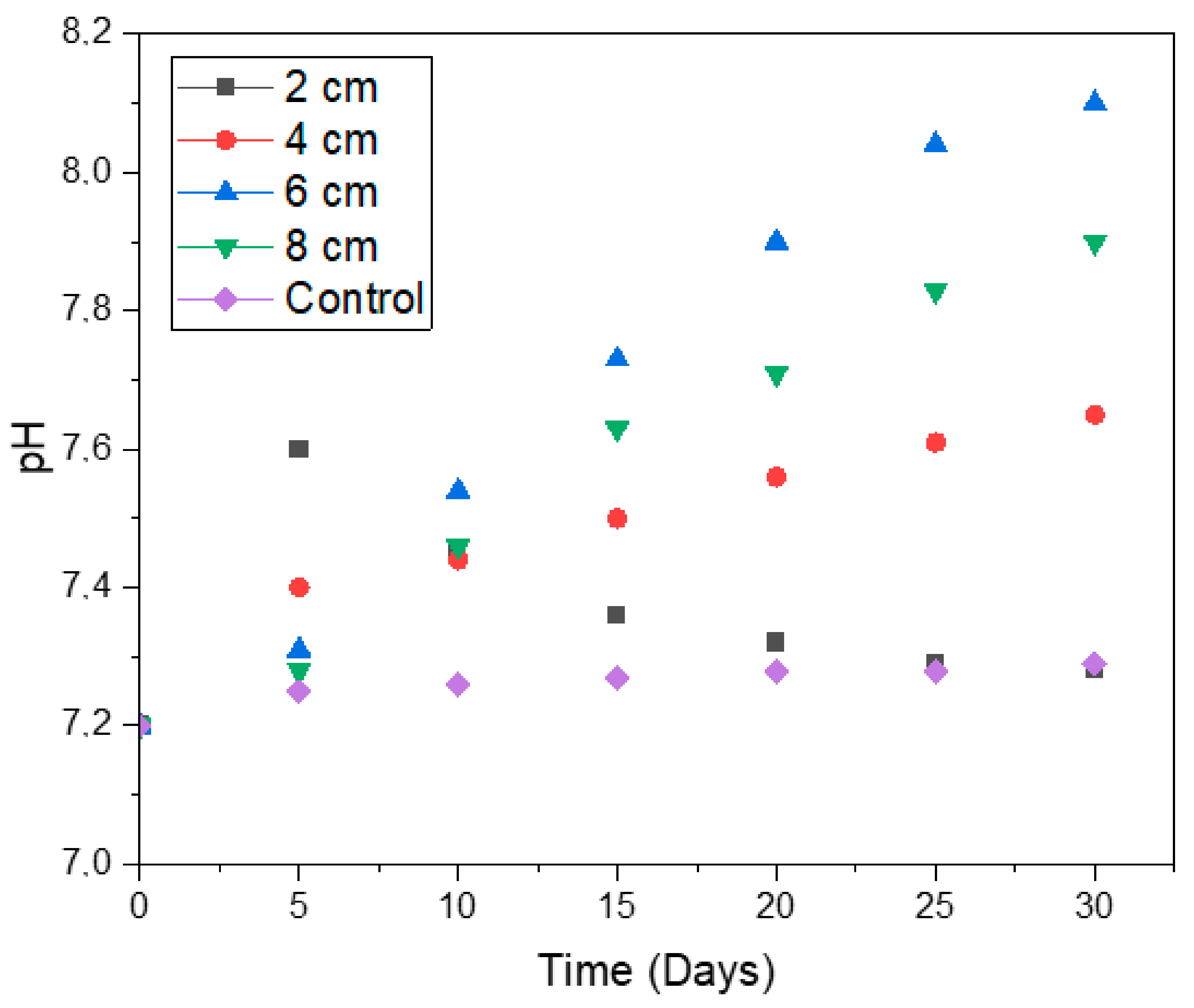
2.4. The pH of the System
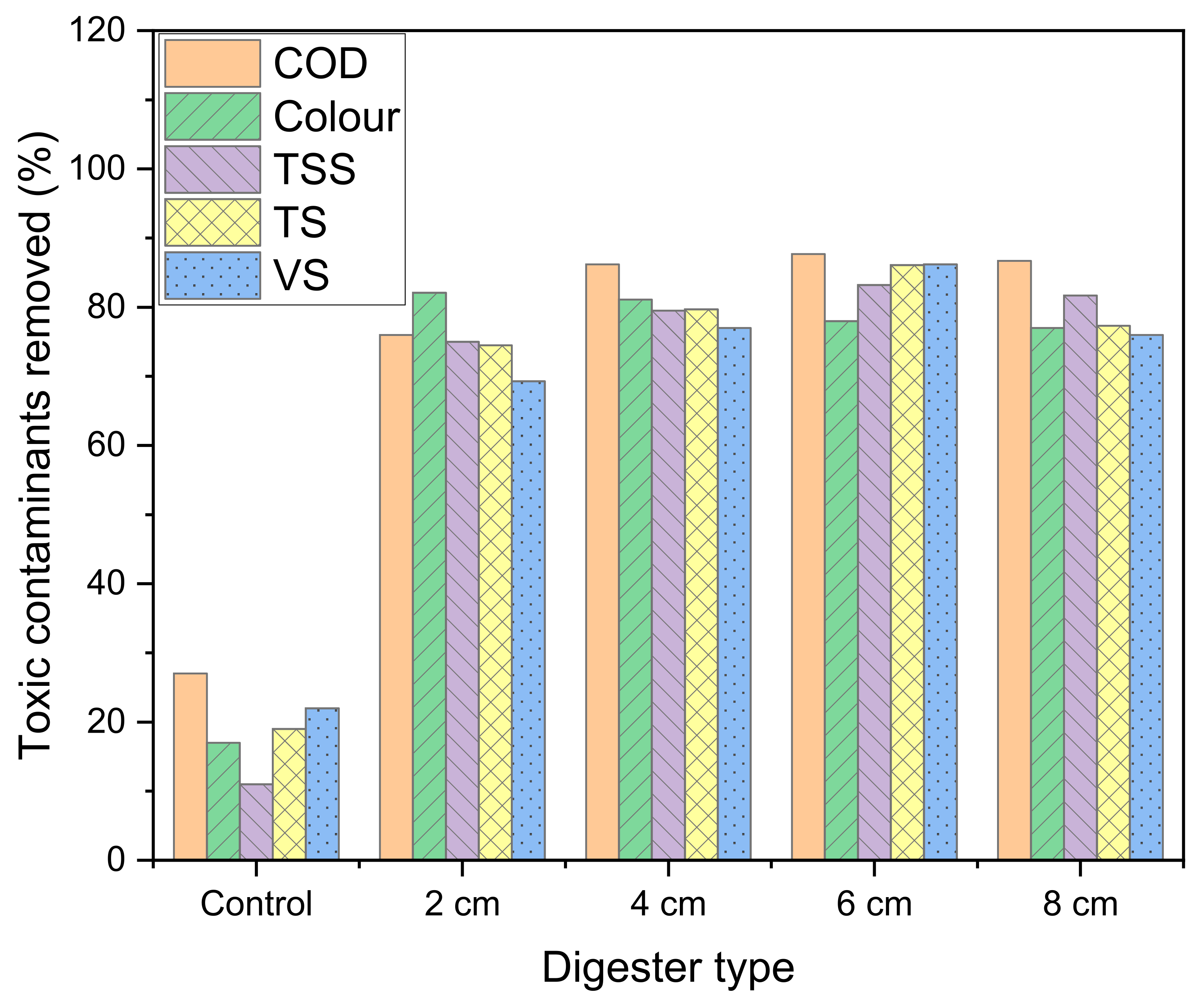
2.5. Removal of Toxic Contaminants
3. Materials and Methods
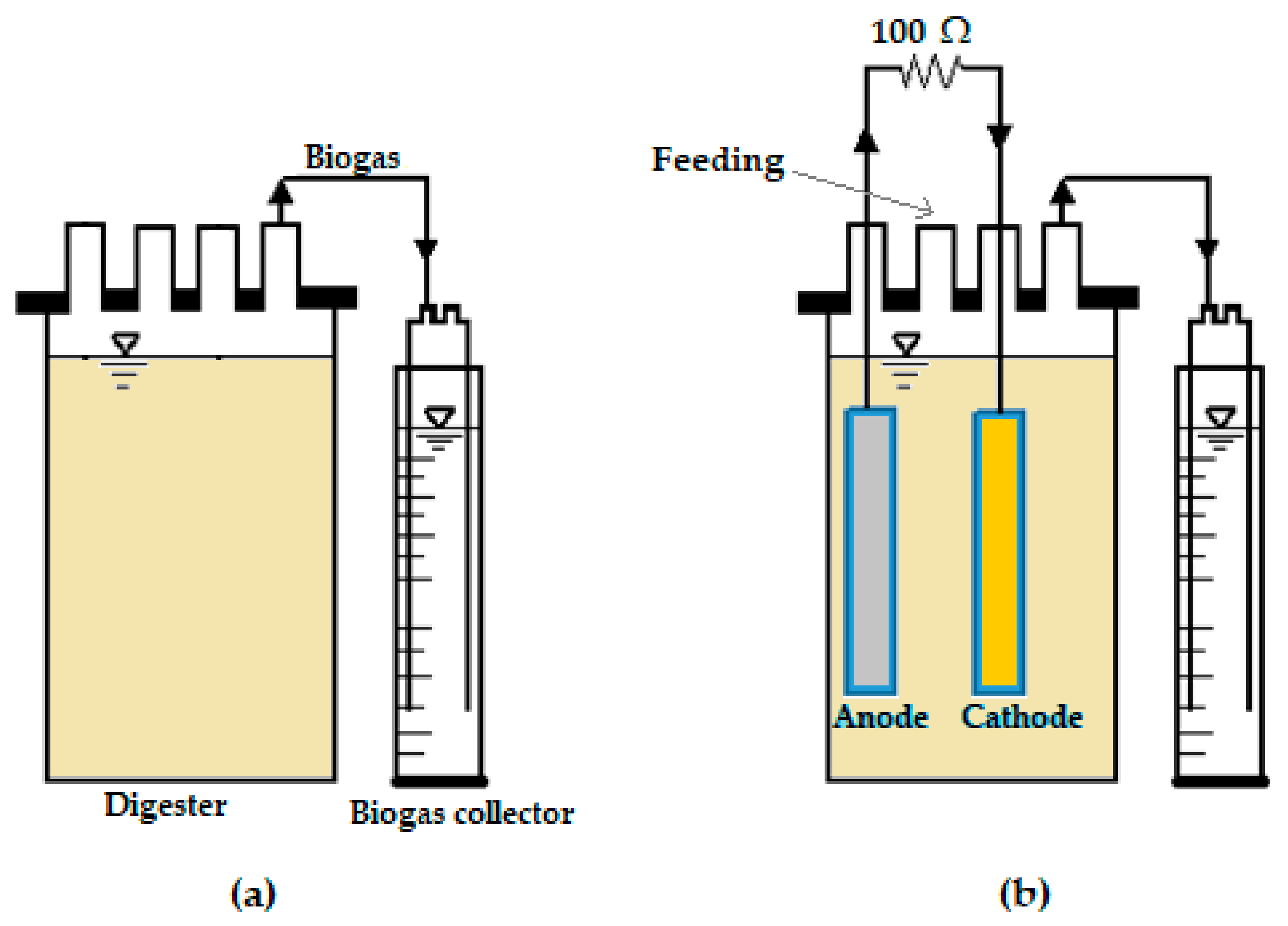
3.1. Equipment Set-Up and Operation
3.2. Sample Analyses and Substrates
3.3. Magnetite-Nanoparticles Synthesis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Avtar, R.; Tripathi, S.; Aggarwal, A.K.; Kumar, P. Population–Urbanization–Energy Nexus: A Review. Resources 2019, 8, 136. [Google Scholar] [CrossRef]
- Garba, N.; Adamu, A. Energy, Environment and Sustainable Development: A Review. Savanna J. Basic Appl. Sci. 2021, 3, 159–163. [Google Scholar]
- Mutezo, G.; Mulopo, J. A review of Africa’s transition from fossil fuels to renewable energy using circular economy principles. Renew. Sustain. Energy Rev. 2021, 137, 110609. [Google Scholar] [CrossRef]
- Perin, G.; Jones, P.R. Economic feasibility and long-term sustainability criteria on the path to enable a transition from fossil fuels to biofuels. Curr. Opin. Biotechnol. 2019, 57, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Beegle, J.R.; Borole, A.P. Energy production from waste: Evaluation of anaerobic digestion and bioelectrochemical systems based on energy efficiency and economic factors. Renew. Sustain. Energy Rev. 2018, 96, 343–351. [Google Scholar] [CrossRef]
- Potrykus, S.; León-Fernández, L.F.; Nieznanski, J.; Karkosinski, D.; Fernandez-Morales, F.J. The Influence of External Load on the Performance of Microbial Fuel Cells. Energies 2021, 14, 612. [Google Scholar] [CrossRef]
- Izam, N.S.M.N.; Itam, Z.; Sing, W.L.; Syamsir, A. Sustainable Development Perspectives of Solar Energy Technologies with Focus on Solar Photovoltaic—A Review. Energies 2022, 15, 2790. [Google Scholar] [CrossRef]
- Kabeyi, M.J.B.; Olanrewaju, O.A. Sustainable Energy Transition for Renewable and Low Carbon Grid Electricity Generation and Supply. Front. Energy Res. 2022, 9, 1032. [Google Scholar] [CrossRef]
- Logan, B.E.; Rabaey, K. Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies. Science 2012, 337, 686–690. [Google Scholar] [CrossRef]
