Electroanalytical Trace Metal Cations Quantification and Speciation in Freshwaters: Historical Overview, Critical Review of the Last Five Years and Road Map for Developing Dynamic Speciation Field Measurements
Abstract
1. Introduction
2. Critical Review
2.1. Theoretical Background
- -
- the free metal in the bulk for inert complexes,
- -
- the total metal in the bulk for the fully labile complexes
- -
- the free metal plus a fraction of the metal complexes, for quasi-labile or non-labile complexes, depending on their degree of lability.
2.2. Electroanalytical Field Measurements and Methodological Developments
2.3. Critical Review of the Last Five Years
3. Road Map
3.1. Total Metal Ion Screening
3.2. Dynamic Speciation Methodology
3.3. Additional Information Necessary for the Sample Characterization
4. Future Work Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buffle, J. Complexation Reactions in Aquatic Systems: An Analytical Approach; Ellis Horwood: Chichester, UK, 1988. [Google Scholar]
- van Leeuwen, H.P.; Town, R.M.; Buffle, J.; Cleven, R.; Davison, W.; Puy, J.; van Riemsdijk, W.H.; Sigg, L. Dynamic Speciation Analysis and Bioavailability of Metals in Aquatic Systems. Environ. Sci. Technol. 2005, 39, 8545–8556. [Google Scholar] [CrossRef]
- Batley, G.E. Trace Element Speciation Analytical Methods and Problems, 1st ed.; CRC Press: Boca Raton, FL, USA, 1989; ISBN 978-0-8493-4712-2. [Google Scholar]
- Florence, T. The Speciation of Trace-Elements in Waters. Talanta 1982, 29, 345–364. [Google Scholar] [CrossRef]
- Nakai, R. Size Matters: Ultra-Small and Filterable Microorganisms in the Environment. Microbes Environ. 2020, 35, ME20025. [Google Scholar] [CrossRef] [PubMed]
- Leenheer, J.A.; Croue, J.P. Characterizing Aquatic Dissolved Organic Matter. Environ. Sci. Technol. 2003, 37, 18A–26A. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Liu, L.; Hua, X.; Lu, Y. Comparison of Lead, Cadmium, Copper and Cobalt Adsorption onto Metal Oxides and Organic Materials in Natural Surface Coatings. Microchem. J. 2007, 85, 270–275. [Google Scholar] [CrossRef]
- Tercier-Waeber, M.-L.; Confalonieri, F.; Abdou, M.; Dutruch, L.; Bossy, C.; Fighera, M.; Bakker, E.; Graziottin, F.; van der Wal, P.; Schafer, J. Advanced Multichannel Submersible Probe for Autonomous High-Resolution in Situ Monitoring of the Cycling of the Potentially Bioavailable Fraction of a Range of Trace Metals. Chemosphere 2021, 282, 131014. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.M.; Aplin, A.C. Role of Colloids and Fine Particles in the Transport of Metals in Rivers Draining Carbonate and Silicate Terrains. Limnol. Oceanogr. 2001, 46, 331–344. [Google Scholar] [CrossRef]
- Kalmykova, Y.; Rauch, S.; Strömvall, A.-M.; Morrison, G.; Stolpe, B.; Hassellöv, M. Colloid-Facilitated Metal Transport in Peat Filters. Water Environ. Res. 2010, 82, 506–511. [Google Scholar] [CrossRef]
- Everett, D.H. Manual of Symbols and Terminology for Physicochemical Quantities and Units, Appendix II: Definitions, Terminology and Symbols in Colloid and Surface Chemistry. Pure Appl. Chem. 1972, 31, 577–638. [Google Scholar] [CrossRef]
- Baxter, D.C.; Frech, W. Speciation of Lead in Environmental and Biological Samples (Technical Report). Pure Appl. Chem. 1995, 67, 615–648. [Google Scholar] [CrossRef]
- Buffle, J.; Horvai, G. Situ Monitoring of Aquatic Systems: Chemical Analysis and Speciation; John Wiley & Sons: New York, NY, USA, 2000. [Google Scholar]
- Davison, W.; Zhang, H. In-Situ Speciation Measurements of Trace Components in Natural-Waters Using Thin-Film Gels. Nature 1994, 367, 546–548. [Google Scholar] [CrossRef]
- Parthasarathy, N.; Pelletier, M.; Tercier-Waeber, M.L.; Buffle, J. On-Line Coupling of Flow through Voltammetric Microcell to Hollow Fiber Permeation Liquid Membrane Device for Subnanomolar Trace Metal Speciation Measurements. Electroanalysis 2001, 13, 1305–1314. [Google Scholar] [CrossRef]
- Temminghoff, E.J.M.; Plette, A.C.C.; Van Eck, R.; Van Riemsdijk, W.H. Determination of the Chemical Speciation of Trace Metals in Aqueous Systems by the Wageningen Donnan Membrane Technique. Anal. Chim. Acta 2000, 417, 149–157. [Google Scholar] [CrossRef]
- Tercier-Waeber, M.-L.; Confalonieri, F.; Koudelka-Hep, M.; Dessureault-Rompre, J.; Graziottin, F.; Buffle, J. Gel-Integrated Voltammetric Microsensors and Submersible Probes as Reliable Tools for Environmental Trace Metal Analysis and Speciation. Electroanalysis 2008, 20, 240–258. [Google Scholar] [CrossRef]
- Tessier, A.; Turner, D.R. Metal Speciation and Bioavailability in Aquatic Systems; Wiley–Blackwell: Chichester, UK; New York, NY, USA, 1995; ISBN 978-0-471-95830-7. [Google Scholar]
- Van Den Berg, C. Potentials and Potentialities of Cathodic Stripping Voltammetry of Trace-Elements in Natural-Waters. Anal. Chim. Acta 1991, 250, 265–276. [Google Scholar] [CrossRef]
- Xue, H.; Sigg, L. A Review of Competitive Ligand-Exchange-Voltammetric Methods for Speciation of Trace Metals in Freshwater. In Environmental Electrochemistry; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2002; Volume 811, pp. 336–370. ISBN 978-0-8412-3774-2. [Google Scholar]
- Galceran, J.; Puy, J. Interpretation of Diffusion Gradients in Thin Films (DGT) Measurements: A Systematic Approach. Environ. Chem. 2015, 12, 112–122. [Google Scholar] [CrossRef]
- Van Der Veeken, P.L.R.; Pinheiro, J.P.; Van Leeuwen, H.P. Metal Speciation by DGT/DET in Colloidal Complex Systems. Environ. Sci. Technol. 2008, 42, 8835–8840. [Google Scholar] [CrossRef]
- Yasadi, K.; Pinheiro, J.P.; Zielinska, K.; Town, R.M.; van Leeuwen, H.P. Partitioning of Humic Acids between Aqueous Solution and Hydrogel. 3. Microelectrodic Dynamic Speciation Analysis of Free and Bound Humic Metal Complexes in the Gel Phase. Langmuir 2015, 31, 1737–1745. [Google Scholar] [CrossRef]
- van Leeuwen, H.P. Revisited: DGT Speciation Analysis of Metal-Humic Acid Complexes. Environ. Chem. 2016, 13, 84–88. [Google Scholar] [CrossRef]
- Zhang, Z.; Buffle, J.; van Leeuwen, H.P. Roles of Dynamic Metal Speciation and Membrane Permeability in Metal Flux through Lipophilic Membranes: General Theory and Experimental Validation with Nonlabile Complexes. Langmuir 2007, 23, 5216–5226. [Google Scholar] [CrossRef]
- Domingos, R.F.; Benedetti, M.F.; Pinheiro, J.P. Application of Permeation Liquid Membrane and Scanned Stripping Chronopotentiometry to Metal Speciation Analysis of Colloidal Complexes. Anal. Chim. Acta 2007, 589, 261–268. [Google Scholar] [CrossRef]
- Kalis, E.J.J.; Weng, L.; Temminghoff, E.J.M.; van Riemsdijk, W.H. Measuring Free Metal Ion Concentrations in Multicomponent Solutions Using the Donnan Membrane Technique. Anal. Chem. 2007, 79, 1555–1563. [Google Scholar] [CrossRef]
- Kalis, E.J.J.; Weng, L.P.; Dousma, F.; Temminghoff, E.J.M.; Van Riemsdijk, W.H. Measuring Free Metal Ion Concentrations in Situ in Natural Waters Using the Donnan Membrane Technique. Environ. Sci. Technol. 2006, 40, 955–961. [Google Scholar] [CrossRef]
- Weng, L.P.; Van Riemsdijk, W.H.; Temminghoff, E.J.M. Kinetic Aspects of Donnan Membrane Technique for Measuring Free Trace Cation Concentration. Anal. Chem. 2005, 77, 2852–2861. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons, Incorporated: New York, NY, USA, 2000; ISBN 978-1-118-31280-3. [Google Scholar]
- Fischer, E.; van den Berg, C.M.G. Determination of Lead Complexation in Lake Water by Cathodic Stripping Voltammetry and Ligand Competition. Anal. Chim. Acta 2001, 432, 11–20. [Google Scholar] [CrossRef]
- Mattsson, G.; Nyholm, L.; Olin, Å.; Örnemark, U. Determination of Selenium in Freshwaters by Cathodic Stripping Voltammetry after UV Irradiation. Talanta 1995, 42, 817–825. [Google Scholar] [CrossRef]
- Meylan, S.; Odzak, N.; Behra, R.; Sigg, L. Speciation of Copper and Zinc in Natural Freshwater: Comparison of Voltammetric Measurements, Diffusive Gradients in Thin Films (DGT) and Chemical Equilibrium Models. Anal. Chim. Acta 2004, 510, 91–100. [Google Scholar] [CrossRef]
- Qian, J.; Xue, H.B.; Sigg, L.; Albrecht, A. Complexation of Cobalt by Natural Ligands in Freshwater. Environ. Sci. Technol. 1998, 32, 2043–2050. [Google Scholar] [CrossRef]
- Xue, H.; Sigg, L. Cadmium Speciation and Complexation by Natural Organic Ligands in Fresh Water. Anal. Chim. Acta 1998, 363, 249–259. [Google Scholar] [CrossRef]
- Xue, H.B.; Jansen, S.; Prasch, A.; Sigg, L. Nickel Speciation and Complexation Kinetics in Freshwater by Ligand Exchange and DPCSV. Environ. Sci. Technol. 2001, 35, 539–546. [Google Scholar] [CrossRef]