- Wang, H.; Ren, Z.J. A comprehensive review of microbial electrochemical systems as a platform technology. Biotechnol. Adv. 2013, 31, 1796–1807. [Google Scholar] [CrossRef]
- Harnisch, F.; Schröder, U. From MFC to MXC: Chemical and biological cathodes and their potential for microbial bioelectrochemical systems. Chem. Soc. Rev. 2010, 39, 4433–4448. [Google Scholar] [CrossRef]
- Zhou, H.; Mei, X.; Liu, B.; Xie, G.; Xing, D. Magnet anode enhances extracellular electron transfer and enrichment of exoelectrogenic bacteria in bioelectrochemical systems. Biotechnol. Biofuels 2019, 12, 133. [Google Scholar] [CrossRef]
- Li, S.; Chen, G. Factors Affecting the Effectiveness of Bioelectrochemical System Applications: Data Synthesis and Meta-Analysis. Batteries 2018, 4, 34. [Google Scholar] [CrossRef]
- Pant, D.; Singh, A.; Van Bogaert, G.; Olsen, S.I.; Nigam, P.S.; Diels, L.; Vanbroekhoven, K. Bioelectrochemical systems (BES) for sustainable energy production and product recovery from organic wastes and industrial wastewater. RSC Adv. 2012, 2, 1248–1263. [Google Scholar] [CrossRef]
- Madondo, N.I.; Tetteh, E.K.; Rathilal, S.; Bakare, B.F. Synergistic Effect of Magnetite and Bioelectrochemical Systems on Anaerobic Digestion. Bioengineering 2021, 8, 198. [Google Scholar] [CrossRef]
- Palanisamy, G.; Jung, H.Y.; Sadhasivam, T.; Kurkuri, M.D.; Kim, S.C.; Roh, S.H. A comprehensive review on microbial fuel cell technologies: Processes, utilization, and advanced developments in electrodes and membranes. J. Clean. Prod. 2019, 221, 598–621. [Google Scholar] [CrossRef]
- Cano, V.; Cano, J.; Nunes, S.C.; Nolasco, M.A. Electricity generation influenced by nitrogen transformations in a microbial fuel cell: Assessment of temperature and external resistance. Renew. Sustain. Energy Rev. 2021, 139, 110590. [Google Scholar] [CrossRef]
- Rousseau, D.P.L.; Louage, F.; Wang, Q.; Zhang, R. Constructed Wetlands for Urban Wastewater Treatment: An Overview. In Encyclopedia of Inland Waters, 2nd ed.; Mehner, T., Tockner, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 4, pp. 272–284. [Google Scholar]