- Laglera, L.M.; Monticelli, D. Iron Detection and Speciation in Natural Waters by Electrochemical Techniques: A Critical Review. Curr. Opin. Electrochem. 2017, 3, 123–129. [Google Scholar] [CrossRef]
- Cuartero, M. Electrochemical Sensors for In-Situ Measurement of Ions in Seawater. Sens. Actuator B-Chem. 2021, 334, 129635. [Google Scholar] [CrossRef]
- Sunda, W.; Guillard, R. Relationship Between Cupric Ion Activity and Toxicity of Copper to Phytoplankton. J. Mar. Res. 1976, 34, 511–529. [Google Scholar]
- Di Toro, D.M.; Allen, H.E.; Bergman, H.L.; Meyer, J.S.; Paquin, P.R.; Santore, R.C. Biotic Ligand Model of the Acute Toxicity of Metals. 1. Technical Basis. Environ. Toxicol. Chem. 2001, 20, 2383–2396. [Google Scholar] [CrossRef]
- Tipping, E. Humic Substances in Soil, Sediment, and Water: Geochemistry, Isolation, and Characterization; Aiken, G.R., Ed.; Wiley: New York, NY, USA, 1985; ISBN 978-0-471-88274-9. [Google Scholar]
- Koopal, L.K.; Saito, T.; Pinheiro, J.P.; van Riemsdijk, W.H. Ion Binding to Natural Organic Matter: General Considerations and the NICA-Donnan Model. Colloid Surf. A-Physicochem. Eng. Asp. 2005, 265, 40–54. [Google Scholar] [CrossRef]
- Tipping, E. WHAMC—A Chemical Equilibrium Model and Computer Code for Waters, Sediments, and Soils Incorporating a Discrete Site/Electrostatic Model of Ion-Binding by Humic Substances. Comput. Geosci. 1994, 20, 973–1023. [Google Scholar] [CrossRef]
- Town, R.M.; van Leeuwen, H.P.; Duval, J.F.L. Rigorous Physicochemical Framework for Metal Ion Binding by Aqueous Nanoparticulate Humic Substances: Implications for Speciation Modeling by the NICA-Donnan and WHAM Codes. Environ. Sci. Technol. 2019, 53, 8516–8532. [Google Scholar] [CrossRef]
- Benedetti, M.F.; vanRiemsdik, W.H.; Koopal, L.K. Humic Substances Considered as a Heterogeneous Donnan Gel Phase. Environ. Sci. Technol. 1996, 30, 1805–1813. [Google Scholar] [CrossRef]
- Milne, C.J.; Kinniburgh, D.G.; van Riemsdijk, W.H.; Tipping, E. Generic NICA−Donnan Model Parameters for Metal-Ion Binding by Humic Substances. Environ. Sci. Technol. 2003, 37, 958–971. [Google Scholar] [CrossRef]
- Janot, N.; Pinheiro, J.P.; Botero, W.G.; Meeussen, J.C.L.; Groenenberg, J.E. PEST-ORCHESTRA, a Tool for Optimising Advanced Ion-Binding Model Parameters: Derivation of NICA-Donnan Model Parameters for Humic Substances Reactivity. Environ. Chem. 2016, 14, 31–38. [Google Scholar] [CrossRef]
- Pinheiro, J.P.; Rotureau, E.; Duval, J.F.L. Addressing the Electrostatic Component of Protons Binding to Aquatic Nanoparticles beyond the Non-Ideal Competitive Adsorption (NICA)-Donnan Level: Theory and Application to Analysis of Proton Titration Data for Humic Matter. J. Colloid Interface Sci. 2021, 583, 642–651. [Google Scholar] [CrossRef]
- Tesfa, M.; Duval, J.F.L.; Marsac, R.; Dia, A.; Pinheiro, J.-P. Absolute and Relative Positioning of Natural Organic Matter Acid-Base Potentiometric Titration Curves: Implications for the Evaluation of the Density of Charged Reactive Sites. Environ. Sci. Technol. 2022, 56, 10494–10503. [Google Scholar] [CrossRef]
- Hudson, R.J.M.; Morel, F.M.M. Lron Transport in Marine Phytoplankton: Kinetics of Cellular and Medium Coordination Reactions. Limnol. Oceanogr. 1990, 35, 1002–1020. [Google Scholar] [CrossRef]
- Morel, F.M.M.; Hudson, R.J.M.; Price, N.M. Limitation of Productivity by Trace Metals in the Sea. Limnol. Oceanogr. 1991, 36, 1742–1755. [Google Scholar] [CrossRef]
- Hudson, R.J.M. Which Aqueous Species Control the Rates of Trace Metal Uptake by Aquatic Biota? Observations and Predictions of Non-Equilibrium Effects. Sci. Total Environ. 1998, 219, 95–115. [Google Scholar] [CrossRef]
- Campbell, P.G.C.; Errécalde, O.; Fortin, C.; Hiriart-Baer, V.P.; Vigneault, B. Metal Bioavailability to Phytoplankton—Applicability of the Biotic Ligand Model. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2002, 133, 189–206. [Google Scholar] [CrossRef]
- Hassler, C.S.; Slaveykova, V.I.; Wilkinson, K.J. Some Fundamental (and Often Overlooked) Considerations Underlying the Free Ion Activity and Biotic Ligand Models. Environ. Toxicol. Chem. 2004, 23, 283–291. [Google Scholar] [CrossRef]
- van Leeuwen, H.P. Revisited: The Conception of Lability of Metal Complexes. Electroanalysis 2001, 13, 826–830. [Google Scholar] [CrossRef]
- Davison, W. Defining Electroanalytically Measured Species in a Natural-Water Sample. J. Electroanal. Chem. 1978, 87, 395–404. [Google Scholar] [CrossRef]
- Vanleeuwen, H. Kinetic Classification of Metal-Complexes in Electroanalytical Speciation. J. Electroanal. Chem. 1979, 99, 93–102. [Google Scholar] [CrossRef]
- Koutecký, J.; Koryta, J. The General Theory of Polarographic Kinetic Currents. Electrochim. Acta 1961, 3, 318–339. [Google Scholar] [CrossRef]
- van Leeuwen, H.P.; Town, R.M. Stripping Chronopotentiometry at Scanned Deposition Potential (SSCP). Part 4. The Kinetic Current Regime. J. Electroanal. Chem. 2004, 561, 67–74. [Google Scholar] [CrossRef]
- Pinheiro, J.P.; Minor, M.; van Leeuwen, H.P. Metal Speciation Dynamics in Colloidal Ligand Dispersions. Langmuir 2005, 21, 8635–8642. [Google Scholar] [CrossRef]
- van Leeuwen, H.P.; Buffle, J. Chemodynamics of Aquatic Metal Complexes: From Small Ligands to Colloids. Environ. Sci. Technol. 2009, 43, 7175–7183. [Google Scholar] [CrossRef]
- Mota, A.M.; Pinheiro, J.P.; Simoes Goncalves, M.L. Electrochemical Methods for Speciation of Trace Elements in Marine Waters. Dynamic Aspects. J. Phys. Chem. A 2012, 116, 6433–6442. [Google Scholar] [CrossRef]
- van Leeuwen, H.P.; Duval, J.F.L.; Pinheiro, J.P.; Blust, R.; Town, R.M. Chemodynamics and Bioavailability of Metal Ion Complexes with Nanoparticles in Aqueous Media. Environ. Sci.-Nano 2017, 4, 2108–2133. [Google Scholar] [CrossRef]
- Puy, J.; Galceran, J. Theoretical Aspects of Dynamic Metal Speciation with Electrochemical Techniques. Curr. Opin. Electrochem. 2017, 1, 80–87. [Google Scholar] [CrossRef]
- Duval, J.F.L.; Town, R.M.; van Leeuwen, H.P. Applicability of the Reaction Layer Principle to Nanoparticulate Metal Complexes at a Macroscopic Reactive (Bio)Interface: A Theoretical Study. J. Phys. Chem. C 2017, 121, 19147–19161. [Google Scholar] [CrossRef]
- Duval, J.F.L.; Town, R.M.; van Leeuwen, H.P. Lability of Nanoparticulate Metal Complexes at a Macroscopic Metal Responsive (Bio)Interface: Expression and Asymptotic Scaling Laws. J. Phys. Chem. C 2018, 122, 6052–6065. [Google Scholar] [CrossRef]
- Duval, J.F.L.; van Leeuwen, H.P.; Town, R.M. Electrostatic Effects on Ligand-Assisted Transfer of Metals to (Bio)Accumulating Interfaces and Metal Complexes (Bioavai)Lability. Colloids Surf. A Physicochem. Eng. Asp. 2023, 658, 130679. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry, 3rd ed.; Wiley-Interscience: NewYork, NY, USA, 1996; ISBN 0-471-51184-6. [Google Scholar]
- Vydra, F.; Stulik, K.; Juláková, E. Electrochemical Stripping Analysis; Ellis Horwood: Chichester, UK, 1976; ISBN 0-470-15131-5. [Google Scholar]
- Barek, J.; Zima, J. Eighty Years of Polarography—History and Future. Electroanalysis 2003, 15, 467–472. [Google Scholar] [CrossRef]
- Nurnberg, H.; Valenta, P.; Mart, L.; Raspor, B.; Sipos, L. Applications of Polarography and Voltammetry to Marine and Aquatic Chemistry. 2. Polarographic Approach to Determination and Speciation of Toxic Trace-Metals in Marine-Environment. Fresenius Z. Fur Anal. Chem. 1976, 282, 357–367. [Google Scholar] [CrossRef]
- Florence, T.; Batley, G. Chemical Speciation in Natural-Waters. Crc Crit. Rev. Anal. Chem. 1980, 9, 219–296. [Google Scholar] [CrossRef]
- Florence, T. Electrochemical Approaches to Trace-Element Speciation in Waters—A Review. Analyst 1986, 111, 489–505. [Google Scholar] [CrossRef]
- Brezonik, P.; Brauner, P.; Stumm, W. Trace-Metal Analysis by Anodic-Stripping Voltammetry—Effect of Sorption by Natural and Model Organic-Compounds. Water Res. 1976, 10, 605–612. [Google Scholar] [CrossRef]
- Buffle, J.; Mota, A.; Goncalves, M. Adsorption of Fulvic-Like Organic-Ligands and Their Cd and Pb Complexes at a Mercury-Electrode. J. Electroanal. Chem. 1987, 223, 235–262. [Google Scholar] [CrossRef]
- Mota, A.; Pinheiro, J.; Goncalves, M. Adsorption of Humic-Acid on a Mercury Aqueous-Solution Interface. Water Res. 1994, 28, 1285–1296. [Google Scholar] [CrossRef]
- Pinheiro, J.; Mota, A.; Goncalves, M.; Vanleeuwen, H. Kinetics of Adsorption of Humic Matter on Mercury. Environ. Sci. Technol. 1994, 28, 2112–2119. [Google Scholar] [CrossRef]