- Koffi, N.J.; Okabe, S. High voltage generation from wastewater by microbial fuel cells equipped with a newly designed low voltage booster multiplier (LVBM). Sci. Rep. 2020, 10, 18985. [Google Scholar] [CrossRef]
- Mateo, S.; Cañizares, P.; Fernandez-Morales, F.J.; Rodrigo, M.A. A critical view on microbial fuel cells: What’s the next stage? ChemSusChem 2018, 11, 4183–4192. [Google Scholar] [CrossRef]
- Duan, X.; Wu, P.; Pi, K.; Zhang, H.; Liu, D.; Gerson, A.R. Application of Modified Electrocoagulation for Efficient Color Removal from Synthetic Methylene Blue Wastewater. Int. J. Electrochem. Sci. 2018, 13, 5575–5588. [Google Scholar] [CrossRef]
- Lyon, D.Y.; Buret, F.; Vogel, T.M.; Monier, J.M. Is resistance futile? Changing external resistance does not improve microbial fuel cell performance. Bioelectrochemistry 2010, 78, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Katuri, K.P.; Scott, K.; Head, I.M.; Picioreanu, C.; Curtis, T.P. Microbial fuel cells meet with external resistance. Bioresour. Technol. 2011, 102, 2758–2766. [Google Scholar] [CrossRef] [PubMed]
- Madondo, N.I.; Rathilal, S.; Bakare, B.F.; Tetteh, E.K. Application of Bioelectrochemical Systems and Anaerobic Additives in Wastewater Treatment: A Conceptual Review. Int. J. Mol. Sci. 2023, 24, 4753. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Xin, H.; Zhong, D.; Qian, F.; Han, H.; Yuan, Y. Effects of different states of Fe on anaerobic digestion: A review. J. Harbin Inst. Technol. 2015, 22, 69–75. [Google Scholar]
- Liu, Y.; Jia, S.; Wu, Q.; Ran, J.; Zhang, W.; Wu, S. Studies of Fe3O4 chitosan nanoparticles prepared by co-precipitation under the magnetic field for lipase immobilization. Catal. Commun. 2011, 12, 717–720. [Google Scholar] [CrossRef]
- Cavalcante, W.A.; Gehring, T.A.; Zaiat, M. Stimulation and inhibition of direct interspecies electron transfer mechanisms within methanogenic reactors by adding magnetite and granular actived carbon. Chem. Eng. J. 2021, 415, 128882. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, C.; Liu, X.; Sun, D.; Li, P.; Qiu, P.; Dang, Y.; Karpinski, N.A.; Smith, J.A.; Holmes, D.E. Magnetite enhances anaerobic digestion of high salinity organic wastewater. Environ. Res. 2020, 189, 109884. [Google Scholar] [CrossRef]
- Cruz Viggi, C.; Casale, S.; Chouchane, H.; Askri, R.; Fazi, S.; Cherif, A.; Zeppilli, M.; Aulenta, F. Magnetite nanoparticles enhance the bioelectrochemical treatment of municipal sewage by facilitating the syntrophic oxidation of volatile fatty acids. J. Chem. Technol. Biotechnol. 2019, 94, 3134–3146. [Google Scholar] [CrossRef]
- Khan, M.E.; Khan, M.M.; Min, B.K.; Cho, M.H. Microbial fuel cell assisted band gap narrowed TiO2 for visible light induced photocatalytic activities and power generation. Sci. Rep. 2018, 8, 1723. [Google Scholar] [CrossRef]
- Wang, C.T.; Li, I.T.; Jang, J.H. Effect of electrode spacing on the performance of microbial fuel cells with a honeycomb flow straightener. Int. J. Energy Res. 2020, 44, 12136–12144. [Google Scholar] [CrossRef]
- Ali, W.; Khan, M.E.; Mohammad, A.; Alhazmi, W. Design and Fabrication of Nano-Structured Materials for Fuel Cell Application. In Environmental Chemistry for a Sustainable World; Rajendran, S., Naushad, M., Vo, D.V.N., Lichtfouse, E., Eds.; Inorganic Materials for Energy Medicine and Environmental Remediation; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Huang, X.; Duan, C.; Duan, W.; Sun, F.; Cui, H.; Zhang, S.; Chen, X. Role of electrode materials on performance and microbial characteristics in the constructed wetland coupled microbial fuel cell (CW-MFC): A review. J. Clean. Prod. 2021, 301, 126951. [Google Scholar] [CrossRef]
- Ghangrekar, M.M.; Shinde, V.B. Performance of membrane-less microbial fuel cell treating wastewater and effect of electrode distance and area on electricity production. Bioresour. Technol. 2007, 98, 2879–2885. [Google Scholar] [CrossRef]