- Ugapo, T.; Pickering, W. Effect of Organic Colloids on Asv Signals of Cd, Pb and Cu. Talanta 1985, 32, 131–138. [Google Scholar] [CrossRef]
- Buffle, J. Calculation of the Surface Concentration of the Oxidized Metal during the Stripping Step in the Anodic-Stripping Techniques and Its Influence on Speciation Measurements in Natural-Waters. J. Electroanal. Chem. 1981, 125, 273–294. [Google Scholar] [CrossRef]
- Mota, A.; Buffle, J.; Kounaves, S.; Goncalves, M. The Importance of Concentration Effects at the Electrode Surface in Anodic-Stripping Voltammetric Measurements of Complexation of Metal-Ions at Natural-Water Concentrations. Anal. Chim. Acta 1985, 172, 13–30. [Google Scholar] [CrossRef]
- Capelo, S.; Mota, A.; Goncalves, M. Complexation of Lead with Humic Matter by Stripping Voltammetry—Prevention of Adsorption on Nafion-Coated Mercury Film Electrode. Electroanalysis 1995, 7, 563–568. [Google Scholar] [CrossRef]
- Wang, J.; Taha, Z. Poly(Ester-Sulfonic Acid)-Coated Mercury Film Electrodes for Anodic-Stripping Voltammetry. Electroanalysis 1990, 2, 383–387. [Google Scholar] [CrossRef]
- Rocha, L.S.; Pinheiro, J.P.; Carapuca, H.M. Ion-Exchange Voltammetry with Nafion/Poly(Sodium 4-Styrenesulfonate) Mixed Coatings on Mercury Film Electrodes: Characterization Studies and Application to the Determination of Trace Metals. Langmuir 2006, 22, 8241–8247. [Google Scholar] [CrossRef] [PubMed]
- Florence, T.; Mann, K. Anodic-Stripping Voltammetry with Medium Exchange in Trace-Element Speciation. Anal. Chim. Acta 1987, 200, 305–312. [Google Scholar] [CrossRef]
- Florence, T. Trace-Element Speciation by Anodic-Stripping Voltammetry. Analyst 1992, 117, 551–553. [Google Scholar] [CrossRef]
- Tercier, M.L.; Buffle, J. Antifouling Membrane-Covered Voltammetric Microsensor for in Situ Measurements in Natural Waters. Anal. Chem. 1996, 68, 3670–3678. [Google Scholar] [CrossRef]
- Belmont-Hebert, C.; Tercier, M.L.; Buffle, J.; Fiaccabrino, G.C.; de Rooij, N.F.; Koudelka-Hep, M. Gel-Integrated Microelectrode Arrays for Direct Voltammetric Measurements of Heavy Metals in Natural Waters and Other Complex Media. Anal. Chem. 1998, 70, 2949–2956. [Google Scholar] [CrossRef]
- Vega, M.; Pardo, R.; Herguedas, M.M.; Barrado, E.; Castrillejo, Y. Pseudopolarographic Determination of Stability Constants of Labile Zinc Complexes in Fresh Water. Anal. Chim. Acta 1995, 310, 131–138. [Google Scholar] [CrossRef]
- Tsang, J.J.; Rozan, T.F.; Hsu-Kim, H.; Mullaugh, K.M.; Luther, G.W. Pseudopolarographic Determination of Cd2+ Complexation in Freshwater. Environ. Sci. Technol. 2006, 40, 5388–5394. [Google Scholar] [CrossRef]
- Tercier-Waeber, M.-L.; Buffle, J. Submersible Online Oxygen Removal System Coupled to an in Situ Voltammetric Probe for Trace Element Monitoring in Freshwater. Environ. Sci. Technol. 2000, 34, 4018–4024. [Google Scholar] [CrossRef]
- Town, R.M.; van Leeuwen, H.P. Fundamental Features of Metal Ion Determination by Stripping Chronopotentiometry. J. Electroanal. Chem. 2001, 509, 58–65. [Google Scholar] [CrossRef]
- van Leeuwen, H.P.; Town, R.M. Stripping Chronopotentiometry at Scanned Deposition Potential (SSCP). Part 1. Fundamental Features. J. Electroanal. Chem. 2002, 536, 129–140. [Google Scholar] [CrossRef]
- Galceran, J.; Companys, E.; Puy, J.; Cecilia, J.; Garces, J.L. AGNES: A New Electroanalytical Technique for Measuring Free Metal Ion Concentration. J. Electroanal. Chem. 2004, 566, 95–109. [Google Scholar] [CrossRef]
- Jagner, D. Potentiometric Stripping Analysis—A Review. Analyst 1982, 107, 593–599. [Google Scholar] [CrossRef]
- Town, R.M.; van Leeuwen, H.P. Effects of Adsorption in Stripping Chronopotentiometric Metal Speciation Analysis. J. Electroanal. Chem. 2002, 523, 1–15. [Google Scholar] [CrossRef]
- Town, R.M.; van Leeuwen, H.P. Depletive Stripping Chronopotentiometry: A Major Step Forward in Electrochemical Stripping Techniques for Metal Ion Speciation Analysis. Electroanalysis 2004, 16, 458–471. [Google Scholar] [CrossRef]
- Town, R.M.; van Leeuwen, H.P. Significance of Wave Form Parameters in Stripping Chronopotentiometric Metal Speciation Analysis. J. Electroanal. Chem. 2002, 535, 11–25. [Google Scholar] [CrossRef]
- Deford, D.; Hume, D. The Determination of Consecutive Formation Constants of Complex Ions from Polarographic Data. J. Am. Chem. Soc. 1951, 73, 5321–5322. [Google Scholar] [CrossRef]
- Town, R.M.; van Leeuwen, H.P. Stripping Chronopotentiometry at Scanned Deposition Potential (SSCP)—Part 2. Determination of Metal Ion Speciation Parameters. J. Electroanal. Chem. 2003, 541, 51–65. [Google Scholar] [CrossRef]
- Pinheiro, J.P.; van Leeuwen, H.P. Scanned Stripping Chronopotentiometry of Metal Complexes: Lability Diagnosis and Stability Computation. J. Electroanal. Chem. 2004, 570, 69–75. [Google Scholar] [CrossRef]
- Serrano, N.; Díaz-Cruz, J.M.; Ariño, C.; Esteban, M.; Puy, J.; Companys, E.; Galceran, J.; Cecilia, J. Full-Wave Analysis of Stripping Chronopotentiograms at Scanned Deposition Potential (SSCP) as a Tool for Heavy Metal Speciation: Theoretical Development and Application to Cd(II)-Phthalate and Cd(II)-Iodide Systems. J. Electroanal. Chem. 2007, 600, 275–284. [Google Scholar] [CrossRef]
- Rocha, L.S.; Pinheiro, J.P.; Carapuca, H.M. Evaluation of Nanometer Thick Mercury Film Electrodes for Stripping Chronopotentiometry. J. Electroanal. Chem. 2007, 610, 37–45. [Google Scholar] [CrossRef]
- Parat, C.; Schneider, A.; Castetbon, A.; Potin-Gautier, M. Determination of Trace Metal Speciation Parameters by Using Screen-Printed Electrodes in Stripping Chronopotentiometry without Deaerating. Anal. Chim. Acta 2011, 688, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Rocha, L.S.; Pereira, E.; Duarte, A.C.; Pinheiro, J.P. Performance of Ex Situ Bismuth Film Rotating Disk Electrode in Trace Metal Analysis by Stripping Chronopotentiometry: Definition of the Depletion Regime and Optimization of Experimental Parameters. Electroanalysis 2011, 23, 1891–1900. [Google Scholar] [CrossRef]
- Pinheiro, J.P.; Rocha, L.S.; Goveia, D.; Town, R.M. Scanned Stripping Chronopotentiometry at Bismuth Film Rotating Disc Electrodes: A Method for Quantitative Dynamic Metal Speciation. Environ. Chem. 2014, 11, 150–157. [Google Scholar] [CrossRef]
- do Nascimento, F.H.; Masini, J.C. Sequential Injection Assisted Stripping Chronopotentiometry at Screen Printed Gold Electrodes for Determination of Hg(II) in Adsorption Studies. Anal. Lett. 2016, 49, 699–710. [Google Scholar] [CrossRef]
- Domingos, R.F.; Huidobro, C.; Companys, E.; Galceran, J.; Puy, J.; Pinheiro, J.P. Comparison of AGNES (Absence of Gradients and Nernstian Equilibrium Stripping) and SSCP (Scanned Stripping Chronopotentiometry) for Trace Metal Speciation Analysis. J. Electroanal. Chem. 2008, 617, 141–148. [Google Scholar] [CrossRef]
- Parat, C.; Authier, L.; Aguilar, D.; Companys, E.; Puy, J.; Galceran, J.; Potin-Gautier, M. Direct Determination of Free Metal Concentration by Implementing Stripping Chronopotentiometry as the Second Stage of AGNES. Analyst 2011, 136, 4337–4343. [Google Scholar] [CrossRef]
- Galceran, J.; Lao, M.; David, C.; Companys, E.; Rey-Castro, C.; Salvador, J.; Puy, J. The Impact of Electrodic Adsorption on Zn, Cd and Pb Speciation Measurements with AGNES. J. Electroanal. Chem. 2014, 722–723, 110–118. [Google Scholar] [CrossRef]
- Rocha, L.S.; Companys, E.; Galceran, J.; Carapuca, H.M.; Pinheiro, J.P. Evaluation of Thin Mercury Film Rotating Disk Electrode to Perform Absence of Gradients and Nernstian Equilibrium Stripping (AGNES) Measurements. Talanta 2010, 80, 1881–1887. [Google Scholar] [CrossRef] [PubMed]
- Rocha, L.S.; Galceran, J.; Puy, J.; Pinheiro, J.P. Determination of the Free Metal Ion Concentration Using AGNES Implemented with Environmentally Friendly Bismuth Film Electrodes. Anal. Chem. 2015, 87, 6071–6078. [Google Scholar] [CrossRef] [PubMed]
- Domingos, R.F.; Carreira, S.; Galceran, J.; Salaun, P.; Pinheiro, J.P. AGNES at Vibrated Gold Microwire Electrode for the Direct Quantification of Free Copper Concentrations. Anal. Chim. Acta 2016, 920, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Sigg, L.; Black, F.; Buffle, J.; Cao, J.; Cleven, R.; Davison, W.; Galceran, J.; Gunkel, P.; Kalis, E.; Kistler, D.; et al. Comparison of Analytical Techniques for Dynamic Trace Metal Speciation in Natural Freshwaters. Environ. Sci. Technol. 2006, 40, 1934–1941. [Google Scholar] [CrossRef]