- Sajana, T.K.; Ghangrekar, M.M.; Mitra, A. Effect of operating parameters on the performance of sediment microbial fuel cell treating aquaculture water. Aquac. Eng. 2014, 61, 17–26. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, J.; Li, Q.; Mo, W.; Wan, R.; Peng, S. Effect of Electrode Distances on Remediation of Eutrophic Water and Sediment by Sediment Microbial Fuel Cell Coupled Floating Beds. Int. J. Environ. Res. Public Health 2022, 19, 10423. [Google Scholar] [CrossRef]
- Emebu, S.; Pecha, J.; Janáčová, D. Review on anaerobic digestion models: Model classification & elaboration of process phenomena. Renew. Sustain. Energy Rev. 2022, 160, 112288. [Google Scholar] [CrossRef]
- Saadi, M.; Pezard, J.; Haddour, N.; Erouel, M.; Vogel, T.M.; Khirouni, K. Stainless steel coated with carbon nanofiber/PDMS composite as anodes in microbial fuel cells. Mater. Res. Express 2020, 7, 025504. [Google Scholar] [CrossRef]
- Simeon, M.I.; Freitag, R. Influence of electrode spacing and fed-batch operation on the maximum performance trend of a soil microbial fuel cell. Int. J. Hydrogen Energy 2022, 47, 12304–12316. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Zhao, Q.; Zhang, Y.; Zhou, Q. In Situ Representation of Soil/Sediment Conductivity Using Electrochemical Impedance Spectroscopy. Sensors 2016, 16, 5. [Google Scholar] [CrossRef]
- Simeon, I.M.; Imoize, A.L.; Freitag, R. Evaluation of the Electrical Performance of a Soil-Type Microbial Fuel Cell Treated with a Substrate at Different Electrode Spacings. In Proceedings of the ICEESEN2020, Kayseri, Turkey, 19–21 March 2020. [Google Scholar]
- Kook, L.; Nemestothy, N.; Belafi-Bako, K.; Bakonyi, P. Investigating the specific role of external load on the performance versus stability trade-off in microbial fuel cells. Bioresour. Technol. 2020, 309, 123313. [Google Scholar] [CrossRef]
- Song, X.; Jo, C.H.; Han, L.; Zhou, M. Recent advance in microbial fuel cell reactor configuration and coupling technologies for removal of antibiotic pollutants. Curr. Opin. Electrochem. 2022, 31, 100833. [Google Scholar] [CrossRef]
- Cheng, S.A.; Liu, H.; Logan, B.E. Increased power generation in a continuous flow MFC with advective flow through the porous anode and reduced electrode spacing. Environ. Sci. Technol. 2006, 40, 2426–2432. [Google Scholar] [CrossRef] [PubMed]
- Song, T.S.; Wang, G.; Wang, H.; Huang, Q.; Xie, J. Experimental evaluation of the influential factors of acetate production driven by a DC power system via CO2 reduction through microbial electrosynthesis. Bioresour. Bioprocess. 2019, 6, 29. [Google Scholar] [CrossRef]
- Greenman, J.; Mendis, B.A.; Gajda, I.; Ieropoulos, I.A. Microbial fuel cell compared to a chemostat. Chemosphere 2022, 296, 133967. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; He, W.; Ren, L.; Stager, J.; Evans, P.J.; Logan, B.E. COD removal characteristics in air cathode microbial fuel cells. Bioresour. Technol. 2015, 176, 23–31. [Google Scholar] [CrossRef]
- Sahu, R.; Parkhey, P. Advancements in bioelectrochemical system-based wastewater treatment: A review on nanocatalytic approach. Sustain. Energy Technol. Assess. 2021, 48, 101558. [Google Scholar] [CrossRef]
- Madondo, N.I.; Kweinor Tetteh, E.; Rathilal, S.; Bakare, B.F. Effect of an Electromagnetic Field on Anaerobic Digestion: Comparing an Electromagnetic System (ES), a Microbial Electrolysis System (MEC), and a Control with No External Force. Molecules 2022, 27, 3372. [Google Scholar] [CrossRef]
- Noveriansyah; Haryati, S.; Bustan, M.D. Effect of Acidity and Electromagnetic Field Strengths on Raw Water Treatment (Turbidity and Color). Int. J. Sci. Technol. Res. 2020, 9, 491–495. [Google Scholar]
- Alabdraba, W.S.; Albayati, M.B.A.; Radeef, A.Y.; Rejab, M.M. Influence of Magnetic Field on The Efficiency of The Coagulation Process to Remove Turbidity from Water. Int. Rev. Chem. Eng. 2013, 5, 8. [Google Scholar]
- Madondo, N.I.; Rathilal, S.; Bakare, B.F. Utilization of Response Surface Methodology in Optimization and Modelling of a Microbial Electrolysis Cell for Wastewater Treatment Using Box–Behnken Design Method. Catalysts 2022, 12, 1052. [Google Scholar] [CrossRef]
- APHA; AWWA; WEF. Standard Methods for Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Madondo, N.I.; Tetteh, E.K.; Rathilal, S.; Bakare, B.F. Application of Bioelectrochemical System and Magnetite Nanoparticles on the Anaerobic Digestion of Sewage Sludge: Effect of Electrode Configuration. Catalysts 2022, 12, 642. [Google Scholar] [CrossRef]