- Pesavento, M.; Alberti, G.; Biesuz, R. Analytical Methods for Determination of Free Metal Ion Concentration, Labile Species Fraction and Metal Complexation Capacity of Environmental Waters: A Review. Anal. Chim. Acta 2009, 631, 129–141. [Google Scholar] [CrossRef]
- Companys, E.; Galceran, J.; Pinheiro, J.P.; Puy, J.; Salaun, P. A Review on Electrochemical Methods for Trace Metal Speciation in Environmental Media. Curr. Opin. Electrochem. 2017, 3, 144–162. [Google Scholar] [CrossRef]
- Illuminati, S.; Annibaldi, A.; Truzzi, C.; Tercier-Waeber, M.-L.; Noel, S.; Braungardt, C.B.; Achterberg, E.P.; Howell, K.A.; Turner, D.; Marini, M.; et al. In-Situ Trace Metal (Cd, Pb, Cu) Speciation along the Po River Plume (Northern Adriatic Sea) Using Submersible Systems. Mar. Chem. 2019, 212, 47–63. [Google Scholar] [CrossRef]
- Tercier-Waeber, M.-L.; Abdou, M.; Fighera, M.; Kowal, J.; Bakker, E.; van der Wal, P. In Situ Voltammetric Sensor of Potentially Bioavailable Inorganic Mercury in Marine Aquatic Systems Based on Gel-Integrated Nanostructured Gold-Based Microelectrode Arrays. ACS Sens. 2021, 6, 925–937. [Google Scholar] [CrossRef]
- Tercier-Waeber, M.-L.; Fighera, M.; Abdou, M.; Bakker, E.; van der Wal, P. Newly Designed Gel-Integrated Nanostructured Gold-Based Interconnected Microelectrode Arrays for Continuous in Situ Arsenite Monitoring in Aquatic Systems. Sens. Actuator B-Chem. 2021, 328, 128996. [Google Scholar] [CrossRef]
- Layglon, N.; Abdou, M.; Massa, F.; Castellano, M.; Bakker, E.; Povero, P.; Tercier-Waeber, M.-L. Speciation of Cu, Cd, Pb and Zn in a Contaminated Harbor and Comparison to Environmental Quality Standards. J. Environ. Manag. 2022, 317, 115375. [Google Scholar] [CrossRef]
- Abdou, M.; Tercier-Waeber, M.-L.; Dutruch, L.; Bossy, C.; Pougnet, F.; Coynel, A.; Bakker, E.; Blanc, G.; Schafer, J. Estuarine Dissolved Speciation and Partitioning of Trace Metals: A Novel Approach to Study Biogeochemical Processes. Environ. Res. 2022, 208, 112596. [Google Scholar] [CrossRef] [PubMed]
- Abdou, M.; Tercier-Waeber, M.-L. New Insights into Trace Metal Speciation and Interaction with Phytoplankton in Estuarine Coastal Waters. Mar. Pollut. Bull. 2022, 181, 113845. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.-L.; Qi, G.-M.; Jiang, T.-J.; Guo, Z.; Yu, D.-Y.; Liu, J.-H.; Huang, X.-J. A Simplified Electrochemical Instrument Equipped with Automated Flow-Injection System and Network Communication Technology for Remote Online Monitoring of Heavy Metal Ions. J. Electroanal. Chem. 2017, 791, 49–55. [Google Scholar] [CrossRef]
- De Vito-Francesco, E.; Farinelli, A.; Yang, Q.; Nagar, B.; Alvarez, R.; Merkoci, A.; Knutz, T.; Haider, A.; Stach, W.; Ziegenbalg, F.; et al. An Innovative Autonomous Robotic System for On-Site Detection of Heavy Metal Pollution Plumes in Surface Water. Environ. Monit. Assess. 2022, 194, 122. [Google Scholar] [CrossRef]
- Hackel, L.; Rotureau, E.; Morrin, A.; Pinheiro, J.P. Developing On-Site Trace Level Speciation of Lead, Cadmium and Zinc by Stripping Chronopotentiometry (SCP): Fast Screening and Quantification of Total Metal Concentrations. Molecules 2021, 26, 5502. [Google Scholar] [CrossRef] [PubMed]
- Rotureau, E.; Gajdar, J.; Herzog, G.; Waldvogel, Y.; Pinheiro, J.-P.; Etienne, M. Electroanalytical Metal Sensor with Built-in Oxygen Filter. Anal. Chim. Acta 2021, 1167, 338544. [Google Scholar] [CrossRef] [PubMed]
- Le, T.X.H.; Etienne, M.; Lapicque, F.; Hehn, A.; Vilà, N.; Walcarius, A. Local Removal of Oxygen for NAD(P)+ Detection in Aerated Solutions. Electrochim. Acta 2020, 353, 136546. [Google Scholar] [CrossRef]
- Rosales-Segovia, K.; Sans-Duno, J.; Companys, E.; Puy, J.; Alcalde, B.; Antico, E.; Fontas, C.; Galceran, J. Effective Concentration Signature of Zn in a Natural Water Derived from Various Speciation Techniques. Sci. Total Environ. 2022, 806, 151201. [Google Scholar] [CrossRef]
- Chen, W.; Gueguen, C.; Smith, D.S.; Galceran, J.; Puy, J.; Companys, E. Metal (Pb, Cd, and Zn) Binding to Diverse Organic Matter Samples and Implications for Speciation Modeling. Environ. Sci. Technol. 2018, 52, 4163–4172. [Google Scholar] [CrossRef]
- Pinheiro, J.P.; Galceran, J.; Rotureau, E.; Companys, E.; Puy, J. Full Wave Analysis of Stripping Chronopotentiometry at Scanned Deposition Potential (SSCP): Obtaining Binding Curves in Labile Heterogeneous Macromolecular Systems for Any Metal-to-Ligand Ratio. J. Electroanal. Chem. 2020, 873, 114436. [Google Scholar] [CrossRef]
- Rotureau, E.; Rocha, L.S.; Goveia, D.; Alves, N.G.; Pinheiro, J.P. Investigating the Binding Heterogeneity of Trace Metal Cations With SiO2 Nanoparticles Using Full Wave Analysis of Stripping Chronopotentiometry at Scanned Deposition Potential. Front. Chem. 2020, 8, 614574. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, M.H.; Companys, E.; Dago, A.; Puy, J.; Galceran, J. Free Indium Concentration Determined with AGNES. Sci. Total Environ. 2018, 612, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, M.H.; Companys, E.; Dago, A.; Puy, J.; Galceran, J. New Methodology to Measure Low Free Indium (III) Concentrations Based on the Determination of the Lability Degree of Indium Complexes. Assessment of In(OH)3 Solubility Product. J. Electroanal. Chem. 2019, 847, 113185. [Google Scholar] [CrossRef]
- Rotureau, E.; Pla-Vilanova, P.; Galceran, J.; Companys, E.; Pinheiro, J.P. Towards Improving the Electroanalytical Speciation Analysis of Indium. Anal. Chim. Acta 2019, 1052, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Rotureau, E.; Pinheiro, J.P.; Duval, J.F.L. On the Evaluation of the Intrinsic Stability of Indium-Nanoparticulate Organic Matter Complexes. Colloid Surf. A-Physicochem. Eng. Asp. 2022, 645, 128859. [Google Scholar] [CrossRef]
- Pla-Vilanova, P.; Galceran, J.; Puy, J.; Companys, E.; Filella, M. Antimony Speciation in Aqueous Solution Followed with AGNES. J. Electroanal. Chem. 2019, 849, 113334. [Google Scholar] [CrossRef]
- Janot, N.; Groenenberg, J.E.; Otero-Farina, A.; Pinheiro, J.P. Free Eu(III) Determination by Donnan Membrane Technique with Electrochemical Detection: Implementation and Evaluation. Aquat. Geochem. 2021, 27, 127–140. [Google Scholar] [CrossRef]
- Borrill, A.J.; Reily, N.E.; Macpherson, J.V. Addressing the Practicalities of Anodic Stripping Voltammetry for Heavy Metal Detection: A Tutorial Review. Analyst 2019, 144, 6834–6849. [Google Scholar] [CrossRef]
- Town, R.M.; van Leeuwen, H.P. Stripping Chronopotentiometry at Scanned Deposition Potential (SSCP): An Effective Methodology for Dynamic Speciation Analysis of Nanoparticulate Metal Complexes. J. Electroanal. Chem. 2019, 853, 113530. [Google Scholar] [CrossRef]
- Lopez-Solis, L.; Galceran, J.; Puy, J.; Companys, E. Absence of Gradients and Nernstian Equilibrium Stripping (AGNES): An Electroanalytical Technique for Chemical Speciation: A Tutorial Review. Chemosensors 2022, 10, 351. [Google Scholar] [CrossRef]
- Van Riemsdijk, W.H.; Koopal, L.K.; Kinniburgh, D.G.; Benedetti, M.F.; Weng, L. Modeling the Interactions between Humics, Ions, and Mineral Surfaces. Environ. Sci. Technol. 2006, 40, 7473–7480. [Google Scholar] [CrossRef] [PubMed]
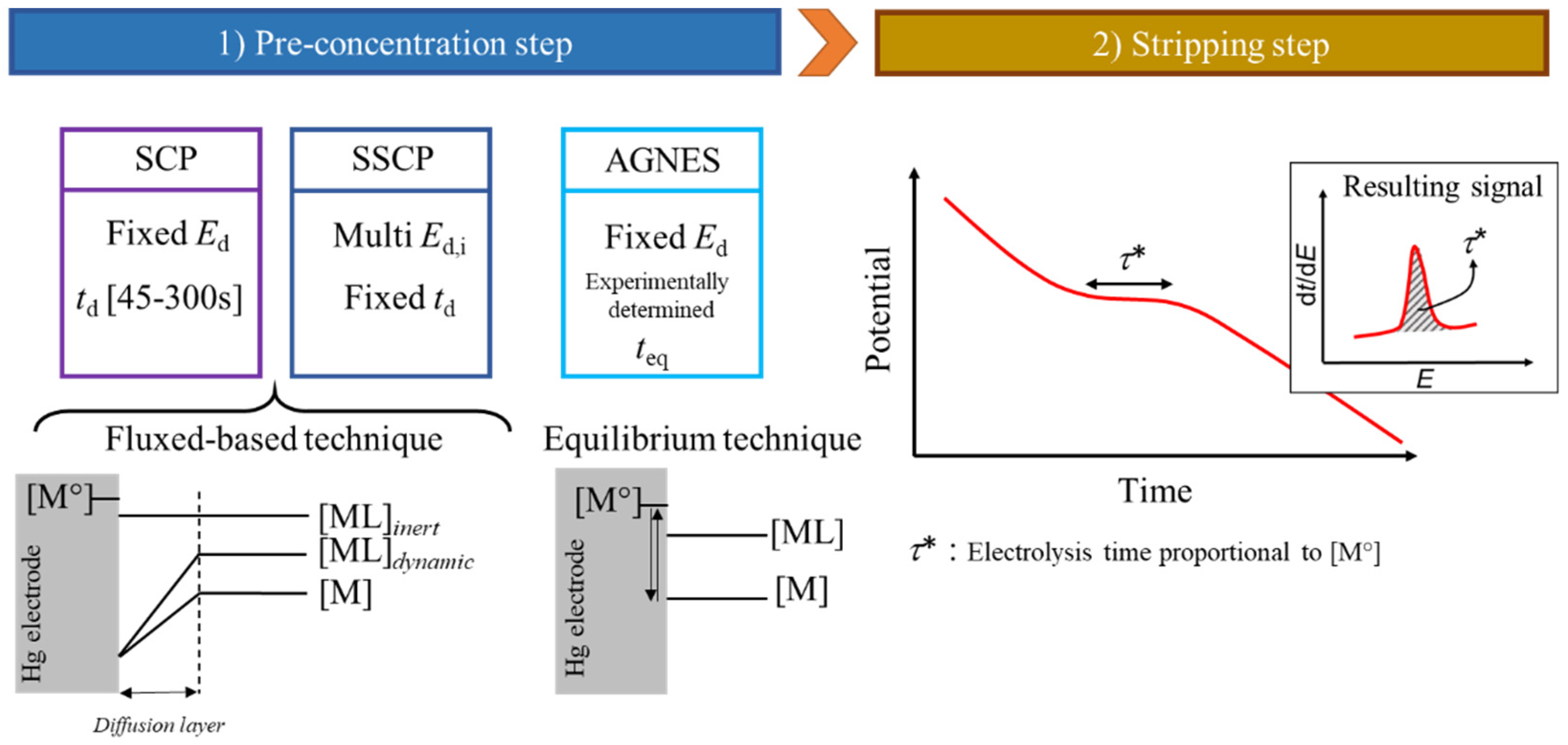
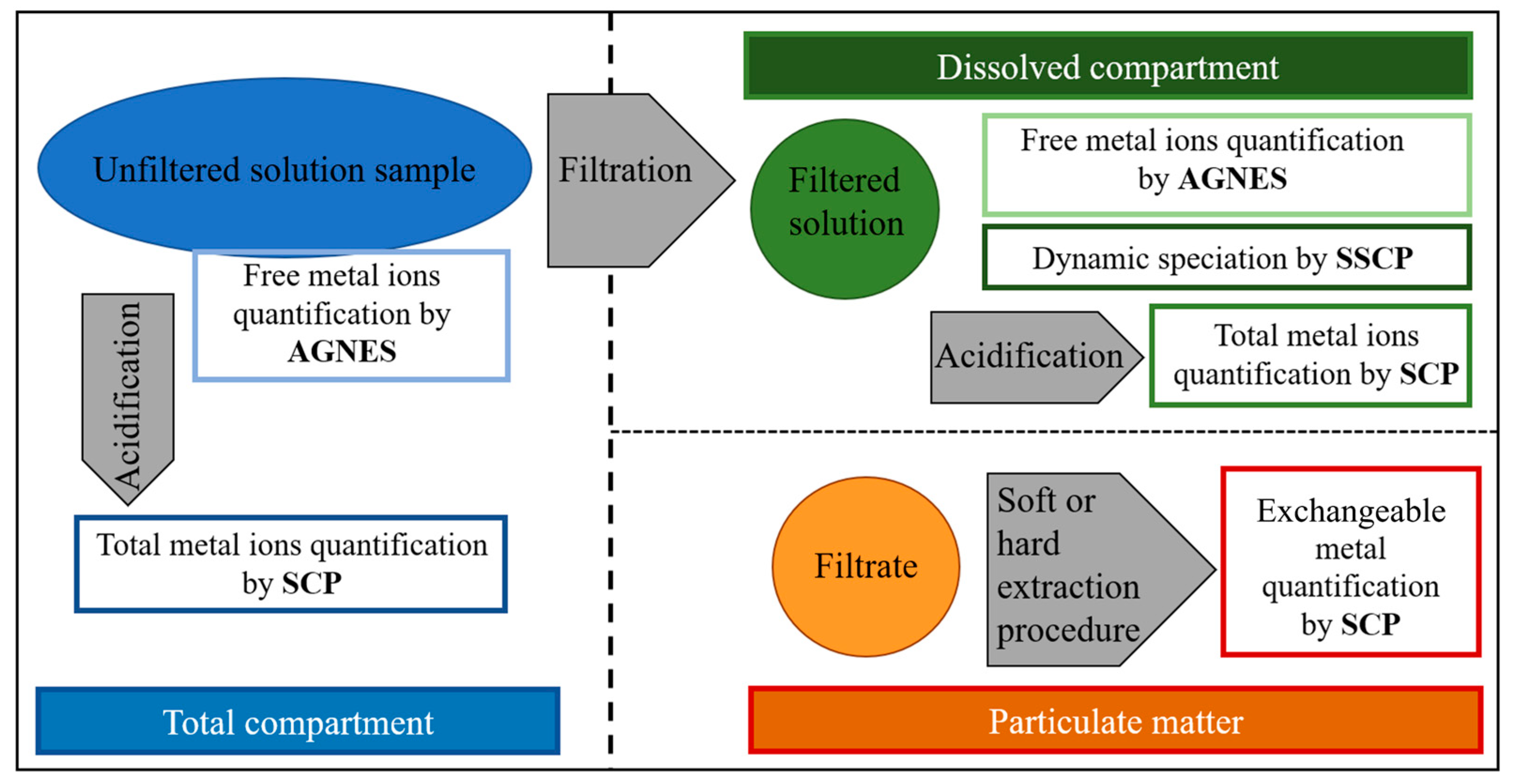
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinheiro, J.P.; Rotureau, E. Electroanalytical Trace Metal Cations Quantification and Speciation in Freshwaters: Historical Overview, Critical Review of the Last Five Years and Road Map for Developing Dynamic Speciation Field Measurements. Molecules 2023, 28, 2831. https://doi.org/10.3390/molecules28062831
Pinheiro JP, Rotureau E. Electroanalytical Trace Metal Cations Quantification and Speciation in Freshwaters: Historical Overview, Critical Review of the Last Five Years and Road Map for Developing Dynamic Speciation Field Measurements. Molecules. 2023; 28(6):2831. https://doi.org/10.3390/molecules28062831
Chicago/Turabian StylePinheiro, José Paulo, and Elise Rotureau. 2023. "Electroanalytical Trace Metal Cations Quantification and Speciation in Freshwaters: Historical Overview, Critical Review of the Last Five Years and Road Map for Developing Dynamic Speciation Field Measurements" Molecules 28, no. 6: 2831. https://doi.org/10.3390/molecules28062831
APA StylePinheiro, J. P., & Rotureau, E. (2023). Electroanalytical Trace Metal Cations Quantification and Speciation in Freshwaters: Historical Overview, Critical Review of the Last Five Years and Road Map for Developing Dynamic Speciation Field Measurements. Molecules, 28(6), 2831. https://doi.org/10.3390/molecules28062831