- Amo-Duodu, G.; Tetteh, E.K.; Rathilal, S.; Chollom, M. Synthesis and characterization of magnetic nanoparticles: Biocatalytic effects on wastewater treatment. Mater. Today Proc. 2022, in press. [CrossRef]




| Type of Bioelectrochemical System | Additive/Device Used for Improved Performance | Reference |
|---|---|---|
| MFC | TiO2 | Khan et al. [30] |
| MFC | Honeycomb type flow straightener | Wang et al. [31] |
| MFC | Metal-metal oxides | Ali et al. [32] |
| Digester Type | Magnetic Field Strength (mT) | Color Removal (%) |
|---|---|---|
| control | 0.30 | 17.0 |
| 8 cm | 4.80 | 77.0 |
| 6 cm | 5.45 | 78.0 |
| 4 cm | 5.89 | 81.1 |
| 2 cm | 6.50 | 82.1 |
| Parameter | Unit | Amount |
|---|---|---|
| pH | - | 7.20 ± 0.32 |
| Color | Pt.Co. | 254.33 ± 6.30 |
| COD | mg/L | 2451.23 ± 200.45 |
| TS | mg/L | 53.34 ± 5.34 |
| VS | mg/L | 45.55 ± 1.12 |
| TSS | mg/L | 39.35 ± 1.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madondo, N.I.; Rathilal, S.; Bakare, B.F.; Tetteh, E.K. Effect of Electrode Spacing on the Performance of a Membrane-Less Microbial Fuel Cell with Magnetite as an Additive. Molecules 2023, 28, 2853. https://doi.org/10.3390/molecules28062853
Madondo NI, Rathilal S, Bakare BF, Tetteh EK. Effect of Electrode Spacing on the Performance of a Membrane-Less Microbial Fuel Cell with Magnetite as an Additive. Molecules. 2023; 28(6):2853. https://doi.org/10.3390/molecules28062853
Chicago/Turabian StyleMadondo, Nhlanganiso Ivan, Sudesh Rathilal, Babatunde Femi Bakare, and Emmanuel Kweinor Tetteh. 2023. "Effect of Electrode Spacing on the Performance of a Membrane-Less Microbial Fuel Cell with Magnetite as an Additive" Molecules 28, no. 6: 2853. https://doi.org/10.3390/molecules28062853
APA StyleMadondo, N. I., Rathilal, S., Bakare, B. F., & Tetteh, E. K. (2023). Effect of Electrode Spacing on the Performance of a Membrane-Less Microbial Fuel Cell with Magnetite as an Additive. Molecules, 28(6), 2853. https://doi.org/10.3390/molecules28062853










